Farnesyl Diphosphate Synthase Gene Associated with Loss of Bone Mass Density and Alendronate Treatment Failure in Patients with Primary Osteoporosis
Abstract
1. Introduction
2. Results
2.1. Comparison between OP Patients and Controls
2.1.1. Clinical Characterization and Biochemical Marker Levels
2.1.2. rs2297480 FDPS SNV and Susceptibility to Developing OP
2.1.3. rs2297480 FDPS SNV and Correlation with Biochemical Markers
2.2. Comparison between Responder and Non-Responder Patients
2.2.1. rs2297480 FDPS SNV and Correlation with Treatment Response
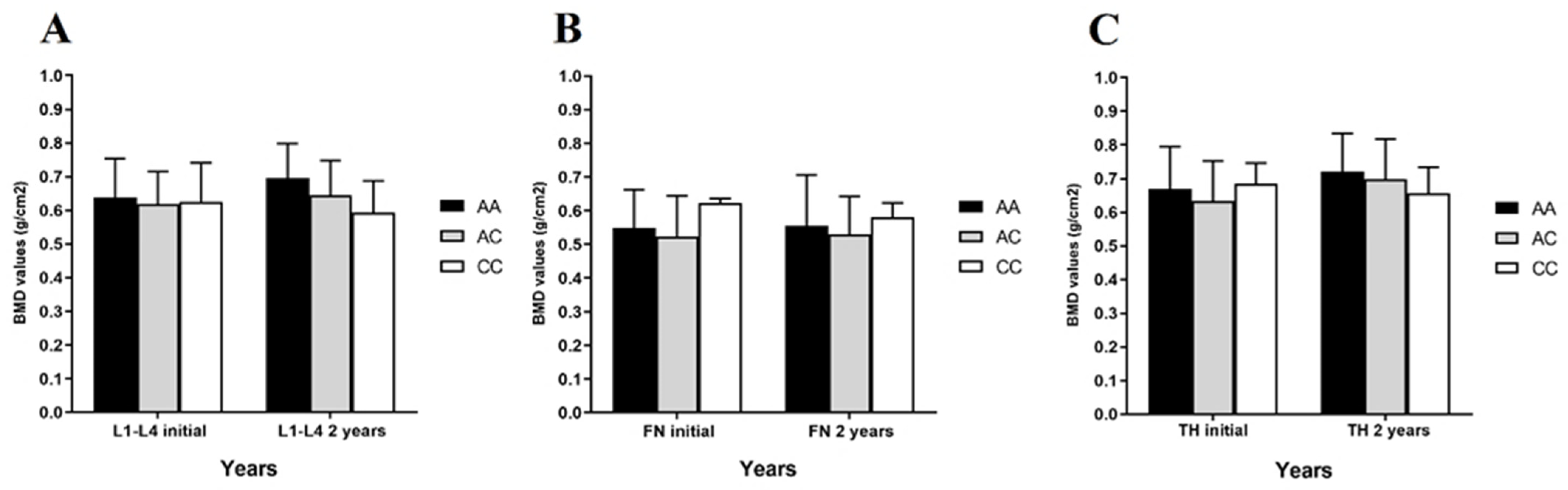
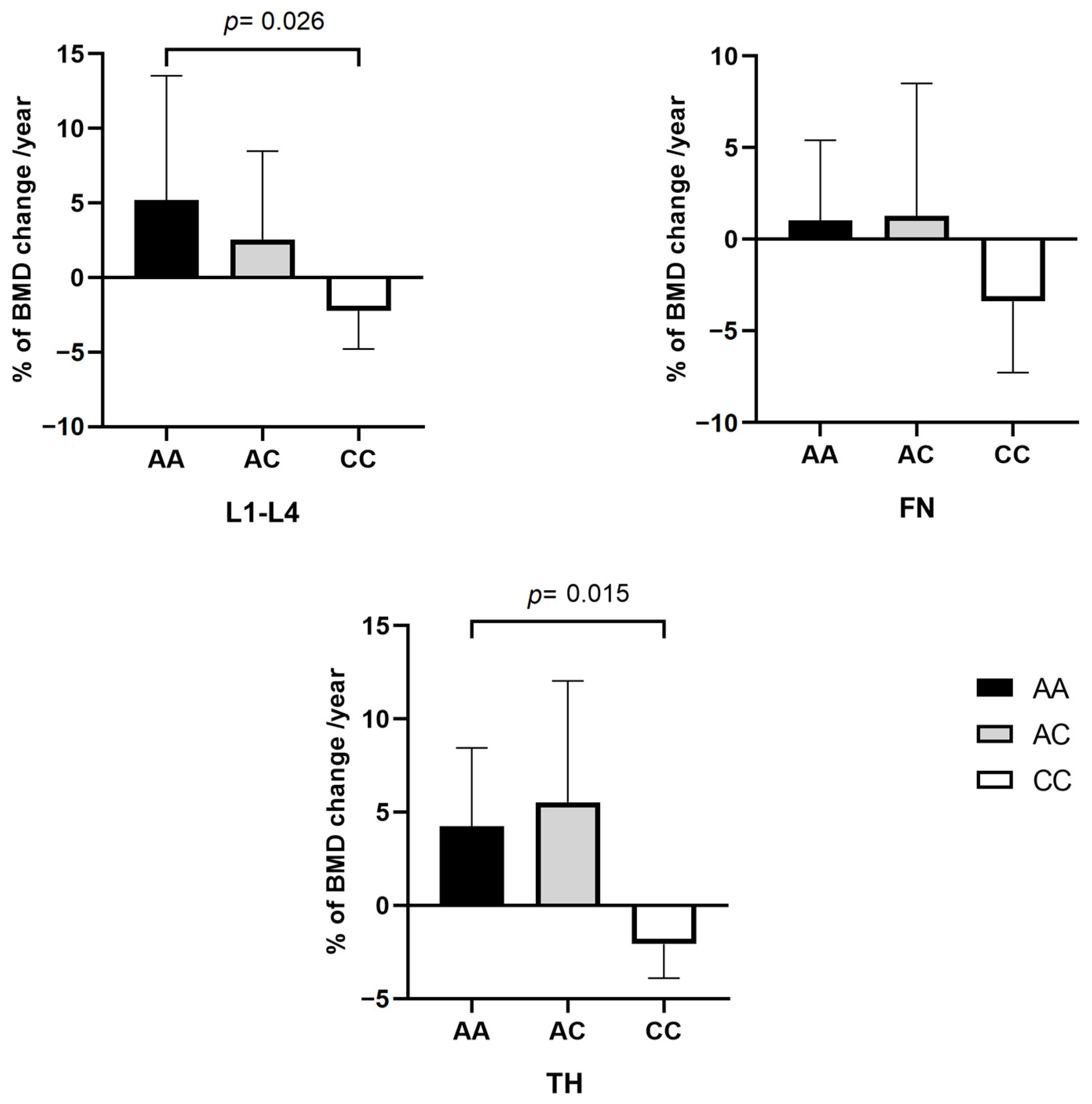
2.2.2. rs2297480 FDPS SNV and Correlation with BMD
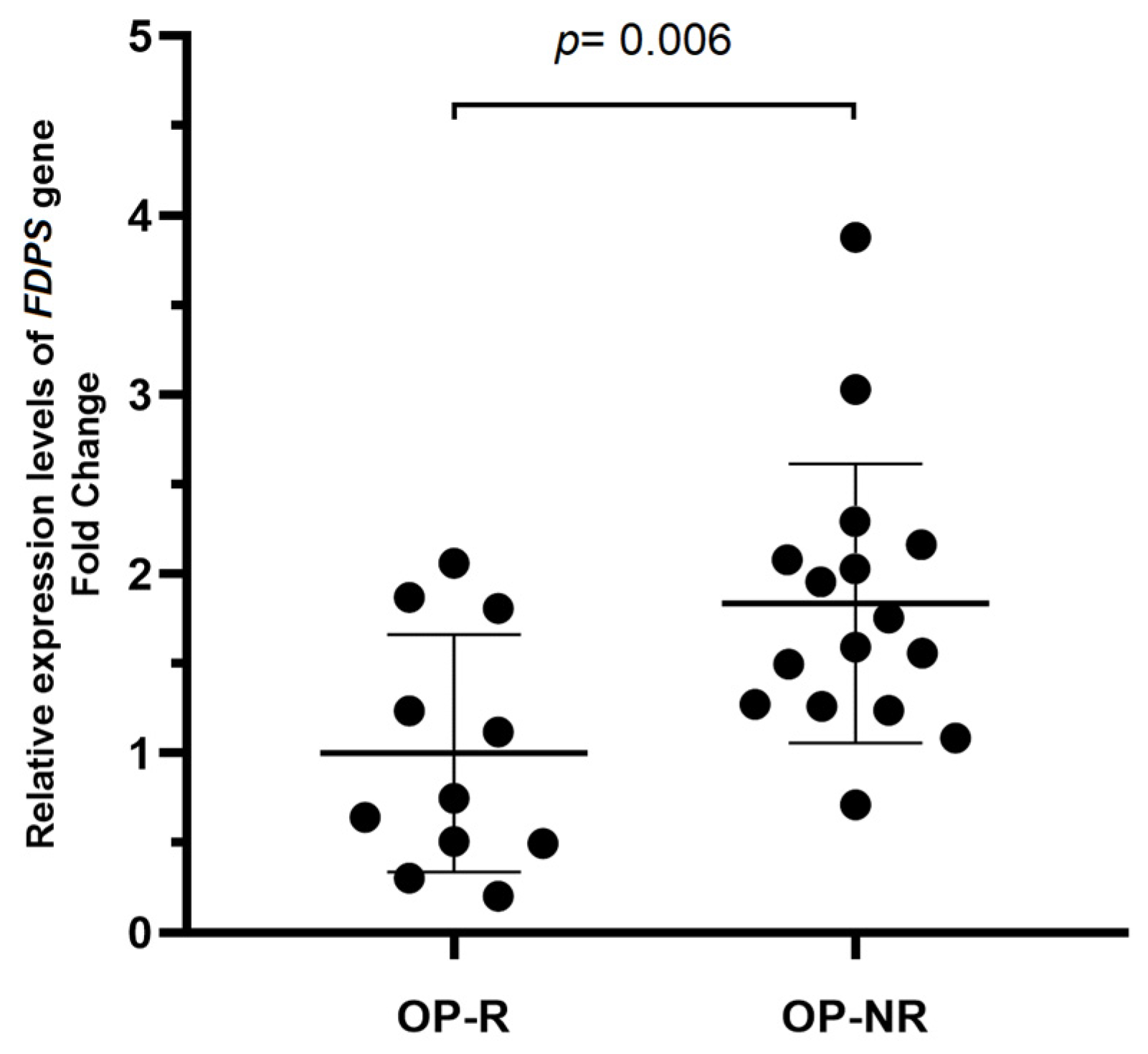
2.3. Expression Analysis of FDPS in Responder and Non-Responder OP Patients
2.3.1. FDPS Gene Expression and Biochemical Marker Correlation
2.3.2. FDPS Gene Expression and SNV rs2297480 Genotypes
3. Discussion
4. Methodology
4.1. Subjects
4.2. Genotyping
4.3. Expression Analysis
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001, 285, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Michael Lewiecki, E.; Wright, N.C.; Curtis, J.R.; Siris, E.; Gagel, R.F.; Saag, K.G.; Singer, A.J.; Steven, P.M.; Adler, R.A. Hip fracture trends in the United States, 2002 to 2015. Osteoporos. Int. 2017, 29, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Roush, K. Prevention and treatment of osteoporosis in postmenopausal women: A review. Am. J. Nurs. 2011, 111, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.T.; Clarke, B.L.; Lewiecki, E.M. The Pathophysiology and Treatment of Osteoporosis. Clin. Ther. 2015, 37, 1837–1850. [Google Scholar] [CrossRef]
- Wasnich, R.D.; Bagger, Y.Z.; Hosking, D.J.; McClung, M.R.; Wu, M.; Mantz, A.M.; Yates, J.J.; Ross, P.D.; Alexandersen, P.; Ravn, P.; et al. Changes in bone density and turnover after alendronate or estrogen withdrawal. Menopause 2004, 11, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.G.G.; Watts, N.B.; Ebetino, F.H.; Rogers, M.J. Mechanisms of action of bisphosphonates: Similarities and differences and their potential influence on clinical efficacy. Osteoporos. Int. 2008, 19, 733–759. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.G.d.; Vieira, J.G.H.; Kunii, I.S.; Lana, J.M.d.; Lazaretti-Castro, M. The effects of discontinuing long term alendronate therapy in a clinical practice setting. Arq. Bras. Endocrinol. Metabol. 2011, 55, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Altman, R.B.; Klein, T.E. Bisphosphonates pathway. Pharmacogenetics Genom. 2011, 21, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Diez-Perez, A.; Adachi, J.D.; Agnusdei, D.; Bilezikian, J.P.; Compston, J.E.; Cummings, S.R.; Eastell, R.; Eriksen, E.F.; Gonzalez-Macias, J.; Liberman, U.A.; et al. Treatment failure in osteoporosis. Osteoporos. Int. 2012, 23, 2769–2774. [Google Scholar] [CrossRef]
- Cairoli, E.; Eller-Vainicher, C.; Ulivieri, F.M.; Zhukouskaya, V.V.; Palmieri, S.; Morelli, V.; Beck-Peccoz, P.; Chiodini, I. Factors associated with bisphosphonate treatment failure in postmenopausal women with primary osteoporosis. Osteoporos. Int. 2014, 25, 1401–1410. [Google Scholar] [CrossRef]
- Levy, M.E.; Parker, R.A.; Ferrell, R.E.; Zmuda, J.M.; Greenspan, S.L. Farnesyl diphosphate synthase: A novel genotype association with bone mineral density in elderly women. Maturitas 2007, 57, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Marini, F.; Falchetti, A.; Silvestri, S.; Bagger, Y.; Luzi, E.; Tanini, A.; Christiansen, C.; Brandi, M.L. Modulatory effect of farnesyl pyrophosphate synthase (FDPS) rs2297480 polymorphism on the response to long-term amino-bisphosphonate treatment in postmenopausal osteoporosis. Curr. Med. Res. Opin. 2008, 24, 2609–2615. [Google Scholar] [CrossRef] [PubMed]
- Olmos, J.M.; Zarrabeitia, M.T.; Hernández, J.L.; Sãudo, C.; González-Macías, J.; Riancho, J.A. Common allelic variants of the farnesyl diphosphate synthase gene influence the response of osteoporotic women to bisphosphonates. Pharmacogenomics J. 2012, 12, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Marozik, P.; Alekna, V.; Rudenko, E.; Tamulaitiene, M.; Rudenka, A.; Mastaviciute, A.; Samokhovec, V.; Cernovas, A.; Kobets, K.; Mosse, I. Bone metabolism genes variation and response to bisphosphonate treatment in women with postmenopausal osteoporosis. PLoS ONE 2019, 14, e0221511. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Cho, E.H.; Ahn, S.H.; Kim, H.M.; Lim, K.H.; Kim, B.J.; Kim, S.W.; Kim, T.H.; Kim, S.Y.; Kim, G.S.; et al. Prediction of future osteoporotic fracture occurrence by genetic profiling: A 6-year follow-up observational study. J. Clin. Endocrinol. Metab. 2016, 101, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- López-Delgado, L.; Riancho-Zarrabeitia, L.; Riancho, J.A. Genetic and acquired factors influencing the effectiveness and toxicity of drug therapy in osteoporosis. Expert Opin. Drug Metab. Toxicol. 2016, 12, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Rivadeneira, F.; Mäkitie, O. Osteoporosis and Bone Mass Disorders: From Gene Pathways to Treatments. Trends Endocrinol. Metab. 2016, 27, 262–281. [Google Scholar] [CrossRef] [PubMed]
- Ralston, S.H.; Uitterlinden, A.G. Genetics of osteoporosis. Endocr. Rev. 2010, 31, 629–662. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, H.; He, J.W.; Zhang, H.; Yue, H.; Hu, W.W.; Gu, J.M.; Shao, C.; Fu, W.Z.; Hu, Y.Q.; et al. Genetic polymorphisms in the mevalonate pathway affect the therapeutic response to alendronate treatment in postmenopausal Chinese women with low bone mineral density. Pharmacogenomics J. 2015, 15, 158–164. [Google Scholar] [CrossRef]
- Pérez-Castrillón, J.L.; Zarrabeitia, M.T.; Abad, L.; Vega, G.; Ruiz-Mambrilla, M.; Gonzalez-Sagredo, M.; Dueñas-Laita, A.; Riancho, J.A. Polymorphisms of the farnesyl diphosphate synthase gene modulate bone changes in response to atorvastatin. Rheumatol. Int. 2014, 34, 1073–1077. [Google Scholar] [CrossRef]
- Ahsan, T.; Urmi, N.J.; Sajib, A.A. Heterogeneity in the distribution of 159 drug-response related SNPs in world populations and their genetic relatedness. PLoS ONE 2020, 15, e0228000. [Google Scholar] [CrossRef] [PubMed]
- Roudsari, J.M.; Mahjoub, S. Quantification and comparison of bone-specific alkaline phosphatase with two methods in normal and paget’s specimens. Casp. J. Intern. Med. 2012, 3, 478–483. [Google Scholar]
- Silva, B.C.; Bilezikian, J.P. Parathyroid hormone: Anabolic and catabolic actions on the skeleton. Curr. Opin. Pharmacol. 2015, 22, 41–50. [Google Scholar] [CrossRef]
- Sugden, C.J.; Roper, J.R.; Williams, J.G. Engineered gene over-expression as a method of drug target identification. Biochem. Biophys. Res. Commun. 2005, 334, 555–560. [Google Scholar] [CrossRef]
- Grove, J.E.; Brown, R.J.; Watts, D.J. The Intracellular Target for the Antiresorptive Aminobisphosphonate Drugs in Dictyostelium discoideum Is the Enzyme Farnesyl Diphosphate Synthase. J. Bone Miner. Res. 2010, 15, 971–981. [Google Scholar] [CrossRef]
- Radominski, S.C.; Bernardo, W.; Paula, A.P.d.; Albergaria, B.-H.; Moreira, C.; Fernandes, C.E.; Castro, C.H.M.; Zerbini, C.A.d.F.; Domiciano, D.S.; Mendonça, L.M.C.; et al. Brazilian guidelines for the diagnosis and treatment of postmenopausal osteoporosis. Rev. Bras. Reumatol. (Engl. Ed.) 2017, 57, 452–466. [Google Scholar] [CrossRef]
- Kanis, J.A. Assessment of Osteoporosis at the Primary Health Care Level; WHO Collaborating Centre for Metabolic Bone Diseases, University of Sheffield Medical School: Brussels, Belgium, 2008. [Google Scholar]
- Francis, R.M. Non-response to osteoporosis treatment. J. Br. Menopause Soc. 2004, 10, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Lakens, D.; Adolfi, F.G.; Albers, C.J.; Anvari, F.; Apps, M.A.J.; Argamon, S.E.; Baguley, T.; Becker, R.B.; Benning, S.D.; Bradford, D.E.; et al. Justify your alpha. Nat. Hum. Behav. 2018, 2, 168–171. [Google Scholar] [CrossRef]
- Lakens, D. Sample Size Justification. Collabra Psychol. 2022, 8, 33267. [Google Scholar] [CrossRef]
- Kang, H. Sample size determination and power analysis using the G*Power software. J. Educ. Eval. Health Prof. 2021, 18, 17. [Google Scholar] [CrossRef]
- Bignon, J.D.; Viña, M.F. 12th IHWC HLA Class II Reference Protocol; HLA et Médecine: Paris, France, 1995. [Google Scholar]
- de Lima, C.A.D.; de Lima, S.C.; Barbosa, A.D.; Sandrin-Garcia, P.; de Barros Pita, W.; de Azevêdo Silva, J.; Crovella, S. Postmenopausal Osteoporosis reference genes for qPCR expression assays. Sci. Rep. 2019, 9, 16533. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]

| Variables | OP (n = 304) | Control (n = 112) | p |
|---|---|---|---|
| Age (years) | 65.20 ± 6.10 | 61.20 ± 5.20 | 0.067 |
| Mean of age at menarche (years) | 13.57 ± 1.84 | 13.68 ± 1.72 | 0.488 |
| Mean of age at menopause (years) | 45.10 ± 7.10 | 44.64 ± 7.14 | 0.422 |
| Lumbar spine (L1-L4) (g/cm2) | 0.626 ± 0.103 | 0.932 ± 0.051 | <0.001 * |
| Total hip (g/cm2) | 0.652 ± 0.117 | 1.058 ± 0.139 | <0.001 * |
| Femoral neck (g/cm2) | 0.540 ± 0.117 | 0.936 ± 0.107 | <0.001 * |
| 25-hydroxyvitamin D (ng/mL) | 29.09 ± 9.24 | 32.26 ± 11.03 | 0.046 * |
| Calcium (mg/dL) | 9.52 ± 1.20 | 9.52 ± 0.87 | 0.622 |
| Alkaline phosphatase (U/L) | 82.20 ± 48.48 | 90.69 ± 62.86 | 0.499 |
| Gamma-glutamyltransferase (U/L) | 22.45 ± 7.44 | 22.22 ± 7.36 | 0.829 |
| Phosphorus (mg/dL) | 3.50 ± 0.67 | 3.59 ± 0.56 | 0.353 |
| Parathyroid hormone (pg/mL) | 56.48 ± 27.07 | 59.13 ± 45.72 | 0.287 |
| Creatinine clearance (ml/min) | 104.00 ± 11.67 | 106.10 ± 10.81 | 0.196 |
| Magnesium (mg/dL) | 2.06 ± 0.28 | 2.02 ± 0.47 | 0.956 |
| Model | Alleles/Genotypes | Controls, n (%) | OP, n (%) | OR | 95% CI | p |
|---|---|---|---|---|---|---|
| A | 159 (71) | 408 (67) | 1 | |||
| C | 65 (29) | 200 (33) | 1.19 | 0.85–1.70 | 0.31 | |
| Codominant | AA | 54 (48.2) | 142 (47) | 1 | 0.15 | |
| AC | 51 (45.5) | 124 (41) | 0.92 | 0.59–1.45 | ||
| CC | 7 (6.2) | 38 (12) | 2.06 | 0.87–4.90 | ||
| Dominant | AA | 54 (48.2) | 142 (46.7) | 1 | ||
| AC-CC | 58 (51.8) | 162 (53.3) | 1.06 | 0.69–1.64 | 0.79 | |
| Recessive | AA-AC | 105 (93.8) | 266 (87.5) | 1 | ||
| CC | 7 (6.2) | 38 (12.5) | 2.14 | 0.93–4.95 | 0.05 | |
| HWE | 0.36 | 0.19 |
| FDPS (rs2297480) | 25(OH)D (ng/mL) | Calcium (mg/dL) | ALP (U/L) | Phosphorus (mg/dL) | PTH (pg/mL) | Mg (mg/dL) |
|---|---|---|---|---|---|---|
| AA | 30.74 ± 10.12 | 9.45 ± 0.91 | 82.22 ± 46.51 | 3.56 ± 0.54 | 48.34 ± 23.42 | 2.10 ± 0.29 |
| AC | 29.94 ± 9.62 | 10.22 ± 7.45 | 95.53 ± 59.80 | 3.44 ± 0.66 | 62.20 ± 39.18 | 1.96 ± 0.39 |
| CC | 27.96 ± 9.13 | 9.39 ± 0.92 | 96.70 ± 42.11 | 3.76 ± 0.84 | 76.45 ± 31.57 | 2.16 ± 0.45 |
| p | 0.523 | 0.726 | 0.020 * | 0.255 | <0.001 * | 0.329 |
| Variables | OP-R (n = 20) | OP-NR (n = 40) | p-Value |
|---|---|---|---|
| Age (years) | 72.76 ± 6.70 | 69.03 ± 7.27 | 0.06 |
| Age at menarche (years) | 14.29 ± 2.24 | 13.59 ± 1.56 | 0.19 |
| Age at menopause (years) | 42.60 ± 8.47 | 45.06 ± 7.81 | 0.28 |
| 25-hydroxyvitamin D (ng/mL) | 32.43 ± 11.71 | 32.17 ± 9.66 | 0.83 |
| Calcium (mg/dL) | 9.55 ± 0.51 | 9.89 ± 0.59 | 0.08 |
| Alkaline phosphatase (U/L) | 114.60 ± 56.81 | 137.80 ± 60.70 | 0.38 |
| Phosphor (mg/dL) | 3.49 ± 0.57 | 3.41 ± 0.45 | 0.67 |
| Parathyroid hormone (pg/mL) | 62.66 ± 32.10 | 57.46 ± 22.53 | 0.83 |
| Magnesium (mg/dL) | 1.76 ± 0.25 | 2.1 ± 0.20 | 0.028 * |
| Model | Alleles/Genotypes | OP-R, n (%) | OP-NR, n (%) | OR | 95% CI | p |
|---|---|---|---|---|---|---|
| A | 24 (67) | 50 (64) | 1 | |||
| C | 12 (33) | 28 (36) | 1.12 | 0.45–2.85 | 0.83 | |
| Codominant | AA | 6 (33.3) | 16 (41) | 1 | 0.08 | |
| AC | 12 (66.7) | 18 (46.2) | 0.56 | 0.17–1.85 | ||
| CC | 0 (0) | 5 (12.8) | ND | 0.00-ND | ||
| Dominant | AA | 6 (33.3) | 16 (41) | 1 | ||
| AC-CC | 12 (67.7) | 23 (59) | 0.72 | 0.22–2.32 | 0.58 | |
| Recessive | AA-AC | 18 (100) | 34 (87.2) | 1 | ||
| CC | 0 (0) | 5 (12.8) | ND | 0.00-ND | 0.045 * | |
| HWE | 0.1 | 1 |
| Gene | Parameters | 25(OH)D | Calcium | ALP | Phosphorus | PTH | Mg |
|---|---|---|---|---|---|---|---|
| OP-R | |||||||
| FDPS | r | −0.0831 | 0.3707 | 0.5821 | −0.6954 | −0.1959 | 0.9262 |
| p-value | 0.80 | 0.26 | 0.10 | 0.017 * | 0.56 | 0.023 * | |
| OP-NR | |||||||
| FDPS | r | −0.1408 | −0.2246 | 0.4351 | −0.0010 | −0.0273 | −0.4878 |
| p-value | 0.61 | 0.36 | 0.15 | 0.99 | 0.92 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guaraná, W.L.; Lima, C.A.D.; Barbosa, A.D.; Crovella, S.; Sandrin-Garcia, P. Farnesyl Diphosphate Synthase Gene Associated with Loss of Bone Mass Density and Alendronate Treatment Failure in Patients with Primary Osteoporosis. Int. J. Mol. Sci. 2024, 25, 5623. https://doi.org/10.3390/ijms25115623
Guaraná WL, Lima CAD, Barbosa AD, Crovella S, Sandrin-Garcia P. Farnesyl Diphosphate Synthase Gene Associated with Loss of Bone Mass Density and Alendronate Treatment Failure in Patients with Primary Osteoporosis. International Journal of Molecular Sciences. 2024; 25(11):5623. https://doi.org/10.3390/ijms25115623
Chicago/Turabian StyleGuaraná, Werbson Lima, Camilla Albertina Dantas Lima, Alexandre Domingues Barbosa, Sergio Crovella, and Paula Sandrin-Garcia. 2024. "Farnesyl Diphosphate Synthase Gene Associated with Loss of Bone Mass Density and Alendronate Treatment Failure in Patients with Primary Osteoporosis" International Journal of Molecular Sciences 25, no. 11: 5623. https://doi.org/10.3390/ijms25115623
APA StyleGuaraná, W. L., Lima, C. A. D., Barbosa, A. D., Crovella, S., & Sandrin-Garcia, P. (2024). Farnesyl Diphosphate Synthase Gene Associated with Loss of Bone Mass Density and Alendronate Treatment Failure in Patients with Primary Osteoporosis. International Journal of Molecular Sciences, 25(11), 5623. https://doi.org/10.3390/ijms25115623






