Unraveling the Microbiome–Human Body Axis: A Comprehensive Examination of Therapeutic Strategies, Interactions and Implications
Abstract
1. Introduction
2. Materials and Methods
3. The Microbiome and Its Implications for Human Health
3.1. The Microbiome Nutrition and Lifestyle: A Complex Symbiosis in Intestinal Health
3.2. Disruptions in Gut Microbiota and Their Impact on Health
| Key Roles of Intestinal Microbiota in Human Health [142] | ||
|---|---|---|
| Metabolic | Immunity | Trophicity |
| The production of short-chain fatty acids, including acetate, butyrate, and propionate, contributes to several beneficial effects on the host organism Vitamin synthesis (vitamins K, B12) [143,144] Increases absorption of calcium, iron and magnesium [145,146,147] Participates in metabolism fatty acids [142] Participates in the degradation of polyphenols [142] Participates in the degradation of choline and amino acids [142] Participates in the production of polyamines [142] Metabolism of xenobiotics (drugs) [142] | Interacts with pathogens for nutrients and attachment sites (receptors) [142] Secretion of antimicrobial components (bacteriocins, lactates) [142] It induces the synthesis of antimicrobial proteins (cathelicidins, C-type lectins, defensins) [142] Stimulates the production of IgA immunoglobulins [142,148] Regulates the development and functioning of the immune system [142] | Regulates intestinal epithelial development through cell proliferation and differentiation (angiogenesis, crypt formation) [149] Stimulates intestinal peristalsis [142] Effects on systems and viscera (central nervous system, liver, heart, lungs) [142] Butyrate resulting from fermentation has an inhibitory effect on neoplastic cells [150] |
4. The Relationship of the Microbiome with Different Parts of the Body
4.1. The Microbiota–Gut–Brain Axis
4.2. The Relationship of the Gut Microbiome to the Cardiovascular System
4.3. The Gut–Muscle Axis
4.4. The Gut–Bone Axis
4.5. The Relationship of the Gut Microbiome to the Immune System
5. Principles of Nutritional Therapy for Balancing the Intestinal Microbiome
- (i.)
- Can be absorbed in the upper gastrointestinal tract without being broken down by gastric acidity or mammalian enzymes;
- (ii.)
- It is fermented by bacterial microflora from intestine;
- (iii.)
- It selectively stimulates the growth and/or activity of saprophytic intestinal bacteria associated with well-being and health [21].
- -
- Resistance of dietary fiber to digestion in the small intestine, enabling its fermentation in the large intestine to generate short-chain fatty acids with known anticancer properties.
- -
- Increase in fecal volume and viscosity due to dietary fiber, reducing the duration of contact between potential carcinogens and mucosal cells.
- -
- Enhanced binding of bile acids to carcinogens facilitated by dietary fiber.
- -
- Elevated levels of antioxidants associated with increased dietary fiber intake.
- -
- Inhibition of estrogen absorption in the intestines leading to increased excretion of estrogen in feces by dietary fiber.
6. The Microbiome and Longevity
7. Fecal Microbiota Transplantation: A Novel Therapy in Dysbiosis-Related Diseases
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moeller, A.H.; Li, Y.; Ngole, E.M.; Ahuka-Mundeke, S.; Lonsdorf, E.V.; Pusey, A.E.; Peeters, M.; Hahn, B.H.; Ochman, H. Rapid Changes in the Gut Microbiome during Human Evolution. Proc. Natl. Acad. Sci. USA 2014, 111, 16431–16435. [Google Scholar] [CrossRef] [PubMed]
- Davenport, E.R.; Sanders, J.G.; Song, S.J.; Amato, K.R.; Clark, A.G.; Knight, R. The Human Microbiome in Evolution. BMC Biol. 2017, 15, 127. [Google Scholar] [CrossRef]
- Sekirov, I.; Russell, S.L.; Caetano, M.; Antunes, L.; Finlay, B.B. Gut Microbiota in Health and Disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the Normal Gut Microbiota. World J. Gastroenterol. WJG 2015, 21, 8787. [Google Scholar] [CrossRef]
- Ioniță-Mîndrican, C.B.; Ziani, K.; Mititelu, M.; Oprea, E.; Neacșu, S.M.; Moroșan, E.; Dumitrescu, D.E.; Roșca, A.C.; Drăgănescu, D.; Negrei, C. Therapeutic Benefits and Dietary Restrictions of Fiber Intake: A State of the Art Review. Nutrients 2022, 14, 2641. [Google Scholar] [CrossRef] [PubMed]
- Hollister, E.B.; Gao, C.; Versalovic, J. Compositional and Functional Features of the Gastrointestinal Microbiome and Their Effects on Human Health. Gastroenterology 2014, 146, 1449–1458. [Google Scholar] [CrossRef]
- Kho, Z.Y.; Lal, S.K. The Human Gut Microbiome–- A Potential Controller of Wellness and Disease. Front. Microbiol. 2018, 9, 1835. [Google Scholar] [CrossRef]
- Ursell, L.K.; Haiser, H.J.; van Treuren, W.; Garg, N.; Reddivari, L.; Vanamala, J.; Dorrestein, P.C.; Turnbaugh, P.J.; Knight, R. The Intestinal Metabolome: An Intersection between Microbiota and Host. Gastroenterology 2014, 146, 1470–1476. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Rani, S.; Sivaranjani, Y.; Kumar, M.; Rao, G. Rhinocerebral Mucormycosis Associated with Actinomycosis in a Diabetic Patient: A Rare Presentation. J. Oral Maxillofac. Pathol. 2019, 23, 122. [Google Scholar] [CrossRef]
- Segata, N.; Haake, S.K.; Mannon, P.; Lemon, K.P.; Waldron, L.; Gevers, D.; Huttenhower, C.; Izard, J. Composition of the Adult Digestive Tract Bacterial Microbiome Based on Seven Mouth Surfaces, Tonsils, Throat and Stool Samples. Genome Biol. 2012, 13, R42. [Google Scholar] [CrossRef]
- Tong, X.; Xu, J.; Lian, F.; Yu, X.; Zhao, Y.; Xu, L.; Zhang, M.; Zhao, X.; Shen, J.; Wu, S.; et al. Structural Alteration of Gut Microbiota during the Amelioration of Human Type 2 Diabetes with Hyperlipidemia by Metformin and a Traditional Chinese Herbal Formula: A Multicenter, Randomized, Open Label Clinical Trial. mBio 2018, 9, e02392-17. [Google Scholar] [CrossRef] [PubMed]
- Dickson, R.P.; Huffnagle, G.B. The Lung Microbiome: New Principles for Respiratory Bacteriology in Health and Disease. PLoS Pathog. 2015, 11, e1004923. [Google Scholar] [CrossRef] [PubMed]
- Dickson, R.P.; Martinez, F.J.; Huffnagle, G.B. The Role of the Microbiome in Exacerbations of Chronic Lung Diseases. Lancet 2014, 384, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Flores, G.E.; Caporaso, J.G.; Henley, J.B.; Rideout, J.R.; Domogala, D.; Chase, J.; Leff, J.W.; Vázquez-Baeza, Y.; Gonzalez, A.; Knight, R.; et al. Temporal Variability Is a Personalized Feature of the Human Microbiome. Genome Biol. 2014, 15, 531. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.A.; Segre, J.A. The Human Microbiome: Our Second Genome. Annu. Rev. Genom. Hum. Genet. 2012, 13, 151–170. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, A.M.; Shanahan, F. The Gut Flora as a Forgotten Organ. EMBO Rep. 2006, 7, 688–693. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome Definition Re-Visited: Old Concepts and New Challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Vernocchi, P.; Del Chierico, F.; Putignani, L. Gut Microbiota Metabolism and Interaction with Food Components. Int. J. Mol. Sci. 2020, 21, 3688. [Google Scholar] [CrossRef]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 2017, 8, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Quaglio, A.E.V.; Grillo, T.G.; De Oliveira, E.C.S.; Di Stasi, L.C.; Sassaki, L.Y. Gut microbiota, inflammatory bowel disease and colorectal cancer. World J. Gastroenterol. 2022, 28, 4053–4060. [Google Scholar] [CrossRef] [PubMed]
- Nova, E.; Gómez-Martinez, S.; González-Soltero, R. The Influence of Dietary Factors on the Gut Microbiota. Microorganisms. 2022, 10, 1368. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ju, D.; Zeng, X. Mechanisms and Clinical Implications of Human Gut Microbiota-Drug Interactions in the Precision Medicine Era. Biomedicines 2024, 12, 194. [Google Scholar] [CrossRef] [PubMed]
- Maftei, N.-M.; Raileanu, C.R.; Balta, A.A.; Ambrose, L.; Boev, M.; Marin, D.B.; Lisa, E.L. The Potential Impact of Probiotics on Human Health: An Update on Their Health-Promoting Properties. Microorganisms 2024, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Jellinger, K.A. Advances in Molecular Medicine: Unravelling Disease Complexity and Pioneering Precision Healthcare. Int. J. Mol. Sci. 2023, 24, 14168. [Google Scholar] [CrossRef]
- Kamel, M.; Aleya, S.; Alsubih, M.; Aleya, L. Microbiome Dynamics: A Paradigm Shift in Combatting Infectious Diseases. J. Pers. Med. 2024, 14, 217. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Z.; Geng, B.; Cai, J. The Bidirectional Signal Communication of Microbiota-Gut-Brain Axis in Hypertension. Int. J. Hypertens. 2021, 2021, 8174789. [Google Scholar] [CrossRef]
- Banaszak, M.; Górna, I.; Woźniak, D.; Przysławski, J.; Drzymała-Czyż, S. Association between Gut Dysbiosis and the Occurrence of SIBO, LIBO, SIFO and IMO. Microorganisms 2023, 11, 573. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Arthur, S.; Haynes, J.; Butts, M.R.; Nepal, N.; Sundaram, U. The Role of Gut Microbiota and Metabolites in Obesity-Associated Chronic Gastrointestinal Disorders. Nutrients 2022, 4, 624. [Google Scholar] [CrossRef]
- Frank, D.N.; St. Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-Phylogenetic Characterization of Microbial Community Imbalances in Human Inflammatory Bowel Diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780. [Google Scholar] [CrossRef] [PubMed]
- le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; et al. Richness of Human Gut Microbiome Correlates with Metabolic Markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Swidsinski, A.; Weber, J.; Loening-Baucke, V.; Hale, L.P.; Lochs, H. Spatial Organization and Composition of the Mucosal Flora in Patients with Inflammatory Bowel Disease. J. Clin. Microbiol. 2005, 43, 3380–3389. [Google Scholar] [CrossRef]
- Joossens, M.; Huys, G.; Cnockaert, M.; de Preter, V.; Verbeke, K.; Rutgeerts, P.; Vandamme, P.; Vermeire, S. Dysbiosis of the Faecal Microbiota in Patients with Crohn’s Disease and Their Unaffected Relatives. Gut 2011, 60, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.; Raes, J.; Pelletier, E.; le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the Human Gut Microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Durbán, A.; Abellán, J.J.; Jiménez-Hernández, N.; Ponce, M.; Ponce, J.; Sala, T.; D’Auria, G.; Latorre, A.; Moya, A. Assessing Gut Microbial Diversity from Feces and Rectal Mucosa. Microb. Ecol. 2011, 61, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Zoetendal, E.G.; von Wright, A.; Vilpponen-Salmela, T.; Ben-Amor, K.; Akkermans, A.D.L.; de Vos, W.M. Mucosa-Associated Bacteria in the Human Gastrointestinal Tract Are Uniformly Distributed along the Colon and Differ from the Community Recovered from Feces. Appl. Environ. Microbiol. 2002, 68, 3401–3407. [Google Scholar] [CrossRef]
- Walter, J.; Ley, R. The Human Gut Microbiome: Ecology and Recent Evolutionary Changes. Annu. Rev. Microbiol. 2011, 65, 411–429. [Google Scholar] [CrossRef]
- Kastl, A.J.; Terry, N.A.; Wu, G.D.; Albenberg, L.G. The Structure and Function of the Human Small Intestinal Microbiota: Current Understanding and Future Directions. Cell. Mol. Gastroenterol. Hepatol. 2020, 9, 33–45. [Google Scholar] [CrossRef]
- Thadepalli, H.; Lou, S.M.A.; Bach, V.T.; Matsui, T.K.; Mandal, A.K. Microflora of the Human Small Intestine. Am. J. Surg. 1979, 138, 845–850. [Google Scholar] [CrossRef]
- Hayashi, H.; Takahashi, R.; Nishi, T.; Sakamoto, M.; Benno, Y. Molecular Analysis of Jejunal, Ileal, Caecal and Recto-Sigmoidal Human Colonic Microbiota Using 16S RRNA Gene Libraries and Terminal Restriction Fragment Length Polymorphism. J. Med. Microbiol. 2005, 54, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.F.; Lindberg, M.; Jakobsson, H.; Bäckhed, F.; Nyrén, P.; Engstrand, L. Comparative Analysis of Human Gut Microbiota by Barcoded Pyrosequencing. PLoS ONE 2008, 3, e2836. [Google Scholar] [CrossRef]
- Pei, Z.; Bini, E.J.; Yang, L.; Zhou, M.; Francois, F.; Blaser, M.J. Bacterial Biota in the Human Distal Esophagus. Proc. Natl. Acad. Sci. USA 2004, 101, 4250–4255. [Google Scholar] [CrossRef] [PubMed]
- Huttenhower, C.; Gevers, D.; Knight, R.; Abubucker, S.; Badger, J.H.; Chinwalla, A.T.; Creasy, H.H.; Earl, A.M.; Fitzgerald, M.G.; Fulton, R.S.; et al. Structure, Function and Diversity of the Healthy Human Microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Justesen, T.; Nielsen, O.H.; Jacobsen, I.E.; Lave, J.; Rasmussen, S.N. The Normal Cultivable Microflora in Upper Jejunal Fluid in Healthy Adults. Scand. J. Gastroenterol. 1984, 19, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Blaser, M.J. Hypothesis: The Changing Relationships of Helicobacter Pylori and Humans: Implications for Health and Disease. J. Infect. Dis. 1999, 179, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Swidsinski, A.; Loening-Baucke, V.; Lochs, H.; Hale, L.P. Spatial Organization of Bacterial Flora in Normal and Inflamed Intestine: A Fluorescence in Situ Hybridization Study in Mice. World J. Gastroenterol. 2005, 11, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The Healthy Human Microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Moodley, Y.; Linz, B.; Yamaoka, Y.; Windsor, H.M.; Breurec, S.; Wu, J.Y.; Maady, A.; Bernhöft, S.; Thiberge, J.M.; Phuanukoonnon, S.; et al. The Peopling of the Pacific from a Bacterial Perspective. Science 2009, 323, 527–530. [Google Scholar] [CrossRef]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikura, N.; Takahashi, S.; Xiao, J.Z.; Abe, F.; Osawa, R. Age-Related Changes in Gut Microbiota Composition from Newborn to Centenarian: A Cross-Sectional Study. BMC Microbiol. 2016, 16, 90. [Google Scholar] [CrossRef]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human Gut Microbiome Viewed across Age and Geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Koenig, J.E.; Spor, A.; Scalfone, N.; Fricker, A.D.; Stombaugh, J.; Knight, R.; Angenent, L.T.; Ley, R.E. Succession of Microbial Consortia in the Developing Infant Gut Microbiome. Proc. Natl. Acad. Sci. USA 2011, 108, 4578–4585. [Google Scholar] [CrossRef] [PubMed]
- de Filippo, C.; Cavalieri, D.; di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of Diet in Shaping Gut Microbiota Revealed by a Comparative Study in Children from Europe and Rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2013, 505, 559–563. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef]
- Tidjani Alou, M.; Lagier, J.C.; Raoult, D. Diet Influence on the Gut Microbiota and Dysbiosis Related to Nutritional Disorders. Hum. Microbiome J. 2016, 1, 3–11. [Google Scholar] [CrossRef]
- Burkitt, D.P. Some diseases characteristic of modern Western civilization. BMJ 1973, 1, 274–278. [Google Scholar] [CrossRef]
- Slavin, J.L. Dietary Fiber: Classification, Chemical Analyses, and Food Sources. J. Am. Diet. Assoc. 1987, 87, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.L. Position of the American Dietetic Association: Health Implications of Dietary Fiber. J. Am. Diet. Assoc. 2008, 108, 1716–1731. [Google Scholar] [CrossRef]
- Weickert, M.O.; Pfeiffer, A.F.H. Metabolic Effects of Dietary Fiber Consumption and Prevention of Diabetes. J. Nutr. 2008, 138, 439–442. [Google Scholar] [CrossRef]
- Lattimer, J.M.; Haub, M.D. Effects of Dietary Fiber and Its Components on Metabolic Health. Nutrients 2010, 2, 1266. [Google Scholar] [CrossRef] [PubMed]
- Nirmala Prasadi, V.P.; Joye, I.J. Dietary Fibre from Whole Grains and Their Benefits on Metabolic Health. Nutrients 2020, 12, 3045. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Hu, Y.; Bruner, D.W. Composition of Gut Microbiota and Its Association with Body Mass Index and Lifestyle Factors in a Cohort of 7–18 Years Old Children from the American Gut Project. Pediatr. Obes. 2019, 14, e12480. [Google Scholar] [CrossRef] [PubMed]
- Monda, V.; Villano, I.; Messina, A.; Valenzano, A.; Esposito, T.; Moscatelli, F.; Viggiano, A.; Cibelli, G.; Chieffi, S.; Monda, M.; et al. Exercise Modifies the Gut Microbiota with Positive Health Effects. Oxidative Med. Cell. Longev. 2017, 2017, 3831972. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and Associated Dietary Extremes Impact on Gut Microbial Diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef]
- Nermes, M.; Endo, A.; Aarnio, J.; Salminen, S.; Isolauri, E. Furry Pets Modulate Gut Microbiota Composition in Infants at Risk for Allergic Disease. J. Allergy Clin. Immunol. 2015, 136, 1688–1690.e1. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Konya, T.; Maughan, H.; Guttman, D.S.; Field, C.J.; Sears, M.R.; Becker, A.B.; Scott, J.A.; Kozyrskyj, A.L. Infant Gut Microbiota and the Hygiene Hypothesis of Allergic Disease: Impact of Household Pets and Siblings on Microbiota Composition and Diversity. Allergy Asthma Clin. Immunol. 2013, 9, 15. [Google Scholar] [CrossRef]
- Ownby, D.R.; Johnson, C.C.; Peterson, E.L. Exposure to Dogs and Cats in the First Year of Life and Risk of Allergic Sensitization at 6 to 7 Years of Age. JAMA 2002, 288, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Tun, H.M.; Konya, T.; Takaro, T.K.; Brook, J.R.; Chari, R.; Field, C.J.; Guttman, D.S.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; et al. Exposure to Household Furry Pets Influences the Gut Microbiota of Infants at 3-4 Months Following Various Birth Scenarios. Microbiome 2017, 5, 40. [Google Scholar] [CrossRef]
- Enck, P.; Zimmermann, K.; Rusch, K.; Schwiertz, A.; Klosterhalfen, S.; Frick, J.S. The Effects of Maturation on the Colonic Microflora in Infancy and childhood. Gastroenterol. Res. Pract. 2009, 2009, 752401. [Google Scholar] [CrossRef][Green Version]
- Mackie, R.I.; Sghir, A.; Gaskins, H.R. Developmental Microbial Ecology of the Neonatal Gastrointestinal Tract. Am. J. Clin. Nutr. 1999, 69, 1035S–1045S. [Google Scholar] [CrossRef] [PubMed]
- Parkin, K.; Christophersen, C.T.; Verhasselt, V.; Cooper, M.N.; Martino, D. Risk Factors for Gut Dysbiosis in Early Life. Microorganisms 2021, 9, 2066. [Google Scholar] [CrossRef]
- Jiménez, E.; Marín, M.L.; Martín, R.; Odriozola, J.M.; Olivares, M.; Xaus, J.; Fernández, L.; Rodríguez, J.M. Is Meconium from Healthy Newborns Actually Sterile? Res. Microbiol. 2008, 159, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Ringel-Kulka, T.; Cheng, J.; Ringel, Y.; Salojärvi, J.; Carroll, I.; Palva, A.; de Vos, W.M.; Satokari, R. Intestinal Microbiota in Healthy U.S. Young Children and Adults—A High Throughput Microarray Analysis. PLoS ONE 2013, 8, e64315. [Google Scholar] [CrossRef]
- Agans, R.; Rigsbee, L.; Kenche, H.; Michail, S.; Khamis, H.J.; Paliy, O. Distal Gut Microbiota of Adolescent Children Is Different from That of Adults. FEMS Microbiol. Ecol. 2011, 77, 404–412. [Google Scholar] [CrossRef]
- Salazar, N.; Arboleya, S.; Fernández-Navarro, T.; de los Reyes-Gavilán, C.G.; Gonzalez, S.; Gueimonde, M. Age-Associated Changes in Gut Microbiota and Dietary Components Related with the Immune System in Adulthood and Old Age: A Cross-Sectional Study. Nutrients 2019, 11, 1765. [Google Scholar] [CrossRef]
- Tiihonen, K.; Ouwehand, A.C.; Rautonen, N. Human Intestinal Microbiota and Healthy Ageing. Ageing Res. Rev. 2010, 9, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Biagi, E.; Nylund, L.; Candela, M.; Ostan, R.; Bucci, L.; Pini, E.; Nikkïla, J.; Monti, D.; Satokari, R.; Franceschi, C.; et al. Through Ageing, and Beyond: Gut Microbiota and Inflammatory Status in Seniors and Centenarians. PLoS ONE 2010, 5, e10667. [Google Scholar] [CrossRef]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’connor, E.M.; Cusack, S.; Harris, H.M.B.; Coakley, M.; Lakshminarayanan, B.; O’sullivan, O.; et al. Gut Microbiota Composition Correlates with Diet and Health in the Elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef]
- Tamboli, C.P.; Neut, C.; Desreumaux, P.; Colombel, J.F. Dysbiosis in Inflammatory Bowel Disease. Gut 2004, 53, 1–4. [Google Scholar] [CrossRef]
- Degruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137. [Google Scholar] [CrossRef] [PubMed]
- Bien, J.; Palagani, V.; Bozko, P. The Intestinal Microbiota Dysbiosis and Clostridium Difficile Infection: Is There a Relationship with Inflammatory Bowel Disease? Ther. Adv. Gastroenterol. 2013, 6, 53–68. [Google Scholar] [CrossRef]
- Knights, D.; Lassen, K.G.; Xavier, R.J. Advances in Inflammatory Bowel Disease Pathogenesis: Linking Host Genetics and the Microbiome. Gut 2013, 62, 1505–1510. [Google Scholar] [CrossRef]
- Shanahan, F.; Bernstein, C.N. The Evolving Epidemiology of Inflammatory Bowel Disease. Curr. Opin. Gastroenterol. 2009, 25, 301–305. [Google Scholar] [CrossRef]
- Clayton, E.M.; Rea, M.C.; Shanahan, F.; Quigley, E.M.M.; Kiely, B.; Hill, C.; Ross, R.P. The Vexed Relationship between Clostridium Difficile and Inflammatory Bowel Disease: An Assessment of Carriage in an Outpatient Setting among Patients in Remission. Am. J. Gastroenterol. 2009, 104, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Butcher, J.; Mack, D.; Stintzi, A. Functional Impacts of the Intestinal Microbiome in the Pathogenesis of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 139–153. [Google Scholar] [CrossRef]
- Magwira, C.A.; Kullin, B.; Lewandowski, S.; Rodgers, A.; Reid, S.J.; Abratt, V.R. Diversity of Faecal Oxalate-Degrading Bacteria in Black and White South African Study Groups: Insights into Understanding the Rarity of Urolithiasis in the Black Group. J. Appl. Microbiol. 2012, 113, 418–428. [Google Scholar] [CrossRef]
- Sidhu, H.; Hoppe, B.; Hesse, A.; Tenbrock, K.; Brömme, S.; Rietschel, E.; Peck, A.B. Absence of Oxalobacter Formigenes in Cystic Fibrosis Patients: A Risk Factor for Hyperoxaluria. Lancet 1998, 352, 1026–1029. [Google Scholar] [CrossRef] [PubMed]
- Buie, T.; Fuchs, G.J.; Furuta, G.T.; Kooros, K.; Levy, J.; Lewis, J.D.; Wershil, B.K.; Winter, H. Recommendations for Evaluation and Treatment of Common Gastrointestinal Problems in Children with ASDs. Pediatrics 2010, 125 (Suppl. S1), S19–S29. [Google Scholar] [CrossRef]
- Mayer, E.A. Gut Feelings: The Emerging Biology of Gut-Brain Communication. Nat. Rev. Neurosci. 2011, 12, 453–466. [Google Scholar] [CrossRef]
- Giongo, A.; Gano, K.A.; Crabb, D.B.; Mukherjee, N.; Novelo, L.L.; Casella, G.; Drew, J.C.; Ilonen, J.; Knip, M.; Hyöty, H.; et al. Toward Defining the Autoimmune Microbiome for Type 1 Diabetes. ISME J. 2011, 5, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Arslan, N. Obesity, Fatty Liver Disease and Intestinal Microbiota. World J. Gastroenterol. 2014, 20, 16452–16463. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, I.; Tap, J.; Roudot-Thoraval, F.; Roperch, J.P.; Letulle, S.; Langella, P.; Gérard, C.; van Nhieu, J.T.; Furet, J.P. Microbial Dysbiosis in Colorectal Cancer (CRC) Patients. PLoS ONE 2011, 6, e16393. [Google Scholar] [CrossRef]
- Schulz, M.D.; Atay, Ç.; Heringer, J.; Romrig, F.K.; Schwitalla, S.; Aydin, B.; Ziegler, P.K.; Varga, J.; Reindl, W.; Pommerenke, C.; et al. High-Fat-Diet-Mediated Dysbiosis Promotes Intestinal Carcinogenesis Independently of Obesity. Nature 2014, 514, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, Y.; Cai, R.; Li, Y.; Gu, B. A Narrative Review of Relationship between Gut Microbiota and Neuropsychiatric Disorders: Mechanisms and Clinical Application of Probiotics and Prebiotics. Ann. Palliat. Med. 2021, 10, 2304–2313. [Google Scholar] [CrossRef]
- Liu, C.; Cheung, W.H.; Li, J.; Chow, S.K.H.; Yu, J.; Wong, S.H.; Ip, M.; Sung, J.J.Y.; Wong, R.M.Y. Understanding the Gut Microbiota and Sarcopenia: A Systematic Review. J. Cachexia Sarcopenia Muscle 2021, 12, 1393–1407. [Google Scholar] [CrossRef] [PubMed]
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Catania, P.; Prati, B.; Tana, C.; Meschi, T. Gut Microbiota, Muscle Mass and Function in Aging: A Focus on Physical Frailty and Sarcopenia. Nutrients 2019, 11, 1633. [Google Scholar] [CrossRef] [PubMed]
- Leiva-Gea, I.; Sánchez-Alcoholado, L.; Martín-Tejedor, B.; Castellano-Castillo, D.; Moreno-Indias, I.; Urda-Cardona, A.; Tinahones, F.J.; Fernández-García, J.C.; Queipo-Ortuño, M.I. Gut Microbiota Differs in Composition and Functionality Between Children with Type 1 Diabetes and MODY2 and Healthy Control Subjects: A Case-Control Study. Diabetes Care 2018, 41, 2385–2395. [Google Scholar] [CrossRef] [PubMed]
- Hugenholtz, F.; de Vos, W.M. Mouse Models for Human Intestinal Microbiota Research: A Critical Evaluation. Cell. Mol. Life Sci. 2018, 75, 149–160. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, E.; Egea-Zorrilla, A.; Plaza-Díaz, J.; Aragón-Vela, J.; Muñoz-Quezada, S.; Tercedor-Sánchez, L.; Abadia-Molina, F. The Gut Microbiota and Its Implication in the Development of Atherosclerosis and Related Cardiovascular Diseases. Nutrients 2020, 12, 605. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, F.; -Or-Rashid, M.H.; Al Mamun, A.; Rahaman, M.S.; Islam, M.M.; Meem, A.F.K.; Sutradhar, P.R.; Mitra, S.; Mimi, A.A.; et al. The Gut Microbiota (Microbiome) in Cardiovascular Disease and Its Therapeutic Regulation. Front. Cell. Infect. Microbiol. 2022, 12, 713. [Google Scholar] [CrossRef]
- Sen, P.; Molinero-Perez, A.; O’Riordan, K.J.; McCafferty, C.P.; O’Halloran, K.D.; Cryan, J.F. Microbiota and Sleep: Awakening the Gut Feeling. Trends Mol. Med. 2021, 27, 935–945. [Google Scholar] [CrossRef]
- Wei, S.; Bahl, M.I.; Baunwall, S.M.D.; Hvas, C.L.; Licht, T.R. Determining Gut Microbial Dysbiosis: A Review of Applied Indexes for Assessment of Intestinal Microbiota Imbalances. Appl. Environ. Microbiol. 2021, 87, e00395-21. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Zhang, X.; Liu, J.; Zhang, Q.; Zhao, Y.; Peng, J.; Feng, Q.; Dai, J.; Sun, S.; et al. Gut Microbial Dysbiosis Is Associated with Altered Hepatic Functions and Serum Metabolites in Chronic Hepatitis B Patients. Front. Microbiol. 2017, 8, 2222. [Google Scholar] [CrossRef]
- Tang, M.L.K.; Mullins, R.J. Food Allergy: Is Prevalence Increasing? Intern. Med. J. 2017, 47, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Henao-Mejia, J.; Henrickson, S.E. Obesity and Immune Status in Children. Curr. Opin. Pediatr. 2020, 32, 805–815. [Google Scholar] [CrossRef]
- Renz, H.; Skevaki, C. Early Life Microbial Exposures and Allergy Risks: Opportunities for Prevention. Nat. Rev. Immunol. 2020, 21, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Dharmage, S.C.; Perret, J.L.; Custovic, A. Epidemiology of Asthma in Children and Adults. Front. Pediatr. 2019, 7, 246. [Google Scholar] [CrossRef]
- Gou, W.; Fu, Y.; Yue, L.; Chen, G.; Cai, X.; Shuai, M.; Xu, F.; Yi, X.; Chen, H.; Zhu, Y.; et al. Gut Microbiota May Underlie the Predisposition of Healthy Individuals to COVID-19. medRxiv 2020. [Google Scholar] [CrossRef]
- Cai, C.; Zhang, X.; Liu, Y.; Shen, E.; Feng, Z.; Guo, C.; Han, Y.; Ouyang, Y.; Shen, H. Gut Microbiota Imbalance in Colorectal Cancer Patients, the Risk Factor of COVID-19 Mortality. Gut Pathog. 2021, 13, 70. [Google Scholar] [CrossRef]
- Borgo, F.; Riva, A.; Benetti, A.; Casiraghi, M.C.; Bertelli, S.; Garbossa, S.; Anselmetti, S.; Scarone, S.; Pontiroli, A.E.; Morace, G.; et al. Microbiota in Anorexia Nervosa: The Triangle between Bacterial Species, Metabolites and Psychological Tests. PLoS ONE 2017, 12, e0179739. [Google Scholar] [CrossRef] [PubMed]
- Armougom, F.; Henry, M.; Vialettes, B.; Raccah, D.; Raoult, D. Monitoring Bacterial Community of Human Gut Microbiota Reveals an Increase in Lactobacillus in Obese Patients and Methanogens in Anorexic Patients. PLoS ONE 2009, 4, e7125. [Google Scholar] [CrossRef]
- Genton, L.; Cani, P.D.; Schrenzel, J. Alterations of Gut Barrier and Gut Microbiota in Food Restriction, Food Deprivation and Protein-Energy Wasting. Clin. Nutr. 2015, 34, 341–349. [Google Scholar] [CrossRef]
- Stecher, B.; Robbiani, R.; Walker, A.W.; Westendorf, A.M.; Barthel, M.; Kremer, M.; Chaffron, S.; Macpherson, A.J.; Buer, J.; Parkhill, J.; et al. Salmonella Enterica Serovar Typhimurium Exploits Inflammation to Compete with the Intestinal Microbiota. PLoS Biol. 2007, 5, 2177–2189. [Google Scholar] [CrossRef]
- Khosravi, A.; Mazmanian, S.K. Disruption of the Gut Microbiome as a Risk Factor for Microbial Infections. Curr. Opin. Microbiol. 2013, 16, 221. [Google Scholar] [CrossRef]
- Lupp, C.; Robertson, M.L.; Wickham, M.E.; Sekirov, I.; Champion, O.L.; Gaynor, E.C.; Finlay, B.B. Host-Mediated Inflammation Disrupts the Intestinal Microbiota and Promotes the Overgrowth of Enterobacteriaceae. Cell Host Microbe 2007, 2, 119–129. [Google Scholar] [CrossRef]
- Sekirov, I.; Tam, N.M.; Jogova, M.; Robertson, M.L.; Li, Y.; Lupp, C.; Finlay, B.B. Antibiotic-Induced Perturbations of the Intestinal Microbiota Alter Host Susceptibility to Enteric Infection. Infect. Immun. 2008, 76, 4726–4736. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humarán, L.G.; Gratadoux, J.J.; Blugeon, S.; Bridonneau, C.; Furet, J.P.; Corthier, G.; et al. Faecalibacterium Prausnitzii Is an Anti-Inflammatory Commensal Bacterium Identified by Gut Microbiota Analysis of Crohn Disease Patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Donat, E.; Ribes-Koninckx, C.; Calabuig, M.; Sanz, Y. Specific Duodenal and Faecal Bacterial Groups Associated with Paediatric Coeliac Disease. J. Clin. Pathol. 2009, 62, 264–269. [Google Scholar] [CrossRef]
- de Palma, G.; Nadal, I.; Medina, M.; Donat, E.; Ribes-Koninckx, C.; Calabuig, M.; Sanz, Y. Intestinal Dysbiosis and Reduced Immunoglobulin-Coated Bacteria Associated with Coeliac Disease in Children. BMC Microbiol. 2010, 10, 63. [Google Scholar] [CrossRef]
- Nadal, I.; Donant, E.; Ribes-Koninckx, C.; Calabuig, M.; Sanz, Y. Imbalance in the Composition of the Duodenal Microbiota of Children with Coeliac Disease. J. Med. Microbiol. 2007, 56, 1669–1674. [Google Scholar] [CrossRef]
- Collado, M.C.; Donat, E.; Ribes-Koninckx, C.; Calabuig, M.; Sanz, Y. Imbalances in Faecal and Duodenal Bifidobacterium Species Composition in Active and Non-Active Coeliac Disease. BMC Microbiol. 2008, 8, 232. [Google Scholar] [CrossRef]
- Wang, T.; Cai, G.; Qiu, Y.; Fei, N.; Zhang, M.; Pang, X.; Jia, W.; Cai, S.; Zhao, L. Structural Segregation of Gut Microbiota between Colorectal Cancer Patients and Healthy Volunteers. ISME J. 2011, 6, 320–329. [Google Scholar] [CrossRef]
- Kostic, A.D.; Gevers, D.; Pedamallu, C.S.; Michaud, M.; Duke, F.; Earl, A.M.; Ojesina, A.I.; Jung, J.; Bass, A.J.; Tabernero, J.; et al. Genomic Analysis Identifies Association of Fusobacterium with Colorectal Carcinoma. Genome Res. 2012, 22, 292–298. [Google Scholar] [CrossRef]
- Belizário, J.E.; Faintuch, J. Microbiome and Gut Dysbiosis. Exp. Suppl. 2018, 109, 459–476. [Google Scholar] [CrossRef]
- Lange, K.; Buerger, M.; Stallmach, A.; Bruns, T. Effects of Antibiotics on Gut Microbiota. Dig. Dis. 2016, 34, 260–268. [Google Scholar] [CrossRef]
- de Jonge, L.; Bos, H.J.; van Langen, I.M.; de Jong-van den Berg, L.T.W.; Bakker, M.K. Antibiotics Prescribed before, during and after Pregnancy in the Netherlands: A Drug Utilization Study. Pharmacoepidemiol. Drug Saf. 2014, 23, 60–68. [Google Scholar] [CrossRef]
- Petersen, I.; Gilbert, R.; Evans, S.; Ridolfi, A.; Nazareth, I. Oral Antibiotic Prescribing during Pregnancy in Primary Care: UK Population-Based Study. J. Antimicrob. Chemother. 2010, 65, 2238–2246. [Google Scholar] [CrossRef]
- Braye, K.; Foureur, M.; de Waal, K.; Jones, M.; Putt, E.; Ferguson, J. Group B Streptococcal Screening, Intrapartum Antibiotic Prophylaxis, and Neonatal Early-Onset Infection Rates in an Australian Local Health District: 2006–2016. PLoS ONE 2019, 14, e0214295. [Google Scholar] [CrossRef]
- Tapiainen, T.; Koivusaari, P.; Brinkac, L.; Lorenzi, H.A.; Salo, J.; Renko, M.; Pruikkonen, H.; Pokka, T.; Li, W.; Nelson, K.; et al. Impact of Intrapartum and Postnatal Antibiotics on the Gut Microbiome and Emergence of Antimicrobial Resistance in Infants. Sci. Rep. 2019, 9, 10635. [Google Scholar] [CrossRef]
- Dethlefsen, L.; Huse, S.; Sogin, M.L.; Relman, D.A. The Pervasive Effects of an Antibiotic on the Human Gut Microbiota, as Revealed by Deep 16S RRNA Sequencing. PLoS Biol. 2008, 6, e280. [Google Scholar] [CrossRef]
- de La Cochetière, M.F.; Durand, T.; Lepage, P.; Bourreille, A.; Galmiche, J.P.; Doré, J. Resilience of the Dominant Human Fecal Microbiota upon Short-Course Antibiotic Challenge. J. Clin. Microbiol. 2005, 43, 5588–5592. [Google Scholar] [CrossRef]
- Raymond, F.; Ouameur, A.A.; Déraspe, M.; Iqbal, N.; Gingras, H.; Dridi, B.; Leprohon, P.; Plante, P.L.; Giroux, R.; Bérubé, È.; et al. The Initial State of the Human Gut Microbiome Determines Its Reshaping by Antibiotics. ISME J. 2015, 10, 707–720. [Google Scholar] [CrossRef]
- Dethlefsen, L.; Relman, D.A. Incomplete Recovery and Individualized Responses of the Human Distal Gut Microbiota to Repeated Antibiotic Perturbation. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4554–4561. [Google Scholar] [CrossRef]
- Löfmark, S.; Jernberg, C.; Jansson, J.K.; Edlund, C. Clindamycin-Induced Enrichment and Long-Term Persistence of Resistant Bacteroides Spp. and Resistance Genes. J. Antimicrob. Chemother. 2006, 58, 1160–1167. [Google Scholar] [CrossRef]
- Jernberg, C.; Löfmark, S.; Edlund, C.; Jansson, J.K. Long-Term Ecological Impacts of Antibiotic Administration on the Human Intestinal Microbiota. ISME J. 2007, 1, 56–66. [Google Scholar] [CrossRef]
- Lindgren, M.; Löfmark, S.; Edlund, C.; Huovinen, P.; Jalava, J. Prolonged Impact of a One-Week Course of Clindamycin on Enterococcus Spp. in Human Normal Microbiota. Scand. J. Infect. Dis. 2009, 41, 215–219. [Google Scholar] [CrossRef]
- Blaser, M. Antibiotic Overuse: Stop the Killing of Beneficial Bacteria. Nature 2011, 476, 393–394. [Google Scholar] [CrossRef]
- Berg, G.; Köberl, M.; Rybakova, D.; Müller, H.; Grosch, R.; Smalla, K. Plant Microbial Diversity Is Suggested as the Key to Future Biocontrol and Health Trends. FEMS Microbiol. Ecol. 2017, 93, 50. [Google Scholar] [CrossRef]
- Schwartz, D.J.; Langdon, A.E.; Dantas, G. Understanding the Impact of Antibiotic Perturbation on the Human Microbiome. Genome Med. 2020, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Proctor, L. Priorities for the next 10 Years of Human Microbiome Research. Nature 2019, 569, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.; Guarner, F.; Bustos Fernandez, L.; Maruy, A.; Sdepanian, V.L.; Cohen, H. Antibiotics as Major Disruptors of Gut Microbiota. Front. Cell. Infect. Microbiol. 2020, 10, 572912. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.J. Intestinal Flora and Endogenous Vitamin Synthesis. Eur. J. Cancer Prev. 1997, 6 (Suppl. S1), S43–S45. [Google Scholar] [CrossRef] [PubMed]
- Conly, J.; Stein, K.; Worobetz, L.; Rutledge-Harding, S. The Contribution of Vitamin K2 (Menaquinones) Produced by the Intestinal Microflora to Human Nutritional Requirements for Vitamin K. Am. J. Gastroenterol. 1994, 89, 915. [Google Scholar] [PubMed]
- Younes, H.; Coudray, C.; Bellanger, J.; Demigné, C.; Rayssiguier, Y.; Rémésy, C. Effects of Two Fermentable Carbohydrates (Inulin and Resistant Starch) and Their Combination on Calcium and Magnesium Balance in Rats. Br. J. Nutr. 2001, 86, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, E.; Iwabuchi, A.; Yoshida, T. Phytate Breakdown and Apparent Absorption of Phosphorus, Calcium and Magnesium in Germfree and Conventionalized Rats. Nutr. Res. 1996, 16, 603–613. [Google Scholar] [CrossRef]
- Roberfroid, M.B.; Bornet, F.; Bouley, C.; Cummings, J.H. Colonic Microflora: Nutrition and Health. Summary and Conclusions of an International Life Sciences Institute (ILSI) [Europe] Workshop Held in Barcelona, Spain. Nutr. Rev. 1995, 53, 127–130. [Google Scholar] [CrossRef]
- Guarner, F.; Malagelada, J.R. Gut Flora in Health and Disease. Lancet 2003, 361, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Midtvedt, T.; Uribe, A. Differential Cell Kinetics in the Ileum and Colon of Germfree Rats. Scand. J. Gastroenterol. 1994, 29, 445–451. [Google Scholar] [CrossRef]
- Gibson, P.R.; Moeller, I.; Kagelari, O.; Folino, M.; Young, G.P. Contrasting Effects of Butyrate on the Expression of Phenotypic Markers of Differentiation in Neoplastic and Non-Neoplastic Colonic Epithelial Cells in Vitro. J. Gastroenterol. Hepatol. 1992, 7, 165–172. [Google Scholar] [CrossRef]
- Bercik, P.; Collins, S.M.; Verdu, E.F. Microbes and the Gut-Brain Axis. Neurogastroenterol. Motil. 2012, 24, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Burokas, A.; Moloney, R.D.; Dinan, T.G.; Cryan, J.F. Microbiota Regulation of the Mammalian Gut-Brain Axis. Adv. Appl. Microbiol. 2015, 91, 1–62. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.T. Dysfunction of the Microbiota-Gut-Brain Axis in Neurodegenerative Disease: The Promise of Therapeutic Modulation with Prebiotics, Medicinal Herbs, Probiotics, and Synbiotics. J. Evid. Based Integr. Med. 2020, 25, 2515690X20957225. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R.; Mazmanian, S.K. Control of Brain Development, Function, and Behavior by the Microbiome. Cell Host Microbe 2015, 17, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Bonaz, B.; Bazin, T.; Pellissier, S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain-Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Ahlman, H.; Nilsson, O. The Gut as the Largest Endocrine Organ in the Body. Ann. Oncol. 2001, 12 (Suppl. S2), S63–S68. [Google Scholar] [CrossRef] [PubMed]
- Erny, D.; de Angelis, A.L.H.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host Microbiota Constantly Control Maturation and Function of Microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Noble, E.E.; Hsu, T.M.; Kanoski, S.E. Gut to Brain Dysbiosis: Mechanisms Linking Western Diet Consumption, the Microbiome, and Cognitive Impairment. Front. Behav. Neurosci. 2017, 11, 9. [Google Scholar] [CrossRef]
- Bourassa, M.W.; Alim, I.; Bultman, S.J.; Ratan, R.R. Butyrate, Neuroepigenetics and the Gut Microbiome: Can a High Fiber Diet Improve Brain Health? Neurosci. Lett. 2016, 625, 56–63. [Google Scholar] [CrossRef]
- Kinross, J.; Nicholson, J.K. Gut Microbiota: Dietary and Social Modulation of Gut Microbiota in the Elderly. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 563–564. [Google Scholar] [CrossRef]
- Claesson, M.J.; Cusack, S.; O’Sullivan, O.; Greene-Diniz, R.; de Weerd, H.; Flannery, E.; Marchesi, J.R.; Falush, D.; Dinan, T.; Fitzgerald, G.; et al. Composition, Variability, and Temporal Stability of the Intestinal Microbiota of the Elderly. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4586–4591. [Google Scholar] [CrossRef]
- Prusiner, S.B. Cell Biology. A Unifying Role for Prions in Neurodegenerative Diseases. Science 2012, 336, 1511–1513. [Google Scholar] [CrossRef]
- Kaufman, S.K.; Sanders, D.W.; Thomas, T.L.; Ruchinskas, A.J.; Vaquer-Alicea, J.; Sharma, A.M.; Miller, T.M.; Diamond, M.I. Tau Prion Strains Dictate Patterns of Cell Pathology, Progression Rate, and Regional Vulnerability In Vivo. Neuron 2016, 92, 796–812. [Google Scholar] [CrossRef]
- Liu, L.; Xu, J.; Zhang, Z.; Ren, D.; Wu, Y.; Wang, D.; Zhang, Y.; Zhao, S.; Chen, Q.; Wang, T. Metabolic Homeostasis of Amino Acids and Diabetic Kidney Disease. Nutrients 2023, 15, 184. [Google Scholar] [CrossRef]
- Gerdes, V.; Gueimonde, M.; Pajunen, L.; Nieuwdorp, M.; Laitinen, K. How Strong Is the Evidence That Gut Microbiota Composition Can Be Influenced by Lifestyle Interventions in a Cardio-Protective Way? Atherosclerosis 2020, 311, 124–142. [Google Scholar] [CrossRef]
- Suganya, K.; Son, T.; Kim, K.W.; Koo, B.S. Impact of Gut Microbiota: How It Could Play Roles beyond the Digestive System on Development of Cardiovascular and Renal Diseases. Microb. Pathog. 2021, 152, 104583. [Google Scholar] [CrossRef]
- Senthong, V.; Wang, Z.; Li, X.S.; Fan, Y.; Wu, Y.; Tang, W.H.W.; Hazen, S.L. Intestinal Microbiota-Generated Metabolite Trimethylamine-N-Oxide and 5-Year Mortality Risk in Stable Coronary Artery Disease: The Contributory Role of Intestinal Microbiota in a COURAGE-Like Patient Cohort. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2016, 5, e002816. [Google Scholar] [CrossRef]
- Roncal, C.; Martínez-Aguilar, E.; Orbe, J.; Ravassa, S.; Fernandez-Montero, A.; Saenz-Pipaon, G.; Ugarte, A.; Estella-Hermoso de Mendoza, A.; Rodriguez, J.A.; Fernández-Alonso, S.; et al. Trimethylamine-N-Oxide (TMAO) Predicts Cardiovascular Mortality in Peripheral Artery Disease. Sci. Rep. 2019, 9, 15580. [Google Scholar] [CrossRef]
- Yao, M.E.; da Liao, P.; Zhao, X.J.; Wang, L. Trimethylamine-N-Oxide Has Prognostic Value in Coronary Heart Disease: A Meta-Analysis and Dose-Response Analysis. BMC Cardiovasc. Disord. 2020, 20, 7. [Google Scholar] [CrossRef]
- Haghikia, A.; Li, X.S.; Liman, T.G.; Bledau, N.; Schmidt, D.; Zimmermann, F.; Kränkel, N.; Widera, C.; Sonnenschein, K.; Haghikia, A.; et al. Gut Microbiota-Dependent TMAO Predicts Risk of Cardiovascular Events in Patients with Stroke and Is Related to Proinflammatory Monocytes. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2225. [Google Scholar] [CrossRef]
- Li, M.; van Esch, B.C.A.M.; Wagenaar, G.T.M.; Garssen, J.; Folkerts, G.; Henricks, P.A.J. Pro- and Anti-Inflammatory Effects of Short Chain Fatty Acids on Immune and Endothelial Cells. Eur. J. Pharmacol. 2018, 831, 52–59. [Google Scholar] [CrossRef]
- Kuipers, F.; Bloks, V.W.; Groen, A.K. Beyond Intestinal Soap—Bile Acids in Metabolic Control. Nat. Rev. Endocrinol. 2014, 10, 488–498. [Google Scholar] [CrossRef]
- Döring, B.; Lütteke, T.; Geyer, J.; Petzinger, E. The SLC10 Carrier Family: Transport Functions and Molecular Structure. Curr. Top. Membr. 2012, 70, 105–168. [Google Scholar] [CrossRef]
- Prasad, K.N.; Bondy, S.C. Dietary Fibers and Their Fermented Short-Chain Fatty Acids in Prevention of Human Diseases. Mech. Ageing Dev. 2018; withdrawn article in press. [Google Scholar] [CrossRef]
- Ramos, S.C.; Fonseca, F.A.; Kasmas, S.H.; Moreira, F.T.; Helfenstein, T.; Borges, N.C.; Moreno, R.A.; Rezende, V.M.; Silva, F.C.; Izar, M.C. The Role of Soluble Fiber Intake in Patients under Highly Effective Lipid-Lowering Therapy. Nutr. J. 2011, 10, 80. [Google Scholar] [CrossRef]
- Brown, L.; Rosner, B.; Willett, W.W.; Sacks, F.M. Cholesterol-Lowering Effects of Dietary Fiber: A Meta-Analysis. Am. J. Clin. Nutr. 1999, 69, 30–42. [Google Scholar] [CrossRef]
- Kim, Y.; Je, Y. Dietary Fibre Intake and Mortality from Cardiovascular Disease and All Cancers: A Meta-Analysis of Prospective Cohort Studies. Arch. Cardiovasc. Dis. 2016, 109, 39–54. [Google Scholar] [CrossRef]
- Soliman, G.A. Dietary Fiber, Atherosclerosis, and Cardiovascular Disease. Nutrients 2019, 11, 1155. [Google Scholar] [CrossRef]
- Rebello, C.J.; O’Neil, C.E.; Greenway, F.L. Dietary Fiber and Satiety: The Effects of Oats on Satiety. Nutr. Rev. 2016, 74, 131–147. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Oniszczuk, T.; Gancarz, M.; Szymańska, J. Role of Gut Microbiota, Probiotics and Prebiotics in the Cardiovascular Diseases. Molecules 2021, 26, 1172. [Google Scholar] [CrossRef]
- Picca, A.; Fanelli, F.; Calvani, R.; Mulè, G.; Pesce, V.; Sisto, A.; Pantanelli, C.; Bernabei, R.; Landi, F.; Marzetti, E. Gut Dysbiosis and Muscle Aging: Searching for Novel Targets against Sarcopenia. Mediat. Inflamm. 2018, 2018, 7026198. [Google Scholar] [CrossRef]
- Bindels, L.B.; Delzenne, N.M. Muscle Wasting: The Gut Microbiota as a New Therapeutic Target? Int. J. Biochem. Cell. Biol. 2013, 45, 2186–2190. [Google Scholar] [CrossRef]
- Ni, Y.; Yang, X.; Zheng, L.; Wang, Z.; Wu, L.; Jiang, J.; Yang, T.; Ma, L.; Fu, Z. Lactobacillus and Bifidobacterium Improves Physiological Function and Cognitive Ability in Aged Mice by the Regulation of Gut Microbiota. Mol. Nutr. Food Res. 2019, 63, e1900603. [Google Scholar] [CrossRef]
- Prokopidis, K.; Giannos, P.; Kirwan, R.; Ispoglou, T.; Galli, F.; Witard, O.C.; Triantafyllidis, K.K.; Kechagias, K.S.; Morwani-Mangnani, J.; Ticinesi, A.; et al. Impact of Probiotics on Muscle Mass, Muscle Strength and Lean Mass: A Systematic Review and Meta-analysis of Randomized Controlled Trials. J. Cachexia Sarcopenia Muscle 2023, 14, 30. [Google Scholar] [CrossRef]
- Lustgarten, M.S. The Role of the Gut Microbiome on Skeletal Muscle Mass and Physical Function: 2019 Update. Front. Physiol. 2019, 10, 500047. [Google Scholar] [CrossRef]
- Flint, H.J. The Impact of Nutrition on the Human Microbiome. Nutr. Rev. 2012, 70 (Suppl. S1), S10–S13. [Google Scholar] [CrossRef]
- Villa, C.R.; Ward, W.E.; Comelli, E.M. Gut Microbiota-Bone Axis. Crit. Rev. Food Sci. Nutr. 2017, 57, 1664–1672. [Google Scholar] [CrossRef]
- Whisner, C.M.; Castillo, L.F. Prebiotics, Bone and Mineral Metabolism. Calcif. Tissue Int. 2018, 102, 443–479. [Google Scholar] [CrossRef]
- Weaver, C.M. Diet, Gut Microbiome, and Bone Health. Curr. Osteoporos. Rep. 2015, 13, 125–130. [Google Scholar] [CrossRef]
- McCabe, L.; Britton, R.A.; Parameswaran, N. Prebiotic and Probiotic Regulation of Bone Health: Role of the Intestine and Its Microbiome. Curr. Osteoporos. Rep. 2015, 13, 363–371. [Google Scholar] [CrossRef]
- Bryk, G.; Coronel, M.Z.; Pellegrini, G.; Mandalunis, P.; Rio, M.E.; de Portela, M.L.P.M.; Zeni, S.N. Effect of a Combination GOS/FOS® Prebiotic Mixture and Interaction with Calcium Intake on Mineral Absorption and Bone Parameters in Growing Rats. Eur. J. Nutr. 2015, 54, 913–923. [Google Scholar] [CrossRef]
- Ibrahim, I.; Syamala, S.; Ayariga, J.A.; Xu, J.; Robertson, B.K.; Meenakshisundaram, S.; Ajayi, O.S. Modulatory Effect of Gut Microbiota on the Gut-Brain, Gut-Bone Axes, and the Impact of Cannabinoids. Metabolites 2022, 12, 1247. [Google Scholar] [CrossRef]
- Pacifici, R. Bone Remodeling and the Microbiome. Cold Spring Harb. Perspect. Med. 2018, 8, a031203. [Google Scholar] [CrossRef]
- Hsu, E.; Pacifici, R. From Osteoimmunology to Osteomicrobiology: How the Microbiota and the Immune System Regulate Bone. Calcif. Tissue Int. 2017, 102, 512–521. [Google Scholar] [CrossRef]
- Hair, R.; Sakaki, J.R.; Chun, O.K. Anthocyanins, Microbiome and Health Benefits in Aging. Molecules 2021, 26, 537. [Google Scholar] [CrossRef]
- Poinsot, P.; Schwarzer, M.; Peretti, N.; Leulier, F. 40 YEARS OF IGF1: The Emerging Connections between IGF1, the Intestinal Microbiome, Lactobacillus Strains and Bone Growth. J. Mol. Endocrinol. 2018, 61, T103–T113. [Google Scholar] [CrossRef]
- Li, J.Y.; Yu, M.; Pal, S.; Tyagi, A.M.; Dar, H.; Adams, J.; Neale Weitzmann, M.; Jones, R.M.; Pacifici, R. Parathyroid Hormone–Dependent Bone Formation Requires Butyrate Production by Intestinal Microbiota. J. Clin. Investig. 2020, 130, 1767–1781. [Google Scholar] [CrossRef]
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How Colonization by Microbiota in Early Life Shapes the Immune System. Science 2016, 352, 539–544. [Google Scholar] [CrossRef]
- Zhao, Q.; Elson, C.O. Adaptive Immune Education by Gut Microbiota Antigens. Immunology 2018, 154, 28–37. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The Microbiome and Innate Immunity. Nature 2016, 535, 65–74. [Google Scholar] [CrossRef]
- Artis, D.; Spits, H. The Biology of Innate Lymphoid Cells. Nature 2015, 517, 293–301. [Google Scholar] [CrossRef]
- Callaway, E. C-Section Babies Are Missing Key Microbes. Nature 2019. [Google Scholar] [CrossRef]
- Darabi, B.; Rahmati, S.; Hafeziahmadi, M.R.; Badfar, G.; Azami, M. The Association between Caesarean Section and Childhood Asthma: An Updated Systematic Review and Meta-Analysis. Allergy Asthma Clin. Immunol. 2019, 15, 1–13. [Google Scholar] [CrossRef]
- Watane, A.; Raolji, S.; Cavuoto, K.; Galor, A. Microbiome and Immune-Mediated Dry Eye: A Review. BMJ Open Ophthalmol. 2022, 7, e000956. [Google Scholar] [CrossRef]
- Gérard, P. Gut Microbiota and Obesity. Cell. Mol. Life Sci. 2015, 73, 147–162. [Google Scholar] [CrossRef]
- Lecomte, V.; Kaakoush, N.O.; Maloney, C.A.; Raipuria, M.; Huinao, K.D.; Mitchell, H.M.; Morris, M.J. Changes in Gut Microbiota in Rats Fed a High Fat Diet Correlate with Obesity-Associated Metabolic Parameters. PLoS ONE 2015, 10, e0126931. [Google Scholar] [CrossRef]
- Cani, P.D. Gut Microbiota and Obesity: Lessons from the Microbiome. Brief. Funct. Genom. 2013, 12, 381–387. [Google Scholar] [CrossRef]
- Million, M.; Angelakis, E.; Maraninchi, M.; Henry, M.; Giorgi, R.; Valero, R.; Vialettes, B.; Raoult, D. Correlation between Body Mass Index and Gut Concentrations of Lactobacillus Reuteri, Bifidobacterium Animalis, Methanobrevibacter Smithii and Escherichia Coli. Int. J. Obes. 2013, 37, 1460–1466. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, M.; Wang, S.; Han, R.; Cao, Y.; Hua, W.; Mao, Y.; Zhang, X.; Pang, X.; Wei, C.; et al. Interactions between Gut Microbiota, Host Genetics and Diet Relevant to Development of Metabolic Syndromes in Mice. ISME J. 2010, 4, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Kasai, C.; Sugimoto, K.; Moritani, I.; Tanaka, J.; Oya, Y.; Inoue, H.; Tameda, M.; Shiraki, K.; Ito, M.; Takei, Y.; et al. Comparison of the Gut Microbiota Composition between Obese and Non-Obese Individuals in a Japanese Population, as Analyzed by Terminal Restriction Fragment Length Polymorphism and next-Generation Sequencing. BMC Gastroenterol. 2015, 15, 100. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-Talk between Akkermansia Muciniphila and Intestinal Epithelium Controls Diet-Induced Obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Sen Gupta, S.; Bhattacharya, T.; Yadav, D.; Barik, A.; Chowdhury, A.; Das, B.; Mande, S.S.; Nair, G.B. Gut Microbiomes of Indian Children of Varying Nutritional Status. PLoS ONE 2014, 9, e95547. [Google Scholar] [CrossRef] [PubMed]
- Million, M.; Diallo, A.; Raoult, D. Gut Microbiota and Malnutrition. Microb. Pathog. 2017, 106, 127–138. [Google Scholar] [CrossRef]
- Monira, S.; Nakamura, S.; Gotoh, K.; Izutsu, K.; Watanabe, H.; Alam, N.H.; Endtz, H.P.; Cravioto, A.; Ali, S.I.; Nakaya, T.; et al. Gut Microbiota of Healthy and Malnourished Children in Bangladesh. Front. Microbiol. 2011, 2, 228. [Google Scholar] [CrossRef]
- Wang, J.; Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; et al. A Metagenome-Wide Association Study of Gut Microbiota in Type 2 Diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut Microbiota in Human Adults with Type 2 Diabetes Differs from Non-Diabetic Adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef]
- Boulangé, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M.E. Impact of the Gut Microbiota on Inflammation, Obesity, and Metabolic Disease. Genome Med. 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Ye, L.; Li, J.; Jin, L.; Wang, W.; Li, S.; Bao, M.; Wu, S.; Li, L.; Geng, B.; et al. Metagenomic and Metabolomic Analyses Unveil Dysbiosis of Gut Microbiota in Chronic Heart Failure Patients. Sci. Rep. 2018, 8, 635. [Google Scholar] [CrossRef]
- Kummen, M.; Mayerhofer, C.C.K.; Vestad, B.; Broch, K.; Awoyemi, A.; Storm-Larsen, C.; Ueland, T.; Yndestad, A.; Hov, J.R.; Trøseid, M. Gut Microbiota Signature in Heart Failure Defined From Profiling of 2 Independent Cohorts. J. Am. Coll. Cardiol. 2018, 71, 1184–1186. [Google Scholar] [CrossRef]
- Katsimichas, T.; Ohtani, T.; Motooka, D.; Tsukamoto, Y.; Kioka, H.; Nakamoto, K.; Konishi, S.; Chimura, M.; Sengoku, K.; Miyawaki, H.; et al. Non-Ischemic Heart Failure with Reduced Ejection Fraction Is Associated with Altered Intestinal Microbiota. Circ. J. 2018, 82, 1640–1650. [Google Scholar] [CrossRef]
- Zhu, Q.; Gao, R.; Zhang, Y.; Pan, D.; Zhu, Y.; Zhang, X.; Yang, R.; Jiang, R.; Xu, Y.; Qin, H. Dysbiosis Signatures of Gut Microbiota in Coronary Artery Disease. Physiol. Genom. 2018, 50, 893–903. [Google Scholar] [CrossRef]
- Li, J.; Zhao, F.; Wang, Y.; Chen, J.; Tao, J.; Tian, G.; Wu, S.; Liu, W.; Cui, Q.; Geng, B.; et al. Gut Microbiota Dysbiosis Contributes to the Development of Hypertension. Microbiome 2017, 5, 14. [Google Scholar] [CrossRef]
- Yan, Q.; Gu, Y.; Li, X.; Yang, W.; Jia, L.; Chen, C.; Han, X.; Huang, Y.; Zhao, L.; Li, P.; et al. Alterations of the Gut Microbiome in Hypertension. Front. Cell. Infect. Microbiol. 2017, 7, 381. [Google Scholar] [CrossRef]
- Jie, Z.; Xia, H.; Zhong, S.L.; Feng, Q.; Li, S.; Liang, S.; Zhong, H.; Liu, Z.; Gao, Y.; Zhao, H.; et al. The Gut Microbiome in Atherosclerotic Cardiovascular Disease. Nat. Commun. 2017, 8, 845. [Google Scholar] [CrossRef]
- Salonen, A.; de Vos, W.M.; Palva, A. Gastrointestinal Microbiota in Irritable Bowel Syndrome: Present State and Perspectives. Microbiology 2010, 156, 3205–3215. [Google Scholar] [CrossRef]
- Bhattarai, Y.; Muniz Pedrogo, D.A.; Kashyap, P.C. Irritable Bowel Syndrome: A Gut Microbiota-Related Disorder? Am. J. Physiol.-Gastrointest. Liver Physiol. 2017, 312, G52–G62. [Google Scholar] [CrossRef]
- Carroll, I.M.; Chang, Y.H.; Park, J.; Sartor, R.B.; Ringel, Y. Luminal and Mucosal-Associated Intestinal Microbiota in Patients with Diarrhea-Predominant Irritable Bowel Syndrome. Gut Pathog. 2010, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Krogius-Kurikka, L.; Lyra, A.; Malinen, E.; Aarnikunnas, J.; Tuimala, J.; Paulin, L.; Mäkivuokko, H.; Kajander, K.; Palva, A. Microbial Community Analysis Reveals High Level Phylogenetic Alterations in the Overall Gastrointestinal Microbiota of Diarrhoea-Predominant Irritable Bowel Syndrome Sufferers. BMC Gastroenterol. 2009, 9, 95. [Google Scholar] [CrossRef]
- Machiels, K.; Joossens, M.; Sabino, J.; de Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; van Immerseel, F.; Verbeke, K.; et al. A Decrease of the Butyrate-Producing Species Roseburia Hominis and Faecalibacterium Prausnitzii Defines Dysbiosis in Patients with Ulcerative Colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef]
- Shen, X.J.; Rawls, J.F.; Randall, T.; Burcal, L.; Mpande, C.N.; Jenkins, N.; Jovov, B.; Abdo, Z.; Sandler, R.S.; Keku, T.O. Molecular Characterization of Mucosal Adherent Bacteria and Associations with Colorectal Adenomas. Gut Microbes 2010, 1, 138. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Heuman, D.M.; Hylemon, P.B.; Sanyal, A.J.; White, M.B.; Monteith, P.; Noble, N.A.; Unser, A.B.; Daita, K.; Fisher, A.R.; et al. Altered Profile of Human Gut Microbiome Is Associated with Cirrhosis and Its Complications. J. Hepatol. 2014, 60, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ji, F.; Guo, J.; Shi, D.; Fang, D.; Li, L. Dysbiosis of Small Intestinal Microbiota in Liver Cirrhosis and Its Association with Etiology. Sci. Rep. 2016, 6, 34055. [Google Scholar] [CrossRef]
- Mohamadkhani, A. On the Potential Role of Intestinal Microbial Community in Hepatocarcinogenesis in Chronic Hepatitis B. Cancer Med. 2018, 7, 3095–3100. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Nakayama, J.; Moriya, K.; Kawaratani, H.; Momoda, R.; Ito, K.; Iio, E.; Nojiri, S.; Fujiwara, K.; Yoneda, M.; et al. Gut Dysbiosis Associated with Hepatitis C Virus Infection. Clin. Infect. Dis. 2018, 67, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Morris, A.; Ghedin, E. The Human Mycobiome in Health and Disease. Genome Med. 2013, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Sanduzzi Zamparelli, M.; Rocco, A.; Compare, D.; Nardone, G. The Gut Microbiota: A New Potential Driving Force in Liver Cirrhosis and Hepatocellular Carcinoma. United Eur. Gastroenterol. J. 2017, 5, 944–953. [Google Scholar] [CrossRef]
- Morita, C.; Tsuji, H.; Hata, T.; Gondo, M.; Takakura, S.; Kawai, K.; Yoshihara, K.; Ogata, K.; Nomoto, K.; Miyazaki, K.; et al. Gut Dysbiosis in Patients with Anorexia Nervosa. PLoS ONE 2015, 10, e0145274. [Google Scholar] [CrossRef] [PubMed]
- Pfleiderer, A.; Lagier, J.C.; Armougom, F.; Robert, C.; Vialettes, B.; Raoult, D. Culturomics Identified 11 New Bacterial Species from a Single Anorexia Nervosa Stool Sample. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1471–1481. [Google Scholar] [CrossRef] [PubMed]
- Finegold, S.M.; Downes, J.; Summanen, P.H. Microbiology of Regressive Autism. Anaerobe 2012, 18, 260–262. [Google Scholar] [CrossRef]
- Finegold, S.M. Desulfovibrio Species Are Potentially Important in Regressive Autism. Med. Hypotheses 2011, 77, 270–274. [Google Scholar] [CrossRef]
- Williams, B.L.; Hornig, M.; Parekh, T.; Ian Lipkin, W. Application of Novel PCR-Based Methods for Detection, Quantitation, and Phylogenetic Characterization of Sutterella Species in Intestinal Biopsy Samples from Children with Autism and Gastrointestinal Disturbances. mBio 2012, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Christophersen, C.T.; Sorich, M.J.; Gerber, J.P.; Angley, M.T.; Conlon, M.A. Low Relative Abundances of the Mucolytic Bacterium Akkermansia Muciniphila and Bifidobacterium Spp. In Feces of Children with Autism. Appl. Environ. Microbiol. 2011, 77, 6718–6721. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Han, Y.; Dy, A.B.C.; Hagerman, R.J. The Gut Microbiota and Autism Spectrum Disorders. Front. Cell Neurosci. 2017, 11, 120. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C.; et al. Association of Brain Amyloidosis with Pro-Inflammatory Gut Bacterial Taxa and Peripheral Inflammation Markers in Cognitively Impaired Elderly. Neurobiol. Aging 2017, 49, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut Microbiome Alterations in Alzheimer’s Disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef] [PubMed]
- Hill-Burns, E.M.; Debelius, J.W.; Morton, J.T.; Wissemann, W.T.; Lewis, M.R.; Wallen, Z.D.; Peddada, S.D.; Factor, S.A.; Molho, E.; Zabetian, C.P.; et al. Parkinson’s Disease and Parkinson’s Disease Medications Have Distinct Signatures of the Gut Microbiome. Mov. Disord. 2017, 32, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Hopfner, F.; Künstner, A.; Müller, S.H.; Künzel, S.; Zeuner, K.E.; Margraf, N.G.; Deuschl, G.; Baines, J.F.; Kuhlenbäumer, G. Gut Microbiota in Parkinson Disease in a Northern German Cohort. Brain Res. 2017, 1667, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.T.; Dowd, S.E.; Galley, J.D.; Hufnagle, A.R.; Allen, R.G.; Lyte, M. Exposure to a Social Stressor Alters the Structure of the Intestinal Microbiota: Implications for Stressor-Induced Immunomodulation. Brain Behav. Immun. 2011, 25, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Irritable Bowel Syndrome: A Microbiome-Gut-Brain Axis Disorder? World J. Gastroenterol. 2014, 20, 14105–14125. [Google Scholar] [CrossRef]
- Campbell, C.; Kandalgaonkar, M.R.; Golonka, R.M.; Yeoh, B.S.; Vijay-Kumar, M.; Saha, P. Crosstalk between Gut Microbiota and Host Immunity: Impact on Inflammation and Immunotherapy. Biomedicines 2023, 11, 294. [Google Scholar] [CrossRef]
- Humayun, M.; Ayuso, J.M.; Park, K.Y.; Di Genova, B.M.; Skala, M.C.; Kerr, S.C.; Knoll, L.J.; Beebe, D.J. Innate Immune Cell Response to Host-Parasite Interaction in a Human Intestinal Tissue Microphysiological System. Sci. Adv. 2022, 8, 8012. [Google Scholar] [CrossRef]
- Noble, A.; Pring, E.T.; Durant, L.; Man, R.; Dilke, S.M.; Hoyles, L.; James, S.A.; Carding, S.R.; Jenkins, J.T.; Knight, S.C. Altered Immunity to Microbiota, B Cell Activation and Depleted Γδ/Resident Memory T Cells in Colorectal Cancer. Cancer Immunology, Immunotherapy 2022, 71, 2619–2629. [Google Scholar] [CrossRef] [PubMed]
- Lyu, M.; Suzuki, H.; Kang, L.; Gaspal, F.; Zhou, W.; Goc, J.; Zhou, L.; Zhou, J.; Zhang, W.; Artis, D.; et al. ILC3s Select Microbiota-Specific Regulatory T Cells to Establish Tolerance in the Gut. Nature 2022, 610, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, M.; Tang, L.; Wang, F.; Huang, S.; Liu, S.; Lei, Y.; Wang, S.; Xie, Z.; Wang, W.; et al. TLR4 Regulates RORγt+ Regulatory T-Cell Responses and Susceptibility to Colon Inflammation through Interaction with Akkermansia Muciniphila. Microbiome 2022, 10, 98. [Google Scholar] [CrossRef]
- Jones, J.M. Dietary Fibre’s Co-Passengers: Is It the Fibre or the Co-Passengers? In Dietary Fibre: New Frontiers for Food and Health; Wageningen Academic: Wageningen, The Netherlands, 2010; pp. 365–378. [Google Scholar]
- Lambeau, K.V.; McRorie, J.W. Fiber Supplements and Clinically Proven Health Benefits: How to Recognize and Recommend an Effective Fiber Therapy. J. Am. Assoc. Nurse Pract. 2017, 29, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Mandaliya, D.; Patel, S.; Seshadri, S. Fiber in Our Diet and Its Role in Health and Disease. In Functional Food and Human Health; Springer: Singapore, 2018; pp. 247–255. [Google Scholar] [CrossRef]
- Năstăsescu, V.; Mititelu, M.; Stanciu, T.I.; Drăgănescu, D.; Grigore, N.D.; Udeanu, D.I.; Stanciu, G.; Neacșu, S.M.; Dinu-Pîrvu, C.E.; Oprea, E.; et al. Food Habits and Lifestyle of Romanians in the Context of the COVID-19 Pandemic. Nutrients 2022, 14, 504. [Google Scholar] [CrossRef]
- Mao, T.; Huang, F.; Zhu, X.; Wei, D.; Chen, L. Effects of Dietary Fiber on Glycemic Control and Insulin Sensitivity in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. J. Funct. Foods 2021, 82, 104500. [Google Scholar] [CrossRef]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic Effects: Metabolic and Health Benefits. Br. J. Nutr. 2010, 104, S1–S63. [Google Scholar] [CrossRef]
- van Loo, J.; Coussement, P.; de Leenheer, L.; Hoebreg, H.; Smits, G. On the Presence of Inulin and Oligofructose as Natural Ingredients in the Western Diet. Crit. Rev. Food Sci. Nutr. 1995, 35, 525–552. [Google Scholar] [CrossRef]
- Amount of Dietary Fiber in Various Foods|Otsuka Pharmaceutical Co., Ltd. Available online: https://www.otsuka.co.jp/en/health-and-illness/fiber/intake/foods-amount/ (accessed on 29 December 2022).
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary Fibre in Foods: A Review. J. Food Sci. Technol. 2012, 49, 255. [Google Scholar] [CrossRef]
- Pino, J.L.; Mujica, V.; Arredondo, M. Effect of Dietary Supplementation with Oat β-Glucan for 3 months in Subjects with Type 2 Diabetes: A Randomized, Double-Blind, Controlled Clinical Trial. J. Funct. Foods 2021, 77, 104311. [Google Scholar] [CrossRef]
- Queenan, K.M.; Stewart, M.L.; Smith, K.N.; Thomas, W.; Fulcher, R.G.; Slavin, J.L. Concentrated Oat Beta-Glucan, a Fermentable Fiber, Lowers Serum Cholesterol in Hypercholesterolemic Adults in a Randomized Controlled Trial. Nutr. J. 2007, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Parnell, J.A.; Reimer, R.A. Weight Loss during Oligofructose Supplementation Is Associated with Decreased Ghrelin and Increased Peptide YY in Overweight and Obese Adults. Am. J. Clin. Nutr. 2009, 89, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Xing, X.; Zhang, L.; Liu, Z.; Zhao, J.; Liu, X. Chitosan Oligosaccharides Show Protective Effects in Coronary Heart Disease by Improving Antioxidant Capacity via the Increase in Intestinal Probiotics. Oxidative Med. Cell. Longev. 2019, 2019, 7658052. [Google Scholar] [CrossRef]
- Lim, S.H. Larch Arabinogalactan Attenuates Myocardial Injury by Inhibiting Apoptotic Cascades in a Rat Model of Ischemia-Reperfusion. J. Med. Food 2017, 20, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Whisner, C.M.; Martin, B.R.; Nakatsu, C.H.; McCabe, G.P.; McCabe, L.D.; Peacock, M.; Weaver, C.M. Soluble Maize Fibre Affects Short-Term Calcium Absorption in Adolescent Boys and Girls: A Randomised Controlled Trial Using Dual Stable Isotopic Tracers. Br. J. Nutr. 2014, 112, 446–456. [Google Scholar] [CrossRef]
- Jakeman, S.A.; Henry, C.N.; Martin, B.R.; McCabe, G.P.; McCabe, L.D.; Jackson, G.S.; Peacock, M.; Weaver, C.M. Soluble Corn Fiber Increases Bone Calcium Retention in Postmenopausal Women in a Dose-Dependent Manner: A Randomized Crossover Trial. Am. J. Clin. Nutr. 2016, 104, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Mazzaferro, S.; Muscaritoli, M.; Mastroluca, D.; Testorio, M.; Perrotta, A.; Esposito, Y.; Carta, M.; Campagna, L.; di Grado, M.; et al. Prebiotic Therapy with Inulin Associated with Low Protein Diet in Chronic Kidney Disease Patients: Evaluation of Nutritional, Cardiovascular and Psychocognitive Parameters. Toxins 2020, 12, 381. [Google Scholar] [CrossRef]
- Vaghef-Mehrabany, E.; Ranjbar, F.; Asghari-Jafarabadi, M.; Hosseinpour-Arjmand, S.; Ebrahimi-Mameghani, M. Calorie Restriction in Combination with Prebiotic Supplementation in Obese Women with Depression: Effects on Metabolic and Clinical Response. Nutr. Neurosci. 2021, 24, 339–353. [Google Scholar] [CrossRef]
- Petry, N.; Egli, I.; Chassard, C.; Lacroix, C.; Hurrell, R. Inulin Modifies the Bifidobacteria Population, Fecal Lactate Concentration, and Fecal PH but Does Not Influence Iron Absorption in Women with Low Iron Status. Am. J. Clin. Nutr. 2012, 96, 325–331. [Google Scholar] [CrossRef]
- Lim, S.H.; Kim, M.Y.; Lee, J. Apple Pectin, a Dietary Fiber, Ameliorates Myocardial Injury by Inhibiting Apoptosis in a Rat Model of Ischemia/Reperfusion. Nutr. Res. Pract. 2014, 8, 391–397. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Machado, A.M.; da Silva, N.B.M.; de Freitas, R.M.P.; de Freitas, M.B.D.; Chaves, J.B.P.; Oliveira, L.L.; Martino, H.S.D.; Alfenas, R.d.C.G. Effects of Yacon Flour Associated with an Energy Restricted Diet on Intestinal Permeability, Fecal Short Chain Fatty Acids, Oxidative Stress and Inflammation Markers Levels in Adults with Obesity or Overweight: A Randomized, Double Blind, Placebo Controlled Clinical Trial. Arch. Endocrinol. Metab. 2021, 64, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Xie, K.; Liu, Z.; Nakasone, Y.; Sakao, K.; Hossain, A.; Hou, D.X. Preventive Effects and Mechanisms of Garlic on Dyslipidemia and Gut Microbiome Dysbiosis. Nutrients 2019, 11, 1225. [Google Scholar] [CrossRef]
- Lee, E.S.; Lee, B.H.; Shin, D.U.; Lim, M.Y.; Chung, W.H.; Park, C.S.; Baik, M.Y.; do Nam, Y.; Seo, D.H. Amelioration of Obesity in High-Fat Diet-Fed Mice by Chestnut Starch Modified by Amylosucrase from Deinococcus Geothermalis. Food Hydrocoll. 2018, 75, 22–32. [Google Scholar] [CrossRef]
- Nihei, N.; Okamoto, H.; Furune, T.; Ikuta, N.; Sasaki, K.; Rimbach, G.; Yoshikawa, Y.; Terao, K. Dietary α-Cyclodextrin Modifies Gut Microbiota and Reduces Fat Accumulation in High-Fat-Diet-Fed Obese Mice. BioFactors 2018, 44, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Scientific Opinion on the Substantiation of Health Claims Related to Beta-Glucans from Oats and Barley and Maintenance of Normal Blood LDL-Cholesterol Concentrations (ID 1236, 1299), Increase in Satiety Leading to a Reduction in Energy Intake (ID 851, 852), Reduction of Post-Prandial Glycaemic Responses (ID 821, 824), and “Digestive Function” (ID 850) Pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2207. [CrossRef]
- Whitehead, A.; Beck, E.J.; Tosh, S.; Wolever, T.M.S. Cholesterol-Lowering Effects of Oat β-Glucan: A Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2014, 100, 1413–1421. [Google Scholar] [CrossRef]
- Singh, A.; Dhanasekaran, D.; Ganamurali, N.; Preethi, L.; Sabarathinam, S. Junk Food-Induced Obesity- a Growing Threat to Youngsters during the Pandemic. Obes. Med. 2021, 26, 100364. [Google Scholar] [CrossRef]
- Busnatu, S.S.; Salmen, T.; Pana, M.A.; Rizzo, M.; Stallone, T.; Papanas, N.; Popovic, D.; Tanasescu, D.; Serban, D.; Stoian, A.P. The Role of Fructose as a Cardiovascular Risk Factor: An Update. Metabolites 2022, 12, 67. [Google Scholar] [CrossRef]
- Bohlouli, J.; Reza Moravejolahkami, A.; Ganjali Dashti, M.; Balouch Zehi, Z.; Ali Hojjati Kermani, M.; Borzoo-Isfahani, M.; Bahreini-Esfahani, N.; Bahreini-, N.; Moravejolahkami, R. COVID-19 and Fast Foods Consumption: A Review. Int. J. Food Prop. 2021, 24, 203–209. [Google Scholar] [CrossRef]
- Mititelu, M.; Nicolescu, T.O.; Ioniţă, C.A.; Nicolescu, F. Study of Heavy Metals and Organic Polluants From Some Fisches of Danube River. J. Enviromental Prot. Ecol. 2012, 13, 869–874. [Google Scholar]
- Mititelu, M.; Nicolescu, T.O.; Ioniţă, C.A.; Nicolescu, F. Heavy Metals Analisys in Some Wild Edible Mushrooms. J. Enviromental Prot. Ecol. 2012, 13, 875–879. [Google Scholar]
- Ioniţă, A.C.; Mititelu, M.; Moroşan, E. Analysis of heavy metals and organic pollutants from some Danube river fishes. Farmacia 2014, 62, 299–305. [Google Scholar]
- Mititelu, M.; Moroşan, E.; Neacsu, S.M.; Ioniţă, E.I. Research regarding the pollution degree from Romanian Black Sea coast. Farmacia 2018, 66, 1059–1063. [Google Scholar] [CrossRef]
- Mititelu, M.; Stanciu, G.; Drăgănescu, D.; Ioniță, A.C.; Neacșu, S.M.; Dinu, M.; Stefanvan Staden, R.-I.; Moroșan, E. Mussel Shells, a Valuable Calcium Resource for the Pharmaceutical Industry. Mar. Drugs 2022, 20, 25. [Google Scholar] [CrossRef] [PubMed]
- Ioniţă, A.C.; Ghica, M.; Moroşan, E.; Nicolescu, F.; Mititelu, M. In vitro effects of some synthesized aminoacetanilide n’-substituted on human leukocytes separated from peripheral blood. Farmacia 2019, 67, 684–690. [Google Scholar] [CrossRef]
- Mititelu, M.; Neacsu, S.M.; Oprea, E.; Dumitrescu, D.-E.; Nedelescu, M.; Drăgănescu, D.; Nicolescu, T.O.; Rosca, A.C.; Ghica, M. Black Sea Mussels Qualitative and Quantitative Chemical Analysis: Nutritional Benefits and Possible Risks through Consumption. Nutrients 2022, 14, 964. [Google Scholar] [CrossRef] [PubMed]
- Ioniță-Mîndrican, C.-B.; Mititelu, M.; Musuc, A.M.; Oprea, E.; Ziani, K.; Neacșu, S.M.; Grigore, N.D.; Negrei, C.; Dumitrescu, D.-E.; Mireșan, H.; et al. Honey and Other Beekeeping Products Intake among the Romanian Population and Their Therapeutic Use. Appl. Sci. 2022, 12, 9649. [Google Scholar] [CrossRef]
- Farvid, M.S.; Eliassen, A.H.; Cho, E.; Liao, X.; Chen, W.Y.; Willett, W.C. Dietary Fiber Intake in Young Adults and Breast Cancer Risk. Pediatrics 2016, 137, e20151226. [Google Scholar] [CrossRef]
- Farvid, M.S.; Spence, N.D.; Holmes, M.D.; Barnett, J.B. Fiber Consumption and Breast Cancer Incidence: A Systematic Review and Meta-Analysis of Prospective Studies. Cancer 2020, 126, 3061–3075. [Google Scholar] [CrossRef]
- Berkey, C.S.; Tamimi, R.M.; Willett, W.C.; Rosner, B.; Hickey, M.; Toriola, A.T.; Frazier, A.L.; Colditz, G.A. Adolescent Alcohol, Nuts, and Fiber: Combined Effects on Benign Breast Disease Risk in Young Women. NPJ Breast Cancer 2020, 6, 61. [Google Scholar] [CrossRef]
- Turner, N.D.; Lupton, J.R. Dietary Fiber. Adv. Nutr. 2011, 2, 151–152. [Google Scholar] [CrossRef]
- Pérez Martínez, G.; Bäuerl, C.; Collado, M.C. Understanding Gut Microbiota in Elderly’s Health Will Enable Intervention through Probiotics. Benef. Microbes 2014, 5, 235–246. [Google Scholar] [CrossRef]
- Lowry, C.A.; Smith, D.G.; Siebler, P.H.; Schmidt, D.; Stamper, C.E.; Hassell, J.E.; Yamashita, P.S.; Fox, J.H.; Reber, S.O.; Brenner, L.A.; et al. The Microbiota, Immunoregulation, and Mental Health: Implications for Public Health. Curr. Environ. Health Rep. 2016, 3, 270. [Google Scholar] [CrossRef]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; de Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zheng, X.; Yuan, Y. Treatment of Insulin Resistance: Straight from the Gut. Drug Discov. Today 2016, 21, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Keenan, M.J.; Marco, M.L.; Ingram, D.K.; Martin, R.J. Improving Healthspan via Changes in Gut Microbiota and Fermentation. Age 2015, 37, 98. [Google Scholar] [CrossRef]
- Patel, R.; Dupont, H.L. New Approaches for Bacteriotherapy: Prebiotics, New-Generation Probiotics, and Synbiotics. Clin. Infect. Dis. 2015, 60 (Suppl. S2), S108–S121. [Google Scholar] [CrossRef] [PubMed]
- Patrignani, P.; Tacconelli, S.; Bruno, A. Gut Microbiota, Host Gene Expression, and Aging. J. Clin. Gastroenterol. 2014, 48 (Suppl. S1), S28–S31. [Google Scholar] [CrossRef]
- Badal, V.D.; Vaccariello, E.D.; Murray, E.R.; Yu, K.E.; Knight, R.; Jeste, D.V.; Nguyen, T.T. The Gut Microbiome, Aging, and Longevity: A Systematic Review. Nutrients 2020, 12, 3759. [Google Scholar] [CrossRef]
- He, D.; Liu, L.; Zhang, Z.; Yang, X.; Jia, Y.; Wen, Y.; Cheng, S.; Meng, P.; Li, C.; Zhang, H.; et al. Association between Gut Microbiota and Longevity: A Genetic Correlation and Mendelian Randomization Study. BMC Microbiol. 2022, 22, 1–12. [Google Scholar] [CrossRef]
- Vaiserman, A.M.; Pasyukova, E.G. Epigenetic Drugs: A Novel Anti-Aging Strategy? Front. Genet. 2012, 3, 224. [Google Scholar] [CrossRef]
- Estaki, M.; Pither, J.; Baumeister, P.; Little, J.P.; Gill, S.K.; Ghosh, S.; Ahmadi-Vand, Z.; Marsden, K.R.; Gibson, D.L. Cardiorespiratory Fitness as a Predictor of Intestinal Microbial Diversity and Distinct Metagenomic Functions. Microbiome 2016, 4, 42. [Google Scholar] [CrossRef]
- Stambler, I. The Unexpected Outcomes of Anti-Aging, Rejuvenation, and Life Extension Studies: An Origin of Modern Therapies. Rejuvenation Res. 2014, 17, 297–305. [Google Scholar] [CrossRef]
- Ok, S.C. Insights into the Anti-Aging Prevention and Diagnostic Medicine and Healthcare. Diagnostics 2022, 12, 819. [Google Scholar] [CrossRef] [PubMed]
- Sbierski-Kind, J.; Grenkowitz, S.; Schlickeiser, S.; Sandforth, A.; Friedrich, M.; Kunkel, D.; Glauben, R.; Brachs, S.; Mai, K.; Thürmer, A.; et al. Effects of Caloric Restriction on the Gut Microbiome Are Linked with Immune Senescence. Microbiome 2022, 10, 57. [Google Scholar] [CrossRef]
- Flanagan, E.W.; Most, J.; Mey, J.T.; Redman, L.M. Calorie Restriction and Aging in Humans. Annu. Rev. Nutr. 2020, 40, 105. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Lattimer, L.D.N.; Stephen, S.; Borum, M.L.; Doman, D.B. Fecal Microbiota Transplantation: A Review of Emerging Indications Beyond Relapsing Clostridium Difficile Toxin Colitis. Gastroenterol. Hepatol. 2015, 11, 24. [Google Scholar]
- Wang, J.W.; Kuo, C.H.; Kuo, F.C.; Wang, Y.K.; Hsu, W.H.; Yu, F.J.; Hu, H.M.; Hsu, P.I.; Wang, J.Y.; Wu, D.C. Fecal Microbiota Transplantation: Review and Update. J. Formos. Med. Assoc. 2019, 118, S23–S31. [Google Scholar] [CrossRef]
- Sepp, E.; Smidt, I.; Rööp, T.; Štšepetova, J.; Kõljalg, S.; Mikelsaar, M.; Soidla, I.; Ainsaar, M.; Kolk, H.; Vallas, M.; et al. Comparative Analysis of Gut Microbiota in Centenarians and Young People: Impact of Eating Habits and Childhood Living Environment. Front. Cell. Infect. Microbiol. 2022, 12, 851404. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, S.; Xu, X.; Xiao, Y.; Yang, M.; Zhao, X.; Jin, C.; Hu, F.; Yang, S.; Tang, B.; et al. Gut Microbiota Associated with Effectiveness and Responsiveness to Mindfulness-Based Cognitive Therapy in Improving Trait Anxiety. Front. Cell. Infect. Microbiol. 2022, 12, 719829. [Google Scholar] [CrossRef]
- Chen, S.S.; Liao, X.M.; Wei, Q.Z.; Zhou, Y.Y.; Su, M.Y.; Hu, Y.; Song, Y.Y.; Zhang, Z.Q.; Liang, J.J. Associations of the Gut Microbiota Composition and Fecal Short-Chain Fatty Acids with Leukocyte Telomere Length in Children Aged 6 to 9 Years in Guangzhou, China: A Cross-Sectional Study. J. Nutr. 2022, 152, 1549–1559. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean Diet Intervention Alters the Gut Microbiome in Older People Reducing Frailty and Improving Health Status: The NU-AGE 1-Year Dietary Intervention across Five European Countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef]
- Allegretti, J.R.; Kassam, Z.; Mullish, B.H.; Chiang, A.; Carrellas, M.; Hurtado, J.; Marchesi, J.R.; McDonald, J.A.K.; Pechlivanis, A.; Barker, G.F.; et al. Effects of Fecal Microbiota Transplantation with Oral Capsules in Obese Patients. Clin. Gastroenterol. Hepatol. 2020, 18, 855–863.e2. [Google Scholar] [CrossRef] [PubMed]
- Allegretti, J.R.; Kassam, Z.; Hurtado, J.; Marchesi, J.R.; Mullish, B.H.; Chiang, A.; Thompson, C.C.; Cummings, B.P. Impact of Fecal Microbiota Transplantation with Capsules on the Prevention of Metabolic Syndrome among Patients with Obesity. Hormones 2021, 20, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.W.; Gao, L.; Stastka, P.; Cheney, M.C.; Mahabamunuge, J.; Soto, M.T.; Ford, C.B.; Bryant, J.A.; Henn, M.R.; Hohmann, E.L. Fecal Microbiota Transplantation for the Improvement of Metabolism in Obesity: The FMT-TRIM Double-Blind Placebo-Controlled Pilot Trial. PLoS Med. 2020, 17, e1003051. [Google Scholar] [CrossRef]
- Crothers, J.W.; Chu, N.D.; Nguyen, L.T.T.; Phillips, M.; Collins, C.; Fortner, K.; Del Rio-Guerra, R.; Lavoie, B.; Callas, P.; Velez, M.; et al. Daily, Oral FMT for Long-Term Maintenance Therapy in Ulcerative Colitis: Results of a Single-Center, Prospective, Randomized Pilot Study. BMC Gastroenterol. 2021, 21, 281. [Google Scholar] [CrossRef]
- Holvoet, T.; Joossens, M.; Vázquez-Castellanos, J.F.; Christiaens, E.; Heyerick, L.; Boelens, J.; Verhasselt, B.; van Vlierberghe, H.; De Vos, M.; Raes, J.; et al. Fecal Microbiota Transplantation Reduces Symptoms in Some Patients with Irritable Bowel Syndrome with Predominant Abdominal Bloating: Short- and Long-Term Results From a Placebo-Controlled Randomized Trial. Gastroenterology 2021, 160, 145–157.e8. [Google Scholar] [CrossRef]
- Kao, D.; Roach, B.; Silva, M.; Beck, P.; Rioux, K.; Kaplan, G.G.; Chang, H.J.; Coward, S.; Goodman, K.J.; Xu, H.; et al. Effect of Oral Capsule– vs. Colonoscopy-Delivered Fecal Microbiota Transplantation on Recurrent Clostridium Difficile Infection: A Randomized Clinical Trial. JAMA 2017, 318, 1985–1993. [Google Scholar] [CrossRef]
- Nicco, C.; Paule, A.; Konturek, P.; Edeas, M. From Donor to Patient: Collection, Preparation and Cryopreservation of Fecal Samples for Fecal Microbiota Transplantation. Diseases 2020, 8, 9. [Google Scholar] [CrossRef]
- Zhang, T.; Lu, G.; Zhao, Z.; Liu, Y.; Shen, Q.; Li, P.; Chen, Y.; Yin, H.; Wang, H.; Marcella, C.; et al. Washed Microbiota Transplantation vs. Manual Fecal Microbiota Transplantation: Clinical Findings, Animal Studies and in Vitro Screening. Protein Cell 2020, 11, 251–266. [Google Scholar] [CrossRef]
- Kim, K.O.; Gluck, M. Fecal Microbiota Transplantation: An Update on Clinical Practice. Clin. Endosc. 2019, 52, 137. [Google Scholar] [CrossRef] [PubMed]
- Rafey, A.; Jahan, S.; Farooq, U.; Akhtar, F.; Irshad, M.; Nizamuddin, S.; Parveen, A. Antibiotics Associated with Clostridium Difficile Infection. Cureus 2023, 15, e39029. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Bahl, M.I.; Baunwall, S.M.D.; Dahlerup, J.F.; Hvas, C.L.; Licht, T.R. Gut Microbiota Differs between Treatment Outcomes Early after Fecal Microbiota Transplantation against Recurrent Clostridioides Difficile Infection. Gut Microbes 2022, 14, 2084306. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, T.M.; Seekatz, A.M.; Markham, N.O.; Yau, T.O.; Hatziapostolou, M.; Jilani, T.; Christodoulou, N.; Roach, B.; Birli, E.; Pomenya, O.; et al. Fecal Microbiota Transplantation for Recurrent Clostridioides Difficile Infection Associates with Functional Alterations in Circulating MicroRNAs. Gastroenterology 2021, 161, 255–270.e4. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Li, P.; Zhu, J.; Cui, B.; Xu, L.; Xiang, J.; Zhang, T.; Long, C.; Huang, G.; Ji, G.; et al. Multiple Fresh Fecal Microbiota Transplants Induces and Maintains Clinical Remission in Crohn’s Disease Complicated with Inflammatory Mass. Sci. Rep. 2017, 7, 4753. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, T.; Xiao, Y.; Tian, L.; Cui, B.; Ji, G.; Liu, Y.Y.; Zhang, F. Timing for the Second Fecal Microbiota Transplantation to Maintain the Long-Term Benefit from the First Treatment for Crohn’s Disease. Appl. Microbiol. Biotechnol. 2019, 103, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Costello, S.P.; Hughes, P.A.; Waters, O.; Bryant, R.V.; Vincent, A.D.; Blatchford, P.; Katsikeros, R.; Makanyanga, J.; Campaniello, M.A.; Mavrangelos, C.; et al. Effect of Fecal Microbiota Transplantation on 8-Week Remission in Patients with Ulcerative Colitis: A Randomized Clinical Trial. JAMA 2019, 321, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Haifer, C.; Paramsothy, S.; Kaakoush, N.O.; Saikal, A.; Ghaly, S.; Yang, T.; Luu, L.D.W.; Borody, T.J.; Leong, R.W. Lyophilised Oral Faecal Microbiota Transplantation for Ulcerative Colitis (LOTUS): A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Gastroenterol. Hepatol. 2022, 7, 141–151. [Google Scholar] [CrossRef]
- El-Salhy, M.; Valeur, J.; Hausken, T.; Gunnar Hatlebakk, J. Changes in Fecal Short-Chain Fatty Acids Following Fecal Microbiota Transplantation in Patients with Irritable Bowel Syndrome. Neurogastroenterol. Motil. 2021, 33, e13983. [Google Scholar] [CrossRef]
- Mazzawi, T.; Hausken, T.; Hov, J.R.; Valeur, J.; Sangnes, D.A.; El-Salhy, M.; Gilja, O.H.; Hatlebakk, J.G.; Lied, G.A. Clinical Response to Fecal Microbiota Transplantation in Patients with Diarrhea-Predominant Irritable Bowel Syndrome Is Associated with Normalization of Fecal Microbiota Composition and Short-Chain Fatty Acid Levels. Scand. J. Gastroenterol. 2019, 54, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Kootte, R.S.; Levin, E.; Salojärvi, J.; Smits, L.P.; Hartstra, A.V.; Udayappan, S.D.; Hermes, G.; Bouter, K.E.; Koopen, A.M.; Holst, J.J.; et al. Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metab. 2017, 26, 611–619.e6. [Google Scholar] [CrossRef] [PubMed]
- De Groot, P.; Scheithauer, T.; Bakker, G.J.; Prodan, A.; Levin, E.; Khan, M.T.; Herrema, H.; Ackermans, M.; Serlie, M.J.M.; De Brauw, M.; et al. Donor Metabolic Characteristics Drive Effects of Faecal Microbiota Transplantation on Recipient Insulin Sensitivity, Energy Expenditure and Intestinal Transit Time. Gut 2020, 69, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, V.; Zhang, Z.; Deehan, E.C.; Kao, D.H.; Hotte, N.; Karmali, S.; Birch, D.W.; Samarasinghe, K.K.; Walter, J.; Madsen, K.L. Fecal Microbial Transplantation and Fiber Supplementation in Patients with Severe Obesity and Metabolic Syndrome: A Randomized Double-Blind, Placebo-Controlled Phase 2 Trial. Nat. Med. 2021, 27, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Allegretti, J.R.; Kassam, Z.; Carrellas, M.; Mullish, B.H.; Marchesi, J.R.; Pechlivanis, A.; Smith, M.; Gerardin, Y.; Timberlake, S.; Pratt, D.S.; et al. Fecal Microbiota Transplantation in Patients with Primary Sclerosing Cholangitis: A Pilot Clinical Trial. Am. J. Gastroenterol. 2019, 114, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Fagan, A.; Gavis, E.A.; Kassam, Z.; Sikaroodi, M.; Gillevet, P.M. Long-Term Outcomes of Fecal Microbiota Transplantation in Patients with Cirrhosis. Gastroenterology 2019, 156, 1921–1923.e3. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Salzman, N.H.; Acharya, C.; Sterling, R.K.; White, M.B.; Gavis, E.A.; Fagan, A.; Hayward, M.; Holtz, M.L.; Matherly, S.; et al. Fecal Microbial Transplant Capsules Are Safe in Hepatic Encephalopathy: A Phase 1, Randomized, Placebo-Controlled Trial. Hepatology 2019, 70, 1690–1703. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Gavis, E.A.; Fagan, A.; Wade, J.B.; Thacker, L.R.; Fuchs, M.; Patel, S.; Davis, B.; Meador, J.; Puri, P.; et al. A Randomized Clinical Trial of Fecal Microbiota Transplant for Alcohol Use Disorder. Hepatology 2021, 73, 1688–1700. [Google Scholar] [CrossRef]
- DeFilipp, Z.; Bloom, P.P.; Torres Soto, M.; Mansour, M.K.; Sater, M.R.A.; Huntley, M.H.; Turbett, S.; Chung, R.T.; Chen, Y.-B.; Hohmann, E.L. Drug-Resistant E. Coli Bacteremia Transmitted by Fecal Microbiota Transplant. New Engl. J. Med. 2019, 381, 2043–2050. [Google Scholar] [CrossRef]
- Biazzo, M.; Deidda, G. Fecal Microbiota Transplantation as New Therapeutic Avenue for Human Diseases. J. Clin. Med. 2022, 11, 4119. [Google Scholar] [CrossRef]
- Desai, D.; Desai, A.; Jamil, A.; Csendes, D.; Gutlapalli, S.D.; Prakash, K.; Swarnakari, K.M.; Bai, M.; Manoharan, M.P.; Raja, R.; et al. Re-Defining the Gut Heart Axis: A Systematic Review of the Literature on the Role of Gut Microbial Dysbiosis in Patients with Heart Failure. Cureus 2023, 15, e34902. [Google Scholar] [CrossRef] [PubMed]
- Antushevich, H. Fecal Microbiota Transplantation in Disease Therapy. Clin. Chim. Acta 2020, 503, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://gastro.org/news/fda-approves-first-fecal-microbiota-transplantation-therapy/ (accessed on 8 May 2024).
- Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-orally-administered-fecal-microbiota-product-prevention-recurrence-clostridioides (accessed on 8 May 2024).
- Available online: https://bellvitgehospital.cat/en/innovation-projects/microbiome-unit (accessed on 8 May 2024).
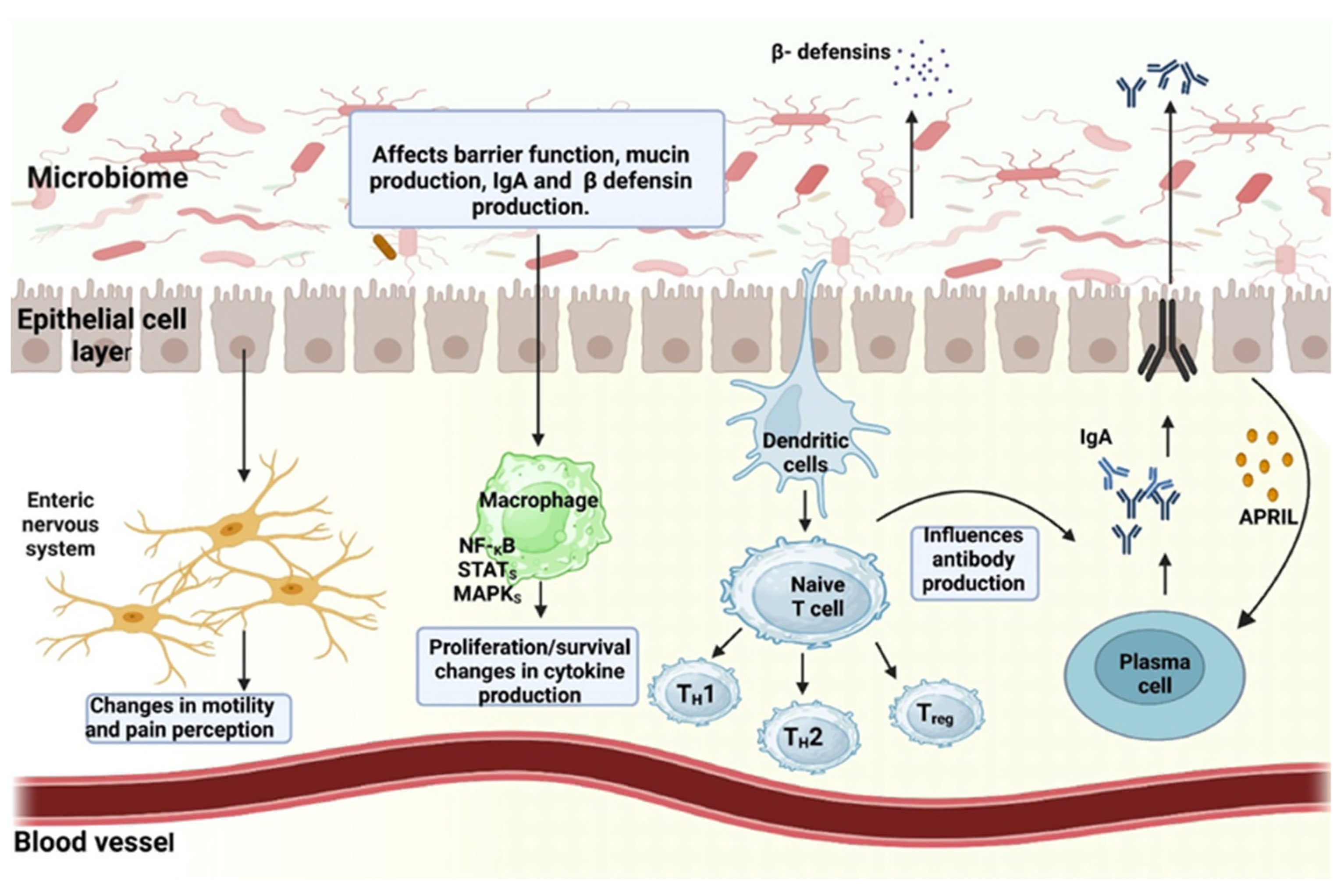
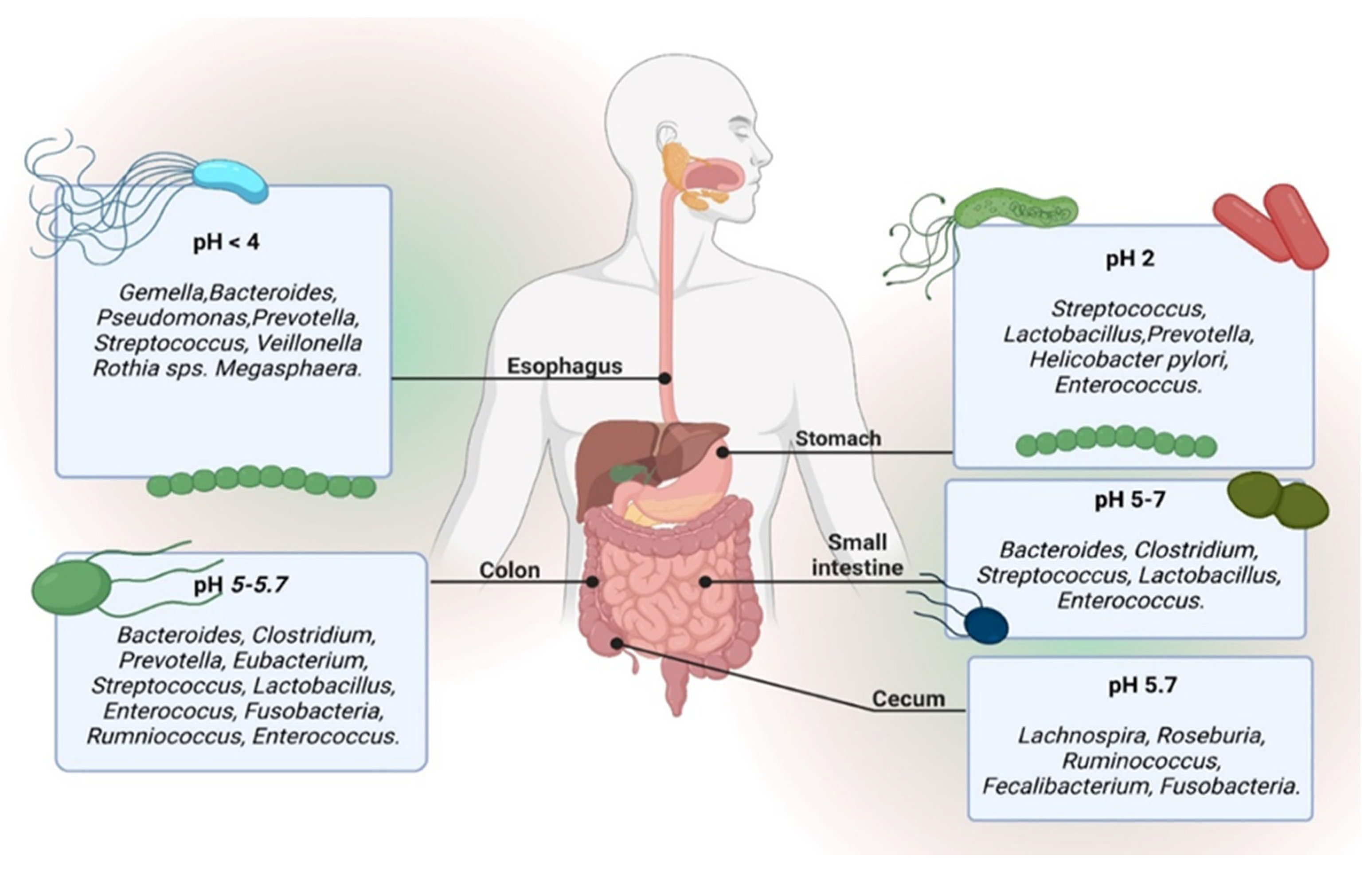

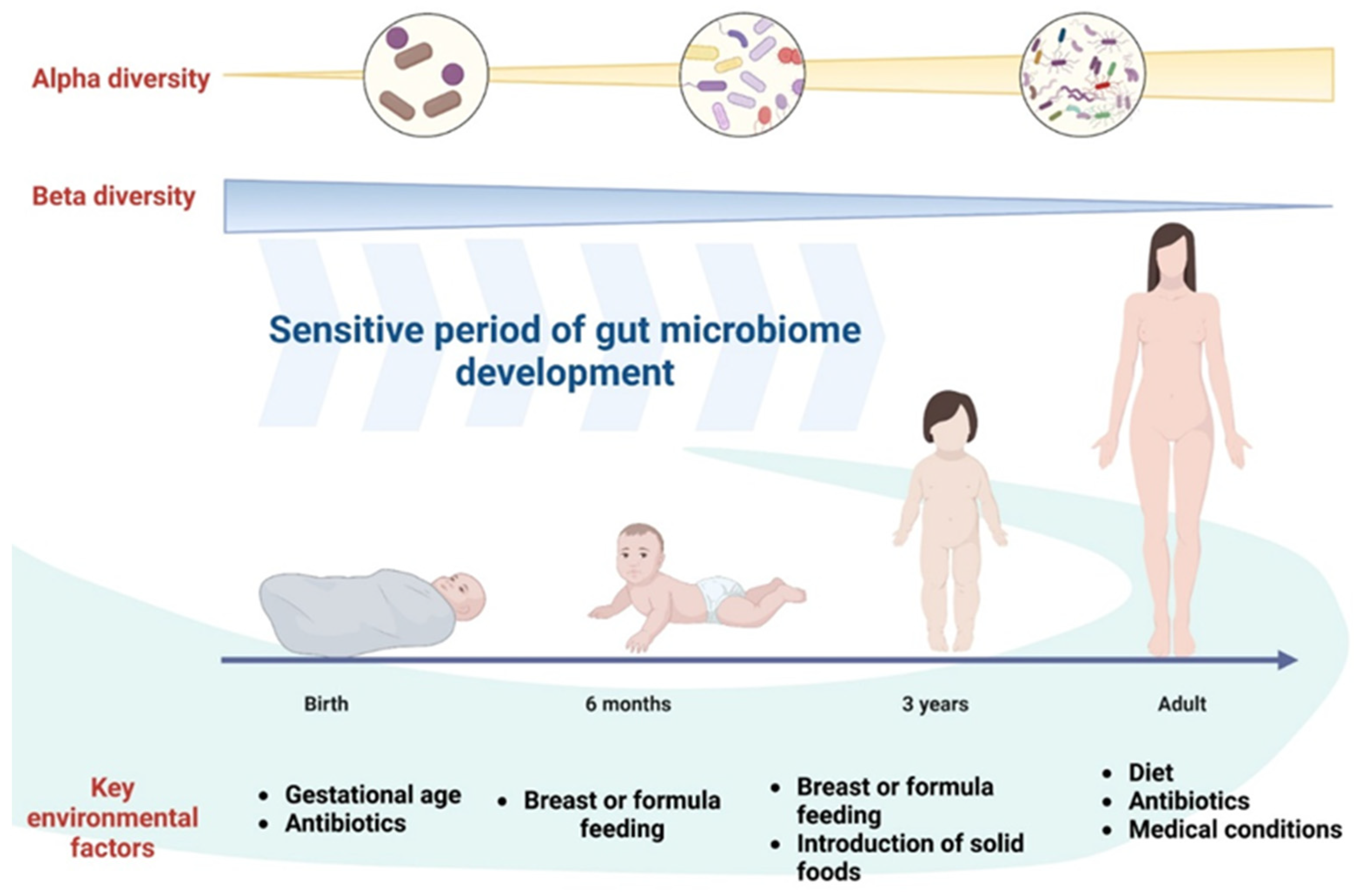
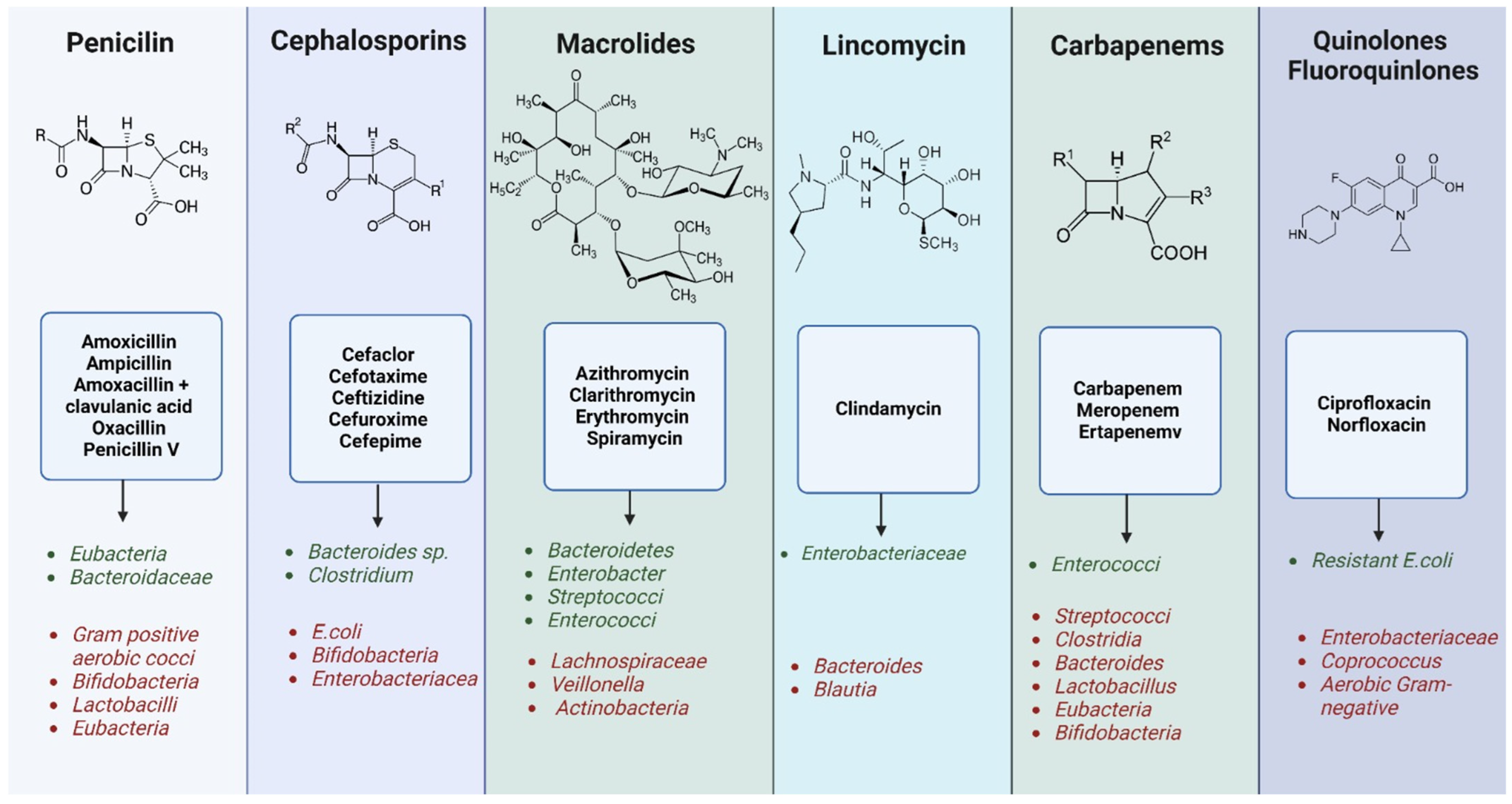
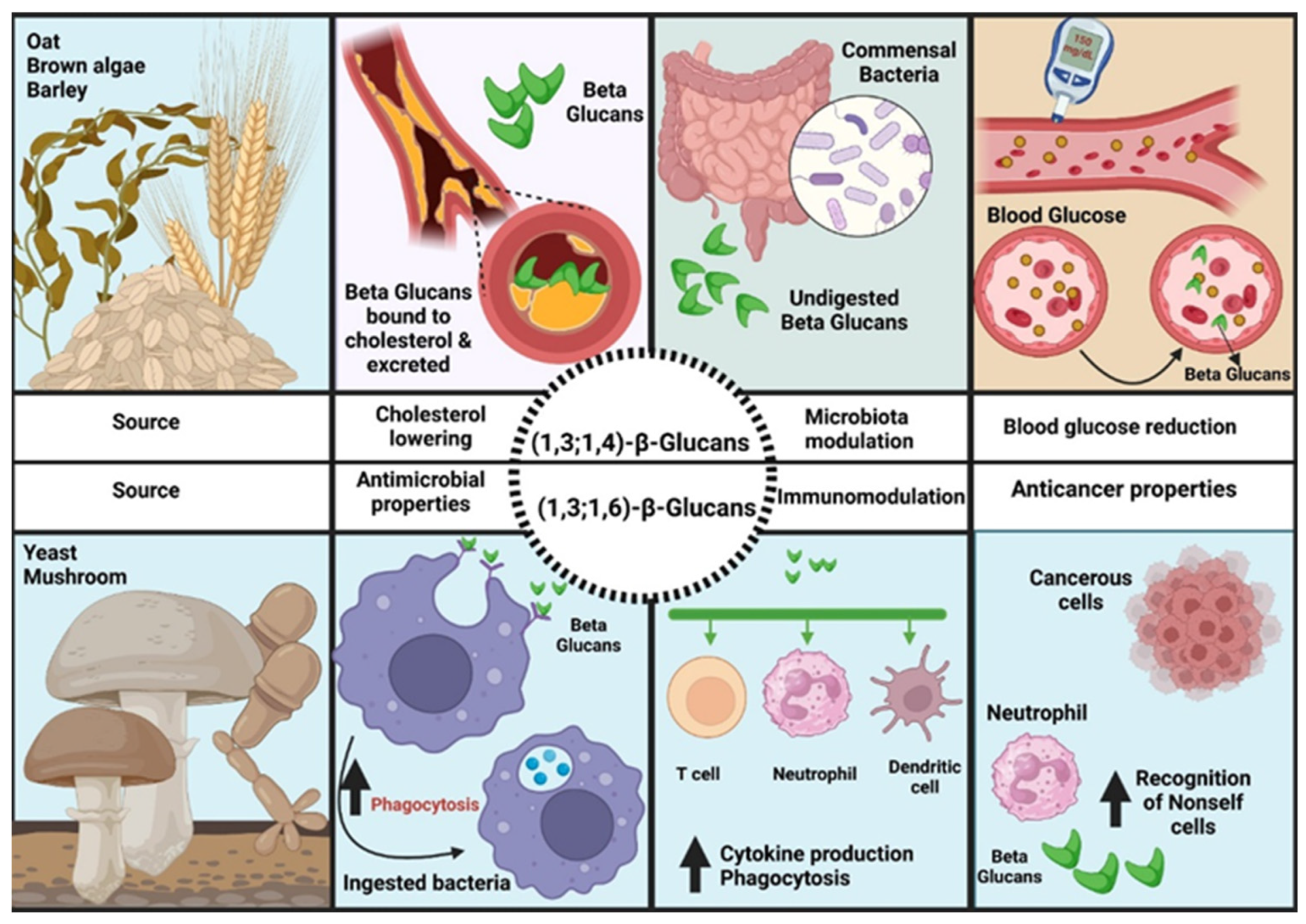
| Pathology | Disease Type | Changes in the Microbiota | References |
|---|---|---|---|
| Obesity | Metabolic disease | ↑ Increased levels: Bacillota phylum:Bacteroides Methanobrevibacter smithii Lactobacillus (Lactobacillus reuteri) Desulfovibrionaceae ↓ Decreased levels: Bifidobacteria Escherichia coli Akkermansia muciniphila | [206,207,208,209,210,211,212] |
| Severe acute malnutrition (SAM) | Metabolic disease | ↑ Increased levels: Proteobacteria Bacteroidaceae Porphyromonadaceae Bilophila Klebsiella Escherichia coli Streptococcus Shigella Enterobacter Veillonella ↓ Decreased levels: Bacteroidetes Roseburia Fecalibacterium Butyribrio Synergistetes Methanobrevibacter smithii | [213,214,215] |
| Type 2 diabetes (T2D) | Metabolic disease | ↑ Increased levels: Bacteroidetes-Bacillota phylum Bacteroidetes-Prevotella Betaproteobacteria Clostridium spp. Bacteroides caccae Desulfovibrionaceae spp. ↓ Decreased levels: Roseburia spp. Bacillota phylum Clostridia | [216,217] |
| Metabolic syndrome | Cardiometabolic disease | ↑ Increased levels: raport Bacillota phylum:Bacteroides Methanobrevibacter smithii Lactobacillus (Lactobacillus reuteri) ↓ Decreased levels: Bifidobacteria Escherichia coli | [211,216,217,218] |
| Ischemic or dilated cardiomyopathy | Cardiovascular disease | ↑ Increased levels: Prevotella Hungatella (Lacnospiraceae) Succiniclasticum Ruminococcus Acinetobacter Veillonella ↓ Decreased levels: Blautia Anaerostipes Fecalibacterium Lachnospiraceae Bifidobacterium Eubacterium Coprococcus Alistipes Oscilibacter | [219,220] |
| Heart failure with reduced ejection fraction | Cardiovascular disease | ↑ Increased levels: Streptococcus Veillonella Eggerthela ↓ Decreased levels: Prevotella SMB53 (Clostridiaceae) | [221] |
| Coronary artery disease | Cardiovascular disease | ↑ Increased levels: Escherichia-Shigella Lactobacillus Enterococcus Streptococcus ↓ Decreased levels: Fecalibacterium Roseburia Eubacterium Subdoligranulum | [222] |
| Hypertension | Cardiovascular disease | ↑ Increased levels: Klebsiella Salmonella Streptococcus Clostridium Parabacteroides Eggerthella Prevotella Porphyromonas ↓ Decreased levels: Fecalibacterium Roseburia Synergistetes Bifidobacterium Oscillibacter Coproroccus Butyrivibrio | [223,224] |
| Atherosclerosis with clinical presentation of stable or unstable angina or myocardial infarction | Cardiovascular disease | ↑ Increased levels: Enterobacteriaceae Streptococcus Lactobacillus salivarius Atopobium parvulum Ruminococcus gnavus Eggerthella lenta ↓ Decreased levels: Roseburia Fecalibacterium | [225] |
| Irritable bowel syndrome (IBS) | Gastrointestinal disease | ↑ Increased levels: Bacillota phylum:Bacteroides Proteobacteria (Enterobacteriaceae spp.) Bacillota phylum Lachnospiraceae Veillonella Streptococci Ruminococcus spp. ↓ Decreased levels: Lactobacillus Actinobacteria (Bifidobacteria, Colinsella) Bacteroidetes Fecalibacterium | [226,227,228,229] |
| Inflammatory bowel disease (IBD) | Gastrointestinal disease | ↑ Increased levels: Proteobacteria ↓ Decreased levels: Lachnospiraceae Bacteroidetes | [31] |
| Crohn’s disease (CRD) | Gastrointestinal disease | ↑ Increased levels: Roseburia hominis Ruminococcus gnavus ↓ Decreased levels: uncharacterized species of Clostridium spp. Fecalibacterium prausnitzii Bifidobacterium adolescentis Dialister invisus | [34,118] |
| Ulcerative colitis (UC) | Gastrointestinal disease | ↓ Decreased levels: Roseburia hominis Fecalibacterium prausnitzii | [230] |
| Celiac disease (CD) | Gastrointestinal disease | ↑ Increased levels: Bacteroides E.coli ↓ Decreased levels: Bifidobacterium longum Clostridium histolyticum C. lituseburense Fecalibacterium prausnitzii | [119,120,121] |
| Colorectal cancer (CRC) | Gastrointestinal disease | ↑ Increased levels: Bacteroides fragilis Enterococcus Escherichia/Shigella Klebsiella Streptococcus Peptostreptococcus Dorea spp. Fecalibacterium spp. Fusobacterium spp. ↓ Decreased levels: Roseburia Lachnospiraceae Bacteroides spp. Coprococcus spp. | [123,124,231] |
| Viral hepatitis | Liver disease | ↑ Increased levels: Enterobacteriaceae Enterococcus fecalis Escherichia coli Fecalibacterium prausnitzii ↓ Decreased levels: Leuconostoc Lactobacillus Weissella Pediococcus | [232,233] |
| Liver cirrhosis secondary to hepatitis B or C virus infection (HBV/HCV) | Liver disease | ↑ Increased levels: Enterobacteriaceae (Neisseria, Gemella) E. Fecalis E. coli F. prausnitzii Candida Veillonella Megasphaera Dialister Atopobium Prevotella ↓ Decreased levels: Bacteroidaceae Ruminococcaceae Lachnospiraceae Bacillota phylum | [233,234,235,236,237] |
| Anorexia nervosa (AN) | Mental illness | ↑ Increased levels: Methanobrevibacter smithii Bacillota phylum Actinobacteria Bacteroidetes ↓ Decreased levels: Lactobacillus plantarum Streptococcus spp. Clostridium coccoides Bacteroides fragilis | [112,113,238,239] |
| Autism spectrum disorders (ASD) | Neurodevelopmental disability | ↑ Increased levels: raport Bacillota phylum-Bacteroidetes Sutterella Lactobacillus Clostridium Bacteroidetes Desulfovibrio Caloramator Sarcina ↓ Decreased levels: Bifidobacterium spp. Bacillota phylum Akkermansia muciniphila | [240,241,242,243,244] |
| Alzheimer’s diseases (AD) | Neurodegenerative disorder | ↑ Increased levels: Escherichia/Shigella Bacteroidetes ↓ Decreased levels: E. rectale Bacillota phylum Bifidobacterium | [245,246] |
| Parkinson’s disease (PD) | Neurodegenerative disorder | ↑ Increased levels: Lactobacillaceae Barnesiellaceae Enterococcaceae Ruminococcaceae Akkermansia ↓ Decreased levels: Lachnospiraceae | [247,248] |
| Stress | Risk factors | ↑ Increased levels: Clostridium spp. Enterobacteriaceae Escherichia coli Pseudomonas spp. ↓ Decreased levels: Bacteroides spp. Lactobacilli spp. | [249,250] |
| Food Product/Supplements | Subjects | Type of Subject | Study Design | Dose/Quantity Administered and Duration of Treatment | Recorded Effects | References |
|---|---|---|---|---|---|---|
| Oat β-glucan | Obese subjects with type 2 diabetes (n = 37; female = 28, male = 9) | Human | Randomized, double-blind, clinical trial Taken as a supplement, daily, with water or milk at room temperature No changes in the subject’s diet | 5 g/day × 12 weeks | Reduction in HbA1c, C peptide; lowering of total cholesterol, VLDL-cholesterol and triglycerides; increased leptin levels decreased GLP-1 (glucagon-like peptide); increase in PYY; decreased populations of Lactobacillus spp. and Bifidobacterium spp.; improvement of intestinal transit | [265] |
| Concentrated oat β-glucan (54%) | Patients with hypercholesterolemia (n = 75; female = 50, male = 25) | Human | Randomized, double-blind, clinical trial Taken as a supplement No changes in the subject’s diet | 6 g/day × 6 weeks | Reduction in total cholesterol and LDL-cholesterol; the treatment group reported increased flatulence as a symptom; increased production of SCFAs (especially butyrate) | [266] |
| Oligofructose (OFS) | Adults with obesity (n = 39; female = 32; male = 7) | Human | Randomized, double-blind, clinical trial No specific dietary instructions | 21 g/day × 12 weeks | Weight loss; glucose levels decreased in the group receiving OFS and increased in the control group | [267] |
| Chitosan oligosaccharides (COS) | Patients with coronary artery disease/CAD (n = 120; female = 61; male = 49) | Human | Randomized clinical trial Hospital diet and normal control diet | 2 g/day × 6 months | Reduction in serum levels of triglycerides, total cholesterol, LDL-cholesterol; decreased levels of transaminases ALT and AST; increase in HDL-cholesterol level Increase levels of antioxidants SOD and GSH; increased bacterial levels of Bacteroides, Megasphaera, Roseburia, Prevotella, Bifidobacterium; decreased bacterial levels of Fecalibacterium alistipes, Escherichia coli | [268] |
| Soluble fiber from corn (SCF) | Boys (n = 15): 13–15 years Girls (n = 9): 12–14 years | Human | Randomized, double-blind, clinical trial Foods usually consumed by teenagers (hamburgers, sandwiches, potato chips and spaghetti). Diet composition (53% carbohydrates, 14% proteins, 33% fats, 15 g of fibers) | 12 g SCF/day × 21 days | Increase in intestinal calcium absorption; decrease in the proportion of the Bacillota phylum phylum; increase in proportion of Bacteroidetes phylum | [269] |
| SCF | Postmenopausal women (n = 12) | Human | Randomized, double-blind, cross-over clinical trial SCF in muffins and fruit-flavored drinks No specific dietary instructions, but the subjects were asked to complete a 4-day diet record | 10–20 g SCF/day × 6 months | Increased calcium retention in bones in postmenopausal women | [270,271] |
| Inulin | Patients with chronic kidney disease/CKD (n = 41; female = 16 male = 25) | Human | Prospective, case-control study Personalized low-protein diet, providing 30–35 kcal/kg/day | 19 g/day × 6 months | Decreased levels of uric acid, serum insulin, glucose, HOMA-IR, CRP, homocysteine, total cholesterol and triglycerides; increase HDL-cholesterol | [272] |
| Inulin | Women with obesity and major depressive disorder (n = 45) | Human | Randomized, double-blind, clinical trial 25% calorie-restricted diet | 10 g/day × 8 weeks | Enhancing the beneficial effects of a calorie-restricted diet on adipose tissue and total cholesterol levels | [273] |
| Inulin (Fibruline Instant) | Women (n = 32): 18–40 years | Human | Randomized, double-blind, cross-over clinical trial Inulin (dissolved in water) was consumed at breakfast, lunch and dinner Test meals (in the morning and 3 h later) with moderate iron bioavailability (cooked rice 50 g dry weight; pureed, boiled vegetable sauce 25 g fresh weight boiled for 4 h) | 20 g/day × 4 weeks | Increasing the concentration of Bifidobacteria; decreased fecal pH | [274,275] |
| Yacon | Women with obesity (n = 26) | Human | Randomized double-blind clinical trial Energy-restricted diet (minus 500 kcal/day) | 25 g/day × 6 weeks | Abdominal discomfort, abdominal pain, and flatulence were observed in the first few days; increasing the antioxidant capacity of plasma; decrease in concentration (protein carbonyl) | [276] |
| Food Product/Supplements | Subjects | Type of Subject | Study Design | Dose/Quantity Administered and Duration of Treatment | Recorded Effects | References |
|---|---|---|---|---|---|---|
| Larch Arabinogalactan (LAG) | Eight-week-old male Sprague Dawley/S | Rat lab | Randomized controlled trial LAG diet + Basal diet | 50 mg/kg/day × 3 days | Reduction in Gelsolin gene and hif1-α gene expression, apoptotic cells, and p38 phosphorylation | [269] |
| Apple pectin (AP) | Eight-week-old male Sprague Dawley/SD (n = 36) | Rat lab | Randomized controlled trial AP diet for the experimental group and basal diet for the control group | 10,40,100 and 400 mg/kg/day × 3 days | Intake of 100 and 400 mg/kg/day of apple pectin reduced infarct size (SI), defined as the ratio of infarct area (IA)/area at risk (AAR), compared to the group receiving an intake of 10 and 40 mg/kg/day; the intake of 100 mg/kg/day reduced the apoptosis process in experimentally induced infarct areas; the intake of 100 mg/kg/day registered a reduction in cleaved caspase-3, which induces apoptosis; the intake of 100 mg/kg/day increased the expression of Bcl-2, but decreased the expression of Bax, compared to the control group; the Bcl-2/Bax ratio (determinant of cell death or survival) was increased in the 100 mg/kg/day group | [275] |
| Garlic | 5 weeks of age male C57BL/6N mice (n = 30) | Rat lab | Randomized clinical trial Normal diet (54% carbohydrate, 21% protein, 6% fat) and high-fat diet (10% carbohydrate, 21% protein, 40% fat) | 5% in the diet for 12 weeks | Reduction in serum levels of GOT and GPT, total cholesterol, triglycerides and LDL, insulin, HOMA-IR; amelioration of high-fat diet-induced dyslipidemia Improved ratio (villus height/crypt depth) Reducing the concentration of branched-chain fatty acids (BCFAs); increasing abundance of Lachnospiraceae; decrease in Prevotella abundance | [277] |
| Amylosucrase-modified chestnut starch | Eight-week-old male C57BL/6N mice | Rat lab | Randomized controlled trial High-fat diet (45% fat of total energy) | 1500 mg/kg bw | Decrease in body weight, white adipose tissue (upregulating SCFAs-GPR43-mediated pathway) | [278] |
| α-cyclodextrin (α-CD) | 5-week-old male C57BL/6JJmsSlc mice (n = 15) | Rat lab | Randomized controlled trial High-fat diet | 5.5% in the diet for 16 weeks | Decrease in body weight and adipose tissue; normalization of adipocyte sizes in epididymal adipose tissue; lowering blood glucose levels; increasing concentrations of Lactobacillales, Bacteroides; decreased abundance for Clostridium; increase in total SCFA content of the cecum; increase in lactic acid levels | [279] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olteanu, G.; Ciucă-Pană, M.-A.; Busnatu, Ș.S.; Lupuliasa, D.; Neacșu, S.M.; Mititelu, M.; Musuc, A.M.; Ioniță-Mîndrican, C.-B.; Boroghină, S.C. Unraveling the Microbiome–Human Body Axis: A Comprehensive Examination of Therapeutic Strategies, Interactions and Implications. Int. J. Mol. Sci. 2024, 25, 5561. https://doi.org/10.3390/ijms25105561
Olteanu G, Ciucă-Pană M-A, Busnatu ȘS, Lupuliasa D, Neacșu SM, Mititelu M, Musuc AM, Ioniță-Mîndrican C-B, Boroghină SC. Unraveling the Microbiome–Human Body Axis: A Comprehensive Examination of Therapeutic Strategies, Interactions and Implications. International Journal of Molecular Sciences. 2024; 25(10):5561. https://doi.org/10.3390/ijms25105561
Chicago/Turabian StyleOlteanu, Gabriel, Maria-Alexandra Ciucă-Pană, Ștefan Sebastian Busnatu, Dumitru Lupuliasa, Sorinel Marius Neacșu, Magdalena Mititelu, Adina Magdalena Musuc, Corina-Bianca Ioniță-Mîndrican, and Steluța Constanța Boroghină. 2024. "Unraveling the Microbiome–Human Body Axis: A Comprehensive Examination of Therapeutic Strategies, Interactions and Implications" International Journal of Molecular Sciences 25, no. 10: 5561. https://doi.org/10.3390/ijms25105561
APA StyleOlteanu, G., Ciucă-Pană, M.-A., Busnatu, Ș. S., Lupuliasa, D., Neacșu, S. M., Mititelu, M., Musuc, A. M., Ioniță-Mîndrican, C.-B., & Boroghină, S. C. (2024). Unraveling the Microbiome–Human Body Axis: A Comprehensive Examination of Therapeutic Strategies, Interactions and Implications. International Journal of Molecular Sciences, 25(10), 5561. https://doi.org/10.3390/ijms25105561










