Alteration of Gut Microbiota Composition and Diversity in Acute and/or Chronic Graft-versus-Host Disease Following Hematopoietic Stem Cell Transplantation: A Prospective Cohort Study
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Abundance Analysis Results
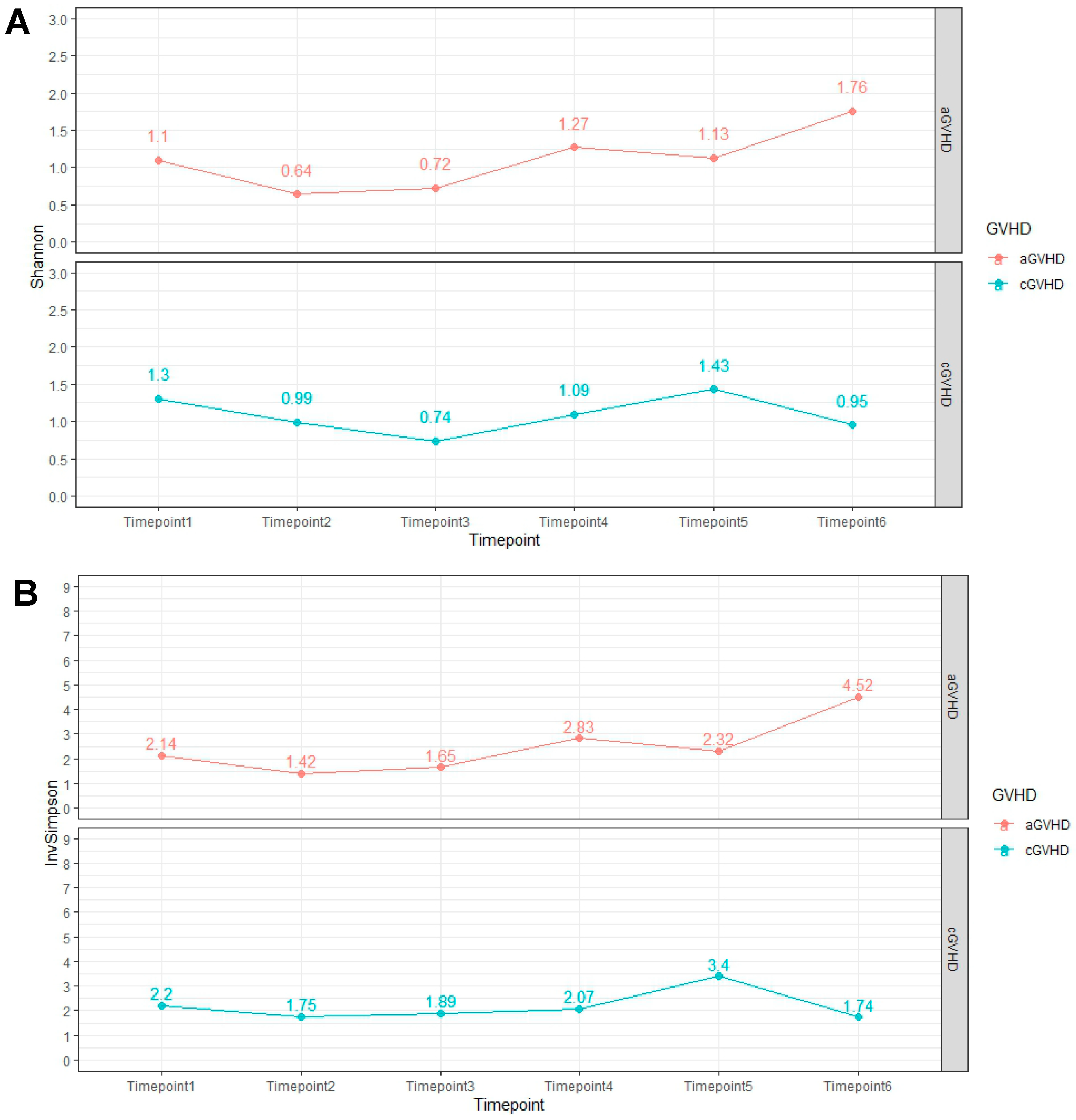
2.3. Alpha Diversity Analysis Results
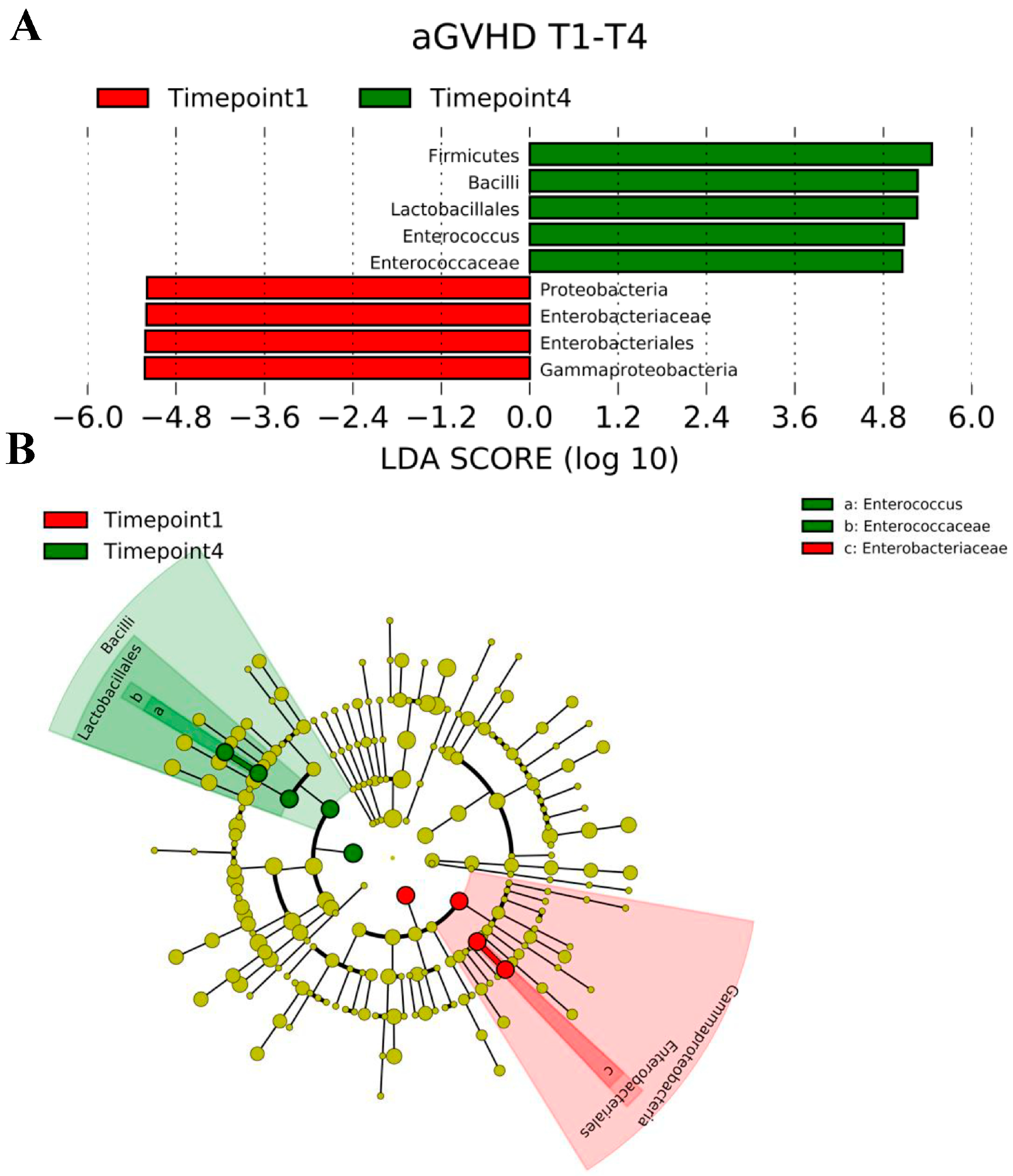
2.4. LEfSe Analysis Results
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Study Endpoint
4.3. Study Population
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loke, J.; Malladi, R.; Moss, P.; Craddock, C. The role of allogeneic stem cell transplantation in the management of acute myeloid leukaemia: A triumph of hope and experience. Br. J. Haematol. 2020, 188, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Malard, F.; Holler, E.; Sandmaier, B.M.; Huang, H.; Mohty, M. Acute graft-versus-host disease. Nat. Rev. Dis. Primers 2023, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Toprak, S.K.; Atilla, P.A.; Atilla, E.; Demirer, T. An overview of infectious complications after allogeneic hematopoietic stem cell transplantation. J. Infect. Chemother. Off. J. Jpn. Soc. Chemother. 2016, 22, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, S.; Weber, D.; Mavin, E.; Wang, X.N.; Dickinson, A.M.; Holler, E. Pathophysiology of GvHD and Other HSCT-Related Major Complications. Front. Immunol. 2017, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Wang, R.; Wang, X.; Yang, S.; Wang, W.; Gao, Q.; Zhang, X. Interplay Between the Intestinal Microbiota and Acute Graft-Versus-Host Disease: Experimental Evidence and Clinical Significance. Front. Immunol. 2021, 12, 644982. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Hayase, E.; Jenq, R.R. The role of microbiota in allogeneic hematopoietic stem cell transplantation. Expert Opin. Biol. Ther. 2021, 21, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.; Nguyen, V.H. Role of gut microbiota in graft-versus-host disease. Leuk. Lymphoma 2011, 52, 1844–1856. [Google Scholar] [CrossRef] [PubMed]
- Staffas, A.; Burgos da Silva, M.; van den Brink, M.R. The intestinal microbiota in allogeneic hematopoietic cell transplant and graft-versus-host disease. Blood 2017, 129, 927–933. [Google Scholar] [CrossRef]
- Shono, Y.; van den Brink, M.R.M. Empiric antibiotic use in allogeneic hematopoietic cell transplantation: Should we avoid anaerobe coverage? Blood Adv. 2017, 1, 2325–2328. [Google Scholar] [CrossRef] [PubMed]
- Taur, Y.; Jenq, R.R.; Perales, M.A.; Littmann, E.R.; Morjaria, S.; Ling, L.; No, D.; Gobourne, A.; Viale, A.; Dahi, P.B.; et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 2014, 124, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Chi, L.; Zhu, Y.; Shi, X.; Tu, P.; Li, B.; Yin, J.; Gao, N.; Shen, W.; Schnabl, B. An Introduction to Next Generation Sequencing Bioinformatic Analysis in Gut Microbiome Studies. Biomolecules 2021, 11, 530. [Google Scholar] [CrossRef] [PubMed]
- Henig, I.; Yehudai-Ofir, D.; Zuckerman, T. The clinical role of the gut microbiome and fecal microbiota transplantation in allogeneic stem cell transplantation. Haematologica 2021, 106, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Peled, J.U.; Gomes, A.L.C.; Devlin, S.M.; Littmann, E.R.; Taur, Y.; Sung, A.D.; Weber, D.; Hashimoto, D.; Slingerland, A.E.; Slingerland, J.B.; et al. Microbiota as Predictor of Mortality in Allogeneic Hematopoietic-Cell Transplantation. N. Engl. J. Med. 2020, 382, 822–834. [Google Scholar] [CrossRef] [PubMed]
- Zeiser, R.; von Bubnoff, N.; Butler, J.; Mohty, M.; Niederwieser, D.; Or, R.; Szer, J.; Wagner, E.M.; Zuckerman, T.; Mahuzier, B.; et al. Ruxolitinib for Glucocorticoid-Refractory Acute Graft-versus-Host Disease. N. Engl. J. Med. 2020, 382, 1800–1810. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, J.; Fowler, D.H.; Bramanti, S.; Pavletic, S.Z. Editorial: Controversies and expectations for prevention and treatment of graft-versus-host-disease: A biological and clinical perspective. Front. Immunol. 2023, 14, 1212756. [Google Scholar] [CrossRef] [PubMed]
- Kasikis, S.; Etra, A.; Levine, J.E. Current and Emerging Targeted Therapies for Acute Graft-Versus-Host Disease. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2021, 35, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Metafuni, E.; Di Marino, L.; Giammarco, S.; Bellesi, S.; Limongiello, M.A.; Sorà, F.; Frioni, F.; Maggi, R.; Chiusolo, P.; Sica, S. The Role of Fecal Microbiota Transplantation in the Allogeneic Stem Cell Transplant Setting. Microorganisms 2023, 11, 2182. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Biliński, J.; Wang, L.; Yang, T.; Luo, R.; Fu, Y.; Yang, G. Safety and efficacy of fecal microbiota transplantation in the treatment of graft-versus-host disease. Bone Marrow Transplant. 2023, 58, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Holler, E.; Butzhammer, P.; Schmid, K.; Hundsrucker, C.; Koestler, J.; Peter, K.; Zhu, W.; Sporrer, D.; Hehlgans, T.; Kreutz, M.; et al. Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: Loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biol. Blood Marrow Transplant. 2014, 20, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Ilett, E.E.; Jørgensen, M.; Noguera-Julian, M.; Nørgaard, J.C.; Daugaard, G.; Helleberg, M.; Paredes, R.; Murray, D.D.; Lundgren, J.; MacPherson, C.; et al. Associations of the gut microbiome and clinical factors with acute GVHD in allogeneic HSCT recipients. Blood Adv. 2020, 4, 5797–5809. [Google Scholar] [CrossRef] [PubMed]
- Taur, Y.; Xavier, J.B.; Lipuma, L.; Ubeda, C.; Goldberg, J.; Gobourne, A.; Lee, Y.J.; Dubin, K.A.; Socci, N.D.; Viale, A.; et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin. Infect. Dis. 2012, 55, 905–914. [Google Scholar] [CrossRef]
- Garrett, W.S. Enterococcus in Graft-versus-Host Disease. N. Engl. J. Med. 2020, 382, 1064–1066. [Google Scholar] [CrossRef]
- Stein-Thoeringer, C.K.; Nichols, K.B.; Lazrak, A.; Docampo, M.D.; Slingerland, A.E.; Slingerland, J.B.; Clurman, A.G.; Armijo, G.; Gomes, A.L.C.; Shono, Y.; et al. Lactose drives Enterococcus expansion to promote graft-versus-host disease. Science 2019, 366, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Kusakabe, S.; Fukushima, K.; Yokota, T.; Hino, A.; Fujita, J.; Motooka, D.; Nakamura, S.; Shibayama, H.; Kanakura, Y. Enterococcus: A Predictor of Ravaged Microbiota and Poor Prognosis after Allogeneic Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2020, 26, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- van Lier, Y.F.; Vos, J.; Blom, B.; Hazenberg, M.D. Allogeneic hematopoietic cell transplantation, the microbiome, and graft-versus-host disease. Gut Microbes 2023, 15, 2178805. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.; Oefner, P.J.; Dettmer, K.; Hiergeist, A.; Koestler, J.; Gessner, A.; Weber, M.; Stämmler, F.; Hahn, J.; Wolff, D.; et al. Rifaximin preserves intestinal microbiota balance in patients undergoing allogeneic stem cell transplantation. Bone Marrow Transplant. 2016, 51, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Jin, H.; Zhou, L.; Zhang, X.; Fan, Z.; Dai, M.; Lin, Q.; Huang, F.; Xuan, L.; Zhang, H.; et al. Intestinal Microbiota at Engraftment Influence Acute Graft-Versus-Host Disease via the Treg/Th17 Balance in Allo-HSCT Recipients. Front. Immunol. 2018, 9, 669. [Google Scholar] [CrossRef]
- Han, L.; Zhang, H.; Chen, S.; Zhou, L.; Li, Y.; Zhao, K.; Huang, F.; Fan, Z.; Xuan, L.; Zhang, X.; et al. Intestinal Microbiota Can Predict Acute Graft-versus-Host Disease Following Allogeneic Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2019, 25, 1944–1955. [Google Scholar] [CrossRef]
- Han, L.; Zhao, K.; Li, Y.; Han, H.; Zhou, L.; Ma, P.; Fan, Z.; Sun, H.; Jin, H.; Jiang, Z.; et al. A gut microbiota score predicting acute graft-versus-host disease following myeloablative allogeneic hematopoietic stem cell transplantation. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2020, 20, 1014–1027. [Google Scholar] [CrossRef]
- Rafei, H.; Jenq, R.R. Microbiome-intestine cross talk during acute graft-versus-host disease. Blood 2020, 136, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.W.; Su, X.H.; Wang, M.Y.; Han, M.Z.; Feng, X.M.; Jiang, E.L. Regulatory T Cells in GVHD Therapy. Front. Immunol. 2021, 12, 697854. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, Y.; Cheng, Q.; Wu, D.; Liu, H. The Role of Intestinal Microbiota in Acute Graft-versus-Host Disease. J. Immunol. Res. 2015, 2015, 145859. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Wu, H.; Wang, L.; Zhao, Y.; Huang, H. Altered intestinal microbiome and epithelial damage aggravate intestinal graft-versus-host disease. Gut Microbes 2023, 15, 2221821. [Google Scholar] [CrossRef] [PubMed]
- Christovich, A.; Luo, X.M. Gut Microbiota, Leaky Gut, and Autoimmune Diseases. Front. Immunol. 2022, 13, 946248. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, E.; Ogawa, Y.; Saijo, Y.; Yamane, M.; Uchino, M.; Kamoi, M.; Fukui, M.; Yang, F.; He, J.; Mukai, S.; et al. Commensal microflora in human conjunctiva; characteristics of microflora in the patients with chronic ocular graft-versus-host disease. Ocul. Surf. 2019, 17, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Hino, A.; Fukushima, K.; Kusakabe, S.; Ueda, T.; Sudo, T.; Fujita, J.; Motooka, D.; Takeda, A.K.; Shinozaki, N.O.; Watanabe, S.; et al. Prolonged gut microbial alterations in post-transplant survivors of allogeneic haematopoietic stem cell transplantation. Br. J. Haematol. 2023, 201, 725–737. [Google Scholar] [CrossRef]
- Bachier, C.R.; Aggarwal, S.K.; Hennegan, K.; Milgroom, A.; Francis, K.; Dehipawala, S.; Rotta, M. Epidemiology and Treatment of Chronic Graft-versus-Host Disease Post-Allogeneic Hematopoietic Cell Transplantation: A US Claims Analysis. Transplant. Cell. Ther. 2021, 27, 504.e1–504.e6. [Google Scholar] [CrossRef] [PubMed]
- Boyiadzis, M.; Arora, M.; Klein, J.P.; Hassebroek, A.; Hemmer, M.; Urbano-Ispizua, A.; Antin, J.H.; Bolwell, B.J.; Cahn, J.Y.; Cairo, M.S.; et al. Impact of Chronic Graft-versus-Host Disease on Late Relapse and Survival on 7489 Patients after Myeloablative Allogeneic Hematopoietic Cell Transplantation for Leukemia. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Gavriilaki, M.; Sakellari, I.; Anagnostopoulos, A.; Gavriilaki, E. The Impact of Antibiotic-Mediated Modification of the Intestinal Microbiome on Outcomes of Allogeneic Hematopoietic Cell Transplantation: Systematic Review and Meta-Analysis. Biol. Blood Marrow Transplant. 2020, 26, 1738–1746. [Google Scholar] [CrossRef] [PubMed]
- Gavriilaki, E.; Mallouri, D.; Laspa, E.; Papakonstantinou, A.; Lazaridou, A.; Varelas, C.; Baldoumi, E.; Giannakopoulou, A.; Demosthenous, C.; Vardi, A.; et al. Open-Label Randomized Controlled Study of Ciprofloxacin vs Rifaximin as Neutropenia Prophylaxis in Allogeneic Hematopoietic Stem Cell Transplantation. Transplant. Proc. 2024, 56, 380–385. [Google Scholar] [CrossRef]
- Sakellari, I.; Gavriilaki, E.; Mallouri, D.; Batsis, I.; Varelas, C.; Tagara, S.; Bousiou, Z.; Papathanasiou, M.; Vardi, A.; Papalexandri, A.; et al. Survival Advantage of Treosulfan Plus Fludarabine Before Allogeneic Hematopoietic Cell Transplantation for Older or Comorbid Patients With Myeloid Malignancies. Transplant. Cell. Ther. 2021, 27, 916.e1–916.e6. [Google Scholar] [CrossRef] [PubMed]
- Glucksberg, H.; Storb, R.; Fefer, A.; Buckner, C.D.; Neiman, P.E.; Clift, R.A.; Lerner, K.G.; Thomas, E.D. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 1974, 18, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Filipovich, A.H.; Weisdorf, D.; Pavletic, S.; Socie, G.; Wingard, J.R.; Lee, S.J.; Martin, P.; Chien, J.; Przepiorka, D.; Couriel, D.; et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol. Blood Marrow Transplant. 2005, 11, 945–956. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2008; ISBN 3-900051-07-0. Available online: https://www.r-project.org/ (accessed on 1 January 2024).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R package version 2.4-2. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 1 January 2024).
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Available online: https://link.springer.com/book/10.1007/978-3-319-24277-4 (accessed on 1 January 2024).
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2008, 26, 32–46. Available online: https://onlinelibrary.wiley.com/doi/10.1111/j.1442-9993.2001.01070.pp.x (accessed on 1 January 2024). [CrossRef]
- McInnes, P. Manual of Procedures for Human Microbiome Project. [online] Hmpdacc.org. 2010. Available online: https://hmpdacc.org/hmp/doc/HMP_MOP_Version12_0_072910.pdf (accessed on 10 October 2023).

| Characteristics | aGVHD | cGVHD | GVHD Total |
|---|---|---|---|
| Total patients | 8 (40) | 12 (60) | 20 (100) |
| Sex (male) | 5 (25) | 9 (45) | 14 (70) |
| Median patient age | 50 | 52.6 | 51.3 |
| Donor | |||
| Related | 3 (15) | 7 (35) | 10 (50) |
| Unrelated (matched) | 4 (20) | 3 (15) | 7 (35) |
| Unrelated (mismatched) | 1 (5) | 2 (10) | 3 (15) |
| Graft source | |||
| Peripheral blood stem cell | 7 (35) | 11 (55) | 18 (90) |
| Bone marrow | 1 (5) | 1 (5) | 2 (10) |
| aGVHD Glucksberg scale | |||
| Grade II | 3 (37.5) | N/A | N/A |
| Grade III | 5 (62.5) | N/A | N/A |
| cGvHD severity | |||
| Moderate | N/A | 8 (66.7) | N/A |
| Severe | N/A | 4 (33.4) | N/A |
| Underlying disease | |||
| Acute myeloid leukemia | 3 (15) | 5 (25) | 8 (40) |
| Acute lymphoblastic leukemia | 3 (15) | 3 (15) | 6 (30) |
| Chronic myelogenous leukemia | 2 (10) | 2 (10) | 4 (20) |
| Myelodysplastic syndrome | 0 (0) | 2 (10) | 2 (10) |
| HCT—CI * | 2.3 | 2.6 | 2.5 |
| EBMT score * | 3.5 | 3.6 | 3.6 |
| DRI | |||
| Intermediate | 3 (15) | 5 (25) | 8 (40) |
| High | 5 (25) | 7 (35) | 12 (60) |
| Conditioning regimen | |||
| Myeloablative | 2 (10) | 4 (20) | 6 (30) |
| Non-myeloablative | 6 (30) | 8 (40) | 14 (70) |
| Level of Analysis | Timepoint 1 * | Level of Analysis | Timepoint 4 * |
|---|---|---|---|
| Phylum | Total Abundance (%) | Phylum | Total Abundance (%) |
| Bacteroidetes | 62.43 | Firmicutes | 75.73 |
| Firmicutes | 22.42 | Bacteroidetes | 18.41 |
| Proteobacteria | 14.22 | Proteobacteria | 4.07 |
| Actinobacteria | 0.92 | Actinobacteria | 1.75 |
| Verrucomicrobia | 0 | Verrucomicrobia | 0.05 |
| Family | Family | ||
| Bacteroidaceae | 41.69 | Erysipelotrichaceae | 18.65 |
| Porphyromonadaceae | 19.94 | Bacteroidaceae | 17.91 |
| Enterobacteriaceae | 13.3 | Lachnospiraceae | 16.38 |
| Clostridiaceae | 4.79 | Enterococcaceae | 11.4 |
| Lachnospiraceae | 4.57 | Streptococcaceae | 8.92 |
| Acidaminococcaceae | 3.38 | Ruminococcaceae | 5.77 |
| Erysipelotrichaceae | 3.15 | Clostridiaceae | 5.22 |
| Ruminococcaceae | 2.94 | Lactobacillaceae | 2.86 |
| unclassified Clostridiales | 1.76 | Hyphomicrobiaceae | 2.73 |
| Veillonellaceae | 1.21 | Aerococcaceae | 1.95 |
| Genus (N) ** | Genus (N) ** | ||
| Bacteroides (6) | 50.75 | Clostridium (7) | 24.62 |
| Parabacteroides (5) | 23.66 | Bacteroides (5) | 19.45 |
| Clostridium (8) | 6.21 | Enterococcus (8) | 12.79 |
| Phascolarctobacterium (1) | 2.22 | Streptococcus (5) | 9.91 |
| Flavonifractor (5) | 2.06 | Ruminococcus (4) | 4.66 |
| Acidaminococcus (1) | 2.03 | Ruminococcus (3) | 4.19 |
| Faecalibacterium (5) | 1.38 | Faecalibacterium (2) | 3.6 |
| Eubacterium (4) | 1.25 | Lactobacillus (4) | 3.31 |
| Ruminococcus (5) | 1.1 | Lachnoclostridium (3) | 3.25 |
| Alistipes (4) | 1.01 | Gemmiger (1) | 3.17 |
| Species (N) ** | Species (N) ** | ||
| Bacteroides vulgatus (4) | 33.86 | Clostridium ramosum (4) | 11.56 |
| Parabacteroides merdae (4) | 23.66 | Clostridium spiroforme (4) | 9.2 |
| Bacteroides dorei (1) | 8.13 | Streptococcus thermophilus (3) | 8.24 |
| Bacteroides thetaiotaomicron (4) | 3.38 | Bacteroides vulgatus (5) | 5.79 |
| Bacteroides uniformis (4) | 3.05 | Ruminococcus gnavus (3) | 5.47 |
| Bacteroides coprocola (1) | 2.8 | Bacteroides intestinalis (3) | 5.44 |
| Phascolarctobacterium faecium (1) | 2.53 | Faecalibacterium prausnitzii (1) | 4.59 |
| Acidaminococcus intestine (1) | 2.34 | Gemmiger formicilis (1) | 4.19 |
| Flavonifractor plautii (5) | 2.1 | Lactobacillus fermentum (2) | 4.09 |
| Eubacterium cylindroides (1) | 1.36 | Ruminococcus gnavus (4) | 4.08 |
| Level of Analysis | Timepoint 1 * | Level of Analysis | Timepoint 4 * |
|---|---|---|---|
| Phylum | Total Abundance (%) | Phylum | Total Abundance (%) |
| Bacteroidetes | 50.31 | Firmicutes | 65.85 |
| Firmicutes | 35.06 | Bacteroidetes | 26.37 |
| Proteobacteria | 13.54 | Proteobacteria | 7.55 |
| Actinobacteria | 1.07 | Actinobacteria | 0.14 |
| Verrucomicrobia | 0.01 | Deferribacteres | 0.05 |
| Verrucomicrobia | 0.03 | ||
| Family | Family | ||
| Bacteroidaceae | 34.96 | Bacteroidaceae | 25.67 |
| Porphyromonadaceae | 14.76 | Erysipelotrichaceae | 17.77 |
| Lachnospiraceae | 13.82 | Clostridiaceae | 12.69 |
| Enterobacteriaceae | 11.84 | Lachnospiraceae | 11.66 |
| Clostridiaceae | 7.37 | Ruminococcaceae | 8.97 |
| Erysipelotrichaceae | 3.29 | unclassified Clostridiales | 7.21 |
| Acidaminococcaceae | 2.64 | Enterobacteriaceae | 5.06 |
| unclassified Clostridiales | 1.9 | Enterococcaceae | 2.4 |
| Ruminococcaceae | 1.79 | Oscillospiraceae | 1.51 |
| Eubacteriaceae | 1.32 | Hyphomicrobiaceae | 1.38 |
| Genus (N) ** | Genus (N) ** | ||
| Bacteroides (9) | 43.24 | Bacteroides (9) | 32.87 |
| Parabacteroides (6) | 17.59 | Clostridium (10) | 31.84 |
| Clostridium (12) | 9.61 | Flavonifractor (7) | 9.36 |
| Roseburia (6) | 3.97 | Ruminococcus (6) | 3.37 |
| Eubacterium (6) | 2.53 | Ruminococcus (6) | 3.07 |
| Lachnoclostridium (8) | 2.47 | Enterococcus (8) | 2.92 |
| Ruminococcus (9) | 2.35 | Faecalibacterium (4) | 2.52 |
| Flavonifractor (8) | 2.21 | Lachnoclostridium (4) | 2.24 |
| Ruminococcus (8) | 2.07 | Subdoligranulum (6) | 2.05 |
| Phascolarctobacterium (2) | 1.71 | Gemmiger (1) | 1.82 |
| Species (N) ** | Species (N) ** | ||
| Bacteroides vulgatus (9) | 35.35 | Bacteroides vulgatus (8) | 22.95 |
| Parabacteroides merdae (5) | 18.36 | Clostridium ramosum (3) | 14.39 |
| Roseburia intestinalis (4) | 3.48 | Flavonifractor plautii (7) | 11.08 |
| Bacteroides coprocola (2) | 2.71 | Clostridium spiroforme (5) | 7.82 |
| Bacteroides thetaiotaomicron (5) | 2.52 | Clostridium aldenense (5) | 5.45 |
| Bacteroides uniformis (5) | 2.5 | Ruminococcus gnavus (5) | 3.73 |
| Flavonifractor plautii (8) | 2.4 | Bacteroides intestinalis (4) | 2.96 |
| Lachnoclostridium clostridioforme (4) | 2.37 | Ruminococcus gnavus (6) | 2.79 |
| Bacteroides fragilis (3) | 2.18 | Faecalibacterium prausnitzii (2) | 2.51 |
| Clostridium spiroforme (8) | 2.04 | Subdoligranulum spp. (5) | 2.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavriilaki, E.; Christoforidi, M.; Ouranos, K.; Minti, F.; Mallouri, D.; Varelas, C.; Lazaridou, A.; Baldoumi, E.; Panteliadou, A.; Bousiou, Z.; et al. Alteration of Gut Microbiota Composition and Diversity in Acute and/or Chronic Graft-versus-Host Disease Following Hematopoietic Stem Cell Transplantation: A Prospective Cohort Study. Int. J. Mol. Sci. 2024, 25, 5789. https://doi.org/10.3390/ijms25115789
Gavriilaki E, Christoforidi M, Ouranos K, Minti F, Mallouri D, Varelas C, Lazaridou A, Baldoumi E, Panteliadou A, Bousiou Z, et al. Alteration of Gut Microbiota Composition and Diversity in Acute and/or Chronic Graft-versus-Host Disease Following Hematopoietic Stem Cell Transplantation: A Prospective Cohort Study. International Journal of Molecular Sciences. 2024; 25(11):5789. https://doi.org/10.3390/ijms25115789
Chicago/Turabian StyleGavriilaki, Eleni, Maria Christoforidi, Konstantinos Ouranos, Fani Minti, Despina Mallouri, Christos Varelas, Andriana Lazaridou, Eirini Baldoumi, Alkistis Panteliadou, Zoi Bousiou, and et al. 2024. "Alteration of Gut Microbiota Composition and Diversity in Acute and/or Chronic Graft-versus-Host Disease Following Hematopoietic Stem Cell Transplantation: A Prospective Cohort Study" International Journal of Molecular Sciences 25, no. 11: 5789. https://doi.org/10.3390/ijms25115789
APA StyleGavriilaki, E., Christoforidi, M., Ouranos, K., Minti, F., Mallouri, D., Varelas, C., Lazaridou, A., Baldoumi, E., Panteliadou, A., Bousiou, Z., Batsis, I., Sakellari, I., & Gioula, G. (2024). Alteration of Gut Microbiota Composition and Diversity in Acute and/or Chronic Graft-versus-Host Disease Following Hematopoietic Stem Cell Transplantation: A Prospective Cohort Study. International Journal of Molecular Sciences, 25(11), 5789. https://doi.org/10.3390/ijms25115789








