Abstract
Cluster of differentiation 19 (CD19) chimeric antigen receptor (CAR) T cells are a highly effective immunotherapy for relapsed and refractory B-cell malignancies, but their utility can be limited by the development of immune effector cell-associated neurotoxicity syndrome (ICANS). The recent discovery of CD19 expression on the pericytes in the blood–brain barrier (BBB) suggests an important off-target mechanism for ICANS development. In addition, the release of systemic cytokines stimulated by the engagement of CD19 with the CAR T cells can cause endothelial activation and decreased expression of tight junction molecules, further damaging the integrity of the BBB. Once within the brain microenvironment, cytokines trigger a cytokine-specific cascade of neuroinflammatory responses, which manifest clinically as a spectrum of neurological changes. Brain imaging is frequently negative or nonspecific, and treatment involves close neurologic monitoring, supportive care, interleukin antagonists, and steroids. The goal of this review is to inform readers about the normal development and microstructure of the BBB, its unique susceptibility to CD19 CAR T cells, the role of individual cytokines on specific elements of the brain’s microstructural environment, and the clinical and imaging manifestations of ICANS. Our review will link cellular pathophysiology with the clinical and radiological manifestations of a complex clinical entity.
1. Introduction
Cluster of differentiation 19 (CD19) is a surface glycoprotein expressed on both physiologic and neoplastic B cells and represents a valuable target for immunotherapies against B-cell malignancies [1]. CD19-directed chimeric antigen receptor (CAR) T cells are a promising therapeutic strategy for both adult and pediatric patients with relapsed or refractory B-cell malignancies. These include patients with B-cell acute lymphoblastic leukemia, chronic lymphocytic leukemia, non-Hodgkin’s lymphoma, and multiple myeloma, who have not responded or relapsed following conventional chemotherapy and/or a hematopoietic stem cell transplant. The T cells are autologously harvested from the patient, stimulated with a cytokine cocktail containing CD8 and CD28, and treated with viral vector agents to express CARs directed against CD19 on their cell surface. The CAR consists of an extracellular domain with a single-chain variable fragment derived from an anti-CD19 antibody, a transmembrane domain, and an intracellular co-stimulatory domain derived from either 4-1BB or CD28 linked to CD3ζ. Once CAR T cells are intravenously infused back into the patient, their engagement with CD19 activates the intracellular co-stimulatory and CD3ζ domains and the synthesis of proteins such as perforin and granzyme. Perforin increases the porosity of neoplastic B cells and granzyme influxes into neoplastic B cells to activate intracellular caspases, thereby initiating apoptosis [2].
Although highly effective in targeting neoplastic B cells, CD19 expression on off-target cells can cause a range of complications. CD19 is also expressed on physiologic B cells, thus their depletion results in hypogammaglobulinemia and increased susceptibility to opportunistic infections [3]. Recent single-cell RNA sequencing (scRNA-seq) analyses identified CD19 on pericytes in the human blood–brain barrier (BBB); pericytes play a critical role in maintaining the integrity of the BBB [4]. This recent discovery has provided important insight into the mechanisms of immune effector cell-associated neurotoxicity syndrome (ICANS), which has been reported with higher frequency and severity in recipients of CD19 CAR T cells, as compared to recipients of CAR T cells with different targets (e.g., CD22) [5]. In addition, CD19 CAR T–cell therapy activates the monocyte/macrophage system and the systemic release of inflammatory cytokines, such as granulocyte monocyte colony-stimulating factor (GM-CSF); interleukins (IL)-1β, IL6, and IL15; and interferon-gamma (IFN-γ), which can further increase the permeability of the BBB [6,7]. Once trafficked across the BBB, these cytokines activate a host of neuroinflammatory responses in the brain microenvironment, which manifests clinically as ICANS [8].
The goal of this narrative review is to systematically consider the microstructure of the BBB, while highlighting the role of each element in the pathogenesis of ICANS and detailing the mechanism of cytokine release after CD19 CAR T–cell infusion, the impact of individual cytokines on the BBB, and the cascade of neuroinflammatory responses that follow. We will conclude our review by linking these cellular mechanisms to the complex and varied clinical and radiological features of ICANS.
2. The Blood–Brain Barrier
The BBB is a complex neurovascular structure composed of an inner layer of highly specialized endothelial cells, surrounded by pericytes embedded in the endothelial basement membrane and an outer layer of astrocyte foot processes [9]. The inner endothelial cells are the first to develop, with immature angioblasts infiltrating the central nervous system (CNS) on approximately embryonic day (E) 12. This is followed by the arrival of pericytes, which stimulate the expression of tight junction strands between the individual endothelial clefts [10]. These tight junction strands anchor to the cytoskeleton of the endothelial cells via tethering to intracellular actin filaments [11]. The tight junction strands consist of three major types of proteins: claudins, MARVEL/D3, and immunoglobulin-like junctional adhesion molecules. Although claudins and MARVEL/D3 proteins play a complementary role in maintaining the transportation of solutes across the cell membrane, the junctional adhesion molecules play a key role in immune cell trafficking across the BBB [11]. Junctional adhesion molecules act in conjunction with basolateral vascular endothelial cadherins and cellular adhesion molecules to facilitate the transport of leukocytes across the BBB. The pro-inflammatory cytokines released during CD19 CAR T–cell therapy, particularly IL-1β, indirectly decrease the expression of these tight junction proteins, thereby disrupting the integrity of the BBB [12].
Immediately external to the vascular endothelium are the pericytes, which play a key role in vessel wall regeneration [13]. Pericytes preferentially contact the endothelial cells at tight junctions, while also positioning themselves at the luminal interfaces of the astrocyte foot processes [14]. A study by Parker et al., employing scRNA-seq analyses of more than 2000 samples of human prefrontal cortex cells, identified CD19 and CD81, the latter of which is essential to cell surface expression of CD19 on human pericytes. CD19 is expressed early in brain development, concurrently with the development of pericytes, and persists throughout adulthood. By comparison, scRNA-seq analysis of mouse mural models demonstrated relatively lower levels of CD19 expression, which most likely accounts for the lower incidence of ICANS in mouse models [4].
The outermost layer of the BBB consists of astrocyte foot processes that ensheath more than 90% of the capillary surface area in the brain. The predominant role of astrocyte foot processes is to maintain the BBB via the wingless-related integration site (Wnt) and Sonic Hedgehog (SHH) pathways [15]. The expression of the Wnt ligand enhances that of glial-derived neurotropic factor, which is critical for maintaining tight junction integrity [16]. In contrast, the SHH ligand binds to cell surface G-protein–coupled receptors and downregulates the expression of pro-inflammatory IL-8 and monocyte chemoattractant protein-1, while upregulating tight junction protein expression [17]. Although the exact mechanism of astrocytic injury in ICANS is yet to be determined, the elevated pro-inflammatory cytokines may disrupt the protective mechanisms of the Wnt- and SHH-signaling pathways, leading to osmotic dysregulation and neurotoxicity [18].
3. The Role of Cytokines in the Breakdown of the Blood–Brain Barrier
Once transfused into the recipient, CAR T cells bind with high affinity to CD19 on the surface of neoplastic B cells. This engagement activates the monocyte/macrophage system, which results in the systemic release of several cytokines, including GM-CSF, IFN-γ, IL-1β, IL-6, IL-10, IL-15, and angiopoietin-2 [19]. The mechanisms of each of these cytokines in the BBB breakdown and subsequent neuroinflammation are briefly summarized in Figure 1 and discussed in greater detail below.
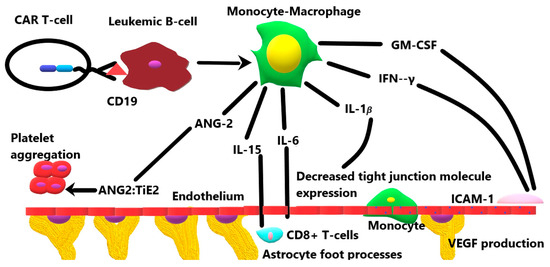
Figure 1.
The role of various cytokines released by the activation of the monocyte–macrophage system in response to CD19 CAR T-cell therapy.
3.1. Granulocyte-Monocyte Colony-Stimulating Factor
The engagement of CD19 with CAR T cells stimulates the release of GM-CSF from the monocyte/macrophage system, which increases both BBB permeability and subsequent neuroinflammation. Studies in preclinical models of neuroinflammation have noted an increased migration of GM-CSF–stimulated monocytes across the BBB, as compared to nonstimulated control monocytes; this increased migration has been linked to a significantly higher secretion of tumor necrosis factor-alpha (TNF-α) by the stimulated monocyte population [20,21]. TNF-α, in turn, increases the expression of leukocyte adhesion molecules, such as intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1), which enable the attachment and migration of stimulated monocytes across the BBB [22]. Once trafficked across the BBB, the GM-CSF–stimulated monocytes mature into macrophages and secrete reactive oxygen species that damage the cytoskeleton of neurons and glial cells [23]. In a preclinical study by Sterner et al., a mouse model implanted with leukemic blasts and treated with human CD19 CAR T cells developed hunched posture and motor weakness, with elevated serum levels of GM-CSF and increased contrast enhancement on brain magnetic resonance images (MRIs), compatible with neurotoxicity. The subsequent administration of lenzilumab, a GM-CSF–neutralizing antibody, decreased the contrast enhancement on brain MRIs and reduced the infiltration of activated macrophages into the brain microenvironment, while preserving the antitumor activity of the CD19 CAR T cells [24]. Clinical studies in both adult and pediatric CD19 CAR T–cell recipients have mirrored these results, with elevated serum levels of GM-CSF noted during the acute neurotoxicity phase [25,26,27]. To date, however, only one clinical study, by Gust et al. in 2019, involving 43 pediatric and young adult recipients of CD19 CAR T–cell therapy, has investigated GM-CSF levels in cerebrospinal fluid during the acute neurotoxicity phase and demonstrated no significant elevation [28]. Early evidence suggests the primary role of GM-CSF as an enabler of activated monocyte/macrophage transport across the BBB, with the subsequent neuroinflammation resulting from the local release of reactive oxygen species. Furthermore, larger-scale clinical studies investigating cerebrospinal fluid levels of GM-CSF are needed to validate this hypothesis. In addition, the potential value of lenzilumab in counteracting neuroinflammation while preserving CAR T–cell function is currently under investigation in a clinical trial (NCT04314843).
3.2. Interferon-γ
IFN-γ is a key cytokine secreted by actively proliferating CD19 CAR T cells. Preclinical models of neuroinflammation have shown that IFN-γ induces gene expression within the endothelial cells of the BBB and increases the expression of ICAM-1 and VCAM-1, which most likely help traffic CD19 CAR T cells into the CNS [29]. Studies of the effects of IFN-γ, once trafficked across the BBB, have yielded conflicting results in preclinical models, depending on the disease process being studied. In models of autoimmune encephalitis, IFN-γ stabilizes the endothelium and decreases leukocyte infiltration; however, in models of reovirus infection, IFN-γ increases permeability [30,31]. Preclinical models of ICANS in which the neuroinflammatory effect of IFN-γ has been evaluated are currently lacking, but indirect evidence suggests that the cytokine has a pro-inflammatory role. An investigation by Zhang et al. in 2022 reported the effects of an IL-6/IFN-γ double-knockout CD19 CAR T–cell product in peripheral monocyte blood cultures; they found that their product induced significantly lower levels of pro-inflammatory cytokines than a standard CD19 CAR T–cell product did, while retaining antitumor activity [32]. More preclinical studies on the effects of IFN-γ on specific elements of the brain microenvironment in ICANS are needed to help clarify the cytokine’s role in this process. In clinical studies, significantly elevated IFN-γ levels have been identified in both the serum and cerebrospinal fluid of CD19 CAR T-cell recipients during the acute phase of neurotoxicity, as compared to those in recipients without neurotoxicity [6,25,26,27,28].
3.3. Interleukin-1β
IL-1β is produced by activated monocytes and macrophages in response to CAR T cells recognizing CD19. IL-1β increases the BBB’s permeability by suppressing the production of SHH by astrocytes and, consequently, downregulating the expression of tight junction proteins [33]. In addition, IL-1β stimulates the release of vascular endothelial growth factor from astrocytes, which exacerbates the BBB disruption [34]. Once trafficked into the CNS, IL-1β stimulates glial cell proliferation by activating the mitogen-activated protein kinase pathway and induces further local cytokine production from both glial cells and microglia [35]. A key preclinical study by Norelli et al., in 2018, in a mouse model of ICANS treated with humanized CD19 CAR T cells, demonstrated that pre-treatment with IL-1β blockade prevented the development of signs of neurotoxicity, including generalized paralysis and seizures [36]. A clinical study of ICANS by Cohen et al., in 2019, reported elevated serum concentrations of IL-1β during the acute neurotoxicity phase, but none of the clinical studies evaluating cytokines in cerebrospinal fluid have identified IL-1β [6,25,26,27,28,37,38]. More clinical studies on the trends in serum and cerebrospinal fluid levels of IL-1β, before and during acute neurotoxicity, are needed to elucidate its role in the pathogenesis of ICANS.
3.4. Interleukin-6
IL-6 is produced by actively proliferating monocytes in CD19 CAR T–cell recipients and has been linked to the symptoms of cytokine release syndrome [19]. Preclinical studies by Saija et al. and Krizanac-Bengez et al. have demonstrated the increased permeability of the BBB in response to systemic IL-6 administration in rats [12,39]. Takeshita et al. investigated the mechanism of the altered permeability in human BBB models, noting a dose- and time-dependent decrease in the endothelial expression of tight junction proteins with IL-6 [40]. Despite this compelling evidence for the role of IL-6 in altering BBB permeability, preclinical models of ICANS have not yet established a direct causative role for IL-6 in ICANS. Norelli et al. demonstrated high serum levels of IL-6 during cytokine release syndrome and amelioration of the syndrome after treatment with the IL-6 blocker tocilizumab in mice engrafted with human leukemic blasts and treated with human CD19 CAR T cells. However, tocilizumab failed to protect mice from ICANS, which was ultimately fatal [36].
Several clinical studies of ICANS in both adult and pediatric patients have demonstrated elevated serum and cerebrospinal fluid levels of IL-6 during the acute neurotoxicity phase [6,25,26,27,28]. Once within the brain microenvironment, IL-6 plays a dual role: it suppresses TNF-α release and neutrophil migration early during inflammation, and it actively recruits monocytes and T cells during the late phase of inflammation. IL-6 also stimulates astrocytosis and angiogenesis, which are required for tissue remodeling during the late phase of inflammation [41].
3.5. Interleukin-10
The role of IL-10 in the pathophysiology of ICANS is somewhat controversial. Studies on inflammatory bowel disease have demonstrated the pro-inflammatory effect of IL-10, as evidenced by the activation of cytotoxic CD8+ T cells and subsequent tissue injury [42,43]. On the other hand, pre-clinical models have established the strong ant-inflammatory response of IL-10 by inhibiting cytokine production by both T-cells and natural killer cells [44]. In three clinical studies investigating the CSF levels of cytokines in patients with ICANS, elevated levels of IL-10 were noted in the post-infusion period, relative to the pre-infusion period. It is hypothesized that IL-10 may play a key role in maintaining the expansion and the activity of CAR T-cells. More detailed clinical studies comparing the CSF levels of IL-10 in patients with and without ICANS, as well as in those with mild vs. severe forms of ICANS may help to more definitively establish the role of IL-10 [6,26,28].
3.6. Interleukin-15
IL-15 plays a key role in the pathogenesis of cytokine release syndrome and ICANS in CD19 CAR T–cell recipients [19]. Preclinical studies on rat brain endothelium by Pan et al. in 2009 and Wu et al. in 2010 demonstrated an increased expression of IL-15 receptors on the surface endothelium in response to the systemic release of pro-inflammatory cytokines such as TNF-α. Increased receptor expression, in turn, leads to the endocytosis of IL-15 and trafficking across the BBB [45,46]. The role of IL-15 in neuroinflammation has been studied in preclinical models of multiple sclerosis. A preclinical study on human fetal neural tissue by Saikali et al. demonstrated an increased recruitment and activation of CD8 T lymphocytes in response to IL-15 and elevated levels of lytic enzymes and antigen-specific cytotoxicity, which play a key role in demyelination [47]. Preclinical models of ICANS which evaluate IL-15 are currently lacking, but the evidence of neuroinflammation in other models and the consistent finding of elevated serum IL-15 levels in patients with ICANS, which has been documented in clinical studies, strongly suggest that this cytokine has a role in in the etiology of ICANS. In addition, one clinical study demonstrated higher levels of IL-15 in cerebrospinal fluid from patients with ICANS, as compared to those at baseline [6]. More preclinical studies exploring the exact mechanism of IL-15 in neuroinflammation and more clinical studies on the changes in IL-15 levels in cerebrospinal fluid are needed to establish its role in the etiology of ICANS.
3.7. Angiopoeitin-2
Under conditions of vascular quiescence, angiopoeitin-1 is bound to the endothelial receptor tyrosine kinase Tie2 and maintains the integrity of the BBB. Angiopoeitin-2 is stored within endothelial Weibel–Palade bodies and is released during endothelial activation in response to pro-inflammatory cytokines. Angiopoietin-2 preferentially binds to Tie2 during periods of stress, thereby promoting platelet aggregation and thrombosis. In animal studies of cerebral malaria and traumatic brain injury, angiopoietin-2/Tie2 binding was associated with the destabilization of the BBB, whereas the manipulation of signaling towards the angiopoietin-1/Tie2 demonstrated a protective effect [48,49]. The role of angiopoietin-2 has also been investigated in CD19 CAR T–cell recipients who developed ICANS. In a study by Gust et al. in 2017, data from 133 adult CD19 CAR T–cell recipients demonstrated a positive correlation between the angiopoietin-2–angiopoietin-1 ratio and the grade of neurotoxicity [6]. In addition, this study demonstrated that patients with grade 4 or higher neurotoxicity have higher serum levels of von Willebrand factor than those with grade 3 or lower neurotoxicity. The von Willebrand factor is stored in the Weibel–Palade bodies of the endothelium with angiopoeitin-2 and is a key stimulator of platelet aggregation [6]. These findings suggest the role of the angiopoietin-2/Tie2 axis in BBB destabilization; however, more prospective clinical studies are needed to establish the role of antiopoeitin-2 in the pathogenesis of ICANS.
4. Clinical and Imaging Features of ICANS
The CAR T–cell- and cytokine-mediated BBB breakdown and the subsequent neuroinflammation can be associated with various clinical features, ranging from mild encephalopathy to seizures and cerebral edema. The development of ICANS is monophasic, with symptoms appearing within 3–6 days post CAR T-cell infusion, peaking at days 7–8 and resolving by days 14–21 [50]. The most frequently reported clinical manifestation of ICANS is cognitive dysfunction, which has been variably described in the literature as delirium, confusion, or encephalopathy [7,26,27]. The cognitive dysfunction in ICANS is often marked by a striking disturbance in speech and handwriting. Although headaches and tremors are non-specific symptoms, they frequently precede or accompany the classic cognitive dysfunction in ICANS [8]. The incidence of seizures in ICANS ranges widely from 5–10%, with a slightly higher incidence rate in recipients of CAR T-cell products with the CD28 co-stimulatory domain, as compared to the 4-1BB co-stimulatory domain [51]. Fatal cerebral edema occurs in 1–2% of CD19 CAR T-cell recipients and is notably absent in recipients of other antigen-specific CAR T-cell products such as CD22. Many of the mechanisms of neurotoxicity, such as the microvascular disruption and endothelial activation described earlier, have been described in cases of fatal cerebral edema and it is unclear whether milder forms of ICANS represent a less profound version of the same mechanism, or a completely different mechanism. More research is necessary to help us to clearly identify the pathophysiologic mechanisms of the varying grades of ICANS [52]. The current grading of ICANS incorporates cognition, consciousness, motor function, seizure activity, and signs of elevated intracranial pressure (Table 1) [8]. Several clinical studies in both adult and pediatric CD19 CAR T-cell recipients have described elevated serum cytokine levels in patients with ICANS, but studies comparing the CSF levels of these cytokines in patients with and without ICANS are currently lacking due to a lack of a clinical indication for obtaining CSF in patients without ICANS [6,26,27]. While obtaining CSF in all CD19 CAR T-cell recipients would certainly help characterize the temporal evolution of inflammation in the CNS better, the practical considerations are currently a limiting factor.

Table 1.
The 2018 American Society of Transplantation and Cellular Therapy criteria for grading of ICANS [8].
Despite the specific and temporal nature of clinical symptoms, conventional MR imaging of the brain is often non-contributory. Most patients with grade 1–2 ICANS have no acute imaging abnormalities in the first 21 days post-infusion, and a few of the patients with grade 3–4 ICANS demonstrate non-specific imaging abnormalities. However, these abnormalities may provide a valuable insight into the underlying pathophysiological mechanisms of ICANS, and radiologists need to be aware of their implications [53]. A small number of patients may present with diffuse leptomeningeal enhancement, a finding which may be seen in infectious or chemical meningitis. In the setting of recent CAR T-cell infusion, however, the findings are likely reflective of a breakdown of the BBB, secondary to the targeting of CD19 on the pericytes and the cytokine-mediated endothelial activation [54]. Another neuroimaging finding reported in ICANS is non-specific hyperintensities on the T2 and fluid attenuated inversion recovery (FLAIR) sequences in the cerebral and cerebellar white matter, which may be linked to the demyelinating effects of IFN-γ. The cytokine induced neuronal excitotoxicity may result in transient restricted diffusion in the cerebral cortex or the splenium of the corpus callosum, due to a higher concentration of excitotoxic neurons in these locations. These findings must be interpreted in the context of recent CAR T-cell infusion and new neurologic symptoms. Other findings reported on MRIs include hyperintensity on the T2 and/or FLAIR sequences in the thalami, brainstem and the external capsules, likely reflecting a combination of local microvascular disruption and excitotoxicity [55]. More research using advanced imaging modalities, such as dynamic susceptibility weighted perfusion imaging to quantify local changes in perfusion and diffusion tensor imaging to quantify changes in white matter anisotropy, are needed to support these hypotheses and establish more definitive imaging criteria for ICANS.
In the 1–2% of CD19 CAR T-cell recipients with fatal cerebral edema, MRI is often not feasible due to clinical instability. A non-contrast head CT is often the only imaging modality that can be performed safely and in a timely manner, and the interpreting radiologist plays a critical role in the management of these patients. Recognizing the early signs of cerebral edema on CT can often alert the clinical team to the possibility of this dreaded complication and implement early therapy with steroids and interleukin antagonists. The early radiographic signs are subtle and non-specific, with mild periventricular white matter hypoattenuation and sulcal effacement suggesting edema development. In the severe form of cerebral edema, there is marked, diffuse cerebral hypoattenuation with the loss of gray-white matter differentiation, effacement of the ventricles, sulci and cisterns, and even frank herniation [55].
5. Conclusions
The BBB is a complex neurovascular unit, with a unique susceptibility to CD19 CAR T–cell therapy at the cellular level. Knowledge of the off-target mechanism of CD19 CAR T cells on pericytes and the role of individual cytokines in decreasing the expression of endothelial tight junction proteins, increasing the expression of cellular adhesion molecules, and trafficking activated monocytes across the BBB has helped further our understanding of the complex neurotoxicity that sometimes accompanies this therapy. Preclinical models of ICANS are particularly needed to help us better understand the role of certain cytokines (e.g., IL-15 and angiopoeitin-2) in BBB breakdown and subsequent neuroinflammation. Furthermore, more prospective clinical studies that investigate the trends in cytokine levels in cerebrospinal fluid are needed to help us understand their temporal relations with the onset and duration of ICANS.
Author Contributions
Conceptualization, S.N.P.; Writing and editing, S.N.P. and G.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We thank Angela McArthur, Department of Scientific Editing, at St. Jude Children’s Research Hospital for editing.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, K.; Wei, G.; Liu, D. CD19: A biomarker for B cell development, lymphoma diagnosis and therapy. Exp. Hematol. Oncol. 2012, 1, 36. [Google Scholar] [CrossRef] [PubMed]
- Larson, R.C.; Maus, M.V. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat. Rev. Cancer 2021, 21, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Doan, A.; Pulsipher, M.A. Hypogammaglobulinemia due to CAR T-cell therapy. Pediatr. Blood Cancer 2018, 65, 10. [Google Scholar] [CrossRef] [PubMed]
- Parker, K.R.; Migliorini, D.; Perkey, E.; Yost, K.E.; Bhaduri, A.; Bagga, P.; Haris, M.; Wilson, N.E.; Liu, F.; Gabunia, K.; et al. Single-Cell Analyses Identify Brain Mural Cells Expressing CD19 as Potential Off-Tumor Targets for CAR-T Immunotherapies. Cell 2020, 183, 126–142 e117. [Google Scholar] [CrossRef] [PubMed]
- Jess, J.; Yates, B.; Dulau-Florea, A.; Parker, K.; Inglefield, J.; Lichtenstein, D.; Schischlik, F.; Ongkeko, M.; Wang, Y.; Shahani, S.; et al. CD22 CAR T-cell associated hematologic toxicities, endothelial activation and relationship to neurotoxicity. J. Immunother. Cancer 2023, 11, e005898. [Google Scholar] [CrossRef] [PubMed]
- Gust, J.; Hay, K.A.; Hanafi, L.A.; Li, D.; Myerson, D.; Gonzalez-Cuyar, L.F.; Yeung, C.; Liles, W.C.; Wurfel, M.; Lopez, J.A.; et al. Endothelial Activation and Blood-Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer Discov. 2017, 7, 1404–1419. [Google Scholar] [CrossRef] [PubMed]
- Gust, J.; Taraseviciute, A.; Turtle, C.J. Neurotoxicity Associated with CD19-Targeted CAR-T Cell Therapies. CNS Drugs 2018, 32, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef]
- Risau, W. Molecular biology of blood-brain barrier ontogenesis and function. Acta Neurochir. Suppl. 1994, 60, 109–112. [Google Scholar] [CrossRef]
- Daneman, R.; Zhou, L.; Kebede, A.A.; Barres, B.A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 2010, 468, 562–566. [Google Scholar] [CrossRef]
- Gerhardt, H.; Liebner, S.; Redies, C.; Wolburg, H. N-cadherin expression in endothelial cells during early angiogenesis in the eye and brain of the chicken: Relation to blood-retina and blood-brain barrier development. Eur. J. Neurosci. 1999, 11, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Saija, A.; Princi, P.; Lanza, M.; Scalese, M.; Aramnejad, E.; De Sarro, A. Systemic cytokine administration can affect blood-brain barrier permeability in the rat. Life Sci. 1995, 56, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Armulik, A.; Genove, G.; Mae, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K.; et al. Pericytes regulate the blood-brain barrier. Nature 2010, 468, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Armulik, A.; Genove, G.; Betsholtz, C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 2011, 21, 193–215. [Google Scholar] [CrossRef] [PubMed]
- Paolinelli, R.; Corada, M.; Orsenigo, F.; Dejana, E. The molecular basis of the blood brain barrier differentiation and maintenance. Is it still a mystery? Pharmacol. Res. 2011, 63, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Guerit, S.; Fidan, E.; Macas, J.; Czupalla, C.J.; Figueiredo, R.; Vijikumar, A.; Yalcin, B.H.; Thom, S.; Winter, P.; Gerhardt, H.; et al. Astrocyte-derived Wnt growth factors are required for endothelial blood-brain barrier maintenance. Prog. Neurobiol. 2021, 199, 101937. [Google Scholar] [CrossRef]
- Alvarez, J.I.; Dodelet-Devillers, A.; Kebir, H.; Ifergan, I.; Fabre, P.J.; Terouz, S.; Sabbagh, M.; Wosik, K.; Bourbonniere, L.; Bernard, M.; et al. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science 2011, 334, 1727–1731. [Google Scholar] [CrossRef]
- Gauthier, J.; Turtle, C.J. Insights into cytokine release syndrome and neurotoxicity after CD19-specific CAR-T cell therapy. Curr. Res. Transl. Med. 2018, 66, 50–52. [Google Scholar] [CrossRef]
- Gust, J.; Ponce, R.; Liles, W.C.; Garden, G.A.; Turtle, C.J. Cytokines in CAR T Cell-Associated Neurotoxicity. Front. Immunol. 2020, 11, 577027. [Google Scholar] [CrossRef]
- Croxford, A.L.; Spath, S.; Becher, B. GM-CSF in Neuroinflammation: Licensing Myeloid Cells for Tissue Damage. Trends Immunol. 2015, 36, 651–662. [Google Scholar] [CrossRef]
- Vogel, D.Y.; Kooij, G.; Heijnen, P.D.; Breur, M.; Peferoen, L.A.; van der Valk, P.; de Vries, H.E.; Amor, S.; Dijkstra, C.D. GM-CSF promotes migration of human monocytes across the blood brain barrier. Eur. J. Immunol. 2015, 45, 1808–1819. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, K.; Poulaki, V.; Doehmen, S.; Welsandt, G.; Radetzky, S.; Lappas, A.; Kociok, N.; Kirchhof, B.; Joussen, A.M. Contribution of TNF-alpha to leukocyte adhesion, vascular leakage, and apoptotic cell death in endotoxin-induced uveitis in vivo. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2184–2191. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, S.; Yamamoto, R.; Murakami, K.; Takahashi, N.; Nishi, R.; Ishii, A.; Nio-Kobayashi, J.; Abe, N.; Tanaka, K.; Jiang, J.J.; et al. GM-CSF Promotes the Survival of Peripheral-Derived Myeloid Cells in the Central Nervous System for Pain-Induced Relapse of Neuroinflammation. J. Immunol. 2023, 211, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Sterner, R.M.; Sakemura, R.; Cox, M.J.; Yang, N.; Khadka, R.H.; Forsman, C.L.; Hansen, M.J.; Jin, F.; Ayasoufi, K.; Hefazi, M.; et al. GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts. Blood 2019, 133, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.D.; Garfall, A.L.; Stadtmauer, E.A.; Melenhorst, J.J.; Lacey, S.F.; Lancaster, E.; Vogl, D.T.; Weiss, B.M.; Dengel, K.; Nelson, A.; et al. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J. Clin. Investig. 2019, 129, 2210–2221. [Google Scholar] [CrossRef] [PubMed]
- Santomasso, B.D.; Park, J.H.; Salloum, D.; Riviere, I.; Flynn, J.; Mead, E.; Halton, E.; Wang, X.; Senechal, B.; Purdon, T.; et al. Clinical and Biological Correlates of Neurotoxicity Associated with CAR T-cell Therapy in Patients with B-cell Acute Lymphoblastic Leukemia. Cancer Discov. 2018, 8, 958–971. [Google Scholar] [CrossRef]
- Shalabi, H.; Martin, S.; Yates, B.; Wolters, P.L.; Kaplan, C.; Smith, H.; Sesi, C.R.; Jess, J.; Toledo-Tamula, M.A.; Struemph, K.; et al. Neurotoxicity following CD19/CD28zeta CAR T-cells in children and young adults with B-cell malignancies. Neuro-Oncology 2022, 24, 1584–1597. [Google Scholar] [CrossRef]
- Gust, J.; Finney, O.C.; Li, D.; Brakke, H.M.; Hicks, R.M.; Futrell, R.B.; Gamble, D.N.; Rawlings-Rhea, S.D.; Khalatbari, H.K.; Ishak, G.E.; et al. Glial injury in neurotoxicity after pediatric CD19-directed chimeric antigen receptor T cell therapy. Ann. Neurol. 2019, 86, 42–54. [Google Scholar] [CrossRef]
- Melrose, J.; Tsurushita, N.; Liu, G.; Berg, E.L. IFN-gamma inhibits activation-induced expression of E- and P-selectin on endothelial cells. J. Immunol. 1998, 161, 2457–2464. [Google Scholar] [CrossRef]
- Li, M.; Cuff, C.F.; Pestka, J.J. T-2 toxin impairment of enteric reovirus clearance in the mouse associated with suppressed immunoglobulin and IFN-gamma responses. Toxicol. Appl. Pharmacol. 2006, 214, 318–325. [Google Scholar] [CrossRef]
- Mangalam, A.K.; Luo, N.; Luckey, D.; Papke, L.; Hubbard, A.; Wussow, A.; Smart, M.; Giri, S.; Rodriguez, M.; David, C. Absence of IFN-gamma increases brain pathology in experimental autoimmune encephalomyelitis-susceptible DRB1*0301.DQ8 HLA transgenic mice through secretion of proinflammatory cytokine IL-17 and induction of pathogenic monocytes/microglia into the central nervous system. J. Immunol. 2014, 193, 4859–4870. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lv, X.; Kong, Q.; Tan, Y. IL-6/IFN-gamma double knockdown CAR-T cells reduce the release of multiple cytokines from PBMCs in vitro. Hum. Vaccin. Immunother. 2022, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Benallegue, N.; Kebir, H.; Kapoor, R.; Crockett, A.; Li, C.; Cheslow, L.; Abdel-Hakeem, M.S.; Gesualdi, J.; Miller, M.C.; Wherry, E.J.; et al. The hedgehog pathway suppresses neuropathogenesis in CD4 T cell-driven inflammation. Brain 2021, 144, 1670–1683. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Choi, H.M.; Yang, H.I.; Yoo, M.C.; Kim, K.S. Increased expression of IL-1 receptors in response to IL-1beta may produce more IL-6, IL-8, VEGF, and PGE(2) in senescent synovial cells induced in vitro than in presenescent cells. Rheumatol. Int. 2012, 32, 2005–2010. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.J.; Shang, C.Z.; Peng, D.W.; Xu, J.; Xu, P.X.; Zhan, L.; Wang, P. Curcumin attenuates ischemia-like injury induced IL-1beta elevation in brain microvascular endothelial cells via inhibiting MAPK pathways and nuclear factor-kappaB activation. Neurol. Sci. 2014, 35, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Norelli, M.; Camisa, B.; Barbiera, G.; Falcone, L.; Purevdorj, A.; Genua, M.; Sanvito, F.; Ponzoni, M.; Doglioni, C.; Cristofori, P.; et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat. Med. 2018, 24, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Kochenderfer, J.N.; Somerville, R.P.T.; Lu, T.; Shi, V.; Bot, A.; Rossi, J.; Xue, A.; Goff, S.L.; Yang, J.C.; Sherry, R.M.; et al. Lymphoma Remissions Caused by Anti-CD19 Chimeric Antigen Receptor T Cells Are Associated With High Serum Interleukin-15 Levels. J. Clin. Oncol. 2017, 35, 1803–1813. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef]
- Krizanac-Bengez, L.; Kapural, M.; Parkinson, F.; Cucullo, L.; Hossain, M.; Mayberg, M.R.; Janigro, D. Effects of transient loss of shear stress on blood-brain barrier endothelium: Role of nitric oxide and IL-6. Brain Res. 2003, 977, 239–246. [Google Scholar] [CrossRef]
- Takeshita, Y.; Fujikawa, S.; Serizawa, K.; Fujisawa, M.; Matsuo, K.; Nemoto, J.; Shimizu, F.; Sano, Y.; Tomizawa-Shinohara, H.; Miyake, S.; et al. New BBB Model Reveals That IL-6 Blockade Suppressed the BBB Disorder, Preventing Onset of NMOSD. Neurol.-Neuroimmunol. Neuroinflamm. 2021, 8. [Google Scholar] [CrossRef]
- Erta, M.; Giralt, M.; Jimenez, S.; Molinero, A.; Comes, G.; Hidalgo, J. Astrocytic IL-6 Influences the Clinical Symptoms of EAE in Mice. Brain Sci. 2016, 6, e1076. [Google Scholar] [CrossRef] [PubMed]
- Mumm, J.B.; Emmerich, J.; Zhang, X.; Chan, I.; Wu, L.; Mauze, S.; Blaisdell, S.; Basham, B.; Dai, J.; Grein, J.; et al. IL-10 elicits IFNgamma-dependent tumor immune surveillance. Cancer Cell 2011, 20, 781–796. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; van Montfrans, C.; van den Ende, A.; Kaser, A.; van Deventer, S.J.; Schreiber, S.; Gregor, M.; Ludwiczek, O.; Rutgeerts, P.; Gasche, C.; et al. Treatment of Crohn’s disease with recombinant human interleukin 10 induces the proinflammatory cytokine interferon gamma. Gut 2002, 50, 191–195. [Google Scholar] [CrossRef]
- Cianciulli, A.; Dragone, T.; Calvello, R.; Porro, C.; Trotta, T.; Lofrumento, D.D.; Panaro, M.A. IL-10 plays a pivotal role in anti-inflammatory effects of resveratrol in activated microglia cells. Int. Immunopharmacol. 2015, 24, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Yu, C.; Hsuchou, H.; Khan, R.S.; Kastin, A.J. Cerebral microvascular IL15 is a novel mediator of TNF action. J. Neurochem. 2009, 111, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Pan, W.; Stone, K.P.; Zhang, Y.; Hsuchou, H.; Kastin, A.J. Expression and signaling of novel IL15Ralpha splicing variants in cerebral endothelial cells of the blood-brain barrier. J. Neurochem. 2010, 114, 122–129. [Google Scholar] [CrossRef]
- Saikali, P.; Antel, J.P.; Pittet, C.L.; Newcombe, J.; Arbour, N. Contribution of astrocyte-derived IL-15 to CD8 T cell effector functions in multiple sclerosis. J. Immunol. 2010, 185, 5693–5703. [Google Scholar] [CrossRef]
- Brickler, T.R.; Hazy, A.; Guilhaume Correa, F.; Dai, R.; Kowalski, E.J.A.; Dickerson, R.; Chen, J.; Wang, X.; Morton, P.D.; Whittington, A.; et al. Angiopoietin/Tie2 Axis Regulates the Age-at-Injury Cerebrovascular Response to Traumatic Brain Injury. J. Neurosci. 2018, 38, 9618–9634. [Google Scholar] [CrossRef]
- Higgins, S.J.; Purcell, L.A.; Silver, K.L.; Tran, V.; Crowley, V.; Hawkes, M.; Conroy, A.L.; Opoka, R.O.; Hay, J.G.; Quaggin, S.E.; et al. Dysregulation of angiopoietin-1 plays a mechanistic role in the pathogenesis of cerebral malaria. Sci. Transl. Med. 2016, 8, 358ra128. [Google Scholar] [CrossRef]
- Fu, C.C.; Wang, R.J.; Wu, D.P. Interpretation of ASTCT Consensus Responses by Chimeric Antigen Receptor T Cell Therapy CRS/ICANS—Review. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2021, 29, 1982–1986. [Google Scholar]
- Saw, J.L.; Sidiqi, M.H.; Ruff, M.; Hocker, S.; Alkhateeb, H.; Ansell, S.M.; Bennani, N.N.; Dingli, D.; Hayman, S.R.; Johnston, P.B.; et al. Acute seizures and status epilepticus in immune effector cell associated neurotoxicity syndrome (ICANS). Blood Cancer J. 2022, 12, 62. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.J.; Svoboda, J.; Chong, E.A.; Nasta, S.D.; Mato, A.R.; Anak, O.; Brogdon, J.L.; Pruteanu-Malinici, I.; Bhoj, V.; Landsburg, D.; et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N. Engl. J. Med. 2017, 377, 2545–2554. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.A.; Kikano, E.; Tirumani, S.H.; de Lima, M.; Caimi, P.; Ramaiya, N.H. Imaging-based Toxicity and Response Pattern Assessment Following CAR T-Cell Therapy. Radiology 2022, 302, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Lapidus, A.H.; Anderson, M.A.; Harrison, S.J.; Dickinson, M.; Kalincik, T.; Lasocki, A. Neuroimaging findings in immune effector cell associated neurotoxicity syndrome after chimeric antigen receptor T-cell therapy. Leuk. Lymphoma 2022, 63, 2364–2374. [Google Scholar] [CrossRef]
- Pinto, S.N.; Liu, C.J.; Nelson, M.D., Jr.; Bluml, S.; Livingston, D.; Tamrazi, B. Neuroimaging of complications arising after CD19 chimeric antigen receptor T-cell therapy: A review. J. Neuroimaging 2023, 33, 703–715. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).