Clinical and Immunological Impact of Ocrelizumab Extended Interval Dosing in Multiple Sclerosis: A Single-Center, Real-World Experience
Abstract
1. Introduction
2. Results
2.1. Study Cohort and Baseline Characteristics
2.2. Ocrelizumab EID Does Not Impact Clinical Outcomes
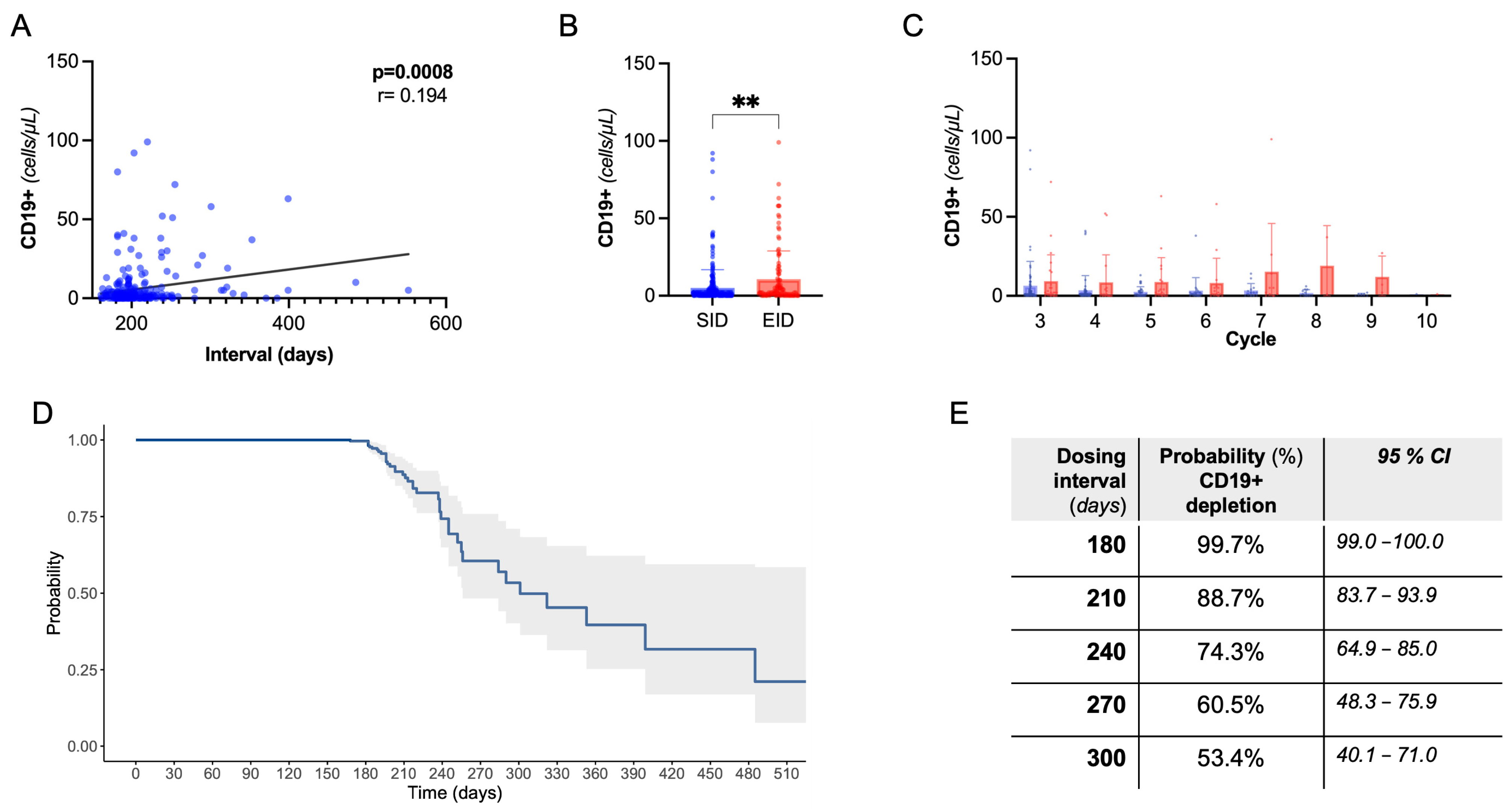
2.3. B-Cell Dynamics Are Affected by Extended Dosing without Clinical and Radiological Implications
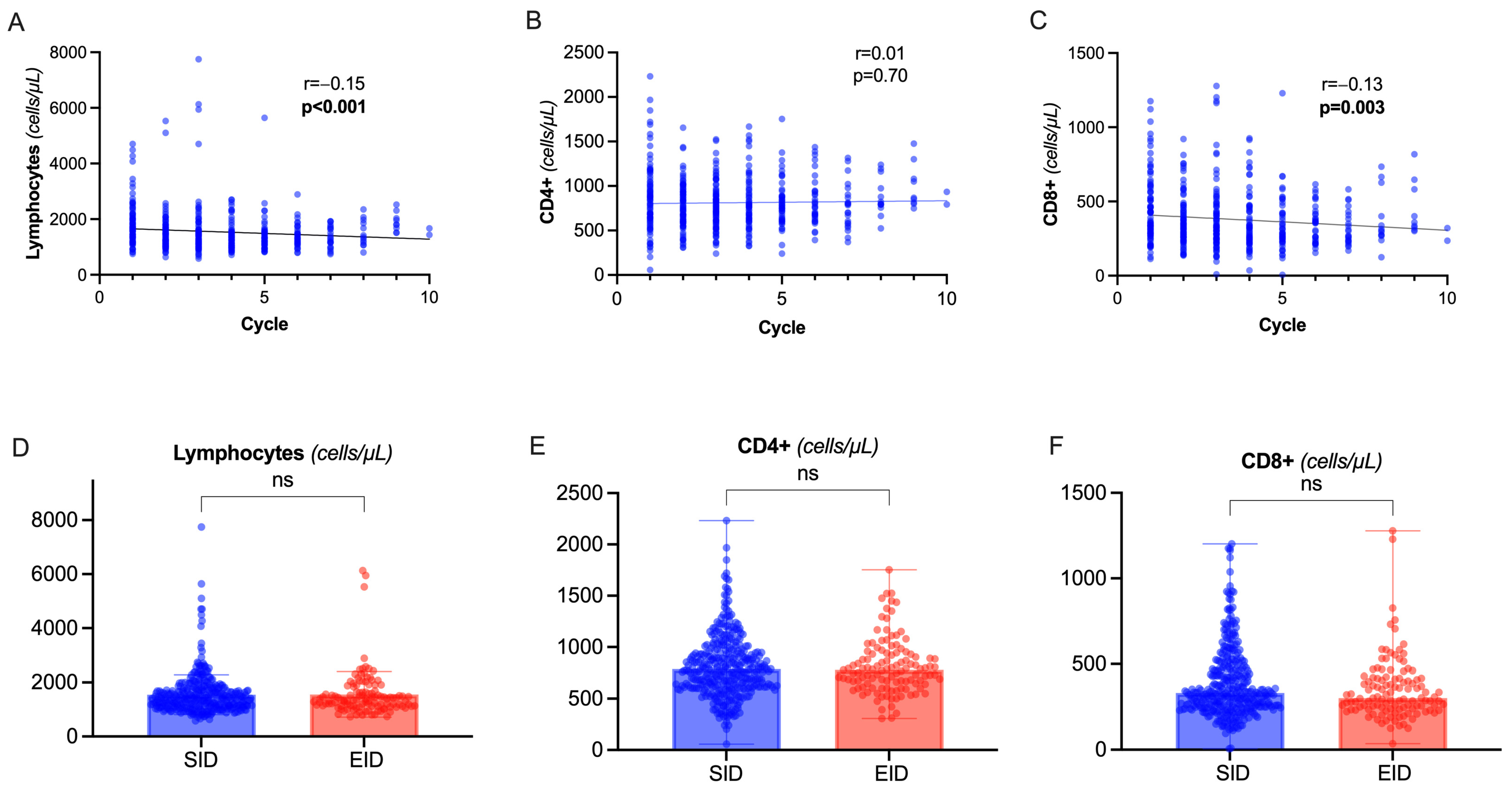
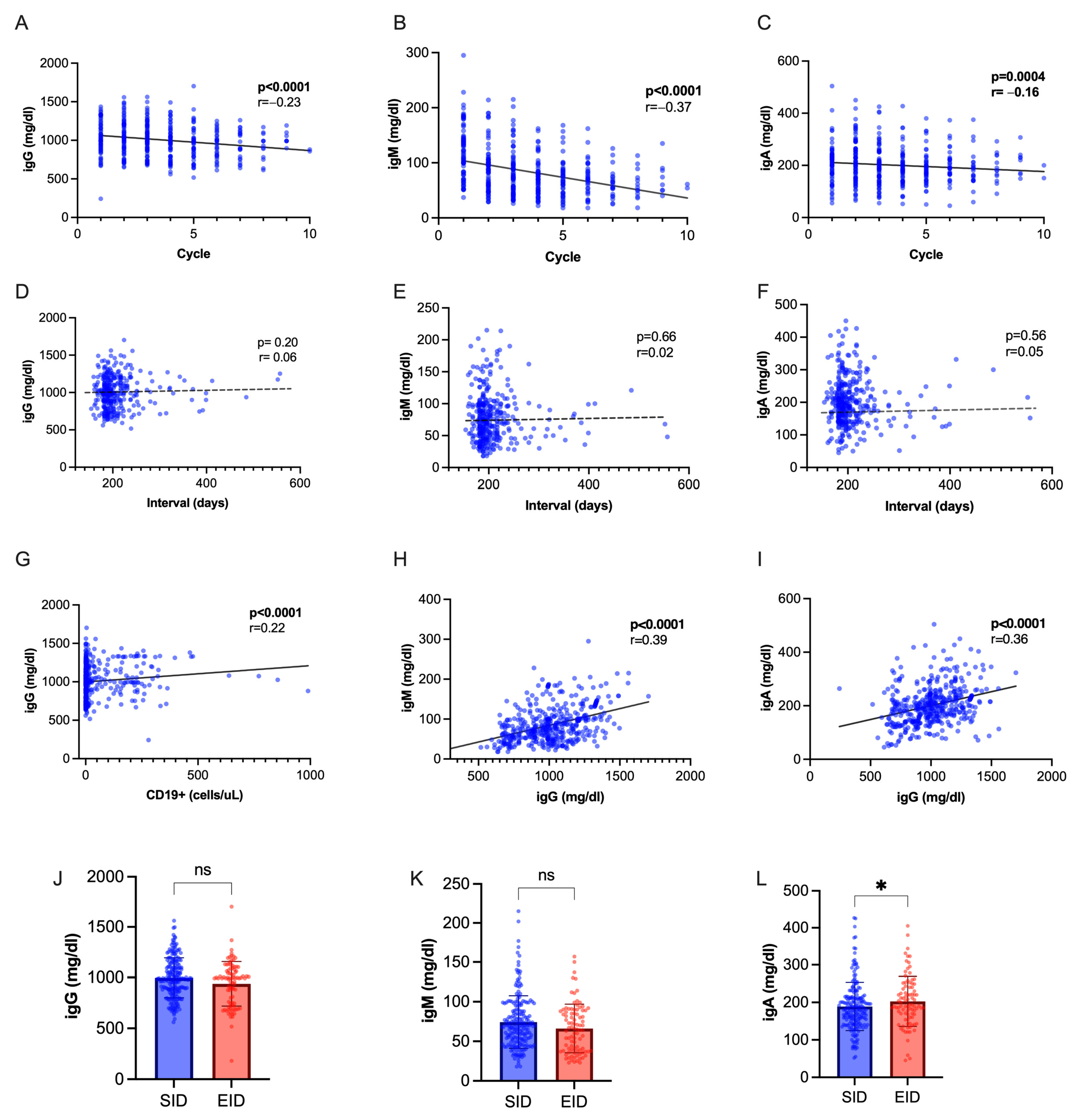
2.4. T Lymphocytes and Immunoglobulin Dynamics Are Not Significantly Different in OCR EID
3. Discussion
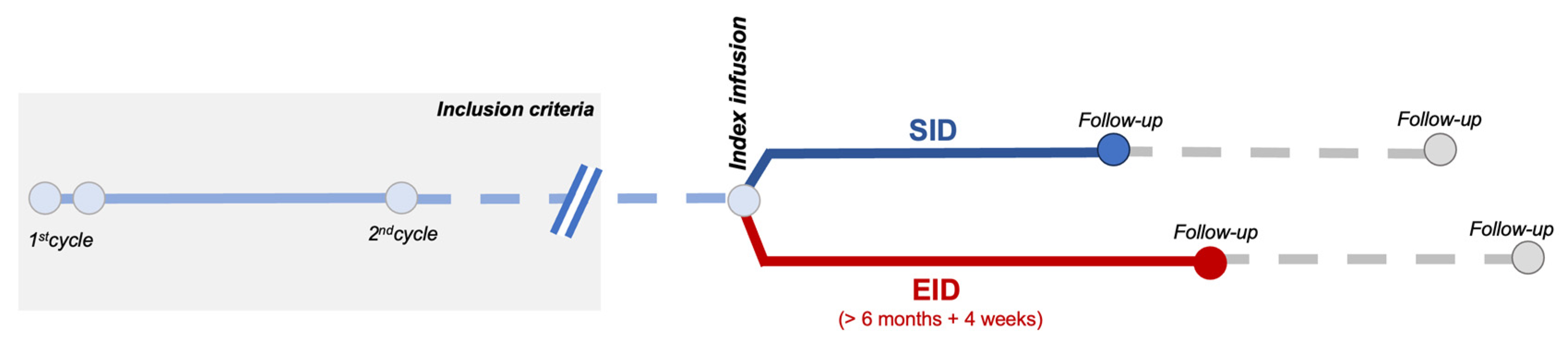
4. Materials and Methods
4.1. Inclusion Criteria and Definitions
4.2. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hauser, S.L.; Bar-Or, A.; Comi, G.; Giovannoni, G.; Hartung, H.-P.; Hemmer, B.; Lublin, F.; Montalban, X.; Rammohan, K.W.; Selmaj, K.; et al. Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N. Engl. J. Med. 2017, 376, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Chisari, C.G.; Bianco, A.; Brescia Morra, V.; Calabrese, M.; Capone, F.; Cavalla, P.; Chiavazza, C.; Comi, C.; Danni, M.; Filippi, M.; et al. Effectiveness of Ocrelizumab in Primary Progressive Multiple Sclerosis: A Multicenter, Retrospective, Real-world Study (OPPORTUNITY). Neurotherapeutics 2023, 20, 1696–1706. [Google Scholar] [CrossRef] [PubMed]
- Bigaut, K.; De Seze, J.; Collongues, N. Ocrelizumab for the treatment of multiple sclerosis. Expert. Rev. Neurother. 2019, 19, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.S. CD20+ T cells: An emerging T cell subset in human pathology. Inflamm. Res. 2022, 71, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Ochs, J.; Nissimov, N.; Torke, S.; Freier, M.; Grondey, K.; Koch, J.; Klein, M.; Feldmann, L.; Gudd, C.; Bopp, T.; et al. Proinflammatory CD20 + T cells contribute to CNS-directed autoimmunity. Sci. Transl. Med. 2022, 14, eabi4632. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.; Pryce, G.; James, L.K.; Marta, M.; Schmierer, K. The ocrelizumab phase II extension trial suggests the potential to improve the risk: Benefit balance in multiple sclerosis. Mult. Scler. Relat. Disord. 2020, 44, 102279. [Google Scholar] [CrossRef] [PubMed]
- Lünemann, J.D.; Ruck, T.; Muraro, P.A.; Bar-Or, A.; Wiendl, H. Immune reconstitution therapies: Concepts for durable remission in multiple sclerosis. Nat. Rev. Neurol. 2020, 16, 56–62. [Google Scholar] [CrossRef]
- Vollmer, B.L.; Wallach, A.I.; Corboy, J.R.; Dubovskaya, K.; Alvarez, E.; Kister, I. Serious safety events in rituximab-treated multiple sclerosis and related disorders. Ann. Clin. Transl. Neurol. 2020, 7, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Tallantyre, E.C.; Whittam, D.H.; Jolles, S.; Paling, D.; Constantinesecu, C.; Robertson, N.P.; Jacob, A. Secondary antibody deficiency: A complication of anti-CD20 therapy for neuroinflammation. J. Neurol. 2018, 265, 1115–1122. [Google Scholar] [CrossRef]
- Guerrieri, S.; Bucca, C.; Nozzolillo, A.; Genchi, A.; Zanetta, C.; Cetta, I.; Rugarli, G.; Gattuso, I.; Azzimonti, M.; Rocca, M.A.; et al. Ocrelizumab extended-interval dosing in multiple sclerosis during SARS-CoV-2 pandemic: A real-world experience. Eur. J. Neurol. 2023, 30, 2859–2864. [Google Scholar] [CrossRef]
- Rolfes, L.; Pawlitzki, M.; Pfeuffer, S.; Nelke, C.; Lux, A.; Pul, R.; Kleinschnitz, C.; Kleinschnitz, K.; Rogall, R.; Pape, K.; et al. Ocrelizumab Extended Interval Dosing in Multiple Sclerosis in Times of COVID-19. Neurol.—Neuroimmunol. Neuroinflamm. 2021, 8, e1035. [Google Scholar] [CrossRef]
- Zanghì, A.; Avolio, C.; Signoriello, E.; Abbadessa, G.; Cellerino, M.; Ferraro, D.; Messina, C.; Barone, S.; Callari, G.; Tsantes, E.; et al. Is It Time for Ocrelizumab Extended Interval Dosing in Relapsing Remitting MS? Evidence from an Italian Multicenter Experience during the COVID-19 Pandemic. Neurotherapeutics 2022, 19, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Schuckmann, A.; Steffen, F.; Zipp, F.; Bittner, S.; Pape, K. Impact of extended interval dosing of ocrelizumab on immunoglobulin levels in multiple sclerosis. Med 2023, 4, 361–372.e3. [Google Scholar] [CrossRef]
- Barun, B.; Gabelić, T.; Adamec, I.; Babić, A.; Lalić, H.; Batinić, D.; Krbot Skorić, M.; Habek, M. Influence of delaying ocrelizumab dosing in multiple sclerosis due to COVID-19 pandemics on clinical and laboratory effectiveness. Mult. Scler. Relat. Disord. 2021, 48, 102704. [Google Scholar] [CrossRef] [PubMed]
- van Lierop, Z.Y.; Toorop, A.A.; van Ballegoij, W.J.; Olde Dubbelink, T.B.; Strijbis, E.M.; de Jong, B.A.; van Oosten, B.W.; Moraal, B.; Teunissen, C.E.; Uitdehaag, B.M.; et al. Personalized B-cell tailored dosing of ocrelizumab in patients with multiple sclerosis during the COVID-19 pandemic. Mult. Scler. J. 2022, 28, 1121–1125. [Google Scholar] [CrossRef]
- Novi, G.; Bovis, F.; Fabbri, S.; Tazza, F.; Gazzola, P.; Maietta, I.; Currò, D.; Bruschi, N.; Roccatagliata, L.; Boffa, G.; et al. Tailoring B cell depletion therapy in MS according to memory B cell monitoring. Neurol.—Neuroimmunol. Neuroinflamm. 2020, 7, e845. [Google Scholar] [CrossRef]
- Sahi, N.K.; Abidi, S.M.A.; Salim, O.; Abraham, R.; Kalra, S.; Al-Araji, A. Clinical impact of Ocrelizumab extended interval dosing during the COVID-19 pandemic and associations with CD19+ B-cell repopulation. Mult. Scler. Relat. Disord. 2021, 56, 103287. [Google Scholar] [CrossRef]
- Smets, I.; Wokke, B.; Smolders, J. Should anti-CD20 be used as an immune reconstitution therapy? Mult. Scler. J. 2023, 29, 308–310. [Google Scholar] [CrossRef]
- Rodriguez-Mogeda, C.; van Lierop, Z.Y.G.J.; van der Pol, S.M.A.; Coenen, L.; Hogenboom, L.; Kamermans, A.; Rodriguez, E.; van Horssen, J.; van Kempen, Z.L.E.; Uitdehaag, B.M.J.; et al. Extended interval dosing of ocrelizumab modifies the repopulation of B cells without altering the clinical efficacy in multiple sclerosis. J. Neuroinflamm. 2023, 20, 215. [Google Scholar] [CrossRef] [PubMed]
- Antulov, R.; Weinstock-Guttman, B.; Cox, J.; Hussein, S.; Durfee, J.; Caiola, C.; Dwyer, M.; Bergsland, N.; Abdelrahman, N.; Stosic, M.; et al. Gender-related differences in MS: A study of conventional and nonconventional MRI measures. Mult. Scler. J. 2009, 15, 345–354. [Google Scholar] [CrossRef]
- Forsthuber, T.G.; Cimbora, D.M.; Ratchford, J.N.; Katz, E.; Stüve, O. B cell-based therapies in CNS autoimmunity: Differentiating CD19 and CD20 as therapeutic targets. Ther. Adv. Neurol. Disord. 2018, 11, 175628641876169. [Google Scholar] [CrossRef] [PubMed]
- AbdelRazek, M.A.; Casasola, M.; Mollashahi, R.; Brodski, A.; Morin, S.; Augustynowicz, A.; Jassim, S.; Matiello, M.; Sloane, J. Extended B-cell depletion beyond 6-months in patients receiving ocrelizumab or rituximab for CNS demyelinating disease. Mult. Scler. Relat. Disord. 2022, 59, 103505. [Google Scholar] [CrossRef] [PubMed]
- Mathias, A.; Pantazou, V.; Perriot, S.; Canales, M.; Jones, S.; Oberholster, L.; Moulin, M.; Fenwick, C.; Bernard-Valnet, R.; Théaudin, M.; et al. Ocrelizumab Impairs the Phenotype and Function of Memory CD8 + T Cells. Neurol.—Neuroimmunol. Neuroinflamm. 2023, 10, e200084. [Google Scholar] [CrossRef]
- Shinoda, K.; Li, R.; Rezk, A.; Mexhitaj, I.; Patterson, K.R.; Kakara, M.; Zuroff, L.; Bennett, J.L.; von Büdingen, H.-C.; Carruthers, R.; et al. Differential effects of anti-CD20 therapy on CD4 and CD8 T cells and implication of CD20-expressing CD8 T cells in MS disease activity. Proc. Natl. Acad. Sci. USA 2023, 120, e2207291120. [Google Scholar] [CrossRef]
- Landi, D.; Grimaldi, A.; Bovis, F.; Ponzano, M.; Fantozzi, R.; Buttari, F.; Signoriello, E.; Lus, G.; Lucchini, M.; Mirabella, M.; et al. Influence of Previous Disease-Modifying Drug Exposure on T-Lymphocyte Dynamic in Patients With Multiple Sclerosis Treated With Ocrelizumab. Neurol.—Neuroimmunol. Neuroinflamm. 2022, 9, e1157. [Google Scholar] [CrossRef]
- Alvarez, E.; Longbrake, E.E.; Rammohan, K.W.; Stankiewicz, J.; Hersh, C.M. Secondary hypogammaglobulinemia in patients with multiple sclerosis on anti-CD20 therapy: Pathogenesis, risk of infection, and disease management. Mult. Scler. Relat. Disord. 2023, 79, 105009. [Google Scholar] [CrossRef] [PubMed]
- Perriguey, M.; Maarouf, A.; Stellmann, J.-P.; Rico, A.; Boutiere, C.; Demortiere, S.; Durozard, P.; Pelletier, J.; Audoin, B. Hypogammaglobulinemia and Infections in Patients With Multiple Sclerosis Treated With Rituximab. Neurol.—Neuroimmunol. Neuroinflamm. 2022, 9, e1115. [Google Scholar] [CrossRef]
- Mears, V.; Jakubecz, C.; Seeco, C.; Woodson, S.; Serra, A.; Abboud, H. Predictors of hypogammaglobulinemia and serious infections among patients receiving ocrelizumab or rituximab for treatment of MS and NMOSD. J. Neuroimmunol. 2023, 377, 578066. [Google Scholar] [CrossRef]
- Otani, I.M.; Lehman, H.K.; Jongco, A.M.; Tsao, L.R.; Azar, A.E.; Tarrant, T.K.; Engel, E.; Walter, J.E.; Truong, T.Q.; Khan, D.A.; et al. Practical guidance for the diagnosis and management of secondary hypogammaglobulinemia: A Work Group Report of the AAAAI Primary Immunodeficiency and Altered Immune Response Committees. J. Allergy Clin. Immunol. 2022, 149, 1525–1560. [Google Scholar] [CrossRef]
| All (N = 93) | SID (N = 29) | EID (N = 64) | p Value | |
|---|---|---|---|---|
| Age (years, mean ± SD) | 46 ± 14 | 46 ± 14 | 45.5 ± 15.3 | 0.845 |
| Male sex (N, %) | 45 (48.4%) | 13 (44.8%) | 32 (50%) | 0.66 |
| RRMS (N, %) PPMS (N%) | 70 (75.3%) 23 (24.7%) | 22 (75.9%) 7 (24.1%) | 48 (75%) 16 (25%) | 1.0 |
| Baseline EDSS | 3.5 (3.5) | 3.5 (3.0) | 3.5 (3.5) | 0.845 |
| Baseline ARR | 0.479 (0.764) | 0.75 (1.04) | 0.472 (0.58) | 0.159 |
| Disease duration (years, mean ± SD) | 7.227 ± 13.3 | 2.97 ± 11.9 | 7.59 ± 13.7 | 0.06 |
| Clinical activity in the year before starting OCR (N, %) | 48 (51.6%) | 14 (48.3%) | 34 (53.1%) | 0.54 |
| MRI activity in the year before starting OCR (N,%) | 53 (56.9%) | 16 (55.2%) | 40 (62.5%) | 0.648 |
| Cumulative EID | Median = 1 | - | Median = 1 | -- |
| Naive (N, %) | 23 (24.7%) | 10 (34.5%) | 13 (20.3%) | 0.194 |
| Last DMT | - | |||
| ALEMTUZUMAB | 7 | 2 | 5 | |
| AZATIOPRINE | 1 | 0 | 1 | |
| CLADRIBINE | 2 | 0 | 2 | |
| DMF | 12 | 4 | 8 | |
| FINGOLIMOD | 11 | 1 | 10 | |
| GA | 10 | 5 | 5 | |
| IFN | 9 | 2 | 7 | |
| NATALIZUMAB | 11 | 5 | 6 | |
| RITUXIMAB | 2 | 0 | 2 | |
| TERIFLUNOMIDE | 5 | 0 | 5 | |
| Reason to switch: | 0.368 | |||
| Efficacy (N, %) | 58 (62.4%) | 16 (55.1%) | 42 (65.6%) | |
| Safety (N, %) | 12 (12.9%) | 3 (10.4%) | 9 (14.1%) | |
| N Previous DMT | 1 (2) | 1 (2) | 2 (2) | 0.09 |
| Follow-up duration after OCR initiation (months, mean ± SD) | 27.2 ± 21.2 | 18.9 ± 6.7 | 33.4 ± 16.2 | <0.001 |
| All (N = 93) | SID (N = 29) | EID (N = 64) | p Value | |
|---|---|---|---|---|
| Lymphocyte count (cells/µL) | 1828 (779) | 2131 (1007) | 1690 (611) | 0.069 |
| CD4+ (cells/µL) | 861 (384) | 947 (433) | 821 (357) | 0.274 |
| CD8+ (cells/µL) | 446 (227) | 495 (247) | 424 (216) | 0.174 |
| CD19+ (cells/µL) | 228 (160) | 291 (225) | 200 (110) | 0.128 |
| igG (mg/dL) | 1065 (219) | 1059 (229) | 1068 (216) | 0.917 |
| igA (mg/dL) | 211 (66) | 212 (79) | 211 (61) | 0.993 |
| igM (mg/dL) | 121 (48) | 117 (42) | 123 (50) | 0.502 |
| Clinical Activity | MRI Activity | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Model | Multivariate Model | Univariate Model | Multivariate Model | |||||
| Covariate | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value |
| Age | 1.02 (0.91–1.17) | 0.63 | 0.96 (0.92–1.01) | 0.87 | ||||
| Sex (male vs. female) | 0.52 (0.02–5.43) | 0.59 | 0.33 (0.07–1.14) | 0.10 | 0.37 (0.14–0.90) | 0.03 | ||
| MS phenotype (PPMS vs. RRMS) | 0.37 (0.01–79.0) | 0.99 | 0.96 (0.25–3.12) | 0.95 | ||||
| No. previous DMTs | 1.17 (0.52–2.06) | 0.63 | 0.98 (0.62–1.40) | 0.91 | ||||
| Naïve at baseline (yes vs. no) | 0.06 (0.02–13.2) | 0.99 | 1.61 (0.42–5.29) | 0.45 | ||||
| EDSS at index infusion | 1.08 (0.60–2.08) | 0.80 | 1.01 (0.76–1.37) | 0.92 | ||||
| Cumulative OCR cycles before index infusion | 0.49 (0.09–1.16) | 0.23 | 0.02 (0.01–37) | 0.99 | 0.86 (0.56–1.21) | 0.43 | ||
| Disease Duration | 1.17 (1.02–1.40) | 0.04 | 1.10 (0.93–1.31) | 0.27 | 1.01 (0.94–1.08) | 0.74 | ||
| EID (yes vs. no) | 1.16 (0.05–12.26) | 0.90 | 1.11 (0.29–3.62) | 0.87 | ||||
| Cumulative EID before index infusion | 0.77 (0.05–2.70) | 0.77 | 1.44 (0.77–2.44) | 0.21 | 1.08 (0.62–1.88) | 0.77 | ||
| Consecutive EID (yes vs. no) | 1.64 (0.08–17.36) | 0.69 | 2.03 (0.44–7.12) | 0.32 | ||||
| B-cell depletion at index infusion (no vs. yes) | 1.26 (0.04–26.7) | 0.99 | 1.45 (0.46–3.75) | 0.47 | ||||
| MRI activity before index infusion (yes vs. no) | 20.0 (0.76–237.0) | 0.04 | 6.56 (0.29–155) | 0.23 | 1.75 (0.09–10.0) | 0.61 | ||
| Clinical activity before index infusion (yes vs. no) | 3.36 (0.15–35.70) | 0.33 | 3.29 (1.28–7.82) | 0.04 | 3.37 (1.30–8.73) | 0.01 | ||
| Loss of NEDA-3 | ||||
|---|---|---|---|---|
| Univariate Model | Multivariate Model | |||
| Covariate | OR (95% CI) | p Value | OR (95% CI) | p Value |
| Age | 0.97 (0.94–0.99) | 0.04 | 0.98 (0.95–1.03) | 0.57 |
| Sex (male vs. female) | 0.62 (0.34–1.11) | 0.11 | 0.84 (0.42–1.72) | 0.64 |
| MS phenotype (PPMS vs. RRMS) | 0.59 (0.29–1.12) | 0.12 | 0.80 (0.33–1.91) | 0.61 |
| No. previous DMTs | 1.05 (0.87–1.25) | 0.56 | ||
| Naïve at baseline (yes vs. no) | 0.94 (0.46–1.79) | 0.85 | ||
| EDSS at index infusion | 0.98 (0.85–1.13) | 0.79 | ||
| Cumulative OCR cycles before index infusion | 0.85 (0.71–1.01) | 0.06 | 0.82 (0.67–0.99) | 0.24 |
| Disease Duration | 1.01 (0.98–1.05) | 0.48 | ||
| EID (yes vs. no) | 1.35 (0.73–2.43) | 0.32 | ||
| Cumulative EID before index infusion | 0.93 (0.62–1.33) | 0.70 | ||
| Consecutive EID (yes vs. no) | 1.24 (0.63–2.31) | 0.52 | ||
| B-cell depletion at index infusion (no vs. yes) | 0.99 (0.41–2.12) | 0.98 | ||
| MRI activity at previous cycle (yes vs. no) | 1.01 (0.15–3.87) | 0.98 | ||
| Clinical activity at previous cycle (yes vs. no) | 3.35 (0.15–35.89) | 0.33 | ||
| Occurrence of Hypo-igG | ||||
|---|---|---|---|---|
| Univariate Model | Multivariate Model | |||
| Covariate | OR (95% CI) | p Value | OR (95% CI) | p Value |
| Age | 1.02 (0.98–1.06) | 0.25 | ||
| Sex (male vs. female) | 0.90 (0.42–1.91) | 0.77 | ||
| MS phenotype (PPMS vs. RRMS) | 0.55 (0.21–1.28) | 0.19 | 0.46 (0.17–1.21) | 0.11 |
| No. previous DMTs | 1.17 (0.92–1.47) | 0.17 | 0.94 (0.71–1.25) | 0.68 |
| Naïve at baseline (yes vs. no) | 0.77 (0.28–1.85) | 0.58 | ||
| EDSS at index infusion | 1.12 (0.92–1.37) | 0.26 | ||
| Cumulative OCR cycles before index infusion | 1.07 (0.81–1.31) | 0.51 | ||
| Disease Duration | 1.05 (1.01–1.10) | 0.02 | 1.04 (0.98–1.09) | 0.11 |
| EID (yes vs. no) | 1.03 (0.94–1.14) | 0.53 | ||
| Cumulative EID before index infusion | 0.99 (0.52–1.90) | 0.90 | ||
| Consecutive EID (yes vs. no) | 1.10 (0.63–1.71) | 0.70 | ||
| B-cell depletion at index infusion (no vs. yes) | 0.98 (0.28–2.70) | 0.96 | ||
| Hypo-IgG at index infusion (yes vs. no) | 4.44 (1.58–11.50) | 0.002 | 4.00 (1.37–11.7) | 0.01 |
| Hypo-IgM at index infusion (yes vs. no) | 1.01 (0.29–2.79) | 0.98 | ||
| Hypo-IgA at previous infusion (yes vs. no) | 3.01 (0.15–24.40) | 0.35 | ||
| Occurrence of Hypo-igM | ||||
|---|---|---|---|---|
| Univariate Model | Multivariate Model | |||
| Covariate | OR (95% CI) | p Value | OR (95% CI) | p Value |
| Age | 1.01 (0.98–1.04) | 0.49 | ||
| Sex (male vs. female) | 3.09 (1.61–6.21) | 0.001 | 3.39 (1.65–6.96) | 0.001 |
| MS phenotype (PPMS vs. RRMS) | 1.02 (0.53–1.93) | 0.94 | ||
| No. previous DMTs | 1.03 (0.83–1.25) | 0.81 | ||
| Naïve at baseline (yes vs. no) | 0.31 (0.10–0.75) | 0.02 | 0.41 (0.15–1.16) | 0.09 |
| EDSS at index infusion | 1.01 (0.87–1.17)) | 0.98 | ||
| Cumulative OCR cycles before index infusion | 1.01 (0.84–1.20) | 0.89 | ||
| Disease Duration | 1.01 (0.97–1.05) | 0.62 | ||
| EID (yes vs. no) | 0.95 (0.51–1.78) | 0.89 | ||
| Cumulative EID before index infusion | 0.99 (0.26–2.38) | 0.98 | ||
| Consecutive EID (yes vs. no) | 1.03 (0.92–1.14 | 0.54 | ||
| B-cell depletion at index infusion (no vs. yes) | 1.61 (0.96–1.51) | 0.19 | 1.23 (0.54–2.80) | 0.61 |
| Hypo-IgG at index infusion (yes vs. no) | 3.47 (1.42–8.45) | 0.006 | 1.96 (0.71–5.41) | 0.19 |
| Hypo-IgM at index infusion (yes vs. no) | 5.40 (2.60–11.26) | <0.001 | 4.67 (2.11–10.67) | <0.001 |
| Hypo-IgA at index infusion (yes vs. no) | 5.20 (0.71–37.77) | 0.10 | 1.80 (0.18–11.70) | 0.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasello, M.; Zancan, V.; Rinaldi, V.; Marrone, A.; Reniè, R.; Diamant, S.; Marconi, M.; Le Mura, L.; Salvetti, M.; Buscarinu, M.C.; et al. Clinical and Immunological Impact of Ocrelizumab Extended Interval Dosing in Multiple Sclerosis: A Single-Center, Real-World Experience. Int. J. Mol. Sci. 2024, 25, 5353. https://doi.org/10.3390/ijms25105353
Nasello M, Zancan V, Rinaldi V, Marrone A, Reniè R, Diamant S, Marconi M, Le Mura L, Salvetti M, Buscarinu MC, et al. Clinical and Immunological Impact of Ocrelizumab Extended Interval Dosing in Multiple Sclerosis: A Single-Center, Real-World Experience. International Journal of Molecular Sciences. 2024; 25(10):5353. https://doi.org/10.3390/ijms25105353
Chicago/Turabian StyleNasello, Martina, Valeria Zancan, Virginia Rinaldi, Antonio Marrone, Roberta Reniè, Selene Diamant, Martina Marconi, Lorenzo Le Mura, Marco Salvetti, Maria Chiara Buscarinu, and et al. 2024. "Clinical and Immunological Impact of Ocrelizumab Extended Interval Dosing in Multiple Sclerosis: A Single-Center, Real-World Experience" International Journal of Molecular Sciences 25, no. 10: 5353. https://doi.org/10.3390/ijms25105353
APA StyleNasello, M., Zancan, V., Rinaldi, V., Marrone, A., Reniè, R., Diamant, S., Marconi, M., Le Mura, L., Salvetti, M., Buscarinu, M. C., & Bellucci, G. (2024). Clinical and Immunological Impact of Ocrelizumab Extended Interval Dosing in Multiple Sclerosis: A Single-Center, Real-World Experience. International Journal of Molecular Sciences, 25(10), 5353. https://doi.org/10.3390/ijms25105353






