Mesothelioma-Associated Fibroblasts Modulate the Response of Mesothelioma Patient-Derived Organoids to Chemotherapy via Interleukin-6
Abstract
1. Introduction
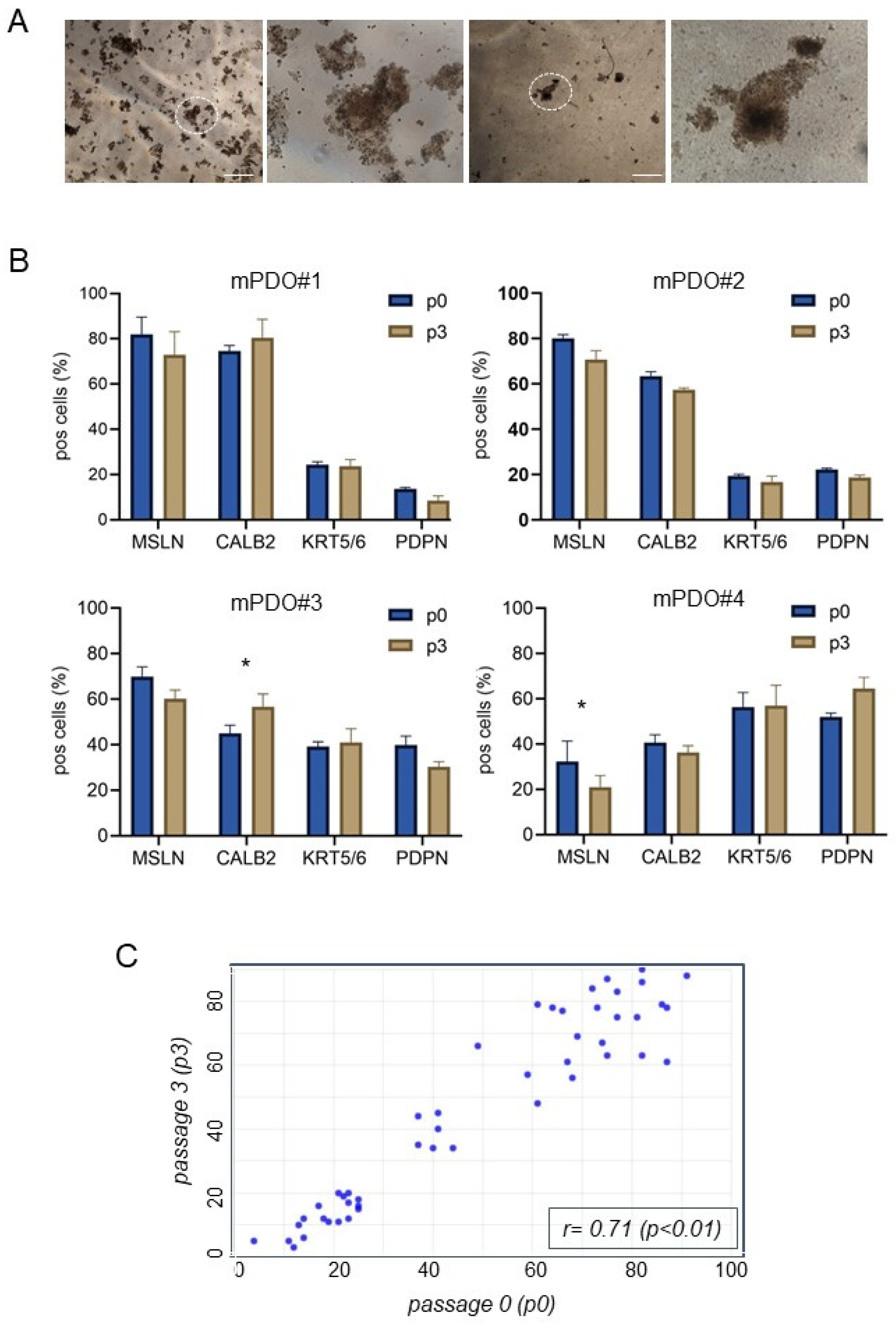
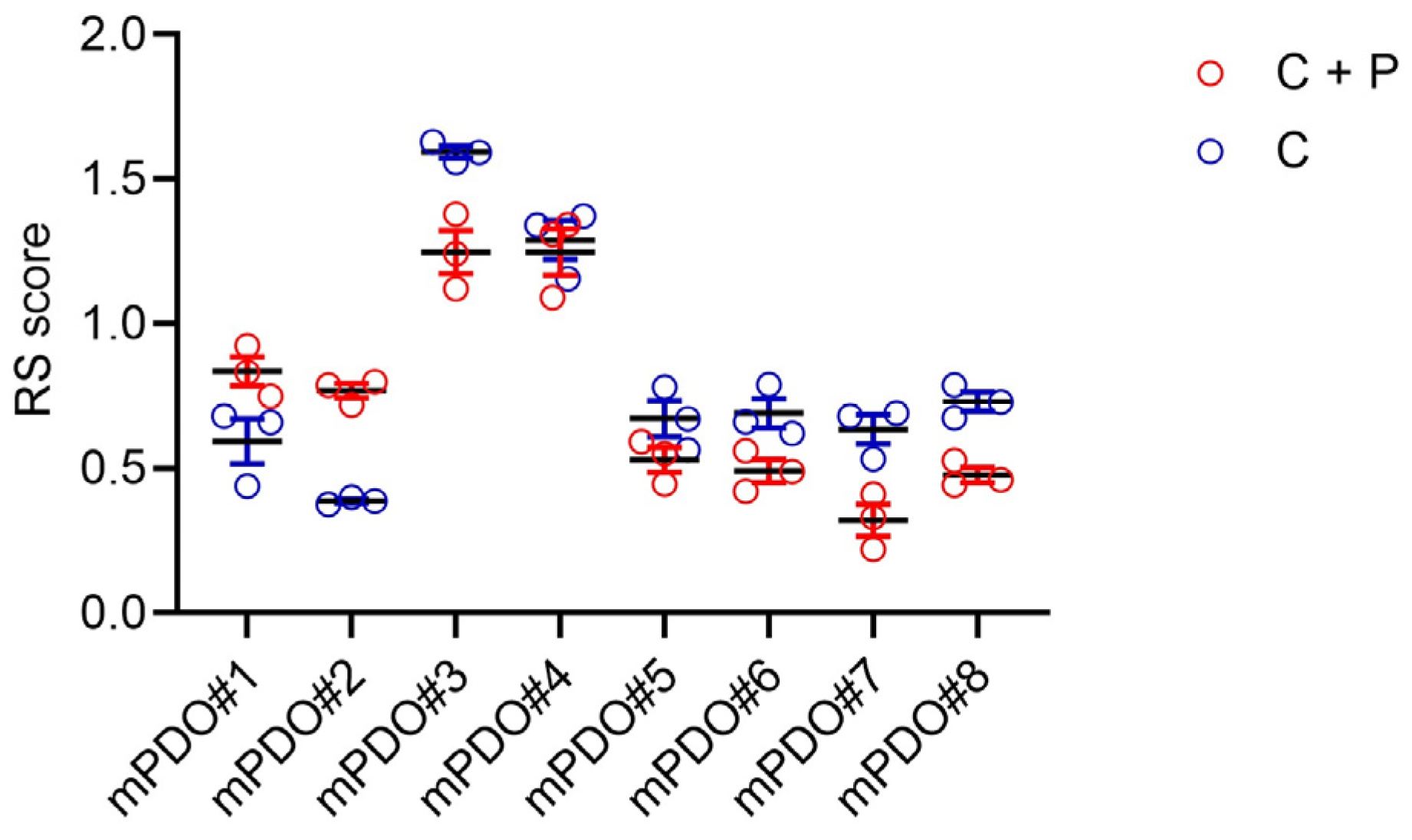
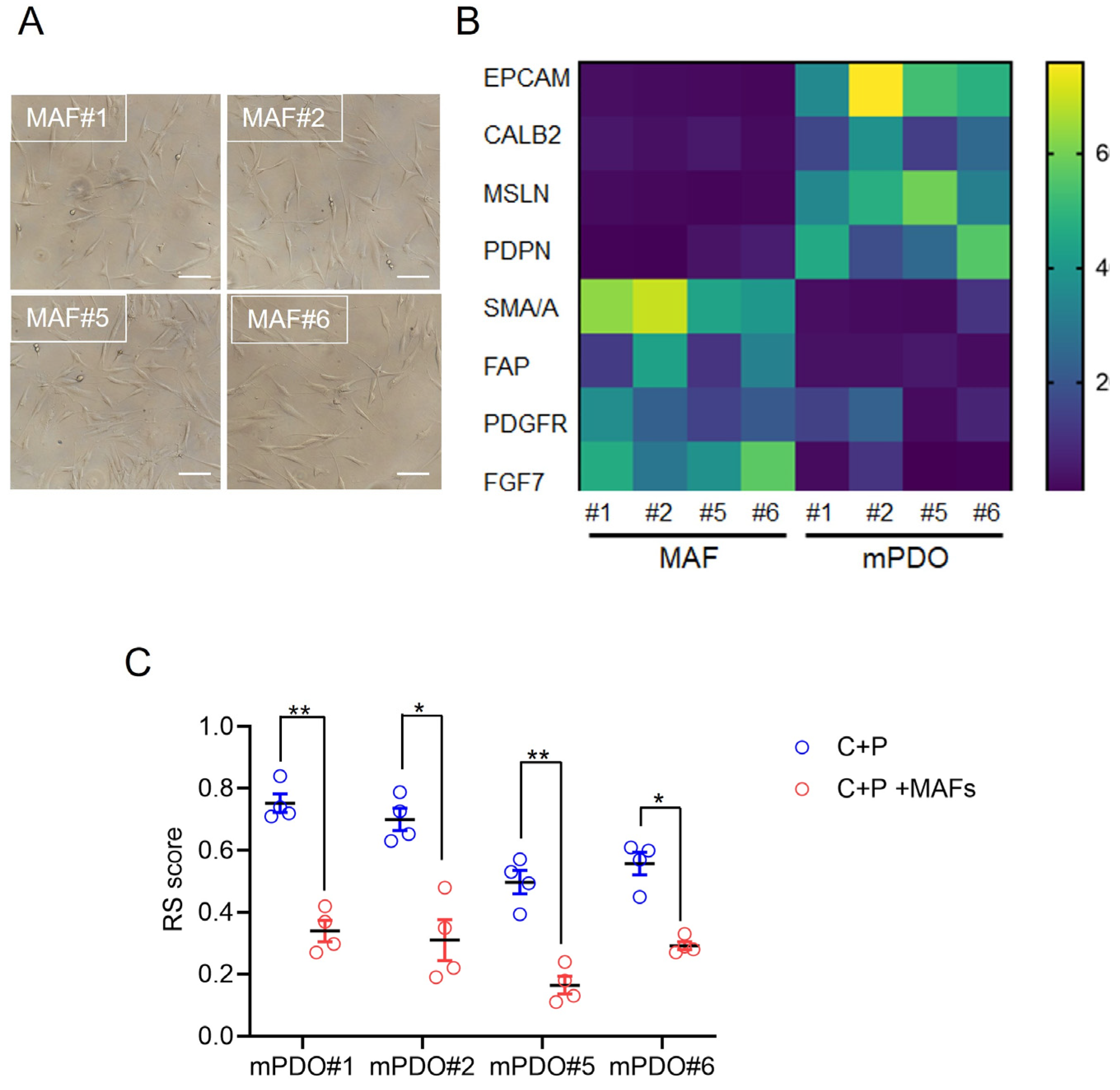
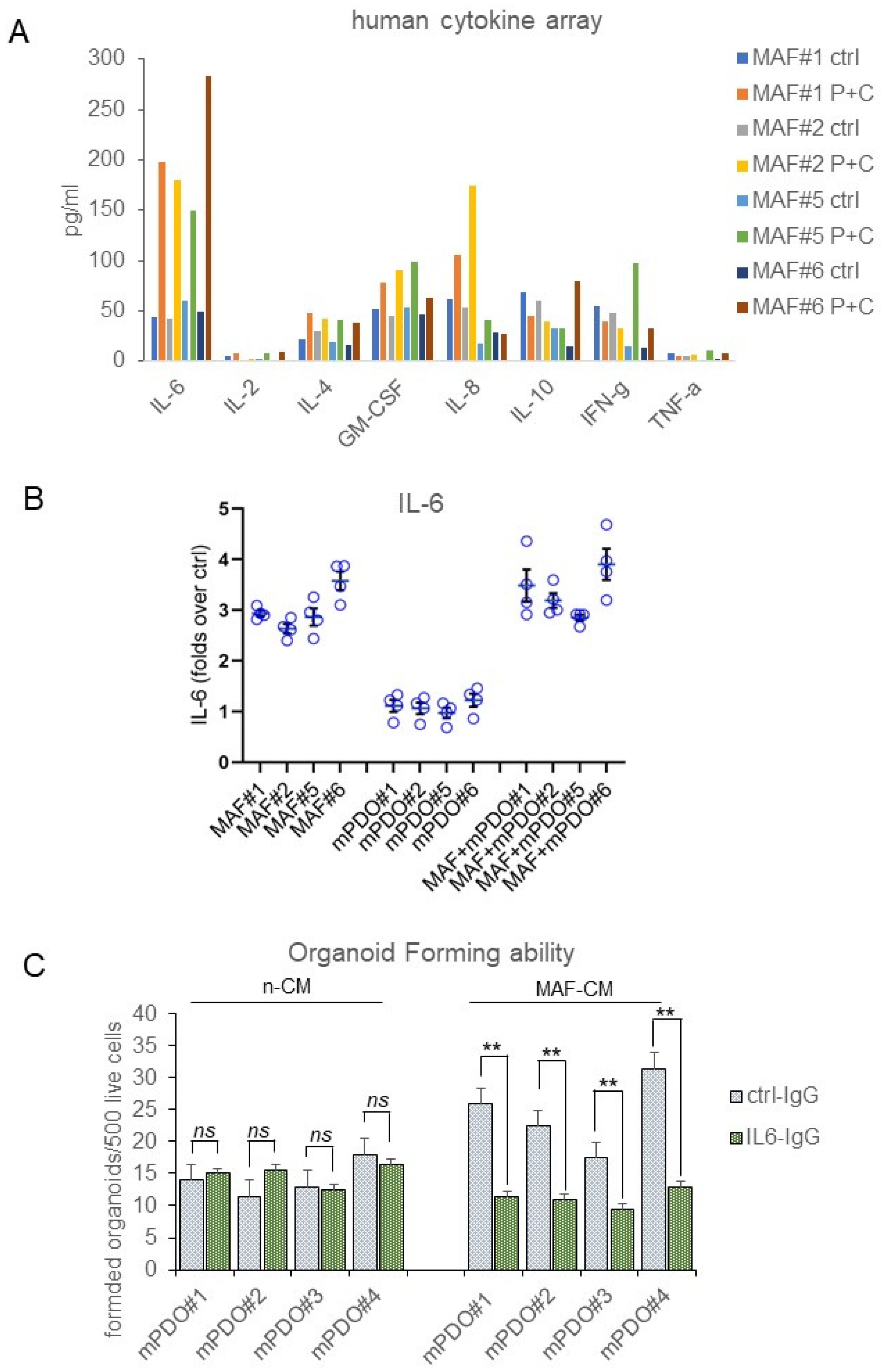
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, A.M.H.; Anwer, S.H.; Sayed, S.; Mansha, M.A.; Bin Kamran, Y.; Khursheed, A.; Haroon, F.; Soomro, N.H.; Idrees, R.; Abbasi, A.N. Comprehensive clinical overview of malignant pleural mesothelioma. Respir. Med. 2024, 222, 107511. [Google Scholar] [CrossRef] [PubMed]
- Baas, P.; Daumont, M.J.; Lacoin, L.; Penrod, J.R.; Carroll, R.; Venkatesan, S.; Ubhi, H.; Calleja, A.; Snee, M. Treatment patterns and outcomes for patients with malignant pleural mesothelioma in England in 2013-2017: A nationwide CAS registry analysis from the I-O Optimise initiative. Lung Cancer 2021, 162, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Meirson, T.; Pentimalli, F.; Cerza, F.; Baglio, G.; Gray, S.G.; Correale, P.; Krstic-Demonacos, M.; Markel, G.; Giordano, A.; Bomze, D.; et al. Comparison of 3 Randomized Clinical Trials of Frontline Therapies for Malignant Pleural Mesothelioma. JAMA Netw. Open. 2022, 5, e221490. [Google Scholar] [CrossRef] [PubMed]
- Correale, P.; Pentimalli, F.; Nardone, V.; Giordano, A.; Mutti, L. CONFIRM trial: What is the real efficacy of second-line immunotherapy in mesothelioma? Lancet Oncol. 2022, 23, e13. [Google Scholar] [CrossRef] [PubMed]
- Fennell, D.A.; Ewings, S.; Ottensmeier, C.; Califano, R.; Hanna, G.G.; Hill, K.; Danson, S.; Steele, N.; Nye, M.; Johnson, L.; et al. Nivolumab versus placebo in patients with relapsed malignant mesothelioma (CONFIRM): A multicentre, double-blind, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 1530–1540. [Google Scholar] [CrossRef] [PubMed]
- Hiddinga, B.I.; Rolfo, C.; van Meerbeeck, J.P. Mesothelioma treatment: Are we on target? A review. J. Adv. Res. 2015, 6, 319–330. [Google Scholar] [CrossRef]
- Mujoomdar, A.A.; Tilleman, T.R.; Richards, W.G.; Bueno, R.; Sugarbaker, D.J. Prevalence of in vitro chemotherapeutic drug resistance in primary malignant pleural mesothelioma: Result. in a cohort of 203 resection specimens. J. Thorac. Cardiovasc. Surg. 2010, 140, 352–355. [Google Scholar] [CrossRef]
- Canino, C.; Mori, F.; Cambria, A.; Diamantini, A.; Germoni, S.; Alessandrini, G.; Borsellino, G.; Galati, R.; Battistini, L.; Blandino, R.; et al. SASP mediates chemoresistance and tumor-initiating-activity of mesothelioma cells. Oncogene 2012, 31, 3148–3163. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Burt, B.M.; Lee, H.-S.; Nguyen, T.T.; Jang, H.-J.; Lee, C.; Hong, W.; Ripley, R.T.; Amos, C.I.; Cheng, C. Clonal gene signatures predict prognosis in mesothelioma and lung adenocarcinoma. NPJ Precis. Oncol. 2024, 8, 47. [Google Scholar] [CrossRef]
- Cioce, M.; Sacconi, A.; Pass, H.I.; Canino, C.; Strano, S.; Blandino, G.; Fazio, V.M. Insights into Intra-Tumoral Heterogeneity: Transcriptional Profiling of Chemoresistant MPM Cell Subpopulations Reveals Involvement of NFkB and DNA Repair Pathways and Contributes a Prognostic Signature. Int. J. Mol. Sci. 2021, 22, 12071. [Google Scholar] [CrossRef]
- Hedayat, S.; Valeri, N. Patient-Derived Organoids: Promises, Hurdles and Potential Clinical Applications. Clin. Oncol. (R Coll Radiol.) 2020, 32, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Porter, R.J.; Murray, G.I.; McLean, M.H. Current concepts in tumour-derived organoids. Br. J. Cancer. 2020, 123, 1209–1218. [Google Scholar] [CrossRef]
- Qu, S.; Xu, R.; Yi, G.; Li, Z.; Zhang, H.; Qi, S.; Huang, G. Patient-derived organoids in human cancer: A platform for fundamental research and precision medicine. Mol. Biomed. 2024, 5, 6. [Google Scholar] [CrossRef]
- Khorsandi, D.; Yang, J.; Foster, S.; Khosravi, S.; Hoseinzadeh, N.; Zarei, F.; Bin Lee, Y.; Runa, F.; Gangrade, A.; Voskanian, L.; et al. Patient-Derived Organoids as Therapy Screening Platforms in Cancer Patients. Adv. Healthc. Mater. 2024, e2302331. [Google Scholar] [CrossRef]
- Donzelli, S.; Cioce, M.; Sacconi, A.; Zanconato, F.; Daralioti, T.; Goeman, F.; Orlandi, G.; Di Martino, S.; Fazio, V.M.; Alessandrini, G.; et al. A PIK3CA-mutant breast cancer metastatic patient-derived organoid approach to evaluate alpelisib treatment for multiple secondary lesions. Mol. Cancer. 2022, 21, 152. [Google Scholar] [CrossRef]
- Papait, A.; Romoli, J.; Stefani, F.R.; Chiodelli, P.; Montresor, M.C.; Agoni, L.; Silini, A.R.; Parolini, O. Fight the Cancer, Hit the CAF! Cancers 2022, 14, 3570. [Google Scholar] [CrossRef]
- Cioce, M.; Fumagalli, M.R.; Donzelli, S.; Goeman, F.; Canu, V.; Rutigliano, D.; Orlandi, G.; Sacconi, A.; Pulito, C.; Palcau, A.C.; et al. Interrogating colorectal cancer metastasis to liver: A search for clinically viable compounds and mechanistic insights in colorectal cancer Patient Derived Organoids. J. Exp. Clin. Cancer Res. 2023, 42, 170. [Google Scholar] [CrossRef] [PubMed]
- Ries, A.; Flehberger, D.; Slany, A.; Pirker, C.; Mader, J.C.; Mohr, T.; Schelch, K.; Sinn, K.; Mosleh, B.; Hoda, M.A.; et al. Mesothelioma-associated fibroblasts enhance proliferation and migration of pleural mesothelioma cells via c-Met/PI3K and WNT signaling but do not protect against cisplatin. J. Exp. Clin. Cancer Res. 2023, 42, 27. [Google Scholar] [CrossRef] [PubMed]
- Chrisochoidou, Y.; Roy, R.; Farahmand, P.; Gonzalez, G.; Doig, J.; Krasny, L.; Rimmer, E.F.; E Willis, A.; MacFarlane, M.; Huang, P.H.; et al. Crosstalk with lung fibroblasts shapes the growth and therapeutic response of mesothelioma cells. Cell Death Dis. 2023, 14, 725. [Google Scholar] [CrossRef]
- Borchert, S.; Mathilakathu, A.; Nath, A.; Wessolly, M.; Mairinger, E.; Kreidt, D.; Steinborn, J.; Walter, R.F.H.; Christoph, D.C.; Kollmeier, J.; et al. Cancer-Associated Fibroblasts Influence Survival in Pleural Mesothelioma: Digital Gene Expression Analysis and Supervised Machine Learning Model. Int. J. Mol. Sci. 2023, 24, 12426. [Google Scholar] [CrossRef]
- Simian, M.; Bissell, M.J. Organoids: A historical perspective of thinking in three dimensions. J. Cell Biol. 2017, 216, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, K.; Katayama, K.; Sugimoto, Y. Human ABC transporter ABCG2/BCRP expression in chemoresistance: Basic and clinical perspectives for molecular cancer therapeutics. Pharmgenomics Pers. Med. 2014, 7, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Rose-John, S.; Jenkins, B.J.; Garbers, C.; Moll, J.M.; Scheller, J. Targeting IL-6 trans-signalling: Past, present and future prospects. Nat. Rev. Immunol. 2023, 23, 666–681. [Google Scholar] [CrossRef]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef]
- Fang, X.; Shu, L.; Chen, T.; Zhao, X.; Yang, L.; Dou, T.; Yang, L.; Li, X.; Feng, M. Organoids derived from patients provide a new opportunity for research and individualized treatment of malignant peritoneal mesothelioma. Mol. Cancer. 2024, 23, 12. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Kruithof-de Julio, M.; Peng, R.W.; Dorn, P. Organoids as a Model for Precision Medicine in Malignant Pleural Mesothelioma: Where Are We Today? Cancers 2022, 14, 3758. [Google Scholar] [CrossRef]
- Mazzocchi, A.R.; Rajan, S.A.P.; Votanopoulos, K.I.; Hall, A.R.; Skardal, A. In vitro patient-derived 3D mesothelioma tumor organoids facilitate patient-centric therapeutic screening. Sci. Rep. 2018, 8, 2886. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.B.; Little, M.H. Organoids are not organs: Sources of variation and misinformation in organoid biology. Stem Cell Rep. 2023, 18, 1255–1270. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Vries, R.G.; Snippert, H.J.; Van De Wetering, M.; Barker, N.; Stange, D.E.; Van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef]
- Cioce, M.; Canino, C.; Pass, H.; Blandino, G.; Strano, S.; Fazio, V.M. Arachidonic acid drives adaptive responses to chemotherapy-induced stress in malignant mesothelioma. J. Exp. Clin. Cancer Res. 2021, 40, 344. [Google Scholar] [CrossRef]
- Dietze, R.; Hammoud, M.K.; Gómez-Serrano, M.; Unger, A.; Bieringer, T.; Finkernagel, F.; Sokol, A.M.; Nist, A.; Stiewe, T.; Reinartz, S.; et al. Phosphoproteomics identify arachidonic-acid-regulated signal transduction pathways modulating macrophage functions with implications for ovarian cancer. Theranostics 2021, 11, 1377–1395. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Saavedra, D.; Stanford, K.I. Lipid Mediators in Cardiovascular Physiology and Disease. In Cardiovascular Signaling in Health and Disease; Parinandi, N.L., Hund, T.J., Eds.; Springer: Cham, Switzerland, 2022; pp. 235–258. [Google Scholar]
- Tallima, H.; El Ridi, R. Arachidonic acid: Physiological roles and potential health benefits—A review. J. Adv. Res. 2018, 11, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, Y.; Weeraratna, A.T. Fibroblasts in cancer: Unity in heterogeneity. Cell 2023, 186, 1580–1609. [Google Scholar] [CrossRef] [PubMed]
- Melchionna, R.; Trono, P.; Di Carlo, A.; Di Modugno, F.; Nistico, P. Transcription factors in fibroblast plasticity and CAF heterogeneity. J. Exp. Clin. Cancer Res. 2023, 42, 347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yue, X.; Chen, Z.; Liu, C.; Wu, W.; Zhang, N.; Liu, Z.; Yang, L.; Jiang, Q.; Cheng, Q.; et al. Define cancer-associated fibroblasts (CAFs) in the tumor microenvironment: New opportunities in cancer immunotherapy and advances in clinical trials. Mol. Cancer 2023, 22, 159. [Google Scholar] [CrossRef] [PubMed]
- Rose-John, S. The biology of interleukin-6 in the 21st century. Semin. Immunol. 2014, 26, 1. [Google Scholar] [CrossRef] [PubMed]
- Orjalo, A.V.; Bhaumik, D.; Gengler, B.K.; Scott, G.K.; Campisi, J. Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc. Natl. Acad. Sci. USA 2009, 106, 17031–17036. [Google Scholar] [CrossRef] [PubMed]
- Kuilman, T.; Michaloglou, C.; Vredeveld, L.C.; Douma, S.; van Doorn, R.; Desmet, C.J.; Aarden, L.A.; Mooi, W.J.; Peeper, D.S. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 2008, 133, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Canino, C.; Luo, Y.; Marcato, P.; Blandino, G.; Pass, H.I.; Cioce, M. A STAT3-NFkB/DDIT3/CEBPbeta axis modulates ALDH1A3 expression in chemoresistant cell subpopulations. Oncotarget 2015, 6, 12637–12653. [Google Scholar] [CrossRef]
- Cioce, M.; Canino, C.; Pulito, C.; Muti, P.; Strano, S.; Blandino, G. Butein impairs the protumorigenic activity of malignant pleural mesothelioma cells. Cell Cycle 2012, 11, 132–140. [Google Scholar] [CrossRef]
- Goldman, M.J.; Craft, B.; Hastie, M.; Repecka, K.; McDade, F.; Kamath, A.; Banerjee, A.; Luo, Y.; Rogers, D.; Brooks, A.N.; et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 2020, 38, 675–678. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cioce, M.; Gatti, V.; Napolitano, F.; Giorgiano, N.M.; Marra, A.; Portella, G.; Fiorelli, A.; Pentimalli, F.; Fazio, V.M. Mesothelioma-Associated Fibroblasts Modulate the Response of Mesothelioma Patient-Derived Organoids to Chemotherapy via Interleukin-6. Int. J. Mol. Sci. 2024, 25, 5355. https://doi.org/10.3390/ijms25105355
Cioce M, Gatti V, Napolitano F, Giorgiano NM, Marra A, Portella G, Fiorelli A, Pentimalli F, Fazio VM. Mesothelioma-Associated Fibroblasts Modulate the Response of Mesothelioma Patient-Derived Organoids to Chemotherapy via Interleukin-6. International Journal of Molecular Sciences. 2024; 25(10):5355. https://doi.org/10.3390/ijms25105355
Chicago/Turabian StyleCioce, Mario, Veronica Gatti, Fabiana Napolitano, Noemi Maria Giorgiano, Andrea Marra, Giuseppe Portella, Alfonso Fiorelli, Francesca Pentimalli, and Vito Michele Fazio. 2024. "Mesothelioma-Associated Fibroblasts Modulate the Response of Mesothelioma Patient-Derived Organoids to Chemotherapy via Interleukin-6" International Journal of Molecular Sciences 25, no. 10: 5355. https://doi.org/10.3390/ijms25105355
APA StyleCioce, M., Gatti, V., Napolitano, F., Giorgiano, N. M., Marra, A., Portella, G., Fiorelli, A., Pentimalli, F., & Fazio, V. M. (2024). Mesothelioma-Associated Fibroblasts Modulate the Response of Mesothelioma Patient-Derived Organoids to Chemotherapy via Interleukin-6. International Journal of Molecular Sciences, 25(10), 5355. https://doi.org/10.3390/ijms25105355









