Apple Glycosyltransferase MdUGT73AR4 Glycosylates ABA to Regulate Stomatal Movement Involved in Drought Stress
Abstract
1. Introduction
2. Results
2.1. Identification of the Apple Glycosyltransferase Gene MdUGT73AR4
2.2. Analysis of the Expression Pattern of MdUGT73AR4 in Different Parts of ‘Gala’
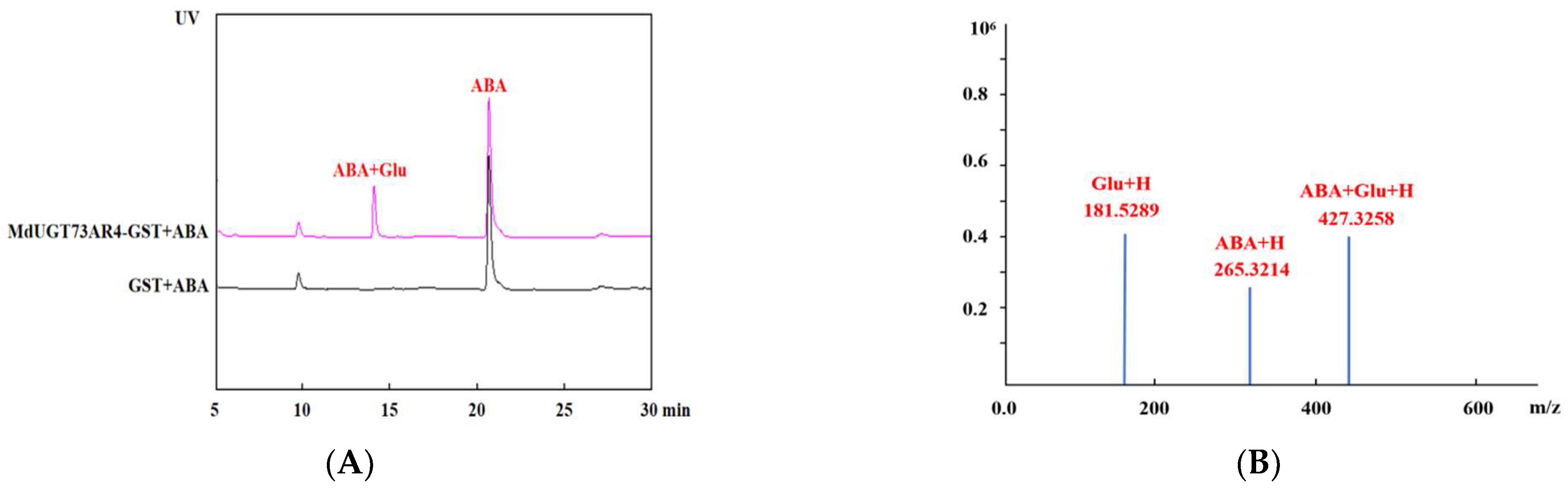
2.3. HPLC Detection of MdUGT73AR4 Glycosylation Modification of ABA
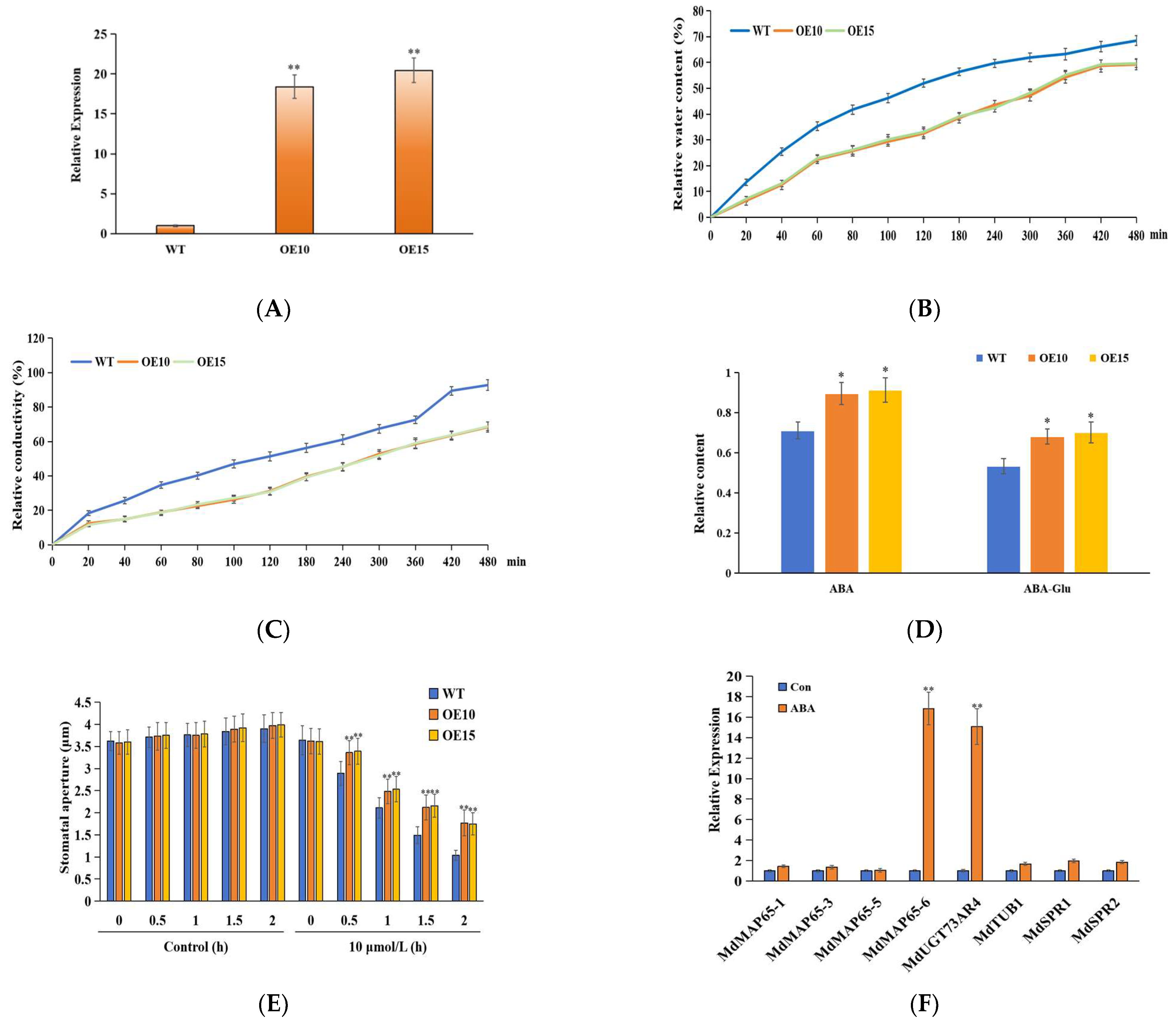
2.4. Acquisition of MdUGT73AR4 Overexpression Lines and Stomatal Opening Measurement
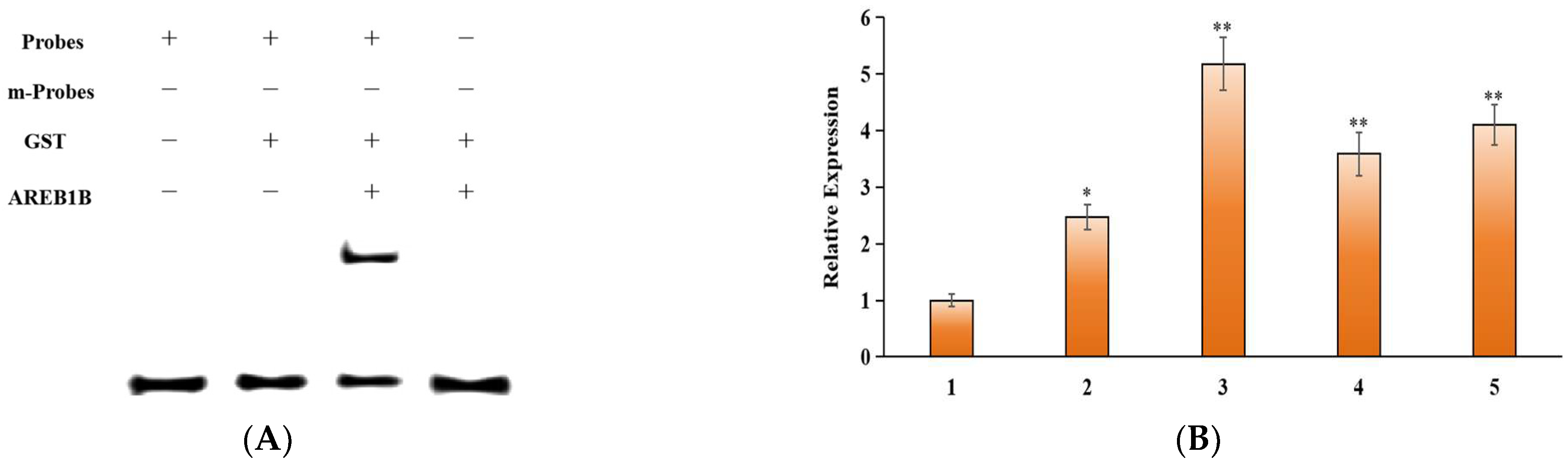
2.5. MdUGT73AR4 Upstream Transcription Factors Identified
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Apple Seedling Stress Treatment
4.3. Real-Time PCR
4.4. Vector Construction
4.5. MdUGT73AR4 Glycosylation Reaction
4.6. Acquisition of Transgenic Plants
4.7. Extraction and HPLC Analysis of ABA Glycosides in Apple Seedlings
4.8. Measurement of Stomatal Opening
4.9. Measurement of Relative Water Content and Relative Conductivity of Leaves as Indicators of Drought Tolerance Physiology
4.10. EMSA and ChIP Experiments
4.11. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, J.; Zhang, A.; Kang, Y.; Han, J.; Yang, B.; Hussain, Q.; Wang, X.; Zhang, M.; Khan, M.A. Biochar promotes soil organic carbon sequestration and reduces net global warming potential in apple orchard: A two-year study in the Loess Plateau of China. Sci. Total Environ. 2022, 803, 150035. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Qin, G.; Cao, F.; He, J.; Shen, X.; Chen, P.; Niu, C.; Zhang, D.; Ren, T.; Zhi, F.; et al. MdZAT5 regulates drought tolerance via mediating accumulation of drought-responsive miRNAs and mRNAs in apple. New Phytol. 2022, 236, 2131–2150. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Fang, X.; Guan, Z.; Yao, Y.; Xu, Z.; Bi, Y.; Ren, K.; Li, J.; Chen, F.; Chen, X.; et al. Functional characterization and structural basis of a reversible glycosyltransferase involves in plant chemical defence. Plant Biotechnol. J. 2023, 21, 2611–2624. [Google Scholar] [CrossRef] [PubMed]
- Meech, R.; Hu, D.G.; McKinnon, R.A.; Mubarokah, S.N.; Haines, A.Z.; Nair, P.C.; Rowland, A.; Mackenzie, P.I. The UDP-Glycosyltransferase (UGT) Superfamily: New Members, New Functions, and Novel Paradigms. Physiol. Rev. 2019, 99, 1153–1222. [Google Scholar] [CrossRef] [PubMed]
- McGraphery, K.; Schwab, W. Comparative Analysis of High-Throughput Assays of Family-1 Plant Glycosyltransferases. Int. J. Mol. Sci. 2020, 21, 2208. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, W.J.; Ratamess, N.A.; Hymer, W.C.; Nindl, B.C.; Fragala, M.S. Growth Hormone(s), Testosterone, Insulin-Like Growth Factors, and Cortisol: Roles and Integration for Cellular Development and Growth With Exercise. Front. Endocrinol. 2020, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Iuchi, S.; Kobayashi, M.; Taji, T.; Naramoto, M.; Seki, M.; Kato, T.; Tabata, S.; Kakubari, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J. 2001, 27, 325–333. [Google Scholar] [CrossRef]
- Qian, J.; Sun, T.; Yan, J.; Hsu, Y.F.; Zheng, M. Arabidopsis glucose-sensitive mutant 3 affects ABA biosynthesis and sensitivity during early seedling development. Plant Physiol. Biochem. 2020, 156, 20–29. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, X.; Shi, M.; Yu, J.; Guo, C. The miR159-MYB33-ABI5 module regulates seed germination in Arabidopsis. Physiol. Plant 2022, 174, e13659. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, T.; Sun, Y.; Zhang, Y.; Radojičić, A.; Ding, Y.; Tian, H.; Huang, X.; Lan, J.; Chen, S.; et al. Diverse Roles of the Salicylic Acid Receptors NPR1 and NPR3/NPR4 in Plant Immunity. Plant Cell 2020, 32, 4002–4016. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Chen, M.Y.; Tsao, H.K.; Sheng, Y.J. Dynamics of bridge-loop transformation in a membrane with mixed monolayer/bilayer structures. Phys. Chem. Chem. Phys. 2018, 20, 6582–6590. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.P.; Onabanjo, C.G.A.; Hardy, K.; Butnev, V.Y.; Bousfield, G.R.; Jonas, K.C. Follicle-Stimulating Hormone Glycosylation Variants Distinctly Modulate Pre-antral Follicle Growth and Survival. Endocrinology 2022, 163, bqac161. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yan, J.P.; Li, D.K.; Luo, Q.; Yan, Q.; Liu, Z.B.; Ye, L.M.; Wang, J.M.; Li, X.F.; Yang, Y. UDP-glucosyltransferase71C5, a Major Glucosyltransferase, Mediates Abscisic Acid Homeostasis in Arabidopsis. Plant Physiol. 2015, 167, 1659–1670. [Google Scholar] [CrossRef] [PubMed]
- Agurla, S.; Gahir, S.; Munemasa, S.; Murata, Y.; Raghavendra, A.S. Mechanism of Stomatal Closure in Plants Exposed to Drought and Cold Stress. Adv. Exp. Med. Biol. 2018, 1081, 215–232. [Google Scholar] [PubMed]
- Liu, H.; Song, S.; Zhang, H.; Li, Y.; Niu, L.; Zhang, J.; Wang, W. Signaling Transduction of ABA, ROS, and Ca(2+) in Plant Stomatal Closure in Response to Drought. Int. J. Mol. Sci. 2022, 23, 14824. [Google Scholar] [CrossRef] [PubMed]
- Pei, D.; Hua, D.; Deng, J.; Wang, Z.; Song, C.; Wang, Y.; Wang, Y.; Qi, J.; Kollist, H.; Yang, S.; et al. Phosphorylation of the plasma membrane H+-ATPase AHA2 by BAK1 is required for ABA-induced stomatal closure in Arabidopsis. Plant Cell 2022, 34, 2708–2729. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.K.; Dubeaux, G.; Takahashi, Y.; Schroeder, J.I. Signaling mechanisms in abscisic acid-mediated stomatal closure. Plant J. 2021, 105, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Eisenach, C.; Baetz, U.; Huck, N.V.; Zhang, J.; De Angeli, A.; Beckers, G.J.M.; Martinoia, E. ABA-Induced Stomatal Closure Involves ALMT4, a Phosphorylation-Dependent Vacuolar Anion Channel of Arabidopsis. Plant Cell 2017, 29, 2552–2569. [Google Scholar] [CrossRef]
- Meng, X.; Wang, H.; He, Y.; Liu, Y.; Walker, J.C.; Torii, K.U.; Zhang, S. A MAPK cascade downstream of ERECTA receptor-like protein kinase regulates Arabidopsis inflorescence architecture by promoting localized cell proliferation. Plant Cell 2012, 24, 4948–4960. [Google Scholar] [CrossRef]
- Xue, X.; Wang, L.; Huang, A.; Liu, Z.; Guo, X.; Sang, Y.; Zhu, J.K.; Xue, H.; Dong, J. Membrane-associated NRPM proteins are novel suppressors of stomatal production in Arabidopsis. Curr. Biol. 2024, 34, 881–894.e7. [Google Scholar] [CrossRef] [PubMed]
- Nadarajah, K.K. ROS Homeostasis in Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, P.; Li, B.; Wang, W.; Zhu, B. Phosphoribosyltransferases and Their Roles in Plant Development and Abiotic Stress Response. Int. J. Mol. Sci. 2023, 24, 11828. [Google Scholar] [CrossRef] [PubMed]
- Gharabli, H.; Della Gala, V.; Welner, D.H. The function of UDP-glycosyltransferases in plants and their possible use in crop protection. Biotechnol. Adv. 2023, 67, 108182. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Dong, G.R.; Ma, Y.Q.; Huang, X.; Mu, T.J.; Huang, X.; Li, Y.J.; Li, X.; Hou, B.K. Glycosyltransferase UGT79B7 Negatively Regulates Hypoxia Stress Response through γ-aminobutyric Acid Homeostasis in Plants. J. Exp. Bot. 2021, 72, 7998–8010. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Xu, Z.Y.; Park, Y.; Kim, D.H.; Lee, Y.; Hwang, I. Abscisic Acid Uridine Diphosphate Glucosyltransferases Play a Crucial Role in Abscisic Acid Homeostasis in Arabidopsis. Plant Physiol. 2014, 165, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Z.; Jin, S.H.; Jiang, X.Y.; Dong, R.R.; Li, P.; Li, Y.J.; Hou, B.K. Ectopic Expression of UGT75D1, a Glycosyltransferase Preferring Indole-3-butyric Acid, Modulates Cotyledon Development and Stress Tolerance in Seed Germination of Arabidopsis thaliana. Plant Mol. Biol. 2016, 90, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.T.; Liu, F.F.; Xiao, D.W.; Jiang, X.Y.; Li, P.; Zhao, S.M.; Hou, B.K.; Li, Y.J. The Arabidopsis UDPglycosyltransferase75B1, Conjugates Abscisic Acid and Affects Plant Response to Abiotic Stresses. Plant Mol. Biol. 2020, 102, 389–401. [Google Scholar] [CrossRef]
- Li, Q.; Yu, H.M.; Meng, X.F.; Lin, J.S.; Li, Y.J.; Hou, B.K. Ectopic Expression of Glycosyltransferase UGT76E11 Increases Flavonoid Accumulation and Enhances Abiotic Stress Tolerance in Arabidopsis. Plant Biol. 2018, 20, 10–19. [Google Scholar] [CrossRef]
- Li, P.; Li, Y.J.; Wang, B.; Yu, H.M.; Li, Q.; Hou, B.K. The Arabidopsis UGT87A2, a Stress-inducible Family 1 Glycosyltransferase, is Involved in the Plant Adaptation to Abiotic Stresses. Physiol. Plant. 2017, 159, 416–432. [Google Scholar] [CrossRef]
- Li, Y.; Liu, F.; Li, P.; Wang, T.; Zheng, C.; Hou, B. An Arabidopsis Cytokinin-modifying Glycosyltransferase UGT76C2 Improves Drought and Salt Tolerance in Rice. Front. Plant Sci. 2020, 11, 560696. [Google Scholar] [CrossRef]
- Wang, T.; Li, P.; Mu, T.; Dong, G.; Zheng, C.; Jin, S.; Chen, T.; Hou, B.; Li, Y. Overexpression of UGT74E2, an Arabidopsis IBA Glycosyltransferase, Enhances Seed Germination and Modulates Stress Tolerance Via ABA Signaling in Rice. Int. J. Mol. Sci. 2020, 20, 7239. [Google Scholar] [CrossRef]
- Komatsu, K.; Takezawa, D.; Sakata, Y. Decoding ABA and osmostress signalling in plants from an evolutionary point of view. Plant Cell Environ. 2020, 43, 2894–2911. [Google Scholar] [CrossRef]
- He, Y.; Sun, S.; Zhao, J.; Huang, Z.; Peng, L.; Huang, C.; Tang, Z.; Huang, Q.; Wang, Z. UDP-glucosyltransferase OsUGT75A promotes submergence tolerance during rice seed germination. Nat. Commun. 2023, 14, 2296. [Google Scholar] [CrossRef]
- Sun, Y.; Ji, K.; Liang, B.; Du, Y.; Jiang, L.; Wang, J.; Kai, W.; Zhang, Y.; Zhai, X.; Chen, P.; et al. Suppressing ABA uridine diphosphate glucosyltransferase (SlUGT75C1) alters fruit ripening and the stress response in tomato. Plant J. 2017, 91, 574–589. [Google Scholar] [CrossRef]
- Uechi, K.; Yaguchi, H.; Tokashiki, J.; Taira, T.; Mizutani, O. Identification of Genes Involved in the Synthesis of the Fungal Cell Wall Component Nigeran and Regulation of Its Polymerization in Aspergillus luchuensis. Appl. Environ. Microbiol. 2021, 87, e0114421. [Google Scholar] [CrossRef]
- Lawson, T.; Vialet-Chabrand, S. Speedy stomata, photosynthesis and plant water use efficiency. New Phytol. 2019, 221, 93–98. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Brookbank, B.P.; Patel, J.; Gazzarrini, S.; Nambara, E. Role of Basal ABA in Plant Growth and Development. Genes 2021, 12, 1936. [Google Scholar] [CrossRef]
- Joo, H.; Lim, C.W.; Lee, S.C. Roles of pepper bZIP transcription factor CaATBZ1 and its interacting partner RING-type E3 ligase CaASRF1 in modulation of ABA signalling and drought tolerance. Plant J. 2019, 100, 399–410. [Google Scholar] [CrossRef]
- Qian, D.; Zhang, Z.; He, J.; Zhang, P.; Ou, X.; Li, T.; Niu, L.; Nan, Q.; Niu, Y.; He, W.; et al. Arabidopsis ADF5 promotes stomatal closure by regulating actin cytoskeleton remodeling in response to ABA and drought stress. J. Exp. Bot. 2019, 70, 435–446. [Google Scholar] [CrossRef]
- Wang, B.; Li, L.; Liu, M.; Peng, D.; Wei, A.; Hou, B.; Lei, Y.; Li, X. TaFDL2-1A confers drought stress tolerance by promoting ABA biosynthesis, ABA responses, and ROS scavenging in transgenic wheat. Plant J. 2022, 112, 722–737. [Google Scholar] [CrossRef]
- Yoshida, T.; Fujita, Y.; Sayama, H.; Kidokoro, S.; Maruyama, K.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AREB1, AREB2 and ABF3 are master transcription factors that cooperatively regulate ABRE-dependent ABA signaling involved in drought stress tolerance and require ABA for full activation. Plant J. 2010, 61, 672–685. [Google Scholar] [CrossRef]
- Lee, M.; Dominguez-Ferreras, A.; Kaliyadasa, E.; Huang, W.J.; Antony, E.; Stevenson, T.; Lehmann, S.; Schäfer, P.; Knight, M.R.; Ntoukakis, V.; et al. Mediator Subunits MED16, MED14, and MED2 Are Required for Activation of ABRE-Dependent Transcription in Arabidopsis. Front. Plant Sci. 2021, 12, 649720. [Google Scholar] [CrossRef]
- Feng, R.J.; Ren, M.Y.; Lu, L.F.; Peng, M.; Guan, X.; Zhou, D.B.; Zhang, M.Y.; Qi, D.F.; Li, K.; Tang, W.; et al. Involvement of abscisic acid-responsive element-binding factors in cassava (Manihot esculenta) dehydration stress response. Sci. Rep. 2019, 9, 12661. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, L.; Wang, X.; Ma, Y.; Zhao, A.; Han, S.; Li, R.; Lei, K.; Ji, L.; Li, P. Apple Glycosyltransferase MdUGT73AR4 Glycosylates ABA to Regulate Stomatal Movement Involved in Drought Stress. Int. J. Mol. Sci. 2024, 25, 5672. https://doi.org/10.3390/ijms25115672
Mu L, Wang X, Ma Y, Zhao A, Han S, Li R, Lei K, Ji L, Li P. Apple Glycosyltransferase MdUGT73AR4 Glycosylates ABA to Regulate Stomatal Movement Involved in Drought Stress. International Journal of Molecular Sciences. 2024; 25(11):5672. https://doi.org/10.3390/ijms25115672
Chicago/Turabian StyleMu, Lijun, Xuekun Wang, Yingxin Ma, Aijuan Zhao, Shibo Han, Ru Li, Kang Lei, Lusha Ji, and Pan Li. 2024. "Apple Glycosyltransferase MdUGT73AR4 Glycosylates ABA to Regulate Stomatal Movement Involved in Drought Stress" International Journal of Molecular Sciences 25, no. 11: 5672. https://doi.org/10.3390/ijms25115672
APA StyleMu, L., Wang, X., Ma, Y., Zhao, A., Han, S., Li, R., Lei, K., Ji, L., & Li, P. (2024). Apple Glycosyltransferase MdUGT73AR4 Glycosylates ABA to Regulate Stomatal Movement Involved in Drought Stress. International Journal of Molecular Sciences, 25(11), 5672. https://doi.org/10.3390/ijms25115672






