The Pivotal Role of Macrophages in the Pathogenesis of Pancreatic Diseases
Abstract
1. Introduction
2. Methodology
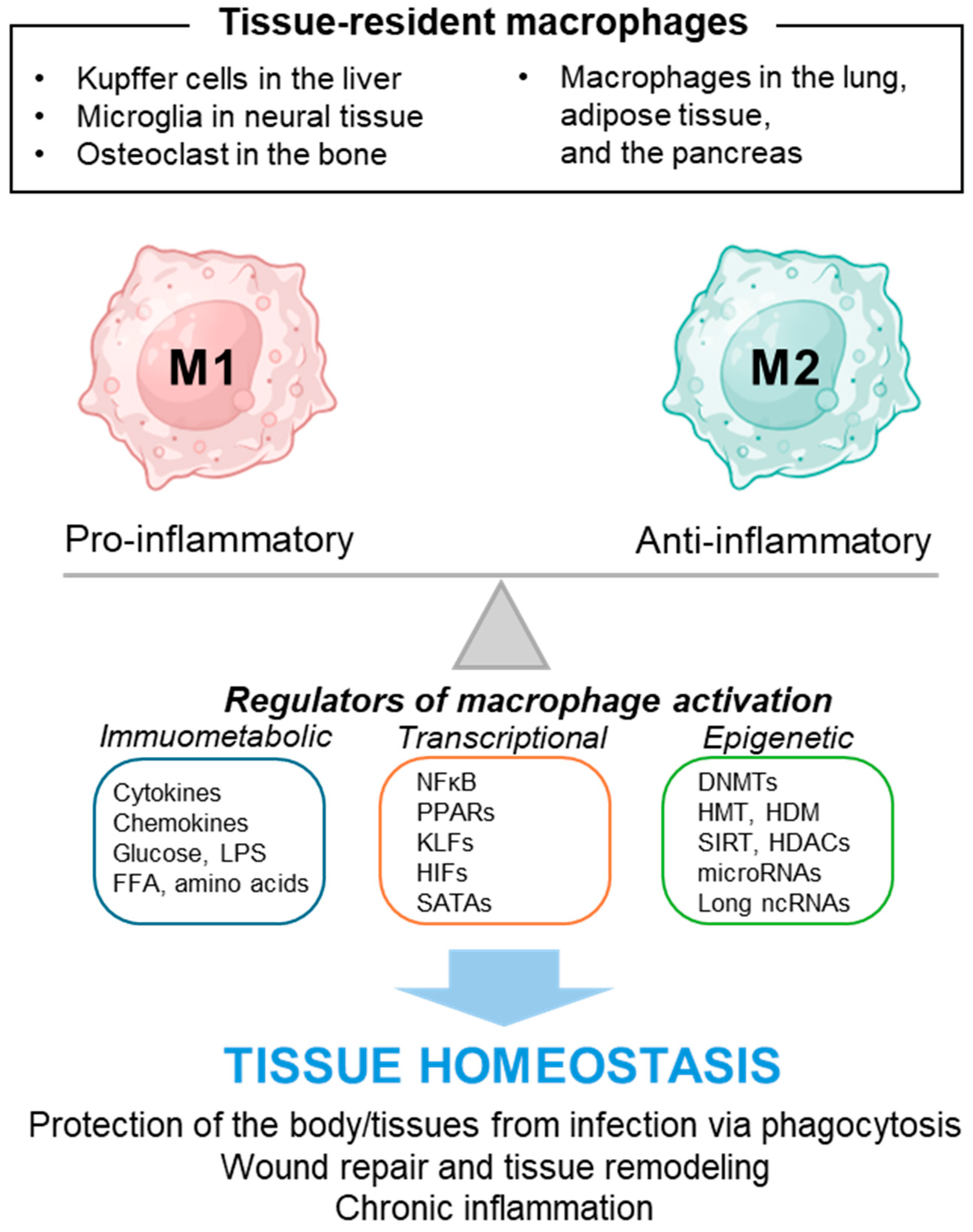
3. Macrophage and Its Polarity
4. Macrophage Activation in Pancreatitis and Its Regulators
4.1. Factors Regulating Macrophage Polarization in AP
4.2. Factors Regulating Macrophage Polarization in CP
5. Macrophage Activation in Diabetes and Its Regulators
6. Macrophage Polarization in PDAC and Its Regulators
7. Discussion
8. Conclusions
Funding
Conflicts of Interest
References
- Leung, P.S. Physiology of the pancreas. Adv. Exp. Med. Biol. 2010, 690, 13–27. [Google Scholar] [PubMed]
- Karpinska, M.; Czauderna, M. Pancreas-Its Functions, Disorders, and Physiological Impact on the Mammals’ Organism. Front. Physiol. 2022, 13, 807632. [Google Scholar] [CrossRef] [PubMed]
- Roder, P.V.; Wu, B.; Liu, Y.; Han, W. Pancreatic regulation of glucose homeostasis. Exp. Mol. Med. 2016, 48, e219. [Google Scholar] [CrossRef] [PubMed]
- Overton, D.L.; Mastracci, T.L. Exocrine-Endocrine Crosstalk: The Influence of Pancreatic Cellular Communications on Organ Growth, Function and Disease. Front. Endocrinol. 2022, 13, 904004. [Google Scholar] [CrossRef] [PubMed]
- Czako, L.; Hegyi, P.; Rakonczay, Z., Jr.; Wittmann, T.; Otsuki, M. Interactions between the endocrine and exocrine pancreas and their clinical relevance. Pancreatology 2009, 9, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Rickels, M.R.; Norris, A.W.; Hull, R.L. A tale of two pancreases: Exocrine pathology and endocrine dysfunction. Diabetologia 2020, 63, 2030–2039. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wang, Z.; Han, T.; Chen, J.; Ou, Y.; Wei, L.; Zhu, X.; Wang, K.; Yan, Z.; Han, Y.P.; et al. Extracellular vesicle-mediated intercellular and interorgan crosstalk of pancreatic islet in health and diabetes. Front. Endocrinol. 2023, 14, 1170237. [Google Scholar] [CrossRef] [PubMed]
- Andersen, D.K.; Korc, M.; Petersen, G.M.; Eibl, G.; Li, D.; Rickels, M.R.; Chari, S.T.; Abbruzzese, J.L. Diabetes, Pancreatogenic Diabetes, and Pancreatic Cancer. Diabetes 2017, 66, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, S.; Dirice, E.; Basile, G.; Diegisser, D.; Alam, J.; Johansson, B.B.; Gupta, M.K.; Hu, J.; Huang, L.; Soh, C.L.; et al. Abnormal exocrine-endocrine cell cross-talk promotes beta-cell dysfunction and loss in MODY8. Nat. Metab. 2022, 4, 76–89. [Google Scholar] [CrossRef]
- Niedzwiedzka-Rystwej, P.; Wolacewicz, M.; Cywoniuk, P.; Klak, M.; Wszola, M. Crosstalk Between Immunity System Cells and Pancreas. Transformation of Stem Cells Used in the 3D Bioprinting Process as a Personalized Treatment Method for Type 1 Diabetes. Arch. Immunol. Ther. Exp. 2020, 68, 13. [Google Scholar] [CrossRef]
- Ying, W.; Fu, W.; Lee, Y.S.; Olefsky, J.M. The role of macrophages in obesity-associated islet inflammation and beta-cell abnormalities. Nat. Rev. Endocrinol. 2020, 16, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.L. Minireview: Emerging Concepts in Islet Macrophage Biology in Type 2 Diabetes. Mol. Endocrinol. 2015, 29, 946–962. [Google Scholar] [CrossRef] [PubMed]
- Storz, P. Roles of differently polarized macrophages in the initiation and progressionof pancreatic cancer. Front. Immunol. 2023, 14, 1237711. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Pacher, P.; Hasko, G. Role of Macrophages in the Endocrine System. Trends Endocrinol. Metab. 2021, 32, 238–256. [Google Scholar] [CrossRef] [PubMed]
- Lehuen, A.; Diana, J.; Zaccone, P.; Cooke, A. Immune cell crosstalk in type 1 diabetes. Nat. Rev. Immunol. 2010, 10, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Jung, S. Monocytes and macrophages: Developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 2014, 14, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Saeed, A.; Liu, Q.; Jiang, Q.; Xu, H.; Xiao, G.G.; Rao, L.; Duo, Y. Macrophages in immunoregulation and therapeutics. Signal Transduct. Target. Ther. 2023, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Liu, J.; Geng, X.; Hou, J.; Wu, G. New insights into M1/M2 macrophages: Key modulators in cancer progression. Cancer Cell Int. 2021, 21, 389. [Google Scholar] [CrossRef]
- Varol, C.; Mildner, A.; Jung, S. Macrophages: Development and tissue specialization. Annu. Rev. Immunol. 2015, 33, 643–675. [Google Scholar] [CrossRef]
- Italiani, P.; Boraschi, D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front. Immunol. 2014, 5, 514. [Google Scholar] [CrossRef]
- Roszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediat. Inflamm. 2015, 2015, 816460. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, A.L.; Costello, S.M.; Schiller, S.M.; Tripet, B.P.; Copie, V. Primary Human M2 Macrophage Subtypes Are Distinguishable by Aqueous Metabolite Profiles. Int. J. Mol. Sci. 2024, 25, 2407. [Google Scholar] [CrossRef] [PubMed]
- Meshkani, R.; Vakili, S. Tissue resident macrophages: Key players in the pathogenesis of type 2 diabetes and its complications. Clin. Chim. Acta 2016, 462, 77–89. [Google Scholar] [CrossRef]
- Gordon, S.; Pluddemann, A.; Martinez Estrada, F. Macrophage heterogeneity in tissues: Phenotypic diversity and functions. Immunol. Rev. 2014, 262, 36–55. [Google Scholar] [CrossRef] [PubMed]
- Mederos, M.A.; Reber, H.A.; Girgis, M.D. Acute Pancreatitis: A Review. JAMA 2021, 325, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Lou, N.; Jiao, J.; Guo, F.; Xiang, H.; Shang, D. Macrophages in pancreatitis: Mechanisms and therapeutic potential. Biomed. Pharmacother. 2020, 131, 110693. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Chari, S.T. Chronic pancreatitis. Lancet 2016, 387, 1957–1966. [Google Scholar] [CrossRef]
- Boxhoorn, L.; Voermans, R.P.; Bouwense, S.A.; Bruno, M.J.; Verdonk, R.C.; Boermeester, M.A.; van Santvoort, H.C.; Besselink, M.G. Acute pancreatitis. Lancet 2020, 396, 726–734. [Google Scholar] [CrossRef]
- Hyun, J.J.; Lee, H.S. Experimental models of pancreatitis. Clin. Endosc. 2014, 47, 212–216. [Google Scholar] [CrossRef]
- Peng, C.; Tu, G.; Wang, J.; Wang, Y.; Wu, P.; Yu, L.; Li, Z.; Yu, X. MLKL signaling regulates macrophage polarization in acute pancreatitis through CXCL10. Cell Death Dis. 2023, 14, 155. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Udari, L.M.; RiveraHernandez, P.; Kwon, J.J.; Willis, B.; Easler, J.J.; Fogel, E.L.; Pandol, S.; Kota, J. Loss of miR-29a/b1 promotes inflammation and fibrosis in acute pancreatitis. JCI Insight 2021, 6, e149539. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Biao, C.; Zhao, Y.Y.; Jun, L.C.; Wei, W.; YLNYZ, A.B.L.Z.; Song, L. Long non-coding RNA MM2P suppresses M1-polarized macrophages-mediated excessive inflammation to prevent sodium taurocholate-induced acute pancreatitis by blocking SHP2-mediated STAT3 dephosphorylation. Clin. Exp. Med. 2023, 23, 3589–3603. [Google Scholar] [CrossRef] [PubMed]
- Aufenanger, J.; Samman, M.; Quintel, M.; Fassbender, K.; Zimmer, W.; Bertsch, T. Pancreatic phospholipase A2 activity in acute pancreatitis: A prognostic marker for early identification of patients at risk. Clin. Chem. Lab. Med. 2002, 40, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Ashley, J.W.; Hancock, W.D.; Nelson, A.J.; Bone, R.N.; Tse, H.M.; Wohltmann, M.; Turk, J.; Ramanadham, S. Polarization of Macrophages toward M2 Phenotype Is Favored by Reduction in iPLA2beta (Group VIA Phospholipase A2). J. Biol. Chem. 2016, 291, 23268–23281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhou, J.; Zhou, J.; Fang, R.H.; Gao, W.; Zhang, L. Lure-and-kill macrophage nanoparticles alleviate the severity of experimental acute pancreatitis. Nat. Commun. 2021, 12, 4136. [Google Scholar] [CrossRef]
- Han, X.; Li, B.; Ye, X.; Mulatibieke, T.; Wu, J.; Dai, J.; Wu, D.; Ni, J.; Zhang, R.; Xue, J.; et al. Dopamine D(2) receptor signalling controls inflammation in acute pancreatitis via a PP2A-dependent Akt/NF-kappaB signalling pathway. Br. J. Pharmacol. 2017, 174, 4751–4770. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Ni, J.; Wu, Z.; Wu, J.; Li, B.; Ye, X.; Dai, J.; Chen, C.; Xue, J.; Wan, R.; et al. Myeloid-specific dopamine D(2) receptor signalling controls inflammation in acute pancreatitis via inhibiting M1 macrophage. Br. J. Pharmacol. 2020, 177, 2991–3008. [Google Scholar] [CrossRef]
- Duan, F.; Wang, X.; Wang, H.; Wang, Y.; Zhang, Y.; Chen, J.; Zhu, X.; Chen, B. GDF11 ameliorates severe acute pancreatitis through modulating macrophage M1 and M2 polarization by targeting the TGFbetaR1/SMAD-2 pathway. Int. Immunopharmacol. 2022, 108, 108777. [Google Scholar] [CrossRef]
- Zhou, L.; Yu, J.; Wang, S.; Ma, Y.; Liu, X.; Zhang, X.; Luo, Y.; Wen, S.; Li, L.; Li, W.; et al. Tectoridin alleviates caerulein-induced severe acute pancreatitis by targeting ERK2 to promote macrophage M2 polarization. Arch. Biochem. Biophys. 2024, 752, 109873. [Google Scholar] [CrossRef]
- Yuan, C.; Xu, X.; Wang, N.; Zhu, Q.; Zhang, J.; Gong, W.; Ding, Y.; Xiao, W.; Chen, W.; Lu, G.; et al. Paeonol protects against acute pancreatitis by inhibiting M1 macrophage polarization via the NLRP3 inflammasomes pathway. Biochem. Biophys. Res. Commun. 2022, 600, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Cheng, X.; Luo, C.; Yang, S.; Li, S.; Wang, B.; Yuan, X.; Yang, Y.; Wen, Y.; Liu, R.; et al. Placental chorionic plate-derived mesenchymal stem cells ameliorate severe acute pancreatitis by regulating macrophage polarization via secreting TSG-6. Stem Cell Res. Ther. 2021, 12, 337. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Zhang, X.; Zhang, X.; Guan, Y.; He, R.; Xue, E.; Zhang, X.; Deng, W.; Yu, J.; Wang, W.; et al. Inhibition of Notch activity suppresses hyperglycemia-augmented polarization of macrophages to the M1 phenotype and alleviates acute pancreatitis. Clin. Sci. 2022, 136, 455–471. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.S.; Cao, F.; Yan, C.S.; Cui, J.T.; Guo, X.Y.; Cheng, L.; Li, L.; Li, Y.L.; Ma, J.M.; Fang, K.; et al. Acinar Cell-Derived Extracellular Vesicle MiRNA-183-5p Aggravates Acute Pancreatitis by Promoting M1 Macrophage Polarization through Downregulation of FoxO1. Front. Immunol. 2022, 13, 869207. [Google Scholar] [CrossRef] [PubMed]
- Habtezion, A. Inflammation in acute and chronic pancreatitis. Curr. Opin. Gastroenterol. 2015, 31, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Sharma, V.; Hsieh, M.H.; Chawla, A.; Murali, R.; Pandol, S.J.; Habtezion, A. Alternatively activated macrophages promote pancreatic fibrosis in chronic pancreatitis. Nat. Commun. 2015, 6, 7158. [Google Scholar] [CrossRef] [PubMed]
- Glaubitz, J.; Wilden, A.; Golchert, J.; Homuth, G.; Volker, U.; Broker, B.M.; Thiele, T.; Lerch, M.M.; Mayerle, J.; Aghdassi, A.A.; et al. In mouse chronic pancreatitis CD25(+)FOXP3(+) regulatory T cells control pancreatic fibrosis by suppression of the type 2 immune response. Nat. Commun. 2022, 13, 4502. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, Y.; Feng, W.; Hua, R.; Zhang, J.; Huo, Y.; Jiang, H.; Yin, B.; Yang, X. Neddylation pathway alleviates chronic pancreatitis by reducing HIF1alpha-CCL5-dependent macrophage infiltration. Cell Death Dis. 2021, 12, 273. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.L.; Liang, X.S.; Zeng, X.P.; Liu, Y.; Li, Z.S.; Wang, L.J.; Hu, L.H. Pirfenidone alleviates chronic pancreatitis via suppressing the activation of pancreatic stellate cells and the M1 polarization of macrophages. Int. Immunopharmacol. 2024, 130, 111691. [Google Scholar] [CrossRef]
- Wang, L.J.; He, L.; Hao, L.; Guo, H.L.; Zeng, X.P.; Bi, Y.W.; Lu, G.T.; Li, Z.S.; Hu, L.H. Isoliquiritigenin ameliorates caerulein-induced chronic pancreatitis by inhibiting the activation of PSCs and pancreatic infiltration of macrophages. J. Cell. Mol. Med. 2020, 24, 9667–9681. [Google Scholar] [CrossRef]
- Zeng, X.P.; Wang, L.J.; Guo, H.L.; He, L.; Bi, Y.W.; Xu, Z.L.; Li, Z.S.; Hu, L.H. Dasatinib ameliorates chronic pancreatitis induced by caerulein via anti-fibrotic and anti-inflammatory mechanism. Pharmacol. Res. 2019, 147, 104357. [Google Scholar] [CrossRef] [PubMed]
- Bansod, S.; Doijad, N.; Godugu, C. Berberine attenuates severity of chronic pancreatitis and fibrosis via AMPK-mediated inhibition of TGF-beta1/Smad signaling and M2 polarization. Toxicol. Appl. Pharmacol. 2020, 403, 115162. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.D.; Harris-Hayes, M.; Schootman, M. Epidemiology of diabetes and diabetes-related complications. Phys. Ther. 2008, 88, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, C.; Regazzi, R. Crosstalk between Macrophages and Pancreatic beta-Cells in Islet Development, Homeostasis and Disease. Int. J. Mol. Sci. 2021, 22, 1765. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, K.; Nagai, R. Islet inflammation in type 2 diabetes and physiology. J. Clin. Investig. 2017, 127, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Gittes, G.K. Concise Review: New Insights Into the Role of Macrophages in beta-Cell Proliferation. Stem Cells Transl. Med. 2015, 4, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Cerf, M.E. Beta cell dysfunction and insulin resistance. Front. Endocrinol. 2013, 4, 37. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Dalmas, E.; Sauter, N.S.; Boni-Schnetzler, M. Inflammation in obesity and diabetes: Islet dysfunction and therapeutic opportunity. Cell Metab. 2013, 17, 860–872. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Mabhida, S.E.; Ziqubu, K.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Hanser, S.; Basson, A.K.; Pheiffer, C.; Kengne, A.P. Pancreatic beta-cell dysfunction in type 2 diabetes: Implications of inflammation and oxidative stress. World J. Diabetes 2023, 14, 130–146. [Google Scholar] [CrossRef]
- Weitz, J.R.; Makhmutova, M.; Almaca, J.; Stertmann, J.; Aamodt, K.; Brissova, M.; Speier, S.; Rodriguez-Diaz, R.; Caicedo, A. Mouse pancreatic islet macrophages use locally released ATP to monitor beta cell activity. Diabetologia 2018, 61, 182–192. [Google Scholar] [CrossRef]
- Brissova, M.; Aamodt, K.; Brahmachary, P.; Prasad, N.; Hong, J.Y.; Dai, C.; Mellati, M.; Shostak, A.; Poffenberger, G.; Aramandla, R.; et al. Islet microenvironment, modulated by vascular endothelial growth factor-A signaling, promotes beta cell regeneration. Cell Metab. 2014, 19, 498–511. [Google Scholar] [CrossRef] [PubMed]
- Saunders, D.C.; Aamodt, K.I.; Richardson, T.M.; Hopkirk, A.J.; Aramandla, R.; Poffenberger, G.; Jenkins, R.; Flaherty, D.K.; Prasad, N.; Levy, S.E.; et al. Coordinated interactions between endothelial cells and macrophages in the islet microenvironment promote beta cell regeneration. NPJ Regen. Med. 2021, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Mukhuty, A.; Fouzder, C.; Kundu, R. Fetuin-A secretion from beta-cells leads to accumulation of macrophages in islets, aggravates inflammation and impairs insulin secretion. J. Cell Sci. 2021, 134, jcs258507. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Hu, Q.; Xu, X.; Niu, Y.; Chen, Y.; Lu, Y.; Su, Q.; Qin, L. Advanced glycation end products enhance M1 macrophage polarization by activating the MAPK pathway. Biochem. Biophys. Res. Commun. 2020, 525, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Kim, D.S.; Gou, W.; Wang, J.; Wang, P.; Wei, Z.; Liu, B.; Li, Z.; Gou, K.; Wang, H. GRP94 regulates M1 macrophage polarization and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E1004–E1013. [Google Scholar] [CrossRef]
- Yin, Y.; Hao, H.; Cheng, Y.; Zang, L.; Liu, J.; Gao, J.; Xue, J.; Xie, Z.; Zhang, Q.; Han, W.; et al. Human umbilical cord-derived mesenchymal stem cells direct macrophage polarization to alleviate pancreatic islets dysfunction in type 2 diabetic mice. Cell Death Dis. 2018, 9, 760. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Sun, H. Glucagon-like peptide-1 modulates RAW264.7 macrophage polarization by interfering with the JNK/STAT3 signaling pathway. Exp. Ther. Med. 2019, 17, 3573–3579. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Y.; Sheng, H.; Liang, C.; Liu, H.; Moran Guerrero, J.A.; Lu, Z.; Mao, W.; Dai, Z.; Liu, X.; et al. Hyperoside Suppresses Renal Inflammation by Regulating Macrophage Polarization in Mice With Type 2 Diabetes Mellitus. Front. Immunol. 2021, 12, 733808. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Le, X.; Zheng, S.; Zhang, K.; He, J.; Liu, M.; Tu, C.; Rao, W.; Du, H.; Ouyang, Y.; et al. MicroRNA-146a-5p-modified human umbilical cord mesenchymal stem cells enhance protection against diabetic nephropathy in rats through facilitating M2 macrophage polarization. Stem Cell Res. Ther. 2022, 13, 171. [Google Scholar] [CrossRef]
- Yu, T.; Gao, M.; Yang, P.; Liu, D.; Wang, D.; Song, F.; Zhang, X.; Liu, Y. Insulin promotes macrophage phenotype transition through PI3K/Akt and PPAR-gamma signaling during diabetic wound healing. J. Cell Physiol. 2019, 234, 4217–4231. [Google Scholar] [CrossRef]
- Li, S.; Ding, X.; Zhang, H.; Ding, Y.; Tan, Q. IL-25 improves diabetic wound healing through stimulating M2 macrophage polarization and fibroblast activation. Int. Immunopharmacol. 2022, 106, 108605. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Huang, Q.; Li, S.; Meng, F.; Li, X.; Gong, X. miR-330-5p/Tim-3 axis regulates macrophage M2 polarization and insulin resistance in diabetes mice. Mol. Immunol. 2018, 95, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Cao, Y.; Zheng, L.; Wang, T.; Zhao, S.; Chen, J.; Pang, C.; Xia, W.; Xia, Z.; Li, N.; et al. Diabetes exacerbated sepsis-induced intestinal injury by promoting M1 macrophage polarization via miR-3061/Snail1 signaling. Front. Immunol. 2022, 13, 922614. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lv, H. PSTPIP2 alleviates obesity associated adipose tissue inflammation and insulin resistance in diabetes mice through promoting M2 macrophage polarization via activation of PPARgamma. J. Diabetes Complicat. 2023, 37, 108479. [Google Scholar] [CrossRef]
- Sun, X.; Ma, Z.; Zhao, X.; Jin, W.; Zhang, C.; Ma, J.; Qiang, L.; Wang, W.; Deng, Q.; Yang, H.; et al. Three-dimensional bioprinting of multicell-laden scaffolds containing bone morphogenic protein-4 for promoting M2 macrophage polarization and accelerating bone defect repair in diabetes mellitus. Bioact. Mater. 2021, 6, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, Q.; Zhang, H.; Wang, S.; Cui, J.; Hu, Y.; Liu, J.; Li, M.; Zhang, K.; Zhou, F.; et al. M2 macrophage-derived exosomes promote diabetic fracture healing by acting as an immunomodulator. Bioact. Mater. 2023, 28, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Ye, Z.; Li, C.; Li, K.; Zhong, X.; Li, H. Mogroside V Inhibits M1 Polarization and Inflammation of Diabetic Mouse Macrophages via p38 MAPK/NF-Kappab Signaling Pathway. Immunol. Investig. 2024, 1–18. [Google Scholar] [CrossRef]
- He, S.; Zhao, Y.; Wang, G.; Ke, Q.; Wu, N.; Lu, L.; Wu, J.; Sun, S.; Jin, W.; Zhang, W.; et al. 4-Octyl itaconate attenuates glycemic deterioration by regulating macrophage polarization in mouse models of type 1 diabetes. Mol. Med. 2023, 29, 31. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, Y.; Xu, Y.; Wang, W.; Zhuang, S.; Wang, R.; Xiao, W. HIIT Ameliorates Inflammation and Lipid Metabolism by Regulating Macrophage Polarization and Mitochondrial Dynamics in the Liver of Type 2 Diabetes Mellitus Mice. Metabolites 2022, 13, 14. [Google Scholar] [CrossRef]
- Karamitopoulou, E. Tumour microenvironment of pancreatic cancer: Immune landscape is dictated by molecular and histopathological features. Br. J. Cancer 2019, 121, 5–14. [Google Scholar] [CrossRef]
- Truong, L.H.; Pauklin, S. Pancreatic Cancer Microenvironment and Cellular Composition: Current Understandings and Therapeutic Approaches. Cancers 2021, 13, 5028. [Google Scholar] [CrossRef]
- Liu, Q.; Liao, Q.; Zhao, Y. Chemotherapy and tumor microenvironment of pancreatic cancer. Cancer Cell Int. 2017, 17, 68. [Google Scholar] [CrossRef]
- Yang, S.; Liu, Q.; Liao, Q. Tumor-Associated Macrophages in Pancreatic Ductal Adenocarcinoma: Origin, Polarization, Function, and Reprogramming. Front. Cell Dev. Biol. 2020, 8, 607209. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J.; Chen, Z.; Luo, J.; Guo, W.; Sun, L.; Lin, L. Targeting M2-like tumor-associated macrophages is a potential therapeutic approach to overcome antitumor drug resistance. NPJ Precis. Oncol. 2024, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, X.; Song, Q.; Liu, L.; Forbes, E.; Tian, W.; Zhang, Z.; Kang, Y.; Wang, H.; Fleming, J.B.; et al. IGFBP2 promotes tumor progression by inducing alternative polarization of macrophages in pancreatic ductal adenocarcinoma through the STAT3 pathway. Cancer Lett. 2021, 500, 132–146. [Google Scholar] [CrossRef]
- Zhou, J.; Lyu, N.; Wang, Q.; Yang, M.; Kimchi, E.T.; Cheng, K.; Joshi, T.; Tukuli, A.R.; Staveley-O’Carroll, K.F.; Li, G. A novel role of TGFBI in macrophage polarization and macrophage-induced pancreatic cancer growth and therapeutic resistance. Cancer Lett. 2023, 578, 216457. [Google Scholar] [CrossRef]
- Hu, H.; Tu, W.; Chen, Y.; Zhu, M.; Jin, H.; Huang, T.; Zou, Z.; Xia, Q. The combination of PKM2 overexpression and M2 macrophages infiltration confers a poor prognosis for PDAC patients. J. Cancer 2020, 11, 2022–2031. [Google Scholar] [CrossRef]
- Hu, W.M.; Liu, S.Q.; Zhu, K.F.; Li, W.; Yang, Z.J.; Yang, Q.; Zhu, Z.C.; Chang, J. The ALOX5 inhibitor Zileuton regulates tumor-associated macrophage M2 polarization by JAK/STAT and inhibits pancreatic cancer invasion and metastasis. Int. Immunopharmacol. 2023, 121, 110505. [Google Scholar] [CrossRef] [PubMed]
- Layton, T.; Stalens, C.; Gunderson, F.; Goodison, S.; Silletti, S. Syk tyrosine kinase acts as a pancreatic adenocarcinoma tumor suppressor by regulating cellular growth and invasion. Am. J. Pathol. 2009, 175, 2625–2636. [Google Scholar] [CrossRef]
- Rohila, D.; Park, I.H.; Pham, T.V.; Weitz, J.; Hurtado de Mendoza, T.; Madheswaran, S.; Ishfaq, M.; Beaman, C.; Tapia, E.; Sun, S.; et al. Syk Inhibition Reprograms Tumor-Associated Macrophages and Overcomes Gemcitabine-Induced Immunosuppression in Pancreatic Ductal Adenocarcinoma. Cancer Res. 2023, 83, 2675–2689. [Google Scholar] [CrossRef]
- Chang, Y.T.; Peng, H.Y.; Hu, C.M.; Huang, S.C.; Tien, S.C.; Jeng, Y.M. Pancreatic cancer-derived small extracellular vesical Ezrin regulates macrophage polarization and promotes metastasis. Am. J. Cancer Res. 2020, 10, 12–37. [Google Scholar] [CrossRef] [PubMed]
- Dai, E.; Han, L.; Liu, J.; Xie, Y.; Kroemer, G.; Klionsky, D.J.; Zeh, H.J.; Kang, R.; Wang, J.; Tang, D. Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein. Autophagy 2020, 16, 2069–2083. [Google Scholar] [CrossRef] [PubMed]
- Chandrakesan, P.; Panneerselvam, J.; May, R.; Weygant, N.; Qu, D.; Berry, W.R.; Pitts, K.; Stanger, B.Z.; Rao, C.V.; Bronze, M.S.; et al. DCLK1-Isoform2 Alternative Splice Variant Promotes Pancreatic Tumor Immunosuppressive M2-Macrophage Polarization. Mol. Cancer Ther. 2020, 19, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wu, D.; Zhou, S.; Wan, F.; Liu, H.; Xu, X.; Xu, X.; Zhao, Y.; Tang, M. The pancreatic cancer secreted REG4 promotes macrophage polarization to M2 through EGFR/AKT/CREB pathway. Oncol. Rep. 2016, 35, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Deng, W.; Zhang, Y.; Chang, Y.; Shelat, V.G.; Tsuchida, K.; Lino-Silva, L.S.; Wang, Z. NLRP3 activation in tumor-associated macrophages enhances lung metastasis of pancreatic ductal adenocarcinoma. Transl. Lung Cancer Res. 2022, 11, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Menjivar, R.E.; Bonilla, M.E.; Steele, N.G.; Kemp, S.B.; Du, W.; Donahue, K.L.; Brown, K.L.; Carpenter, E.S.; Avritt, F.R.; et al. Notch Signaling Regulates Immunosuppressive Tumor-Associated Macrophage Function in Pancreatic Cancer. Cancer Immunol. Res. 2024, 12, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ge, W.L.; Wang, S.J.; Liu, Y.Y.; Zhang, Z.H.; Hua, Y.; Zhang, X.F.; Zhang, J.J. MiR-548t-5p regulates pancreatic ductal adenocarcinoma metastasis through an IL-33-dependent crosstalk between cancer cells and M2 macrophages. Cell Cycle 2024, 23, 169–187. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Han, Y.; Chen, J.; Liang, X.; Sun, L. MiR-506 Promotes Antitumor Immune Response in Pancreatic Cancer by Reprogramming Tumor-Associated Macrophages toward an M1 Phenotype. Biomedicines 2023, 11, 2874. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Gao, R.; Luo, M.; Li, D.; Guo, L.; Yu, Z.; Xiong, F.; Wei, C.; Wu, B.; Xu, Z.; et al. Exosomal LINC00460/miR-503-5p/ANLN positive feedback loop aggravates pancreatic cancer progression through regulating T cell-mediated cytotoxicity and PD-1 checkpoint. Cancer Cell Int. 2022, 22, 390. [Google Scholar] [CrossRef]
- He, Z.; Wang, J.; Zhu, C.; Xu, J.; Chen, P.; Jiang, X.; Chen, Y.; Jiang, J.; Sun, C. Exosome-derived FGD5-AS1 promotes tumor-associated macrophage M2 polarization-mediated pancreatic cancer cell proliferation and metastasis. Cancer Lett. 2022, 548, 215751. [Google Scholar] [CrossRef]
- Bhoopathi, P.; Kumar, A.; Pradhan, A.K.; Maji, S.; Mannangatti, P.; Windle, J.J.; Subler, M.A.; Zhang, D.; Vudatha, V.; Trevino, J.G.; et al. Cytoplasmic-delivery of polyinosine-polycytidylic acid inhibits pancreatic cancer progression increasing survival by activating Stat1-CCL2-mediated immunity. J. Immunother. Cancer 2023, 11, e007624. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Sun, S.; Zhang, Y.; Xie, F.; Li, S. The role of irreversible electroporation in promoting M1 macrophage polarization via regulating the HMGB1-RAGE-MAPK axis in pancreatic cancer. Oncoimmunology 2021, 10, 1897295. [Google Scholar] [CrossRef] [PubMed]
- Gea-Sorli, S.; Closa, D. Role of macrophages in the progression of acute pancreatitis. World J. Gastrointest. Pharmacol. Ther. 2010, 1, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Yu, H.; Zhou, Q.; Wu, Y.; Ren, J.; Zhao, Z.; Tao, X.; Dong, D. Macrophages: A rising star in immunotherapy for chronic pancreatitis. Pharmacol. Res. 2022, 185, 106508. [Google Scholar] [CrossRef] [PubMed]
- Poh, A.R.; Ernst, M. Tumor-Associated Macrophages in Pancreatic Ductal Adenocarcinoma: Therapeutic Opportunities and Clinical Challenges. Cancers 2021, 13, 2860. [Google Scholar] [CrossRef] [PubMed]
- Kraakman, M.J.; Murphy, A.J.; Jandeleit-Dahm, K.; Kammoun, H.L. Macrophage polarization in obesity and type 2 diabetes: Weighing down our understanding of macrophage function? Front. Immunol. 2014, 5, 470. [Google Scholar] [CrossRef]
- Boicean, A.; Ichim, C.; Todor, S.B.; Anderco, P.; Popa, M.L. The Importance of Microbiota and Fecal Microbiota Transplantation in Pancreatic Disorders. Diagnostics 2024, 14, 861. [Google Scholar] [CrossRef]
- Thomas, R.M.; Jobin, C. Microbiota in pancreatic health and disease: The next frontier in microbiome research. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Schepis, T.; De Lucia, S.S.; Nista, E.C.; Manilla, V.; Pignataro, G.; Ojetti, V.; Piccioni, A.; Gasbarrini, A.; Franceschi, F.; Candelli, M. Microbiota in Pancreatic Diseases: A Review of the Literature. J. Clin. Med. 2021, 10, 5920. [Google Scholar] [CrossRef]
- Boicean, A.; Birlutiu, V.; Ichim, C.; Todor, S.B.; Hasegan, A.; Bacila, C.; Solomon, A.; Cristian, A.; Dura, H. Predictors of Post-ERCP Pancreatitis (P.E.P.) in Choledochal Lithiasis Extraction. J. Pers. Med. 2023, 13, 1356. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as tools and targets in cancer therapy. Nat. Rev. Drug Discov. 2022, 21, 799–820. [Google Scholar] [CrossRef] [PubMed]
- Kuntzel, T.; Bagnard, D. Manipulating Macrophage/Microglia Polarization to Treat Glioblastoma or Multiple Sclerosis. Pharmaceutics 2022, 14, 344. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Kapate, N.; Shields, C.W., IV; Mitragotri, S. Drug delivery to macrophages: A review of targeting drugs and drug carriers to macrophages for inflammatory diseases. Adv. Drug Deliv. Rev. 2020, 165–166, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Van de Sande, B.; Lee, J.S.; Mutasa-Gottgens, E.; Naughton, B.; Bacon, W.; Manning, J.; Wang, Y.; Pollard, J.; Mendez, M.; Hill, J.; et al. Applications of single-cell RNA sequencing in drug discovery and development. Nat. Rev. Drug Discov. 2023, 22, 496–520. [Google Scholar] [CrossRef] [PubMed]
- Loscalzo, J. Molecular interaction networks and drug development: Novel approach to drug target identification and drug repositioning. FASEB J. 2023, 37, e22660. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.; Park, H.; Park, J.; Park, S.B. Recent advances in identifying protein targets in drug discovery. Cell Chem. Biol. 2021, 28, 394–423. [Google Scholar] [CrossRef] [PubMed]
- Ardavin, C.; Alvarez-Ladron, N.; Ferriz, M.; Gutierrez-Gonzalez, A.; Vega-Perez, A. Mouse Tissue-Resident Peritoneal Macrophages in Homeostasis, Repair, Infection, and Tumor Metastasis. Adv. Sci. 2023, 10, e2206617. [Google Scholar] [CrossRef] [PubMed]
- Louwe, P.A.; Badiola Gomez, L.; Webster, H.; Perona-Wright, G.; Bain, C.C.; Forbes, S.J.; Jenkins, S.J. Recruited macrophages that colonize the post-inflammatory peritoneal niche convert into functionally divergent resident cells. Nat. Commun. 2021, 12, 1770. [Google Scholar] [CrossRef] [PubMed]
- Zinselmeyer, B.H.; Vomund, A.N.; Saunders, B.T.; Johnson, M.W.; Carrero, J.A.; Unanue, E.R. The resident macrophages in murine pancreatic islets are constantly probing their local environment, capturing beta cell granules and blood particles. Diabetologia 2018, 61, 1374–1383. [Google Scholar] [CrossRef]
- Baer, J.M.; Zuo, C.; Kang, L.I.; de la Lastra, A.A.; Borcherding, N.C.; Knolhoff, B.L.; Bogner, S.J.; Zhu, Y.; Yang, L.; Laurent, J.; et al. Fibrosis induced by resident macrophages has divergent roles in pancreas inflammatory injury and PDAC. Nat. Immunol. 2023, 24, 1443–1457. [Google Scholar] [CrossRef]
| Regulators | Mφ Polarization | Effect on Pancreatitis | Ref. |
|---|---|---|---|
| MLKL ↓ in acinar cell | M1 polarization ↓ | AP promotion ↓ | [31] |
| miR-29a/b1 ↓ | M1 polarization ↑ | AP promotion ↑ | [32] |
| MM2P | M1 polarization ↓ | AP promotion ↓ | [33] |
| PLA2 inhibitor | M1 polarization ↓ | AP promotion ↓ | [36] |
| Dopamine & D2 receptor | M1 polarization ↓ | AP promotion ↓ | [37,38] |
| GDF11 | M1 polarization ↓ | AP promotion ↓ | [39] |
| Tectoridin | M1 polarization ↓ | AP promotion ↓ | [40] |
| Paeonol | M1 polarization ↓ | AP promotion ↓ | [41] |
| CP-MSCs | M2 polarization ↑ | AP promotion ↓ | [42] |
| Hyperglycemia | M1 polarization ↑ | AP promotion ↑ | [43] |
| miR-183-5p in acinar EVs | M1 polarization ↑ | AP promotion ↑ | [44] |
| IL-4/13 in PSCs IL-4/13 in T cells | M2 polarization ↑ | CP promotion ↑ | [46,47] |
| MLN4924 | M2 polarization ↑ | CP promotion ↑ | [48] |
| Pirfenidone | M1 polarization ↓ PSC activation ↓ | CP promotion ↓ | [49] |
| Isoliquiritigenin | M1 polarization ↓ PSC activation ↓ | CP promotion ↓ | [50] |
| Dasatinib | M1 polarization ↓ PSC activation ↓ | CP promotion ↓ | [51] |
| Berberine | M2 polarization ↓ PSC activation ↓ | CP promotion ↓ | [52] |
| Tissue/Cell | Regulators | Mφ Condition | Effect on Diabetes | Ref. |
|---|---|---|---|---|
| Pancreatic islet | Several factors from β cell (insulin, ATP, etc.) | Mφ crosstalk with β cell | β cell integrity Islet inflammation | Reviewed in [54,55] |
| Pancreatic islet | Fetuin-A from β cell | Mφ accumulation in islet | Intra-islet inflammation | [63] |
| β cell | AGEs | M1 polarization ↑ | β cell dysfunction | [64] |
| Systemic | GRP94 | M1 polarization ↑ | Insulin resistance ↑ | [65] |
| β cell | US-MSC | M2 polarization ↑ | β cell protection | [66] |
| β cell | GLP-1 | M2 polarization ↑ | β cell protection | [67] |
| Kidney | HPS | M2 polarization ↑ | Diabetic nephropathy protection | [68] |
| Kidney | miR-146a-5p from US-MSC | M2 polarization ↑ | Renal injury recovery | [69] |
| Skin | Insulin, IL-25 | M2 polarization ↑ | Wound healing | [70,71] |
| Systemic | Anti-miR-330-5p | M2 polarization ↑ | Insulin resistance ↓ | [72] |
| Intestine in sepsis | miR-3061 ↓ | M1 polarization ↑ | Intestinal injury ↑ | [73] |
| Adipose tissue | PSTPIP2 | M2 polarization ↑ | Inflammation, Insulin resistance ↓ | [74] |
| Bone | BMP-4 | M2 polarization ↑ | Bone defect repair ↑ | [75] |
| Bone | M2-derived exosome | M2 polarization ↑ | Diabetic fracture healing ↑ | [76] |
| Systemic | Mogroside V | M1 polarization ↓ | Inflammation ↓ | [77] |
| Systemic | 4-Octyl itaconate | M1 polarization ↓ | Glucose metabolism ↑ | [78] |
| Liver | HIIT | M1 polarization ↓ | Inflammation ↓ | [79] |
| Regulators | Mφ Polarization | Effect on PDAC | Ref. |
|---|---|---|---|
| IGFBP2 | M2 polarization ↑ | PDAC progression ↑ | [85] |
| TGFBI | M2 polarization ↑ | PDAC progression ↑ | [86] |
| PKM2 | M2 polarization ↑ | PDAC progression ↑ | [87] |
| Zileuton (ALOX5 inhibitor) | M2 polarization ↓ | PDAC progression ↓ | [88] |
| R788 (Syk inhibitor) | M2 polarization ↓ | PDAC progression ↓ | [90] |
| sEV-EZR | M2 polarization ↑ | PDAC metastasis ↑ | [91] |
| Oncogenic KRAS | M2 polarization ↑ | PDAC progression ↑ | [92] |
| DCLK1-iso2 | M2 polarization ↑ | PDAC progression ↑ | [93] |
| REG4 | M2 polarization ↑ | PDAC progression ↑ | [94] |
| NLRP3 | M2a/c/d polarization ↑ | PDAC progression ↑ | [95] |
| Notch signaling pathway | M2 polarization ↑ | PDAC progression ↑ | [96] |
| miR-548t-5p | M2 polarization ↓ | PDAC progression ↓ | [97] |
| miR-506 | M2 polarization ↓ | PDAC progression ↓ | [98] |
| Exosomal-LINK00460 | M2 polarization ↑ | PDAC progression ↑ | [99] |
| Exosomal-FGD5-AS1 | M2 polarization ↑ | PDAC progression ↑ | [100] |
| Cytoplasmic delivery of pIC | M1 polarization ↑ | PDAC progression ↓ | [101] |
| Irreversible electroporation (IRE) | M1 polarization ↑ | PDAC progression ↓ | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, S.; Lee, E.K. The Pivotal Role of Macrophages in the Pathogenesis of Pancreatic Diseases. Int. J. Mol. Sci. 2024, 25, 5765. https://doi.org/10.3390/ijms25115765
Ryu S, Lee EK. The Pivotal Role of Macrophages in the Pathogenesis of Pancreatic Diseases. International Journal of Molecular Sciences. 2024; 25(11):5765. https://doi.org/10.3390/ijms25115765
Chicago/Turabian StyleRyu, Seungyeon, and Eun Kyung Lee. 2024. "The Pivotal Role of Macrophages in the Pathogenesis of Pancreatic Diseases" International Journal of Molecular Sciences 25, no. 11: 5765. https://doi.org/10.3390/ijms25115765
APA StyleRyu, S., & Lee, E. K. (2024). The Pivotal Role of Macrophages in the Pathogenesis of Pancreatic Diseases. International Journal of Molecular Sciences, 25(11), 5765. https://doi.org/10.3390/ijms25115765





