Annona squamosa Fruit Extract Ameliorates Lead Acetate-Induced Testicular Injury by Modulating JAK-1/STAT-3/SOCS-1 Signaling in Male Rats
Abstract
1. Introduction
2. Results
2.1. Phytochemical Constituents of ASFE
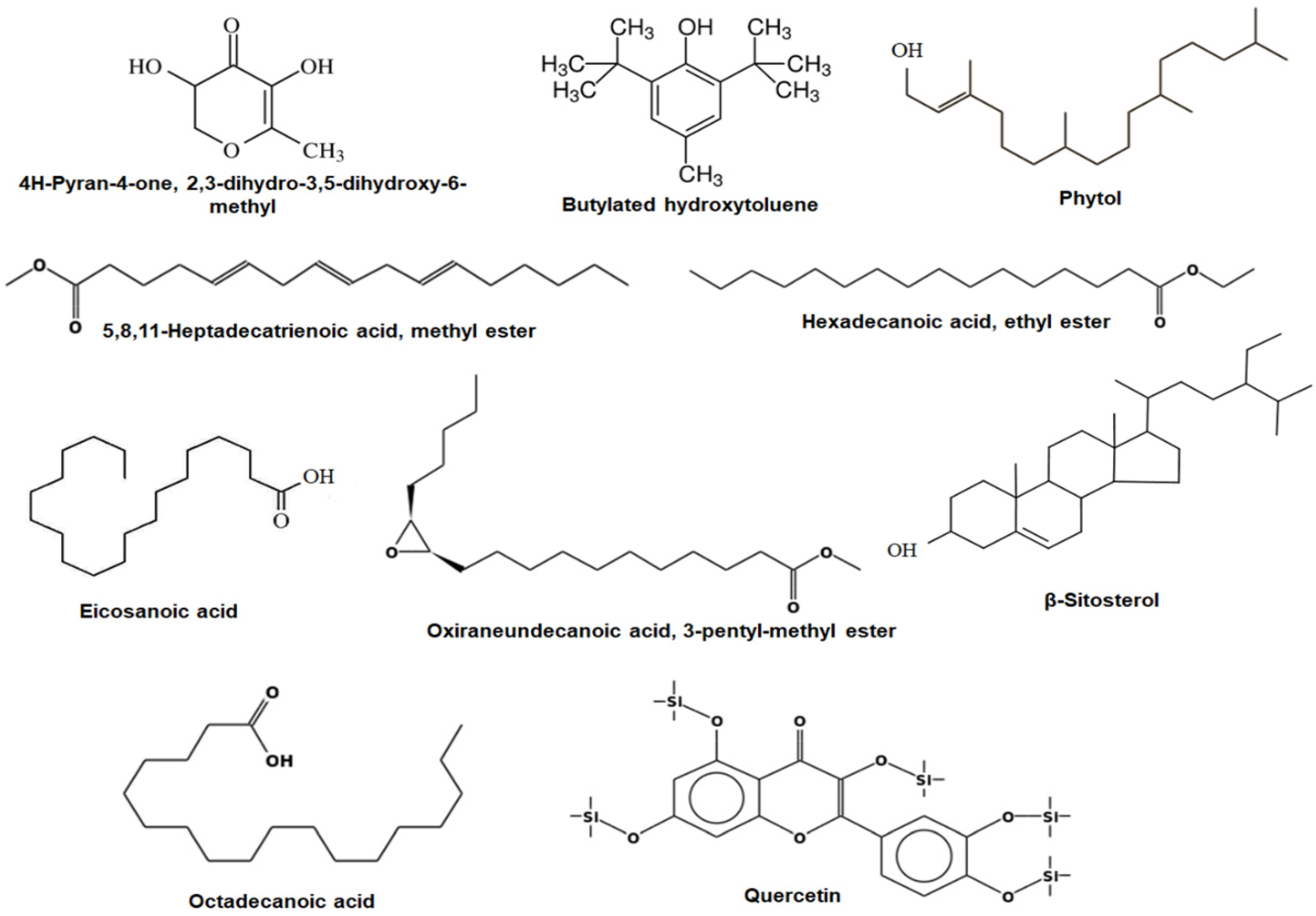
2.2. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis of ASFE
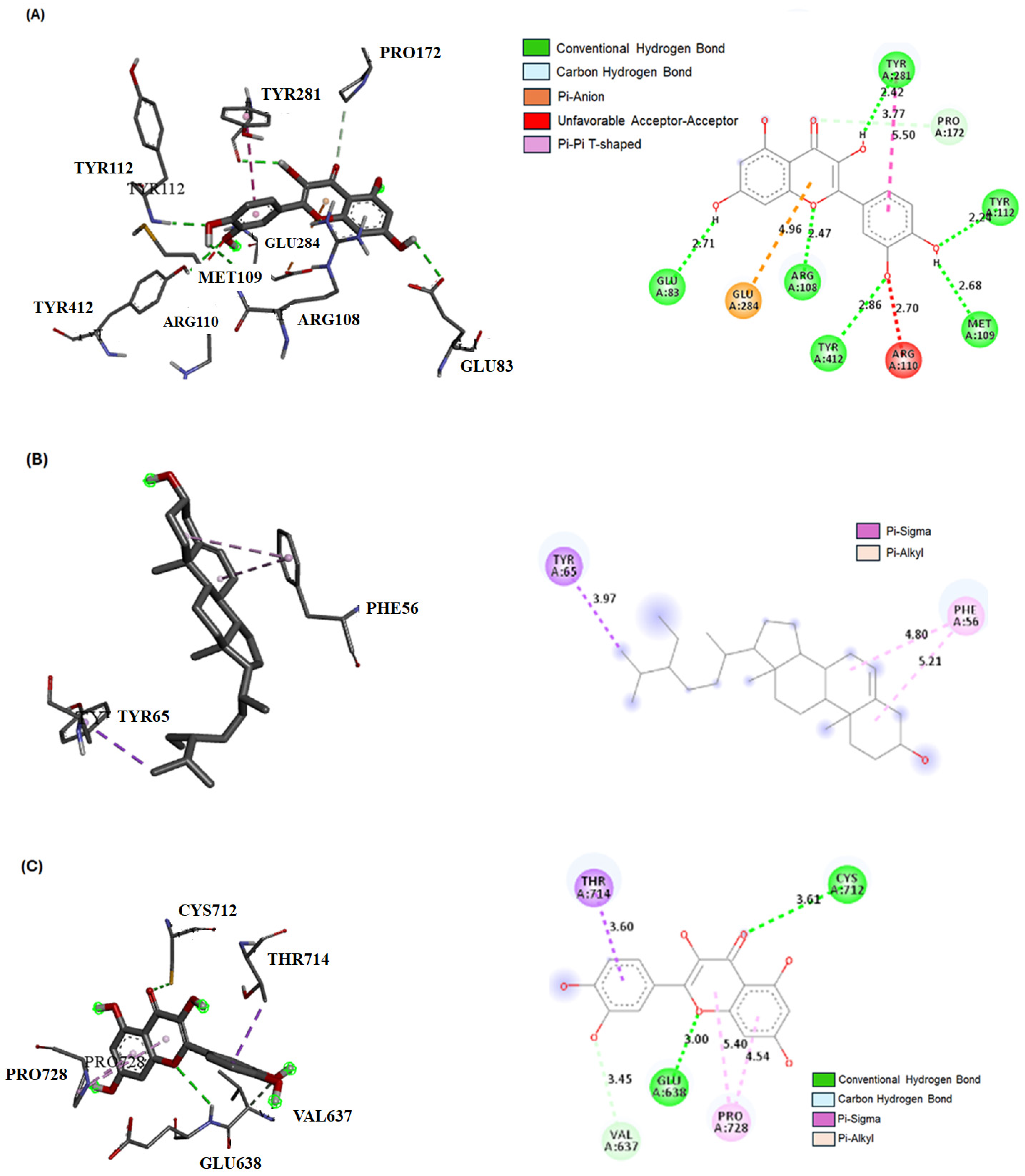
2.3. Bioactive Compound Interactions with JAK-1, STAT-3, and SOCS-1 Proteins by Molecular Docking
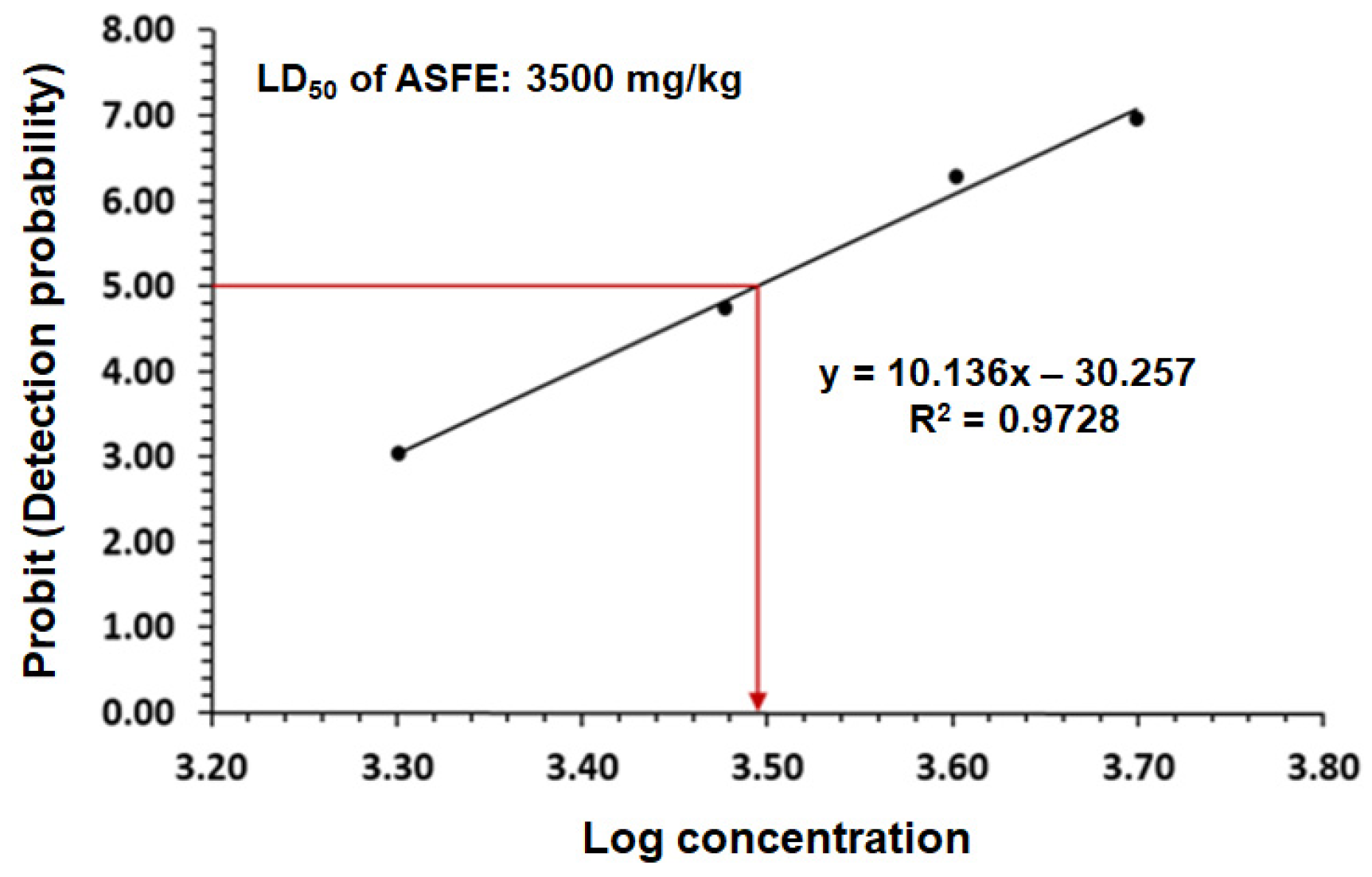
2.4. The LD50 of ASFE in Rats
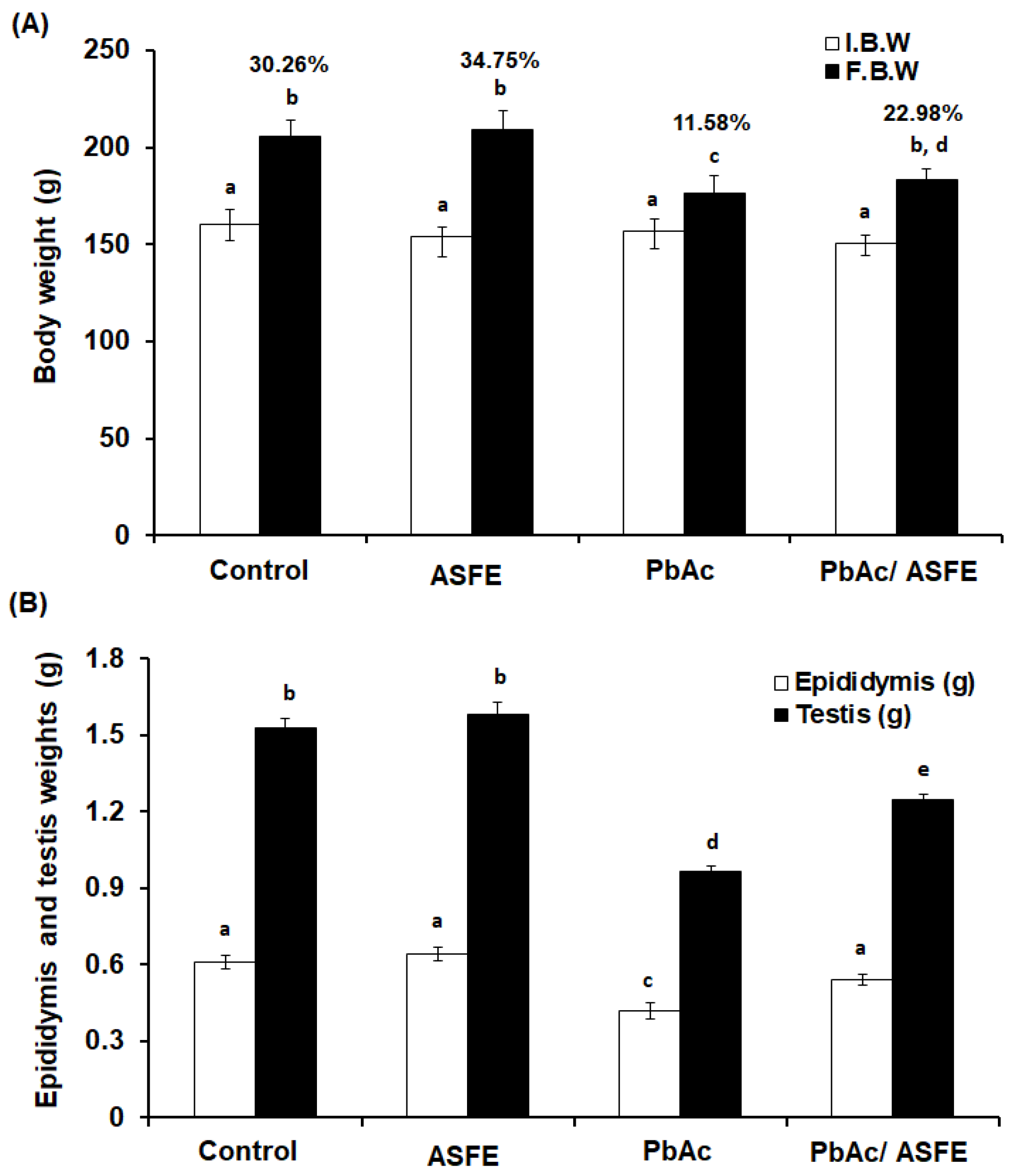
2.5. Effect of ASFE Treatment on Body Weight, Epididymis, and Testis Weights of Rats Given Lead Acetate
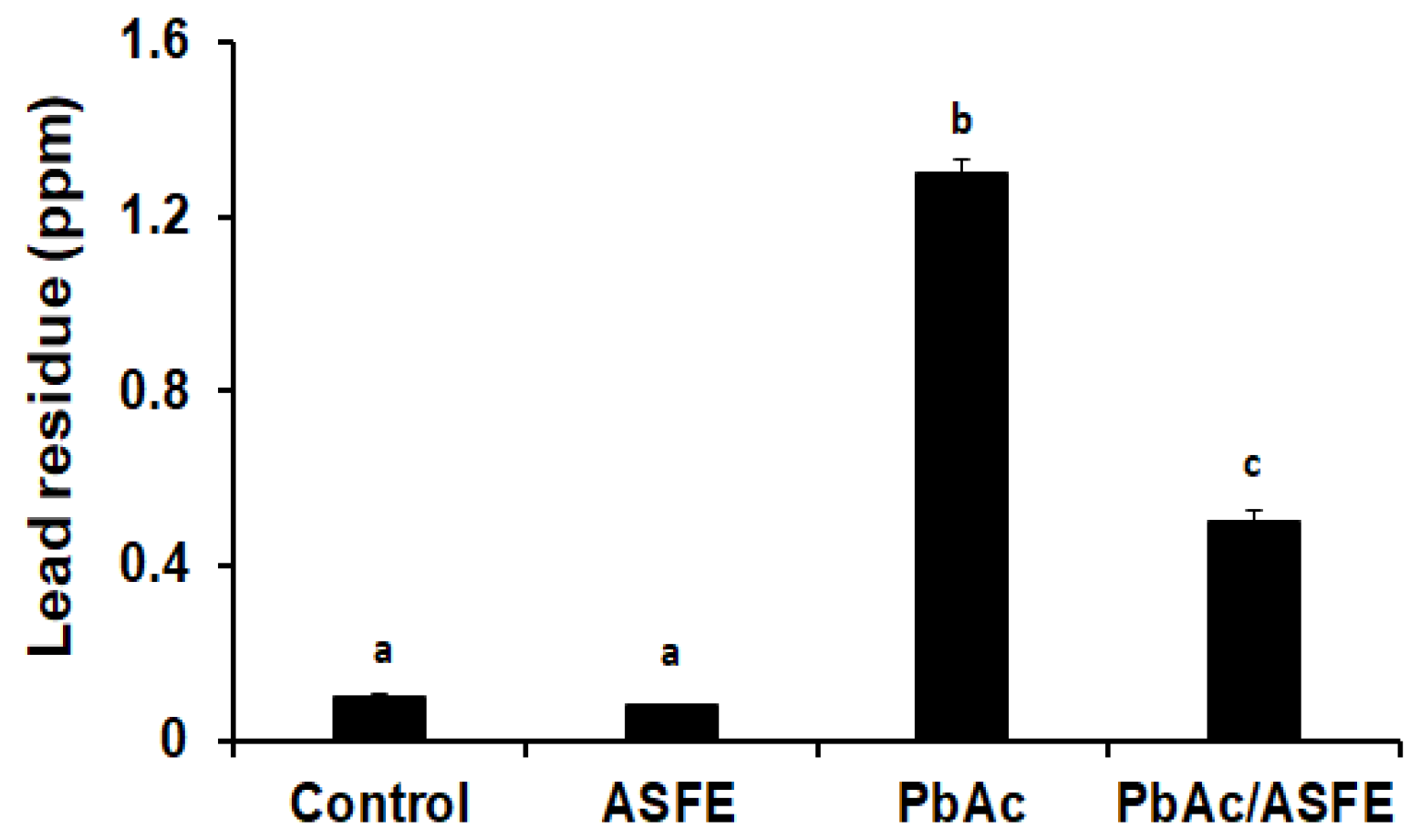
2.6. Effect of ASFE Treatment on Lead Residues in Testis of PbAc-Intoxicated Rats
2.7. Effect of ASFE Treatment on Spermatological Parameters of PbAc-Intoxicated Rats
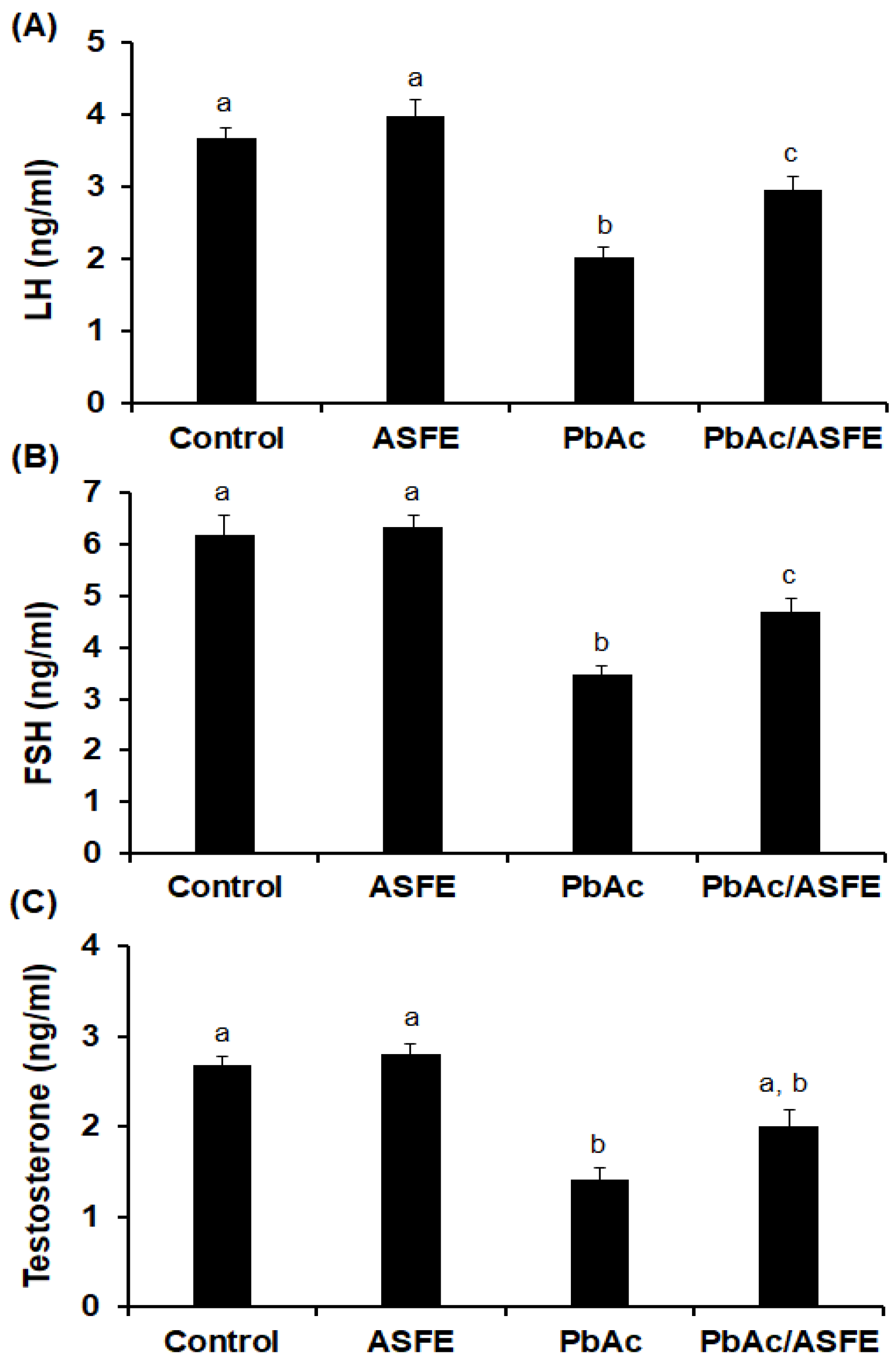
2.8. Effect of ASFE on Levels of Reproductive Hormonal in Male Rats Given Lead Acetate
2.9. Treatment of PbAc-Intoxicated Rats with ASFE Alleviated Oxidative Stress on Testicular Tissues
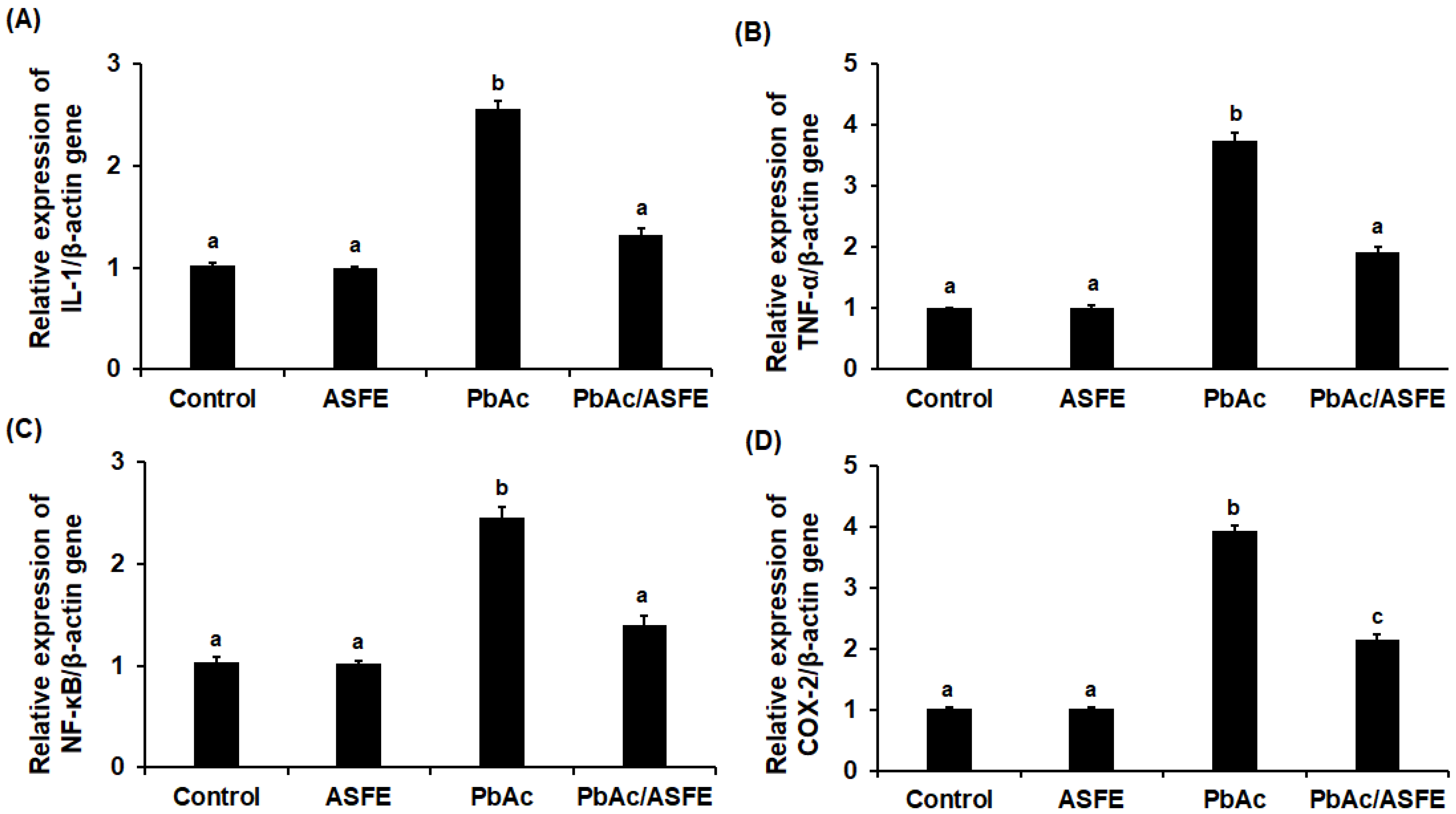
2.10. Effects of ASFE Treatment on Inflammatory Cytokines of PbAc-Intoxicated Rats
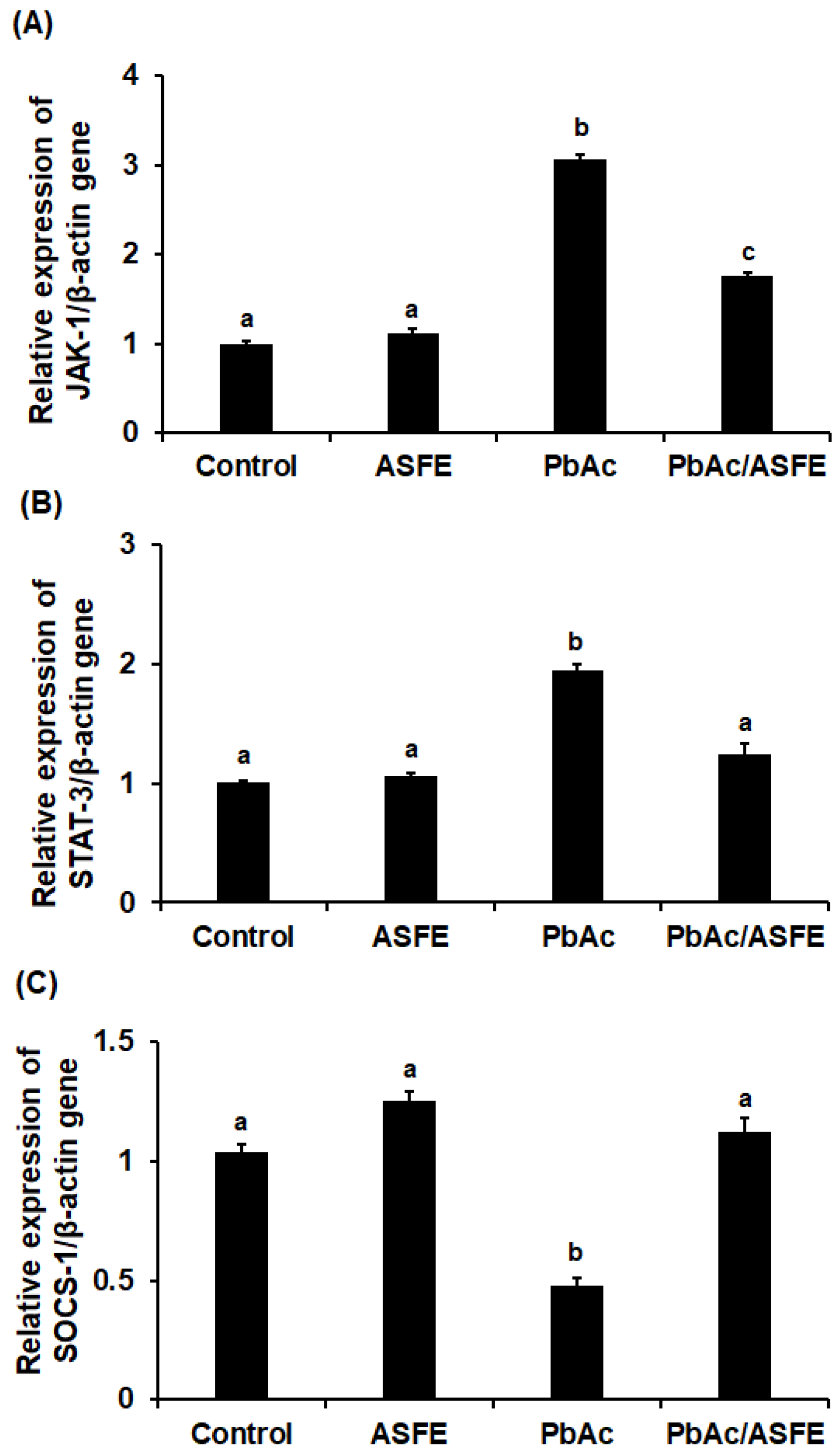
2.11. Treatment with ASFE Mitigated Inflammation by Modulating JAK-1/STAT-3/SOCS-1 Signaling in PbAc-Intoxicated Rats
2.12. Treatment with ASFE Significantly Improved Testicular Histopathological Damage Induced by PbAc in Rats
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Collection and Preparation of Plant Materials
4.3. Phytochemical Analysis of ASFE
4.4. Gas Chromatography and Mass Spectrum (GC-MS) Profiling of ASFE
4.5. Molecular Docking Analysis
4.6. Determination of the Median Lethal Dose (LD50) of ASFE
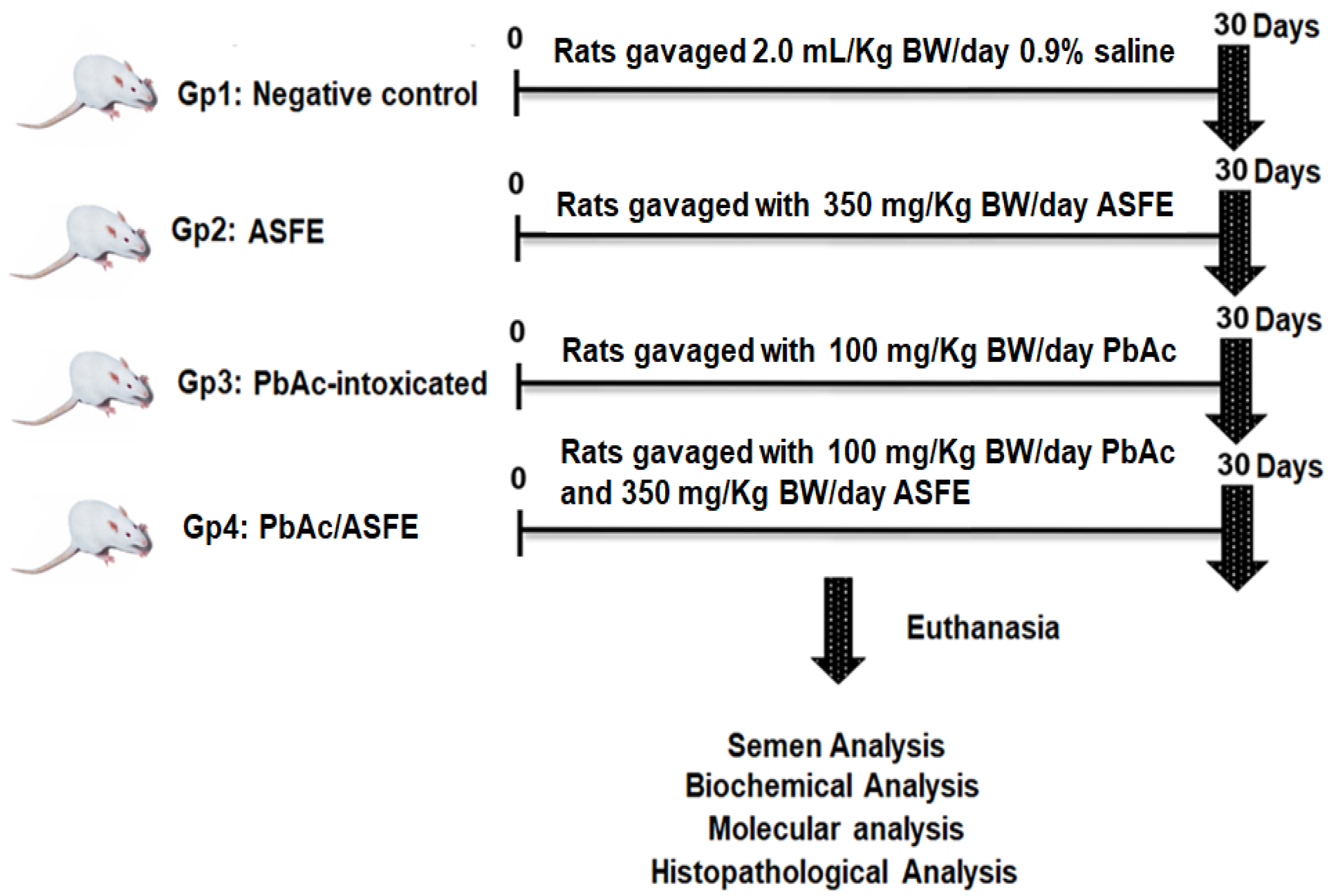
4.7. Animals and Experimental Design
4.8. Semen Analysis
4.9. Biochemical Analysis
4.10. Molecular Analysis
4.11. Histopathological Investigations
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Babakhanzadeh, E.; Nazari, M.; Ghasemifar, S.; Khodadadian, A. Some of the factors involved in male infertility: A prospective review. Int. J. Gen. Med. 2020, 13, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.H.; Zhang, Y.; Dong, P.Y.; Gurunathan, S.; Zhang, X.F. Comprehensive review on the positive and negative effects of various important regulators on male spermatogenesis and fertility. Front. Nutr. 2023, 9, 1063510. [Google Scholar] [CrossRef] [PubMed]
- El-Magd, M.A.; Kahilo, K.A.; Nasr, N.E.; Kamal, T.; Shukry, M.; Saleh, A.A. A potential mechanism associated with lead-induced testicular toxicity in rats. Andrologia 2017, 49, e12750. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Collin, M.S.; Venkatraman, S.K.; Vijayakumar, N.; Kanimozhi, V.; Arbaaz, S.M.; Sibiya Stacey, R.G.; Anusha, J.; Choudhary, R.; Lvov, V.; Tovar, G.I.; et al. Bioaccumulation of lead (Pb) and its effects on human: A review. J. Hazard. Mater. Adv. 2022, 7, 100094. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic mechanisms of five heavy metals: Mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. Occupational and Environmental Exposure to Lead and Reproductive Health Impairment: An Overview. Indian J. Occup. Environ. Med. 2018, 22, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Abu-Khudir, R.; Almutairi, H.H.; Abd El-Rahman, S.S.; El-Said, K.S. The palliative and antioxidant effects of hesperidin against lead-acetate-induced testicular injury in male Wistar rats. Biomedicines 2023, 11, 2390. [Google Scholar] [CrossRef] [PubMed]
- Anjum, M.R.; Madhu, P.; Reddy, K.P.; Reddy, P.S. The protective effects of zinc in lead-induced testicular and epididymal toxicity in Wistar rats. Toxicol. Ind. Health. 2017, 33, 265–276. [Google Scholar] [CrossRef] [PubMed]
- El Barky, A.R.; El-Said, K.S.; Sadek, M.E.; Mohamed, T.M. Anti-diabetic activity of Egyptian celery apigenin. Asian J. Dairy Food Res. 2019, 38, 341–346. [Google Scholar] [CrossRef]
- El-Said, K.S.; Haidyrah, A.S.; Mobasher, M.A.; Khayyat, A.I.A.; Shakoori, A.; Al-Sowayan, N.S.; Barnawi, I.O.; Mariah, R.A. Artemisia annua extract attenuate doxorubicin-induced hepatic injury via PI-3K/Akt/Nrf-2-mediated signaling pathway in rats. Int. J. Mol. Sci. 2023, 24, 15525. [Google Scholar] [CrossRef] [PubMed]
- Khafaji, S.S. Antioxidant, anti-inflammatory, and anti-reprotoxic effects of kaempferol and vitamin E on lead acetate-induced testicular toxicity in male rats. Open Vet. J. 2023, 13, 1683–1695. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Chen, Y.; Chen, J.; Li, X.; Chen, Y. A Review on Annona squamosa L.: Phytochemicals and Biological Activities. Am. J. Chin. Med. 2017, 45, 933–964. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Prakash, S.; Kumar, M.; Radha; Zhang, B.; Sheri, V.; Rais, N.; Chandran, D.; Dey, A.; Sarkar, T.; et al. Seed waste from custard apple (Annona squamosa L.): A comprehensive insight on bioactive compounds, health promoting activity and safety profile. Processes 2022, 10, 2119. [Google Scholar] [CrossRef]
- Mutakin, M.; Fauziati, R.; Fadhilah, F.N.; Zuhrotun, A.; Amalia, R.; Hadisaputri, Y.E. Pharmacological activities of soursop (Annona muricata Lin.). Molecules 2022, 27, 1201. [Google Scholar] [CrossRef] [PubMed]
- Zahid, M.; Arif, M.; Rahman, M.A.; Mujahid, M. Hepatoprotective and antioxidant activities of Annona squamosa seed extract against alcohol-induced liver injury in Sprague Dawley rats. Drug Chem. Toxicol. 2020, 43, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Kaleem, M.; Medha, P.; Ahmed, Q.U.; Asif, M.; Bano, B. Beneficial effects of Annona squamosa extract in streptozotocin-induced diabetic rats. Singap. Med. J. 2008, 49, 800–804. [Google Scholar]
- Dholvitayakhun, A.; Trachoo, N.; Sakee, U.; Cushnie, T.P. Potential applications for Annona squamosa leaf extract in the treatment and prevention of foodborne bacterial disease. Nat. Prod. Commun. 2013, 8, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Monem, D.D.; Elwakeel, S.H.B. Protective effect of graviola (Annona muricata) leaves extract against cadmium induced testicular toxicity in male albino rats. Egypt. Acad. J. Biol. Sci. 2020, 12, 139–154. [Google Scholar] [CrossRef]
- Alsenosy, A.A.; El-Far, A.H.; Sadek, K.M.; Ibrahim, S.A.; Atta, M.S.; Sayed-Ahmed, A.; Al Jaouni, S.K.; Mousa, S.A. Graviola (Annona muricata) attenuates behavioral alterations and testicular oxidative stress induced by streptozotocin in diabetic rats. PLoS ONE 2019, 14, e0222410. [Google Scholar] [CrossRef] [PubMed]
- O’Bryan, M.K.; Hedger, M.P. Inflammatory networks in the control of spermatogenesis: Chronic inflammation in an immunologically privileged tissue? Adv. Exp. Med. Biol. 2008, 636, 92–114. [Google Scholar] [PubMed]
- Liongue, C.; Ward, A.C. Evolution of the JAK-STAT pathway. JAKSTAT 2013, 2, e22756. [Google Scholar] [CrossRef]
- Liau, N.P.D.; Laktyushin, A.; Lucet, I.S.; Murphy, J.M.; Yao, S.; Whitlock, E.; Callaghan, K.; Nicola, N.A.; Kershaw, N.J.; Babon, J.J. The molecular basis of JAK/STAT inhibition by SOCS1. Nat. Commun. 2018, 9, 1558. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lin, H.; Wu, G.; Zhu, M.; Li, M. IL-6/STAT3 is a promising therapeutic target for hepatocellular carcinoma. Front. Oncol. 2021, 11, 760971. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Xie, L.; Li, L.; Li, X.; Li, H.; Liu, J.; Chen, X.; Mao, B.; Song, T.; et al. Interleukin 6 inhibits the differentiation of rat stem Leydig cells. Mol. Cell. Endocrinol. 2018, 472, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Alves-Silva, T.; Freitas, G.A.; Húngaro, T.G.R.; Arruda, A.C.; Oyama, L.M.; Avellar, M.C.W.; Araujo, R.C. Interleukin-6 deficiency modulates testicular function by increasing the expression of suppressor of cytokine signaling 3 (SOCS3) in mice. Sci. Rep. 2021, 11, 11456. [Google Scholar] [CrossRef] [PubMed]
- Somade, O.T.; Oyinloye, B.E.; Ajiboye, B.O.; Osukoya, O.A. Syringic acid demonstrates an anti-inflammatory effect via modulation of the NF-κB-iNOS-COX-2 and JAK-STAT signaling pathways in methyl cellosolve-induced hepato-testicular inflammation in rats. Biochem. Biophys. Rep. 2023, 34, 101484. [Google Scholar] [CrossRef] [PubMed]
- Kolawole, T.A.; Asiwe, J.N.; Buduburisi, B.R.; Akintade, V.A.; Adebayo, O.G.; Ojetola, A.A.; Dapper, D.V. Cabbage (Brassica oleracea) mitigates lead (II) acetate-induced testicular dysfunction in Wistar rats via up-regulation of Bcl-2 protein expression, pituitary-testicular hormonal axis, and down-regulation of oxido-inflammatory reactions. Andrologia 2022, 54, e14476. [Google Scholar] [CrossRef] [PubMed]
- Bentaiba, K.; Belhocine, M.; Chougrani, F.; Bououdina, M.; Mostari, A.; Fernini, M.; Bouzouina, M. Effectiveness of Withania frutescens root extract on testicular damage induced by lead acetate in adult albino rats. Reprod. Toxicol. 2023, 115, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Hussein, S.; Ben Bacha, A.; Alonazi, M.; Alwaili, M.A.; Mobasher, M.A.; Alburae, N.A.; Banjabi, A.A.; El-Said, K.S. Urtica pilulifera leaves extract mitigates cadmium induced hepatotoxicity via modulation of antioxidants, inflammatory markers and Nrf-2 signaling in mice. Front. Mol. Biosci. 2024, 11, 1365440. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Qi, X.; Zhang, M.; Tong, X.; Jiang, N.; Pan, W.; Xiong, W.; Li, Y.; Xu, J.; Shen, J.; et al. Efficient decontamination of heavy metals from aqueous solution using pullulan/polydopamine hydrogels. Int. J. Biol. Macromol. 2020, 145, 1049–1058. [Google Scholar] [CrossRef]
- Qi, X.; Lin, L.; Shen, L.; Li, Z.; Qin, T.; Qian, Y.; Wu, X.; Wei, X.; Gong, Q.; Shen, J. Efficient decontamination of lead ions from wastewater by salecan polysaccharide-based hydrogels. ACS Sustain. Chem. Eng. 2019, 7, 11014–11023. [Google Scholar] [CrossRef]
- Balachandar, R.; Bagepally, B.S.; Kalahasthi, R.; Haridoss, M. Blood lead levels and male reproductive hormones: A systematic review and meta-analysis. Toxicology 2020, 443, 152574. [Google Scholar] [CrossRef] [PubMed]
- Flora, S.J.; Pachauri, V. Chelation in metal intoxication. Int. J. Environ. Res. Public Health 2010, 7, 2745–2788. [Google Scholar] [CrossRef] [PubMed]
- Mobasher, M.A.; Germoush, M.O.; El-Tantawi, H.G.; El-Said, K.S. Metformin improves biochemical and pathophysiological changes in hepatocellular carcinoma with pre-existed diabetes mellitus rats. Pathogens 2021, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Al Kazman, B.S.M.; Harnett, J.E.; Hanrahan, J.R. Traditional uses, phytochemistry and pharmacological activities of Annonacae. Molecules 2022, 27, 3462. [Google Scholar] [CrossRef] [PubMed]
- Zubaidi, S.N.; Mohd Nani, H.; Ahmad Kamal, M.S.; Abdul Qayyum, T.; Maarof, S.; Afzan, A.; Mohmad Misnan, N.; Hamezah, H.S.; Baharum, S.N.; Mediani, A. Annona muricata: Comprehensive review on the ethnomedicinal, phytochemistry, and pharmacological aspects focusing on antidiabetic properties. Life 2023, 13, 353. [Google Scholar] [CrossRef] [PubMed]
- Vikas, B.; Akhil, B.S.R.; Sujathan, K. Free radical scavenging properties of Annona squamosa. Asian Pac. J. Cancer Prev. 2017, 18, 2725–2731. [Google Scholar]
- Loizzo, M.R.; Tundis, R.; Bonesi, M.; Menichini, F.; Mastellone, V.; Avallone, L.; Menichini, F. Radical scavenging, antioxidant, and metal chelating activities of Annona cherimola Mill. (cherimoya) peel and pulp in relation to their total phenolic and total flavonoid contents. Food Comp. Anal. 2012, 25, 179–184. [Google Scholar] [CrossRef]
- Parthiban, E.; Arokiyaraj, C.; Janarthanan, S.; Ramanibai, R. Antioxidant and GC–MS analysis of Annona reticulata leaves extract against unsecure free radicals. SN Appl. Sci. 2019, 1, 349. [Google Scholar] [CrossRef]
- Nasim, N.; Sandeep, I.S.; Mohanty, S. Plant-derived natural products for drug discovery: Current approaches and prospects. Nucleus (Calcutta) 2022, 65, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, Q.; Zhao, Z.; Bai, B.; Sun, Z.; Cai, L.; Fu, Y.; Ma, Y.; Wang, Q.; Xi, G. Effect of hydroxyl on antioxidant properties of 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one to scavenge free radicals. RSC Adv. 2021, 11, 34456–34461. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Kawata, A.; Katayama, T.; Fujisawa, S. Anti-inflammatory activity of the artificial antioxidants 2-Tert-butyl-4-methoxyphenol (BHA), 2,6-Di-tert-butyl-4- methylphenol (BHT) and 2,4,6-Tri-tert-butylphenol (TBP), and their various combinations. In Vivo 2015, 29, 197–206. [Google Scholar] [PubMed]
- Islam, M.T.; Ali, E.S.; Uddin, S.J.; Shaw, S.; Islam, M.A.; Ahmed, M.I.; Chandra Shill, M.; Karmakar, U.K.; Yarla, N.S.; Khan, I.N.; et al. Phytol: A review of biomedical activities. Food Chem. Toxicol. 2018, 121, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.; Nag, A.; Khatua, S.; Sen, S.; Chakraborty, N.; Naskar, A.; Acharya, K.; Calina, D.; Sharifi-Rad, J. Anticancer activity and other biomedical properties of β-sitosterol: Bridging phytochemistry and current pharmacological evidence for future translational approaches. Phytother. Res. 2024, 38, 592–619. [Google Scholar] [CrossRef] [PubMed]
- El-Said, K.S.; Ali, E.M.; Kanehira, K.; Taniguchi, A. Effects of toll-like receptors 3 and 4 induced by titanium dioxide nanoparticles in DNA damage-detecting sensor cells. J. Biosens. Bioelectron. 2013, 4, 2. [Google Scholar] [CrossRef]
- El-Said, K.S.; Atta, A.; Mobasher, M.A.; Germoush, M.O.; Mohamed, T.M.; Salem, M.M. Quercetin mitigates rheumatoid arthritis by inhibiting adenosine deaminase in rats. Mol. Med. 2022, 28, 24. [Google Scholar] [CrossRef] [PubMed]
- Konate, A.; Sawadogo, W.R.; Dubruc, F.; Caillard, O.; Ouedraogo, M.; Guissou, I.P. Phytochemical and anticonvulsant properties of Annona senegalensis Pers. (Annonaceae), plant used in burkina folk medicine to treat epilepsy and convulsions. Br. J. Pharmacol. Toxicol. 2012, 3, 245–250. [Google Scholar]
- Onwusonye, J.C.; Uwakwe, A.; Patrick, A.; Iwuanyanwu, K.P. Acute and sub-acute toxicity studies of methanol leaf extracts of Annona squamosa Linn. in mice. Sky J. Biochem. Res. 2014, 3, 53–59. [Google Scholar]
- Osowski, A.; Fedoniuk, L.; Bilyk, Y.; Fedchyshyn, O.; Sas, M.; Kramar, S.; Lomakina, Y.; Fik, V.; Chorniy, S.; Wojtkiewicz, J. Lead exposure assessment and its impact on the structural organization and morphological peculiarities of rat ovaries. Toxics 2023, 11, 769. [Google Scholar] [CrossRef] [PubMed]
- Ramah, A.; EL-shwarby, R.M.; Nabila, M.A.; El-shewey, E.A. The effect of lead toxicity on male albino rats reproduction with ameliorate by vitamin E and pumpkin seeds oil. Benha Vet. Med. J. 2015, 28, 43–52. [Google Scholar] [CrossRef]
- Shalan, M.; Al-Bakry, N.; Rashwan, H. The ameliorative effects of lactoferrin against lead acetate toxicity in female albino rat. Egypt J. Zool. 2023, 80, 35–49. [Google Scholar] [CrossRef]
- Marchlewicz, M.; Michalska, T.; Wiszniewska, B. Detection of lead-induced oxidative stress in lead the rat epididymis by chemiluminescence. Chemosphere 2004, 57, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Offor, S.J.; Mbagwu, H.O.; Orisakwe, O.E. Improvement of lead acetate-induced testicular injury and sperm quality deterioration by Solanum Anomalum Thonn. Ex. schumach fruit extracts in albino rats. J. Fam. Reprod. Health 2019, 13, 98–108. [Google Scholar]
- Behairy, A.; Hashem, M.M.; Abo-El-Sooud, K.; El-Metwally, A.E.; Hassan, B.A.; Abd-Elhakim, Y.M. Quercetin abates aluminum trioxide nanoparticles and lead acetate induced altered sperm quality, testicular oxidative damage, and sexual hormones disruption in male rats. Antioxidants 2022, 11, 2133. [Google Scholar] [CrossRef] [PubMed]
- Wahab, O.A.; Princely, A.C.; Oluwadamilare, A.A.; Ore-Oluwapo, D.O.; Blessing, A.O.; Alfred, E.F. Clomiphene citrate ameliorated lead acetate-induced reproductive toxicity in male Wistar rats. JBRA. Assist. Reprod. 2019, 23, 336–343. [Google Scholar]
- Sudjarwo, S.A.; Sudjarwo, G.W. Koerniasari Protective effect of curcumin on lead acetate-induced testicular toxicity in Wistar rats. Res. Pharm. Sci. 2017, 12, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Emam, R.A.; Ahmed, E.A. Ameliorative effect of L-carnitine on chronic lead-induced reproductive toxicity in male rats. Vet. Med. Sci. 2021, 7, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Oyeyemi, W.A.; Akinola, A.O.; Daramola, O.O.; Aikpitanyi, I.; Durotoluwa, O.T.; Alele, P.O.; Olohigbe, P.; Ogieriakhi, I.; Okoro, T. Vitamin E and quercetin attenuated the reproductive toxicity mediated by lead acetate in male Wistar. Bull. Natl. Res. Cent. 2022, 46, 22. [Google Scholar] [CrossRef]
- El-Said, K.S.; Hussein, S.; Alrashdi, B.M.; Mahmoud, H.A.; Ibrahim, M.A.; Elbakry, M.; El-Tantawy, H.; Kabil, D.I.; El-Naggar, S.A. Musa sp. leaves extract ameliorates the hepato-renal toxicities induced by cadmium in mice. Molecules 2022, 27, 559. [Google Scholar] [CrossRef] [PubMed]
- Elsheikh, N.A.; Omer, N.A.; Yi-Ru, W.; Mei-Qian, K.; Ilyas, A.; Abdurahim, Y.; Wang, G.L. Protective effect of betaine against lead-induced testicular toxicity in male mice. Andrologia 2020, 52, e13600. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P.; Slama, P.; Roychoudhury, S. Oxidative Stress, testicular inflammatory pathways, and male reproduction. Int. J. Mol. Sci. 2021, 22, 10043. [Google Scholar] [CrossRef]
- Tekin, S.; Çelebi, F. Investigation of the effect of hesperidin on some reproductive parameters in testicular toxicity induced by Bisphenol A. Andrologia 2022, 54, e14562. [Google Scholar] [CrossRef] [PubMed]
- Hussein, S.; Kamel, G.A.M. Pioglitazone ameliorates cisplatin-induced testicular toxicity by attenuating oxidative stress and inflammation via TLR4/MyD88/NF-κB signaling pathway. J. Trace Elem. Med. Biol. 2023, 80, 127287. [Google Scholar] [CrossRef] [PubMed]
- Adeyi, O.E.; Somade, O.T.; Ajayi, B.O.; James, A.S.; Adeboye, T.R.; Olufemi, D.A.; Oyinlola, E.V.; Sanyaolu, E.T.; Mufutau, I.O. The anti-inflammatory effect of ferulic acid is via the modulation of NFκB-TNF-α-IL-6 and STAT1-PIAS1 signaling pathways in 2-methoxyethanol-induced testicular inflammation in rats. Phytomed. Plus 2023, 3, 100464. [Google Scholar] [CrossRef]
- O’Shea, J.J.; Holland, S.M.; Staudt, L.M. JAKs and STATs in immunity, immunodeficiency, and cancer. N. Engl. J. Med. 2013, 368, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; Almeida, L.; Barbosa, R.M.; Laranjinha, J. Luteolin suppresses the JAK/ STAT pathway in a cellular model of intestinal inflammation. Food Funct. 2017, 8, 387–396. [Google Scholar] [CrossRef]
- Shuai, K.; Liu, B. Regulation of JAK–STAT signaling in the immune system. Nat. Rev. Immunol. 2003, 3, 900–911. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Larkin, J. Therapeutic implication of SOCS1 modulation in the treatment of autoimmunity and cancer. Front. Pharmacol. 2019, 10, 324. [Google Scholar] [CrossRef] [PubMed]
- Oatley, J.M.; Kaucher, A.V.; Avarbock, M.R.; Brinster, R.L. Regulation of mouse spermatogonial stem cell diferentiation by STAT3 signaling. Biol. Reprod. 2010, 83, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Gulzar, S.; Karim, R.H. Effect of vitamin C against lead acetate toxicity on sperm count, sperm morphology and testis tissue in the rat before and in recovery period. ZANCO J. Pure App. Sci. 2020, 32, 127–138. [Google Scholar]
- Ragab, S.M.M.; Almohaimeed, H.M.; Alghriany, A.A.I.; Khalil, N.S.A.; Abd-Allah, E.A. Protective effect of Moringa oleifera leaf ethanolic extract against uranyl acetate-induced testicular dysfunction in rats. Sci. Rep. 2024, 14, 932. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimzadeh, M.A.; Pourmorad, F.; Bekhradnia, A.R. Iron chelating activity, phenol, and flavonoid content of some medicinal plants from Iran. Afr. J. Biotechnol. 2008, 7, 3188–3192. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Hiai, S.; Oura, H.; Odaka, Y.; Nakajima, T.A. Colorimetric estimation of Ginseng saponins. Planta Med. 1975, 28, 363–369. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Jiménez, J.; Doerr, S.; Martínez-Rosell, G.; Rose, A.S.; De Fabritiis, G. DeepSite: Protein-binding site predictor using 3D-convolutional neural networks. Bioinformatics 2017, 33, 3036–3042. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Finney, D.J.; Stevens, W.L. A table for the calculation of working probits and weight in propit analysis. Biometrika 1948, 35, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Ayuba, Y.; Ekanem, A.U.; Garba, S.H. Effect of oral administration of lead acetate exposure on the histology of the testis and testicular sperm concentration in Wistar albino rats. Schol. J. App. Med. Sci. 2017, 5, 2337–2344. [Google Scholar]
- Wells, M.; Awam, O. New technique for assessing acrosomal characteristics of spermatozoa1. J. Dairy Sci. 1970, 53, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, J.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: Philadelphia, PA, USA, 2008. [Google Scholar]
- Teixeira, T.A.; Pariz, J.R.; Dutra, R.T.; Saldiva, P.H.; Costa, E.; Hallak, J. Cut-off values of the Johnsen score and Copenhagen index as histopathological prognostic factors for postoperative semen quality in selected infertile patients undergoing microsurgical correction of bilateral subclinical varicocele. Transl. Androl. Urol. 2019, 8, 346–355. [Google Scholar] [CrossRef] [PubMed]

| Phytochemical Analysis | ASF |
|---|---|
| Metal-chelating activity (MCA) (%) | 82 ± 3.87 |
| EC50 of MCA (μg/mL) | 383.65 ± 4.95 |
| Total phenolic content (mg GAE/g DW) | 25.48 ± 2.56 |
| Total flavonoid contents (mg QE/g DW) | 16.75 ± 1.87 |
| Total antioxidant capacity (TAC) (mg AAE/g DW) | 67.78 ± 3.58 |
| Saponin (mg/g DW) | 392 ± 3.85 |
| Anthocyanin (mg ECG/g DW) | 3.79 ± 0.29 |
| DPPH scavenging activity (%) | 86 ± 4.02 |
| IC50 of DPPH (mg/mL) | 5.81 ± 0.80 |
| No. | RT (min.) | Name | MF | PA (%) |
|---|---|---|---|---|
| 1 | 2.25 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl | C6H8O4 | 6.17 |
| 2 | 5.18 | 2-Methoxy-4-vinylphenol | C9H10O2 | 3.23 |
| 3 | 7.34 | Hydroxylamine, O-decyl | C10H23NO | 2.00 |
| 4 | 7.64 | Caryophyllene | C15H24 | 2.72 |
| 5 | 8.73 | Butylated hydroxytoluene | C15H24O | 4.80 |
| 6 | 10.58 | Hexadecanol | C16H32O2 | 2.35 |
| 7 | 11.82 | Pentadecanoic acid, 14-methyl-, methyl ester | C17H34O2 | 2.14 |
| 8 | 17.27 | 9,12-Octadecadienoic acid, methyl ester | C19H34O2 | 2.66 |
| 9 | 18.59 | 5,8,11-Heptadecatrienoic acid, methyl ester | C18H30O2 | 8.61 |
| 10 | 19.35 | Phytol | C20H40O | 5.36 |
| 11 | 22.85 | Oleic acid | C18H34O2 | 2.63 |
| 12 | 24.43 | Eicosanoic acid | C20H40O2 | 12.51 |
| 13 | 25.76 | Oxiraneundecanoic acid, 3-pentyl-, methyl ester | C19H36O3 | 6.42 |
| 14 | 27.68 | β-Sitosterol | C29H50O | 7.53 |
| 15 | 33.57 | Bis(2-Ethylhexyl) phthalate | C24H38O4 | 2.19 |
| 16 | 39.72 | Hexadecanoic acid, ethyl ester | C18H36O2 | 12.36 |
| 17 | 42.69 | 9,12,15-Octadecatrienoic acid, methyl ester | C19H32O2 | 2.65 |
| 18 | 43.58 | Octadecanoic acid | C18H36O2 | 5.74 |
| 19 | 45.63 | Quercetin derivative | C30H50O7Si5 | 7.85 |
| Compound | JAK-1 | STAT-3 | SOCS-1 |
|---|---|---|---|
| 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl | −5.8 | −4.6 | −4.9 |
| 2-Methoxy-4-vinylphenol | −5.4 | −5.0 | −5.3 |
| Hydroxylamine, O-decyl | −4.9 | −4.0 | −4.4 |
| Caryophyllene | −6.5 | −6.4 | −6.5 |
| Butylated hydroxytoluene | −6.0 | −5.9 | −6.2 |
| Hexadecanol | −4.2 | −4.0 | −3.8 |
| Pentadecanoic acid, 14-methyl-, methyl ester | −4.8 | −3.8 | −4.4 |
| 9,12-Octadecadienoic acid, methyl ester | −4.9 | −3.8 | −4.7 |
| 5,8,11-Heptadecatrienoic acid, methyl ester | −5.0 | −4.7 | −4.7 |
| Phytol | −5.1 | −3.8 | −4.5 |
| Oleic acid | −5.3 | −4.5 | −3.9 |
| Eicosanoic acid | −6.0 | −3.8 | −3.8 |
| Oxiraneundecanoic acid, 3-pentyl-, methyl ester | −5.4 | −5.1 | −5.0 |
| β-Sitosterol | −7.9 | −6.8 | −7.1 |
| Bis(2-Ethylhexyl) phthalate | −6.3 | −5.9 | −4.8 |
| Hexadecanoic acid, ethyl ester | −4.6 | −4.0 | −4.5 |
| 9,12,15-Octadecatrienoic acid, methyl ester | −5.0 | −4.6 | −4.5 |
| Octadecanoic acid | −5.1 | −3.9 | −4.5 |
| Quercetin derivative | −9.1 | −7.9 | −6.9 |
| Groups | Abnormality (%) | Count (×106/mL) | Viability (%) | Motility (%) |
|---|---|---|---|---|
| Control | 11.82 ± 1.55 a | 68.91 ± 3.87 a | 64.31 ± 4.65 a | 53.66 ± 4.56 a |
| ASFE | 10.21 ± 2.01 a | 71.24 ± 4.58 a | 61.53 ± 3.93 a | 55.79 ± 3.93 a |
| PbAc | 19.62 ± 2.59 b | 31.77 ± 2.79 b | 27.82 ± 2.95 b | 28.21 ± 3.15 b |
| PbAc/ASFE | 13.54 ± 2.89 a | 55.69 ± 3.48 a,c | 50.75 ± 3.86 a | 47.84 ± 3.79 a |
| Groups | MDA (nmol/g Tissue) | PC (nmol/mg Protein) | SOD (U/mg Protein) | CAT (U/mg Protein) | GSH (mmol/g Tissue) |
|---|---|---|---|---|---|
| Control | 21.73 ± 1.95 a | 2.80 ± 0.38 a | 0.67 ± 0.05 a | 3.88 ± 0.29 a | 31.21 ± 2.96 a |
| ASFE | 17.92 ± 1.45 a | 2.57 ± 0.26 a | 0.89 ± 0.07 a | 4.24 ± 0.38 a | 33.47 ± 3.12 a |
| PbAc | 44.67 ± 3.86 b | 6.87 ± 0.49 b | 0.39 ± 0.06 b | 1.95 ± 0.18 b | 17.89 ± 1.84 a |
| PbAc/ASFE | 26.84 ± 2.74 a | 3.72 ± 0.31 c | 0.58 ± 0.04 a | 2.87 ± 0.25 a,c | 24.75 ± 1.95 a,c |
| Groups | IL-6 (Pg/mL/mg Protein) | TNF-α (Pg/mL/mg Protein) | NF-κB (ng/mg Tissue) | COX-2 (ng/g Tissue) |
|---|---|---|---|---|
| Control | 28.44 ± 1.49 a | 58.47 ± 1.86 a | 18.65 ± 0.97 a | 225.53 ± 4.61 a |
| ASFE | 26.21 ± 1.23 a | 53.98 ± 1.39 a | 16.43 ± 1.11 a | 215.42 ± 5.82 a |
| PbAc | 69.87 ± 3.14 b | 84.29 ± 2.17 b | 37.69 ± 1.74 c | 359.86 ± 6.27 b |
| PbAc/ASFE | 34.65 ± 2.15 a | 64.38 ± 0.04 a | 27.55 ± 1.07 d | 302.17 ± 5.31 e |
| Gene | Accession Number | Forward Sequence (5′–3′) | Reverse Sequence (5′–3′) |
|---|---|---|---|
| JAK-1 | NM_053466 | GAATGTACTGGGCGTCTTGG | TCAAGGAGTGGGGTTGCTTC |
| STAT-3 | NM_012747 | CACCCTGAAGCTGACCCAG | TATTGCTGCAGGTCGTTGGT |
| SOCS-1 | NM_145879 | TAACCCGGTACTCCGTGACT | CTCCCACGTGGTTCCAGAAA |
| IL-6 | NM_012589 | CACAAGTCCGGAGAGGAGAC | TCACAAACTCCAGGTAGAAACG |
| TNF-α | NM_012675 | CTGAACTTCGGGGTGATCG | GCTTGGTGGTTTGCTACGAC |
| NF-κB | NM_001415012 | CCTAGCTTTCTCTGAACTGCAAA | GGGTCAGAGGCCAATAGAGA |
| COX-2 | NM_017232 | CTACACCAGGGCCCTTCC | TCCAGAACTTCTTTTGAATCAGG |
| β-actin | NM_031144.3 | ATCGCTGACAGGATGCAGAAG | AGAGCCACCAATCCACACAGA |
| Score | Description |
|---|---|
| 10 | Complete spermatogenesis with many spermatozoa. Germinal epithelium organized in a regular thickness leaving an open lumen. |
| 9 | Many spermatozoa are present but germinal epithelium disorganized with marked sloughing or obliteration of lumen. |
| 8 | Only a few spermatozoa are present in the section. |
| 7 | No spermatozoa but many spermatids are present. |
| 6 | No spermatozoa and only a few spermatids present. |
| 5 | No spermatozoa, no spermatids but several or many spermatocytes present. |
| 4 | Only few spermatocytes and no spermatids or spermatozoa present. |
| 3 | Spermatogonia are the only germ cells present. |
| 2 | No germ cells but Sertoli cells are present. |
| 1 | No cells in the tubular section |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mobasher, M.A.; Alsirhani, A.M.; Alwaili, M.A.; Baakdah, F.; Eid, T.M.; Alshanbari, F.A.; Alzahri, R.Y.; Alkhodair, S.A.; El-Said, K.S. Annona squamosa Fruit Extract Ameliorates Lead Acetate-Induced Testicular Injury by Modulating JAK-1/STAT-3/SOCS-1 Signaling in Male Rats. Int. J. Mol. Sci. 2024, 25, 5562. https://doi.org/10.3390/ijms25105562
Mobasher MA, Alsirhani AM, Alwaili MA, Baakdah F, Eid TM, Alshanbari FA, Alzahri RY, Alkhodair SA, El-Said KS. Annona squamosa Fruit Extract Ameliorates Lead Acetate-Induced Testicular Injury by Modulating JAK-1/STAT-3/SOCS-1 Signaling in Male Rats. International Journal of Molecular Sciences. 2024; 25(10):5562. https://doi.org/10.3390/ijms25105562
Chicago/Turabian StyleMobasher, Maysa A., Alaa Muqbil Alsirhani, Maha Abdullah Alwaili, Fadi Baakdah, Thamir M Eid, Fahad A. Alshanbari, Reem Yahya Alzahri, Sahar Abdulrahman Alkhodair, and Karim Samy El-Said. 2024. "Annona squamosa Fruit Extract Ameliorates Lead Acetate-Induced Testicular Injury by Modulating JAK-1/STAT-3/SOCS-1 Signaling in Male Rats" International Journal of Molecular Sciences 25, no. 10: 5562. https://doi.org/10.3390/ijms25105562
APA StyleMobasher, M. A., Alsirhani, A. M., Alwaili, M. A., Baakdah, F., Eid, T. M., Alshanbari, F. A., Alzahri, R. Y., Alkhodair, S. A., & El-Said, K. S. (2024). Annona squamosa Fruit Extract Ameliorates Lead Acetate-Induced Testicular Injury by Modulating JAK-1/STAT-3/SOCS-1 Signaling in Male Rats. International Journal of Molecular Sciences, 25(10), 5562. https://doi.org/10.3390/ijms25105562






