Identification and Expression Profile of NCED Genes in Arachis hypogaea L. during Drought Stress
Abstract
1. Introduction
2. Results
2.1. Identification and Characterisation of AhNCEDs
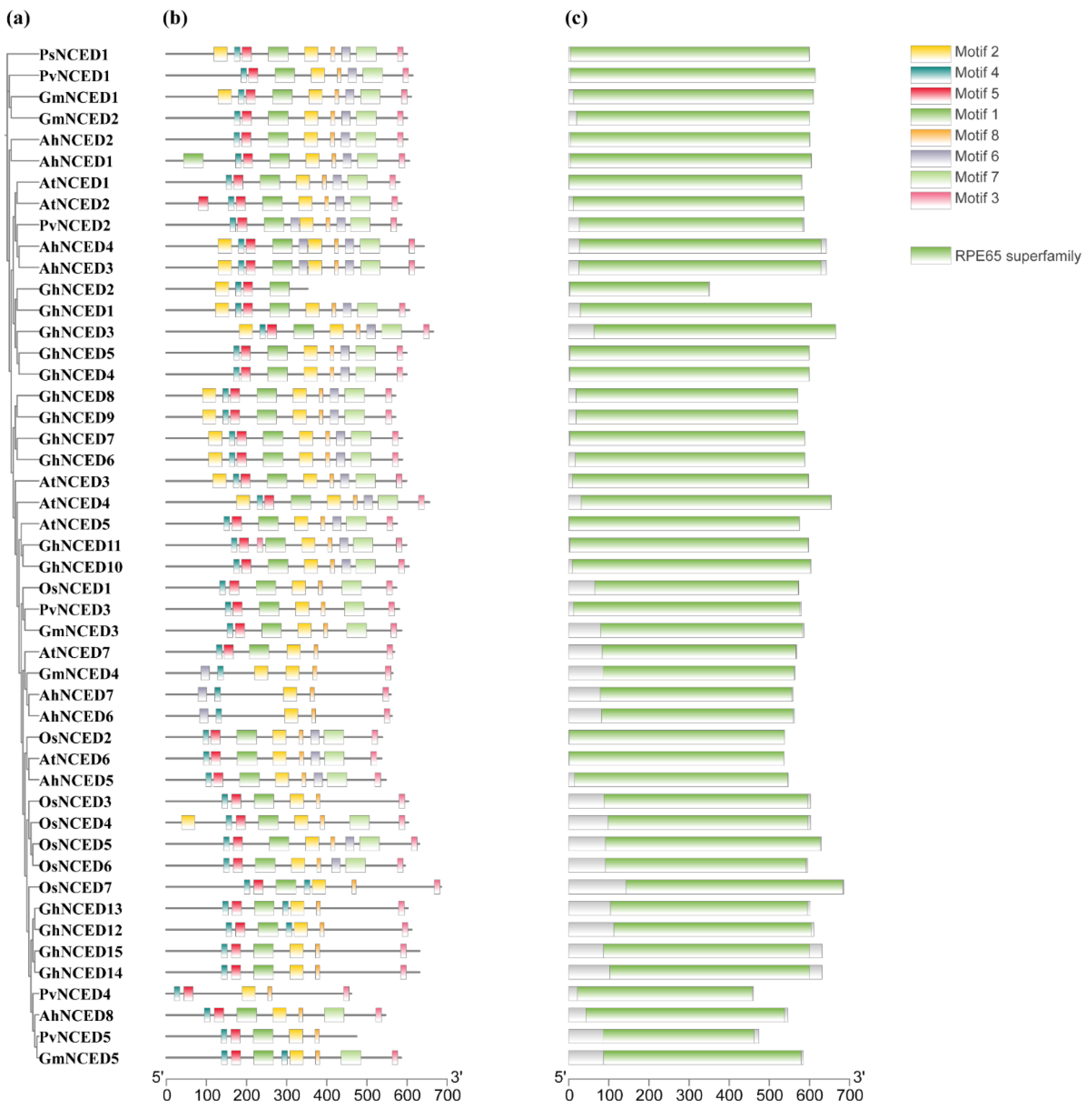
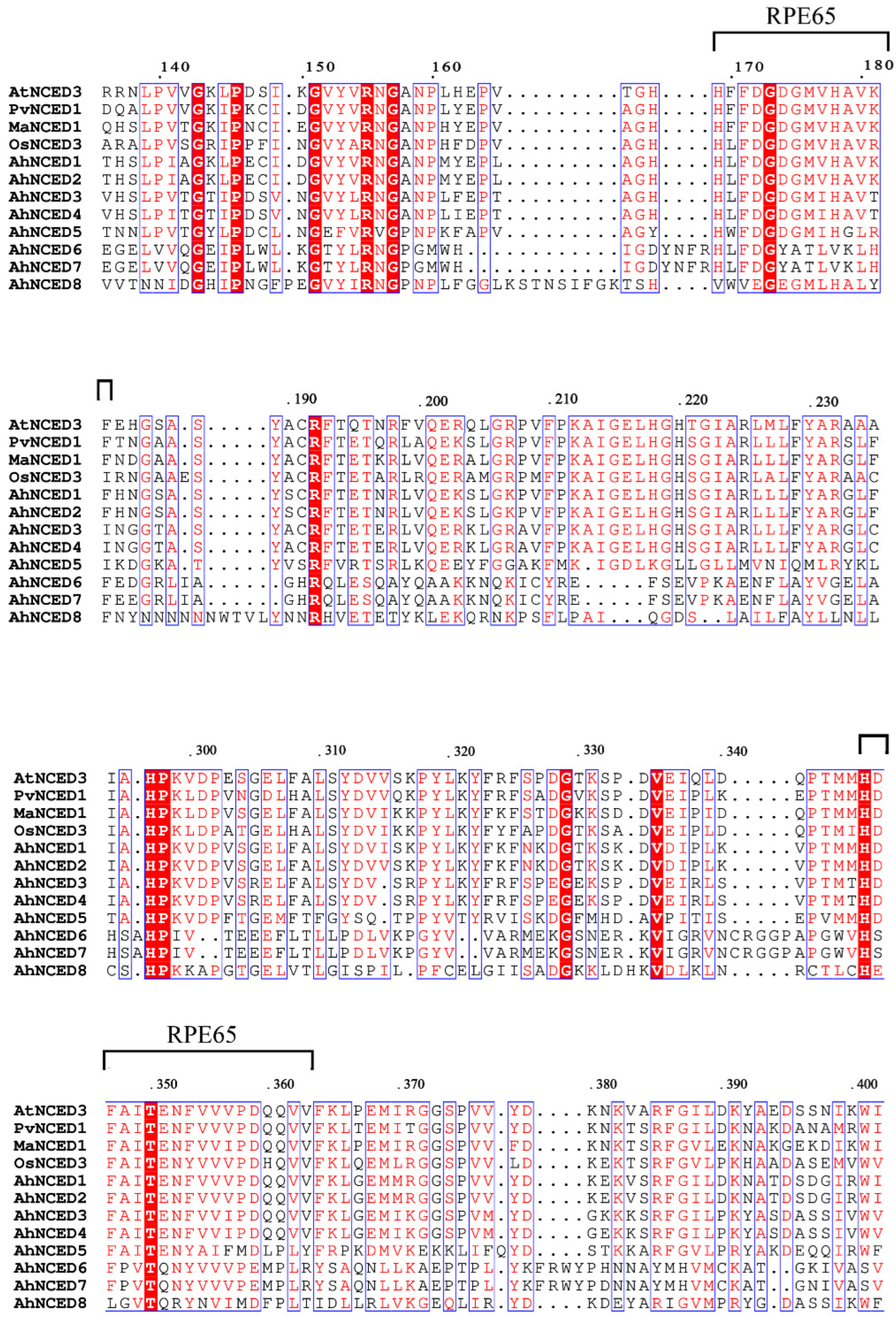
2.2. Analysis of Conserved Motifs and Domains of AhNCEDs
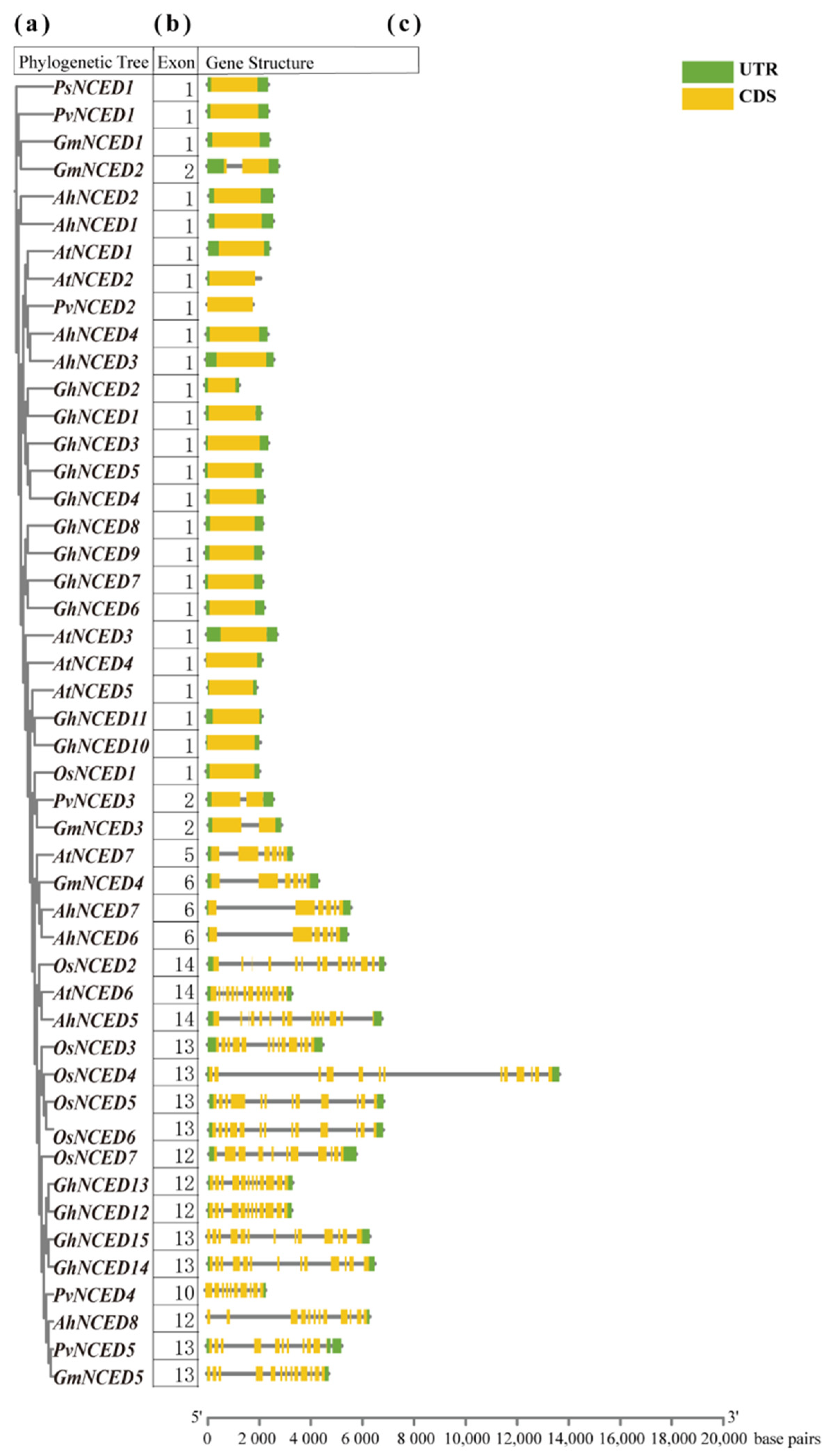
2.3. Gene Structure Prediction of AhNCEDs
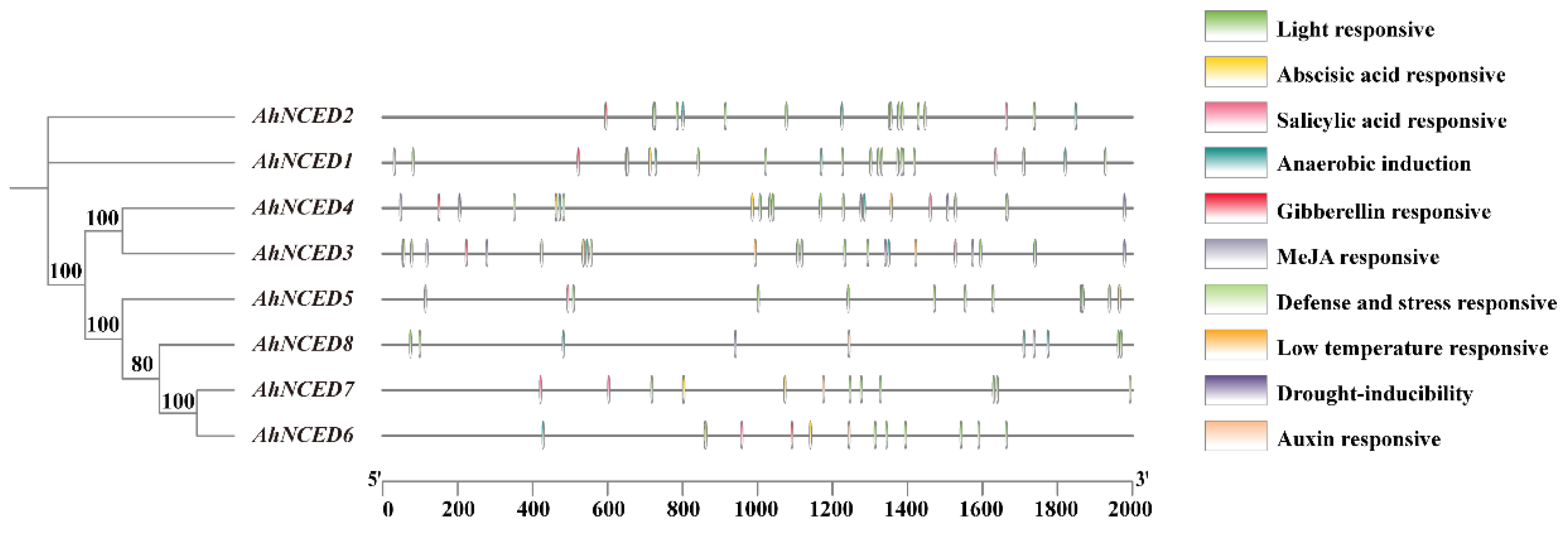
2.4. Stress-Related Cis-Elements in the Promoters of AhNCEDs
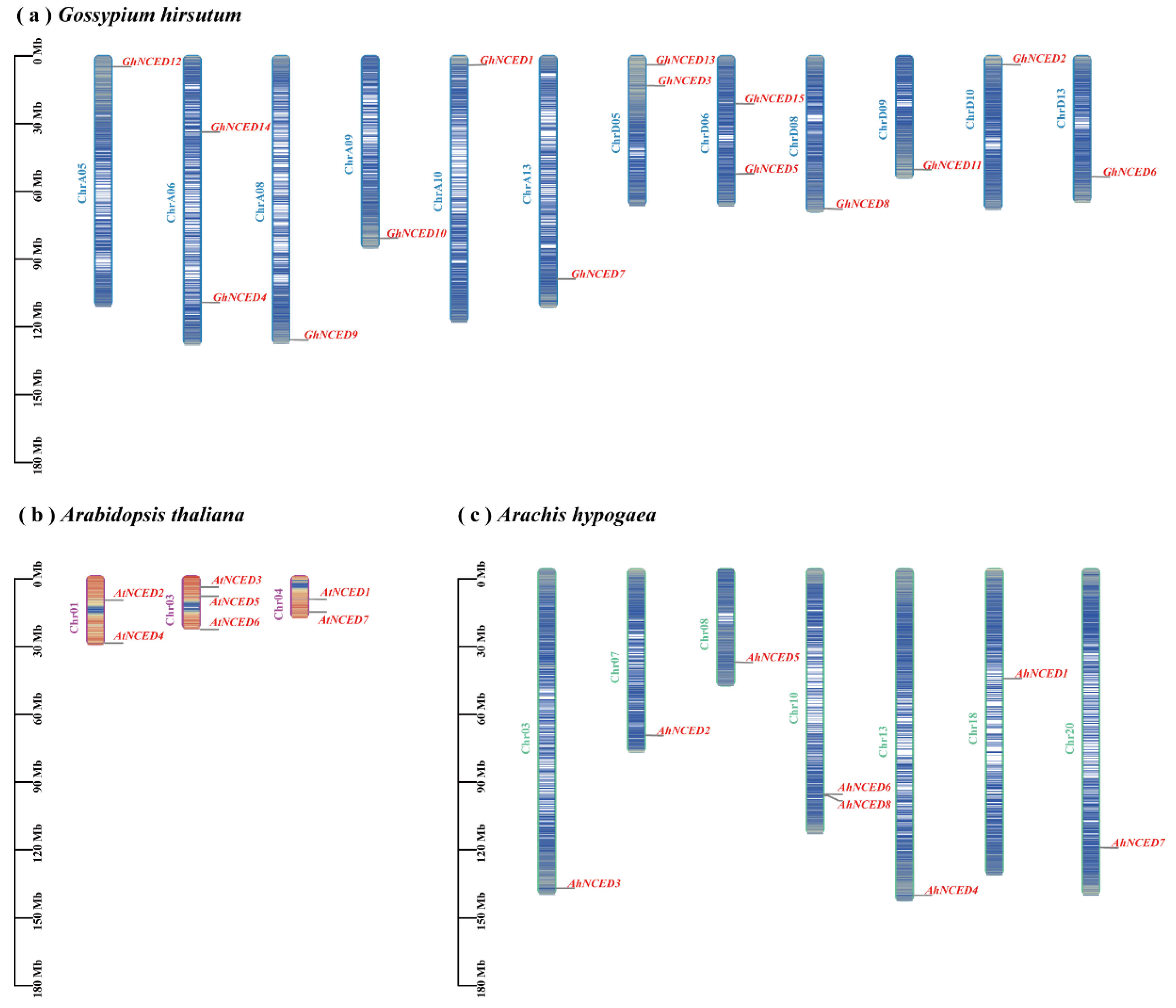
2.5. Chromosomal Localisation of AhNCEDs
2.6. Collinearity Analysis of AhNCEDs
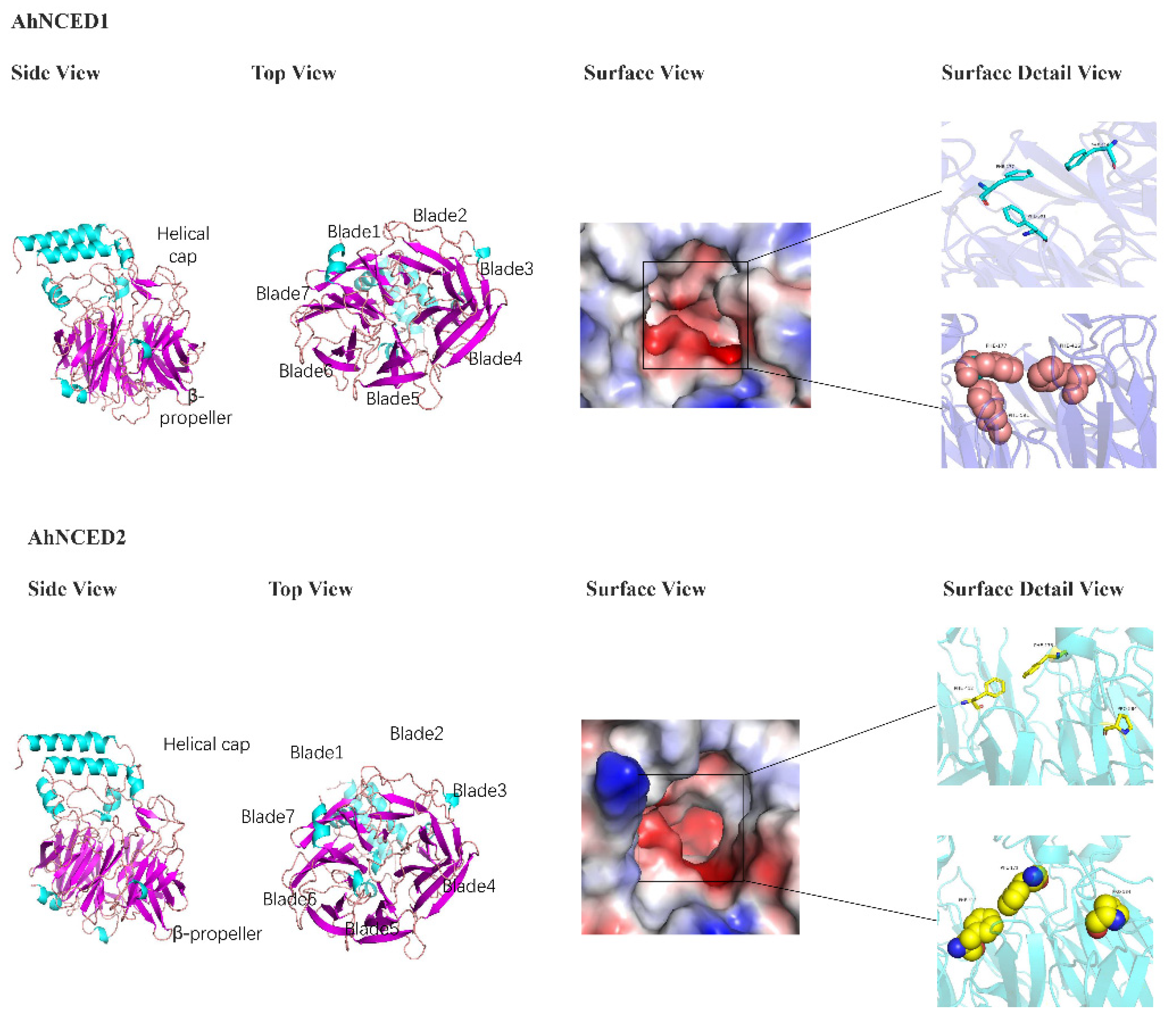
2.7. Prediction of AhNCED Proteins Structures
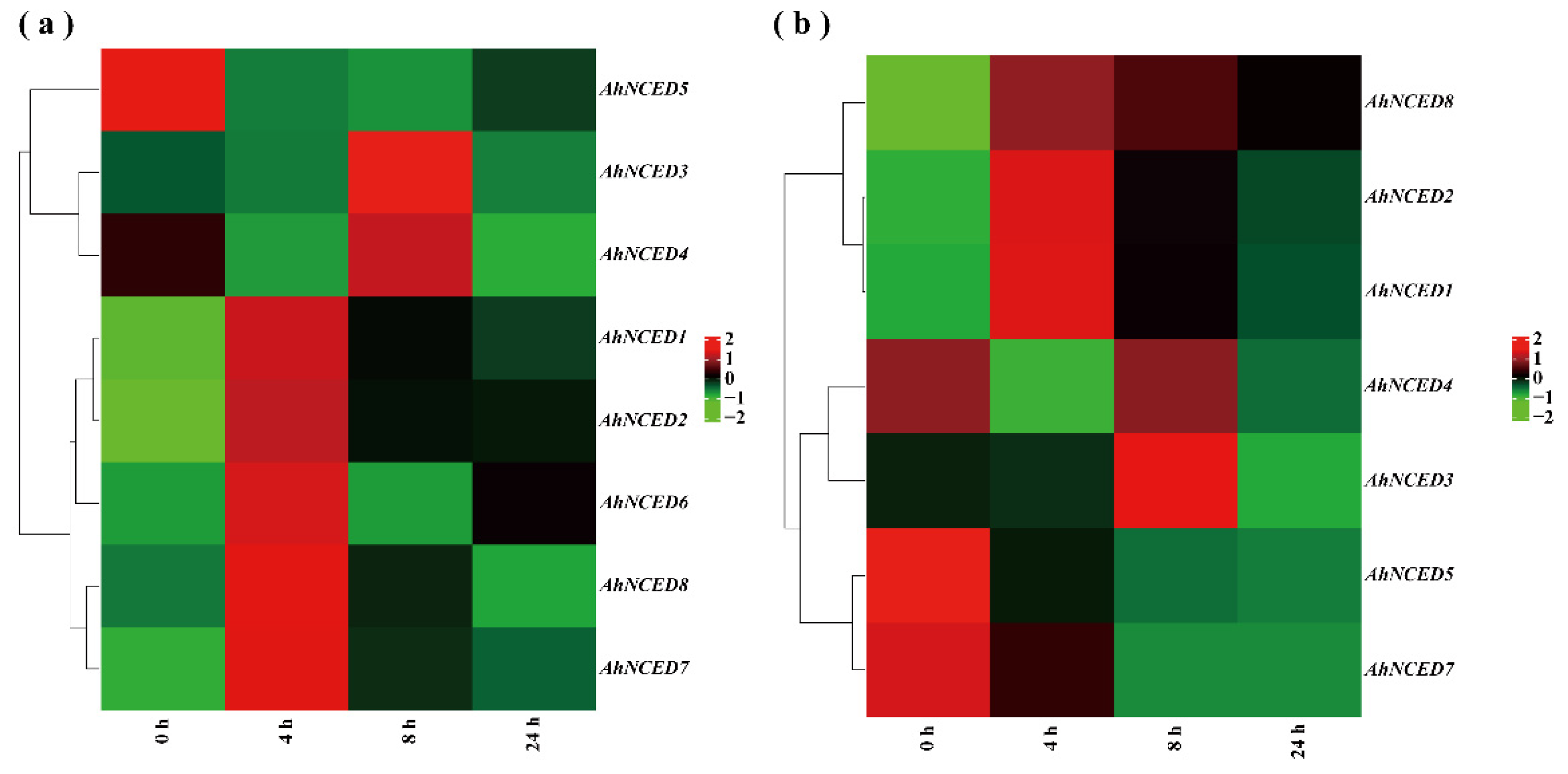
2.8. Expression Profile of AhNCEDs under Drought Stress
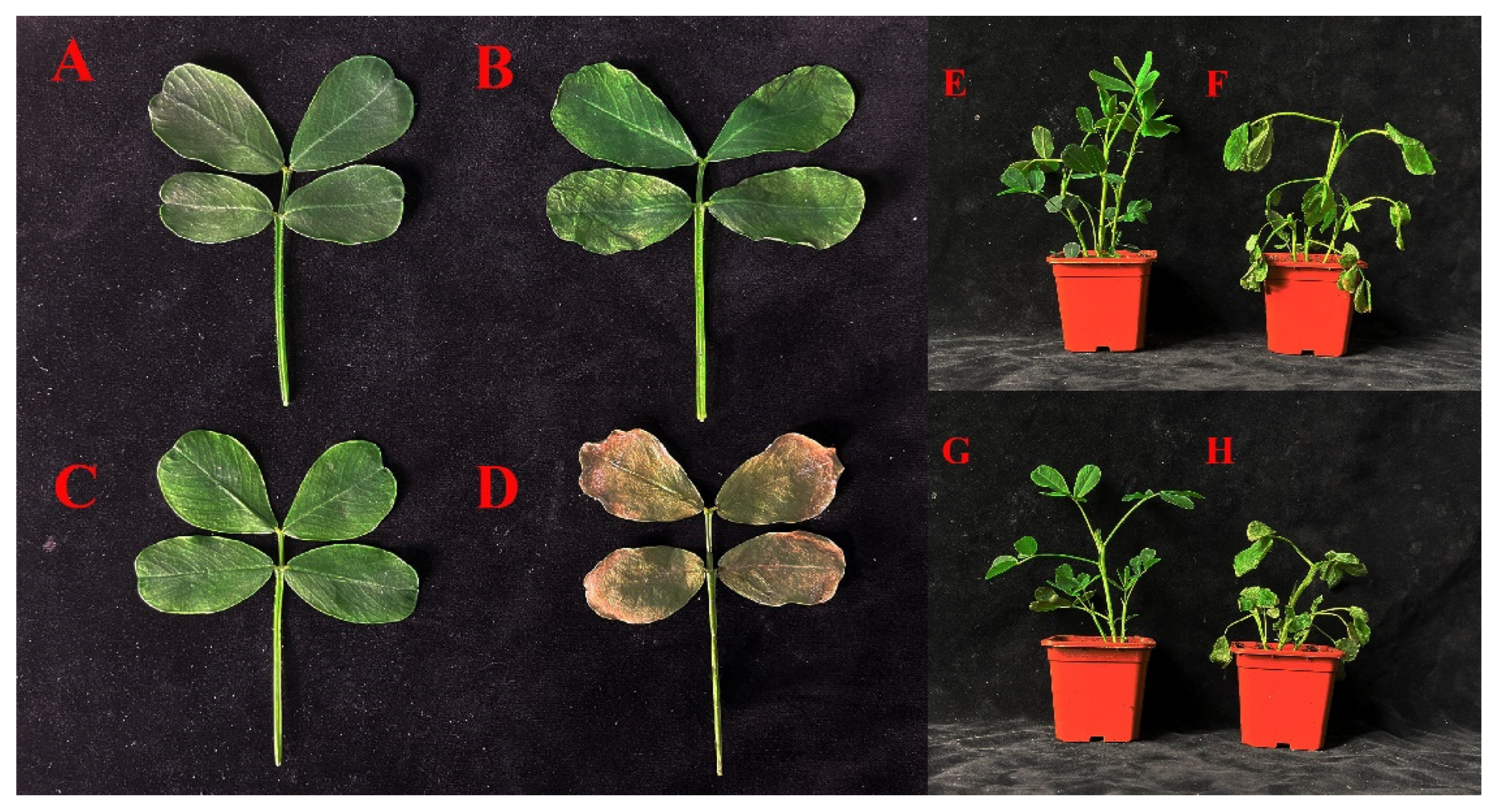
2.9. Phenotyping of Two Arachis Hypogaea Varieties under Drought Stress
2.10. Expression Analysis of AhNCEDs in Arachis Hypogaea Varieties under Drought Stress
3. Discussion
4. Materials and Methods
4.1. Plant Material and Treatments
4.2. Sequence Retrieval
4.3. Phylogenetic Analysis of NCED Protein Sequences
4.4. Protein Motif and Domain Analysis and Multiple Sequence Alignments of AhNCEDs
4.5. Evolutionary Analysis of NCED Genes
4.6. Cis-Elements Analysis of AhNCEDs Promoters
4.7. Chromosomal Localisation of AhNCEDs
4.8. Synteny Analysis of AhNCEDs
4.9. Modelling of AhNCEDs Proteins
4.10. Heatmap of AhNCEDs Gene Expression Patterns
4.11. RNA Extraction and Gene Expression Analysis
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Golfazani, M.M.; Taghvaei, M.M.; Lahiji, H.S.; Ashery, S.; Raza, A. Investigation of protein’ interaction network and the expression pattern of genes involved in the ABA biogenesis and antioxidant system under methanol spray in drought-stressed rapeseed. 3 Biotech 2022, 12, 217. [Google Scholar]
- Hou, P.; Wang, F.; Luo, B.; Li, A.; Wang, C.; Shabala, L.; Ahmed, H.A.I.; Deng, S.; Zhang, H.; Song, P.; et al. Antioxidant enzymatic activity and osmotic adjustment as components of the drought tolerance mechanism in Carex duriuscula. Plants 2021, 10, 436. [Google Scholar] [CrossRef] [PubMed]
- Trovato, M.; Brini, F.; Mseddi, K.; Rhizopoulou, S.; Jones, M.A. A holistic and sustainable approach linked to drought tolerance of Mediterranean crops. Front. Plant Sci. 2023, 14, 1167376. [Google Scholar] [CrossRef] [PubMed]
- García-Gómez, B.E.; Salazar, J.A.; Nicolás-Almansa, M.; Razi, M.; Rubio, M.; Ruiz, D.; Martínez-Gómez, P. Molecular bases of fruit quality in Prunus species: An Integrated Genomic, Transcriptomic, and Metabolic Review with a Breeding Perspective. Int. J. Mol. Sci. 2020, 22, 333. [Google Scholar] [CrossRef] [PubMed]
- García-Pastor, M.E.; Giménez, M.J.; Serna-Escolano, V.; Guillén, F.; Valero, D.; Serrano, M.; García-Martínez, S.; Terry, L.A.; Alamar, M.C.; Zapata, P.J. Oxalic acid preharvest treatment improves colour and quality of seedless table grape ‘magenta’ upregulating on-vine abscisic acid metabolism, relative VvNCED1 gene expression, and the antioxidant system in berries. Front. Plant Sci. 2021, 12, 740240. [Google Scholar] [CrossRef] [PubMed]
- Ke, D.; Guo, J.; Li, K.; Wang, Y.; Han, X.; Fu, W.; Miao, Y.; Jia, K.P. Carotenoid-derived bioactive metabolites shape plant root architecture to adapt to the rhizospheric environments. Front. Plant Sci. 2022, 13, 986414. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Cao, J.; Ge, K.; Li, L. The site of water stress governs the pattern of ABA synthesis and transport in peanut. Sci. Rep. 2016, 6, 32143. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signalling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic acid-induced stomatal closure: An important component of plant defense against abiotic and biotic stress. Front. Plant Sci. 2021, 12, 615114. [Google Scholar] [CrossRef]
- Tang, X.; Fei, X.; Sun, Y.; Shao, H.; Zhu, J.; He, X.; Wang, X.; Yong, B.; Tao, X. Abscisic acid-polyacrylamide (ABA-PAM) treatment enhances forage grass growth and soil microbial diversity under drought stress. Front. Plant Sci. 2022, 13, 973665. [Google Scholar] [CrossRef]
- Wu, C.; Lin, M.; Chen, F.; Chen, J.; Liu, S.; Yan, H.; Xiang, Y. Homologous Drought-Induced 19 Proteins, PtDi19-2 and PtDi19-7, enhance drought tolerance in transgenic plants. Int. J. Mol. Sci. 2022, 23, 3371. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, X.; Wei, J.; Zuo, Y.; Wang, L. The regulatory networks of the circadian clock involved in plant adaptation and crop yield. Plants 2023, 12, 1897. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yu, X.; Chen, L.; Zhao, G.; Li, S.; Zhou, H.; Dai, Y.; Sun, N.; Xie, Y.; Gao, J.; et al. Genome-wide identification and expression analysis of the NCED family in cotton (Gossypium hirsutum L.). PLoS ONE 2021, 16, e0246021. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Jiao, Y.; Xie, N.; Guo, Y.; Zhang, F.; Xiang, Z.; Wang, R.; Wang, F.; Gao, Q.; Tian, L.; et al. OsNCED5, a 9-cis-epoxycarotenoid dioxygenase gene, regulates salt and water stress tolerance and leaf senescence in rice. Plant Sci. 2019, 287, 110188. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.U.; Mun, B.G.; Bae, E.K.; Kim, J.Y.; Kim, H.H.; Shahid, M.; Choi, Y.I.; Hussain, A.; Yun, B.W. Drought stress-mediated transcriptome profile reveals nced as a key player modulating drought tolerance in Populus davidiana. Front. Plant Sci. 2021, 12, 755539. [Google Scholar] [CrossRef]
- Avico, E.H.; Acevedo, R.M.; Duarte, M.J.; Rodrigues Salvador, A.; Nunes-Nesi, A.; Ruiz, O.A.; Sansberro, P.A. Integrating Transcriptional, Metabolic, and Physiological Responses to Drought Stress in Ilex paraguariensis Roots. Plants 2023, 12, 2404. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yoo, E.; Lee, S.; Sung, J.; Noh, H.J.; Hwang, S.J.; Desta, K.T.; Lee, G.A. Seed weight and genotype influence the total oil content and fatty acid composition of peanut seeds. Foods 2022, 11, 3463. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Huai, D.; Zhang, Z.; Cheng, K.; Kang, Y.; Wan, L.; Yan, L.; Jiang, H.; Lei, Y.; Liao, B. Development of a high-density genetic map based on specific length amplified fragment sequencing and its application in quantitative trait loci analysis for yield-related traits in cultivated peanut. Front. Plant Sci. 2018, 9, 827. [Google Scholar] [CrossRef]
- Qin, M.; Li, X.; Tang, S.; Huang, Y.; Li, L.; Hu, B. Expression of AhATL1, an ABA Transport Factor Gene from Peanut, Is Affected by Altered Memory Gene Expression Patterns and Increased Tolerance to Drought Stress in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 3398. [Google Scholar] [CrossRef]
- Hu, B.; Hong, L.; Liu, X.; Xiao, S.N.; Lv, Y.; Li, L. Identification of different ABA biosynthesis sites at seedling and fruiting stages in Arachis hypogaea L. following water stress. Plant Growth Regul. 2013, 70, 131–140. [Google Scholar] [CrossRef]
- Pruthvi, V.; Narasimhan, R.; Nataraja, K.N. Simultaneous expression of abiotic stress responsive transcription factors, AtDREB2A, AtHB7 and AtABF3 improves salinity and drought tolerance in peanut (Arachis hypogaea L.). PLoS ONE 2014, 9, e111152. [Google Scholar] [CrossRef] [PubMed]
- Razi, K.; Muneer, S. Drought stress-induced physiological mechanisms, signaling pathways and molecular response of chloroplasts in common vegetable crops. Crit. Rev. Biotechnol. 2021, 41, 669–691. [Google Scholar] [CrossRef] [PubMed]
- Dorner, J.W.; Cole, R.J.; Sanders, T.H.; Blankenship, P.D. Interrelationship of kernel water activity, soil temperature, maturity, and phytoalexin production in preharvest aflatoxin contamination of drought-stressed peanuts. Mycopathologia 1989, 105, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Ratnakumar, P.; Vadez, V. Groundnut (Arachis hypogaea) genotypes tolerant to intermittent drought maintain a high harvest index and have small leaf canopy under stress. Funct. Plant Biol. 2011, 38, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Zhang, Z.; Kang, T.; Dai, L.; Ci, D.; Qin, F.; Song, W. Rooting traits of peanut genotypes differing in drought tolerance under drought stress. Int. J. Plant Prod. 2017, 11, 349–360. [Google Scholar]
- Qin, X.; Zeevaart, J.A. The 9-cis-epoxycarotenoid cleavage reaction is the key regulatory step of abscisic acid biosynthesis in water-stressed bean. Proc. Natl. Acad. Sci. USA 1999, 96, 15354–15361. [Google Scholar] [CrossRef] [PubMed]
- Iuchi, S.; Kobayashi, M.; Taji, T.; Naramoto, M.; Seki, M.; Kato, T.; Tabata, S.; Kakubari, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J. 2001, 27, 325–333. [Google Scholar] [CrossRef]
- Hwang, S.; Chen, H.; Huang, W.; Chu, Y.; Shii, C.; Cheng, W. Ectopic expression of rice OsNCED3 in Arabidopsis increases ABA level and alters leaf morphology. Plant Sci. 2010, 178, 12–22. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, X.; Quan, S.; Zhang, M.; Kang, C.; Guo, C.; Zhang, Z.; Niu, J. Genome-wide identification and expression analysis of nced gene family in pear and its response to exogenous gibberellin and paclobutrazol. Int. J. Mol. Sci. 2023, 24, 7566. [Google Scholar] [CrossRef]
- Zhu, P.; Li, R.; Fan, W.; Xia, Z.; Li, J.; Wang, C.; Zhao, A. A mulberry 9-cis-epoxycarotenoid dioxygenase gene MaNCED1 is involved in plant growth regulation and confers salt and drought tolerance in transgenic tobacco. Front. Plant Sci. 2023, 14, 1228902. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-Ⅱ: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Jiang, C.; Li, X.; Zou, J.; Ren, J.; Jin, C.; Zhang, H.; Yu, H.; Jin, H. Comparative transcriptome analysis of genes involved in the drought stress response of two peanut (Arachis hypogaea L.) varieties. BMC Plant Biol, 2021; 21, 64. [Google Scholar]
- Flavell, R.B. Perspective: 50 years of plant chromosome biology. Plant Physiol. 2021, 185, 731–753. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.A.; Woollard, A. The road less travelled? Exploring the nuanced evolutionary consequences of duplicated genes. Essays Biochem. 2022, 66, 737–744. [Google Scholar] [PubMed]
- Bavishi, A.; Lin, L.; Schroeder, K.; Peters, A.; Cho, H.; Choudhary, M. The prevalence of gene duplications and their ancient origin in Rhodobacter sphaeroides 2.4.1. BMC Microbiol. 2010, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yegorov, S.; Good, S. Using paleogenomics to study the evolution of gene families: Origin and duplication history of the relaxin family hormones and their receptors. PLoS ONE 2012, 7, e32923. [Google Scholar] [CrossRef] [PubMed]
- Flagel, L.E.; Wendel, J.F. Gene duplication and evolutionary novelty in plants. New Phytol. 2009, 183, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.S.; Sintaha, M.; Cheung, M.Y.; Lam, H.M. Plant hormone signaling crosstalks between biotic and abiotic stress responses. Int. J. Mol. Sci. 2018, 19, 3206. [Google Scholar] [CrossRef] [PubMed]
- Degenkolbe, T.; Do, P.T.; Kopka, J.; Zuther, E.; Hincha, D.K.; Köhl, K.I. Identification of drought tolerance markers in a diverse population of rice cultivars by expression and metabolite profiling. PLoS ONE 2013, 8, e63637. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Liang, W.; Yan, K.; Xu, D.; Qin, T.; Fiaz, S.; Kear, P.; Bi, Z.; Liu, Y.; Liu, Z.; et al. Expression of potato StDRO1 in Arabidopsis alters root architecture and drought tolerance. Front. Plant Sci. 2022, 13, 836063. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, 293–296. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Messing, S.A.; Gabelli, S.B.; Echeverria, I.; Vogel, J.T.; Guan, J.C.; Tan, B.C.; Klee, H.J.; McCarty, D.R.; Amzel, L.M. Structural insights into maize viviparous14, a key enzyme in the biosynthesis of the phytohormone abscisic acid. Plant Cell 2010, 22, 2970–2980. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]



| Protein | Accession Numbers | Encoding Amino Acid No. | Molecular Weight (kD) | Theoretical (pI) | GRAVY | Instability Index | Aliphatic Index |
|---|---|---|---|---|---|---|---|
| AhNCED1 | AH18G19580.1 | 605 | 6.71 | 8.49 | −0.38 | 42.04 | 75.44 |
| AhNCED2 | AH07G23190.1 | 601 | 6.68 | 8.49 | −0.39 | 42.98 | 75.12 |
| AhNCED3 | AH03G48330.1 | 642 | 7.12 | 6.39 | −0.40 | 39.93 | 76.68 |
| AhNCED4 | AH13G50990.1 | 642 | 7.14 | 6.39 | −0.41 | 41.83 | 77.13 |
| AhNCED5 | AH08G22610.1 | 547 | 6.16 | 5.66 | −0.32 | 31.72 | 82.10 |
| AhNCED6 | AH10G21080.1 | 562 | 6.27 | 6.99 | −0.34 | 42.71 | 79.27 |
| AhNCED7 | AH20G27640.1 | 559 | 6.22 | 7.59 | −0.36 | 43.00 | 77.26 |
| AhNCED8 | AH10G21240.1 | 546 | 6.17 | 5.54 | −0.26 | 35.49 | 82.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, A.; Li, J.; Wang, H.; Zhao, P. Identification and Expression Profile of NCED Genes in Arachis hypogaea L. during Drought Stress. Int. J. Mol. Sci. 2024, 25, 5564. https://doi.org/10.3390/ijms25105564
Chen A, Li J, Wang H, Zhao P. Identification and Expression Profile of NCED Genes in Arachis hypogaea L. during Drought Stress. International Journal of Molecular Sciences. 2024; 25(10):5564. https://doi.org/10.3390/ijms25105564
Chicago/Turabian StyleChen, Ao, Jingyan Li, Heping Wang, and Puyan Zhao. 2024. "Identification and Expression Profile of NCED Genes in Arachis hypogaea L. during Drought Stress" International Journal of Molecular Sciences 25, no. 10: 5564. https://doi.org/10.3390/ijms25105564
APA StyleChen, A., Li, J., Wang, H., & Zhao, P. (2024). Identification and Expression Profile of NCED Genes in Arachis hypogaea L. during Drought Stress. International Journal of Molecular Sciences, 25(10), 5564. https://doi.org/10.3390/ijms25105564





