Health-Promoting Properties of Natural Flavonol Glycosides Isolated from Staphylea pinnata L.
Abstract
1. Introduction
2. Results
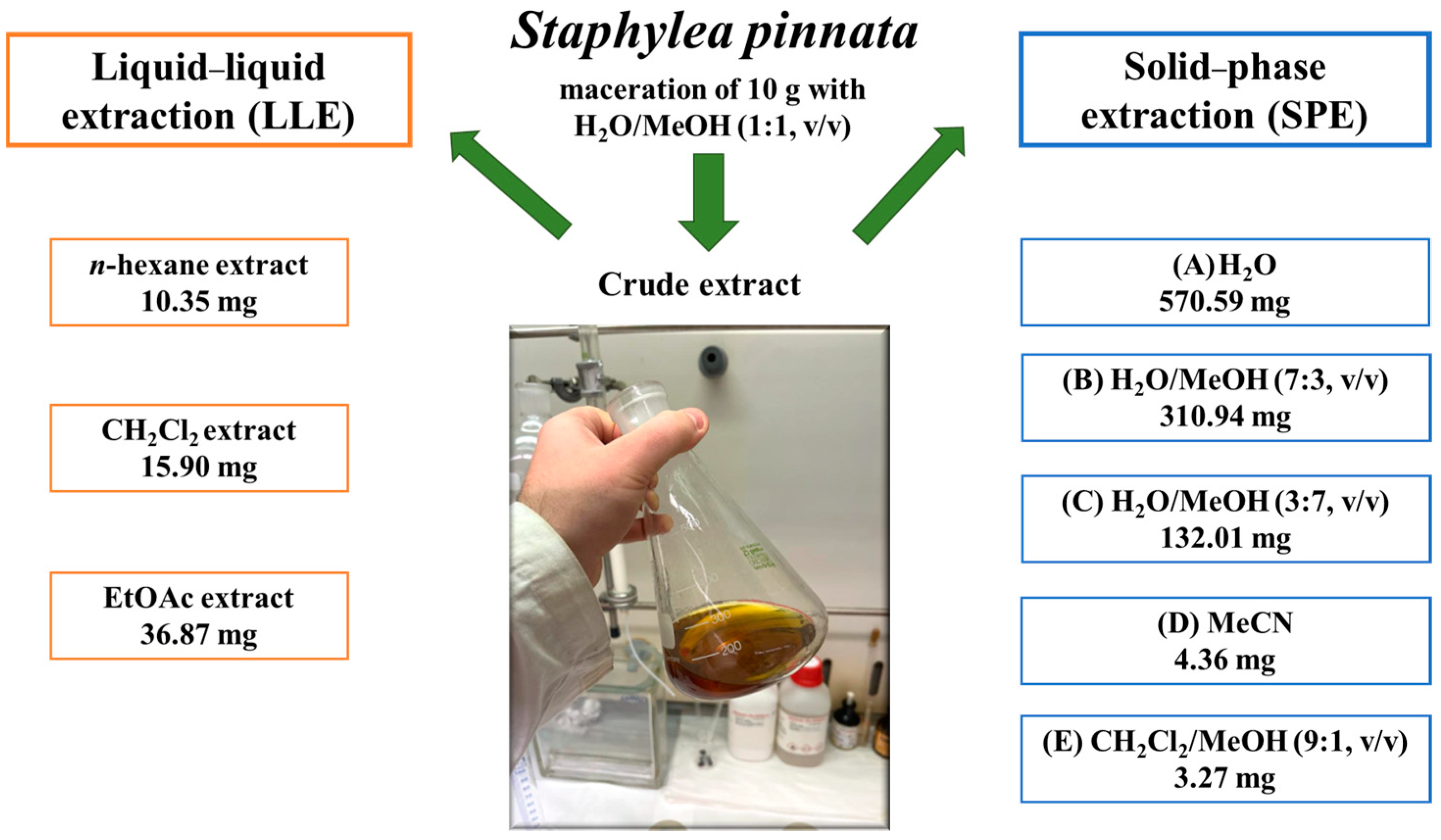
2.1. Extraction of S. pinnata’s Aerial Parts
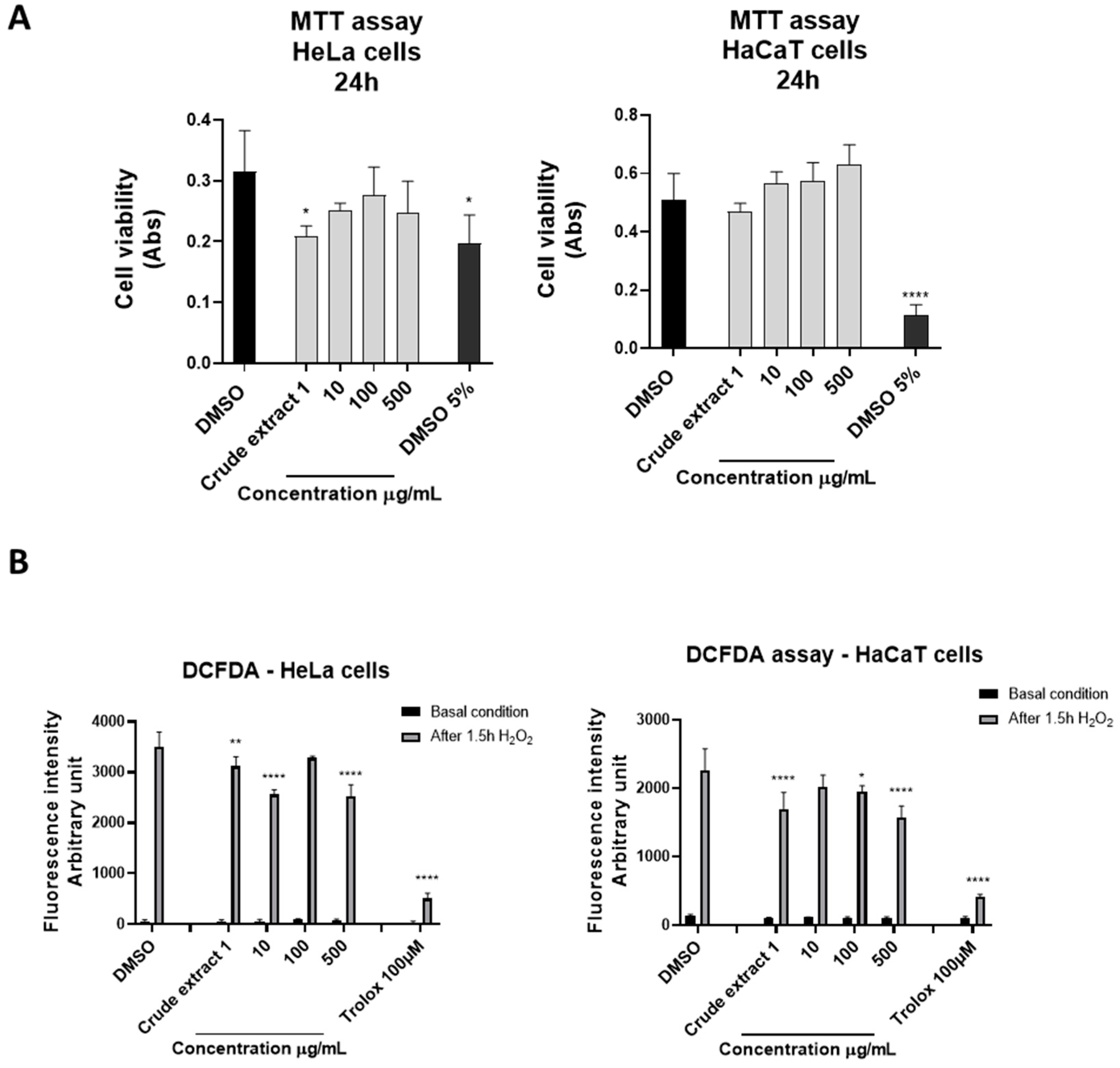
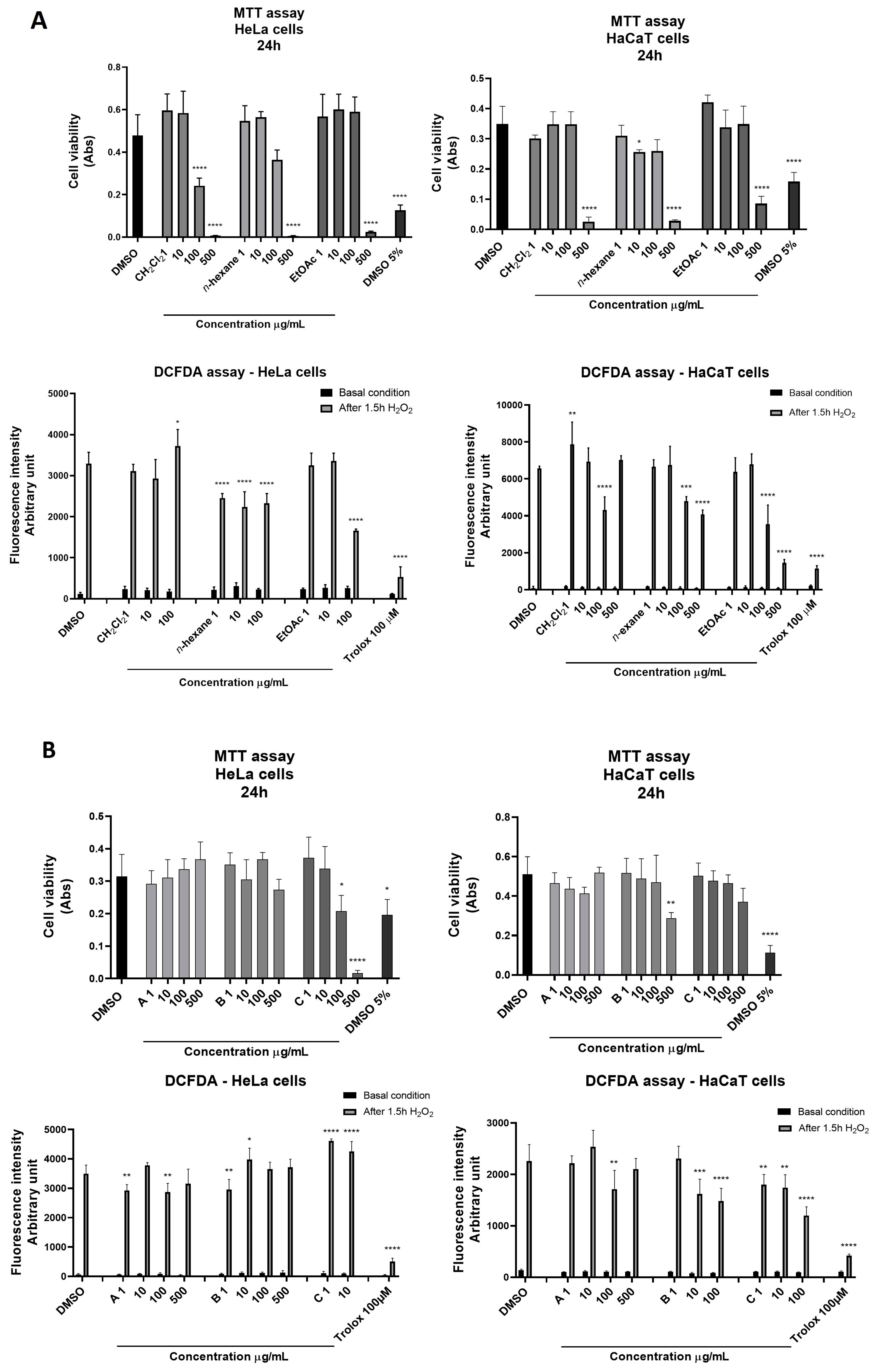
2.2. Cytotoxicity and Antioxidant Properties of S. pinnata’s Crude Hydroalcoholic Extract and the LLE and SPE Fractions
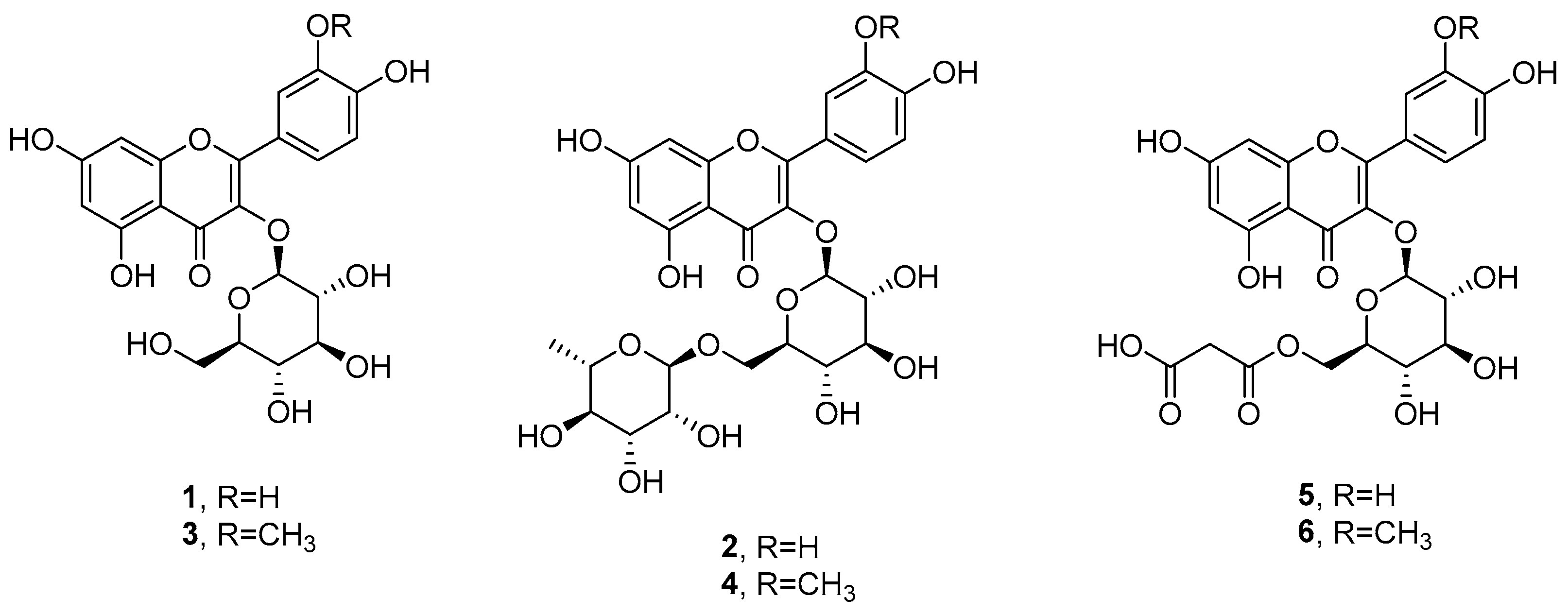
2.3. Purification and Identification of Pure Metabolites from S. pinnata
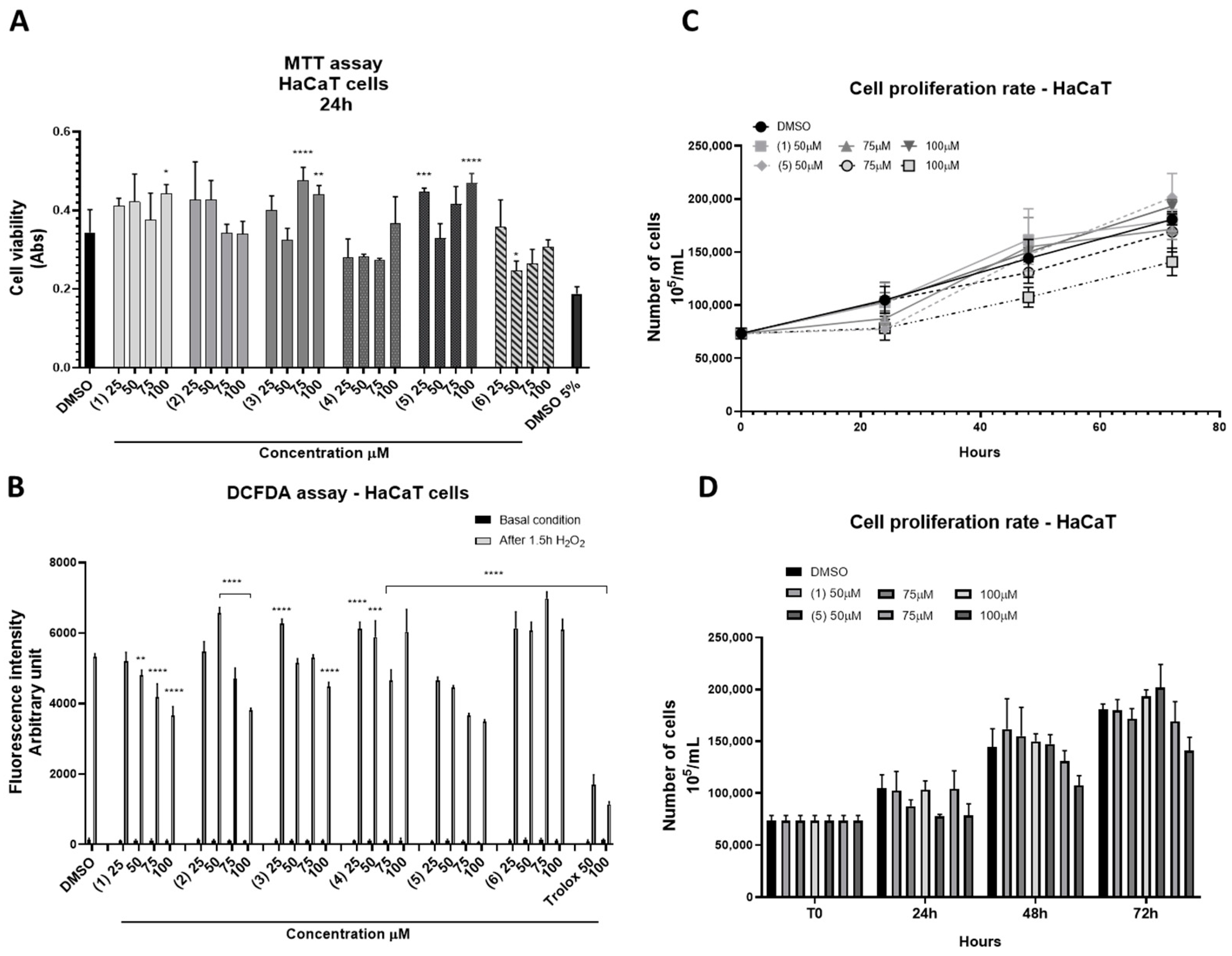
2.4. Cytotoxicity and Antioxidant Properties of the Pure Metabolites from S. pinnata
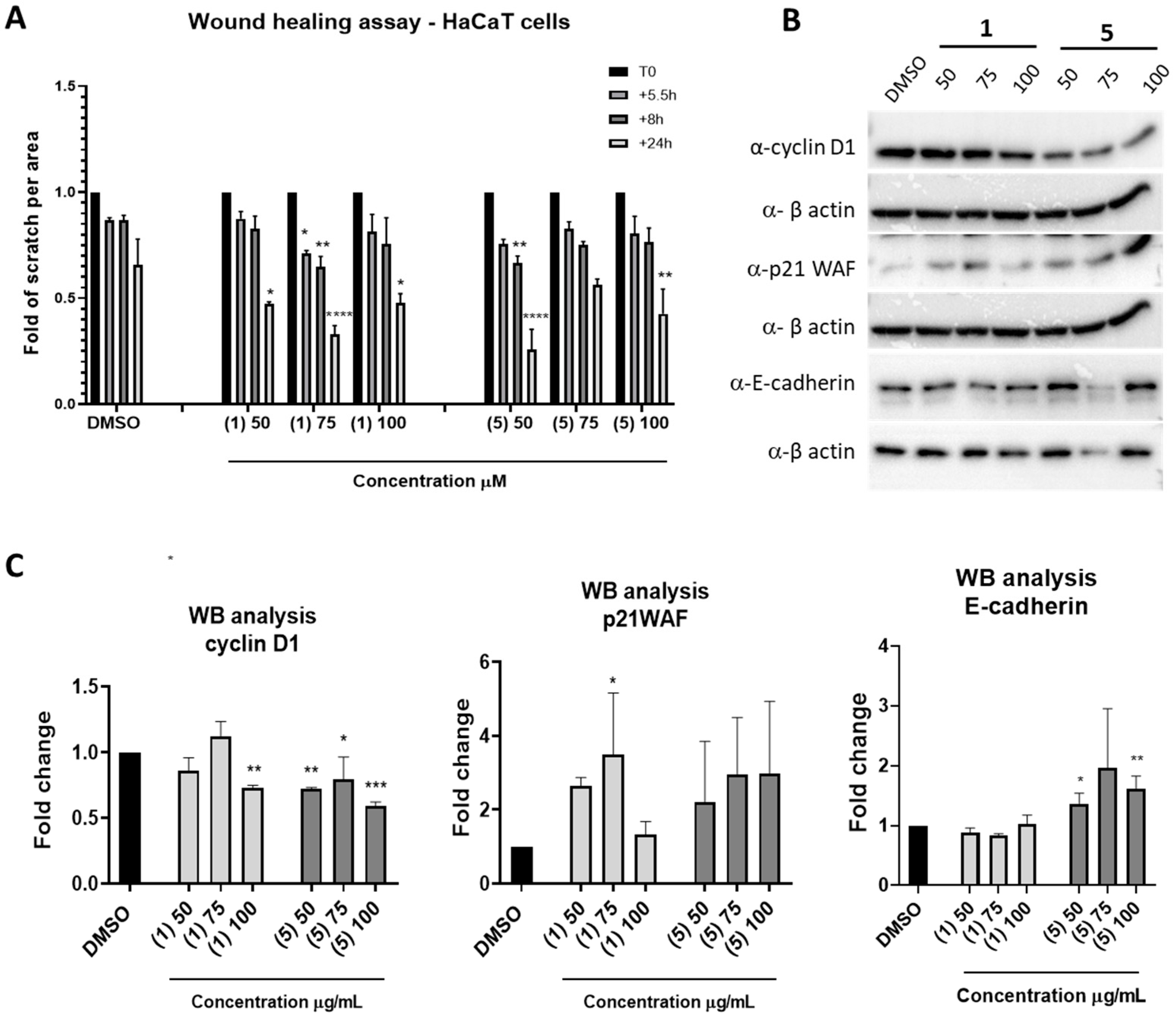
2.5. In Vitro Wound Healing Assay
2.6. Antimicrobial and Microbicidal Assays
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. General Experimental Procedures
4.3. Extraction and Purification of the Metabolites
4.4. Cell Culture and Reagents
4.5. Western Blot Analysis
4.6. DCFDA Assay
4.7. Cell Viability Assay
4.8. Cell Growth Profile
4.9. Wound Healing Assay
4.10. Antimicrobial Assays
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mukherjee, P.K.; Maity, N.; Nema, N.K.; Sarkar, B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine 2011, 19, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, D.F.; Cervantes, E.L.; Luna-Vital, D.A. Food-derived bioactive compounds with anti-aging potential for nutricosmetic and cosmeceutical products. Crit. Rev. Food Sci. Nutr. 2021, 61, 3740–3755. [Google Scholar] [CrossRef]
- Bjørklund, G.; Shanaida, M.; Lysiuk, R.; Butnariu, M.; Peana, M.; Sarac, I.; Strus, O.; Smetanina, K.; Chirumbolo, S. Natural compounds and products from an anti-aging perspective. Molecules 2022, 27, 7084. [Google Scholar] [CrossRef] [PubMed]
- Kepekçi, R.A.; İlçe, B.Y.; Kanmazalp, S.D. Plant-derived biomaterials for wound healing. Stud. Nat. Prod. Chem. 2021, 70, 227–264. [Google Scholar]
- Madeja, J.; Harmata, K.; Kołaczek, P.; Karpińska-Kołaczek, M.; Piątek, K.; Naks, P. Bracken (Pteridium aquilinum (L.) Kuhn), mistletoe (Viscum album (L.)) and bladder-nut (Staphylea pinnata (L.))-mysterious plants with unusual applications. Cultural and ethnobotanical studies. Cult. Ethnobot. Stud. Plants Cult. Seeds Cult. Herit. Eur. 2009, 207–214. [Google Scholar]
- Lacikova, L.; Jancova, M.; Muselik, J.; Masterova, I.; Grancai, D.; Fickova, M. Antiproliferative, cytotoxic, antioxidant activity and polyphenols contents in leaves of four Staphylea L. species. Molecules 2009, 14, 3259–3267. [Google Scholar] [CrossRef] [PubMed]
- Gostyńska, M. Zwyczaje i obrzędy ludowe w Polsce związane z kłokoczką południową (Staphylea pinnata L.). Rocz. Dendrol. 1962, 16, 113–120. [Google Scholar]
- Jantova, S.; Nagy, M.; Ružeková, L.; Grancai, D. Cytotoxic effects of plant extracts from the families Fabaceae, Oleaceae, Philadelphaceae, Rosaceae and Staphyleaceae. Phytother. Res. 2001, 15, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Lacikova, L.; Muselik, J.; Masterova, I.; Grancai, D. Antioxidant activity and total phenols in different extracts of four Staphylea L. species. Molecules 2007, 12, 98–102. [Google Scholar] [CrossRef]
- Laciková, L.; Švajdlenka, E.; Mašterová, I.; Grančai, D. Isolation and identification of flower oil components from four Staphylea L. species. Chem. Pap. 2007, 61, 512–514. [Google Scholar] [CrossRef]
- Lacikova, L.; Pferschy-Wenzig, E.M.; Masterova, I.; Grancai, D.; Bauer, R. Antiinflammatory potential and fatty acid content of lipophilic leaf extracts of four Staphylea L. species. Nat. Prod. Commun. 2009, 4, 1934578X0900400420. [Google Scholar] [CrossRef]
- Sohn, S.J.; Kwon, Y.S.; Kim, S.S.; Chun, W.J.; Kim, C.M. Chemical constituents of the leaves of Staphylea bumalda. Nat. Prod. Sci. 2004, 10, 173–176. [Google Scholar]
- Šircelj, H.; Vidrih, R.; Veberič, R.; Mikulic-Petkovsek, M. Evaluation of bioactive constituents in European bladdernut (Staphylea pinnata L.) seed kernels. J. Food Compos. Anal. 2019, 78, 33–41. [Google Scholar] [CrossRef]
- He, Z.; Lian, W.; Liu, J.; Zheng, R.; Xu, H.; Du, G.; Liu, A. Isolation, structural characterization and neuraminidase inhibitory activities of polyphenolic constituents from Flos caryophylli. Phytochem. Lett. 2017, 19, 160–167. [Google Scholar] [CrossRef]
- Kazuma, K.; Noda, N.; Suzuki, M. Malonylated flavonol glycosides from the petals of Clitoria ternatea. Phytochemistry 2003, 62, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Eskalieva, B.K.; Akhmed, A.; Burasheva, G.S.; Abilov, Z.A.; Akhmad, V.U. Biologically active compounds from Climacoptera. Chem. Nat. Compd. 2004, 40, 87–88. [Google Scholar] [CrossRef]
- Wald, B.; Wray, V.; Galensa, R.; Herrmann, K. Malonated flavonol glycosides and 3,5-dicaffeoylquinic acid from pears. Phytochemistry 1989, 28, 663–664. [Google Scholar] [CrossRef]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Sarkhail, P.; Navidpour, L.; Rahimifard, M.; Hosseini, N.M.; Souri, E. Bioassay-guided fractionation and identification of wound healing active compound from Pistacia vera L. hull extract. J. Ethnopharmacol. 2020, 248, 112335. [Google Scholar] [CrossRef]
- Dewick, P.M. Medicinal Natural Products, 3rd ed.; John Wiley and Sons Ltd.: Chicester, UK, 2009. [Google Scholar]
- Daryanavard, H.; Postiglione, A.E.; Mühlemann, J.K.; Muday, G.K. Flavonols modulate plant development, signaling, and stress responses. Curr. Opin. Plant Biol. 2023, 72, 102350. [Google Scholar] [CrossRef]
- Barreca, D.; Trombetta, D.; Smeriglio, A.; Mandalari, G.; Romeo, O.; Felice, M.R.; Gattuso, G.; Nabavi, S.M. Food flavonols: Nutraceuticals with complex health benefits and functionalities. Trends Food Sci. Technol. 2021, 117, 194–204. [Google Scholar] [CrossRef]
- Jucá, M.M.; Cysne Filho, F.M.S.; de Almeida, J.C.; Mesquita, D.D.S.; Barriga, J.R.M.; Dias, K.C.F.; Barbosa, T.M.; Vasconcelos, L.C.; Leal, L.K.A.M.; Ribeiro, J.E.; et al. Flavonoids: Biological activities and therapeutic potential. Nat. Prod. Res. 2020, 34, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Oberley, L.W.; Oberley, T.D.; Buettner, G.R. Cell division in normal and transformed cells: The possible role of superoxide and hydrogen peroxide. Med. Hypotheses 1981, 7, 21–42. [Google Scholar] [CrossRef] [PubMed]
- Tunggal, J.A.; Helfrich, I.; Schmitz, A.; Schwarz, H.; Günzel, D.; Fromm, M.; Kemler, R.; Krieg, T.; Niessen, C.M. E-cadherin is essential for in vivo epidermal barrier function by regulating tight junctions. EMBO J. 2005, 24, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Vipin, C.; Saptami, K.; Fida, F.; Mujeeburahiman, M.; Rao, S.S.; Athmika; Arun, A.B.; Rekha, P.D. Potential synergistic activity of quercetin with antibiotics against multidrug-resistant clinical strains of Pseudomonas aeruginosa. PLoS ONE 2020, 15, e0241304. [Google Scholar] [CrossRef] [PubMed]
- Kwun, M.S.; Lee, D.G. Quercetin-induced yeast apoptosis through mitochondrial dysfunction under the accumulation of magnesium in Candida albicans. Fungal Biol. 2020, 124, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Lin, Q.; Yao, J.; Zhang, G.; Peng, X.; Tian, J. In vitro outcomes of quercetin on Candida albicans planktonic and biofilm cells and in vivo effects on vulvovaginal candidiasis. Evidences of its mechanisms of action. Phytomedicine 2023, 114, 154800. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.A.; Famuyide, I.; Wooding, M.; McGaw, L.J.; Mühling, K.H. Secondary metabolite profile and pharmacological opportunities of lettuce plants following selenium and sulfur enhancement. Pharmaceutics 2022, 14, 2267. [Google Scholar] [CrossRef] [PubMed]
- Pagano, C.; Perioli, L.; Baiocchi, C.; Bartoccini, A.; Beccari, T.; Blasi, F.; Calarco, P.; Ceccarini, M.R.; Cossignani, L.; di Michele, A.; et al. Preparation and characterization of polymeric microparticles loaded with Moringa oleifera leaf extract for exuding wound treatment. Int. J. Pharm. 2020, 587, 119700. [Google Scholar] [CrossRef]
- Mugo, S.M.; Njenga, P.K. Chemical composition, antioxidant potential and antimicrobial activities of Ixora scheffleri subspecies keniensis essential oil. J. Med. Plant Econ. Dev. 2020, 4, a58. [Google Scholar]
- Ly, T.N.; Shimoyamada, M.; Yamauchi, R. Isolation and characterization of rosmarinic acid oligomers in Celastrus hindsii Benth leaves and their antioxidative activity. J. Agric. Food Chem. 2006, 54, 3786–3793. [Google Scholar] [CrossRef]
- Kim, J.S.; Kwon, Y.S.; Sa, Y.J.; Kim, M.J. Isolation and identification of sea buckthorn (Hippophae rhamnoides) phenolics with antioxidant activity and α-glucosidase inhibitory effect. J. Agric. Food Chem. 2011, 59, 138–144. [Google Scholar] [CrossRef]
- DuPont, M.S.; Mondin, Z.; Williamson, G.; Price, K.R. Effect of variety, processing, and storage on the flavonoid glycoside content and composition of lettuce and endive. J. Agric. Food Chem. 2000, 48, 3957–3964. [Google Scholar] [CrossRef]
- Hasle Enerstvedt, K.; Lundberg, A.; Jordheim, M. Characterization of polyphenolic content in the aquatic plants Ruppia cirrhosa and Ruppia maritima—A source of nutritional natural products. Molecules 2017, 23, 16. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Powolny, A.A.; Singh, S.V. Benzyl isothiocyanate targets mitochondrial respiratory chain to trigger reactive oxygen species-dependent apoptosis in human breast cancer cells. J. Biol. Chem. 2008, 283, 30151–30163. [Google Scholar] [CrossRef]
- Van Meerloo, J.; Kaspers, G.J.L.; Cloos, J. Cell sensitivity assays: The MTT assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar]
- Fontana, R.; Guidone, D.; Sangermano, F.; Calabrò, V.; Pollice, A.; La Mantia, G.; Vivo, M. PKC dependent p14ARF phosphorylation on threonine 8 drives cell proliferation. Sci. Rep. 2018, 8, 7056. [Google Scholar] [CrossRef]
- ISO 6888-1:2021; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus aureus and Other Species)—Part 1: Method Using Baird-Parker Agar Medium. International Organization for Standardization: Geneva, Switzerland, 2021. Available online: https://www.iso.org/standard/76672.html (accessed on 12 February 2024).
- ISO 13720:2010; Meat and Meat Products Enumeration of Presumptive Pseudomonas spp. International Organization for Standardization: Geneva, Switzerland, 2010. Available online: https://www.iso.org/standard/45099.html (accessed on 12 February 2024).
- ISO 21527-1:2008; Microbiology of Food and Animal Feeding Stuffs. ISO 21527-1:2008; Microbiology of Food and Animal Feeding Stuffs. Horizontal Method for the Enumeration of Yeasts and Moulds. International Organization for Standardization: Geneva, Switzerland, 2008. Available online: https://www.iso.org/standard/38275.html (accessed on 12 February 2024).
- UNI EN 1276:2020; Chemical Disinfectants and Antiseptics-Quantitative Suspension Test for the Evaluation of Bactericidal Activity of Chemical Disinfectants and Antiseptics Used in Food, Industrial, Domestic and Institutional Areas-Test Method and Requirements (Phase 2, Step 1). European Standards: Bruxelles, Belgium, 2020. Available online: https://www.en-standard.eu/ (accessed on 12 February 2024).
- Alrashidi, A.; Jafar, M.; Higgins, N.; Mulligan, C.; Varricchio, C.; Moseley, R.; Celiksoy, V.; Houston, D.M.J.; Heard, C.M. A time-kill assay study on the synergistic bactericidal activity of pomegranate rind extract and Zn (II) against methicillin-resistant Staphylococcus aureus (MRSA), Staphylococcus epidermidis, Escherichia coli, and Pseudomonas aeruginosa. Biomolecules 2021, 11, 1889. [Google Scholar] [CrossRef]

| Sample | Die-Off Concentration (Mean ± SE) [CFU/mL] | Log CFU Reduction | Die-Off Rate [%] | p-Value |
|---|---|---|---|---|
| Negative control [50 µL DMSO] | 1,750,000 ± 50 | 2 × 105 | 7.89 | - |
| Positive control [100 µg/mL chloramphenicol] | 5500 ± 15 | 2 × 106 | 99.71 | ≤0.0001 |
| Fraction C [150 µg/mL] | 57,525 ± 4 | 2 × 106 | 96.97 | ≤0.0001 ** |
| Fraction C [100 µg/mL] | 172,267 ± 15 | 2 × 106 | 90.93 | ≤0.0001 ** |
| Isoquercetin (1) [100 µM] | 1,708,000 ± 125 | 2 × 105 | 10.11 | >0.05 * |
| Quercetin malonyl glucoside (5) [100 µM] | 1,742,333 ± 89 | 2 × 105 | 8.30 | >0.05 * |
| Sample | Die-Off Concentration (Mean ± SE) [CFU/mL] | Log CFU Reduction | Die-Off Rate [%] | p-Value |
|---|---|---|---|---|
| Negative control [50 µL DMSO] | 1,636,667 ± 79 | 4 × 104 | 2.58 | - |
| Positive control [100 µg/mL ehloramphenicol] | 5083 ± 13 | 2 × 106 | 99.70 | ≤0.0001 |
| Fraction C [150 µg/mL] | 1,013,283 ± 101 | 7 × 105 | 39.69 | ≤0.0001 ** |
| Fraction C [100 µg/mL] | 1,586,000 ± 225 | 9 × 104 | 5.60 | >0.05 * |
| Isoquercetin (1) [100 µM] | 239,000 ± 107 | 1 × 106 | 85.77 | ≤0.0001 ** |
| Quercetin malonyl glucoside (5) [100 µM] | 455,700 ± 68 | 1 × 106 | 72.88 | ≤0.0001 ** |
| Sample | Die-Off Concentration (Mean ± SE) [CFU/mL] | Log CFU Reduction | Die-Off Rate [%] | p-Value |
|---|---|---|---|---|
| Negative control [50 µL DMSO] | 1,400,000 ± 71 | 8 × 104 | 5.41 | - |
| Positive control [100 µg/mL econazole] | 46,913 ± 9 | 1 × 106 | 96.83 | ≤0.0001 |
| Fraction C [150 µg/mL] | 700,333 ± 72 | 8 × 105 | 52.68 | ≤0.0001 ** |
| Fraction C [100 µg/mL] | 1,168,867 ± 220 | 3 × 105 | 21.02 | <0.05 * |
| Isoquercetin (1) [100 µM] | 1,391,500 ± 122 | 9 × 104 | 5.98 | >0.05 * |
| Quercetin malonyl glucoside (5) [100 µM] | 1,359,667 ± 73 | 1 × 105 | 8.13 | >0.05 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paolillo, I.; Roscigno, G.; Innangi, M.; Zorrilla, J.G.; Petraglia, G.; Russo, M.T.; Carraturo, F.; Guida, M.; Pollice, A.; Cimmino, A.; et al. Health-Promoting Properties of Natural Flavonol Glycosides Isolated from Staphylea pinnata L. Int. J. Mol. Sci. 2024, 25, 5582. https://doi.org/10.3390/ijms25115582
Paolillo I, Roscigno G, Innangi M, Zorrilla JG, Petraglia G, Russo MT, Carraturo F, Guida M, Pollice A, Cimmino A, et al. Health-Promoting Properties of Natural Flavonol Glycosides Isolated from Staphylea pinnata L. International Journal of Molecular Sciences. 2024; 25(11):5582. https://doi.org/10.3390/ijms25115582
Chicago/Turabian StylePaolillo, Ida, Giuseppina Roscigno, Michele Innangi, Jesús G. Zorrilla, Gianmarco Petraglia, Maria Teresa Russo, Federica Carraturo, Marco Guida, Alessandra Pollice, Alessio Cimmino, and et al. 2024. "Health-Promoting Properties of Natural Flavonol Glycosides Isolated from Staphylea pinnata L." International Journal of Molecular Sciences 25, no. 11: 5582. https://doi.org/10.3390/ijms25115582
APA StylePaolillo, I., Roscigno, G., Innangi, M., Zorrilla, J. G., Petraglia, G., Russo, M. T., Carraturo, F., Guida, M., Pollice, A., Cimmino, A., Masi, M., & Calabrò, V. (2024). Health-Promoting Properties of Natural Flavonol Glycosides Isolated from Staphylea pinnata L. International Journal of Molecular Sciences, 25(11), 5582. https://doi.org/10.3390/ijms25115582












