Those That Remain Caught in the “Organic Matter Trap”: Sorption/Desorption Study for Levelling the Fate of Selected Neonicotinoids
Abstract
1. Introduction
2. Results
2.1. Physico-Chemical Characteristics of Experimental Soil
2.2. Estimation of Nonlinear Sorption/Desorption Model in Describing the Behavior of Acetamiprid, Imidacloprid, and Thiacloprid in Soil
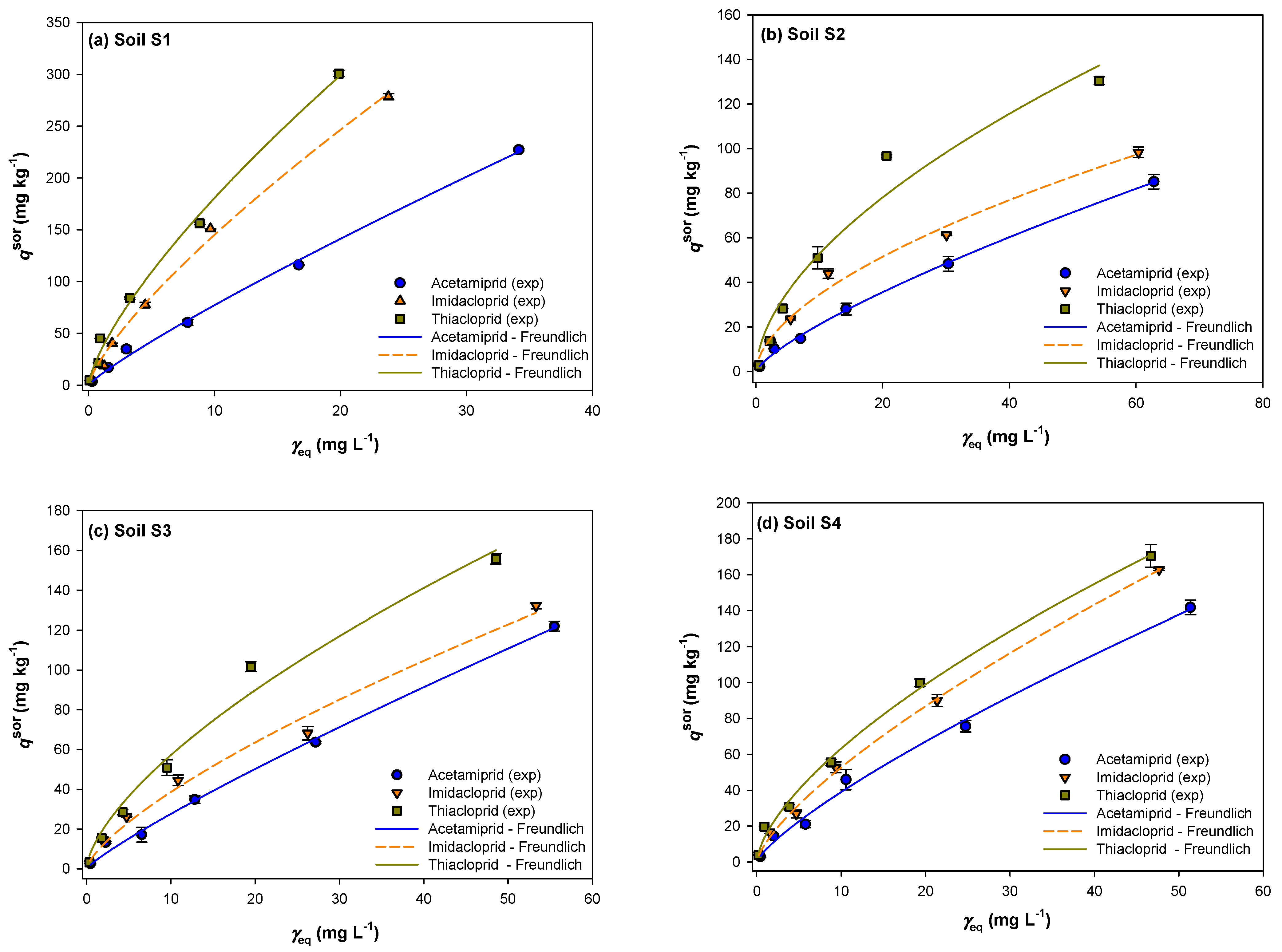
2.3. Sorption Equilibrium Study
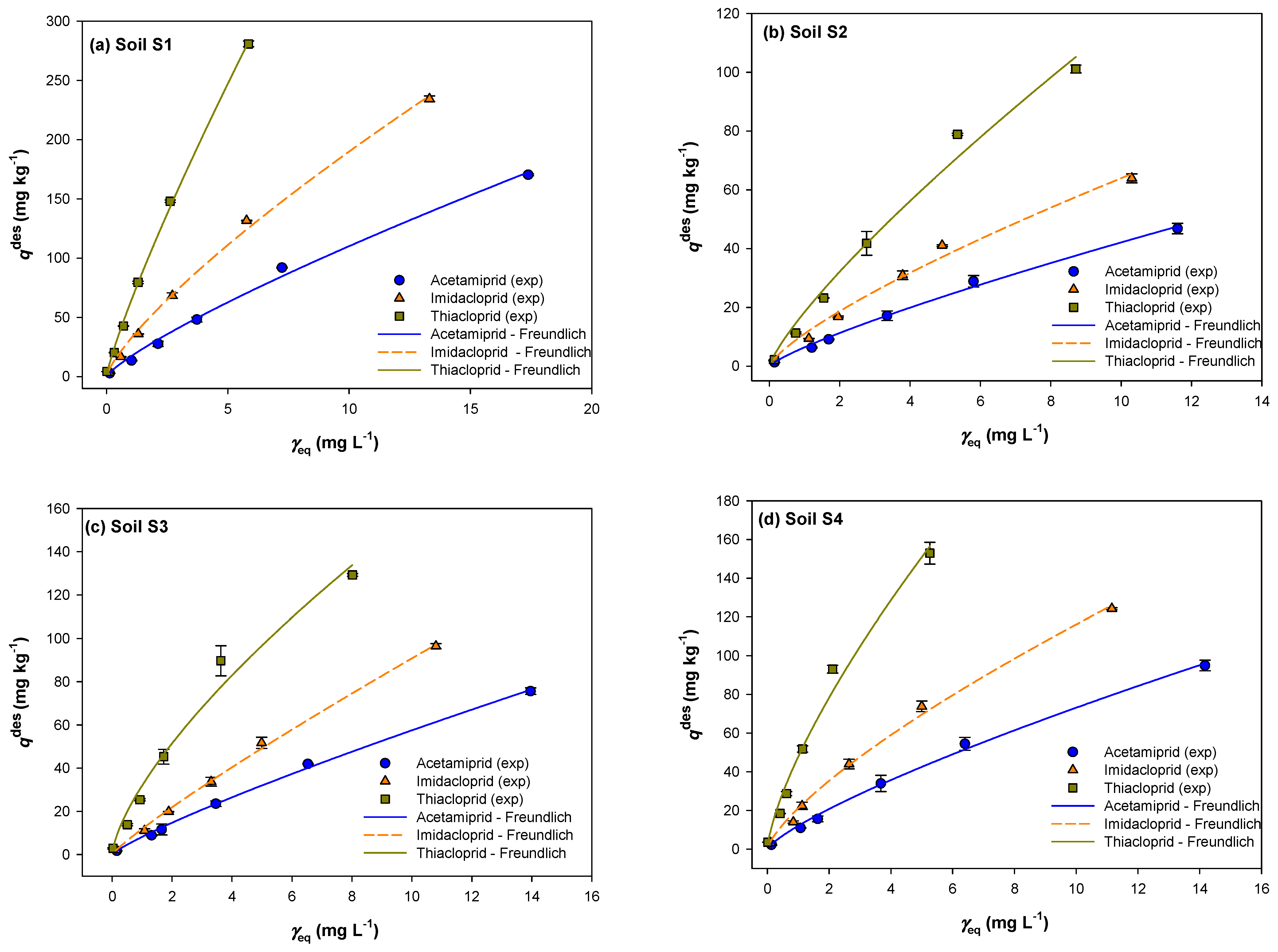
2.4. Desorption Equilibrium Study
2.5. Sorption and Desorption Isotherms
2.6. Effect of Physico-Chemical Soil Characteristics on Acetamiprid, Imidacloprid, and Thiacloprid Sorption/Desorption Parameters
2.7. Determination of the Dominant Physico-Chemical Soil Characteristics on the Sorption/Desorption Processes of Acetamiprid, Imidacloprid, and Thiacloprid
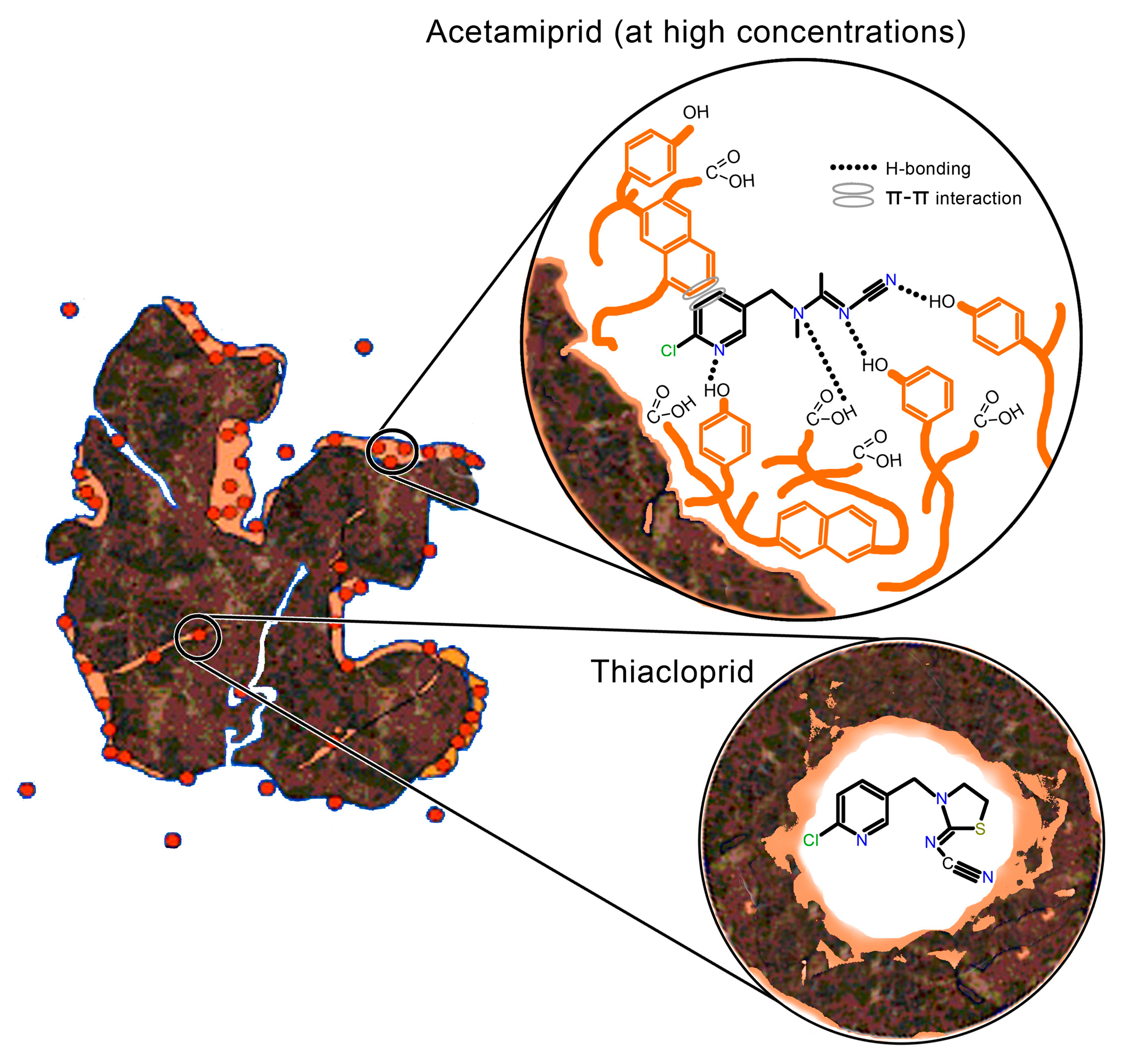
3. Discussion
4. Materials and Methods
4.1. Soil Sampling and Physico-Chemical Soil Properties
4.2. Sorption/Desorption Equilibrium Experiments
4.2.1. Sorption Equilibrium Experiments of Acetamiprid, Thiacloprid, and Imidacloprid in Soil
4.2.2. Desorption Equilibrium Experiments of Acetamiprid, Thiacloprid, and Imidacloprid in Soil
4.3. Analytical Methods
4.3.1. Analysis of Ca2+, Mg2+, Na+, and K+ on AAS
4.3.2. Analysis of Insecticides on HPLC-MS/MS
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pingali, P.L. Green Revolution: Impacts, Limits, and the Path Ahead. Proc. Natl. Acad. Sci. USA 2012, 109, 12302–12308. [Google Scholar] [CrossRef]
- Farm to Fork Strategy European Comision. Available online: https://commission.europa.eu/document/cac217cc-ca81-4d6b-abcf-d14343aefc5b_en (accessed on 20 May 2024).
- Comunication from the Commission to the European Parlament, the Council, the European Economic and Social Committee and the Committee of the Regions EU Biodiversity Strategy for 2030. Available online: https://eur-lex.europa.eu/legal-content/en/txt/?uri=celex:52020dc0380 (accessed on 20 May 2024).
- The Common Agricultural Policy: 2023-27. European Comision. Available online: https://agriculture.ec.europa.eu/common-agricultural-policy/cap-overview/cap-2023-27_en (accessed on 20 May 2024).
- Jeschke, P.; Nauen, R. Neonicotinoids-from Zero to Hero in Insecticide Chemistry. Pest Manag. Sci. 2008, 64, 1084–1098. [Google Scholar] [CrossRef]
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The Global Status of Insect Resistance to Neonicotinoid Insecticides. Pestic. Biochem. Physiol. 2015, 121, 78–87. [Google Scholar] [CrossRef]
- Klara, B.; Renata, B.; Ana, P. Potrošnja Pesticida u Hrvatskoj Poljoprivredi u Razdoblju Od 2012. Do 2017. Godine. Available online: https://hrcak.srce.hr/file/344383 (accessed on 20 May 2024).
- Simon-Delso, N.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D.W.; Giorio, C.; Girolami, V.; et al. Systemic Insecticides (Neonicotinoids and Fipronil): Trends, Uses, Mode of Action and Metabolites. Environ. Sci. Pollut. Res. 2015, 22, 5–34. [Google Scholar] [CrossRef]
- Goulson, D. REVIEW: An Overview of the Environmental Risks Posed by Neonicotinoid Insecticides. J. Appl. Ecol. 2013, 50, 977–987. [Google Scholar] [CrossRef]
- Thompson, D.A.; Lehmler, H.-J.; Kolpin, D.W.; Hladik, M.L.; Vargo, J.D.; Schilling, K.E.; LeFevre, G.H.; Peeples, T.L.; Poch, M.C.; LaDuca, L.E.; et al. A Critical Review on the Potential Impacts of Neonicotinoid Insecticide Use: Current Knowledge of Environmental Fate, Toxicity, and Implications for Human Health. Environ. Sci. Process. Impacts 2020, 22, 1315–1346. [Google Scholar] [CrossRef]
- Bonmatin, J.-M.; Giorio, C.; Girolami, V.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; Long, E.; Marzaro, M.; Mitchell, E.A.D.; et al. Environmental Fate and Exposure; Neonicotinoids and Fipronil. Environ. Sci. Pollut. Res. 2015, 22, 35–67. [Google Scholar] [CrossRef]
- PubChem—Substance and Compound Databases. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 20 May 2024).
- Maienfisch, P.; Angst, M.; Brandl, F.; Fischer, W.; Hofer, D.; Kayser, H.; Kobel, W.; Rindlisbacher, A.; Senn, R.; Steinemann, A.; et al. Chemistry and Biology of Thiamethoxam: A Second Generation Neonicotinoid. Pest Manag. Sci. 2001, 57, 906–913. [Google Scholar] [CrossRef]
- Robin, S. Stork Andreas Uptake, Translocation and Metabolism of Imidacloprid in Plants. Bull. Insectology 2003, 56, 35–40. [Google Scholar]
- Tomizawa, M.; Casida, J.E. Neonicotinoid Insecticide Toxicology: Mechanisms of Selective Action. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 247–268. [Google Scholar] [CrossRef]
- Pisa, L.W.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Downs, C.A.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; McField, M.; et al. Effects of Neonicotinoids and Fipronil on Non-Target Invertebrates. Environ. Sci. Pollut. Res. 2015, 22, 68–102. [Google Scholar] [CrossRef]
- Stokstad, E. Pesticides under Fire for Risks to Pollinators. Science 2013, 340, 674–676. [Google Scholar] [CrossRef]
- Pisa, L.; Goulson, D.; Yang, E.-C.; Gibbons, D.; Sánchez-Bayo, F.; Mitchell, E.; Aebi, A.; Van Der Sluijs, J.; MacQuarrie, C.J.K.; Giorio, C.; et al. An Update of the Worldwide Integrated Assessment (WIA) on Systemic Insecticides. Part 2: Impacts on Organisms and Ecosystems. Environ. Sci. Pollut. Res. 2021, 28, 11749–11797. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, Z.; Teng, Y.; Christie, P.; Wang, J.; Ren, W.; Luo, Y.; Li, Z. Non-Target Effects of Repeated Chlorothalonil Application on Soil Nitrogen Cycling: The Key Functional Gene Study. Sci. Total Environ. 2016, 543, 636–643. [Google Scholar] [CrossRef]
- Zhang, P.; Ren, C.; Sun, H.; Min, L. Sorption, Desorption and Degradation of Neonicotinoids in Four Agricultural Soils and Their Effects on Soil Microorganisms. Sci. Total Environ. 2018, 615, 59–69. [Google Scholar] [CrossRef]
- Craddock, H.A.; Huang, D.; Turner, P.C.; Quirós-Alcalá, L.; Payne-Sturges, D.C. Trends in Neonicotinoid Pesticide Residues in Food and Water in the United States, 1999–2015. Environ. Health 2019, 18, 7. [Google Scholar] [CrossRef]
- Lu, C.; Chang, C.-H.; Palmer, C.; Zhao, M.; Zhang, Q. Neonicotinoid Residues in Fruits and Vegetables: An Integrated Dietary Exposure Assessment Approach. Environ. Sci. Technol. 2018, 52, 3175–3184. [Google Scholar] [CrossRef]
- Bakırcı, G.T.; Yaman Acay, D.B.; Bakırcı, F.; Ötleş, S. Pesticide Residues in Fruits and Vegetables from the Aegean Region, Turkey. Food Chem. 2014, 160, 379–392. [Google Scholar] [CrossRef]
- Kapoor, U.; Srivastava, M.K.; Srivastava, A.K.; Patel, D.K.; Garg, V.; Srivastava, L.P. Analysis of Imidacloprid Residues in Fruits, Vegetables, Cereals, Fruit Juices, and Baby Foods, and Daily Intake Estimation in and around Lucknow, India. Environ. Toxicol. Chem. 2013, 32, 723–727. [Google Scholar] [CrossRef]
- Juraske, R.; Castells, F.; Vijay, A.; Muñoz, P.; Antón, A. Uptake and Persistence of Pesticides in Plants: Measurements and Model Estimates for Imidacloprid after Foliar and Soil Application. J. Hazard. Mater. 2009, 165, 683–689. [Google Scholar] [CrossRef]
- Kimura-Kuroda, J.; Komuta, Y.; Kuroda, Y.; Hayashi, M.; Kawano, H. Nicotine-Like Effects of the Neonicotinoid Insecticides Acetamiprid and Imidacloprid on Cerebellar Neurons from Neonatal Rats. PLoS ONE 2012, 7, e32432. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Plant Protection Products and their Residues (PPR). Scientific Opinion on the Developmental Neurotoxicity Potential of Acetamiprid and Imidacloprid. EFSA J. 2013, 11, 3471. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Peer Review of the Pesticide Risk Assessment of the Active Substance Acetamiprid. EFSA J. 2016, 14, e4610. [Google Scholar] [CrossRef]
- European Commission. Directorate-General for Health and Food Safety Commission Implementing Regulation (EU) 2018/113. Available online: https://eur-lex.europa.eu/eli/reg_impl/2018/113/oj (accessed on 20 May 2024).
- Lewis, K.A.; Tzilivakis, J.; Warner, D.J.; Green, A. An International Database for Pesticide Risk Assessments and Management. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 1050–1064. [Google Scholar] [CrossRef]
- European Commission. Directorate-General for Health and Food Safety Commission Implementing Regulation (EU) 2018/784. Available online: https://eur-lex.europa.eu/eli/reg_impl/2018/784/oj (accessed on 20 May 2024).
- European Commission. Directorate-General for Health and Food Safety Commission Implementing Regulation (EU) 2018/785. Available online: https://eur-lex.europa.eu/eli/reg_impl/2018/785/oj (accessed on 20 May 2024).
- European Commission. Directorate-General for Health and Food Safety Commission Implementing Regulation (EU) 2020/23 of 13 January 2020 Concerning the Non-Renewal of the Approval of the Active Substance Thiacloprid, in Accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council Concerning the Placing of Plant Protection Products on the Market, and Amending the Annex to Commission Implementing Regulation (EU) No 540/2011. Available online: https://eur-lex.europa.eu/eli/reg_impl/2020/23/oj (accessed on 20 May 2024).
- Klingelhöfer, D.; Braun, M.; Brüggmann, D.; Groneberg, D.A. Neonicotinoids: A Critical Assessment of the Global Research Landscape of the Most Extensively Used Insecticide. Environ. Res. 2022, 213, 113727. [Google Scholar] [CrossRef] [PubMed]
- Stehle, S.; Ovcharova, V.; Wolfram, J.; Bub, S.; Herrmann, L.Z.; Petschick, L.L.; Schulz, R. Neonicotinoid Insecticides in Global Agricultural Surface Waters—Exposure, Risks and Regulatory Challenges. Sci. Total Environ. 2023, 867, 161383. [Google Scholar] [CrossRef] [PubMed]
- Strouhova, A.; Velisek, J.; Stara, A. Selected Neonicotinoids and Associated Risk for Aquatic Organisms. Vet. Med. 2023, 68, 313–336. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, D.; Kania, J.; Kmiecik, E.; Malina, G.; Wątor, K. Fate of Selected Neonicotinoid Insecticides in Soil–Water Systems: Current State of the Art and Knowledge Gaps. Chemosphere 2020, 255, 126981. [Google Scholar] [CrossRef]
- Pietrzak, D.; Kania, J.; Malina, G.; Kmiecik, E.; Wątor, K. Pesticides from the EU First and Second Watch Lists in the Water Environment. Clean Soil Air Water 2019, 47, 1800376. [Google Scholar] [CrossRef]
- European Environment Agency Pesticides in Rivers, Lakes and Groundwater in Europe. Available online: https://www.eea.europa.eu/en/european-zero-pollution-dashboards/indicators/pesticides-in-rivers-lakes-and-groundwater-in-europe (accessed on 20 May 2024).
- Morrissey, C.A.; Mineau, P.; Devries, J.H.; Sanchez-Bayo, F.; Liess, M.; Cavallaro, M.C.; Liber, K. Neonicotinoid Contamination of Global Surface Waters and Associated Risk to Aquatic Invertebrates: A Review. Environ. Int. 2015, 74, 291–303. [Google Scholar] [CrossRef]
- Gupta, S.; Gajbhiye, V.T. Persistence of Acetamiprid in Soil. Bull. Environ. Contam. Toxicol. 2007, 78, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Liébana, J.A.; Mingorance, M.D.; Peña, A. Thiacloprid Adsorption and Leaching in Soil: Effect of the Composition of Irrigation Solutions. Sci. Total Environ. 2018, 610–611, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Petković Didović, M.; Kowalkowski, T.; Broznić, D. Emerging Contaminant Imidacloprid in Mediterranean Soils: The Risk of Accumulation Is Greater than the Risk of Leaching. Toxics 2022, 10, 358. [Google Scholar] [CrossRef] [PubMed]
- Aseperi, A.K.; Busquets, R.; Hooda, P.S.; Cheung, P.C.W.; Barker, J. Behaviour of Neonicotinoids in Contrasting Soils. J. Environ. Manag. 2020, 276, 111329. [Google Scholar] [CrossRef] [PubMed]
- Broznić, D.; Marinić, J.; Tota, M.; Jurešić, G.Č.; Petković, O.; Milin, Č. Hysteretic Behavior of Imidacloprid Sorption-Desorption in Soils of Croatian Coastal Regions. Soil Sediment Contam. Int. J. 2012, 21, 850–871. [Google Scholar] [CrossRef]
- Coquet, Y. Variation of Pesticide Sorption Isotherm in Soil at the Catchment Scale. Pest Manag. Sci. 2003, 59, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Redlich, O.; Peterson, D.L. A Useful Adsorption Isotherm. J. Phys. Chem. 1959, 63, 1024. [Google Scholar] [CrossRef]
- Kandil, M.M.; El-Aswad, A.F.; Koskinen, W.C. Sorption–Desorption of Imidacloprid onto a Lacustrine Egyptian Soil and Its Clay and Humic Acid Fractions. J. Environ. Sci. Health Part B 2015, 50, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Dankyi, E.; Gordon, C.; Carboo, D.; Apalangya, V.A.; Fomsgaard, I.S. Sorption and Degradation of Neonicotinoid Insecticides in Tropical Soils. J. Environ. Sci. Health Part B 2018, 53, 587–594. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, W.; Ma, Y.; Liu, K. Sorption and Degradation of Imidacloprid in Soil and Water. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2006, 41, 623–634. [Google Scholar] [CrossRef]
- Nemeth-Konda, L.; Füleky, G.; Morovjan, G.; Csokan, P. Sorption Behaviour of Acetochlor, Atrazine, Carbendazim, Diazinon, Imidacloprid and Isoproturon on Hungarian Agricultural Soil. Chemosphere 2002, 48, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.; Bi, G.; Ward, T.J.; Li, L. Adsorption and Degradation of Neonicotinoid Insecticides in Agricultural Soils. Environ. Sci. Pollut. Res. 2023, 30, 47516–47526. [Google Scholar] [CrossRef] [PubMed]
- Sinčić Modrić, G.; Petković Didović, M.; Dubrović, I.; Žurga, P.; Broznić, D. Those That Remain: Sorption/Desorption Behaviour and Kinetics of the Neonicotinoids Still in Use. Int. J. Mol. Sci. 2023, 24, 6548. [Google Scholar] [CrossRef] [PubMed]
- EPA Integrated Pest Management (IPM) Principles. Available online: https://food.ec.europa.eu/plants/pesticides/sustainable-use-pesticides/integrated-pest-management-ipm (accessed on 20 May 2024).
- Li, Z. Prioritizing Agricultural Pesticides to Protect Human Health: A Multi-Level Strategy Combining Life Cycle Impact and Risk Assessments. Ecotoxicol. Environ. Saf. 2022, 242, 113869. [Google Scholar] [CrossRef] [PubMed]
- Zebec, V.; Semialjac, Z.; Marković, M.; Tadić, V.; Radić, D.; Rastija, D. Influence of Physical and Chemical Properties of Different Soil Types on Optimal Soil Moisture for Tillage. Agriculture 2017, 23, 10–18. [Google Scholar] [CrossRef]
- Broznić, D.; Milin, Č. Imidacloprid—Olive Orchard “Guardian”. Medicina 2009, 45, 119–126. [Google Scholar] [PubMed]
- Rubinić, V.; Husnjak, S. Clay and Humus Contents Have the Key Impact on Physical Properties of Croatian Pseudogleys. Agric. Conspec. Sci. 2016, 81, 187–191. [Google Scholar]
- Broznić, D.; Milin, Č. Effects of Temperature on Sorption-Desorption Processes of Imidacloprid in Soils of Croatian Coastal Regions. J. Environ. Sci. Health Part B 2012, 47, 779–794. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, D.; Kania, J.; Kmiecik, E. Transport Parameters of Selected Neonicotinoids in Different Aquifer Materials Using Batch Sorption Tests. Geol. Geophys. Environ. 2022, 48, 367–379. [Google Scholar] [CrossRef]
- Xu, Z.; Qian, X.; Wang, C.; Zhang, C.; Tang, T.; Zhao, X.; Li, L. Environmentally Relevant Concentrations of Microplastic Exhibits Negligible Impacts on Thiacloprid Dissipation and Enzyme Activity in Soil. Environ. Res. 2020, 189, 109892. [Google Scholar] [CrossRef]
- Carbo, L.; Martins, E.L.; Dores, E.F.G.C.; Spadotto, C.A.; Weber, O.L.S.; De-Lamonica-Freire, E.M. Acetamiprid, Carbendazim, Diuron and Thiamethoxam Sorption in Two Brazilian Tropical Soils. J. Environ. Sci. Health Part B 2007, 42, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Olivier, D.P.; Kookana, R.S.; Quintana, B. Sorption of Pesticides in Tropical and Temperate Soils from Australia and the Philippines. J. Agric. Food Chem. 2005, 53, 6420–6425. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-Y.; Mu, C.-L.; Gu, C.; Liu, C.; Liu, X.-J. Impact of Woodchip Biochar Amendment on the Sorption and Dissipation of Pesticide Acetamiprid in Agricultural Soils. Chemosphere 2011, 85, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Kodešová, R.; Kočárek, M.; Kodeš, V.; Drábek, O.; Kozák, J.; Hejtmánková, K. Pesticide Adsorption in Relation to Soil Properties and Soil Type Distribution in Regional Scale. J. Hazard. Mater. 2011, 186, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.; Koskinen, W.C.; Yen, P.Y. Sorption−Desorption of Imidacloprid and Its Metabolites in Soils. J. Agric. Food Chem. 1997, 45, 1468–1472. [Google Scholar] [CrossRef]
- McCall, P.J.; Laskowski, D.A.; Swann, R.L.; Dishburger, H.J. Measurements of Sorption Coefficients of Organic Chemicals and Their Use in Environmental Fate Analysis. In: Test Protocols for Environmental Fate and Movement of Toxicants. In Proceedings of the 94th Annual Meeting of the American Association of Official Analytical Chemists (AOAC), Washington, DC, USA, 21–22 October 1980. [Google Scholar]
- Sheng, G.Y.; Johnston, C.T.; Teppen, B.J.; Boyd, S.A. Potential Contributions of Smectite Clays and Organic Matter to Pesticide Retention in Soils. J. Agric. Food Chem. 2001, 49, 2899–2907. [Google Scholar] [CrossRef] [PubMed]
- Reddy, T.V.; Chauhan, S.; Chakraborty, S. Adsorption Isotherm and Kinetics Analysis of Hexavalent Chromium and Mercury on Mustard Oil Cake. Environ. Eng. Res. 2016, 22, 95–107. [Google Scholar] [CrossRef]
- Carrizosa, M.J.; Rice, P.J.; Koskinen, W.C.; Carrizosa, I.; Del Hermosin, M.C. Sorption of Isoxaflutole and DKN on Organoclays. Clays Clay Miner. 2004, 52, 341–349. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, H.; Min, L.; Ren, C. Biochars Change the Sorption and Degradation of Thiacloprid in Soil: Insights into Chemical and Biological Mechanisms. Environ. Pollut. 2018, 236, 158–167. [Google Scholar] [CrossRef]
- Giles, C.H.; Smith, D.; Huitson, A. A General Treatment and Classification of the Solute Adsorption Isotherm. I. Theoretical. J. Colloid Interface Sci. 1974, 47, 755–765. [Google Scholar] [CrossRef]
- Xing, B.; Pignatello, J.J. Dual-Mode Sorption of Low-Polarity Compounds in Glassy Poly(Vinyl Chloride) and Soil Organic Matter. Environ. Sci. Technol. 1997, 31, 792–799. [Google Scholar] [CrossRef]
- Gunasekara, A.S.; Xing, B. Sorption and Desorption of Naphthalene by Soil Organic Matter: Importance of Aromatic and Aliphatic Components. J. Environ. Qual. 2003, 32, 240–246. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, H.; Mittelmeijer-Hazeleger, M.C. Adsorption of CO2 and N2 on Soil Organic Matter: Nature of Porosity, Surface Area, and Diffusion Mechanisms. Environ. Sci. Technol. 1996, 30, 408–413. [Google Scholar] [CrossRef]
- Xing, B.; McGill, W.B.; Dudas, M.J. Sorption of α-Naphthol onto Organic Sorbents Varying in Polarity and Aromaticity. Chemosphere 1994, 28, 145–153. [Google Scholar] [CrossRef]
- Kang, S.; Xing, B. Phenanthrene Sorption to Sequentially Extracted Soil Humic Acids and Humins. Environ. Sci. Technol. 2005, 39, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Perminova, I.V.; Grechishcheva, N.Y.; Kovalevskii, D.V.; Kudryavtsev, A.V.; Petrosyan, V.S.; Matorin, D.N. Quantification and Prediction of the Detoxifying Properties of Humic Substances Related to Their Chemical Binding to Polycyclic Aromatic Hydrocarbons. Environ. Sci. Technol. 2001, 35, 3841–3848. [Google Scholar] [CrossRef]
- Kukkonen, J. Bioavailability of Organic Pollutants in Boreal Waters with Varying Levels of Dissolved Organic Material. Water Res. 1991, 25, 455–463. [Google Scholar] [CrossRef]
- Schlautman, M.A.; Morgan, J.J. Effects of Aqueous Chemistry on the Binding of Polycyclic Aromatic Hydrocarbons by Dissolved Humic Materials. Environ. Sci. Technol. 1993, 27, 961–969. [Google Scholar] [CrossRef]
- Jin, J.; Kang, M.; Sun, K.; Pan, Z.; Wu, F.; Xing, B. Properties of Biochar-Amended Soils and Their Sorption of Imidacloprid, Isoproturon, and Atrazine. Sci. Total Environ. 2016, 550, 504–513. [Google Scholar] [CrossRef]
- Huang, W.; Peng, P.; Yu, Z.; Fu, J. Effects of Organic Matter Heterogeneity on Sorption and Desorption of Organic Contaminants by Soils and Sediments. Appl. Geochem. 2003, 18, 955–972. [Google Scholar] [CrossRef]
- Prashar, P.; Shah, S. Impact of Fertilizers and Pesticides on Soil Microflora in Agriculture. In Sustainable Agriculture Reviews; Lichtfouse, E., Ed.; Springer International Publishing: Cham, Switzerland, 2016; Volume 19, pp. 331–361. ISBN 978-3-319-26776-0. [Google Scholar]
- Mörtl, M.; Kereki, O.; Darvas, B.; Klátyik, S.; Vehovszky, Á.; Győri, J.; Székács, A. Study on Soil Mobility of Two Neonicotinoid Insecticides. J. Chem. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Singh, N.S.; Mukherjee, I.; Das, S.K.; Varghese, E. Leaching of Clothianidin in Two Different Indian Soils: Effect of Organic Amendment. Bull. Environ. Contam. Toxicol. 2018, 100, 553–559. [Google Scholar] [CrossRef]
- US EPA. Archive Document Standard Operating Procedures Soil Sampling; US EPA: Washington, DC, USA, 2012.
- OECD. OECD Test. No. 106: Adsorption—Desorption Using. a Batch Equilibrium Method; OECD Guidelines for the Testing of Chemicals, Section 1; OECD: Paris, France, 2000; ISBN 978-92-64-06960-2. [Google Scholar]
- EN 12393-1:2013; Foods of Plant Origin—Multiresidue Methods for the Determination of Pesticide Residues by GC or LC-MS/MS 2013. European Union: Brussels, Belgium, 2013.
- EN 12393-2:2013; Foods of Plant Origin—Multiresidue Methods for the Determination of Pesticide Residues by GC or LC-MS/MS—Part 2: Methods for Extraction and Clean-Up 2013. European Union: Brussels, Belgium, 2013.
- EN 12393-3:2013; Foods of Plant Origin—Multiresidue Methods for the Determination of Pesticide Residues by GC or LC-MS/MS—Part 3: Determination and Confirmatory Tests (EN 12393-3:2013). European Union: Brussels, Belgium, 2013.
- Hinz, C. Description of Sorption Data with Isotherm Equations. Geoderma 2001, 99, 225–243. [Google Scholar] [CrossRef]
- Selim, H.M.; Zhu, H. Atrazine Sorption-Desorption Hysteresis by Sugarcane Mulch Residue. J. Environ. Qual. 2005, 34, 325–335. [Google Scholar] [CrossRef]

| Properties | Acetamiprid | Imidacloprid | Thiacloprid |
|---|---|---|---|
| Chemical structure | 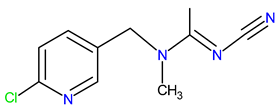 | 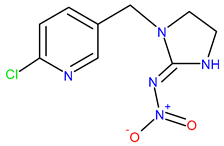 | 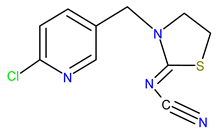 |
| IUPAC name | N-[(6-chloropyridin-3-yl)methyl]-N′-cyano-N-methylethanimidamide | (NE)-N-[1-[(6-chloropyridin-3-yl)methyl]imidazolidin-2-ylidene]nitramide | [3-[(6-chloropyridin-3-yl)methyl]-1.3-thiazolidin-2-ylidene]cyanamide |
| Molecular formula | C10H11ClN4 | C9H10ClN5O2 | C10H9ClN4S |
| Molar mass (g/mol) | 222.67 | 255.66 | 255.72 |
| Melting point (°C) | 98.9 | 144 | 136.0 |
| Vapor pressure (mPa) | 5.81 (25 °C) | 4 × 10−7 (20 °C) | 7.99 × 10−7 (20 °C) |
| Water solubility (g/L) | 4.25 (25 °C) | 0.61 (20 °C) | 0.19 (20 °C) |
| KOW | 6.31 (25 °C) | 3.72 (21 °C) | 18.20 (20 °C) |
| pKa | 0.7 | pKa1 = 1.56; pKa2 = 11.12 | no dissociation |
| DT50 (day) | 1–8.2 | 48–190 | 12–142 |
| Hydrogen bond donor count | 0 | 1 | 0 |
| Hydrogen bond acceptor count | 3 | 4 | 4 |
| Topological polar surface area (Å2) | 52.3 | 86.3 | 77.6 |
| Physico-Chemical Characteristics | Soil | |||
|---|---|---|---|---|
| S1 | S2 | S3 | S4 | |
| Location | Pakrac | Lipik | Ploštine | Kutina |
| GCS | 45°49′ N 17°08′ E | 45°42′ N 17°13′ E | 45°29′ N 17°07′ E | 45°47′ N 16°48′ E |
| Textural classes | Clay loam | Clay loam | Clay loam | Clay loam |
| pH (a) | 4.94 (±0.11) | 5.29 (±0.06) | 5.25 (±0.04) | 5.55 (±0.04) |
| HA (b) (cmol/kg) | 13.39 (±1.02) | 4.62 (±0.46) | 4.59 (±0.44) | 6.59 (±0.26) |
| CEC (c) (cmol/kg) | 60.76 (±4.26) | 48.28 (±1.54) | 49.76 (±1.91) | 49.59 (±1.69) |
| Clay (%) | 30.75 (±1.25) | 35.26 (±0.86) | 36.62 (±0.67) | 37.60 (±1.07) |
| Ca2+ (mg/100 g) | 38.9 (±0.6) | 25.7 (±1.9) | 20.4 (±3.9) | 23.0 (±2.9) |
| Mg2+ (mg/100 g) | 450.8 (±33.8) | 401.1 (±21.6) | 447.0 (±34.8) | 352.4 (±24.4) |
| Na+ (mg/100 g) | 23.4 (±57.2) | 30.9 (±4.5) | 28.5 (±8.7) | 31.5 (±5.4) |
| K+ (mg/100 g) | 286.7 (±32.9) | 315.1 (±46.4) | 240.8 (±29.1) | 449.5 (±5.4) |
| TOC (d) (%) | 2.59 (±0.10) | 1.06 (±0.15) | 1.71 (±0.01) | 2.21 (±0.05) |
| CoxHa (e) (%) | 0.56 (±0.06) | 0.42 (±0.06) | 0.74 (±0.14) | 0.47 (±0.10) |
| CoxFa (f) (%) | 1.06 (±0.08) | 0.32 (±0.03) | 0.10 (±0.01) | 0.70 (±0.03) |
| N (%) | 0.221 (±0.009) | 0.128 (±0.002) | 0.175 (±0.002) | 0.224 (±0.011) |
| C (%) | 2.128 (±0.014) | 0.946 (±0.018) | 1.283 (±0.005) | 1.728 (±0.040) |
| H (%) | 0.595 (±0.005) | 0.373 (±0.005) | 0.456 (±0.009) | 0.492 (±0.014) |
| S (%) | 0.0242 (±0.0011) | 0.0128 (±0.0004) | 0.0174 (±0.0006) | 0.0253 (±0.0008) |
| O (%) | 97.032 (±0.09) | 98.540 (±0.13) | 98.068 (±0.06) | 97.531 (±0.20) |
| Ratio H/C | 3.33 (±0.02) | 4.70 (±0.05) | 4.24 (±0.07) | 3.39 (±0.03) |
| Ratio N/C | 0.089 (±0.004) | 0.116 (±0.052) | 0.117 (±0.013) | 0.111 (±0.023) |
| Ratio S/C | 0.0043 (±0.0003) | 0.0051 (±0.0001) | 0.0051 (±0.0006) | 0.0055 (±0.0002) |
| Ratio O/C | 34.23 (±0.02) | 78.31 (±0.06) | 57.38 (±0.03) | 42.48 (±0.01) |
| Ratio (N + O)/C | 34.32 (±0.06) | 78.20 (±0.08) | 57.38 (±0.04) | 42.37 (±0.05) |
| Ratio E465/E665 | 8.20 (±0.31) | 5.45 (±0.30) | 6.76 (±0.09) | 7.19 (±0.15) |
| Sorption | Desorption | |||||||
|---|---|---|---|---|---|---|---|---|
| Fitted/Statistical Parameter | S1 | S2 | S3 | S4 | S1 | S2 | S3 | S4 |
| Acetamiprid | ||||||||
| KFsor/des (a,b) [(mg/kg)/(mg/L)]1/n | 11.31 (±1.51) | 3.56 (±0.28) | 4.98 (±0.67) | 6.46 (±1.09) | 15.27 (±2.29) | 6.73 (±0.90) | 9.49 (±0.81) | 11.65 (±1.31) |
| 1/nsor/des (c,d) | 0.848 (±0.030) | 0.772 (±0.011) | 0.777 (±0.026) | 0.784 (±0.031) | 0.848 (±0.039) | 0.765 (±0.035) | 0.732 (±0.025) | 0.779 (±0.030) |
| R2 (e) | 0.9991 | 0.9999 | 0.9994 | 0.9990 | 0.9987 | 0.9988 | 0.9985 | 0.9992 |
| SRMSE (f) | 0.0422 | 0.0165 | 0.0360 | 0.0449 | 0.0641 | 0.0542 | 0.0395 | 0.0491 |
| err-% (g) | 3.36 | 1.31 | 2.87 | 3.57 | 5.09 | 4.31 | 3.15 | 3.90 |
| m (h) | 4 (χ2tab = 9.488 at p = 0.05) | |||||||
| Imidacloprid | ||||||||
| KFsor/des (a,b) [(mg/kg)/(mg/L)]1/n | 18.61 (±3.01) | 5.68 (±0.94) | 6.83 (±1.67) | 10.53 (±0.96) | 26.70 (±2.67) | 10.76 (±2.33) | 13.50 (±1.12) | 20.93 (±2.48) |
| 1/nsor/des (c,d) | 0.895 (±0.033) | 0.740 (±0.019) | 0.757 (±0.043) | 0.704 (±0.020) | 0.881 (±0.028) | 0.738 (±0.059) | 0.749 (±0.027) | 0.699 (±0.037) |
| R2 (e) | 0.9985 | 0.9994 | 0.9979 | 0.9995 | 0.9977 | 0.9979 | 0.9953 | 0.9991 |
| SRMSE (f) | 0.0560 | 0.0328 | 0.0649 | 0.0314 | 0.0486 | 0.0926 | 0.0414 | 0.0667 |
| err-% (g) | 3.48 | 2.61 | 5.16 | 2.50 | 3.87 | 7.36 | 3.29 | 5.31 |
| m (h) | 4 (χ2tab = 9.488 at p = 0.05) | |||||||
| Thiacloprid | ||||||||
| KFsor/des (a,b) [(mg/kg)/(mg/L)]1/n | 32.60 (±4.42) | 6.71 (±3.13) | 8.54 (±2.44) | 13.74 (±1.49) | 64.45 (±4.81) | 17.26 (±2.74) | 29.60 (±4.86) | 46.51 (±5.21) |
| 1/nsor/des (c,d) | 0.755 (±0.039) | 0.829 (±0.049) | 0.791 (±0.042) | 0.665 (±0.024) | 0.753 (±0.039) | 0.826 (±0.049) | 0.706 (±0.058) | 0.640 (±0.053) |
| R2 (e) | 0.9999 | 0.9959 | 0.9978 | 0.9999 | 0.9959 | 0.9978 | 0.9969 | 0.9949 |
| SRMSE (f) | 0.0657 | 0.0944 | 0.0736 | 0.0408 | 0.0652 | 0.0753 | 0.0821 | 0.0993 |
| err-% (g) | 5.22 | 7.51 | 5.85 | 3.24 | 5.18 | 5.99 | 6.55 | 7.90 |
| m (h) | 4 (χ2tab = 9.488 at p = 0.05) | |||||||
| Parameters | Acetamiprid | Imidacloprid | Thiacloprid | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S1 | S2 | S3 | S4 | S1 | S2 | S3 | S4 | |
| KOC (L/kg) | 436.85 (±47.86) | 284.79 (±21.86) | 292.16 (±48.87) | 292.46 (±21.84) | 718.51 (±24.11) | 454.52 (±42.94) | 399.52 (±6.78) | 476.90 (±44.51) | 1258.10 (±24.00) | 537.09 (±10.87) | 499.49 (±83.05) | 621.38 (±19.55) |
| ΔG (kJ/mol) | −14.81 (±0.27) | −13.77 (±0.22) | −13.82 (±4.09) | −13.83 (±1.82) | −16.03 (±0.81) | −14.91 (±0.23) | −14.56 (±0.41) | −15.03 (±2.27) | −17.39 (±0.46) | −15.32 (±0.49) | −15.13 (±4.07) | −15.68 (±0.77) |
| H | 0.904 (±0.032) | 0.991 (±0.004) | 0.940 (±0.011) | 0.913 (±0.001) | 0.967 (±0.001) | 0.869 (±0.015) | 0.862 (±0.003) | 0.869 (±0.012) | 0.988 (±0.013) | 0.996 (±0.006) | 0.891 (±0.033) | 0.905 (±0.020) |
| λ | 0.046 (±0.016) | 0.004 (±0.002) | 0.027 (±0.006) | 0.040 (±0.001) | 0.016 (±0.001) | 0.059 (±0.009) | 0.063 (±0.002) | 0.057 (±0.007) | 0.005 (±0.001) | 0.002 (±0.001) | 0.051 (±0.018) | 0.040 (±0.009) |
| Variable | 1/nsor (g) | 1/ndes (i) | KOC (j) | ΔG (k) | H (l) | λ (m) | ||
|---|---|---|---|---|---|---|---|---|
| pH | −0.63 | −0.69 | −0.46 | −0.63 | −0.77 (p = 0.025) | 0.75 (p = 0.031) | 0.17 | −0.21 |
| HA (a) | 0.96 (p < 0.001) | 0.88 (p = 0.004) | 0.92 (p = 0.001) | 0.35 | 0.90 (p = 0.002 | −0.89 (p = 0.003) | −0.65 | 0.68 |
| CEC (b) | 0.94 (p < 0.001) | 0.89 (p = 0.003) | 0.87 (p = 0.005) | 0.40 | 0.92 (p = 0.001) | −0.91 (p = 0.002) | −0.60 | 0.64 |
| Clay | −0.76 (p = 0.028) | −0.79 (p = 0.019) | −0.62 | −0.66 | −0.86 (p = 0.006) | 0.85 (p = 0.008) | 0.25 | −0.30 |
| TOC (c) | 0.91 (p = 0.002) | 0.74 (p = 0.035) | 0.98 (p < 0.001) | −0.08 | 0.71 (p = 0.049) | −0.71 | −0.90 (p = 0.002) | 0.91 (p = 0.002) |
| CoxHa (d) | 0.10 | 0.08 | 0.09 | −0.20 | 0.08 | −0.07 | −0.28 | 0.27 |
| CoxFa (e) | 0.87 (p = 0.005) | 0.76 (p = 0.030) | 0.89 (p = 0.003) | 0.21 | 0.75 (p = 0.031) | −0.75 (p = 0.032) | −0.64 | 0.67 |
| Ratio E465/E665 | 0.91 (p = 0.001) | 0.75 (p = 0.030) | 0.97 (p < 0.001) | −0.07 | 0.73 (p = 0.040) | −0.72 (p = 0.042) | −0.91 (p = 0.002) | 0.91 (p = 0.002) |
| Ratio H/C | −0.82 (p = 0.013) | −0.63 | −0.93 (p = 0.001) | 0.19 | −0.58 | 0.58 | 0.88 (p = 0.003) | −0.88 (p = 0.003) |
| Ratio N/C | −0.95 (p < 0.001) | −0.86 (p = 0.006) | −0.92 (p = 0.001) | −0.33 | −0.87 (p = 0.005) | 0.86 (p = 0.006) | 0.64 | −0.67 |
| Ratio S/C | −0.78 (p = 0.023) | −0.80 (p = 0.018) | −0.64 | −0.56 | −0.86 (p = 0.06) | 0.84 (p = 0.09) | 0.36 | −0.39 |
| Ratio O/C | −0.86 (p = 0.006) | −0.68 | −0.95 (p < 0.001) | 0.19 | −0.63 | 0.63 | 0.93 (p = 0.001) | −0.93 (p = 0.001) |
| Ratio (N + O)/C | −0.86 (p = 0.006) | −0.68 | −0.95 (p < 0.001) | 0.19 | −0.63 | 0.63 | 0.93 (p = 0.001) | −0.93 (p = 0.001) |
| Variable | 1/nsor (g) | 1/ndes (i) | KOC (j) | ΔG (k) | H (l) | λ (m) | ||
|---|---|---|---|---|---|---|---|---|
| pH | −0.61 | −0.94 (p = 0.001) | −0.27 | −0.91 (p = 0.001) | −0.71 (p = 0.047) | 0.67 | −0.82 (p = 0.013) | 0.78 (p = 0.022) |
| HA (a) | 0.98 (p < 0.001) | 0.87 (p = 0.005) | 0.85 (p = 0.007) | 0.93 (p = 0.001) | 0.97 (p < 0.001) | −0.96 (p < 0.001) | 0.97 (p < 0.001) | −0.97 (p < 0.001) |
| CEC (b) | 0.95 (p < 0.001) | 0.94 (p = 0.001) | 0.77 (p = 0.027) | 0.98 (p < 0.001) | 0.94 (p = 0.001) | −0.92 (p = 0.001) | 0.98 (p < 0.001) | −0.96 (p < 0.001) |
| Clay | −0.78 (p = 0.023) | −0.95 (p < 0.001) | −0.48 | −0.97 (p < 0.001) | −0.89 (p = 0.003) | 0.87 (p = 0.005) | −0.94 (p = 0.001) | 0.92 (p = 0.001) |
| TOC (c) | 0.92 (p = 0.001) | 0.58 | 0.99 (p < 0.001) | 0.66 | 0.75 (p = 0.030) | −0.75 (p = 0.033) | 0.73 (p = 0.039) | −0.75 (p = 0.034) |
| CoxHa (d) | 0.01 | 0.22 | −0.02 | 0.14 | −0.15 | 0.20 | −0.01 | 0.04 |
| CoxFa (e) | 0.93 (p = 0.001) | 0.62 | 0.91 (p = 0.002) | 0.71 (p = 0.047) | 0.90 (p = 0.003) | −0.91 (p = 0.002) | 0.83 (p = 0.011) | −0.85 (p = 0.008) |
| Ratio E465/E665 | 0.90 (p = 0.002) | 0.65 | 0.94 (p = 0.001) | 0.70 | 0.73 (p = 0.042) | −0.71 (p = 0.050) | 0.74 (p = 0.037) | −0.74 (p = 0.035) |
| Ratio H/C | −0.84 (p = 0.009) | −0.39 | −0.98 (p < 0.001) | −0.49 | −0.66 | 0.67 | −0.60 | 0.63 |
| Ratio N/C | −0.98 (p < 0.001) | −0.81 (p = 0.016) | −0.87 (p = 0.004) | −0.87 (p < 0.001) | −0.97 (p < 0.001)) | 0.96 (p < 0.001)) | −0.94 (p < 0.001) | 0.95 (p < 0.001) |
| Ratio S/C | −0.76 (p = 0.027) | −0.98 (p < 0.001) | −0.47 | −0.97 (p < 0.001) | −0.82 (p = 0.013) | 0.78 (p = 0.022) | −0.90 (p = 0.002) | 0.88 (p = 0.004) |
| Ratio O/C | −0.86 (p = 0.006) | −0.50 | −0.97 (p < 0.001) | −0.57 | −0.66 | 0.65 | −0.64 | 0.65 |
| Ratio (N + O)/C | −0.86 (p = 0.007) | −0.50 | −0.96 (p < 0.001) | −0.57 | −0.66 | 0.65 | −0.64 | 0.65 |
| Variable | 1/nsor (g) | 1/ndes (i) | KOC (j) | ΔG (k) | H (l) | λ (m) | ||
|---|---|---|---|---|---|---|---|---|
| pH | −0.69 | −0.42 | −0.41 | −0.56 | −0.76 (p = 0.029) | 0.70 | −0.54 | 0.50 |
| HA (a) | 0.99 (p < 0.001) | −0.25 | 0.90 (p = 0.002) | −0.01 | 0.99 (p < 0.001) | −0.98 (p < 0.001) | 0.42 | −0.44 |
| CEC (b) | 0.98 (p < 0.001) | −0.11 | 0.85 (p = 0.008) | 0.09 | 0.98 (p < 0.001) | −0.96 (p < 0.001) | 0.41 | −0.41 |
| Clay | −0.84 (p = 0.009) | −0.22 | −0.58 | −0.48 | −0.91 (p = 0.002) | 0.87 (p = 0.005) | −0.70 | 0.68 |
| TOC (c) | 0.88 (p = 0.004) | −0.63 | 0.99 (p < 0.001) | −0.51 | 0.80 (p = 0.018) | −0.81 (p = 0.015) | −0.05 | 0.01 |
| CoxHa (d) | 0.03 | 0.15 | 0.07 | −0.17 | −0.03 | 0.09 | −0.55 | 0.59 |
| CoxFa (e) | 0.90 (p = 0.002) | −0.49 | 0.89 (p = 0.003) | −0.19 | 0.88 (p = 0.004) | −0.91 (p = 0.002) | 0.40 | −0.45 |
| Ratio E465/E665 | 0.87 (p = 0.005) | −0.54 | 0.97 (p < 0.001) | −0.47 | 0.79 (p = 0.020) | −0.79 (p = 0.020) | −0.11 | 0.08 |
| Ratio H/C | −0.79 (p = 0.022) | 0.77 (p = 0.026) | −0.95 (p < 0.001) | 0.63 | −0.69 | 0.72 (p = 0.042) | 0.10 | −0.04 |
| Ratio N/C | −0.98 (p < 0.001) | 0.32 | −0.91 (p = 0.002) | 0.04 | −0.97 (p < 0.001) | 0.98 (p < 0.001) | −0.44 | 0.47 |
| Ratio S/C | −0.83 (p = 0.01) | −0.23 | −0.60 | −0.38 | −0.87 (p = 0.005) | 0.82 (p = 0.012) | −0.49 | 0.46 |
| Ratio O/C | −0.81 (p = 0.015) | 0.68 | −0.97 (p < 0.001) | 0.62 | −0.71 (p = 0.05) | 0.72 (p = 0.04) | 0.19 | −0.14 |
| Ratio (N + O)/C | −0.81 (p = 0.015) | 0.68 | −0.97 (p < 0.001) | 0.62 | −0.71 (p = 0.05) | 0.72 (p = 0.04) | 0.19 | −0.14 |
| Principal Component | PC1 | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| Eigenvalue | 11.52 | 3.94 | 2.58 | 1.70 |
| % Total variance | 54.87 | 18.75 | 12.28 | 8.08 |
| Cumulative % | 54.87 | 73.62 | 85.90 | 93.98 |
| Eigenvectors | ||||
| HA (a) | 0.289 | −0.031 | 0.071 | 0.073 |
| pH | −0.203 | 0.287 | −0.072 | 0.305 |
| CEC (b) | 0.340 | −0.078 | 0.095 | −0.066 |
| clay | −0.243 | 0.251 | −0.026 | 0.057 |
| TOC (c) | 0.258 | 0.207 | 0.128 | 0.082 |
| CoxHa (d) | 0.019 | 0.058 | 0.206 | −0.647 |
| CoxFa (e) | 0.259 | 0.064 | 0.015 | 0.333 |
| Ratio E465/E665 | 0.258 | 0.184 | 0.161 | −0.059 |
| Ratio H/C | −0.227 | −0.290 | −0.096 | −0.221 |
| Ratio N/C | −0.284 | 0.009 | −0.049 | −0.166 |
| Ratio S/C | −0.242 | 0.212 | −0.092 | 0.248 |
| Ratio O/C | −0.238 | −0.255 | −0.146 | −0.043 |
| Ratio (N + O)/C | −0.238 | −0.254 | −0.146 | −0.043 |
| KFsor (f) | 0.287 | 0.059 | −0.272 | −0.087 |
| 1/nsor (g) | 0.112 | −0.360 | 0.304 | −0.010 |
| KFdes (h) | 0.188 | 0.162 | −0.406 | −0.133 |
| 1/ndes (i) | 0.127 | −0.360 | 0.275 | 0.154 |
| KOC (j) | 0.220 | 0.012 | −0.381 | −0.137 |
| ΔG (k) | −0.194 | 0.002 | 0.366 | 0.223 |
| λ (l) | −0.084 | 0.311 | 0.284 | −0.217 |
| H (m) | 0.089 | −0.323 | −0.255 | 0.220 |
| Insecticide | Regression Equation | R2 | p |
|---|---|---|---|
| Acetamiprid | 0.937 | 0.0024 | |
| 0.642 | 0.0736 | ||
| 0.985 | 0.0001 | ||
| 0.412 | 0.1863 | ||
| Imidacloprid | 0.986 | 0.0001 | |
| 0.943 | 0.0020 | ||
| 0.991 | <0.0001 | ||
| 0.993 | <0.0001 | ||
| Thiacloprid | 0.998 | <0.0001 | |
| 0.752 | 0.0356 | ||
| 0.999 | <0.0001 | ||
| 0.962 | 0.0009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinčić Modrić, G.; Marinić, J.; Karleuša, R.; Dubrović, I.; Kosobucki, P.; Broznić, D. Those That Remain Caught in the “Organic Matter Trap”: Sorption/Desorption Study for Levelling the Fate of Selected Neonicotinoids. Int. J. Mol. Sci. 2024, 25, 5700. https://doi.org/10.3390/ijms25115700
Sinčić Modrić G, Marinić J, Karleuša R, Dubrović I, Kosobucki P, Broznić D. Those That Remain Caught in the “Organic Matter Trap”: Sorption/Desorption Study for Levelling the Fate of Selected Neonicotinoids. International Journal of Molecular Sciences. 2024; 25(11):5700. https://doi.org/10.3390/ijms25115700
Chicago/Turabian StyleSinčić Modrić, Gordana, Jelena Marinić, Romano Karleuša, Igor Dubrović, Przemysław Kosobucki, and Dalibor Broznić. 2024. "Those That Remain Caught in the “Organic Matter Trap”: Sorption/Desorption Study for Levelling the Fate of Selected Neonicotinoids" International Journal of Molecular Sciences 25, no. 11: 5700. https://doi.org/10.3390/ijms25115700
APA StyleSinčić Modrić, G., Marinić, J., Karleuša, R., Dubrović, I., Kosobucki, P., & Broznić, D. (2024). Those That Remain Caught in the “Organic Matter Trap”: Sorption/Desorption Study for Levelling the Fate of Selected Neonicotinoids. International Journal of Molecular Sciences, 25(11), 5700. https://doi.org/10.3390/ijms25115700







