Osteophyte Cartilage as a Potential Source for Minced Cartilage Implantation: A Novel Approach for Articular Cartilage Repair in Osteoarthritis
Abstract
1. Introduction
2. Results
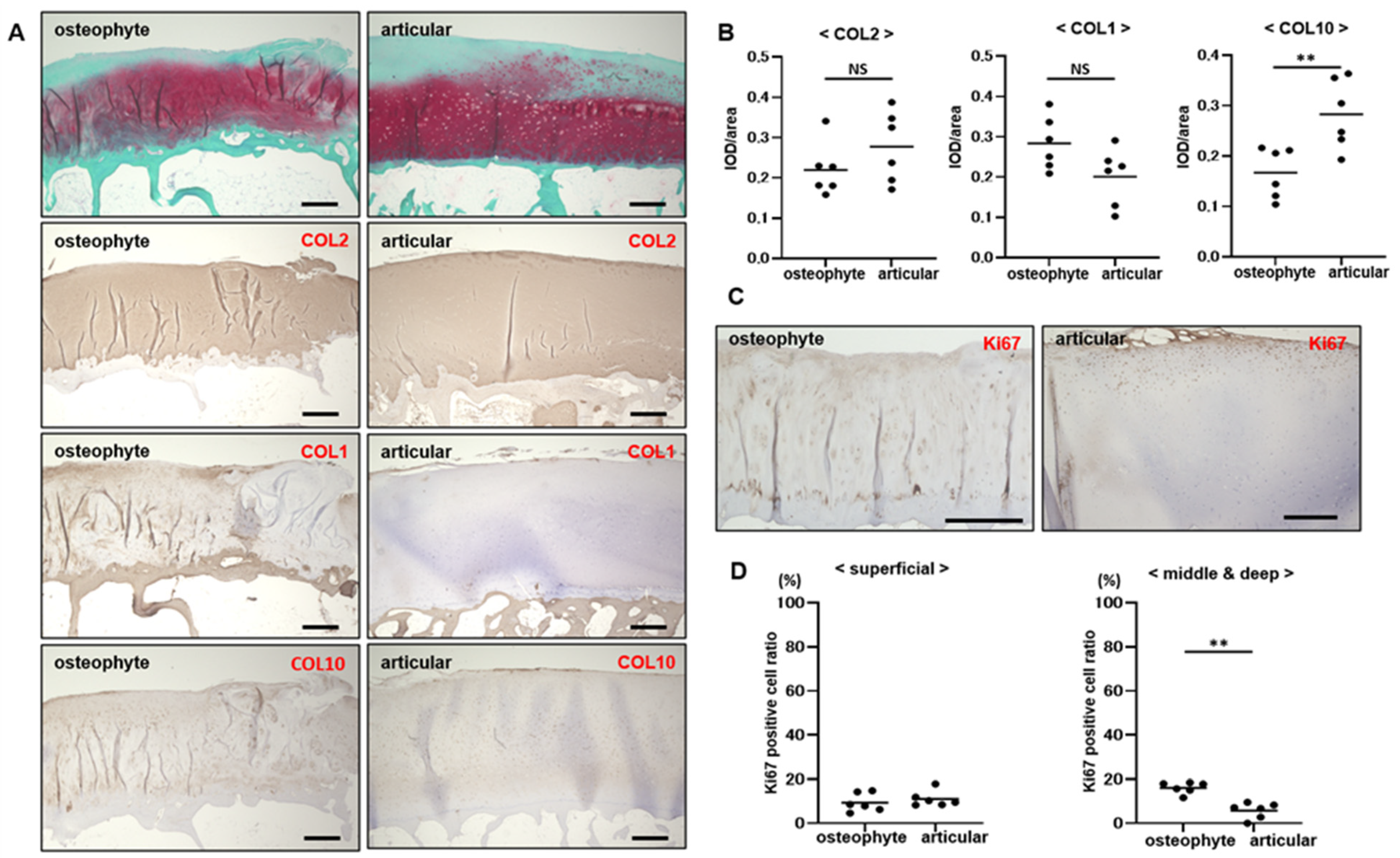
2.1. Osteophyte Cartilage Has Similar Properties to Articular Cartilage
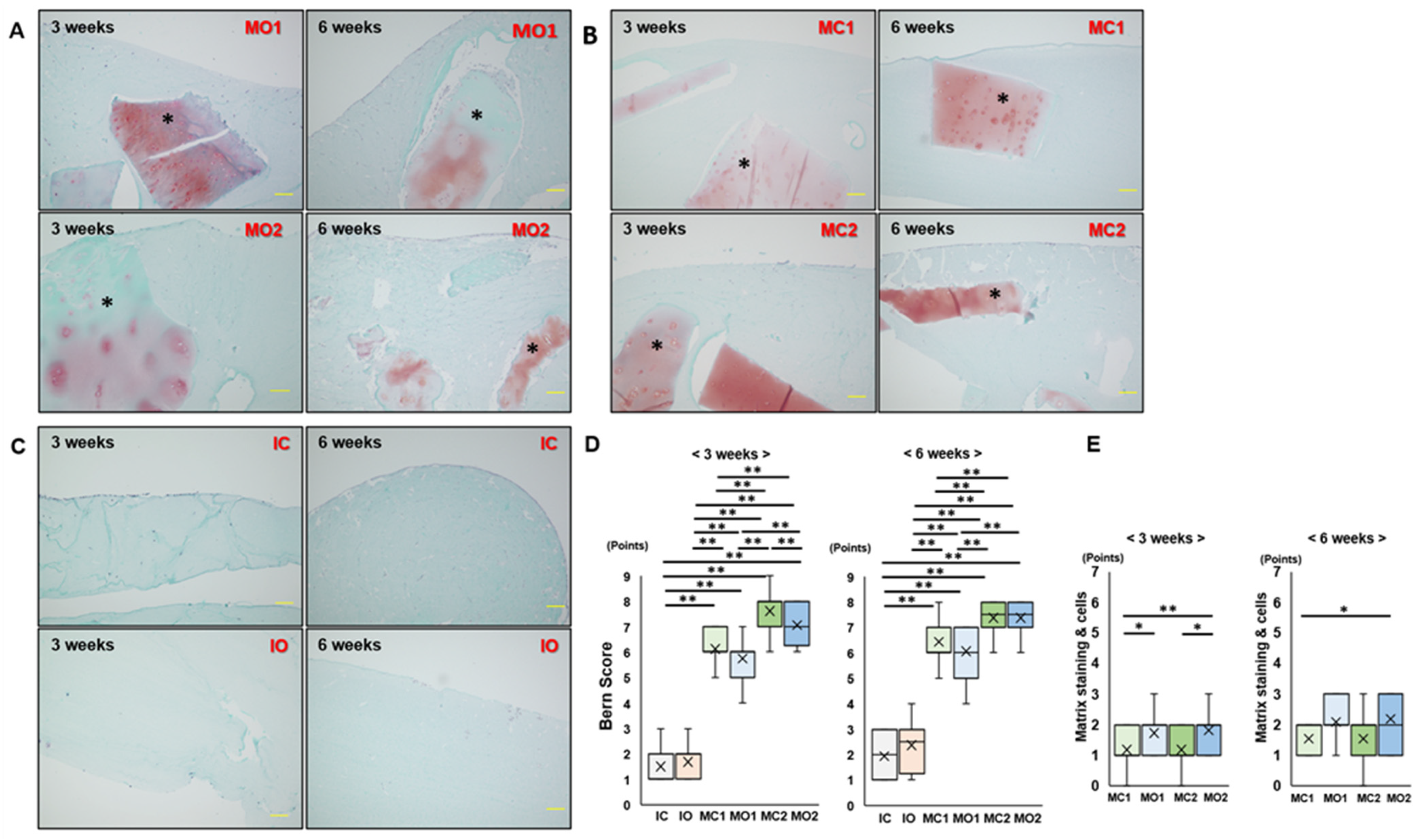
2.2. Matrix Staining of Osteophyte and Articular Cartilage Fragments in Atelocollagen Gel Is Maintained after 6 Weeks of Culture
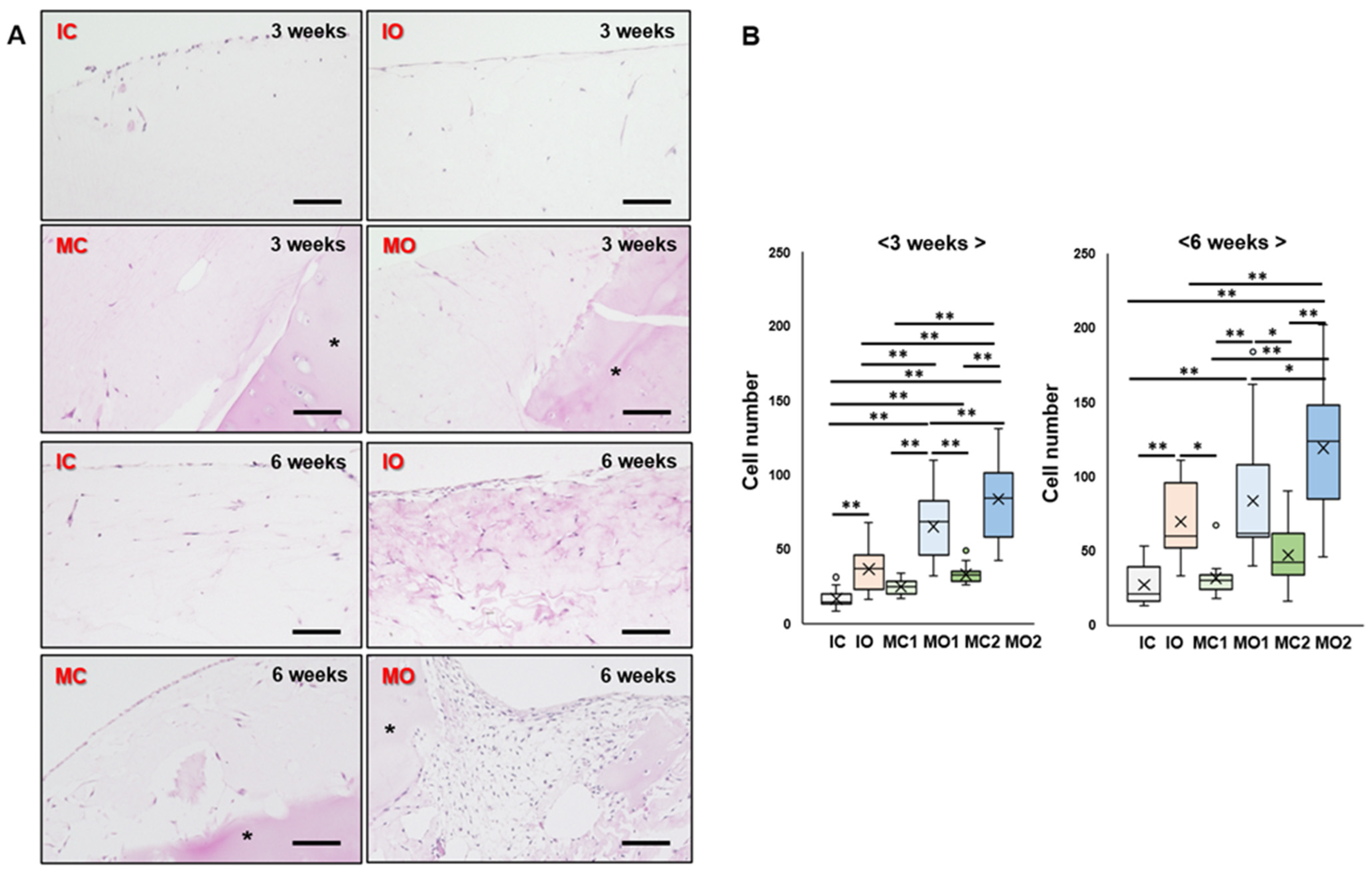
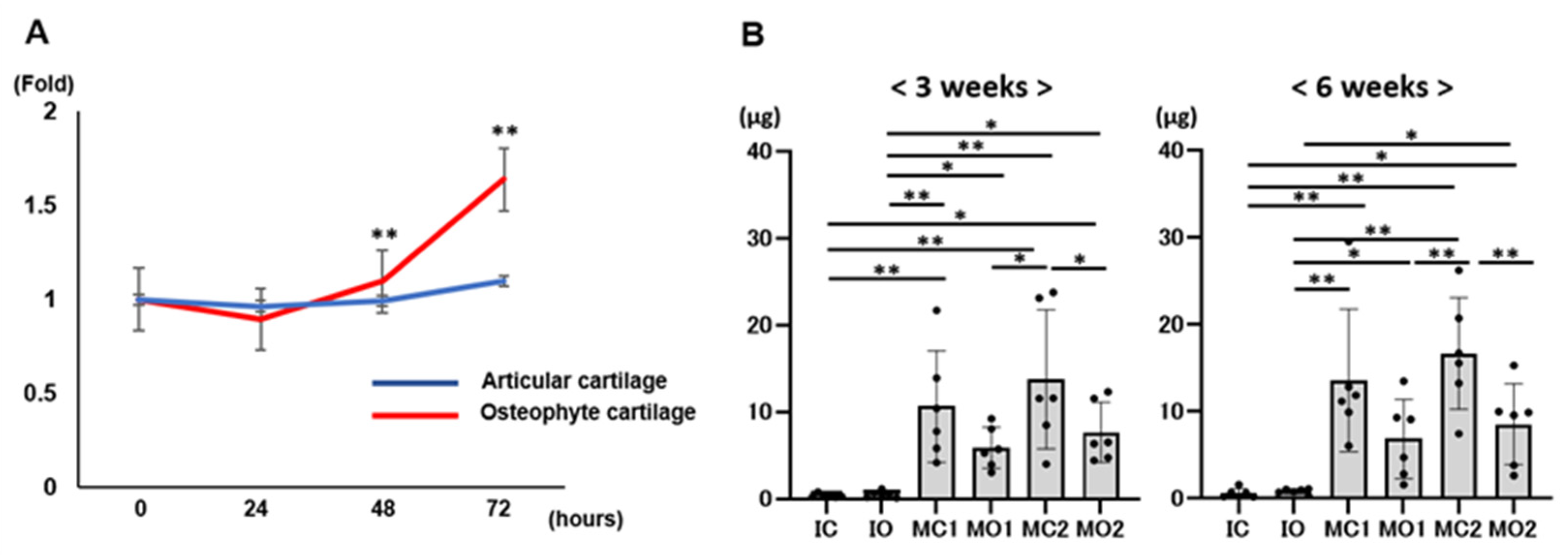
2.3. Osteophyte Cartilage Has Better Cell Migration and Proliferation Abilities in the Gel than Articular Cartilage
2.4. Migrated and Proliferated Cells in the Gel Are Chondrocytes from Osteophyte Cartilage
2.5. Osteophyte Chondrocytes Have GAG Production Ability and Better Proliferation Potential than Articular Chondrocytes
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Preparation of Cartilage/Atelocollagen Composite
4.3. Histological Analysis
4.4. Immunohistochemistry
4.5. Cell Proliferation Assay
4.6. Evaluation of GAG Contents
4.7. RNA Sequence
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, L. Osteoarthritis of the knee. N. Eng. J. Med. 2021, 384, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Eng. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Colombini, A.; Libonati, F.; Lopa, S.; Peretti, G.M.; Moretti, M.; de Girolamo, L. Autologous chondrocyte implantation provides good long-term clinical results in the treatment of knee osteoarthritis: A systemic review. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 2338–2348. [Google Scholar] [CrossRef] [PubMed]
- Salzmann, G.M.; Ossendorff, R.; Gilat, R.; Cole, B.J. Autologous minced cartilage implantation for treatment of chondral and osteochondral lesions in the knee joint: An overview. Cartilage 2021, 13 (Suppl. S1), 1124S–1136S. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Dhanaraj, S.; Wang, Z.; Bradley, D.M.; Bowman, S.M.; Cole, B.J.; Binette, F. Minced cartilage without cell culture serves as an effective intraoperative cell source for cartilage repair. J. Orthop. Res. 2006, 24, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, R.; Nakasa, T.; Ishikawa, M.; Tsuyuguchi, Y.; Matsubara, N.; Miyaki, S.; Adachi, N. Repair of an osteochondral defect with minced cartilage embedded in atelocollagen gel: A rabbit model. Am. J. Sports Med. 2019, 47, 2216–2224. [Google Scholar] [CrossRef]
- Cole, B.J.; Farr, J.; Winalski, C.S.; Hosea, T.; Richmond, J.; Mandelbaum, B.; De Deyne, P.G. Outcomes after a single-stage procedure for cell-based cartilage repair: A prospective clinical safety trial with 2-year follow-up. Am. J. Sports Med. 2011, 39, 1170–1179. [Google Scholar] [CrossRef]
- Christensen, B.B.; Foldager, C.B.; Jensen, J.; Lind, M. Autologous dual-tissue transplantation for osteochondral repair: Early clinical and radiological results. Cartilage 2015, 6, 166–173. [Google Scholar] [CrossRef]
- Massen, F.K.; Inauen, C.R.; Harder, L.P.; Runer, A.; Preiss, S.; Saltzmann, G.M. One-step autologous minced cartilage procedure for the treatment of knee joint chondral and osteochondral lesions: A series of 27 patients with 2-year follow-up. Orthop. J. Sports Med. 2019, 7, 2325967119853773. [Google Scholar] [CrossRef]
- Wong, S.H.J.; Chiu, K.; Yan, C.H. Review article: Osteophyte. J. Orthop. Surg. 2016, 24, 403–410. [Google Scholar] [CrossRef]
- Roelofs, A.J.; Zupan, J.; Riemen, A.H.K.; Kania, K.; Ansboro, S.; White, N.; Clark, S.M.; De Bari, C. Joint morphogenetic cells in the adult mammalian synovium. Nat. Commun. 2017, 8, 15040. [Google Scholar] [CrossRef] [PubMed]
- Gelse, K.; Ekici, A.B.; Cipa, F.; Swoboda, B.; Carl, H.D.; Olk, A.; Hennig, F.F.; Klinger, P. Molecular differentiation between osteophytic and articular cartilage-clues for a transient and permanent chondrocyte phenotype. Osteoarthr. Cartil. 2012, 20, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, A.E.M.; Agergaard, J.; Schjerling, P.; Heinemeier, K.M.; van Hall, G.; Kjaer, M. The regional turnover of cartilage collagen matrix in late-stage human knee osteoarthritis. Osteoarthr. Cartil. 2022, 30, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, F.H. Closure of joint cartilage defects using cartilage fragments and fibrin glue. Fortschr. Med. 1983, 101, 1650–1652. [Google Scholar] [PubMed]
- Bonasia, D.E.; Marmotti, A.; Rosso, F.; Collo, G.; Rossi, R. Use of chondral fragments for one stage cartilage repair: A systematic review. World J. Orthop. 2015, 6, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Angele, P.; Fritz, J.; Albrecht, D.; Koh, J.; Zellner, J. Defect type, localization and marker gene expression determines early adverse events of matrix-associated autologous chondrocyte implantation. Injury 2015, 46 (Suppl. S4), S2–S9. [Google Scholar] [CrossRef] [PubMed]
- Ossendorf, R.; Grede, L.; Scheidt, S.; Strauss, A.C.; Burger, C.; Wirtz, D.; Saltzmann, G.M.; Schildberg, F.A. Comparison of minced cartilage implantation with autologous chondrocyte transplantation in an in vitro inflammation model. Cells 2024, 13, 546. [Google Scholar] [CrossRef] [PubMed]
- Gelse, K.; Söder, S.; Eger, W.; Diemtar, T.; Aigner, T. Osteophyte development. Molecular characterization of differentiation stages. Osteoarthr. Cartil. 2003, 11, 141–148. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Siebuhr, A.S.; Brandt-Hansen, N.U.; Wang, J.; Su, D.; Zheng, Q.; Simonsen, O.; Petersen, K.K.; Arendt-Nielsen, L.; Eskehave, T.; et al. Type X collagen levels are elevated in serum from human osteoarthritis patients and associated with biomarkers of cartilage degradation and inflammation. BMC Musculoskelet. Disord. 2014, 15, 309. [Google Scholar] [CrossRef]
- Walker, G.D.; Fischer, M.; Gannon, J.; Thompson, R.C., Jr.; Oegema, T.R., Jr. Expression of type-X collagen in osteoarthritis. J. Orthop. Res. 1995, 13, 4–12. [Google Scholar] [CrossRef]
- Jeanne, M.; Jorgensen, J.; Gould, D.B. Molecular and genetic analyses of collagen type Ⅳ mutant mouse models of spontaneous intracerebral hemorrhage identify mechanisms for stroke prevention. Circulation 2015, 131, 1555–1565. [Google Scholar] [CrossRef]
- Wen, Y.; Yang, H.; Wu, J.; Wang, A.; Chen, X.; Hu, S.; Zhang, Y.; Bai, D.; Jin, Z. COL4A2 in the tissue-specific extracellular matrix plays important role on osteogenic differentiation of periodontal ligament stem cells. Theranostics 2019, 9, 4265–4286. [Google Scholar] [CrossRef] [PubMed]
- Dunn, L.L.; de Valence, S.; Tille, J.-C.; Hammel, P.; Walpoth, B.H.; Stocker, R.; Imhof, B.A.; Miljkovic-Licina, M. Biodegradable and plasma-treated electrospun scaffolds coated with recombinant Olfactomedin-like 3 for accelerating wound healing and tissue regeneration. Wound Rep. Reg. 2016, 24, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Anuj, A.; Reuven, N.; Roberts, S.G.E.; Elson, A. BASP1 down-regulates RANKL-induced osteoclastogenesis. Exp. Cell Res. 2023, 431, 113758. [Google Scholar] [CrossRef]
- Zhu, L.; Weng, Z.; Shen, P.; Zhou, J.; Zheng, J.; Weng, F.; Zhang, X.; Yang, H. S100B regulates inflammatory response during osteoarthritis via fibroblast growth factor receptor 1 signaling. Mol. Med. Rep. 2018, 18, 4855–4864. [Google Scholar] [CrossRef]
- Liu, D.; Li, R.; Shi, M.; Kuang, Y.; Wang, J.; Shen, C.; Qiu, Q.; Liang, L.; Xiao, Y.; Xu, H. SMOC2 promotes aggressive behavior of fibroblast-like synoviocytes in rheumatoid arthritis through transcriptional and post-transcriptional regulating MYO1C. Cell Death Dis. 2022, 13, 1035. [Google Scholar] [CrossRef] [PubMed]
- Zebboudj, A.F.; Imura, M.; Boström, K.; Matrix, G.L.A. protein, a regulatory protein for bone morphogenetic protein-2. J. Biol. Chem. 2002, 277, 4388–4394. [Google Scholar] [CrossRef] [PubMed]
- Tuckermann, J.P.; Pittois, K.; Partridge, N.C.; Merregaert, J.; Angel, P. Collagenase-3 (MMP-13) and integral membrane protein 2a (Itm2a) are marker genes of chondrogenic/osteoblastic cells in bone formation: Sequential temporal, and spatial expression of Itm2a, alkaline phosphatase, MMP-13, and osteocalcin in the mouse. J. Bone Miner. Res. 2000, 15, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Boeuf, S.; Börger, M.; Hennig, A.; Kasten, P.; Richter, W. Enhanced ITM2A expression inhibits chondrogenic differentiation of mesenchymal stem cells. Differentiation 2009, 70, 108–115. [Google Scholar] [CrossRef]
- Santo, V.E.; Gomes, M.E.; Mano, J.F.; Reis, R.L. Controlled release strategies for bone, cartilage, and osteochondral engineering-part I: Recapitulation of native tissue healing and variables for the design of delivery systems. Tissue Eng. B Rev. 2013, 19, 308–326. [Google Scholar] [CrossRef]
- Nekomoto, A.; Nakasa, T.; Ikuta, Y.; Ding, C.; Miyaki, S.; Adachi, N. Feasibility administration of calcitonin gene-related peptide receptor antagonist on attenuation of pain and progression in osteoarthritis. Sci. Rep. 2023, 13, 15354. [Google Scholar] [CrossRef] [PubMed]
- Tsuyuguchi, Y.; Nakasa, T.; Ishikawa, M.; Miyaki, S.; Matsushita, R.; Kanemitsu, M.; Adachi, N. The benefit of minced cartilage over isolated chondrocytes in atelocollagen gel on chondrocyte proliferation and migration. Cartilage 2021, 12, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Muraki, S.; Oka, H.; Akune, T.; Mabuchi, A.; En-Yo, Y.; Yoshida, M.; Saika, A.; Suzuki, T.; Yoshida, H.; Ishibashi, H.; et al. Prevalence of radiographic knee osteoarthritis and its association with knee pain in the elderly of Japanese population-based cohorts: The ROAD study. Osteoarthr. Cartil. 2009, 17, 1137–1143. [Google Scholar] [CrossRef]
- Martin, J.A.; Buckwalter, J.A. Telomere erosion and senescence in human articular cartilage chondrocytes. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, B172–B179. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, T.; Muneta, T.; Sakaguchi, Y.; Nimura, A.; Yokogawa, A.; Koga, H.; Sekiya, I. Higher chondrogenic potential of fibrous- and adipose synovium-derived cells compared with subcutaneous fat-derived cells: Distinguishing properties of mesenchymal stem cells in humans. Arthritis Rheum. 2006, 54, 843–853. [Google Scholar] [CrossRef]
- Buckwalter, J.A. Articular cartilage injuries. Clin. Orthop. Relat. Res. 2002, 402, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Grogan, S.P.; Barbero, A.; Winkelmann, V.; Rieser, F.; Fitzsimmons, J.S.; O’Driscoll, S.; Martin, I.; Mainil-Varlet, P. Visual histological grading system for the evaluation of in vitro generated neocartilage. Tissue Eng. 2006, 12, 2141–2149. [Google Scholar] [CrossRef] [PubMed]
- Mankin, H.J.; Dorfman, H.; Lippiello, L.; Zarins, A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J. Bone Jt. Surg. Am. 1971, 53, 522–537. [Google Scholar] [CrossRef]
- Zhao, W.; Zou, T.; Cui, H.; Lv, Y.; Gao, D.; Ruan, C.; Zhang, X.; Zhang, Y. Parathyroid hormone (1–34) promotes the effects of 3D printed scaffold-seeded bone marrow mesenchymal stem cells on meniscus regeneration. Stem Cell Res. Ther. 2020, 11, 328. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of system-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
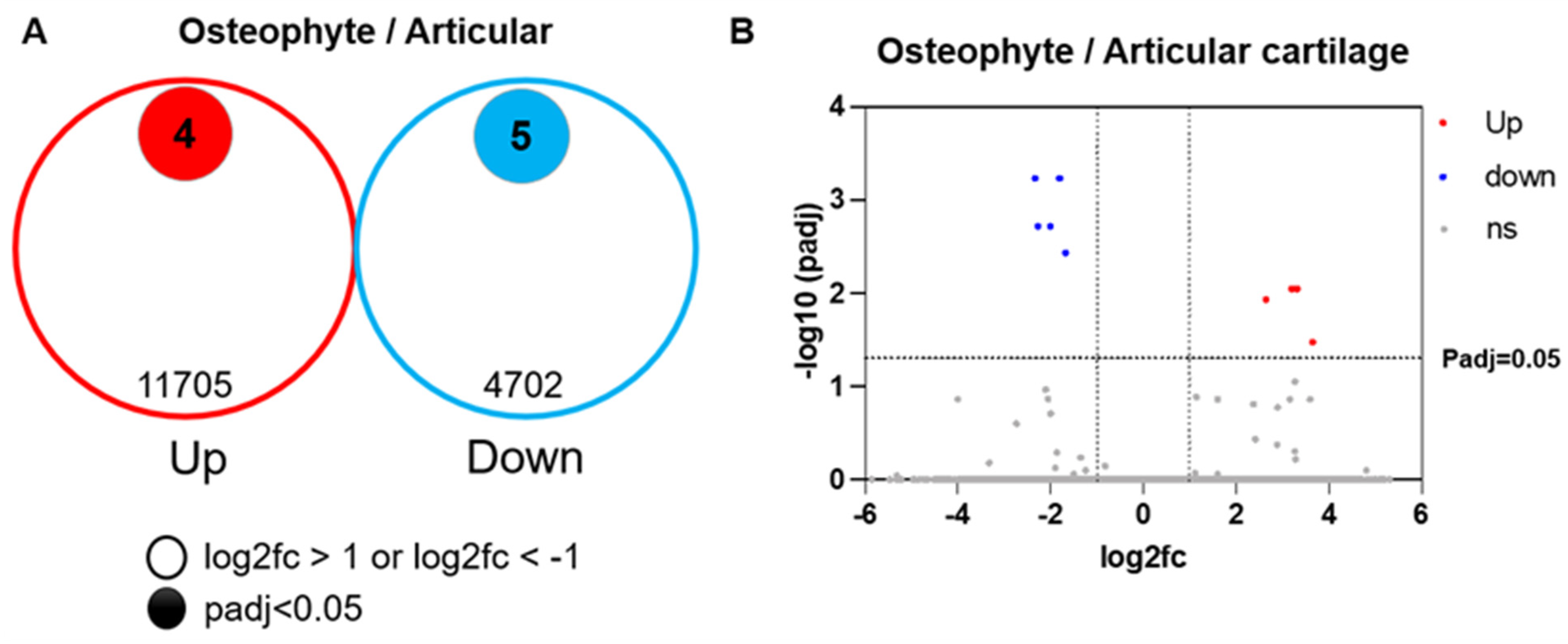

| GeneID | gene_sym | log2FoldChange (Osteophyte/Articular) | p-Value | padj | |
|---|---|---|---|---|---|
| UP | ENSG00000108821 | COL1A1 | 3.654 | <0.01 | 0.034 |
| ENSG00000134871 | COL4A2 | 3.32 | <0.01 | 0.009 | |
| ENSG00000116774 | OLFML3 | 3.201 | <0.01 | 0.009 | |
| ENSG00000176788 | BASP1 | 2.642 | <0.01 | 0.012 | |
| down | ENSG00000111341 | MGP | −1.676 | <0.01 | 0.004 |
| ENSG00000078596 | ITM2A | −1.805 | <0.01 | 0.001 | |
| ENSG00000112562 | SMOC2 | −2.004 | <0.01 | 0.002 | |
| ENSG00000160307 | S100B | −2.273 | <0.01 | 0.002 | |
| ENSG00000125845 | BMP2 | −2.336 | <0.01 | 0.002 | |
| no difference | ENSG00000139219 | COL2A1 | −0.083 | 0.891 | 0.9998 |
| ENSG00000157766 | ACAN | −0.671 | 0.123 | 0.9998 | |
| ENSG00000234899 | SOX9-AS1 | −0.239 | 0.855 | 0.9998 |
| Groups | Contents | Cell Number or Weight |
|---|---|---|
| IC | isolated chondrocyte from articular cartilage | 2.0 × 105 |
| IO | isolated chondrocyte from osteophyte cartilage | 2.0 × 105 |
| MC1 | minced cartilage from articular cartilage | 12.5 mg |
| MO1 | minced cartilage from osteophyte cartilage | 12.5 mg |
| MC2 | minced cartilage from articular cartilage | 25 mg |
| MO2 | minced cartilage from osteophyte cartilage | 25 mg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawabata, S.; Nakasa, T.; Nekomoto, A.; Yimiti, D.; Miyaki, S.; Adachi, N. Osteophyte Cartilage as a Potential Source for Minced Cartilage Implantation: A Novel Approach for Articular Cartilage Repair in Osteoarthritis. Int. J. Mol. Sci. 2024, 25, 5563. https://doi.org/10.3390/ijms25105563
Kawabata S, Nakasa T, Nekomoto A, Yimiti D, Miyaki S, Adachi N. Osteophyte Cartilage as a Potential Source for Minced Cartilage Implantation: A Novel Approach for Articular Cartilage Repair in Osteoarthritis. International Journal of Molecular Sciences. 2024; 25(10):5563. https://doi.org/10.3390/ijms25105563
Chicago/Turabian StyleKawabata, Shingo, Tomoyuki Nakasa, Akinori Nekomoto, Dilimulati Yimiti, Shigeru Miyaki, and Nobuo Adachi. 2024. "Osteophyte Cartilage as a Potential Source for Minced Cartilage Implantation: A Novel Approach for Articular Cartilage Repair in Osteoarthritis" International Journal of Molecular Sciences 25, no. 10: 5563. https://doi.org/10.3390/ijms25105563
APA StyleKawabata, S., Nakasa, T., Nekomoto, A., Yimiti, D., Miyaki, S., & Adachi, N. (2024). Osteophyte Cartilage as a Potential Source for Minced Cartilage Implantation: A Novel Approach for Articular Cartilage Repair in Osteoarthritis. International Journal of Molecular Sciences, 25(10), 5563. https://doi.org/10.3390/ijms25105563




