Identification of 3-Aryl-1-benzotriazole-1-yl-acrylonitrile as a Microtubule-Targeting Agent (MTA) in Solid Tumors
Abstract
1. Introduction
2. Results
2.1. Cytotoxic Effect of 34 in Different Solid Tumor Cell Lines
2.2. 34 Inhibits Colony Formation in Cancer Cells
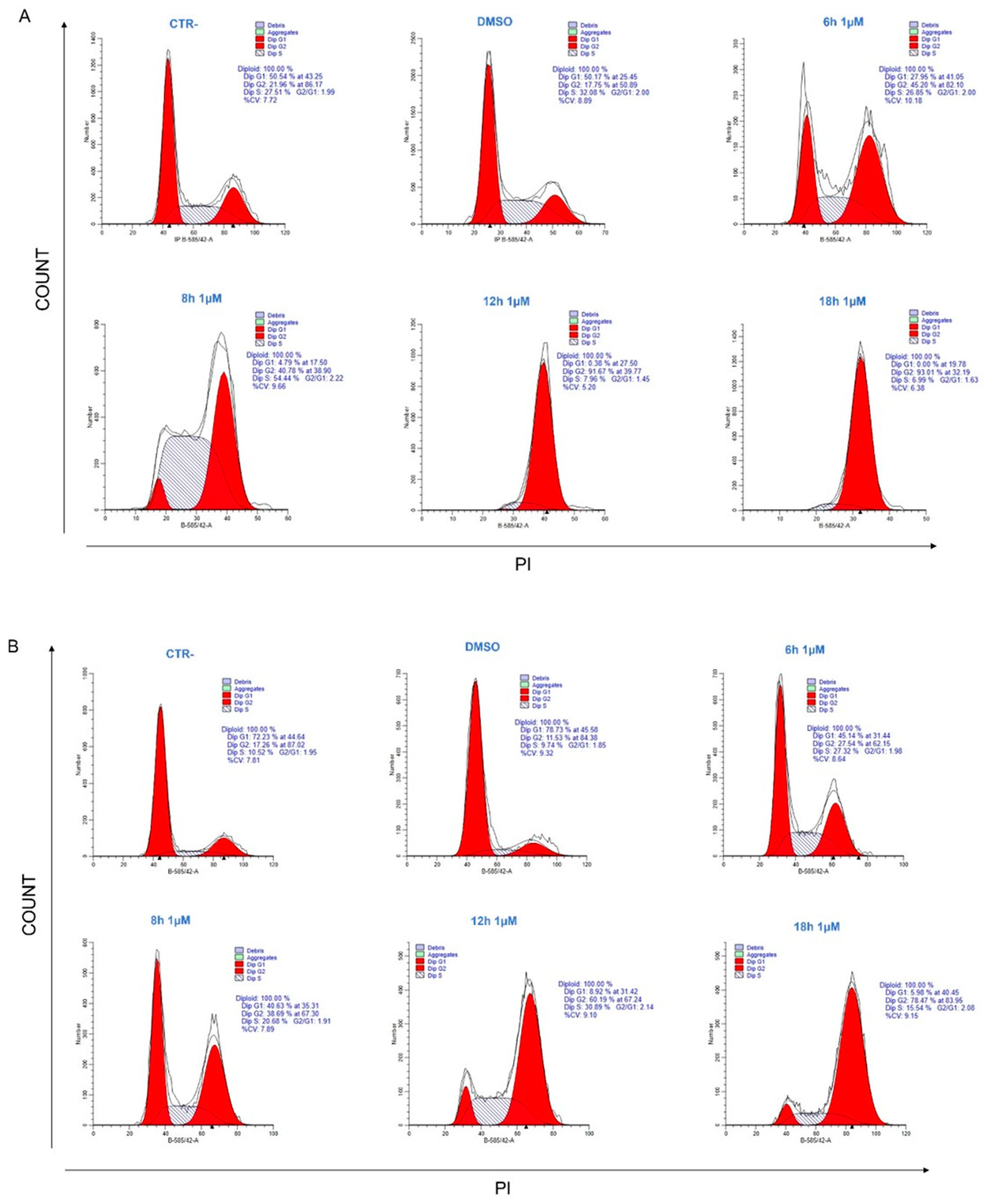
2.3. 34 Induces Cell Cycle Arrest in G2/M Phase
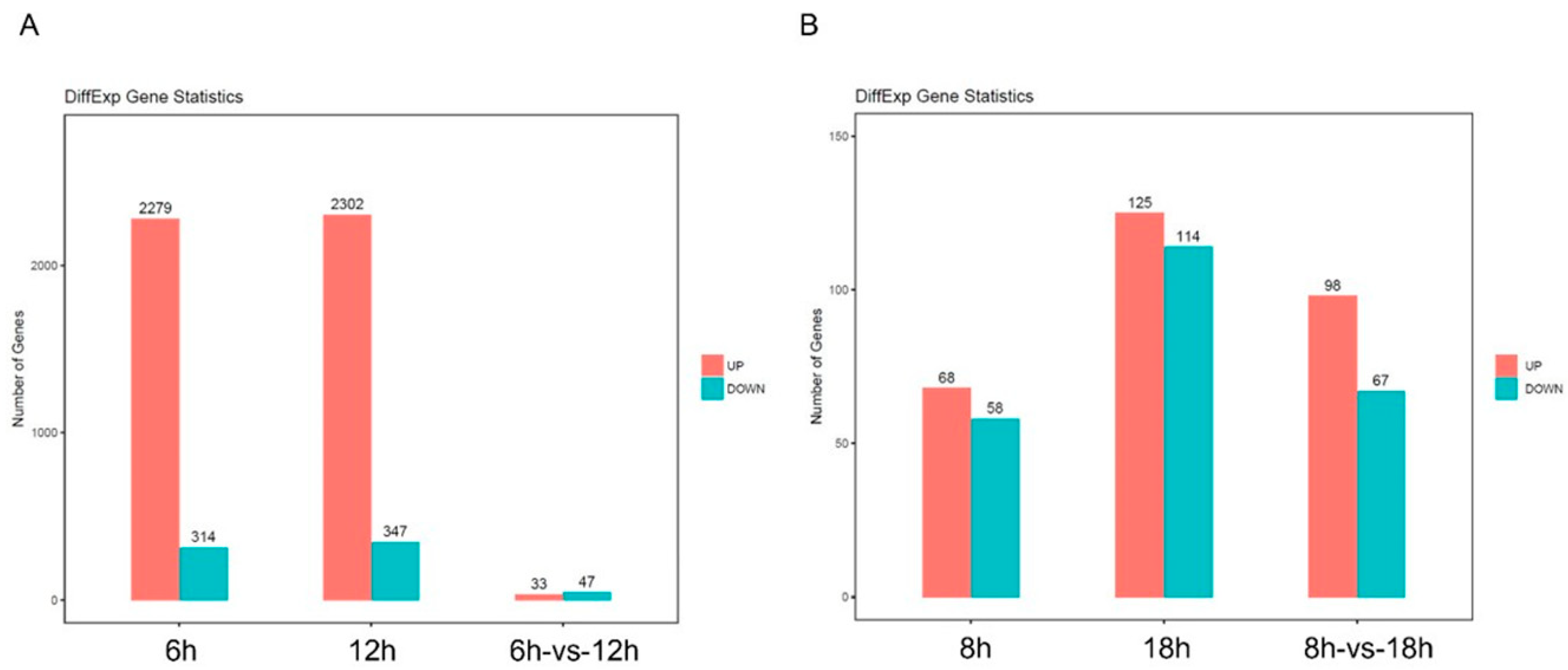
2.4. Analysis of Differently Expressed Genes after 34 Treatment by RNAseq
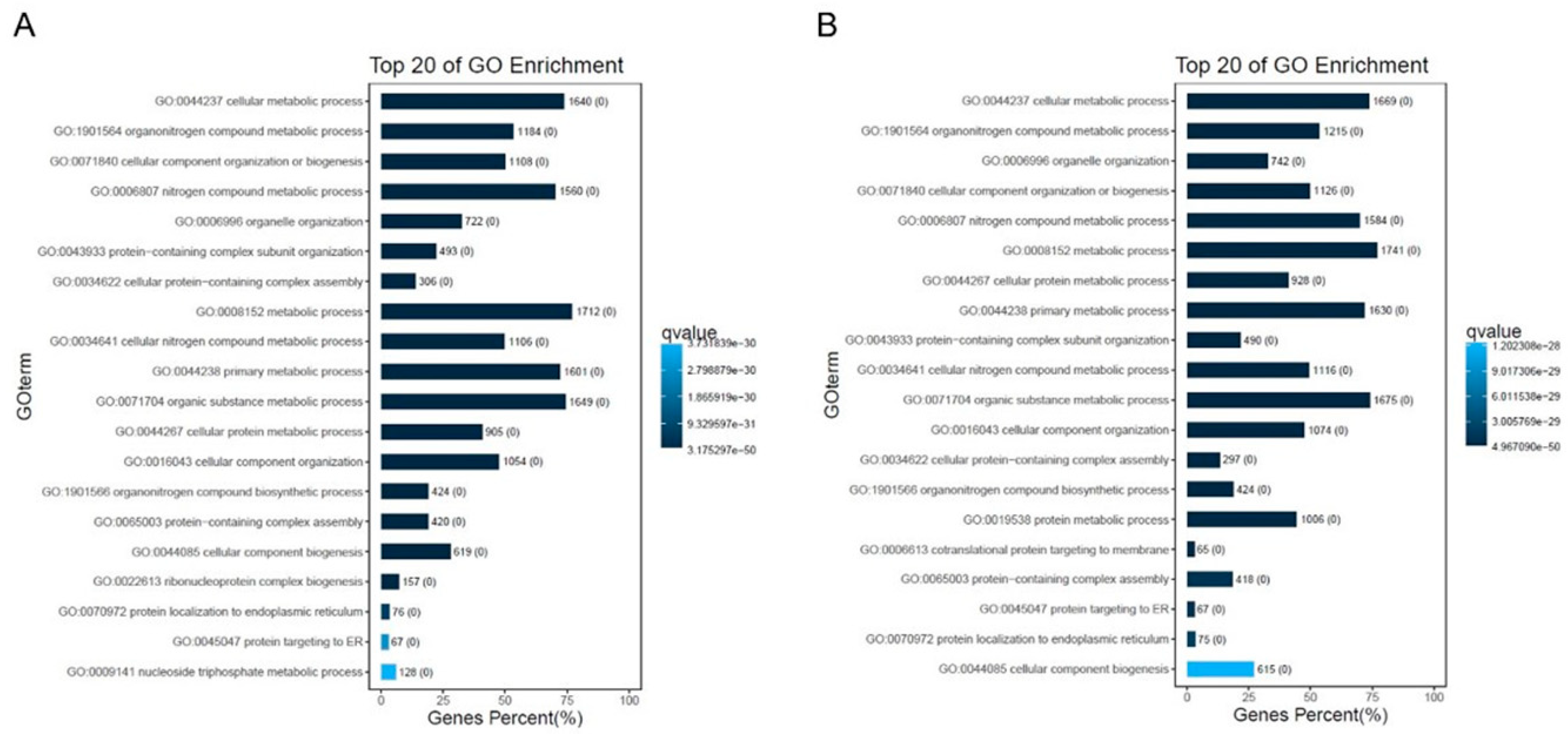
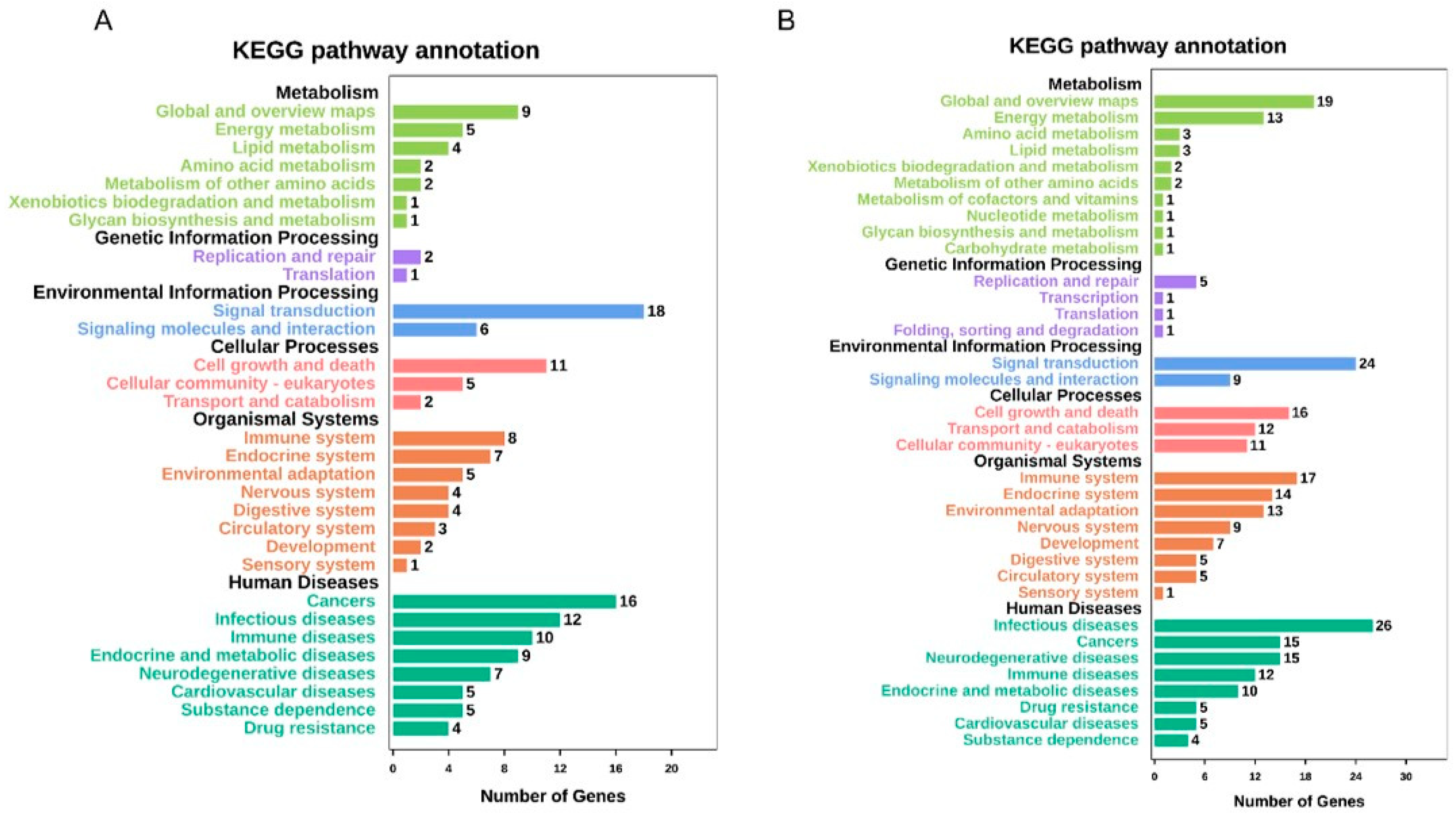
2.5. GO Mapping and KEGG Pathway Analyses of DEGs
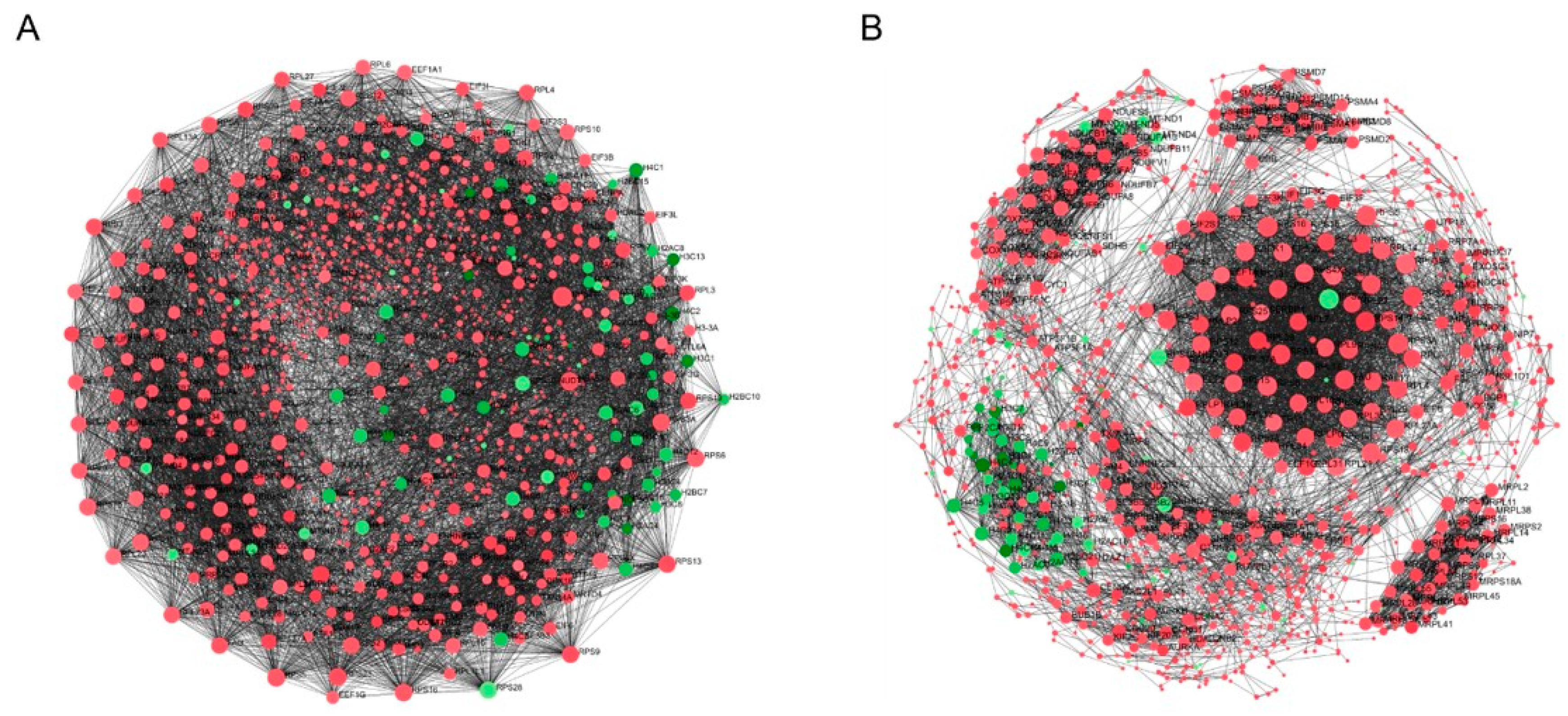
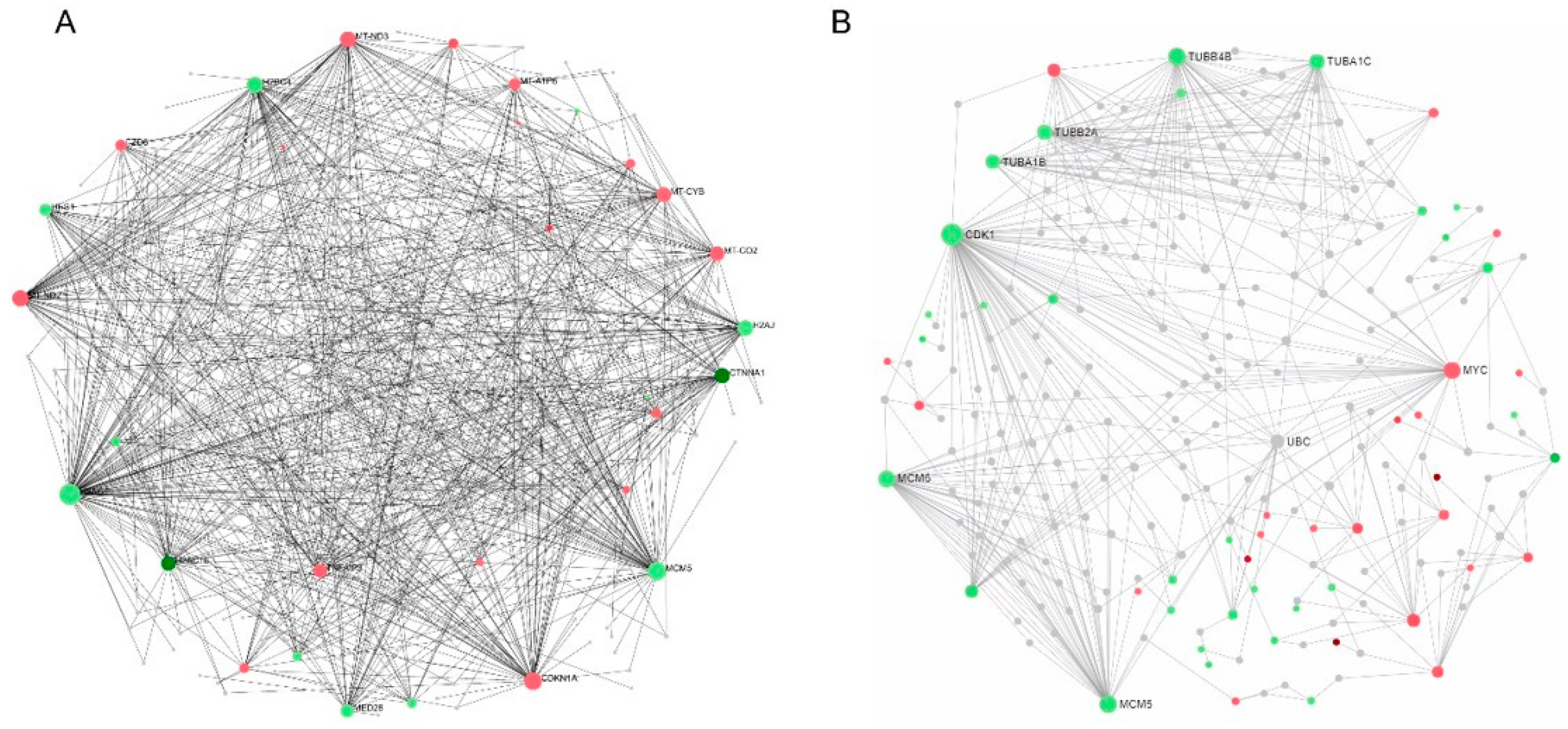
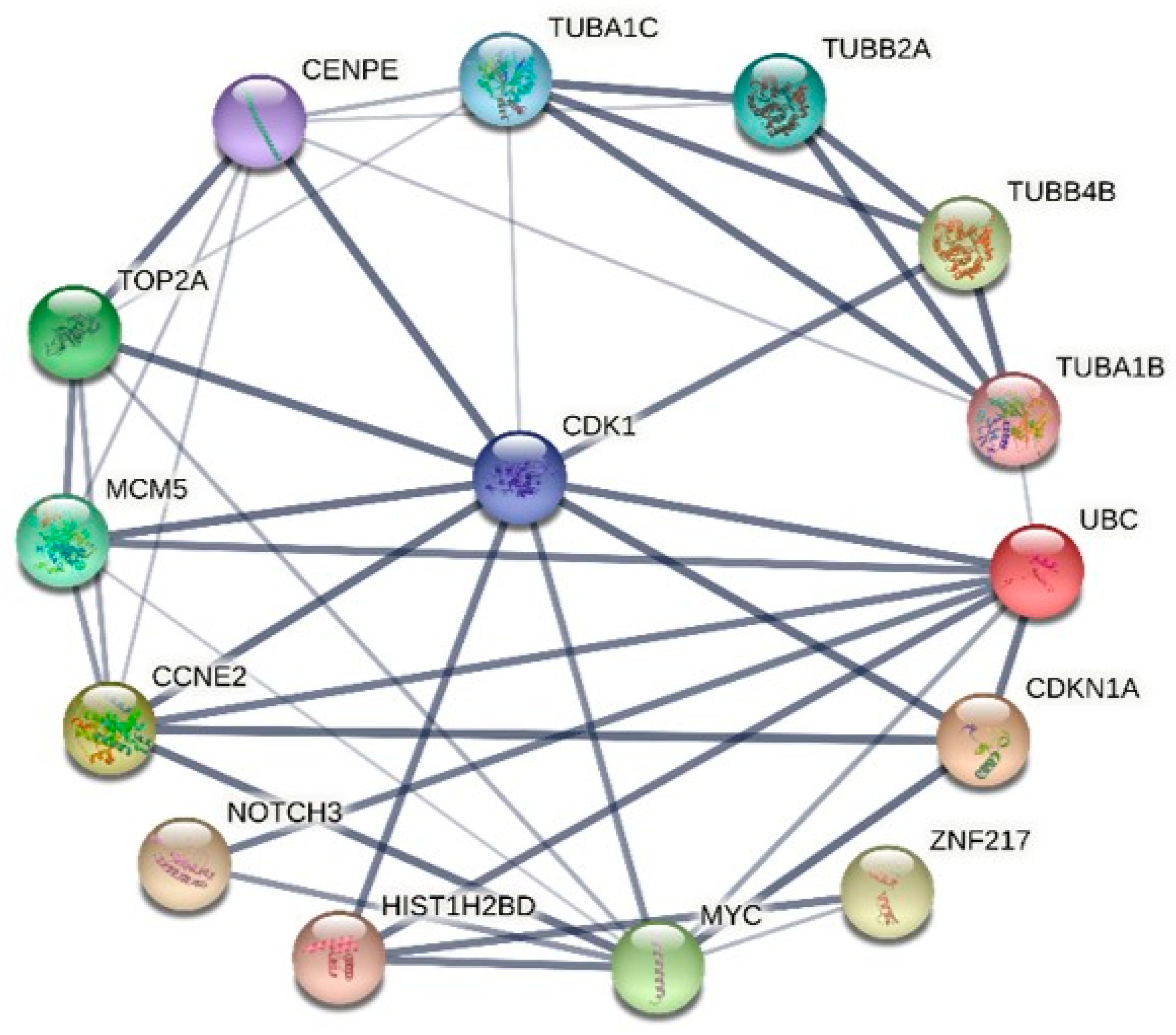
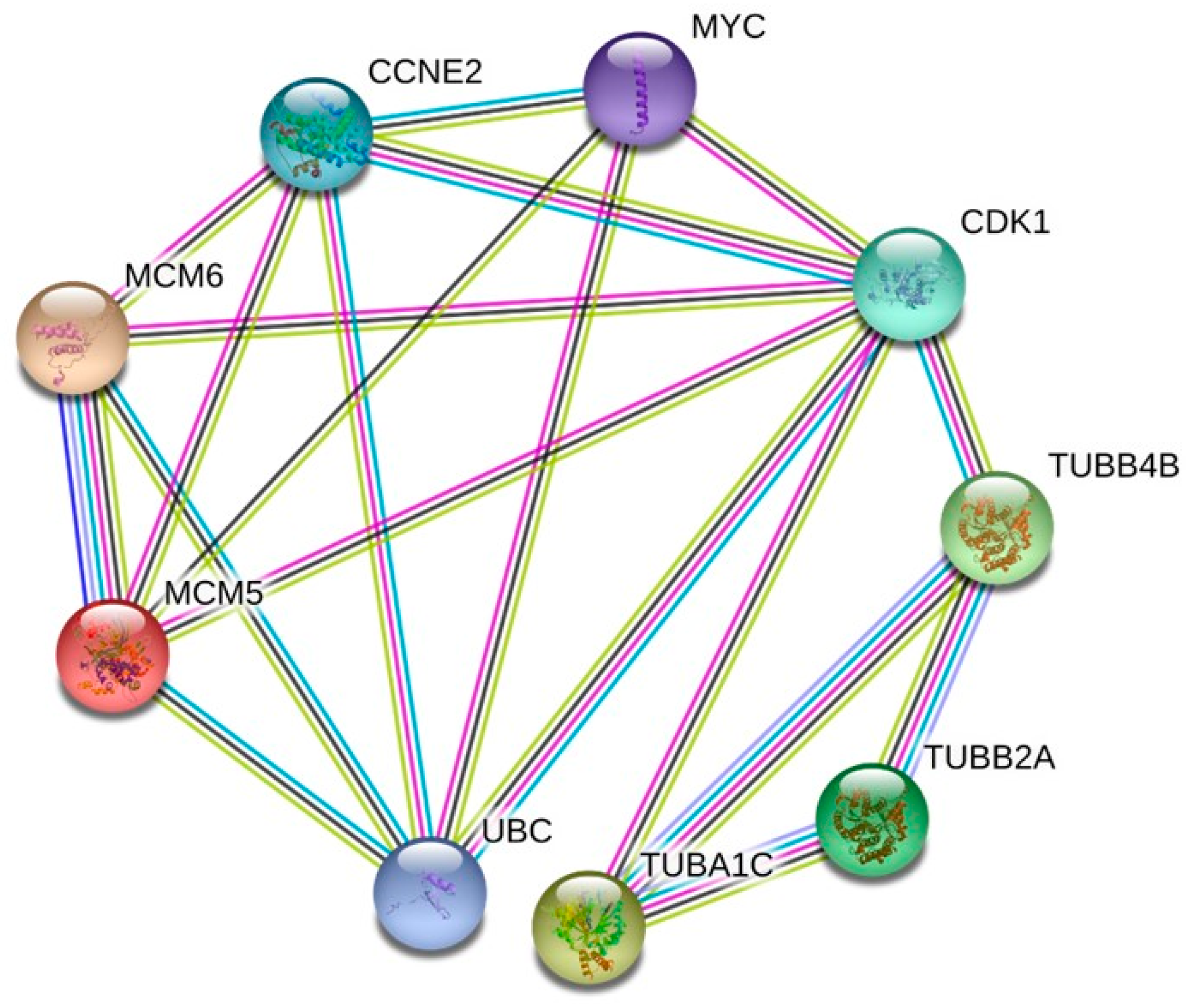
2.6. Protein–Protein Interaction (PPI) Network Analysis and Hub Genes Screening
2.7. Connectivity Map and Drugbank Analysis of DEGs
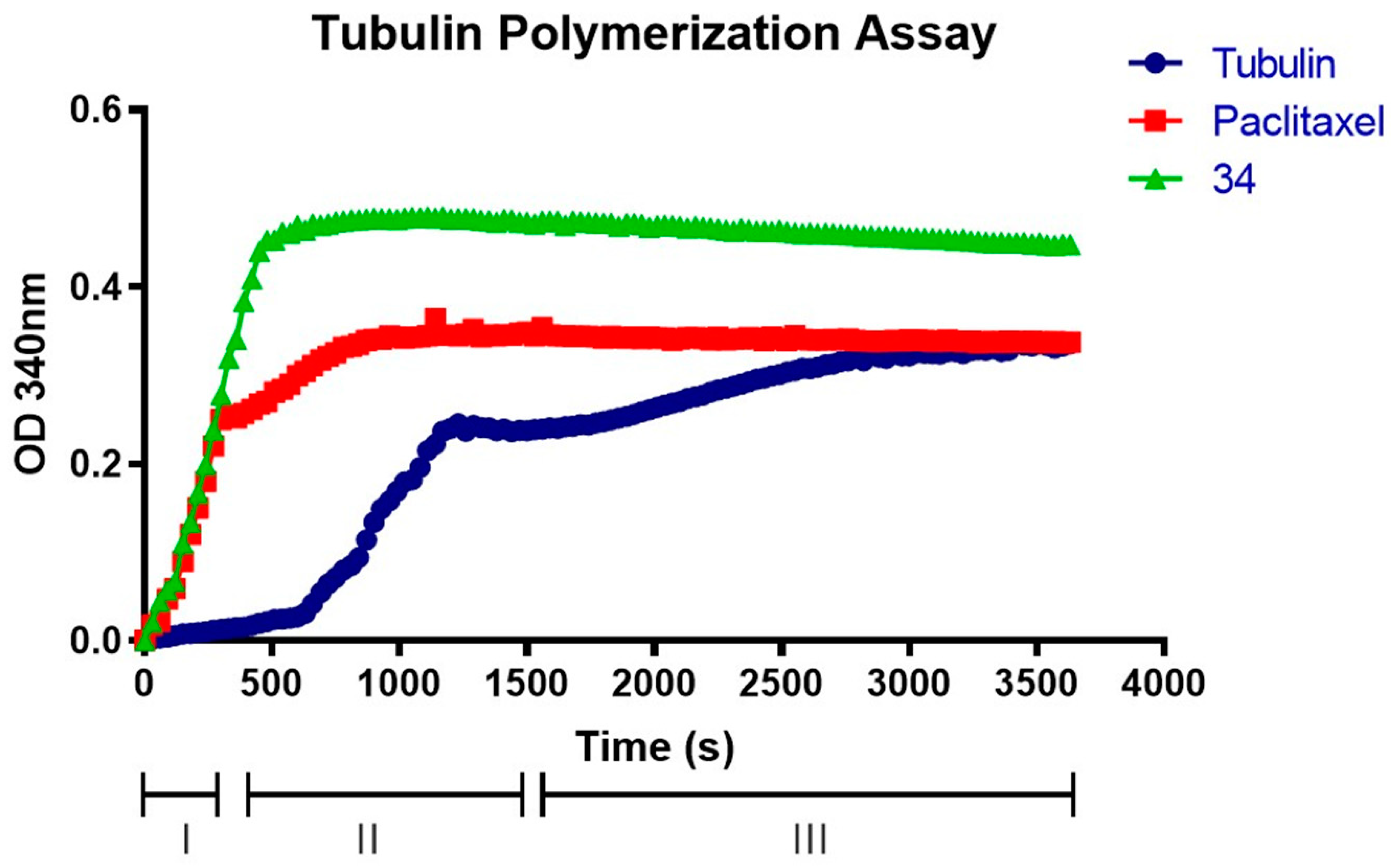
2.8. Compound 34 Induces Tubulin Polymerization
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. MTT Assay
4.3. Cell Cycle Analysis
4.4. Colony Formation Assay
4.5. RNAseq Library Preparation and Sequencing
4.6. Differentially Expressed Gene Analysis (DEGs)
4.7. GO Enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Analysis for DEG Characterization
4.8. Hub Genes and Key Modules
4.9. Connectivity Map (CMap) and Drugbank Analysis of DEGs
4.10. Tubulin Assay
4.11. Datasets
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Albahde, M.A.H.; Abdrakhimov, B.; Li, G.-Q.; Zhou, X.; Zhou, D.; Xu, H.; Qian, H.; Wang, W. The Role of Microtubules in Pancreatic Cancer: Therapeutic Progress. Front. Oncol. 2021, 11, 640863. [Google Scholar] [CrossRef] [PubMed]
- Brouhard, G.J.; Rice, L.M. Microtubule dynamics: An interplay of biochemistry and mechanics. Nat. Rev. Mol. Cell Biol. 2018, 19, 451–463. [Google Scholar] [CrossRef]
- Hemmat, M.; Castle, B.T.; Odde, D.J. Microtubule dynamics: Moving toward a multi-scale approach. Curr. Opin. Cell Biol. 2018, 50, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Checchi, P.M.; Nettles, J.H.; Zhou, J.; Snyder, J.P.; Joshi, H.C. Microtubule-interacting drugs for cancer treatment. Trends Pharmacol. Sci. 2003, 24, 361–365. [Google Scholar] [CrossRef]
- Florian, S.; Mitchison, T.J. Anti-Microtubule Drugs. Methods Mol. Biol. 2016, 1413, 403–421. [Google Scholar] [CrossRef] [PubMed]
- Prosser, S.L.; Pelletier, L. Mitotic spindle assembly in animal cells: A fine balancing act. Nat. Rev. Mol. Cell Biol. 2017, 18, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, M.O.; Prota, A.E. Microtubule-Targeting Agents: Strategies To Hijack the Cytoskeleton. Trends Cell Biol. 2018, 28, 776–792. [Google Scholar] [CrossRef] [PubMed]
- Kerssemakers, J.W.J.; Munteanu, E.L.; Laan, L.; Noetzel, T.L.; Janson, M.E.; Dogterom, M. Assembly dynamics of microtubules at molecular resolution. Nature 2006, 442, 709–712. [Google Scholar] [CrossRef]
- Howard, J.; Hyman, A.A. Microtubule polymerases and depolymerases. Curr. Opin. Cell Biol. 2007, 19, 31–35. [Google Scholar] [CrossRef]
- Panda, D.; Miller, H.P.; Wilson, L. Determination of the size and chemical nature of the stabilizing “cap” at microtubule ends using modulators of polymerization dynamics. Biochemistry 2002, 41, 1609–1617. [Google Scholar] [CrossRef]
- Kaul, R.; Risinger, A.L.; Mooberry, S.L. Microtubule-Targeting Drugs: More than Antimitotics. J. Nat. Prod. 2019, 82, 680–685. [Google Scholar] [CrossRef]
- Shuai, W.; Wang, G.; Zhang, Y.; Bu, F.; Zhang, S.; Miller, D.D.; Li, W.; Ouyang, L.; Wang, Y. Recent Progress on Tubulin Inhibitors with Dual Targeting Capabilities for Cancer Therapy. J. Med. Chem. 2021, 64, 7963–7990. [Google Scholar] [CrossRef] [PubMed]
- Dhyani, P.; Quispe, C.; Sharma, E.; Bahukhandi, A.; Sati, P.; Attri, D.C.; Szopa, A.; Sharifi-Rad, J.; Docea, A.O.; Mardare, I.; et al. Anticancer potential of alkaloids: A key emphasis to colchicine, vinblastine, vincristine, vindesine, vinorelbine and vincamine. Cancer Cell Int. 2022, 22, 206. [Google Scholar] [CrossRef] [PubMed]
- Yassine, F.; Salibi, E.; Gali-Muhtasib, H. Overview of the Formulations and Analogs in the Taxanes’ Story. Curr. Med. Chem. 2016, 23, 4540–4558. [Google Scholar] [CrossRef] [PubMed]
- Rida, P.C.G.; Livecche, D.; Ogden, A.; Zhou, J.; Aneja, R. The Noscapine Chronicle: A Pharmaco-Historic Biography of the Opiate Alkaloid Family and its Clinical Applications. Med. Res. Rev. 2015, 35, 1072–1096. [Google Scholar] [CrossRef] [PubMed]
- Tomar, V.; Kukreti, S.; Prakash, S.; Madan, J.; Chandra, R. Noscapine and its Analogs as Chemotherapeutic Agent: Current updates. Curr. Top. Med. Chem. 2017, 17, 174–188. [Google Scholar] [CrossRef]
- Čermák, V.; Dostál, V.; Jelínek, M.; Libusová, L.; Kovář, J.; Rösel, D.; Brábek, J. Microtubule-targeting agents and their impact on cancer treatment. Eur. J. Cell Biol. 2020, 99, 151075. [Google Scholar] [CrossRef] [PubMed]
- Barreca, M.; Spano, V.; Rocca, R.; Bivacqua, R.; Gualtieri, G.; Raimondi, M.V.; Gaudio, E.; Bortolozzi, R.; Manfreda, L.; Bai, R.; et al. Identification of pyrrolo[3′,4’:3,4]cyclohepta[1,2-d][1,2]oxazoles as promising new candidates for the treatment of lymphomas. Eur. J. Med. Chem. 2023, 254, 115372. [Google Scholar] [CrossRef]
- Wang, X.; Gigant, B.; Zheng, X.; Chen, Q. Microtubule-targeting agents for cancer treatment: Seven binding sites and three strategies. MedComm–Oncology 2023, 2, e46. [Google Scholar] [CrossRef]
- Barreca, M.; Lang, N.; Tarantelli, C.; Spriano, F.; Barraja, P.; Bertoni, F. Antibody-drug conjugates for lymphoma patients: Preclinical and clinical evidences. Explor. Target. Antitumor Ther. 2022, 3, 763–794. [Google Scholar] [CrossRef]
- Carta, A.; Briguglio, I.; Piras, S.; Boatto, G.; La Colla, P.; Loddo, R.; Tolomeo, M.; Grimaudo, S.; Di Cristina, A.; Pipitone, R.M.; et al. 3-Aryl-2-[1H-benzotriazol-1-yl]acrylonitriles: A novel class of potent tubulin inhibitors. Eur. J. Med. Chem. 2011, 46, 4151–4167. [Google Scholar] [CrossRef] [PubMed]
- Riu, F.; Sanna, L.; Ibba, R.; Piras, S.; Bordoni, V.; Scorciapino, M.A.; Lai, M.; Sestito, S.; Bagella, L.; Carta, A. A comprehensive assessment of a new series of 5’,6’-difluorobenzotriazole-acrylonitrile derivatives as microtubule targeting agents (MTAs). Eur. J. Med. Chem. 2021, 222, 113590. [Google Scholar] [CrossRef]
- Carta, A.; Sanna, P.; Palomba, M.; Vargiu, L.; La Colla, M.; Loddo, R. Synthesis and antiproliferative activity of 3-aryl-2-(1H-benzotriazol-1-yl)acrylonitriles. Part III. Eur. J. Med. Chem. 2003, 37, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Riu, F.; Ibba, R.; Zoroddu, S.; Sestito, S.; Lai, M.; Piras, S.; Sanna, L.; Bordoni, V.; Bagella, L.; Carta, A. Design, synthesis, and biological screening of a series of 4’-fluoro-benzotriazole-acrylonitrile derivatives as microtubule-destabilising agents (MDAs). J. Enzym. Inhib. Med. Chem. 2022, 37, 2223–2240. [Google Scholar] [CrossRef] [PubMed]
- Risinger, A.; Li, J.; Bennett, M.; Rohena, C.; Peng, J.; Schriemer, D.; Mooberry, S. Taccalonolide binding to tubulin imparts microtubule stability and potent in vivo activity. Cancer Res. 2013, 73, 6870–6892. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Gao, L.; Yang, F.; Gao, G.; Li, L. GSK-3β Inhibitor Alsterpaullone Attenuates MPP+-Induced Cell Damage in a c-Myc-Dependent Manner in SH-SY5Y Cells. Front. Cell Neurosci. 2018, 12, 283. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Li, H.; Liu, W.; Mishra, S.K.; Li, C. Andrographolide Induces Apoptosis in Gastric Cancer Cells through Reactivation of p53 and Inhibition of Mdm-2. Dokl. Biochem. Biophys. 2021, 500, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Yang, R.; Li, H.; Ke, K.; Luo, C.; Yang, F.; Shi, X.-N.; Zhu, Y.; Liu, X.; Wong, M.-H.; et al. Econazole nitrate inhibits PI3K activity and promotes apoptosis in lung cancer cells. Sci. Rep. 2017, 7, 17987. [Google Scholar] [CrossRef]
- Byrem, T.M.; Beermann, D.H.; Robinson, T.F. The beta-agonist cimaterol directly enhances chronic protein accretion in skeletal muscle. J. Anim. Sci. 1998, 76, 988–998. [Google Scholar] [CrossRef]
- Friedli, M.J.; Inestrosa, N.C. Huperzine A and Its Neuroprotective Molecular Signaling in Alzheimer’s Disease. Molecules 2021, 26, 6531. [Google Scholar] [CrossRef]
- Hyun, S.Y.; Le, H.T.; Min, H.-Y.; Pei, H.; Lim, Y.; Song, I.; Nguyen, Y.T.K.; Hong, S.; Han, B.W.; Lee, H.-Y. Evodiamine inhibits both stem cell and non-stem-cell populations in human cancer cells by targeting heat shock protein 70. Theranostics 2021, 11, 2932. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ikuno, Y.; Ohj, M.; Kusaka, S.; Jiang, R.; Çekiç, O.; Sawa, M.; Tano, Y. Platelet-derived growth factor receptor kinase inhibitor AG1295 and inhibition of experimental proliferative vitreoretinopathy. Jpn. J. Ophthalmol. 2003, 47, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Sutter, A.P.; Maaser, K.; Gerst, B.; Krahn, A.; Zeitz, M.; Scherübl, H. Enhancement of peripheral benzodiazepine receptor ligand-induced apoptosis and cell cycle arrest of esophageal cancer cells by simultaneous inhibition of MAPK/ERK kinase. Biochem. Pharmacol. 2004, 67, 1701–1710. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.-J.; Lin, S.-S.; Wu, W.-R.; Chen, L.-R.; Li, C.-F.; Chen, H.-D.; Chou, C.-T.; Chen, Y.-C.; Liang, S.-S.; Chien, S.-T.; et al. A microtubule inhibitor, ABT-751, induces autophagy and delays apoptosis in Huh-7 cells. Toxicol. Appl. Pharmacol. 2016, 311, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-H.; Wei, S.-H.; Jiang, J.-H.; Chang, Y.-S.; Liu, M.-Y.; Fu, S.-L.; Huang, C.-Y.F.; Lin, W.-J. Perturbation of p38α MAPK as a Novel Strategy to Effectively Sensitize Chronic Myeloid Leukemia Cells to Therapeutic BCR-ABL Inhibitors. Int. J. Mol. Sci. 2021, 22, 12573. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Preuss, C.V. Inamrinone; StatPearls: Tampa, FL, USA, 2022. [Google Scholar]
- Mónica, F.Z.; De Nucci, G. Tadalafil for the treatment of benign prostatic hyperplasia. Expert. Opin. Pharmacother. 2019, 20, 929–937. [Google Scholar] [CrossRef]
- Maegawa, S.; Gopalakrishnan, V. PLK inhibitors come of age in pediatric brain tumors. Neuro Oncol. 2022, 24, 427. [Google Scholar] [CrossRef] [PubMed]
- Škubník, J.; Pavlíčková, V.S.; Ruml, T.; Rimpelová, S. Vincristine in Combination Therapy of Cancer: Emerging Trends in Clinics. Biology 2021, 10, 849. [Google Scholar] [CrossRef]
- Hseu, Y.-C.; Chiang, Y.-C.; Gowrisankar, Y.V.; Lin, K.-Y.; Huang, S.-T.; Shrestha, S.; Chang, G.-R.; Yang, H.-L. The In Vitro and In Vivo Anticancer Properties of Chalcone Flavokawain B through Induction of ROS-Mediated Apoptotic and Autophagic Cell Death in Human Melanoma Cells. Cancers 2020, 12, 2936. [Google Scholar] [CrossRef]
- Hou, Z.-J.; Luo, X.; Zhang, W.; Peng, F.; Cui, B.; Wu, S.-J.; Zheng, F.-M.; Xu, J.; Xu, L.-Z.; Long, Z.-J.; et al. Flubendazole, FDA-approved anthelmintic, targets breast cancer stem-like cells. Oncotarget 2015, 6, 6326–6340. [Google Scholar] [CrossRef]
- Jayasooriya, R.G.P.T.; Molagoda, I.M.N.; Park, C.; Jeong, J.-W.; Choi, Y.H.; Moon, D.-O.; Kim, M.-O.; Kim, G.-Y. Molecular chemotherapeutic potential of butein: A concise review. Food Chem. Toxicol. 2018, 112, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Campanacci, V.; Urvoas, A.; Cantos-Fernandes, S.; Aumont-Nicaise, M.; Arteni, A.-A.; Velours, C.; Valerio-Lepiniec, M.; Dreier, B.; Plückthun, A.; Pilon, A.; et al. Insight into microtubule nucleation from tubulin-capping proteins. Proc. Natl. Acad. Sci. USA 2019, 116, 9859–9864. [Google Scholar] [CrossRef]
- Zoroddu, S.; Corona, P.; Sanna, L.; Borghi, F.; Bordoni, V.; Asproni, B.; Pinna, G.A.; Bagella, L.; Murineddu, G. Novel 1,3,4-oxadiazole chalcogen analogues: Synthesis and cytotoxic activity. Eur. J. Med. Chem. 2022, 238, 114440. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Zoroddu, S.; Sanna, L.; Bordoni, V.; Lyu, W.; Murineddu, G.; Pinna, G.A.; Forcales, S.V.; Sala, A.; Kelvin, D.J.; Bagella, L. RNAseq Analysis of Novel 1,3,4-Oxadiazole Chalcogen Analogues Reveals Anti-Tubulin Properties on Cancer Cell Lines. Int. J. Mol. Sci. 2023, 24, 11263. [Google Scholar] [CrossRef] [PubMed]
- Sarhadi, V.K.; Armengol, G. Molecular Biomarkers in Cancer. Biomolecules 2022, 12, 1021. [Google Scholar] [CrossRef]
- Hozhabri, H.; Dehkohneh, R.S.G.; Razavi, S.M.; Salarian, F.; Rasouli, A.; Azami, J.; Shiran, M.G.; Kardan, Z.; Farrokhzad, N.; Namini, A.M.; et al. Comparative analysis of protein-protein interaction networks in metastatic breast cancer. PLoS ONE 2022, 17, e0260584. [Google Scholar] [CrossRef] [PubMed]
- Sobierajska, K.; Ciszewski, W.M.; Wawro, M.E.; Wieczorek-Szukała, K.; Boncela, J.; Papiewska-Pajak, I.; Niewiarowska, J.; Kowalska, M.A. TUBB4B Downregulation Is Critical for Increasing Migration of Metastatic Colon Cancer Cells. Cells 2019, 8, 810. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Zhang, C.; Lee, S.; Sjöstedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357, 2507. [Google Scholar] [CrossRef]
- Prassanawar, S.S.; Panda, D. Tubulin heterogeneity regulates functions and dynamics of microtubules and plays a role in the development of drug resistance in cancer. Biochem. J. 2019, 476, 1359–1376. [Google Scholar] [CrossRef]
- Jayathirtha, M.; Whitham, D.; Alwine, S.; Donnelly, M.; Neagu, A.-N.; Darie, C.C. Investigating the Function of Human Jumping Translocation Breakpoint Protein (hJTB) and Its Interacting Partners through In-Solution Proteomics of MCF7 Cells. Molecules 2022, 27, 8301. [Google Scholar] [CrossRef] [PubMed]
- Cushion, T.D.; Paciorkowski, A.R.; Pilz, D.T.; Mullins, J.G.; Seltzer, L.E.; Marion, R.W.; Tuttle, E.; Ghoneim, D.; Christian, S.L.; Chung, S.-K.; et al. De Novo Mutations in the Beta-Tubulin Gene TUBB2A Cause Simplified Gyral Patterning and Infantile-Onset Epilepsy. Am. J. Hum. Genet. 2014, 94, 634. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhu, H.; Chen, B.; He, X.; Shen, Y.; Zhang, X.; Xu, Y.; Xu, X. The oncogenic role of tubulin alpha-1c chain in human tumours. BMC Cancer 2022, 22, 498. [Google Scholar] [CrossRef] [PubMed]
- Bian, T.; Zheng, M.; Jiang, D.; Liu, J.; Sun, H.; Li, X.; Liu, L.; Zhang, J.; Liu, Y. Prognostic biomarker TUBA1C is correlated to immune cell infiltration in the tumor microenvironment of lung adenocarcinoma. Cancer Cell Int. 2021, 21, 144. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Hu, X.; Gu, L.; Jian, Z.; Li, L.; Hu, S.; Qiu, S.; Xiong, X. TUBA1C is a Prognostic Marker in Low-grade Glioma and Correlates with Immune Cell Infiltration in the Tumor Microenvironment. Front. Genet. 2021, 12, 1950. [Google Scholar] [CrossRef]
- Saito, Y.; Santosa, V.; Ishiguro, K.I.; Kanemaki, M.T. MCMBP promotes the assembly of the MCM2–7 hetero-hexamer to ensure robust DNA replication in human cells. Elife 2022, 11, e77393. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Lerman, L.O.; Lerman, A. Ubiquitin and Ubiquitin-Like Proteins in Protein Regulation. Circ. Res. 2007, 100, 1276–1291. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, W. Progress in Anticancer Drug Development Targeting Ubiquitination-Related Factors. Int. J. Mol. Sci. 2022, 23, 15104. [Google Scholar] [CrossRef] [PubMed]
- Kalous, J.; Jansová, D.; Šušor, A. Role of Cyclin-Dependent Kinase 1 in Translational Regulation in the M-Phase. Cells 2020, 9, 1568. [Google Scholar] [CrossRef]
- Fagundes, R.; Teixeira, L.K. Cyclin E/CDK2: DNA Replication, Replication Stress and Genomic Instability. Front. Cell Dev. Biol. 2021, 9, 774845. [Google Scholar] [CrossRef]
- García-Gutiérrez, L.; Bretones, G.; Molina, E.; Arechaga, I.; Symonds, C.; Acosta, J.C.; Blanco, R.; Fernández, A.; Alonso, L.; Sicinski, P.; et al. Myc stimulates cell cycle progression through the activation of Cdk1 and phosphorylation of p27. Sci. Rep. 2019, 9, 18693. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Bu, W.; Lv, G.; Xu, L.; Hou, D.; Wang, J.; Liu, X.; Yang, T.; Zhang, X.; Liu, Q.; et al. Disrupted mitochondrial homeostasis coupled with mitotic arrest generates antineoplastic oxidative stress. Oncogene 2021, 41, 427–443. [Google Scholar] [CrossRef] [PubMed]
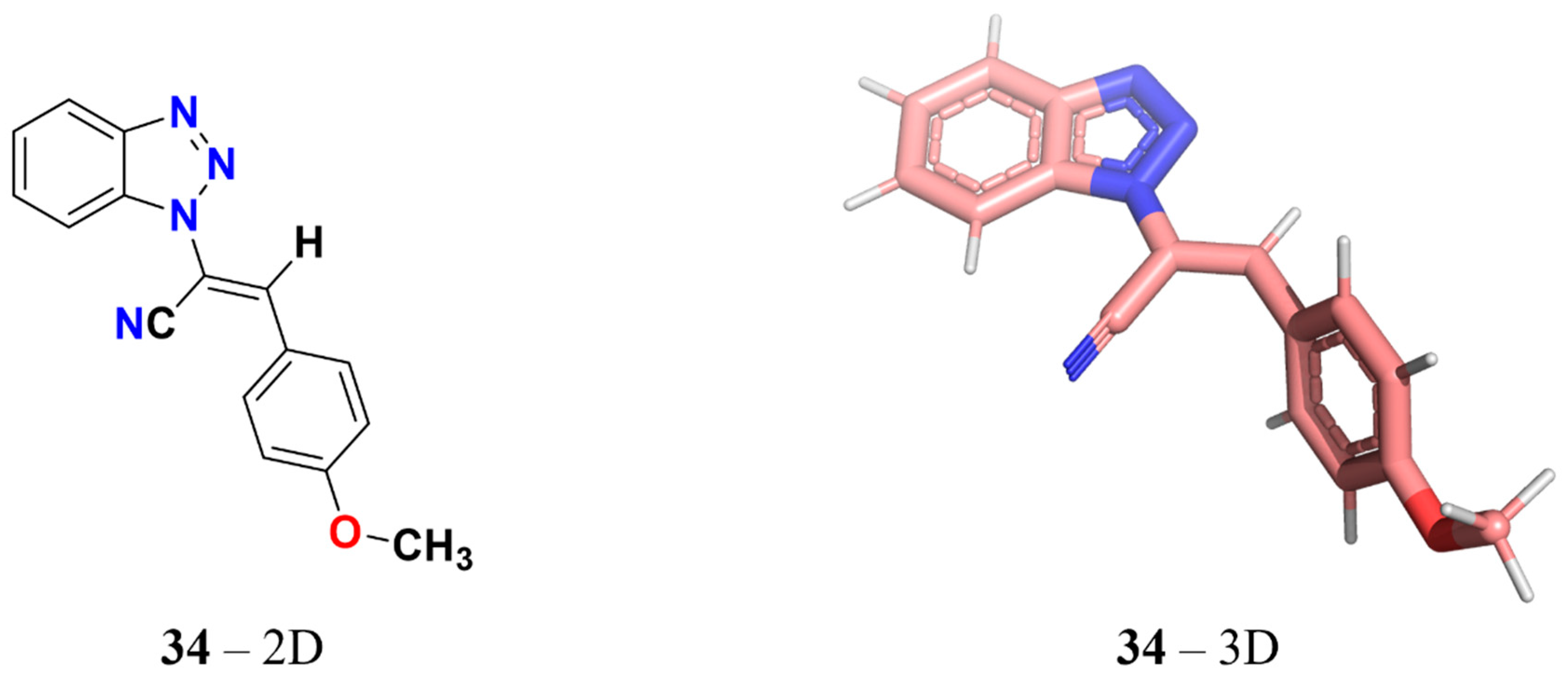



| IC50 a 34 (µM) Mean ± SD | Cell Lines |
|---|---|
| * 0.2 ± 0.0173 | SKMEL28 b |
| * 0.1 ± 0.0141 | MCF7 c |
| * 0.6 ± 0.0169 | SKMES-1 d |
| * 0.8 ± 0.0412 | HepG1 e |
| 0.02 ± 0.0071 | HeL af |
| 0.08 ± 0.0264 | PC3 g |
| Subnetwork Number | No. of Nodes | GO-BP | False Discovery Rate |
|---|---|---|---|
| 1 | 103 | protein-containing complex subunit organization | 1.69 × 10−6 |
| 2 | 92 | chromosome organization | 1.03 × 10−15 |
| 3 | 40 | negative regulation of gene expression, epigenetic | 6.49 × 10−28 |
| 4 | 49 | DNA conformation change | 1.56 × 10−18 |
| 5 | 24 | regulation of gene silencing | 2.55 × 10−10 |
| 6 | 376 | metabolic process | 2.61 × 10−5 |
| 7 | 47 | nucleosome assembly | 7.55 × 10−26 |
| Category | Term | Description | Gene Count | p Value |
|---|---|---|---|---|
| Biological Process (GO) | GO:0044772 | mitotic cell cycle phase transition | 6 | 3.34 × 10−7 |
| Biological Process (GO) | GO:0000082 | G1/S transition of mitotic cell cycle | 6 | 3.29 × 10−5 |
| Biological Process (GO) | GO:0006270 | DNA replication initiation | 3 | 4.52 × 10−5 |
| Biological Process (GO) | GO:0007017 | microtubule-based process | 5 | 0.0054 |
| KEGG_PATHWAY | hsa04110 | cell cycle | 5 | 6.27 × 10−8 |
| KEGG_PATHWAY | hsa03030 | DNA replication | 2 | 0.0010 |
| KEGG_PATHWAY | hsa04115 | p53 signaling pathway | 2 | 0.0029 |
| KEGG_PATHWAY | hsa04218 | cellular senescence | 2 | 0.00040 |
| Score | Target | Name | Description |
|---|---|---|---|
| 99.51 | GSK3 | Alsterpaullone [26] | Glycogen synthase kinase inhibitor |
| 98.03 | p53-HDM-2 | RITA [27] | MDM inhibitor |
| 97.67 | Cytochrome P-450 51 | Econazole [28] | Bacterial cell wall synthesis inhibitor |
| 97.5 | β-adrenergic receptors | Cimaterol [29] | Adrenergic receptor agonist |
| 97.37 | AChEIs | Huperzine-A [30] | Acetylcholinesterase inhibitor |
| 97.19 | HSP70 | Evodiamine [31] | ATPase inhibitor |
| 96.51 | PDGF | Tyrphostin-AG-1295 [32] | PDGFR receptor inhibitor |
| 95.95 | MEK1, MEK2 | U-0124 [33] | MEK inhibitor |
| 95.77 | β-tubulin | ABT-751 [34] | Tubulin inhibitor |
| 95.74 | p38 MAPK | TAK-715 [35] | p38 MAPK inhibitor |
| Score | Target | Name | Description |
|---|---|---|---|
| 99.68 | PDE-3, PDE-4, PDE-5 | Amrinone [36], Tadalafil [37] | Phosphodiesterase inhibitor |
| 99.50 | PLK1, PLK3 | Volasertib [38] | PLK inhibitor |
| 99.48 | TUBB, TUBA4A | Vincristine [39] | Tubulin inhibitor, microtubule inhibitor |
| 99.07 | HIF1A | Flavokavain-B [40] | Carcinoma cell growth inhibitor, hypoxia inducible factor inhibitor |
| 99.00 | TUBB | Flubendazole [41] | Acetylcholinesterase inhibitor, microtubule inhibitor |
| 98.28 | ACE, CXCL8, IL6, SIRT1, SRD5A1, SRD5A2, TNF | Butein [42] | Angiotensin-converting enzyme inhibitor, EGFR inhibitor, interleukin synthesis inhibitor, NFkB pathway inhibitor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zoroddu, S.; Sanna, L.; Bordoni, V.; Weidong, L.; Gadau, S.D.; Carta, A.; Kelvin, D.J.; Bagella, L. Identification of 3-Aryl-1-benzotriazole-1-yl-acrylonitrile as a Microtubule-Targeting Agent (MTA) in Solid Tumors. Int. J. Mol. Sci. 2024, 25, 5704. https://doi.org/10.3390/ijms25115704
Zoroddu S, Sanna L, Bordoni V, Weidong L, Gadau SD, Carta A, Kelvin DJ, Bagella L. Identification of 3-Aryl-1-benzotriazole-1-yl-acrylonitrile as a Microtubule-Targeting Agent (MTA) in Solid Tumors. International Journal of Molecular Sciences. 2024; 25(11):5704. https://doi.org/10.3390/ijms25115704
Chicago/Turabian StyleZoroddu, Stefano, Luca Sanna, Valentina Bordoni, Lyu Weidong, Sergio Domenico Gadau, Antonio Carta, David J. Kelvin, and Luigi Bagella. 2024. "Identification of 3-Aryl-1-benzotriazole-1-yl-acrylonitrile as a Microtubule-Targeting Agent (MTA) in Solid Tumors" International Journal of Molecular Sciences 25, no. 11: 5704. https://doi.org/10.3390/ijms25115704
APA StyleZoroddu, S., Sanna, L., Bordoni, V., Weidong, L., Gadau, S. D., Carta, A., Kelvin, D. J., & Bagella, L. (2024). Identification of 3-Aryl-1-benzotriazole-1-yl-acrylonitrile as a Microtubule-Targeting Agent (MTA) in Solid Tumors. International Journal of Molecular Sciences, 25(11), 5704. https://doi.org/10.3390/ijms25115704







