Identifying the Morphological and Molecular Features of a Cell-Based Orthotopic Pancreatic Cancer Mouse Model during Growth over Time
Abstract
1. Introduction
2. Results
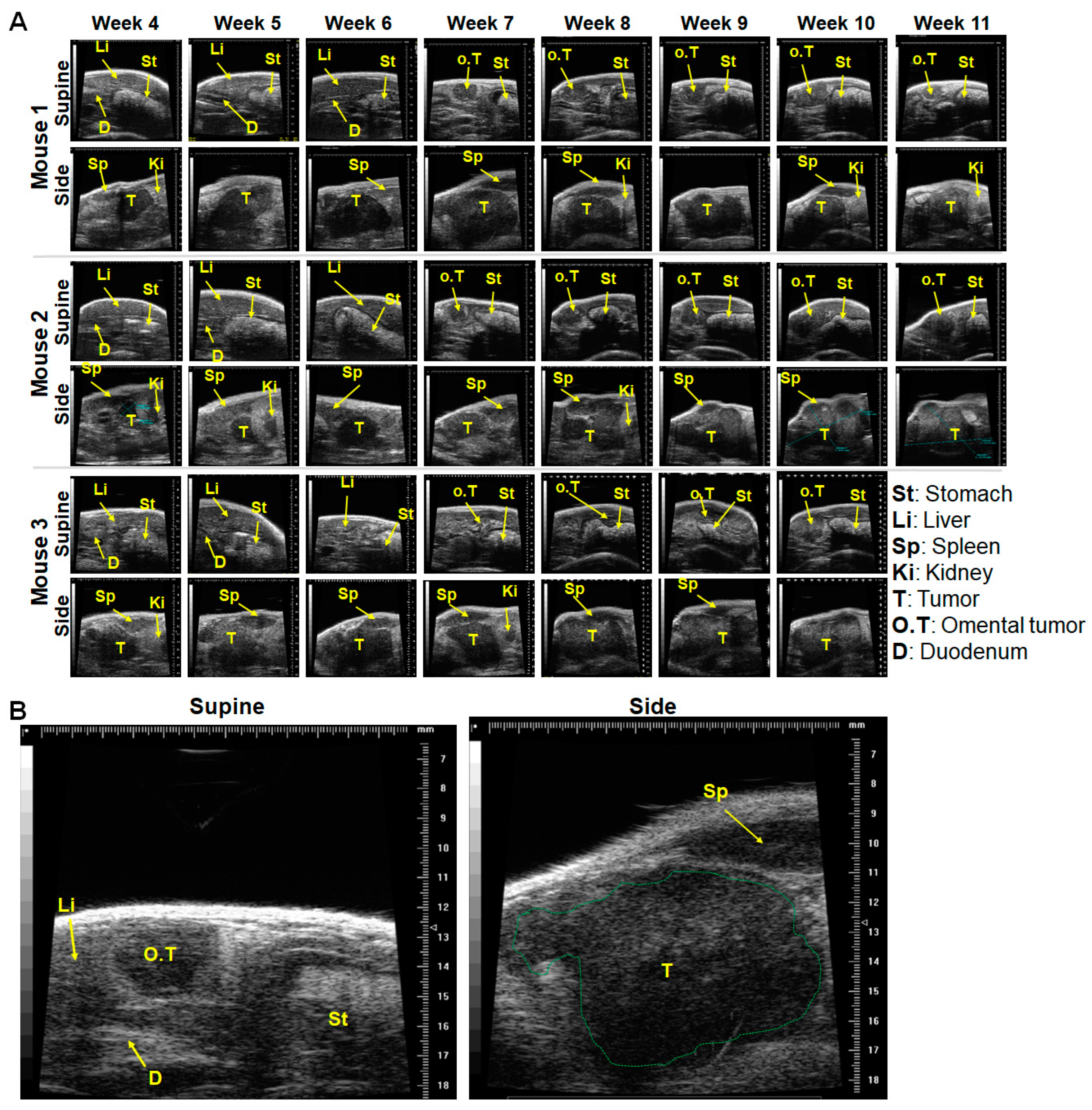
2.1. In a Minor Number of Mice, Implanted Tumors Remained Secluded within the Pancreas, and Almost Outgrew the Pancreas over Time
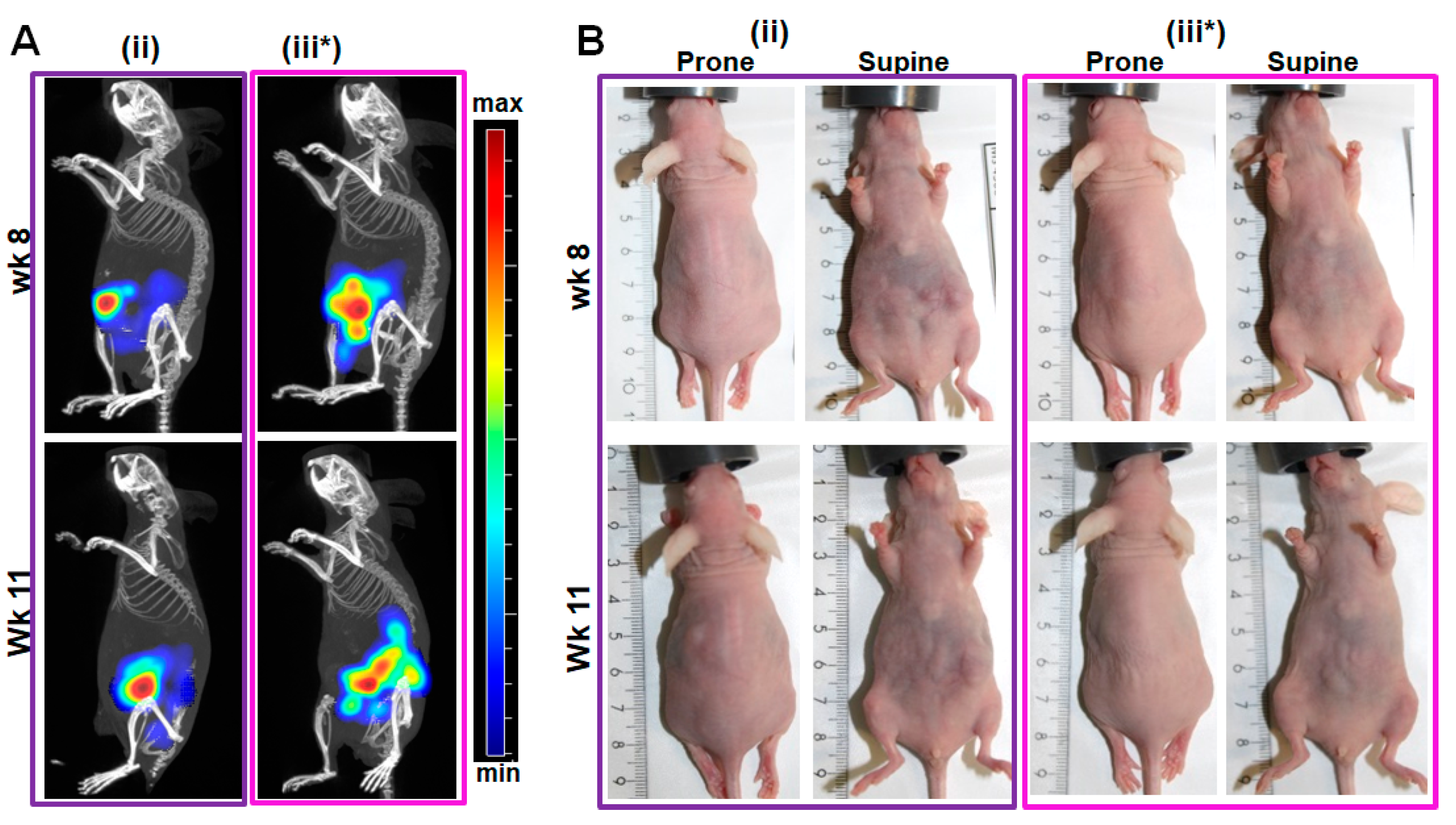
2.2. Tumor Infiltration of Vital Organs and Disease Progression
2.3. Validation of the In Situ Positions of Tumors and Organs by Near-Infrared (NIR) Fluorescence Imaging
2.4. Comparative Validation of the Mouse Pancreas Anatomy with Surrounding Organs, and Excised Tumors
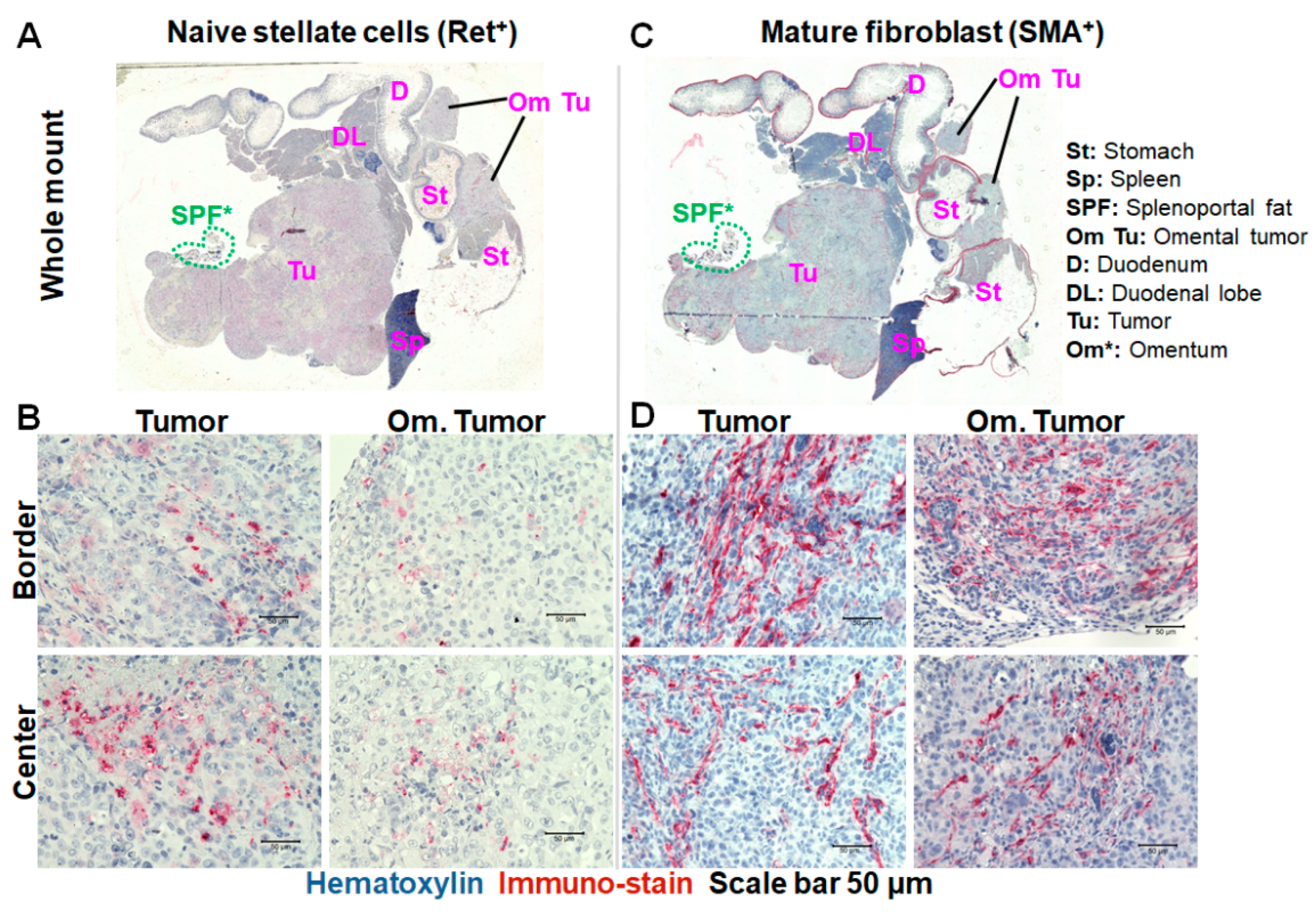
2.5. Immunohistochemistry Revealed Differences in the Levels of Naïve Pancreatic Stellate Cells and Mature Fibroblasts in the Primary and Omental Tumors over Growth Time
2.6. Collagen Fiber Levels Support Progression-Related Differences in the Tumors
3. Discussion
Summary and Suggestions for the Designing, Monitoring, and Selection of Cell- or Graft-Based Orthothopic Pancreatic Tumor Models Based on Growth-Dependent Tumor Parameters Prior to Preclinical Drug Testing
- i
- For the preparation of cells and tumor fragments for implantation, a low concentration (2–4% (v/v) depending on the tumor model in question) of a supporting matrix should be added before implantation, and the implantation should be performed directly into the pancreas body (splenic lobe).
- ii
- Ultrasound imaging should be carried out preferentially both on the side and supine position in order to monitor the primary tumor and to pinpoint the onset of the infiltration of the omentum and/or stomach wall and metastasis. Furthermore, the use of imaging systems that have higher penetration and/or additionally provide functional and molecular information of the tumors [54] should be considered.
- iii
- For therapy efficacy testing purposes, a designated “tumor growth stage” based on the progression-dependent characteristics of the model in question (see Table 1 above) should be selected in order to define suitable therapeutic drugs efficient for the parameter based early, advanced, or late stages.
4. Materials and Methods
4.1. Cell lines and Cell Culture
4.2. Use of Animals
Immune Deficient Mice
4.3. Generation of Pancreatic Cancer Models in Mice
Microsurgical Procedure for Tumor Cell Implantation into the Pancreas and Expected Duration for Tumor Development
4.4. Monitoring the Orthotopic Pancreatic Tumor Growth by Ultrasound (US) Imaging
4.5. Whole Body Fluorescence Imaging of Mice
4.6. Histological Analysis of Tumor Fibroblast and Collagen Levels
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Constantinescu, A.; Sandru, V.; Ilie, M.; Ungureanu, B.S.; Gheonea, D.I.; Ciurea, T.; Cazacu, S.M.; Vere, C.C.; Constantinescu, G. Biliary Stenting for Malignant Biliary Obstruction Secondary to Pancreatic Cancer. Curr. Health Sci. J. 2020, 46, 323–328. [Google Scholar] [CrossRef]
- Franck, C.; Muller, C.; Rosania, R.; Croner, R.S.; Pech, M.; Venerito, M. Advanced Pancreatic Ductal Adenocarcinoma: Moving Forward. Cancers 2020, 12, 1955. [Google Scholar] [CrossRef]
- Michelakos, T.; Pergolini, I.; Castillo, C.F.; Honselmann, K.C.; Cai, L.; Deshpande, V.; Wo, J.Y.; Ryan, D.P.; Allen, J.N.; Blaszkowsky, L.S.; et al. Predictors of Resectability and Survival in Patients With Borderline and Locally Advanced Pancreatic Cancer who Underwent Neoadjuvant Treatment With FOLFIRINOX. Ann. Surg. 2019, 269, 733–740. [Google Scholar] [CrossRef]
- Mollberg, N.; Rahbari, N.N.; Koch, M.; Hartwig, W.; Hoeger, Y.; Buchler, M.W.; Weitz, J. Arterial resection during pancreatectomy for pancreatic cancer: A systematic review and meta-analysis. Ann. Surg. 2011, 254, 882–893. [Google Scholar] [CrossRef]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef]
- Lockhart, A.C.; Rothenberg, M.L.; Berlin, J.D. Treatment for pancreatic cancer: Current therapy and continued progress. Gastroenterology 2005, 128, 1642–1654. [Google Scholar] [CrossRef]
- Tansi, F.L.; Maduabuchi, W.O.; Hirsch, M.; Southern, P.; Hattersley, S.; Quaas, R.; Teichgraber, U.; Pankhurst, Q.A.; Hilger, I. Deep-tissue localization of magnetic field hyperthermia using pulse sequencing. Int. J. Hyperth. 2021, 38, 743–754. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Rangarajan, A.; Weinberg, R.A. Opinion: Comparative biology of mouse versus human cells: Modelling human cancer in mice. Nat. Rev. Cancer 2003, 3, 952–959. [Google Scholar] [CrossRef]
- Sastra, S.A.; Olive, K.P. Quantification of murine pancreatic tumors by high-resolution ultrasound. Methods Mol. Biol. 2013, 980, 249–266. [Google Scholar] [CrossRef]
- Ito, M.; Hiramatsu, H.; Kobayashi, K.; Suzue, K.; Kawahata, M.; Hioki, K.; Ueyama, Y.; Koyanagi, Y.; Sugamura, K.; Tsuji, K.; et al. NOD/SCID/gamma(c)(null) mouse: An excellent recipient mouse model for engraftment of human cells. Blood 2002, 100, 3175–3182. [Google Scholar] [CrossRef]
- Trevino, J.G.; Summy, J.M.; Lesslie, D.P.; Parikh, N.U.; Hong, D.S.; Lee, F.Y.; Donato, N.J.; Abbruzzese, J.L.; Baker, C.H.; Gallick, G.E. Inhibition of SRC expression and activity inhibits tumor progression and metastasis of human pancreatic adenocarcinoma cells in an orthotopic nude mouse model. Am. J. Pathol. 2006, 168, 962–972. [Google Scholar] [CrossRef]
- Hoover, M.; Adamian, Y.; Brown, M.; Maawy, A.; Chang, A.; Lee, J.; Gharibi, A.; Katz, M.H.; Fleming, J.; Hoffman, R.M.; et al. A novel method for RNA extraction from FFPE samples reveals significant differences in biomarker expression between orthotopic and subcutaneous pancreatic cancer patient-derived xenografts. Oncotarget 2017, 8, 5885–5894. [Google Scholar] [CrossRef]
- Zhan, B.; Wen, S.; Lu, J.; Shen, G.; Lin, X.; Feng, J.; Huang, H. Identification and causes of metabonomic difference between orthotopic and subcutaneous xenograft of pancreatic cancer. Oncotarget 2017, 8, 61264–61281. [Google Scholar] [CrossRef]
- Erstad, D.J.; Sojoodi, M.; Taylor, M.S.; Ghoshal, S.; Razavi, A.A.; Graham-O’Regan, K.A.; Bardeesy, N.; Ferrone, C.R.; Lanuti, M.; Caravan, P.; et al. Orthotopic and heterotopic murine models of pancreatic cancer and their different responses to FOLFIRINOX chemotherapy. Dis. Model. Mech. 2018, 11, dmm034793. [Google Scholar] [CrossRef]
- Kim, M.P.; Evans, D.B.; Wang, H.; Abbruzzese, J.L.; Fleming, J.B.; Gallick, G.E. Generation of orthotopic and heterotopic human pancreatic cancer xenografts in immunodeficient mice. Nat. Protoc. 2009, 4, 1670–1680. [Google Scholar] [CrossRef]
- Kota, J.; Hancock, J.; Kwon, J.; Korc, M. Pancreatic cancer: Stroma and its current and emerging targeted therapies. Cancer Lett. 2017, 391, 38–49. [Google Scholar] [CrossRef]
- Park, J.Y.; Hiroshima, Y.; Lee, J.Y.; Maawy, A.A.; Hoffman, R.M.; Bouvet, M. MUC1 selectively targets human pancreatic cancer in orthotopic nude mouse models. PLoS ONE 2015, 10, e0122100. [Google Scholar] [CrossRef]
- Maawy, A.A.; Hiroshima, Y.; Kaushal, S.; Luiken, G.A.; Hoffman, R.M.; Bouvet, M. Comparison of a chimeric anti-carcinoembryonic antigen antibody conjugated with visible or near-infrared fluorescent dyes for imaging pancreatic cancer in orthotopic nude mouse models. J. Biomed. Opt. 2013, 18, 126016. [Google Scholar] [CrossRef]
- Lafaro, K.J.; Melstrom, L.G. The Paradoxical Web of Pancreatic Cancer Tumor Microenvironment. Am. J. Pathol. 2019, 189, 44–57. [Google Scholar] [CrossRef]
- Peng, X.; Wang, R.; Wang, T.; Yang, W.; Wang, H.; Gu, W.; Ye, L. Carbon Dots/Prussian Blue Satellite/Core Nanocomposites for Optical Imaging and Photothermal Therapy. ACS Appl. Mater. Interfaces 2018, 10, 1084–1092. [Google Scholar] [CrossRef]
- Maebayashi, T.; Ishibashi, N.; Aizawa, T.; Sakaguchi, M.; Sato, T.; Kawamori, J.; Tanaka, Y. Treatment outcomes of concurrent hyperthermia and chemoradiotherapy for pancreatic cancer: Insights into the significance of hyperthermia treatment. Oncol. Lett. 2017, 13, 4959–4964. [Google Scholar] [CrossRef] [PubMed]
- Tansi, F.L.; Fröbel, F.; Maduabuchi, W.O.; Steiniger, F.; Westermann, M.; Quaas, R.; Teichgräber, U.K.; Hilger, I. Effect of Matrix-Modulating Enzymes on The Cellular Uptake of Magnetic Nanoparticles and on Magnetic Hyperthermia Treatment of Pancreatic Cancer Models In Vivo. Nanomaterials 2021, 11, 438. [Google Scholar] [CrossRef] [PubMed]
- Arcot, R.; Potts, B.A.; Polascik, T.J. Focal Cryoablation of Image-Localized Prostate Cancer. J. Endourol. 2021, 35, S17–S23. [Google Scholar] [CrossRef]
- Dolenšek, J.; Rupnik, M.S.; Stožer, A. Structural similarities and differences between the human and the mouse pancreas. Islets 2015, 7, e1024405. [Google Scholar] [CrossRef]
- Samulitis, B.K.; Pond, K.W.; Pond, E.; Cress, A.E.; Patel, H.; Wisner, L.; Patel, C.; Dorr, R.T.; Landowski, T.H. Gemcitabine resistant pancreatic cancer cell lines acquire an invasive phenotype with collateral hypersensitivity to histone deacetylase inhibitors. Cancer Biol. Ther. 2015, 16, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Lieber, M.; Mazzetta, J.; Nelson-Rees, W.; Kaplan, M.; Todaro, G. Establishment of a continuous tumor-cell line (panc-1) from a human carcinoma of the exocrine pancreas. Int. J. Cancer 1975, 15, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.H.; Nowak, N.J.; Loor, R.; Ochi, H.; Sandberg, A.A.; Lopez, C.; Pickren, J.W.; Berjian, R.; Douglass, H.O., Jr.; Chu, T.M. Characterization of a new primary human pancreatic tumor line. Cancer Investig. 1986, 4, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Deer, E.L.; Gonzalez-Hernandez, J.; Coursen, J.D.; Shea, J.E.; Ngatia, J.; Scaife, C.L.; Firpo, M.A.; Mulvihill, S.J. Phenotype and genotype of pancreatic cancer cell lines. Pancreas 2010, 39, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Luchini, C.; Paolino, G.; Mattiolo, P.; Piredda, M.L.; Cavaliere, A.; Gaule, M.; Melisi, D.; Salvia, R.; Malleo, G.; Shin, J.I.; et al. KRAS wild-type pancreatic ductal adenocarcinoma: Molecular pathology and therapeutic opportunities. J. Exp. Clin. Cancer Res. 2020, 39, 227. [Google Scholar] [CrossRef]
- Öhlund, D.; Handly-Santana, A.; Biffi, G.; Elyada, E.; Almeida, A.S.; Ponz-Sarvise, M.; Corbo, V.; Oni, T.E.; Hearn, S.A.; Lee, E.J.; et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med. 2017, 214, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Malchiodi, Z.X.; Weiner, L.M. Understanding and Targeting Natural Killer Cell-Cancer-Associated Fibroblast Interactions in Pancreatic Ductal Adenocarcinoma. Cancers 2021, 13, 405. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Clark, R.; Chekmareva, M.; Johnson, A.; George, S.; Shaw, P.; Seewaldt, V.; Rinker-Schaeffer, C. In Vivo and Ex Vivo Approaches to Study Ovarian Cancer Metastatic Colonization of Milky Spot Structures in Peritoneal Adipose. J. Vis. Exp. 2015, 104, e52721. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Yu, D.H.; Chen, Y.; Zhao, C.Y.; Zhang, J.; Liu, Q.H.; Ni, C.R.; Zhu, M.H. Expression of fibroblast activation protein in human pancreatic adenocarcinoma and its clinicopathological significance. World J. Gastroenterol. 2012, 18, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Brocks, B.; Garin-Chesa, P.; Behrle, E.; Park, J.E.; Rettig, W.J.; Pfizenmaier, K.; Moosmayer, D. Species-Crossreactive scFv Against the Tumor Stroma Marker “Fibroblast Activation Protein” Selected by Phage Display From an Immunized FAP−/− Knock-Out Mouse. Mol. Med. 2001, 7, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.A.; Shea, A.A.; Heffron, C.L.; Schmelz, E.M.; Roberts, P.C. Intra-abdominal fat depots represent distinct immunomodulatory microenvironments: A murine model. PLoS ONE 2013, 8, e66477. [Google Scholar] [CrossRef]
- Kersy, O.; Loewenstein, S.; Lubezky, N.; Sher, O.; Simon, N.B.; Klausner, J.M.; Lahat, G. Omental Tissue-Mediated Tumorigenesis of Gastric Cancer Peritoneal Metastases. Front. Oncol. 2019, 9, 1267. [Google Scholar] [CrossRef] [PubMed]
- Meza-Perez, S.; Randall, T.D. Immunological Functions of the Omentum. Trends Immunol. 2017, 38, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Litbarg, N.O.; Gudehithlu, K.P.; Sethupathi, P.; Arruda, J.A.; Dunea, G.; Singh, A.K. Activated omentum becomes rich in factors that promote healing and tissue regeneration. Cell Tissue Res. 2007, 328, 487–497. [Google Scholar] [CrossRef]
- Clark, R.; Krishnan, V.; Schoof, M.; Rodriguez, I.; Theriault, B.; Chekmareva, M.; Rinker-Schaeffer, C. Milky spots promote ovarian cancer metastatic colonization of peritoneal adipose in experimental models. Am. J. Pathol. 2013, 183, 576–591. [Google Scholar] [CrossRef]
- Peixoto, R.D.; Speers, C.; McGahan, C.E.; Renouf, D.J.; Schaeffer, D.F.; Kennecke, H.F. Prognostic factors and sites of metastasis in unresectable locally advanced pancreatic cancer. Cancer Med. 2015, 4, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Feygenzon, V.; Loewenstein, S.; Lubezky, N.; Pasmanic-Chor, M.; Sher, O.; Klausner, J.M.; Lahat, G. Unique cellular interactions between pancreatic cancer cells and the omentum. PLoS ONE 2017, 12, e0179862. [Google Scholar] [CrossRef] [PubMed]
- Mikuła-Pietrasik, J.; Sosińska, P.; Maksin, K.; Kucińska, M.G.; Piotrowska, H.; Murias, M.; Woźniak, A.; Szpurek, D.; Książek, K. Colorectal cancer-promoting activity of the senescent peritoneal mesothelium. Oncotarget 2015, 6, 29178–29195. [Google Scholar] [CrossRef]
- Mikuła-Pietrasik, J.; Uruski, P.; Kucińska, M.; Tykarski, A.; Książek, K. The protective activity of mesothelial cells against peritoneal growth of gastrointestinal tumors: The role of soluble ICAM-1. Int. J. Biochem. Cell Biol. 2017, 86, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Thomassen, I.; van Gestel, Y.R.; van Ramshorst, B.; Luyer, M.D.; Bosscha, K.; Nienhuijs, S.W.; Lemmens, V.E.; de Hingh, I.H. Peritoneal carcinomatosis of gastric origin: A population-based study on incidence, survival and risk factors. Int. J. Cancer 2014, 134, 622–628. [Google Scholar] [CrossRef]
- Liu, X.; Cai, H.; Sheng, W.; Wang, Y. Long-term results and prognostic factors of gastric cancer patients with microscopic peritoneal carcinomatosis. PLoS ONE 2012, 7, e37284. [Google Scholar] [CrossRef]
- Stähle, M.; Veit, C.; Bachfischer, U.; Schierling, K.; Skripczynski, B.; Hall, A.; Gierschik, P.; Giehl, K. Mechanisms in LPA-induced tumor cell migration: Critical role of phosphorylated ERK. J. Cell Sci. 2003, 116, 3835–3846. [Google Scholar] [CrossRef] [PubMed]
- Cannistra, M.; Ruggiero, M.; Zullo, A.; Serafini, S.; Grande, R.; Nardo, B. Metastases of pancreatic adenocarcinoma: A systematic review of literature and a new functional concept. Int. J. Surg. 2015, 21 (Suppl. 1), S15–S21. [Google Scholar] [CrossRef]
- Oda; Kondo, H.; Yamao, T.; Saito, D.; Ono, H.; Gotoda, T.; Yamaguchi, H.; Yoshida, S.; Shimoda, T. Metastatic tumors to the stomach: Analysis of 54 patients diagnosed at endoscopy and 347 autopsy cases. Endoscopy 2001, 33, 507–510. [Google Scholar] [CrossRef]
- Metildi, C.A.; Kaushal, S.; Hoffman, R.M.; Bouvet, M. In vivo serial selection of human pancreatic cancer cells in orthotopic mouse models produces high metastatic variants irrespective of Kras status. J. Surg. Res. 2013, 184, 290–298. [Google Scholar] [CrossRef]
- Sherman, M.H. Stellate Cells in Tissue Repair, Inflammation, and Cancer. Annu. Rev. Cell Dev. Biol. 2018, 34, 333–355. [Google Scholar] [CrossRef] [PubMed]
- Apte, M.; Pirola, R.C.; Wilson, J.S. Pancreatic stellate cell: Physiologic role, role in fibrosis and cancer. Curr. Opin. Gastroenterol. 2015, 31, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Omary, M.B.; Lugea, A.; Lowe, A.W.; Pandol, S.J. The pancreatic stellate cell: A star on the rise in pancreatic diseases. J. Clin. Investig. 2007, 117, 50–59. [Google Scholar] [CrossRef] [PubMed]
- James, M.L.; Gambhir, S.S. A molecular imaging primer: Modalities, imaging agents, and applications. Physiol. Rev. 2012, 92, 897–965. [Google Scholar] [CrossRef] [PubMed]
- Shcherbo, D.; Murphy, C.S.; Ermakova, G.V.; Solovieva, E.A.; Chepurnykh, T.V.; Shcheglov, A.S.; Verkhusha, V.V.; Pletnev, V.Z.; Hazelwood, K.L.; Roche, P.M.; et al. Far-red fluorescent tags for protein imaging in living tissues. Biochem. J. 2009, 418, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Feldman, J.P.; Goldwasser, R.; Mark, S.; Schwartz, J.; Orion, I. A Mathematical Model for Tumor Volume Evaluation using Two-Dimensions. J. Appl. Quant. Methods 2009, 4, 8. [Google Scholar]
- Crowe, A.R.; Yue, W. Semi-quantitative Determination of Protein Expression Using Immunohistochemistry Staining and Analysis: An Integrated Protocol. Bio-Protoc. 2019, 9, e3465. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Q.; Xu, C.B. A convenient method for quantifying collagen fibers in atherosclerotic lesions by ImageJ software. Int. J. Clin. Exp. Med. 2017, 10, 7. [Google Scholar]






| Tumor Progression * | Estimated Time Post Induction | US Detection of Primary Tumor | US Detection of Infiltration | Histological Validations | |||||
|---|---|---|---|---|---|---|---|---|---|
| Side Position | Supine Position | Side Position | Supine Position | PSCs Vit-A | myCAF aSMA | iCAFs FAP (Stroma) | Fibrosis Collagen | ||
| Early | 2–4 weeks | Small tumor secluded in pancreas | No peculiarity | No peculiarity | No peculiarity | Very low <5% oval cells | >20% | −/+ | ≤20% |
| Advanced | 5–7 weeks | Large tumor | Growth towards gastric lobe/omentum | Growth towards gastric lobe/omentum | Omental infiltration | Low, <10% mix of oval/elongated cells | ≥40% | + | ≥40% |
| Late | ≥7 weeks | -Very large tumor -Metastasis in surrounding organs | -Pressure on stomach -Tumor in omentum | -Metastasis in surrounding organs, e.g., spleen | -Pressure on stomach -Omental/or stomach wall infiltration -Metastasis | >10% Mostly elongated | >40% | +++ | > 60% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tansi, F.L.; Schrepper, A.; Schwarzer, M.; Teichgräber, U.; Hilger, I. Identifying the Morphological and Molecular Features of a Cell-Based Orthotopic Pancreatic Cancer Mouse Model during Growth over Time. Int. J. Mol. Sci. 2024, 25, 5619. https://doi.org/10.3390/ijms25115619
Tansi FL, Schrepper A, Schwarzer M, Teichgräber U, Hilger I. Identifying the Morphological and Molecular Features of a Cell-Based Orthotopic Pancreatic Cancer Mouse Model during Growth over Time. International Journal of Molecular Sciences. 2024; 25(11):5619. https://doi.org/10.3390/ijms25115619
Chicago/Turabian StyleTansi, Felista L., Andrea Schrepper, Michael Schwarzer, Ulf Teichgräber, and Ingrid Hilger. 2024. "Identifying the Morphological and Molecular Features of a Cell-Based Orthotopic Pancreatic Cancer Mouse Model during Growth over Time" International Journal of Molecular Sciences 25, no. 11: 5619. https://doi.org/10.3390/ijms25115619
APA StyleTansi, F. L., Schrepper, A., Schwarzer, M., Teichgräber, U., & Hilger, I. (2024). Identifying the Morphological and Molecular Features of a Cell-Based Orthotopic Pancreatic Cancer Mouse Model during Growth over Time. International Journal of Molecular Sciences, 25(11), 5619. https://doi.org/10.3390/ijms25115619






