DFT and TD-DFT Investigations for the Limitations of Lengthening the Polyene Bridge between N,N-dimethylanilino Donor and Dicyanovinyl Acceptor Molecules as a D-π-A Dye-Sensitized Solar Cell
Abstract
1. Introduction
2. Results and Discussion
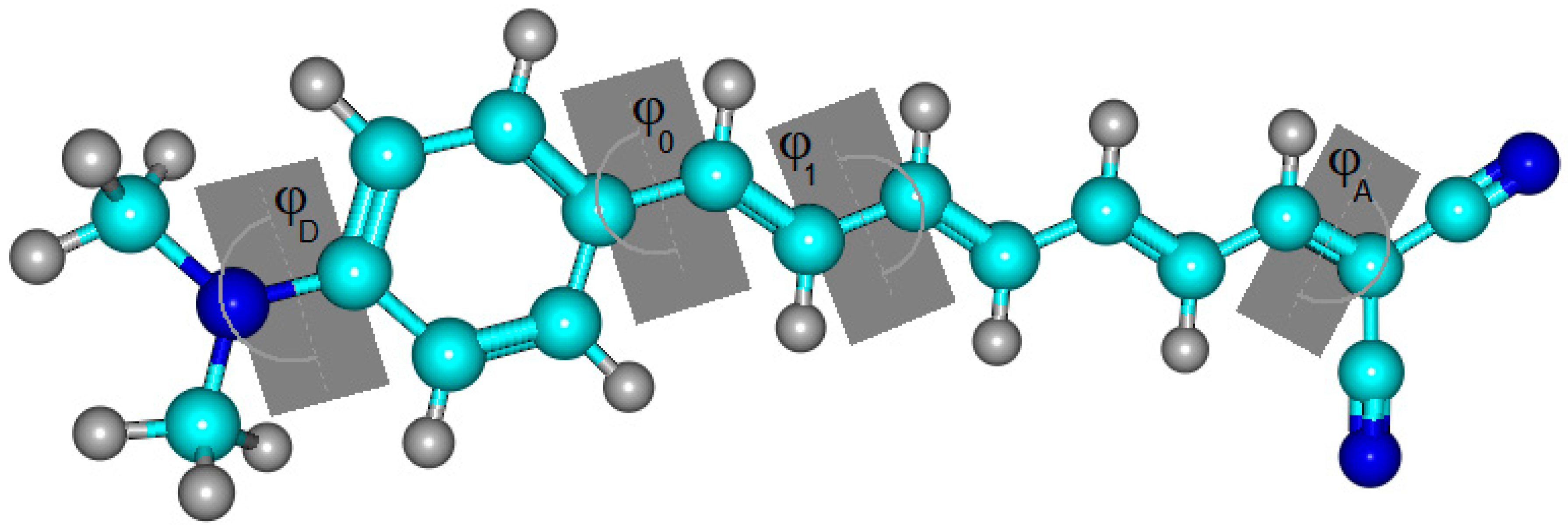
2.1. Optimized Molecular Structure
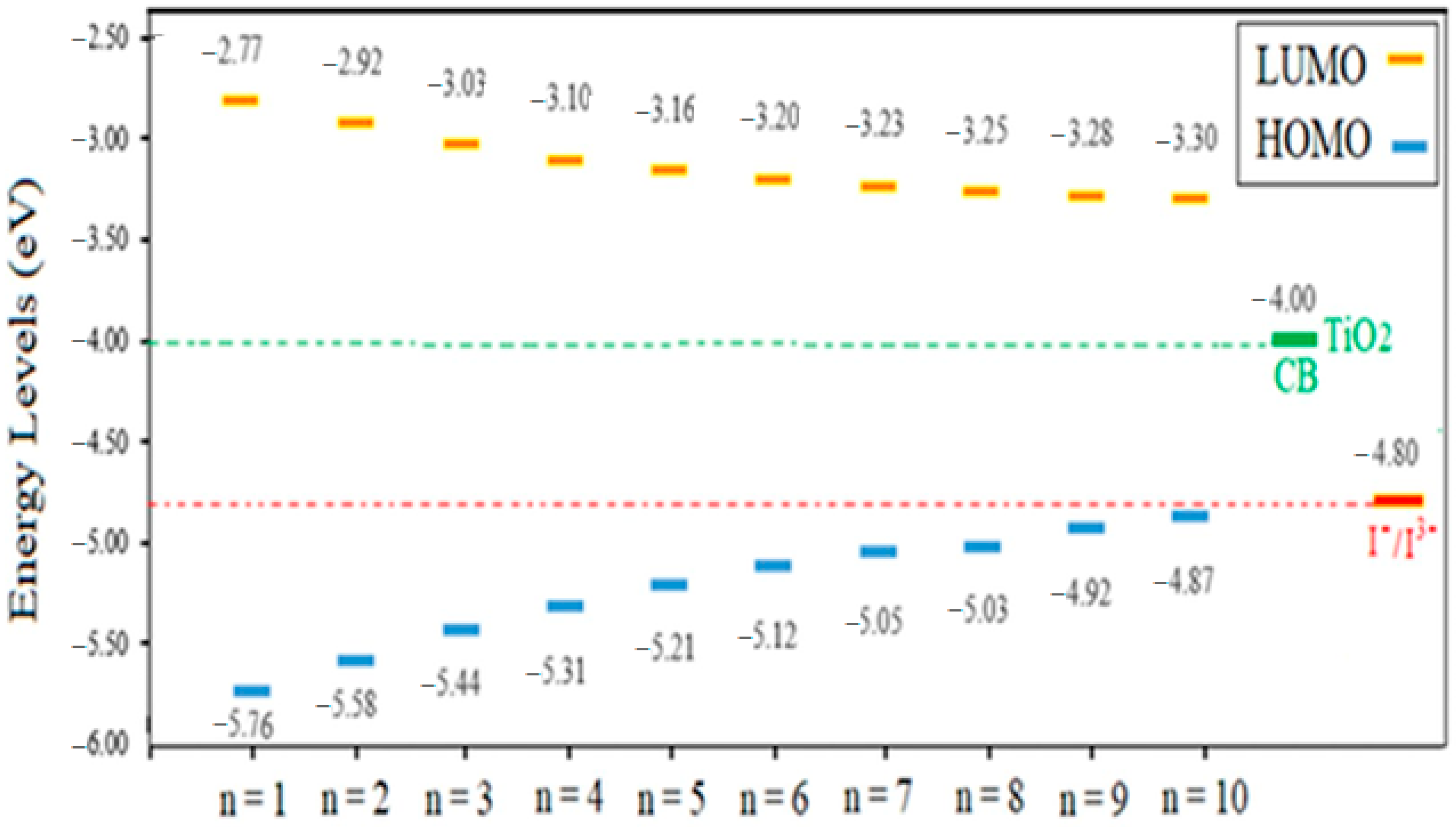
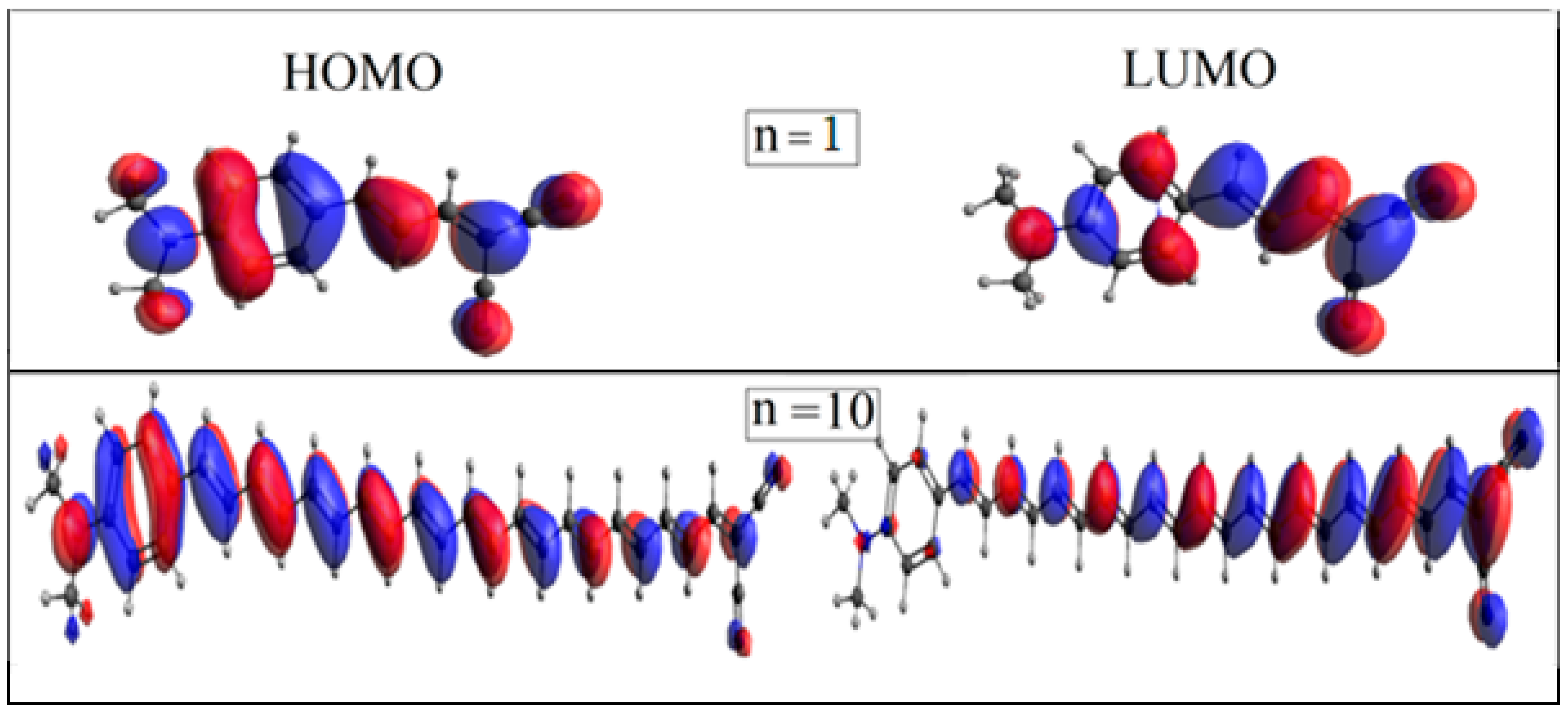
2.2. Electronic Properties
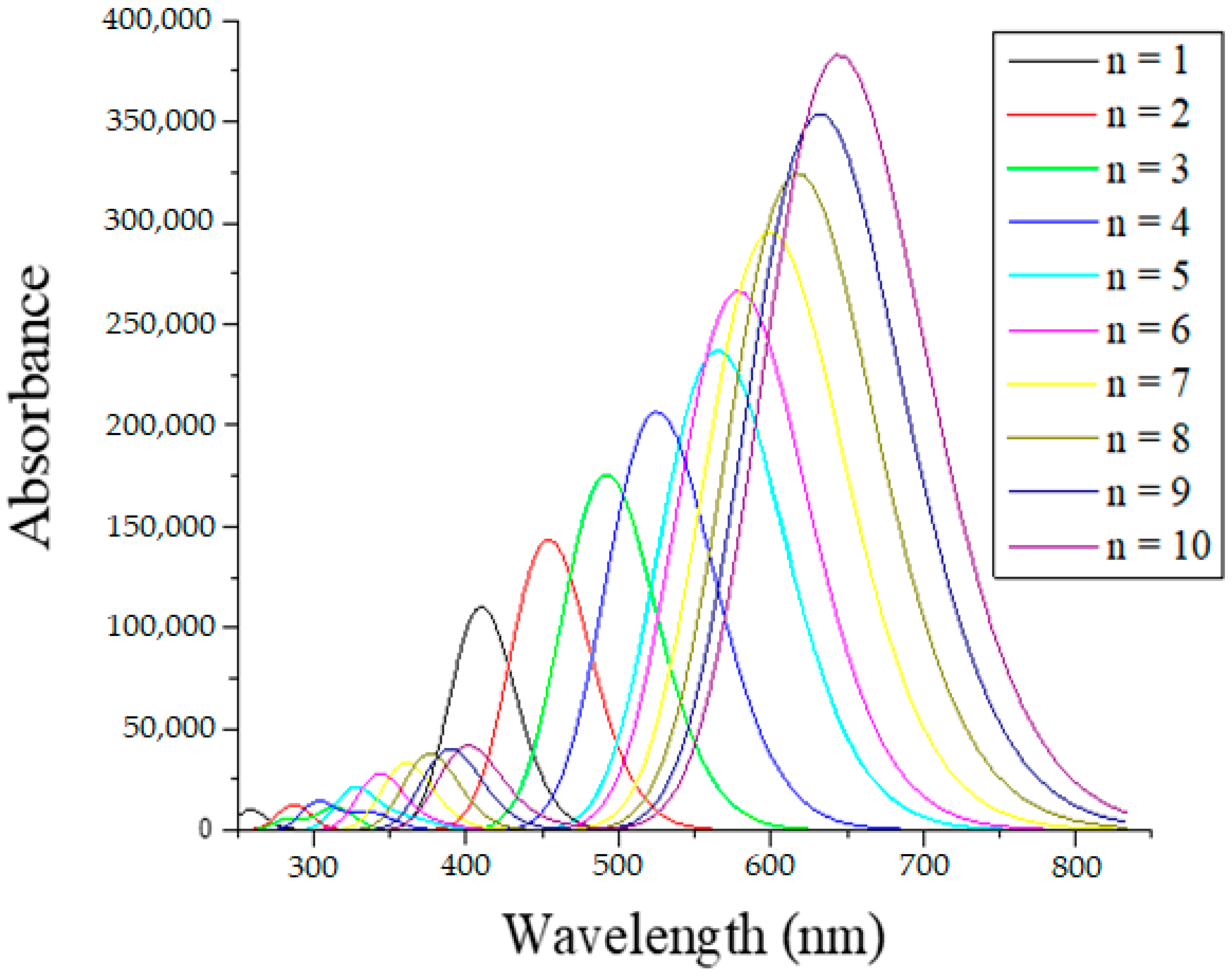
2.3. Absorption Spectra
2.4. Photovoltaic Properties
3. Materials and Methods
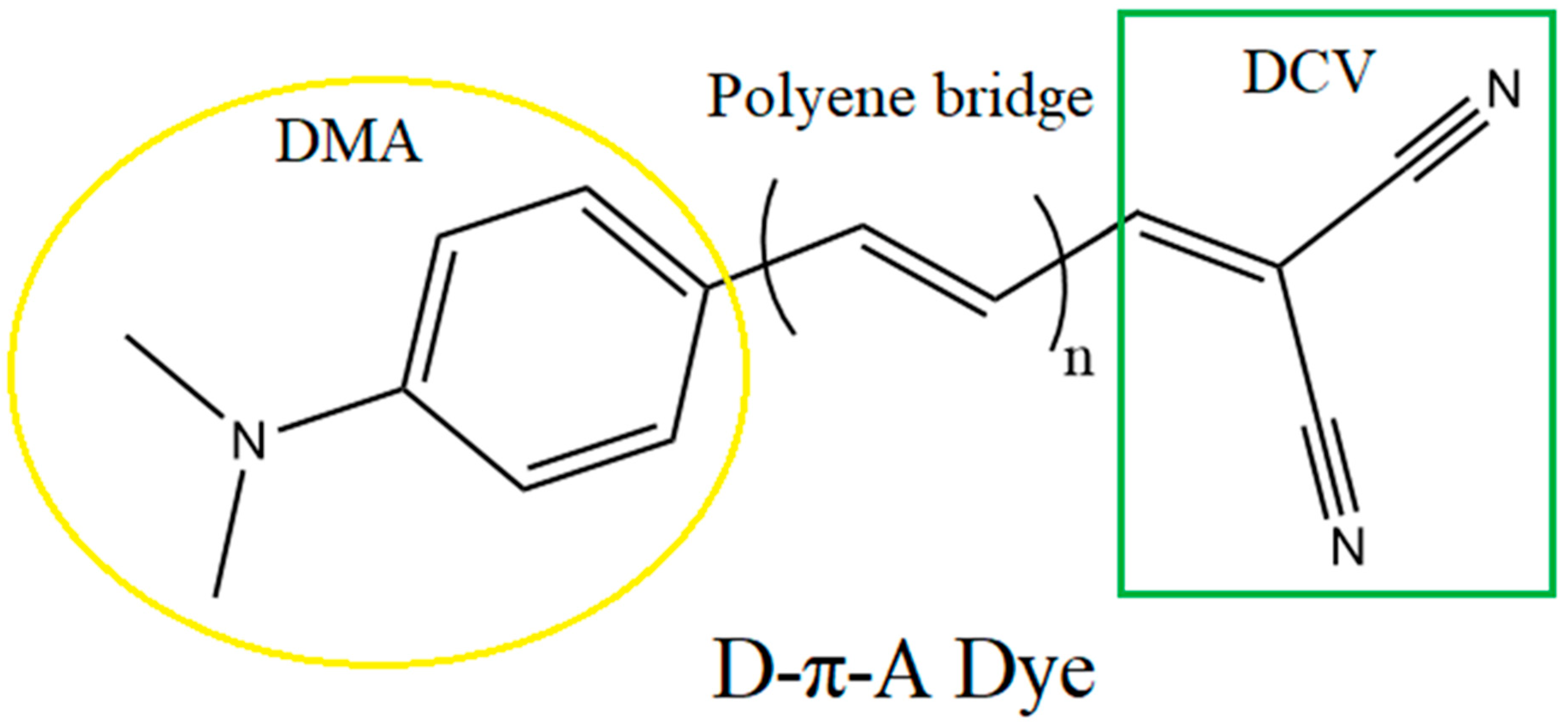
3.1. Chemistry and Molecular Design
3.2. Computational Methods
3.3. Photovoltaic Parameters
| (1) | is the ground-state oxidation potential of dye | |
| (2) | is the oxidation potential of the excited dye is the lowest excitation energy that corresponds to λmax | |
| (3) | is the reduction potential of the conduction band edge of TiO2, (−4.0 eV) | |
| (4) | is the redox potential I−/I3− (−4.8 V) | |
| (5) | ||
| (6) | ||
| (7) | ||
| (8) |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.-H.; Kim, D.-H.; So, J.-H.; Koo, H.-J. Toward Eco-Friendly Dye-Sensitized Solar Cells (DSSCs): Natural Dyes and Aqueous Electrolytes. Energies 2021, 15, 219. [Google Scholar] [CrossRef]
- Mariotti, N.; Bonomo, M.; Fagiolari, L.; Barbero, N.; Gerbaldi, C.; Bella, F.; Barolo, C. Recent advances in eco-friendly and cost-effective materials towards sustainable dye-sensitized solar cells. Green Chem. 2020, 22, 7168–7218. [Google Scholar] [CrossRef]
- Andualem, A.; Demiss, S. Review on Dye-Sensitized Solar Cells (DSSCs). J. Heterocycl. 2018, 1, 29–34. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Datta, J. Wide-low energy coupled semi-conductor layers of TiO2–CdX boosting the performance of DSSC. Sol. Energy 2020, 208, 674–687. [Google Scholar] [CrossRef]
- Aslam, A.; Mehmood, U.; Arshad, M.H.; Ishfaq, A.; Zaheer, J.; Khan, A.U.H.; Sufyan, M. Dye-sensitized solar cells (DSSCs) as a potential photovoltaic technology for the self-powered internet of things (IoTs) applications. Sol. Energy 2020, 207, 874–892. [Google Scholar] [CrossRef]
- Kumavat, P.P.; Sonar, P.; Dalal, D.S. An overview on basics of organic and dye sensitized solar cells, their mechanism and recent improvements. Renew. Sustain. Energy Rev. 2017, 78, 1262–1287. [Google Scholar] [CrossRef]
- Al-Alwani, M.A.; Mohamad, A.B.; Ludin, N.A.; Kadhum, A.A.H.; Sopian, K. Dye-sensitised solar cells: Development, structure, operation principles, electron kinetics, characterisation, synthesis materials and natural photosensitisers. Renew. Sustain. Energy Rev. 2016, 65, 183–213. [Google Scholar] [CrossRef]
- Karthick, S.N.; Hemalatha, K.V.; Balasingam, S.K.; Manik Clinton, F.; Akshaya, S.; Kim, H.J. Dye-Sensitized Solar Cells: History, Components, Configuration, and Working Principle. In Interfacial Engineering in Functional Materials for Dye-Sensitized Solar Cells, 1st ed.; Pandikumar, A., Jothivenkatachalam, K., Bhojanaa, K., Eds.; Wiley: Hoboken, NJ, USA, 2019; pp. 1–16. [Google Scholar] [CrossRef]
- Durrant, J.R. Molecular approaches to solar energy conversion: The energetic cost of charge separation from molecular-excited states. Philos. Trans. R. Soc. A 2013, 371, 20120195. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Haleem, A.; Siddiq, M.; Hussain, M.K.; Qamar, S.; Hameed, S.; Waris, M. Research on dye sensitized solar cells: Recent advancement toward the various constituents of dye sensitized solar cells for efficiency enhancement and future prospects. RSC Adv. 2023, 13, 19508–19529. [Google Scholar] [CrossRef] [PubMed]
- Almansour, A.I.; Kumar, R.S.; Al-Shemaimari, K.I.; Arumugam, N. Highly Efficient DSSCs Sensitized Using NIR Responsive Bacteriopheophytine-a and Its Derivatives Extracted from Rhodobacter Sphaeroides Photobacteria. Molecules 2024, 29, 931. [Google Scholar] [CrossRef]
- Hosseinnezhad, M.; Ghahari, M.; Mobarhan, G.; Fathi, M.; Palevicius, A.; Nutalapati, V.; Janusas, G.; Nasiri, S. New Insights into Improving the Photovoltaic Performance of Dye-Sensitized Solar Cells by Removing Platinum from the Counter Electrode Using a Graphene-MoS2 Composite or Hybrid. Micromachines 2023, 14, 2161. [Google Scholar] [CrossRef] [PubMed]
- Nowsherwan, G.A.; Iqbal, M.A.; Rehman, S.U.; Zaib, A.; Sadiq, M.I.; Dogar, M.A.; Azhar, M.; Maidin, S.S.; Hussain, S.S.; Morsy, K.; et al. Numerical optimization and performance evaluation of ZnPC:PC70BM based dye-sensitized solar cell. Sci. Rep. 2023, 13, 10431. [Google Scholar] [CrossRef] [PubMed]
- Asiam, F.K.; Hao, N.H.; Kaliamurthy, A.K.; Kang, H.C.; Yoo, K.; Lee, J.-J. Preliminary Investigation on Vacancy Filling by Small Molecules on the Performance of Dye-Sensitized Solar Cells: The Case of a Type-II Absorber. Front. Chem. 2021, 9, 701781. [Google Scholar] [CrossRef] [PubMed]
- Yin, N.; Wang, L.; Lin, Y.; Yi, J.; Yan, L.; Dou, J.; Yang, H.-B.; Zhao, X.; Ma, C.-Q. Effect of the π-conjugation length on the properties and photovoltaic performance of A–π–D–π–A type oligothiophenes with a 4,8-bis(thienyl)benzo [1,2-b:4,5-b′]dithiophene core. Beilstein J. Org. Chem. 2016, 12, 1788–1797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cole, J.M. Anchoring Groups for Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 3427–3455. [Google Scholar] [CrossRef] [PubMed]
- Cowper, P.; Pockett, A.; Kociok-Köhn, G.; Cameron, P.J.; Lewis, S.E. Azulene—Thiophene—Cyanoacrylic acid dyes with donor-π-acceptor structures. Synthesis, characterisation and evaluation in dye-sensitized solar cells. Tetrahedron 2018, 74, 2775–2786. [Google Scholar] [CrossRef]
- Hosseinnezhad, M.; Moradian, S.; Gharanjig, K. Novel Organic Dyes Based on Thioindigo for Dye-Sensitized Solar Cells. Dye. Pigment. 2015, 123, 147–153. [Google Scholar] [CrossRef]
- Fernandes, S.S.M.; Castro, M.C.R.; Pereira, A.I.; Mendes, A.; Serpa, C.; Pina, J.; Justino, L.L.G.; Burrows, H.D.; Raposo, M.M.M. Optical and Photovoltaic Properties of Thieno[3,2-b]thiophene-Based Push–Pull Organic Dyes with Different Anchoring Groups for Dye-Sensitized Solar Cells. ACS Omega 2017, 2, 9268–9279. [Google Scholar] [CrossRef] [PubMed]
- Reginato, G.; Calamante, M.; Zani, L.; Mordini, A.; Franchi, D. Design and synthesis of organic sensitizers with enhanced anchoring stability in dye-sensitized solar cells. Pure Appl. Chem. 2018, 90, 363–376. [Google Scholar] [CrossRef]
- Faccio, R.; Fernandez-Werner, L.; Pardo, H.; Mombru, A.W. Current Trends in Materials for Dye Sensitized Solar Cells. Recent Pat. Nanotechnol. 2011, 5, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Dikbıyık, D.E.; Karakurt, O.; Cevher, D.; Ozsoy, G.H.; Yıldırım, E.; Cirpan, A. How do we get across? Impact of bridging aromatic units on the optical, electronic, and photovoltaic properties of donor-acceptor polymers. Polymer 2024, 294, 126696. [Google Scholar] [CrossRef]
- Zubair, H.; Mahmood, R.F.; Waqas, M.; Ishtiaq, M.; Iqbal, J.; Ibrahim, M.A.A.; Sayed, S.R.M.; Noor, S.; Khera, R.A. Effect of tailoring π-linkers with extended conjugation on the SJ-IC molecule for achieving high VOC and improved charge mobility towards enhanced photovoltaic applications. RSC Adv. 2023, 13, 26050–26068. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Qu, J.; He, F. Chlorination: An Effective Strategy for High-Performance Organic Solar Cells. Adv. Sci. 2020, 7, 2000509. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Huang, W.; Yang, C.; Zhang, J.; Zhang, Q. Recent advances on π-conjugated polymers as active elements in high performance organic field-effect transistors. Front. Phys. 2021, 16, 33500. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, M.J.; Song, H.M.; Song, B.J.; Seo, K.D.; Pastore, M.; Anselmi, C.; Fantacci, S.; De Angelis, F.; Nazeeruddin, M.K.; et al. Organic dyes incorporating low-band-gap chromophores based on π-extended benzothiadiazole for dye-sensitized solar cells. Dye. Pigment. 2011, 91, 192–198. [Google Scholar] [CrossRef]
- Bureš, F.; Schweizer, W.B.; May, J.C.; Boudon, C.; Gisselbrecht, J.; Gross, M.; Biaggio, I.; Diederich, F. Property Tuning in Charge-Transfer Chromophores by Systematic Modulation of the Spacer between Donor and Acceptor. Chem.—A Eur. J. 2007, 13, 5378–5387. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, J.; Hickel, T. Density functional theory in materials science. WIREs Comput. Mol. Sci. 2013, 3, 438–448. [Google Scholar] [CrossRef]
- Aljaafreh, M.J.; Hussein, R.K. Substituent effect on the electronic and optical properties of newly designed pyrrole derivatives using density functional theory. Open Phys. 2024, 22, 20240012. [Google Scholar] [CrossRef]
- Mohamed Bouzzine, S.; Abdelaaziz, A.; Zaid, F.; Hamidi, M.; Al-Zahrani, F.A.M.; El-Shishtawy, R.M. Investigation of the structural and optoelectronic characteristics in coumarin-based dye-sensitized solar cells, both in isolation and with TiO2 adsorption. Comput. Mater. Sci. 2024, 241, 113043. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, V.; Sharma, S.S. Dye-Sensitized Solar Cells: Fundamentals and Current Status. Nanoscale Res. Lett. 2018, 13, 381. [Google Scholar] [CrossRef] [PubMed]
- Bozkaya, U. Accurate Electron Affinities from the Extended Koopmans’ Theorem Based on Orbital-Optimized Methods. J. Chem. Theory Comput. 2014, 10, 2041–2048. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.J.; Mori-Sánchez, P.; Yang, W. Challenges for Density Functional Theory. Chem. Rev. 2011, 112, 289–320. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A. Astaxanthin interacting with metal clusters: Free radical scavenger and photovoltaic materials. Theor. Chem. Acc. 2016, 135, 130. [Google Scholar] [CrossRef]
- Xie, S.; Xia, Y.; Zheng, Z.; Zhang, X.; Yuan, J.; Zhou, H.; Zhang, Y. Effects of Nonradiative Losses at Charge Transfer States and Energetic Disorder on the Open-Circuit Voltage in Nonfullerene Organic Solar Cells. Adv. Funct. Mater. 2018, 28, 1705659. [Google Scholar] [CrossRef]
- Katoh, R.; Furube, A. Electron injection efficiency in dye-sensitized solar cells. J. Photochem. Photobiol. C Photochem. Rev. 2014, 20, 1–16. [Google Scholar] [CrossRef]
- Samanta, P.N.; Majumdar, D.; Roszak, S.; Leszczynski, J. First-Principles Approach for Assessing Cold Electron Injection Efficiency of Dye-Sensitized Solar Cell: Elucidation of Mechanism of Charge Injection and Recombination. J. Phys. Chem. C 2020, 124, 2817–2836. [Google Scholar] [CrossRef]
- Daeneke, T.; Mozer, A.J.; Uemura, Y.; Makuta, S.; Fekete, M.; Tachibana, Y.; Koumura, N.; Bach, U.; Spiccia, L. Dye Regeneration Kinetics in Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2012, 134, 16925–16928. [Google Scholar] [CrossRef] [PubMed]
- Chaitanya, K.; Ju, X.-H.; Heron, B.M. Theoretical study on the light harvesting efficiency of zinc porphyrin sensitizers for DSSCs. RSC Adv. 2014, 4, 26621–26634. [Google Scholar] [CrossRef]
- Banerjee, T.; Podjaski, F.; Kroeger, J.; Biswal, B.P.; Lotsch, B. Polymer photocatalysts for solar-to-chemical energy conversion. Nat. Rev. Mater. 2020, 6, 168–190. [Google Scholar] [CrossRef]
- Ooyama, Y.; Harima, Y. Molecular Designs and Syntheses of Organic Dyes for Dye-Sensitized Solar Cells. Eur. J. Org. Chem. 2009, 2009, 2903–2934. [Google Scholar] [CrossRef]
- Elegbeleye, I.F.; Maluta, N.E.; Maphanga, R.R. Density functional theory study of promising polyene-diphenylaniline organic chromophores for dye-sensitized solar cell applications. Cogent Eng. 2018, 5, 1532778. [Google Scholar] [CrossRef]
- Bogdanov, G.; Tillotson, J.P.; Khrustalev, V.N.; Rigin, S.; Timofeeva, T.V. Synthesis, crystal structure studies and solvatochromic behaviour of two 2-{5-[4-(dimethylamino)phenyl]penta-2,4-dien-1-ylidene}malononitrile derivatives. Acta Crystallogr. Sect. C Struct. Chem. 2019, 75, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Nurazzi, N.M.; Harussani, M.M.; Demon SZ, N.; Halim, N.A.; Mohamad, I.S.; Bahruji, H.; Abdullah, N. Research Progress on Polythiophene and Its Application as Chemical Sensor. Zulfaqar J. Def. Sci. Eng. Technol. 2022, 5, 48–68. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Altürk, S.; Avcı, D.; Tamer, Ö.; Atalay, Y. Comparison of different hybrid DFT methods on structural, spectroscopic, electronic and NLO parameters for a potential NLO material. Comput. Theor. Chem. 2017, 1100, 34–45. [Google Scholar] [CrossRef]
- Emel’yanova, N.; Sanina, N.; Krivenko, A.; Manzhos, R.; Bozhenko, K.; Aldoshin, S. Comparison of pure and hybrid DFT functionals for geometry optimization and calculation of redox potentials for iron nitrosyl complexes with “μ-SCN” bridging ligands. Theor. Chem. Acc. 2013, 132, 1316. [Google Scholar] [CrossRef]
- Jacquemin, D.; Perpète, E.A.; Scuseria, G.E.; Ciofini, I.; Adamo, C. TD-DFT Performance for the Visible Absorption Spectra of Organic Dyes: Conventional versus Long-Range Hybrids. J. Chem. Theory Comput. 2008, 4, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision A. 1; Gaussian Inc.: Wallingford, UK, 2009. [Google Scholar]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhurko, G.; Zhurko, D. Chemcraft Graphical Program for Visualization of Computed Results. 2015. Available online: http://www.chemcraftprog.com/ (accessed on 2 March 2024).
- Deligkaris, C.; Rodriguez, J.H. Correction to DFT interaction energies by an empirical dispersion term valid for a range of intermolecular distances. Phys. Chem. Chem. Phys. 2012, 14, 3414–3424. [Google Scholar] [CrossRef] [PubMed]
- Katoh, R.; Furube, A.; Yoshihara, T.; Hara, K.; Fujihashi, G.; Takano, S.; Murata, S.; Arakawa, H.; Tachiya, M. Efficiencies of Electron Injection from Excited N3 Dye into Nanocrystalline Semiconductor (ZrO2, TiO2, ZnO, Nb2O5, SnO2, In2O3) Films. J. Phys. Chem. B 2004, 108, 4818–4822. [Google Scholar] [CrossRef]
- Xie, M.; Wang, J.; Xia, H.-Q.; Bai, F.-Q.; Jia, R.; Rim, J.-G.; Zhang, H.-X. Theoretical studies on the spectroscopic properties of porphyrin derivatives for dye-sensitized solar cell application. RSC Adv. 2015, 5, 33653–33665. [Google Scholar] [CrossRef]
- Sang-Aroon, W.; Saekow, S.; Amornkitbamrung, V. Density functional theory study on the electronic structure of Monascus dyes as photosensitizer for dye-sensitized solar cells. J. Photochem. Photobiol. A Chem. 2013, 236, 35–40. [Google Scholar] [CrossRef]
- Preat, J.; Michaux, C.; Jacquemin, D.; Perpète, E.A. Enhanced Efficiency of Organic Dye-Sensitized Solar Cells: Triphenylamine Derivatives. J. Phys. Chem. C 2009, 113, 16821–16833. [Google Scholar] [CrossRef]
| Compounds | Eg (eV) | IP (eV) | EA (eV) |
|---|---|---|---|
| n = 1 | 2.99 | 5.76 | 2.77 |
| n = 2 | 2.66 | 5.58 | 2.92 |
| n = 3 | 2.41 | 5.44 | 3.03 |
| n = 4 | 2.21 | 5.31 | 3.10 |
| n = 5 | 2.05 | 5.21 | 3.16 |
| n = 6 | 1.92 | 5.12 | 3.20 |
| n = 7 | 1.82 | 5.05 | 3.23 |
| n = 8 | 1.78 | 5.03 | 3.25 |
| n = 9 | 1.65 | 4.92 | 3.28 |
| n = 10 | 1.58 | 4.87 | 3.30 |
| Compounds | E eX (eV) | Wavelength (nm) | Oscillator Strength (ƒ) | Transition | Major Contribution |
|---|---|---|---|---|---|
| n = 1 | 2.98 | 415.594 | 1.52 | HOMO→LUMO | 99% |
| n = 2 | 2.69 | 461.024 | 1.98 | HOMO→LUMO | 94% |
| n = 3 | 2.48 | 500.477 | 2.43 | HOMO→LUMO | 92% |
| n = 4 | 2.32 | 534.918 | 2.86 | HOMO→LUMO | 90% |
| n = 5 | 2.20 | 564.767 | 3.27 | HOMO→LUMO | 87% |
| n = 6 | 2.10 | 590.397 | 3.68 | HOMO→LUMO | 85% |
| n = 7 | 2.06 | 612.294 | 4.08 | HOMO→LUMO | 82% |
| n = 8 | 1.97 | 630.830 | 4.49 | HOMO→LUMO | 79% |
| n = 9 | 1.92 | 646.487 | 4.89 | HOMO→LUMO | 75% |
| n = 10 | 1.88 | 659.626 | 5.29 | HOMO→LUMO | 71% |
| Compounds | E eX (eV) | E dyes (eV) | E dyes* (eV) | ΔG inject (eV) | ΔG reg (eV) |
|---|---|---|---|---|---|
| n = 1 | 2.98 | 5.76 | 3.03 | −1.22 | 0.96 |
| n = 2 | 2.69 | 5.58 | 2.89 | −1.11 | 0.78 |
| n = 3 | 2.48 | 5.44 | 2.96 | −1.04 | 0.64 |
| n = 4 | 2.32 | 5.31 | 2.99 | −1.01 | 0.51 |
| n = 5 | 2.20 | 5.21 | 3.02 | −0.99 | 0.41 |
| n = 6 | 2.10 | 5.12 | 3.02 | −0.98 | 0.32 |
| n = 7 | 2.03 | 5.05 | 3.03 | −0.98 | 0.25 |
| n = 8 | 1.97 | 5.03 | 3.06 | −0.94 | 0.23 |
| n = 9 | 1.91 | 4.92 | 3.00 | −1.00 | 0.12 |
| n = 10 | 1.88 | 4.87 | 2.99 | −1.01 | 0.07 |
| Compounds | LHE | VOC (eV) | τ (ns) |
|---|---|---|---|
| n = 1 | 0.9698 | 1.23 | 1.71 |
| n = 2 | 0.989529 | 1.08 | 1.61 |
| n = 3 | 0.996285 | 0.97 | 1.54 |
| n = 4 | 0.99862 | 0.9 | 1.50 |
| n = 5 | 0.999463 | 0.84 | 1.46 |
| n = 6 | 0.999791 | 0.8 | 1.42 |
| n = 7 | 0.999917 | 0.77 | 1.33 |
| n = 8 | 0.999968 | 0.75 | 1.32 |
| n = 9 | 0.999987 | 0.72 | 1.28 |
| n = 10 | 0.999995 | 0.70 | 1.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu Alrub, S.; Ali, A.I.; Hussein, R.K.; Alghamdi, S.K.; Eladly, S.A. DFT and TD-DFT Investigations for the Limitations of Lengthening the Polyene Bridge between N,N-dimethylanilino Donor and Dicyanovinyl Acceptor Molecules as a D-π-A Dye-Sensitized Solar Cell. Int. J. Mol. Sci. 2024, 25, 5586. https://doi.org/10.3390/ijms25115586
Abu Alrub S, Ali AI, Hussein RK, Alghamdi SK, Eladly SA. DFT and TD-DFT Investigations for the Limitations of Lengthening the Polyene Bridge between N,N-dimethylanilino Donor and Dicyanovinyl Acceptor Molecules as a D-π-A Dye-Sensitized Solar Cell. International Journal of Molecular Sciences. 2024; 25(11):5586. https://doi.org/10.3390/ijms25115586
Chicago/Turabian StyleAbu Alrub, Sharif, Ahmed I. Ali, Rageh K. Hussein, Suzan K. Alghamdi, and Sally A. Eladly. 2024. "DFT and TD-DFT Investigations for the Limitations of Lengthening the Polyene Bridge between N,N-dimethylanilino Donor and Dicyanovinyl Acceptor Molecules as a D-π-A Dye-Sensitized Solar Cell" International Journal of Molecular Sciences 25, no. 11: 5586. https://doi.org/10.3390/ijms25115586
APA StyleAbu Alrub, S., Ali, A. I., Hussein, R. K., Alghamdi, S. K., & Eladly, S. A. (2024). DFT and TD-DFT Investigations for the Limitations of Lengthening the Polyene Bridge between N,N-dimethylanilino Donor and Dicyanovinyl Acceptor Molecules as a D-π-A Dye-Sensitized Solar Cell. International Journal of Molecular Sciences, 25(11), 5586. https://doi.org/10.3390/ijms25115586






