Utilizing the Banana S-Adenosyl-L-Homocysteine Hydrolase Allergen to Identify Cross-Reactive IgE in Ryegrass-, Latex-, and Kiwifruit-Allergic Individuals
Abstract
1. Introduction
2. Results
2.1. Protein Profiling and SAHH Activity in Allergen Extracts
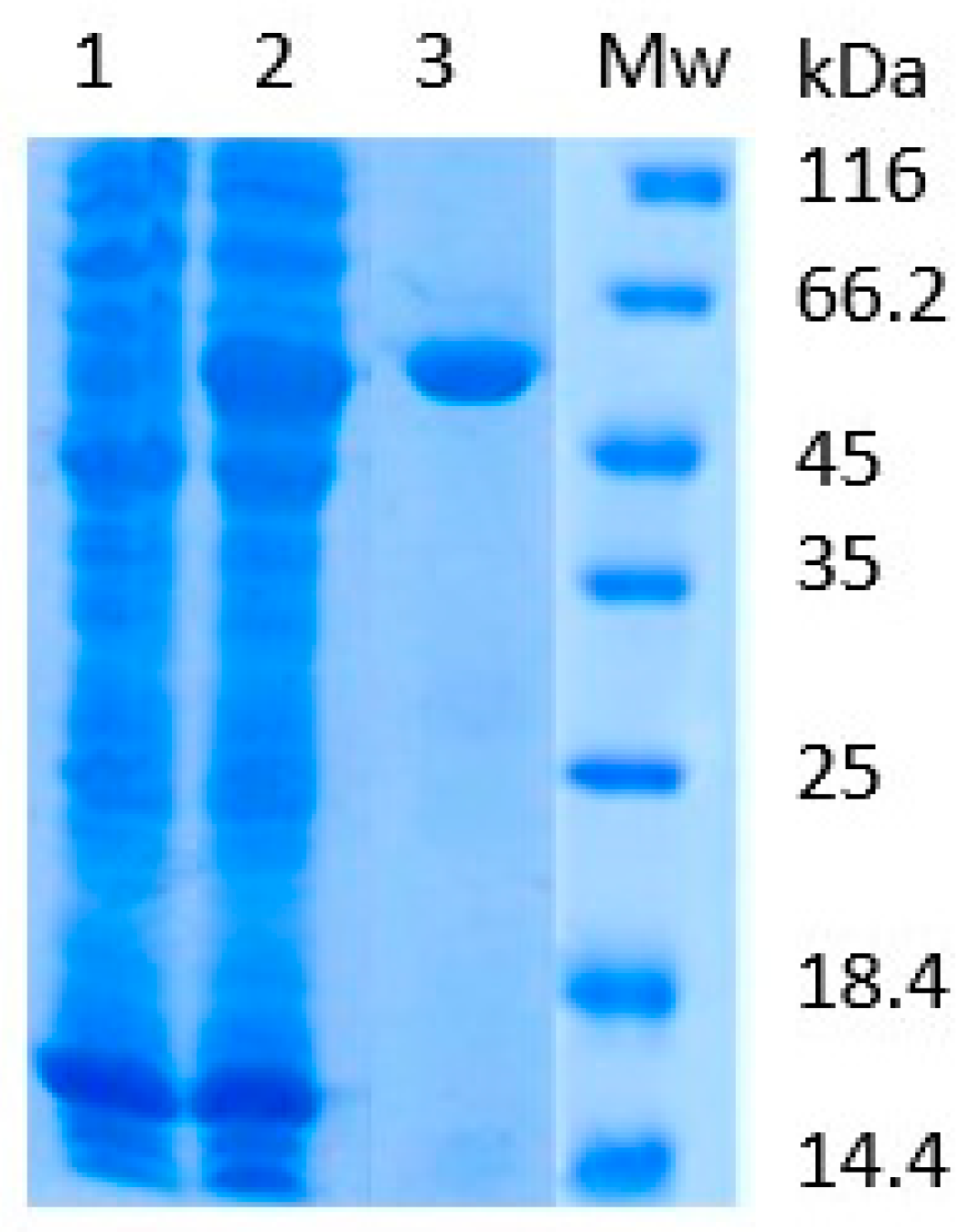
2.2. Isolation of Recombinant Banana SAHH
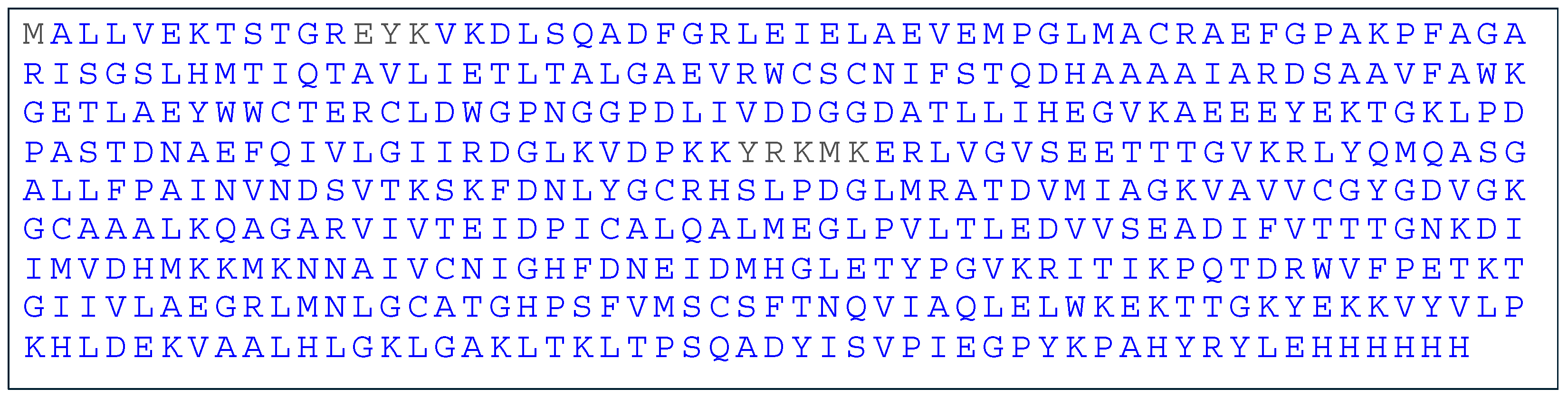
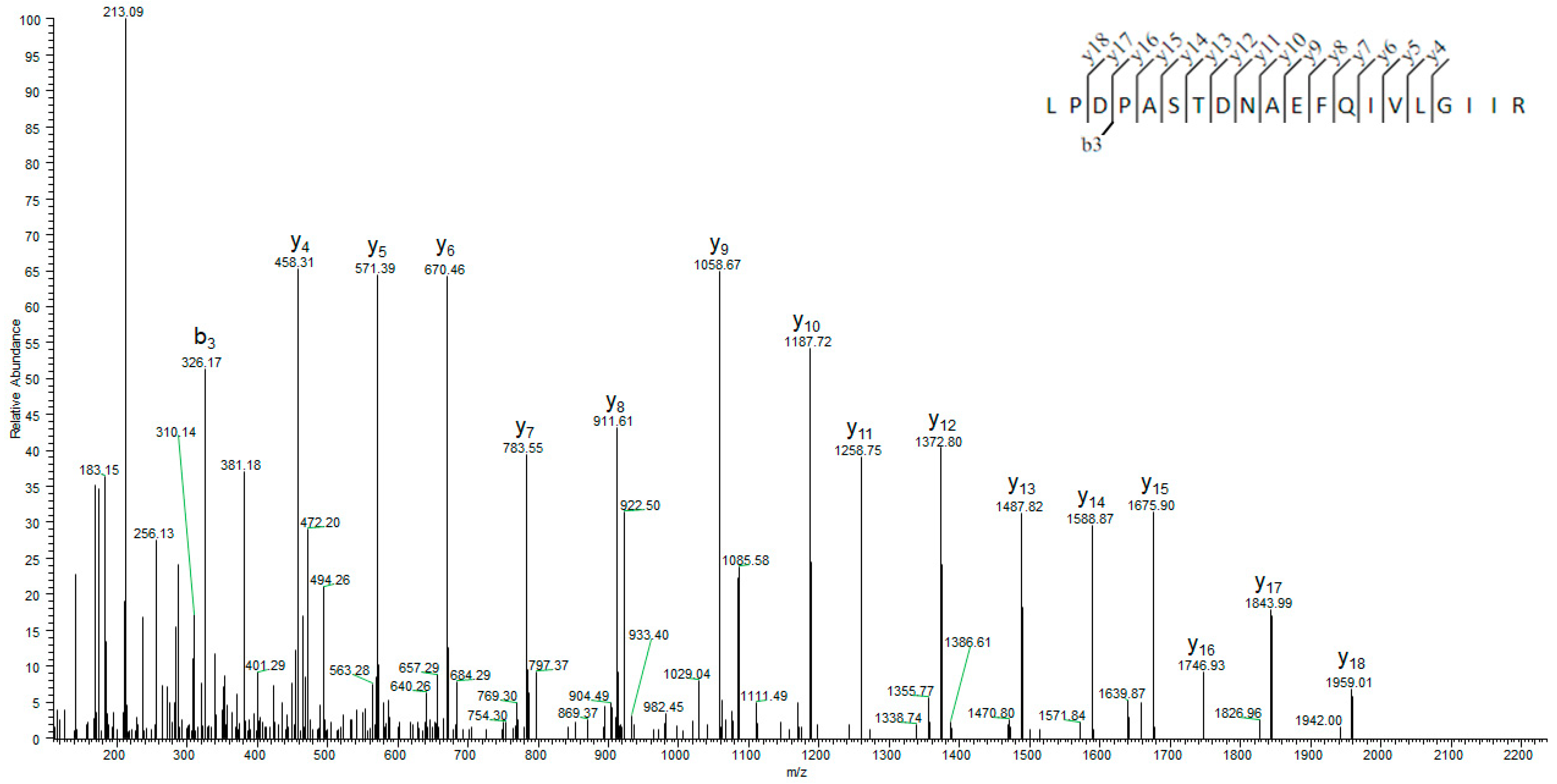
2.3. Mass Spectrometry Analysis of Recombinant Banana SAHH
2.4. Bioinformatics Analysis of SAHH Amino Acid Sequence
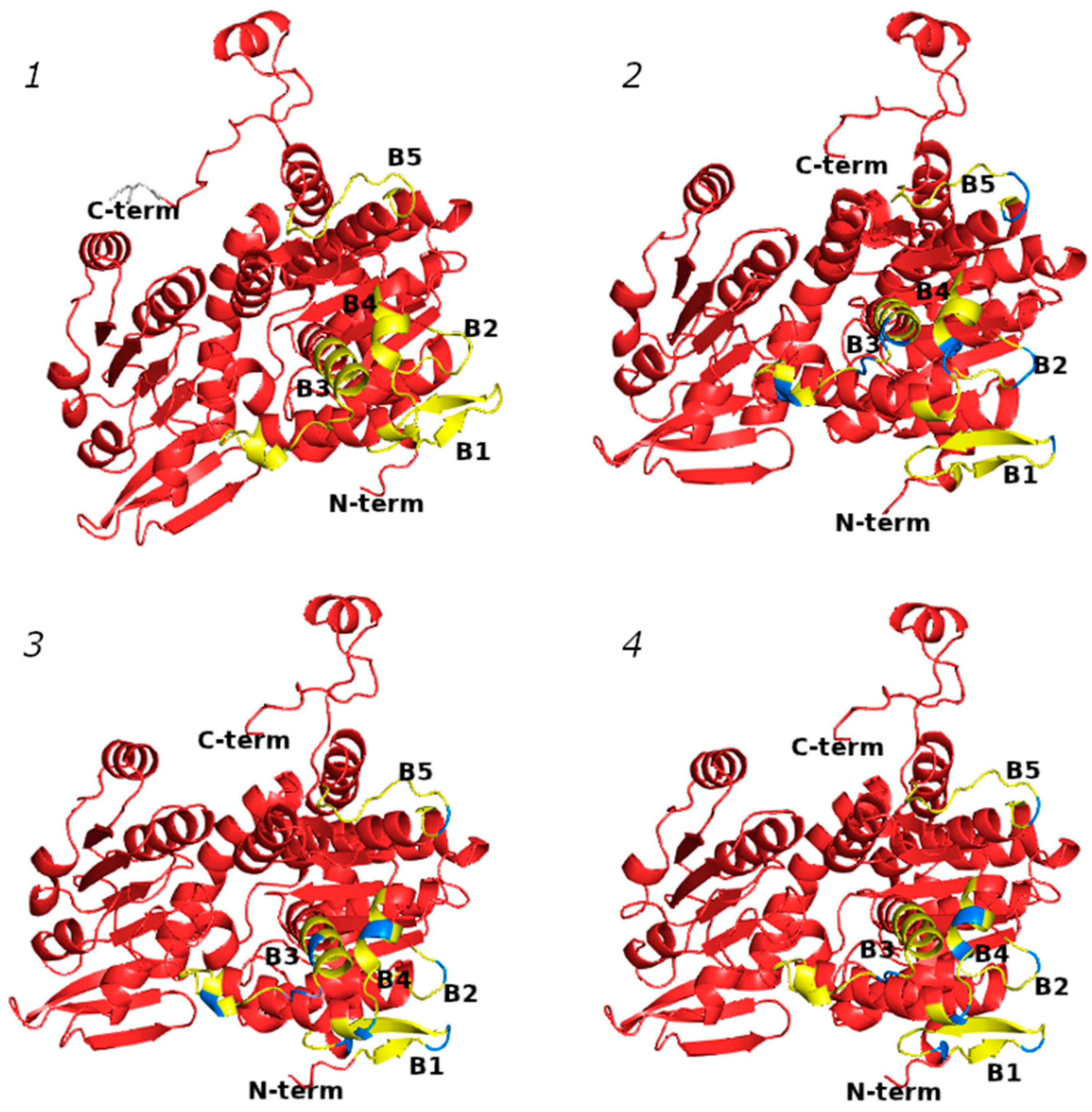
2.5. Profiling of B-Cell Epitopes
2.6. Profiling of T-Cell Epitopes
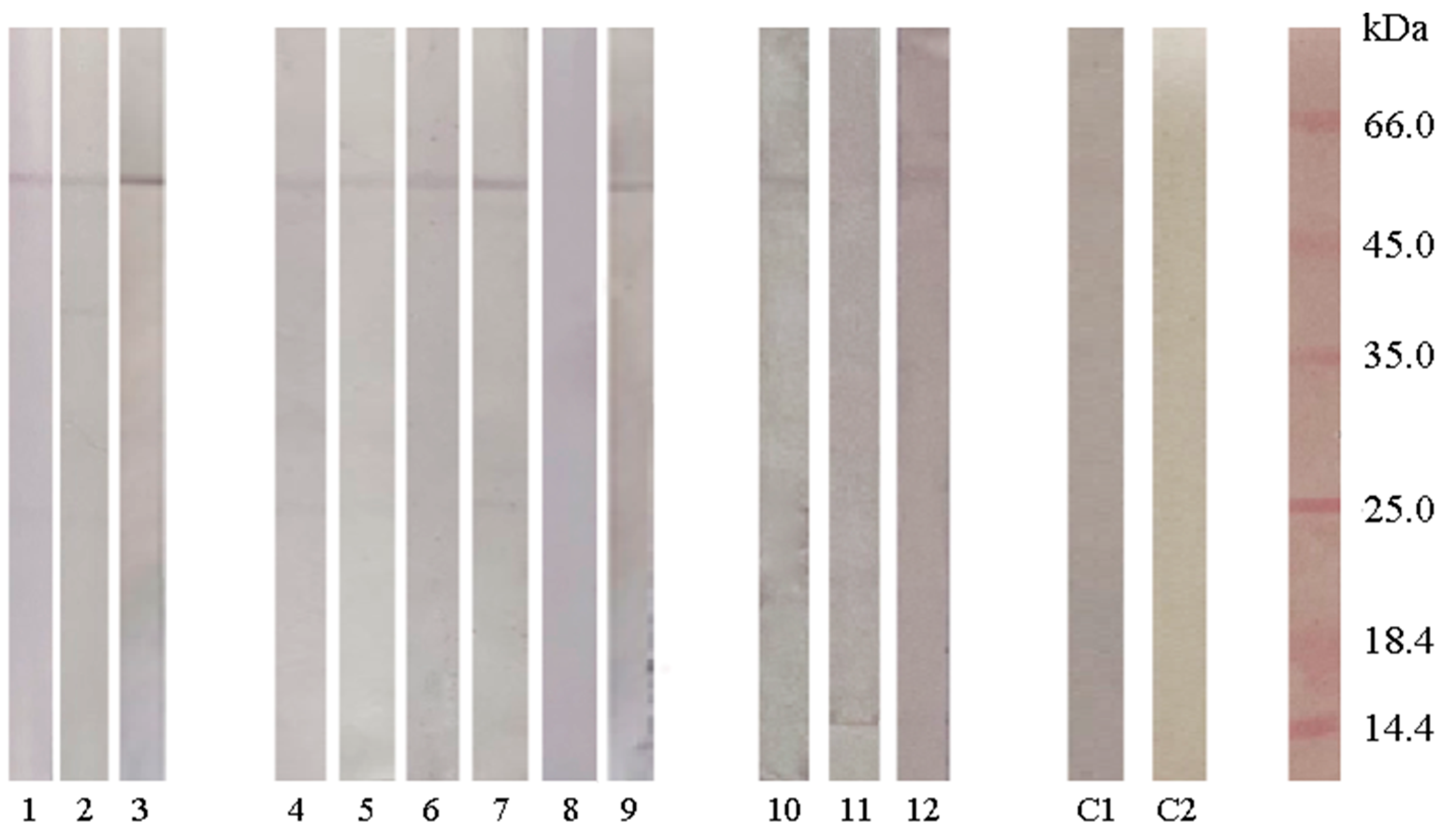
2.7. Detection of SAHH Cross-Reactive IgE in Ryegrass-, Latex-, and Kiwifruit-Allergic Individuals
3. Discussion
4. Materials and Methods
4.1. Preparation of Allergen Extracts
4.2. Detection of SAHH Activity in Allergen Extracts
4.3. Recombinant SAHH Protein Expression and Purification
4.4. Mass Spectrometry Analysis of Recombinant SAHH
4.5. Bioinformatics Analysis, 3D Modeling, and In Silico B- and T-Cell Epitope Mapping
4.6. Patients’ Sera
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verhoeckx, K.; Lindholm Bøgh, K.; Constable, A.; Epstein, M.M.; Hoffmann Sommergruber, K.; Holzhauser, T.; Houben, G.; Kuehn, A.; Roggen, E.; O’Mahony, L.; et al. COST Action “ImpARAS”: What Have We Learnt to Improve Food Allergy Risk Assessment. A Summary of a 4 Year Networking Consortium. Clin. Transl. Allergy 2020, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Bright, D.M.; Stegall, H.L.; Slawson, D.C. Food Allergies: Diagnosis, Treatment, and Prevention. Am. Fam. Physician 2023, 108, 159–165. [Google Scholar] [PubMed]
- Parisi, C.A.S.; Kelly, K.J.; Ansotegui, I.J.; Gonzalez-Díaz, S.N.; Bilò, M.B.; Cardona, V.; Park, H.S.; Braschi, M.C.; Macias-Weinmann, A.; Piga, M.A.; et al. Update on Latex Allergy: New Insights into an Old Problem. World Allergy Organ. J. 2021, 14, 100569. [Google Scholar] [CrossRef] [PubMed]
- Anto, J.M.; Bousquet, J.; Akdis, M.; Auffray, C.; Keil, T.; Momas, I.; Postma, D.S.; Valenta, R.; Wickman, M.; Cambon-Thomsen, A.; et al. Mechanisms of the Development of Allergy (MeDALL): Introducing Novel Concepts in Allergy Phenotypes. J. Allergy Clin. Immunol. 2017, 139, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Barre, A.; Pichereaux, C.; Simplicien, M.; Burlet-Schiltz, O.; Benoist, H.; Rougé, P. A Proteomic- and Bioinformatic-Based Identification of Specific Allergens from Edible Insects: Probes for Future Detection as Food Ingredients. Foods 2021, 10, 280. [Google Scholar] [CrossRef] [PubMed]
- Valenta, R.; Campana, R.; Niederberger, V. Recombinant Allergy Vaccines Based on Allergen-Derived B Cell Epitopes. Immunol. Lett. 2017, 189, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Candreva, Á.M.; Smaldini, P.L.; Cauerhff, A.; Petruccelli, S.; Docena, G.H. A Novel Approach to Ameliorate Experimental Milk Allergy Based on the Oral Administration of a Short Soy Cross-Reactive Peptide. Food Chem. 2021, 346, 128926. [Google Scholar] [CrossRef]
- Ferreira, F.; Hawranek, T.; Gruber, P.; Wopfner, N.; Mari, A. Allergic Cross-Reactivity: From Gene to the Clinic. Allergy Eur. J. Allergy Clin. Immunol. 2004, 59, 243–267. [Google Scholar] [CrossRef]
- Hauser, M.; Roulias, A.; Ferreira, F.; Egger, M. Panallergens and Their Impact on the Allergic Patient. Allergy Asthma Clin. Immunol. 2010, 6, 1. [Google Scholar] [CrossRef]
- Wawrzeńczyk, A.; Rawicka, E.; Napiórkowska-Baran, K.; Alska, E.; Bartuzi, Z. Cross-Reactive Aeroallergens–the Main Cause of Food Allergy. Food Agric. Immunol. 2023, 34, 1–9. [Google Scholar] [CrossRef]
- Palmer, L.K.; Marsh, J.T.; Lu, M.; Goodman, R.E.; Zeece, M.G.; Johnson, P.E. Shellfish Tropomyosin IgE Cross-Reactivity Differs among Edible Insect Species. Mol. Nutr. Food Res. 2020, 64, 1–7. [Google Scholar] [CrossRef]
- Pali-Schöll, I.; Meinlschmidt, P.; Larenas-Linnemann, D.; Purschke, B.; Hofstetter, G.; Rodríguez-Monroy, F.A.; Einhorn, L.; Mothes-Luksch, N.; Jensen-Jarolim, E.; Jäger, H. Edible Insects: Cross-Recognition of IgE from Crustacean- and House Dust Mite Allergic Patients, and Reduction of Allergenicity by Food Processing. World Allergy Organ. J. 2019, 12, 100006. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; Food and Agriculture Organization of the United Nations. Evaluation of Allergenicity of Genetically Modified Foods: Report of a Joint FAO/WHO Expert Consultation on Allergenicity of Foods Derived from Biotechnology; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy; WHO: Geneva, Switzerland, 2001. [Google Scholar]
- Bianco, M.; Ventura, G.; Calvano, C.D.; Losito, I.; Cataldi, T.R.I. Discovery of Marker Peptides of Spirulina Microalga Proteins for Allergen Detection in Processed Foodstuffs. Food Chem. 2022, 393, 133319. [Google Scholar] [CrossRef]
- Bianco, M.; Ventura, G.; Calvano, C.D.; Losito, I.; Cataldi, T.R.I. Food Allergen Detection by Mass Spectrometry: From Common to Novel Protein Ingredients. Proteomics 2023, 23, 2200427. [Google Scholar] [CrossRef]
- Aleksic, I.; Popovic, M.; Dimitrijevic, R.; Andjelkovic, U.; Vassilopoulou, E.; Sinaniotis, A.; Atanaskovic-Markovic, M.; Lindner, B.; Petersen, A.; Papadopoulos, N.G.; et al. Molecular and Immunological Characterization of Mus a 5 Allergen from Banana Fruit. Mol. Nutr. Food Res. 2012, 56, 446–453. [Google Scholar] [CrossRef]
- Palacin, A.; Quirce, S.; Sanchez-Monge, R.; Bobolea, I.; Diaz-Perales, A.; Martin-Munoz, F.; Pascual, C.; Salcedo, G. Sensitization Profiles to Purified Plant Food Allergens among Pediatric Patients with Allergy to Banana. Pediatr. Allergy Immunol. 2011, 22, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Reindl, J.; Rihs, H.P.; Scheurer, S.; Wangorsch, A.; Haustein, D.; Vieths, S. IgE Reactivity to Profilin in Pollen-Sensitized Subjects with Adverse Reactions to Banana and Pineapple. Int. Arch. Allergy Immunol. 2002, 128, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Bublin, M.; Pfister, M.; Radauer, C.; Oberhuber, C.; Bulley, S.; DeWitt, Å.M.; Lidholm, J.; Reese, G.; Vieths, S.; Breiteneder, H.; et al. Component-Resolved Diagnosis of Kiwifruit Allergy with Purified Natural and Recombinant Kiwifruit Allergens. J. Allergy Clin. Immunol. 2010, 125, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Asero, R. Allergy to Kiwi: Is Component-Resolved Diagnosis in Routine Clinical Practice Really Impossible? Eur. Ann. Allergy Clin. Immunol. 2012, 44, 42–47. [Google Scholar]
- Bernardi, M.L.; Giangrieco, I.; Camardella, L.; Ferrara, R.; Palazzo, P.; Panico, M.R.; Crescenzo, R.; Carratore, V.; Zennaro, D.; Liso, M.; et al. Allergenic Lipid Transfer Proteins from Plant-Derived Foods Do Not Immunologically and Clinically Behave Homogeneously: The Kiwifruit LTP as a Model. PLoS ONE 2011, 6, e27856. [Google Scholar] [CrossRef]
- Polikovsky, M.; Fernand, F.; Sack, M.; Frey, W.; Müller, G.; Golberg, A. In Silico Food Allergenic Risk Evaluation of Proteins Extracted from Macroalgae Ulva sp. with Pulsed Electric Fields. Food Chem. 2019, 276, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Bianco, M.; Ventura, G.; Calvano, C.D.; Losito, I.; Cataldi, T.R.I. A New Paradigm to Search for Allergenic Proteins in Novel Foods by Integrating Proteomics Analysis and in Silico Sequence Homology Prediction: Focus on Spirulina and Chlorella Microalgae. Talanta 2022, 240, 123188. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Ojalvo, D.; Benedé, S.; Antunes, C.M.; Bavaro, S.L.; Bouchaud, G.; Costa, A.; Denery-Papini, S.; Díaz-Perales, A.; Garrido-Arandia, M.; Gavrovic-Jankulovic, M.; et al. Applying the Adverse Outcome Pathway (AOP) for Food Sensitization to Support in Vitro Testing Strategies. Trends Food Sci. Technol. 2019, 85, 307–319. [Google Scholar] [CrossRef]
- Đurašinović, T.; Lopandić, Z.; Protić-Rosić, I.; Nešić, A.; Trbojević-Ivić, J.; Jappe, U.; Gavrović-Jankulović, M. Identification of S-Adenosyl-L-Homocysteine Hydrolase from Banana Fruit as a Novel Plant Panallergen. Food Chem. 2024, 437, 137782. [Google Scholar] [CrossRef]
- Brzezinski, K. S-Adenosyl-l-Homocysteine Hydrolase: A Structural Perspective on the Enzyme with Two Rossmann-Fold Domains. Biomolecules 2020, 10, 1682. [Google Scholar] [CrossRef] [PubMed]
- Crespo, J.F.; Bueno, C.; Villalba, M.; Monaci, L.; Cuadrado, C.; Novak, N.; Cabanillas, B. Epitope Mapping of the Major Allergen 2S Albumin from Pine Nut. Food Chem. 2021, 339, 127895. [Google Scholar] [CrossRef] [PubMed]
- Tahmasian, A.; Drew, R.; Broadbent, J.A.; Juhász, A.; Nye-Wood, M.; Colgrave, M.L. Conventional Solid-State Fermentation Impacts the White Lupin Proteome Reducing the Abundance of Allergenic Peptides. Food Chem. 2023, 426, 136622. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, H.F.; Zhang, Y.X.; Chen, H.L.; Cao, M.J.; Li, M.S.; Zhang, M.L.; He, X.R.; Liu, G.M. Expression and Epitope Identification of Myosin Light Chain Isoform 1, an Allergen in Procambarus Clarkii. Food Chem. 2020, 317, 126422. [Google Scholar] [CrossRef]
- Werfel, T.; Asero, R.; Ballmer-Weber, B.K.; Beyer, K.; Enrique, E.; Knulst, A.C.; Mari, A.; Muraro, A.; Ollert, M.; Poulsen, L.K.; et al. Position Paper of the EAACI: Food Allergy Due to Immunological Cross-Reactions with Common Inhalant Allergens. Allergy Eur. J. Allergy Clin. Immunol. 2015, 70, 1079–1090. [Google Scholar] [CrossRef]
- McKenna, O.E.; Asam, C.; Araujo, G.R.; Roulias, A.; Goulart, L.R.; Ferreira, F. How Relevant Is Panallergen Sensitization in the Development of Allergies? Pediatr. Allergy Immunol. 2016, 27, 560–568. [Google Scholar] [CrossRef]
- Peveri, S.; Pattini, S.; Costantino, M.T.; Incorvaia, C.; Montagni, M.; Roncallo, C.; Villalta, D.; Savi, E. Molecular Diagnostics Improves Diagnosis and Treatment of Respiratory Allergy and Food Allergy with Economic Optimization and Cost Saving. Allergol. Immunopathol. 2019, 47, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Bublin, M.; Breiteneder, H. Cross-Reactivities of Non-Homologous Allergens. Allergy Eur. J. Allergy Clin. Immunol. 2020, 75, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Furci, F.; Ricciardi, L. Plant Food Allergy Improvement after Grass Pollen Sublingual Immunotherapy: A Case Series. Pathogens 2021, 10, 1412. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, H.; Yokooji, T.; Taogoshi, T. Common Food Allergens and Their IgE-Binding Epitopes. Allergol. Int. 2015, 64, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Curin, M.; Garmatiuk, T.; Resch-Marat, Y.; Chen, K.W.; Hofer, G.; Fauland, K.; Keller, W.; Hemmer, W.; Vrtala, S.; Focke-Tejkl, M.; et al. Similar Localization of Conformational IgE Epitopes on the House Dust Mite Allergens Der p 5 and Der p 21 despite Limited IgE Cross-reactivity. Allergy 2018, 73, 1653–1661. [Google Scholar] [CrossRef] [PubMed]
- Saetang, J.; Tipmanee, V.; Benjakul, S. In Silico Prediction of Cross-Reactive Epitopes of Tropomyosin from Shrimp and Other Arthropods Involved in Allergy. Molecules 2022, 27, 2667. [Google Scholar] [CrossRef] [PubMed]
- Borres, M.P.; Maruyama, N.; Sato, S.; Ebisawa, M. Recent Advances in Component Resolved Diagnosis in Food Allergy. Allergol. Int. 2016, 65, 378–387. [Google Scholar] [CrossRef]
- Martínez, D.; Fang, L.; Meza-Torres, C.; Garavito, G.; López-Lluch, G.; Egea, E. Toward Consensus Epitopes B and T of Tropomyosin Involved in Cross-Reactivity across Diverse Allergens: An in Silico Study. Biomedicines 2024, 12, 884. [Google Scholar] [CrossRef] [PubMed]
- Sewell, A.K. Why Must T Cells Be Cross-Reactive? Nat. Rev. Immunol. 2012, 12, 669–677. [Google Scholar] [CrossRef]
- Kamath, S.D.; Bublin, M.; Kitamura, K.; Matsui, T.; Ito, K.; Lopata, A.L. Cross-Reactive Epitopes and Their Role in Food Allergy. J. Allergy Clin. Immunol. 2023, 151, 1178–1190. [Google Scholar] [CrossRef]
- Aalberse, R.C. Assessment of Allergen Cross-Reactivity. Clin. Mol. Allergy 2007, 5, 2. [Google Scholar] [CrossRef]
- Cong, Y.; Li, Y.; Li, L. Immunoglobulin E and Immunoglobulin G Cross-Reactive Allergens and Epitopes between Cow Milk AS1-Casein and Soybean Proteins. J. Dairy. Sci. 2020, 103, 9815–9824. [Google Scholar] [CrossRef]
- Gavrović-Jankulović, M.; Ćirković, T.; Vučković, O.; Atanasković-Marković, M.; Petersen, A.; Gojgić, G.; Burazer, L.; Jankov, R.M. Isolation and Biochemical Characterization of a Thaumatin-like Kiwi Allergen. J. Allergy Clin. Immunol. 2002, 110, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Gavrović-Jankulović, M.; Cirković, T.; Burazer, L.; Vucković, O.; Jankov, R.M. IgE Cross-Reactivity between Meadow Fescue Pollen and Kiwi Fruit in Patients’ Sera with Sensitivity to Both Extracts. J. Investig. Allergol. Clin. Immunol. 2002, 12, 279–286. [Google Scholar]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Lozada-Ramírez, J.D.; Martínez-Martínez, I.; García-Carmona, F.; Sánchez-Ferrer, A. Cloning, Overexpression, Purification, and Characterization of S-Adenosylhomocysteine Hydrolase from Corynebacterium Efficiens YS-314. Biotechnol. Prog. 2008, 24, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, A.; Tomas, H.; Havliš, J.; Olsen, J.V.; Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2007, 1, 2856–2860. [Google Scholar] [CrossRef]
- Altschul, S.F.; Wootton, J.C.; Gertz, E.M.; Agarwala, R.; Morgulis, A.; Schäffer, A.A.; Yu, Y.K. Protein Database Searches Using Compositionally Adjusted Substitution Matrices. FEBS J. 2005, 272, 5101–5109. [Google Scholar] [CrossRef]
- Madeira, F.; Pearce, M.; Tivey, A.R.N.; Basutkar, P.; Lee, J.; Edbali, O.; Madhusoodanan, N.; Kolesnikov, A.; Lopez, R. Search and Sequence Analysis Tools Services from EMBL-EBI in 2022. Nucleic Acids Res. 2022, 50, W276–W279. [Google Scholar] [CrossRef]
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L. ColabFold: Making Protein Folding Accessible to All. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef]
- Odorico, M.; Pellequer, J.L. BEPITOPE: Predicting the Location of Continuous Epitopes and Patterns in Proteins. J. Mol. Recognit. 2003, 16, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, M.C.; Peters, B.; Nielsen, M.; Marcatili, P. BepiPred-2.0: Improving Sequence-Based B-Cell Epitope Prediction Using Conformational Epitopes. Nucleic Acids Res. 2017, 45, W24–W29. [Google Scholar] [CrossRef] [PubMed]
- Reynisson, B.; Barra, C.; Kaabinejadian, S.; Hildebrand, W.H.; Peters, B.; Peters, B.; Nielsen, M.; Nielsen, M. Improved Prediction of MHC II Antigen Presentation through Integration and Motif Deconvolution of Mass Spectrometry MHC Eluted Ligand Data. J. Proteome Res. 2020, 19, 2304–2315. [Google Scholar] [CrossRef] [PubMed]


| Position | MHC II | Epitope | %Rank |
|---|---|---|---|
| 182 | DRB1_0301 | RDGLKVDPKKYRKMK | 0.04 |
| 96 | DRB1_0301 | AAAIARDSAAVFAWK | 0.31 |
| 328 | DRB1_0301 | KDIIMVDHMKKMKNN | 0.98 |
| 317 | DRB1_0701 | EADIFVTTTGNKDII | 0.44 |
| 278 | DRB1_0701 | AAALKQAGARVIVTE | 0.79 |
| 103 | DRB1_1501 | SAAVFAWKGETLAEY | 0.39 |
| 142 | DRB1_1501 | DATLLIHEGVKAEEE | 0.91 |
| 96 | DRB3_0101 | AAAIARDSAAVFAWK | 0.09 |
| 341 | DRB3_0202 | NNAIVCNIGHFDNEI | 0.39 |
| 170 | DRB5_0101 | DNAEFQIVLGIIRDG | 0.57 |
| 189 | DRB5_0101 | PKKYRKMKERLVGVS | 0.79 |
| Position | MHC II | Epitope | %Rank |
|---|---|---|---|
| 182 | DRB1_0301 | RDGLKTDPKRYHKMK | 0.21 |
| 96 | DRB1_0301 | ASAIARDSAAVFAWK | 0.31 |
| 328 | DRB1_0301 | KDIIMVDHMKKMKNN | 0.98 |
| 317 | DRB1_0701 | ETDIFVTTTGNKDII | 0.42 |
| 278 | DRB1_0701 | AAALKQAGARVIVTE | 0.79 |
| 103 | DRB1_1501 | SAAVFAWKGETLQEY | 0.38 |
| 142 | DRB1_1501 | DATLLIHEGVKAEEE | 0.91 |
| 96 | DRB3_0101 | ASAIARDSAAVFAWK | 0.09 |
| 341 | DRB3_0202 | NNAIVCNIGHFDNEI | 0.39 |
| 170 | DRB5_0101 | DNAEFQIVLTIIRDG | 0.52 |
| Position | MHC II | Epitope | %Rank |
|---|---|---|---|
| 182 | DRB1_0301 | RDGLKTDVRRYRKMK | 0.16 |
| 96 | DRB1_0301 | ASAIARDSAAVFAWK | 0.31 |
| 317 | DRB1_0701 | DADIFVTTTGNKDII | 0.44 |
| 278 | DRB1_0701 | AAALKQAGARVIVTE | 0.79 |
| 103 | DRB1_1501 | SAAVFAWKGETLEEY | 0.38 |
| 142 | DRB1_1501 | DATLLIHEGVKAEEE | 0.91 |
| 96 | DRB3_0101 | AAAIARDSAAVFAWK | 0.09 |
| 341 | DRB3_0202 | NNAIVCNIGHFDNEI | 0.39 |
| 170 | DRB5_0101 | DNPEFKIVLTIIRDG | 0.07 |
| Position | MHC II | Epitope | %Rank |
|---|---|---|---|
| 182 | DRB1_0301 | RDGLKTDPKKYHKMK | 0.16 |
| 96 | DRB1_0301 | AAAIARDSASVFAWK | 0.52 |
| 317 | DRB1_0701 | ETDIFVTTTGNKDII | 0.44 |
| 278 | DRB1_0701 | AAALKQAGARVIVTE | 0.79 |
| 103 | DRB1_1501 | SASVFAWKGETLQEY | 0.39 |
| 96 | DRB3_0101 | AAAIARDSASVFAWK | 0.11 |
| 341 | DRB3_0202 | NNAIVCNIGHFDNEI | 0.39 |
| 170 | DRB5_0101 | DNAEFQIVLTIIRDG | 0.52 |
| # | Sex | Years | sIgE |
|---|---|---|---|
| 1. | Male | 25 | Latex 2.46 kU/L |
| 2. | Male | 25 | Latex 48.2 kU/L |
| 3. | Female | 4 | Latex 3.149 kU/L |
| 4. | Male | 11 | Ryegrass 41.05 kU/L |
| 5. | Female | 28 | Ryegrass 37.4 kU/L |
| 6. | Male | 19 | Ryegrass 10.4 kU/L |
| 7. | Male | 13 | Ryegrass 112.2 kU/L |
| 8. | Female | 29 | Ryegrass 24.4 kU/L |
| 9. | Male | 13 | Ryegrass 6.01 kU/L |
| 10. | Female | 39 | Kiwifruit 4.31 kU/L |
| 11. | Male | 10 | Kiwifruit 1.14 kU/L |
| 12. | Male | Kiwifruit 0.67 kU/mL | |
| c1 | Male | 30 | House Dust Mites 4.26 kU/L |
| c2 | Control of secondary antibodies | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Đurašinović, T.; Lopandić, Z.; Protić-Rosić, I.; Ravnsborg, T.; Blagojević, G.; Burazer, L.; Jensen, O.N.; Gavrović-Jankulović, M. Utilizing the Banana S-Adenosyl-L-Homocysteine Hydrolase Allergen to Identify Cross-Reactive IgE in Ryegrass-, Latex-, and Kiwifruit-Allergic Individuals. Int. J. Mol. Sci. 2024, 25, 5800. https://doi.org/10.3390/ijms25115800
Đurašinović T, Lopandić Z, Protić-Rosić I, Ravnsborg T, Blagojević G, Burazer L, Jensen ON, Gavrović-Jankulović M. Utilizing the Banana S-Adenosyl-L-Homocysteine Hydrolase Allergen to Identify Cross-Reactive IgE in Ryegrass-, Latex-, and Kiwifruit-Allergic Individuals. International Journal of Molecular Sciences. 2024; 25(11):5800. https://doi.org/10.3390/ijms25115800
Chicago/Turabian StyleĐurašinović, Tatjana, Zorana Lopandić, Isidora Protić-Rosić, Tina Ravnsborg, Gordan Blagojević, Lidija Burazer, Ole N. Jensen, and Marija Gavrović-Jankulović. 2024. "Utilizing the Banana S-Adenosyl-L-Homocysteine Hydrolase Allergen to Identify Cross-Reactive IgE in Ryegrass-, Latex-, and Kiwifruit-Allergic Individuals" International Journal of Molecular Sciences 25, no. 11: 5800. https://doi.org/10.3390/ijms25115800
APA StyleĐurašinović, T., Lopandić, Z., Protić-Rosić, I., Ravnsborg, T., Blagojević, G., Burazer, L., Jensen, O. N., & Gavrović-Jankulović, M. (2024). Utilizing the Banana S-Adenosyl-L-Homocysteine Hydrolase Allergen to Identify Cross-Reactive IgE in Ryegrass-, Latex-, and Kiwifruit-Allergic Individuals. International Journal of Molecular Sciences, 25(11), 5800. https://doi.org/10.3390/ijms25115800





