Statins—From Fungi to Pharmacy
Abstract
1. Introduction
2. The Discovery of Statins
3. Fungal-Derived Statins
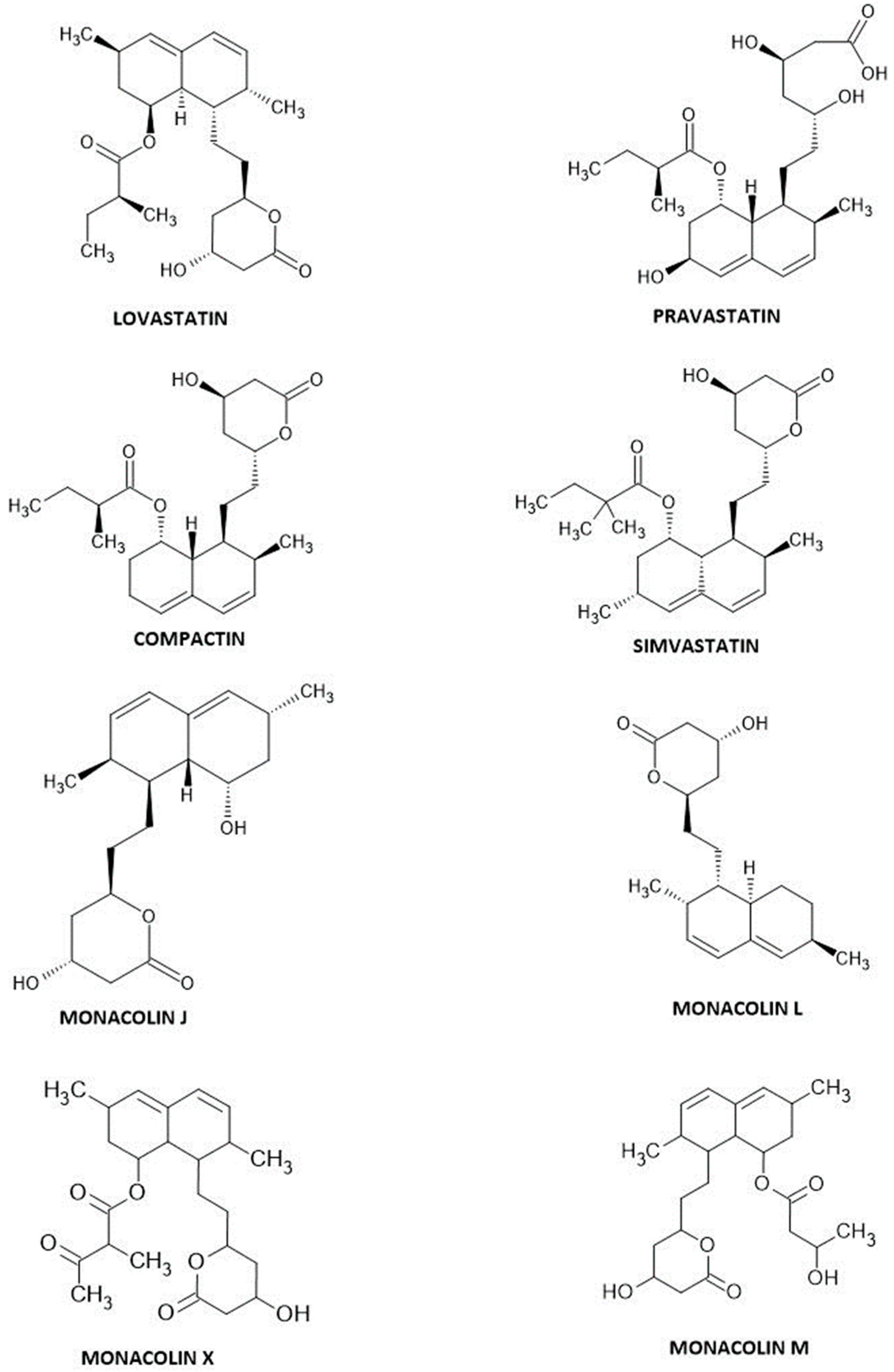
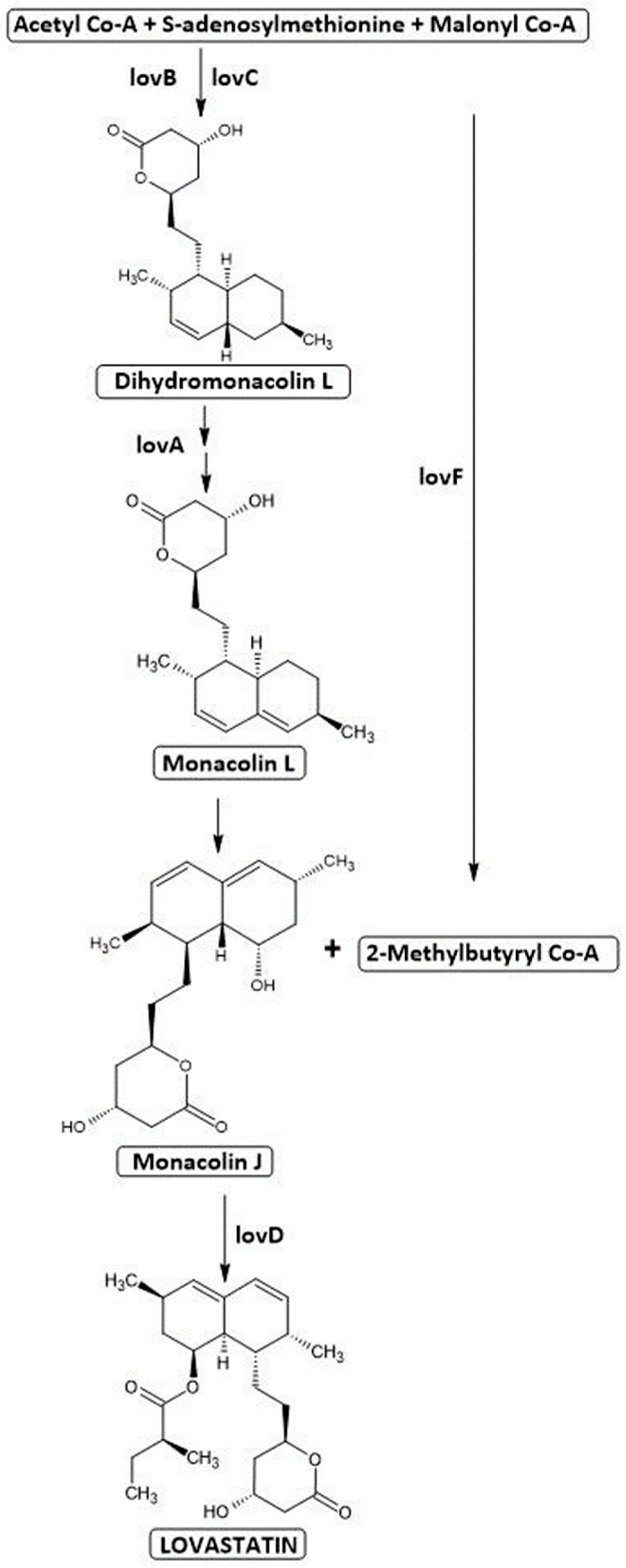
3.1. Lovastatin
3.2. Simvastatin
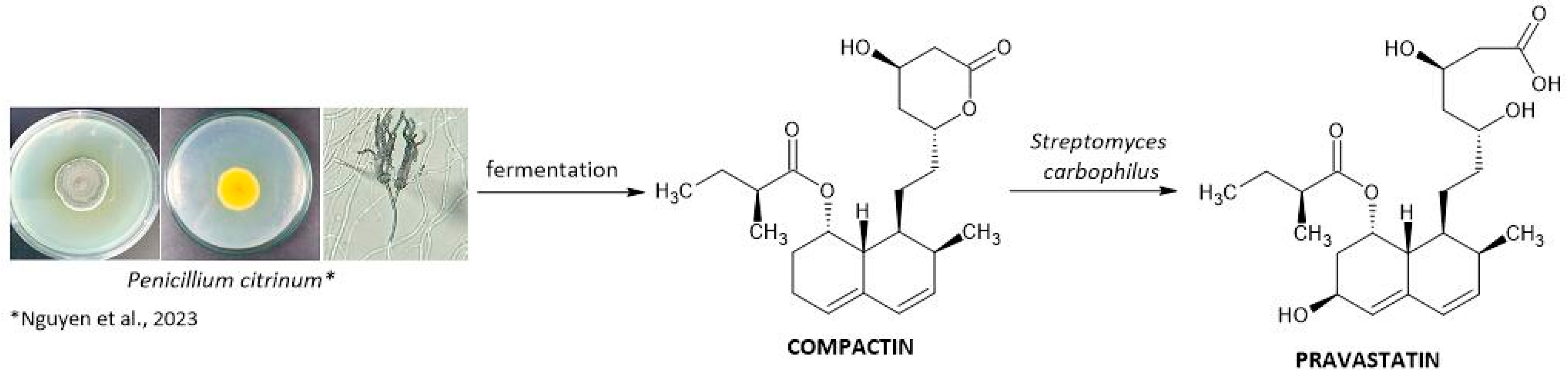
3.3. Pravastatin
- -
- A spore suspension or vegetative mycelium as the inoculum for the seed culture.
- -
- Of 5% malt extract and peptone in the seed medium.
- -
- pH of the seed medium between 6.0 and 7.5 before sterilization.
- -
- Incubation of the seed medium at temperatures ranging from 25 to 35 °C for about 40 to 55 h.
- -
- Selection of components such as dextrose monohydrate, peptone, and yeast extract for the production medium.
- -
- Incubation of the production medium at temperatures between 24 and 35 °C for approximately 48 to 148 h.
- -
- Using compactin, compactin salt, or compactin derivative as the substrate for bioconversion.
- -
- pH regulation by feeding with a carbon source chosen from saccharides or glycerol [53].
4. Synthetic Statins
5. Mechanism of Action
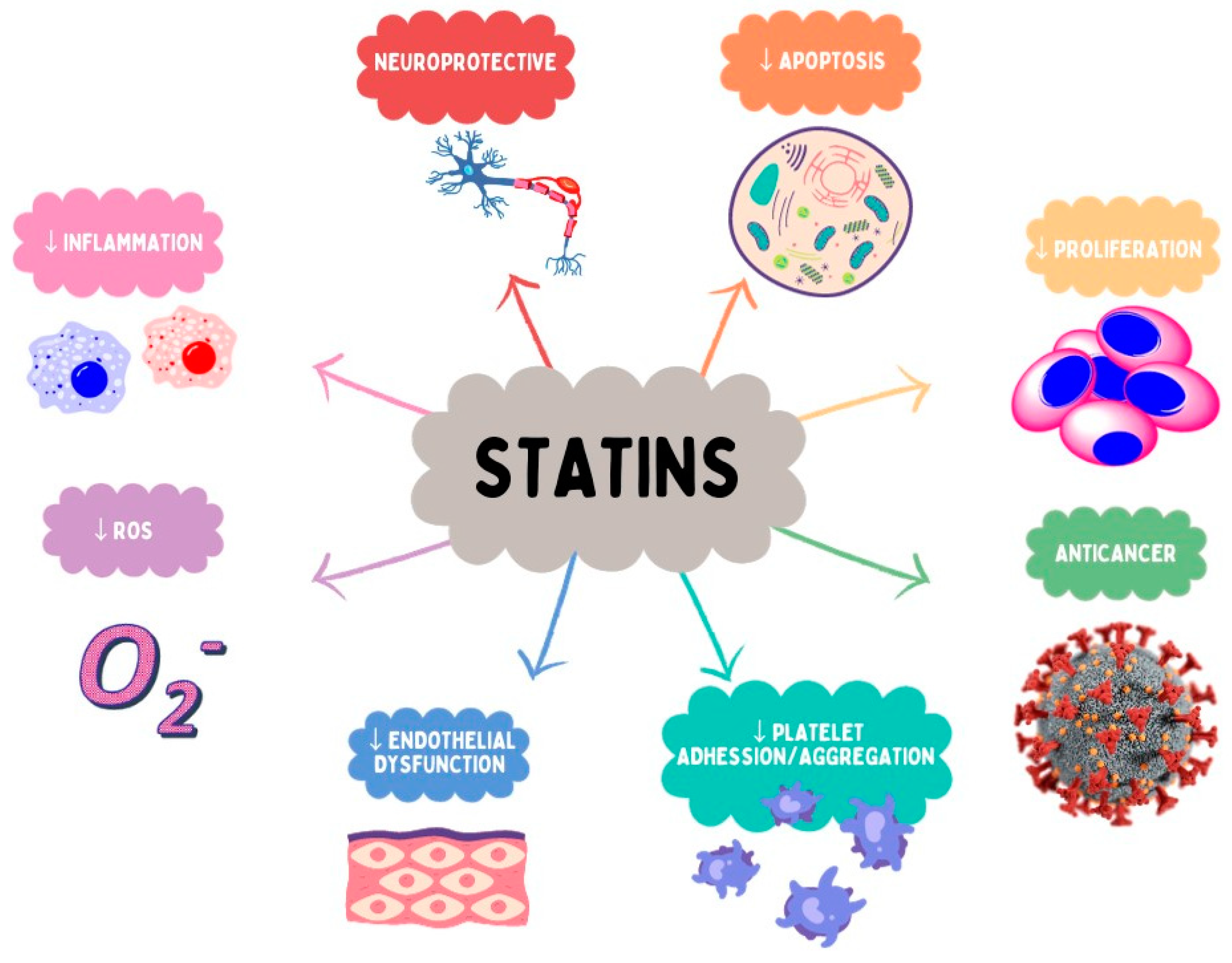
6. Pleiotropic Activity of Statins
7. Selected Aspects of Statin Pharmacokinetics
| Drug | Prodrug | Solubility | Bioavailability (%) | Metabolism by Cytochrome P450 Enzymes | OATP Transport | Substrates of P-glycoprotein | Metabolites | Protein Binding | T1/2 (h) |
|---|---|---|---|---|---|---|---|---|---|
| Type I—fungal derived statins | |||||||||
| lovastatin | Yes | Lipophilic | <5 | CYP3A4 | OATP1B1 | Yes | Active metabolites | >96% | 2–4 |
| pravastatin | No | Hydrophilic | 17 | Sulfation | OATP1B1 OATP1B3 OATP2B1 | No | Not active metabolites | 50% | 1–3 |
| simvastatin | Yes | Lipophilic | <5 | CYP3A4 | OATP1B1 | Yes | Active metabolites | >95% | 2–3 |
| Type II—synthetically derived statins | |||||||||
| fluvastatin | No | Lipophilic | 24 | CYP2C9 | OATP1B1 OATP1B3 OATP2B1 | No | Not active metabolites | >98% | 0.5–3 |
| atorvastatin | No | Lipophilic | ~12 | CYP3A4 | OATP1B1 OATP2B1 | Yes | Active metabolites | >98% | 15–30 |
| cerivastatin | No | Lipophilic | 60 | CYP3A4 CYP2C8 | OATP1B1 | No | Active metabolites | >99% | 2–3 |
| pitavastatin | No | Lipophilic | ~60 | Main: lactonization/glucuronidation Minimal: CYP2C8 CYP2C9 | OATP1B1 OATP1A2 OATP1B3 | Yes | Minimally metabolized | 96% | 10–12 |
| rosuvastatin | No | Hydrophilic | 20 | Minimal: CYP2C9 CYP2C19 | OATP1B1 OATP1A2 OATP1B3 OATP2B1 | No | Minimally metabolized | 88% | 19–20 |
8. Interactions
9. Adverse Reactions
10. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chester, A. From Fleming to Endo: The Discovery of Statins. GCSP 2022, 2021, e202132. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Endo, A. A Historical Perspective on the Discovery of Statins. Proc. Jpn. Acad. Ser. B 2010, 86, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Stossel, T.P. The Discovery of Statins. Cell 2008, 134, 903–905. [Google Scholar] [CrossRef] [PubMed]
- Endo, A. Discovery and Development of Statins. Nat. Prod. Commun. 2017, 12, 1934578X1701200. [Google Scholar] [CrossRef]
- Musset, L.; Allenbach, Y.; Benveniste, O.; Boyer, O.; Bossuyt, X.; Bentow, C.; Phillips, J.; Mammen, A.; Van Damme, P.; Westhovens, R.; et al. Anti-HMGCR Antibodies as a Biomarker for Immune-Mediated Necrotizing Myopathies: A History of Statins and Experience from a Large International Multi-Center Study. Autoimmun. Rev. 2016, 15, 983–993. [Google Scholar] [CrossRef]
- Alberts, A.W.; Chen, J.; Kuron, G.; Hunt, V.; Huff, J.; Hoffman, C.; Rothrock, J.; Lopez, M.; Joshua, H.; Harris, E.; et al. Mevinolin: A Highly Potent Competitive Inhibitor of Hydroxymethylglutaryl-Coenzyme A Reductase and a Cholesterol-Lowering Agent. Proc. Natl. Acad. Sci. USA 1980, 77, 3957–3961. [Google Scholar] [CrossRef] [PubMed]
- Barrios-González, J.; Miranda, R.U. Biotechnological Production and Applications of Statins. Appl. Microbiol. Biotechnol. 2010, 85, 869–883. [Google Scholar] [CrossRef]
- Endo, A. The Discovery and Development of HMG-CoA Reductase Inhibitors. J. Lipid Res. 1992, 33, 1569–1582. [Google Scholar] [CrossRef]
- Endo, A. A Gift from Nature: The Birth of the Statins. Nat. Med. 2008, 14, 1050–1052. [Google Scholar] [CrossRef]
- Mabuchi, H.; Haba, T.; Tatami, R.; Miyamoto, S.; Sakai, Y.; Wakasugi, T.; Watanabe, A.; Koizumi, J.; Takeda, R. Effects of an Inhibitor of 3-Hydroxy-3-Methylglutaryl Coenzyme a Reductase on Serum Lipoproteins and Ubiquinone-10 Levels in Patients with Familial Hypercholesterolemia. N. Engl. J. Med. 1981, 305, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, F.; Wang, Z.; Hu, Z. Identification and Chemical Profiling of Monacolins in Red Yeast Rice Using High-Performance Liquid Chromatography with Photodiode Array Detector and Mass Spectrometry. J. Pharm. Biomed. Anal. 2004, 35, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Endo, A.; Komagata, D.; Shimada, H. Monacolin M, a New Inhibitor of Cholesterol Biosynthesis. J. Antibiot. 1986, 39, 1670–1673. [Google Scholar] [CrossRef] [PubMed]
- Vendruscolo, F.; Bühler, R.M.M.; De Carvalho, J.C.; De Oliveira, D.; Moritz, D.E.; Schmidell, W.; Ninow, J.L. Monascus: A Reality on the Production and Application of Microbial Pigments. Appl. Biochem. Biotechnol. 2016, 178, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, Z.; Chi, J.; Wang, W.; Su, M.; Kou, W.; Yu, P.; Yu, L.; Chen, L.; Zhu, J.-S.; et al. Multicenter Clinical Trial of the Serum Lipid-Lowering Effects of a Monascus Purpureus (Red Yeast) Rice Preparation from Traditional Chinese Medicine. Curr. Ther. Res. 1997, 58, 964–978. [Google Scholar] [CrossRef]
- Li, C.; Zhu, Y.; Wang, Y.; Zhu, J.-S.; Chang, J.; Kritchevsky, D. Monascus Purpureus-Fermented Rice (Red Yeast Rice): A Natural Food Product That Lowers Blood Cholesterol in Animal Models of Hypercholesterolemia. Nutr. Res. 1998, 18, 71–81. [Google Scholar] [CrossRef]
- Lin, Y.-L.; Wang, T.-H.; Lee, M.-H.; Su, N.-W. Biologically Active Components and Nutraceuticals in the Monascus-Fermented Rice: A Review. Appl. Microbiol. Biotechnol. 2008, 77, 965–973. [Google Scholar] [CrossRef]
- Pérez-Jiménez, F.; Pascual, V.; Meco, J.F.; Pérez Martínez, P.; Delgado Lista, J.; Domenech, M.; Estruch, R.; León-Acuña, A.; López-Miranda, J.; Sánchez-Ramos, A.; et al. Documento de recomendaciones de la SEA 2018. El estilo de vida en la prevención cardiovascular. Clínica E Investig. En Arterioscler. 2018, 30, 280–310. [Google Scholar] [CrossRef]
- Divsalar, P.; Noorbala, A.A.; Moazen-Zadeh, E.; Jafarinia, M.; Shakiba, M.; Shahmansouri, N.; Ghazizadeh-Hashemi, M.; Etesam, F.; Akhondzadeh, S. Red Yeast Rice as an Adjunct to Sertraline for Treatment of Depression in Patients with Percutaneous Coronary Intervention: Placebo-Controlled Trial. Adv. Integr. Med. 2018, 5, 69–74. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, H.; Zhou, X.; Luo, H.; Tang, F.; Yang, J.; Alterovitz, G.; Cheng, L.; Ren, B. Lovastatin Synergizes with Itraconazole against Planktonic Cells and Biofilms of Candida Albicans through the Regulation on Ergosterol Biosynthesis Pathway. Appl. Microbiol. Biotechnol. 2018, 102, 5255–5264. [Google Scholar] [CrossRef]
- Chen, C.-C.; Liu, T.-Y.; Huang, S.-P.; Ho, C.-T.; Huang, T.-C. Differentiation and Apoptosis Induction by Lovastatin and γ-Tocotrienol in HL-60 Cells via Ras/ERK/NF-κB and Ras/Akt/NF-κB Signaling Dependent down-Regulation of Glyoxalase 1 and HMG-CoA Reductase. Cell. Signal. 2015, 27, 2182–2190. [Google Scholar] [CrossRef] [PubMed]
- Klawitter, J.; Shokati, T.; Moll, V.; Christians, U.; Klawitter, J. Effects of Lovastatin on Breast Cancer Cells: A Proteo-Metabonomic Study. Breast Cancer Res. 2010, 12, R16. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Cao, X.; Wen, Q.; Chen, Z.; Cheng, Z.; Huang, X.; Zhang, Y.; Long, C.; Zhang, Y.; Huang, Z. An Overview of the Bioactivity of Monacolin K / Lovastatin. Food Chem. Toxicol. 2019, 131, 110585. [Google Scholar] [CrossRef]
- Malec, M. Monacolin K—A Natural Statin. Farm. Pol. 2019, 75, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Stefanutti, C.; Mazza, F.; Mesce, D.; Morozzi, C.; Di Giacomo, S.; Vitale, M.; Pergolini, M. Monascus Purpureus for Statin and Ezetimibe Intolerant Heterozygous Familial Hypercholesterolaemia Patients: A Clinical Study. Atheroscler. Suppl. 2017, 30, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Krieger, J.; Li, D.; Papanikolaou, D. Missing Novelty in Drug Development. Rev. Financ. Stud. 2022, 35, 636–679. [Google Scholar] [CrossRef]
- Barylski, M. Atorvastatin and Rosuvastatin in Cardiovascular System Diseases—Why, When and Whom They Should Be Given? Geriatria 2012, 6, 166–182. [Google Scholar]
- Chruściel, P.; Banach, M. Atorvastatin in Patients with Overweight and Obesity. Choroby Serca i Naczyń 2016, 13, 5–14. [Google Scholar]
- Furberg, C.D.; Pitt, B. Withdrawal of Cerivastatin from the World Market. Curr. Control. Trials Cardiovasc. Med. 2001, 2, 205. [Google Scholar] [CrossRef]
- Newman, C.B.; Preiss, D.; Tobert, J.A.; Jacobson, T.A.; Page, R.L.; Goldstein, L.B.; Chin, C.; Tannock, L.R.; Miller, M.; Raghuveer, G.; et al. Statin Safety and Associated Adverse Events: A Scientific Statement From the American Heart Association. ATVB 2019, 39, E38–E81. [Google Scholar] [CrossRef]
- Ose, L. Pitavastatin: Finding Its Place in Therapy. Ther. Adv. Chronic Dis. 2011, 2, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Mulder, K.C.L.; Mulinari, F.; Franco, O.L.; Soares, M.S.F.; Magalhães, B.S.; Parachin, N.S. Lovastatin Production: From Molecular Basis to Industrial Process Optimization. Biotechnol. Adv. 2015, 33, 648–665. [Google Scholar] [CrossRef] [PubMed]
- Barrios-González, J.; Baños, J.G.; Covarrubias, A.A.; Garay-Arroyo, A. Lovastatin Biosynthetic Genes of Aspergillus terreus Are Expressed Differentially in Solid-State and in Liquid Submerged Fermentation. Appl. Microbiol. Biotechnol. 2008, 79, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Baños, J.G.; Tomasini, A.; Szakács, G.; Barrios-González, J. High Lovastatin Production by Aspergillus terreus in Solid-State Fermentation on Polyurethane Foam: An Artificial Inert Support. J. Biosci. Bioeng. 2009, 108, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Barrios-González, J.; Pérez-Sánchez, A.; Bibián, M.E. New Knowledge about the Biosynthesis of Lovastatin and Its Production by Fermentation of Aspergillus terreus. Appl. Microbiol. Biotechnol. 2020, 104, 8979–8998. [Google Scholar] [CrossRef]
- Kennedy, J.; Auclair, K.; Kendrew, S.G.; Park, C.; Vederas, J.C.; Richard Hutchinson, C. Modulation of Polyketide Synthase Activity by Accessory Proteins during Lovastatin Biosynthesis. Science 1999, 284, 1368–1372. [Google Scholar] [CrossRef]
- Osman, M.E.; Khattab, O.H.; Zaghlol, G.M.; Abd El-Hameed, R.M. Optimization of Some Physical and Chemical Factors for Lovastatin Productivity by Local Strain of Aspergillus terreus. Aust. J. Basic. Appl. Sci. 2011, 5, 718–732. [Google Scholar]
- Bizukojc, M.; Pawlak, M.; Boruta, T.; Gonciarz, J. Effect of pH on Biosynthesis of Lovastatin and Other Secondary Metabolites by Aspergillus terreus ATCC 20542. J. Biotechnol. 2012, 162, 253–261. [Google Scholar] [CrossRef]
- Hajjaj, H.; Niederberger, P.; Duboc, P. Lovastatin Biosynthesis by Aspergillus terreus in a Chemically Defined Medium. Appl. Environ. Microbiol. 2001, 67, 2596–2602. [Google Scholar] [CrossRef]
- Bizukojc, M.; Pecyna, M. Lovastatin and (+)-geodin Formation by Aspergillus terreus ATCC 20542 in a Batch Culture with the Simultaneous Use of Lactose and Glycerol as Carbon Sources. Eng. Life Sci. 2011, 11, 272–282. [Google Scholar] [CrossRef]
- Lai, L.-S.T.; Pan, C.-C.; Tzeng, B.-K. The Influence of Medium Design on Lovastatin Production and Pellet Formation with a High-Producing Mutant of Aspergillus terreus in Submerged Cultures. Process Biochem. 2003, 38, 1317–1326. [Google Scholar] [CrossRef]
- Talreja, O.; Kerndt, C.C.; Cassagnol, M. Simvastatin. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Murthy, K.; Horne, S.; Weeratunga, G.; Yang, S. Process for Producing Simvastatin. US Patent No. 6,307,066, 23 October 2001. [Google Scholar]
- Hoyos, P.; Pace, V.; Alcántara, A. Biocatalyzed Synthesis of Statins: A Sustainable Strategy for the Preparation of Valuable Drugs. Catalysts 2019, 9, 260. [Google Scholar] [CrossRef]
- Xie, X.; Tang, Y. Efficient Synthesis of Simvastatin by Use of Whole-Cell Biocatalysis. Appl. Env. Microbiol. 2007, 73, 2054–2060. [Google Scholar] [CrossRef] [PubMed]
- Syed, M.B.; Ponnusamy, T. Bioconversion of Mevastatin to Pravastatin by Various Microorganisms and Its Applications—A Review. Biocatal. Agric. Biotechnol. 2018, 13, 62–74. [Google Scholar] [CrossRef]
- Chakravarti, R.; Sahai, V. Compactin? A Review. Appl. Microbiol. Biotechnol. 2004, 64, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Shaligram, N.S.; Singh, S.K.; Singhal, R.S.; Pandey, A.; Szakacs, G. Compactin Production Studies Using Penicillium Brevicompactum under Solid-State Fermentation Conditions. Appl. Biochem. Biotechnol. 2009, 159, 505–520. [Google Scholar] [CrossRef]
- Tobert, J.A. New Developments in Lipid-Lowering Therapy: The Role of Inhibitors of Hydroxymethylglutaryl-Coenzyme A Reductase. Circulation 1987, 76, 534–538. [Google Scholar] [CrossRef]
- Hamelin, B. Hydrophilicity/Lipophilicity: Relevance for the Pharmacology and Clinical Effects of HMG-CoA Reductase Inhibitors. Trends Pharmacol. Sci. 1998, 19, 26–37. [Google Scholar] [CrossRef]
- Matsuoka, T.; Miyakoshi, S.; Tanzawa, K.; Nakahara, K.; Hosobuchi, M.; Serizawa, N. Purification and Characterization of Cytochrome P-450sca from Streptomyces carbophilus. ML-236B (Compactin) Induces a Cytochrome P-450sca in Streptomyces carbophilus That Hydroxylates ML-236B to Pravastatin Sodium (CS-514), a Tissue-Selective Inhibitor of 3-Hydroxy-3-Methylglutaryl-Coenzyme-A Reductase. Eur. J. Biochem. 1989, 184, 707–713. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Lin, K.-H.; Nguyen, T.P.; Le, H.S.; Ngo, K.N.; Pham, D.C.; Tran, T.N.; Su, C.-H.; Barrow, C.J. Isolation and Cultivation of Penicillium citrinum for Biological Control of Spodoptera litura and Plutella xylostella. Fermentation 2023, 9, 438. [Google Scholar] [CrossRef]
- Gururaja, R.; Goel, A.; Sridharan, M.; Melarkode, R.S.; Kulkarni, M.; Poornaprajna, A.; Sathyanathan, D.; Ganesh, S.; Suryanarayan, S. Process for Producing Pravastatin Sodium Salt Using Streptomyces flavidovirens Dsm 14455. U.S. Patent No. 7,189,558, 13 March 2007. [Google Scholar]
- Tsujita, Y.; Kuroda, M.; Shimada, Y.; Tanzawa, K.; Arai, M.; Kaneko, I.; Tanaka, M.; Masuda, H.; Tarumi, C.; Watanabe, Y.; et al. CS-514, a Competitive Inhibitor of 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase: Tissue-Selective Inhibition of Sterol Synthesis and Hypolipidemic Effect on Various Animal Species. Biochim. et Biophys. Acta BBA-Lipids Lipid Metab. 1986, 877, 50–60. [Google Scholar] [CrossRef]
- Rollini, M.M.M. Biosynthesis and Biotechnological Production of Statins by Filamentous Fungi and Application of These Cholesterol-Lowering Drugs. Appl. Microbiol. Biotechnol. 2002, 58, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Sit, S.Y.; Parker, R.A.; Motoc, I.; Han, W.; Balasubramanian, N.; Catt, J.D.; Brown, P.J.; Harte, W.E.; Thompson, M.D.; Wright, J.J. Synthesis, Biological Profile, and Quantitative Structure-Activity Relationship of a Series of Novel 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase Inhibitors. J. Med. Chem. 1990, 33, 2982–2999. [Google Scholar] [CrossRef]
- Tse, F.L.S.; Smith, H.T.; Ballard, F.H.; Nicoletti, J. Disposition of Fluvastatin, an Inhibitor of HMG-CoA Reductase, in Mouse, Rat, Dog, and Monkey. Biopharm. Drug Disp. 1990, 11, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Quirk, J.; Thornton, M.; Kirkpatrick, P. Rosuvastatin Calcium. Nat. Rev. Drug Discov. 2003, 2, 769–770. [Google Scholar] [CrossRef] [PubMed]
- Kajinami, K.; Mabuchi, H.; Saito, Y. NK-104: A Novel Synthetic HMG-CoA Reductase Inhibitor. Expert. Opin. Investig. Drugs 2000, 9, 2653–2661. [Google Scholar] [CrossRef]
- Istvan, E.S.; Deisenhofer, J. Structural Mechanism for Statin Inhibition of HMG-CoA Reductase. Science 2001, 292, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Matsui, M.; Miyamura, Y.; Takeda, I.; Ishii, J.; Kumagai, T.; Machida, M.; Shibata, T.; Arita, M. Biosynthesis of Novel Statins by Combining Heterologous Genes from Xylaria and Aspergillus. ACS Synth. Biol. 2018, 7, 2783–2789. [Google Scholar] [CrossRef]
- McKenney, J.M. Pharmacologic Characteristics of Statins. Clin. Cardiol. 2003, 26, 32–38. [Google Scholar] [CrossRef]
- Duan, Y.; Gong, K.; Xu, S.; Zhang, F.; Meng, X.; Han, J. Regulation of Cholesterol Homeostasis in Health and Diseases: From Mechanisms to Targeted Therapeutics. Signal Transduct. Target. Ther. 2022, 7, 265. [Google Scholar] [CrossRef]
- Blumenthal, R.S. Statins: Effective Antiatherosclerotic Therapy. Am. Heart J. 2000, 139, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Newby, L.K.; Arnold, S.V.; Bittner, V.; Brewer, L.C.; Demeter, S.H.; Dixon, D.L.; Fearon, W.F.; Hess, B.; Johnson, H.M.; et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients With Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2023, 148, E9–E119. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.H.; Seeger, J.D.; Franklin, C. Clinically Relevant Differences between the Statins: Implications for Therapeutic Selection. Am. J. Med. 2001, 111, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, R.S.; Tangney, C.C. Antiatherothrombotic Properties of Statins: Implications for Cardiovascular Event Reduction. JAMA 1998, 279, 1643. [Google Scholar] [CrossRef]
- Ahmadi, Y.; Fard, J.K.; Ghafoor, D.; Eid, A.H.; Sahebkar, A. Paradoxical Effects of Statins on Endothelial and Cancer Cells: The Impact of Concentrations. Cancer Cell Int. 2023, 23, 43. [Google Scholar] [CrossRef]
- Miller, L.J.; Chacko, R. The Role of Cholesterol and Statins in Alzheimer’s Disease. Ann. Pharmacother. 2004, 38, 91–98. [Google Scholar] [CrossRef]
- Haag, M.D.M.; Hofman, A.; Koudstaal, P.J.; Stricker, B.H.C.; Breteler, M.M.B. Statins Are Associated with a Reduced Risk of Alzheimer Disease Regardless of Lipophilicity. The Rotterdam Study. J. Neurol. Neurosurg. Psychiatry 2009, 80, 13–17. [Google Scholar] [CrossRef]
- Kurata, T.; Kawai, H.; Miyazaki, K.; Kozuki, M.; Morimoto, N.; Ohta, Y.; Ikeda, Y.; Abe, K. Statins Have Therapeutic Potential for the Treatment of Alzheimer’s Disease, Likely via Protection of the Neurovascular Unit in the AD Brain. J. Neurol. Sci. 2012, 322, 59–63. [Google Scholar] [CrossRef]
- Stein, E.A.; Illingworth, D.R.; Kwiterovich, P.O., Jr.; Liacouras, C.A.; Siimes, M.A.; Jacobson, M.S.; Brewster, T.G.; Hopkins, P.; Davidson, M.; Graham, K.; et al. Efficacy and Safety of Lovastatin in Adolescent Males With Heterozygous Familial Hypercholesterolemia: A Randomized Controlled Trial. JAMA 1999, 281, 137. [Google Scholar] [CrossRef]
- Duong, H.; Bajaj, T. Lovastatin. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Castaño, G.; Menéndez, R.; Más, R.; Amor, A.; Fernández, J.L.; González, R.L.; Lezcay, M.; Alvarez, E. Effects of Policosanol and Lovastatin on Lipid Profile and Lipid Peroxidation in Patients with Dyslipidemia Associated with Type 2 Diabetes Mellitus. Int. J. Clin. Pharmacol. Res. 2002, 22, 89–99. [Google Scholar]
- Berglund, L.; Witztum, J.L.; Galeano, N.F.; Khouw, A.S.; Ginsberg, H.N.; Ramakrishnan, R. Three-Fold Effect of Lovastatin Treatment on Low Density Lipoprotein Metabolism in Subjects with Hyperlipidemia: Increase in Receptor Activity, Decrease in apoB Production, and Decrease in Particle Affinity for the Receptor. Results from a Novel Triple-Tracer Approach. J. Lipid Res. 1998, 39, 913–924. [Google Scholar]
- Wielka Baza ChPL. Available online: http://chpl.com.pl/#detail=1808!70199529 (accessed on 23 November 2023).
- Knipscheer, H.C.; Boelen, C.C.A.; Kastelein, J.J.P.; Van Diermen, D.E.; Groenemeijer, B.E.; Van Den Ende, A.; Büller, H.R.; Bakker, H.D. Short-Term Efficacy and Safety of Pravastatin in 72 Children with Familial Hypercholesterolemia. Pediatr. Res. 1996, 39, 867–871. [Google Scholar] [CrossRef]
- Gaddi, A.; Arca, M.; Ciarrocchi, A.; Fazio, S.; D’Alò, G.; Tiozzo, R.; Descovich, G.C.; Calandra, S. Pravastatin in Heterozygous Familial Hypercholesterolemia: Low-Density Lipoprotein (LDL) Cholesterol-Lowering Effect and LDL Receptor Activity on Skin Fibroblasts. Metabolism 1991, 40, 1074–1078. [Google Scholar] [CrossRef]
- Wielka Baza ChPL. Available online: http://chpl.com.pl/#detail=7352917!79769687 (accessed on 23 November 2023).
- Isley, W.L.; Miles, J.M.; Patterson, B.W.; Harris, W.S. The Effect of High-Dose Simvastatin on Triglyceride-Rich Lipoprotein Metabolism in Patients with Type 2 Diabetes Mellitus. J. Lipid Res. 2006, 47, 193–200. [Google Scholar] [CrossRef][Green Version]
- Barter, P.J.; Brandrup-Wognsen, G.; Palmer, M.K.; Nicholls, S.J. Effect of Statins on HDL-C: A Complex Process Unrelated to Changes in LDL-C: Analysis of the VOYAGER Database. J. Lipid Res. 2010, 51, 1546–1553. [Google Scholar] [CrossRef]
- Wielka Baza ChPL. Available online: http://chpl.com.pl/#detail=3484678!71508361 (accessed on 23 November 2023).
- Simcovas-Informacje o Leku-Opis-Leku-Dawki-Działanie-Skład-Interakcje-Inn-Atc. Available online: https://chpl.com.pl/informacja-o-produkcie-Simcovas-11632392.html (accessed on 28 November 2023).
- Adams, S.P.; Sekhon, S.S.; Tsang, M.; Wright, J.M. Fluvastatin for Lowering Lipids. Cochrane Database Syst. Rev. 2018, 2018, CD012282. [Google Scholar] [CrossRef]
- Langtry, H.D.; Markham, A. Fluvastatin: A Review of Its Use in Lipid Disorders. Drugs 1999, 57, 583–606. [Google Scholar] [CrossRef]
- Wielka Baza ChPL. Available online: http://chpl.com.pl/#detail=1771!9071 (accessed on 23 November 2023).
- Davidson, M.; Ma, P.; Stein, E.A.; Gotto, A.M.; Raza, A.; Chitra, R.; Hutchinson, H. Comparison of Effects on Low-Density Lipoprotein Cholesterol and High-Density Lipoprotein Cholesterol with Rosuvastatin versus Atorvastatin in Patients with Type IIa or IIb Hypercholesterolemia. Am. J. Cardiol. 2002, 89, 268–275. [Google Scholar] [CrossRef]
- Adams, S.P.; Tsang, M.; Wright, J.M. Atorvastatin for Lowering Lipids. Cochrane Database Syst. Rev. 2015, 2017, CD008226. [Google Scholar] [CrossRef]
- Adams, S.P.; Tsang, M.; Wright, J.M. Lipid Lowering Efficacy of Atorvastatin. In Cochrane Database of Systematic Reviews; The Cochrane Collaboration, Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2012; p. CD008226.pub2. [Google Scholar]
- Jones, P.H.; Davidson, M.H.; Stein, E.A.; Bays, H.E.; McKenney, J.M.; Miller, E.; Cain, V.A.; Blasetto, J.W. Comparison of the Efficacy and Safety of Rosuvastatin versus Atorvastatin, Simvastatin, and Pravastatin across Doses (STELLAR**STELLAR = Statin Therapies for Elevated Lipid Levels Compared Across Doses to Rosuvastatin. Trial). Am. J. Cardiol. 2003, 92, 152–160. [Google Scholar] [CrossRef]
- Wielka Baza ChPL. Available online: http://chpl.com.pl/#detail=10378843!71508377 (accessed on 23 November 2023).
- Atoris®-Informacje o Leku-Opis-Leku-Dawki-Działanie-Skład-Interakcje-Inn-Atc. Available online: https://chpl.com.pl/informacja-o-produkcie-Atoris--18273550.html (accessed on 28 November 2023).
- Adams, S.P.; Tiellet, N.; Alaeiilkhchi, N.; Wright, J.M. Cerivastatin for Lowering Lipids. Cochrane Database Syst. Rev. 2020, 1, CD012501. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rifai, N.; Lowenthal, S.P. Rapid Reduction in C-Reactive Protein With Cerivastatin Among 785 Patients With Primary Hypercholesterolemia. Circulation 2001, 103, 1191–1193. [Google Scholar] [CrossRef]
- Adams, S.P.; Alaeiilkhchi, N.; Wright, J.M. Pitavastatin for Lowering Lipids. Cochrane Database Syst. Rev. 2020, 2020, CD012735. [Google Scholar] [CrossRef]
- Pirillo, A.; Catapano, A.L. Pitavastatin and HDL: Effects on Plasma Levels and Function(s). Atheroscler. Suppl. 2017, 27, e1–e9. [Google Scholar] [CrossRef]
- Wielka Baza ChPL. Available online: http://chpl.com.pl/#detail=11613129!5767 (accessed on 23 November 2023).
- Saito, Y. Critical Appraisal of the Role of Pitavastatin in Treating Dyslipidemias and Achieving Lipid Goals. VHRM 2009, 5, 921–936. [Google Scholar] [CrossRef][Green Version]
- Wożakowska-Kapłon, B. Terapia Hipercholesterolemii w Schorzeniach Układu Sercowo-Naczyniowego—Jaki Cel, Jaka Statyna, Jaka Dawka? Folia Cardiologica 2014, 9, 55–56. [Google Scholar]
- Adams, S.P.; Sekhon, S.S.; Wright, J.M. Rosuvastatin for Lowering Lipids. Cochrane Database Syst. Rev. 2014, 2017, CD010254. [Google Scholar] [CrossRef]
- Wielka Baza ChPL. Available online: http://chpl.com.pl/#detail=10756408!76312630 (accessed on 23 November 2023).
- Bajaj, T.; Giwa, A.O. Rosuvastatin. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Hansson, G.K.; Libby, P. The Immune Response in Atherosclerosis: A Double-Edged Sword. Nat. Rev. Immunol. 2006, 6, 508–519. [Google Scholar] [CrossRef]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-Converting Enzyme 2 Is a Functional Receptor for the SARS Coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef]
- Tai, W.; He, L.; Zhang, X.; Pu, J.; Voronin, D.; Jiang, S.; Zhou, Y.; Du, L. Characterization of the Receptor-Binding Domain (RBD) of 2019 Novel Coronavirus: Implication for Development of RBD Protein as a Viral Attachment Inhibitor and Vaccine. Cell. Mol. Immunol. 2020, 17, 613–620. [Google Scholar] [CrossRef]
- Pawlik, L.; Śpiołek, E.; Fichna, J.; Tarasiuk, A. Charakterystyka Wirusa SARS-CoV-2 i Potencjalne Farmakologiczne Sposoby Leczenia. Postep. Biochem. 2020, 66, 1–8. [Google Scholar] [CrossRef]
- Daniels, L.B.; Ren, J.; Kumar, K.; Bui, Q.M.; Zhang, J.; Zhang, X.; Sawan, M.A.; Eisen, H.; Longhurst, C.A.; Messer, K. Relation of Prior Statin and Anti-Hypertensive Use to Severity of Disease among Patients Hospitalized with COVID-19: Findings from the American Heart Association’s COVID-19 Cardiovascular Disease Registry. PLoS ONE 2021, 16, e0254635. [Google Scholar] [CrossRef]
- Kow, C.S.; Hasan, S.S. Meta-Analysis of Effect of Statins in Patients with COVID-19. Am. J. Cardiol. 2020, 134, 153–155. [Google Scholar] [CrossRef]
- Onorato, D.; Pucci, M.; Carpene, G.; Henry, B.M.; Sanchis-Gomar, F.; Lippi, G. Protective Effects of Statins Administration in European and North American Patients Infected with COVID-19: A Meta-Analysis. Semin. Thromb. Hemost. 2021, 47, 392–399. [Google Scholar] [CrossRef]
- German, C.A.; Liao, J.K. Understanding the Molecular Mechanisms of Statin Pleiotropic Effects. Arch. Toxicol. 2023, 97, 1529–1545. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chang, C.-H.; Tai, C.-H.; Cheng, M.-F.; Chen, Y.-C.; Chao, Y.-T.; Huang, T.-L.; Yen, R.-F.; Wu, R.-M. A Double-Blind, Randomized, Controlled Trial of Lovastatin in Early-Stage Parkinson’s Disease. Mov. Disord. 2021, 36, 1229–1237. [Google Scholar] [CrossRef]
- Santa Lucia, G.; Snyder, A.; Lateef, A.; Drohan, A.; Gregoski, M.J.; Barton, V.; Elston, D.M. Safety and Efficacy of Topical Lovastatin Plus Cholesterol Cream vs Topical Lovastatin Cream Alone for the Treatment of Disseminated Superficial Actinic Porokeratosis: A Randomized Clinical Trial. JAMA Dermatol. 2023, 159, 488–495. [Google Scholar] [CrossRef]
- Jung, N.H.; Egert-Schwender, S.; Schossow, B.; Kehl, V.; Wahlländer, U.; Brich, L.; Janke, V.; Blankenstein, C.; Zenker, M.; Mall, V. Improvement of Synaptic Plasticity and Cognitive Function in RASopathies-a Monocentre, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled, Cross-over Clinical Trial (SynCoRAS). Trials 2023, 24, 383. [Google Scholar] [CrossRef]
- Bernardino, I.; Dionísio, A.; Castelo-Branco, M. Cortical Inhibition in Neurofibromatosis Type 1 Is Modulated by Lovastatin, as Demonstrated by a Randomized, Triple-Blind, Placebo-Controlled Clinical Trial. Sci. Rep. 2022, 12, 13814. [Google Scholar] [CrossRef]
- Jayawardana, K.S.; Mundra, P.A.; Giles, C.; Barlow, C.K.; Nestel, P.J.; Barnes, E.H.; Kirby, A.; Thompson, P.; Sullivan, D.R.; Alshehry, Z.H.; et al. Changes in Plasma Lipids Predict Pravastatin Efficacy in Secondary Prevention. JCI Insight 2019, 4, e128438. [Google Scholar] [CrossRef]
- Costantine, M.M.; West, H.; Wisner, K.L.; Caritis, S.; Clark, S.; Venkataramanan, R.; Stika, C.S.; Rytting, E.; Wang, X.; Ahmed, M.S.; et al. A Randomized Pilot Clinical Trial of Pravastatin versus Placebo in Pregnant Patients at High Risk of Preeclampsia. Am. J. Obs. Gynecol. 2021, 225, 666.e1–666.e15. [Google Scholar] [CrossRef]
- Wada, S.; Koga, M.; Minematsu, K.; Toyoda, K.; Suzuki, R.; Kagimura, T.; Nagai, Y.; Aoki, S.; Nezu, T.; Hosomi, N.; et al. Baseline Carotid Intima-Media Thickness and Stroke Recurrence during Secondary Prevention With Pravastatin. Stroke 2019, 50, 1586–1589. [Google Scholar] [CrossRef]
- Hosomi, N.; Kitagawa, K.; Nagai, Y.; Nakagawa, Y.; Aoki, S.; Nezu, T.; Kagimura, T.; Maruyama, H.; Origasa, H.; Minematsu, K.; et al. Different Influences of Statin Treatment in Preventing At-Risk Stroke Subtypes: A Post Hoc Analysis of J-STARS. J. Atheroscler. Thromb. 2020, 27, 449–460. [Google Scholar] [CrossRef]
- Kofink, D.; Eppinga, R.N.; van Gilst, W.H.; Bakker, S.J.L.; Dullaart, R.P.F.; van der Harst, P.; Asselbergs, F.W. Statin Effects on Metabolic Profiles: Data From the PREVEND IT (Prevention of Renal and Vascular End-Stage Disease Intervention Trial). Circ. Cardiovasc. Genet. 2017, 10, e001759. [Google Scholar] [CrossRef]
- Kitagawa, K.; Hosomi, N.; Nagai, Y.; Kagimura, T.; Ohtsuki, T.; Origasa, H.; Minematsu, K.; Uchiyama, S.; Nakamura, M.; Matsumoto, M.; et al. Reduction in High-Sensitivity C-Reactive Protein Levels in Patients with Ischemic Stroke by Statin Treatment: Hs-CRP Sub-Study in J-STARS. J. Atheroscler. Thromb. 2017, 24, 1039–1047. [Google Scholar] [CrossRef]
- Ghosh, C.C.; Thamm, K.; Berghelli, A.V.; Schrimpf, C.; Maski, M.R.; Abid, T.; Milam, K.E.; Rajakumar, A.; Santel, A.; Kielstein, J.T.; et al. Drug Repurposing Screen Identifies Foxo1-Dependent Angiopoietin-2 Regulation in Sepsis. Crit. Care Med. 2015, 43, e230–e240. [Google Scholar] [CrossRef]
- Vogt, N.M.; Hunt, J.F.V.; Ma, Y.; Van Hulle, C.A.; Adluru, N.; Chappell, R.J.; Lazar, K.K.; Jacobson, L.E.; Austin, B.P.; Asthana, S.; et al. Effects of Simvastatin on White Matter Integrity in Healthy Middle-Aged Adults. Ann. Clin. Transl. Neurol. 2021, 8, 1656–1667. [Google Scholar] [CrossRef]
- Rao, A.K.; Del Carpio-Cano, F.; Janapati, S.; Zhao, H.; Voelker, H.; Lu, X.; Criner, G. NIH COPD Clinical Research Network, the Canadian Institute of Health Research Investigators Effects of Simvastatin on Tissue Factor Pathway of Blood Coagulation in STATCOPE (Simvastatin in the Prevention of COPD Exacerbations) Trial. J. Thromb. Haemost. 2021, 19, 1709–1717. [Google Scholar] [CrossRef]
- De Giorgi, R.; Quinton, A.M.G.; Waters, S.; Cowen, P.J.; Harmer, C.J. An Experimental Medicine Study of the Effects of Simvastatin on Emotional Processing, Reward Learning, Verbal Memory, and Inflammation in Healthy Volunteers. Psychopharmacology 2022, 239, 2635–2645. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, S.-H.; Lee, G.K.; Lim, E.J.; Han, J.-Y. A Randomized Phase II Study of Irinotecan Plus Cisplatin with or without Simvastatin in Ever-Smokers with Extended Disease Small Cell Lung Cancer. Cancer Res. Treat. 2023, 55, 885–893. [Google Scholar] [CrossRef]
- Badreldeen, A.; El Razaky, O.; Erfan, A.; El-Bendary, A.; El Amrousy, D. Comparative Study of the Efficacy of Captopril, Simvastatin, and L-Carnitine as Cardioprotective Drugs in Children with Type 1 Diabetes Mellitus: A Randomised Controlled Trial. Cardiol. Young 2021, 31, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Sommer, I.E.; Gangadin, S.S.; de Witte, L.D.; Koops, S.; van Baal, C.; Bahn, S.; Drexhage, H.; van Haren, N.E.M.; Veling, W.; Bruggeman, R.; et al. Simvastatin Augmentation for Patients With Early-Phase Schizophrenia-Spectrum Disorders: A Double-Blind, Randomized Placebo-Controlled Trial. Schizophr. Bull. 2021, 47, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D.E.; Mehta, R.; Garcia-Tsao, G.; Albrecht, J.; Aytaman, A.; Baffy, G.; Bajaj, J.; Hernaez, R.; Hunt, K.; Ioannou, G.; et al. SACRED: Effect of Simvastatin on Hepatic Decompensation and Death in Subjects with High-Risk Compensated Cirrhosis: Statins and Cirrhosis: Reducing Events of Decompensation. Contemp. Clin. Trials 2021, 104, 106367. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yang, Y.; Wang, J.; Dong, Z.; Niu, X.; Liu, E.; Liu, T.; Li, L.; Liang, Y.; Li, G. Combined Treatment with Valsartan and Fluvastatin to Delay Disease Progression in Nonpermanent Atrial Fibrillation with Hypertension: A Clinical Trial. Clin. Cardiol. 2020, 43, 1592–1600. [Google Scholar] [CrossRef] [PubMed]
- Potena, L.; Grigioni, F.; Ortolani, P.; Magnani, G.; Fabbri, F.; Masetti, M.; Coccolo, F.; Fallani, F.; Russo, A.; Ionico, T.; et al. Safety and Efficacy of Early Aggressive versus Cholesterol-Driven Lipid-Lowering Strategies in Heart Transplantation: A Pilot, Randomized, Intravascular Ultrasound Study. J. Heart Lung Transpl. 2011, 30, 1305–1311. [Google Scholar] [CrossRef]
- Song, M.; Li, A.; Gong, J.; Yang, D.; Ma, L.; Zhou, X.; Yan, Y.; Xie, Y. Effects of Combined Prednisone + Fluvastatin on Cholesterol and Bilirubin in Pediatric Patients with Minimal Change Nephropathy. Clin. Ther. 2013, 35, 286–293. [Google Scholar] [CrossRef]
- Kronborg, T.M.; Schierwagen, R.; Trošt, K.; Gao, Q.; Moritz, T.; Bendtsen, F.; Gantzel, R.H.; Andersen, M.L.; Teisner, A.S.; Grønbæk, H.; et al. Atorvastatin for Patients with Cirrhosis. A Randomized, Placebo-Controlled Trial. Hepatol. Commun. 2023, 7, e0332. [Google Scholar] [CrossRef]
- Saghafi, F.; Ramezani, V.; Jafari-Nedooshan, J.; Zarekamali, J.; Kargar, S.; Tabatabaei, S.M.; Sahebnasagh, A. Efficacy of Topical Atorvastatin-Loaded Emulgel and Nano-Emulgel 1% on Post-Laparotomy Pain and Wound Healing: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Int. Wound J. 2023, 20, 4006–4014. [Google Scholar] [CrossRef]
- Lo, J.; Lu, M.T.; Ihenachor, E.J.; Wei, J.; Looby, S.E.; Fitch, K.V.; Oh, J.; Zimmerman, C.O.; Hwang, J.; Abbara, S.; et al. Effects of Statin Therapy on Coronary Artery Plaque Volume and High-Risk Plaque Morphology in HIV-Infected Patients with Subclinical Atherosclerosis: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet HIV 2015, 2, e52–e63. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Hong, S.-J.; Kang, W.C.; Hong, B.-K.; Lee, J.-Y.; Lee, J.-B.; Cho, H.-J.; Yoon, J.; Lee, S.-J.; Ahn, C.-M.; et al. Rosuvastatin versus Atorvastatin Treatment in Adults with Coronary Artery Disease: Secondary Analysis of the Randomised LODESTAR Trial. BMJ 2023, 383, e075837. [Google Scholar] [CrossRef]
- Yunihastuti, E.; Rusdi, L.; Syahrir Azizi, M.; Estiasari, R.; Jasirwan, C.O.M.; Wulandari, E.A.T.; Purnamasari, D.; Shinta Noviar, M.; Aman Nasution, S. Effect of Atorvastatin on Subclinical Atherosclerosis in Virally-Suppressed HIV-Infected Patients with CMV Seropositivity: A Randomized Double-Blind Placebo-Controlled Trial. F1000Research 2021, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Neilan, T.G.; Quinaglia, T.; Onoue, T.; Mahmood, S.S.; Drobni, Z.D.; Gilman, H.K.; Smith, A.; Heemelaar, J.C.; Brahmbhatt, P.; Ho, J.S.; et al. Atorvastatin for Anthracycline-Associated Cardiac Dysfunction: The STOP-CA Randomized Clinical Trial. JAMA 2023, 330, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi Kebar, S.; Atighi, E.; Hosseninia, S.; Babapour, B. Preventive Effects of Nicorandil and Atorvastatin in Contrastinduced Nephropathy in Patients with Renal Dysfunction Undergoing Coronary Artery Angiography: A Double Blind, Randomized, Controlled Clinical Trial. Iran. J. Kidney Dis. 2023, 17, 205–214. [Google Scholar] [PubMed]
- Tsujita, K.; Yokote, K.; Ako, J.; Tanigawa, R.; Tajima, S.; Suganami, H. Efficacy and Safety of Pitavastatin/Ezetimibe Fixed-Dose Combination vs. Pitavastatin: Phase III, Double-Blind, Randomized Controlled Trial. J. Atheroscler. Thromb. 2023, 30, 1580–1600. [Google Scholar] [CrossRef] [PubMed]
- Hamada, C.; Okuda, M.; Tomino, Y. Pitavastatin Compared with Differential Intervention Trial by Standard Therapy on Cardiovascular Events in Patients with Dyslipidemia on Chronic Hemodialysis (DIALYSIS): A Randomized Controlled Trial. Blood Purif. 2023, 52, 483–492. [Google Scholar] [CrossRef]
- Jeong, H.S.; Hong, S.J.; Cho, J.-M.; Han, K.H.; Cha, D.-H.; Jo, S.-H.; Kang, H.-J.; Choi, S.-Y.; Choi, C.U.; Cho, E.J.; et al. A Multicenter, Randomized, Double-Blind, Active-Controlled, Factorial Design, Phase III Clinical Trial to Evaluate the Efficacy and Safety of Combination Therapy of Pitavastatin and Ezetimibe Versus Monotherapy of Pitavastatin in Patients With Primary Hypercholesterolemia. Clin. Ther. 2022, 44, 1310–1325. [Google Scholar] [CrossRef]
- Grinspoon, S.K.; Fitch, K.V.; Zanni, M.V.; Fichtenbaum, C.J.; Umbleja, T.; Aberg, J.A.; Overton, E.T.; Malvestutto, C.D.; Bloomfield, G.S.; Currier, J.S.; et al. Pitavastatin to Prevent Cardiovascular Disease in HIV Infection. N. Engl. J. Med. 2023, 389, 687–699. [Google Scholar] [CrossRef]
- deFilippi, C.; Toribio, M.; Wong, L.P.; Sadreyev, R.; Grundberg, I.; Fitch, K.V.; Zanni, M.V.; Lo, J.; Sponseller, C.A.; Sprecher, E.; et al. Differential Plasma Protein Regulation and Statin Effects in Human Immunodeficiency Virus (HIV)-Infected and Non-HIV-Infected Patients Utilizing a Proteomics Approach. J. Infect. Dis. 2020, 222, 929–939. [Google Scholar] [CrossRef]
- Srichatrapimuk, S.; Wongsa, A.; Sungkanuparph, S.; Kiertiburanakul, S.; Tassaneetrithep, B.; Phuphuakrat, A. Effects of Pitavastatin on Atherosclerotic-Associated Inflammatory Biomarkers in People Living with HIV with Dyslipidemia and Receiving Ritonavir-Boosted Atazanavir: A Randomized, Double-Blind, Crossover Study. AIDS Res. Ther. 2023, 20, 13. [Google Scholar] [CrossRef]
- Braun, L.R.; Feldpausch, M.N.; Czerwonka, N.; Weiss, J.; Branch, K.; Lee, H.; Martinez-Salazar, E.L.; Torriani, M.; Sponseller, C.A.; Grinspoon, S.K.; et al. Effects of Pitavastatin on Insulin Sensitivity and Liver Fat: A Randomized Clinical Trial. J. Clin. Endocrinol. Metab. 2018, 103, 4176–4186. [Google Scholar] [CrossRef]
- Cha, J.-J.; Hong, S.J.; Kim, J.H.; Lim, S.; Joo, H.J.; Park, J.H.; Yu, C.W.; Lee, P.H.; Lee, S.W.; Lee, C.W.; et al. Effect of Rosuvastatin 20 Mg versus Rosuvastatin 5 Mg plus Ezetimibe on Statin Side-Effects in Elderly Patients with Atherosclerotic Cardiovascular Disease: Rationale and Design of a Randomized, Controlled SaveSAMS Trial. Am. Heart J. 2023, 261, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-K.; Hong, S.-J.; Lee, Y.-J.; Hong, S.J.; Yun, K.H.; Hong, B.-K.; Heo, J.H.; Rha, S.-W.; Cho, Y.-H.; Lee, S.-J.; et al. Long-Term Efficacy and Safety of Moderate-Intensity Statin with Ezetimibe Combination Therapy versus High-Intensity Statin Monotherapy in Patients with Atherosclerotic Cardiovascular Disease (RACING): A Randomised, Open-Label, Non-Inferiority Trial. Lancet 2022, 400, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.W.; Kim, C.-Y.; Hong, S.-P.; Bae, H.-J.; Choi, J.Y.; Ryu, J.K.; Lee, J.-B.; Lee, K.-H.; Han, K.-R.; Yang, D.-H.; et al. Randomized, Multicenter, Parallel, Open, Phase 4 Study to Compare the Efficacy and Safety of Rosuvastatin/Amlodipine Polypill versus Atorvastatin/Amlodipine Polypill in Hypertension Patient with Dyslipidemia. J. Clin. Hypertens. 2023, 25, 828–844. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.J.; Jeong, H.S.; Ahn, J.C.; Cha, D.-H.; Won, K.H.; Kim, W.; Cho, S.K.; Kim, S.-Y.; Yoo, B.-S.; Sung, K.C.; et al. A Phase III, Multicenter, Randomized, Double-Blind, Active Comparator Clinical Trial to Compare the Efficacy and Safety of Combination Therapy With Ezetimibe and Rosuvastatin Versus Rosuvastatin Monotherapy in Patients With Hypercholesterolemia: I-ROSETTE (Ildong Rosuvastatin & Ezetimibe for Hypercholesterolemia) Randomized Controlled Trial. Clin. Ther. 2018, 40, 226–241.e4. [Google Scholar] [CrossRef] [PubMed]
- Hougaard Christensen, M.M.; Bruun Haastrup, M.; Øhlenschlæger, T.; Esbech, P.; Arnspang Pedersen, S.; Bach Dunvald, A.; Bjerregaard Stage, T.; Pilsgaard Henriksen, D.; Thestrup Pedersen, A.J. Interaction Potential between Clarithromycin and Individual Statins—A Systematic Review. Basic Clin. Pharmacol. Toxicol. 2020, 126, 307–317. [Google Scholar] [CrossRef]
- Egom, E.E.A.; Hafeez, H. Biochemistry of Statins. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2016; Volume 73, pp. 127–168. ISBN 978-0-12-804690-6. [Google Scholar]
- Climent, E.; Benaiges, D.; Pedro-Botet, J. Hydrophilic or Lipophilic Statins? Front. Cardiovasc. Med. 2021, 8, 687585. [Google Scholar] [CrossRef]
- GISSI-HF investigators Effect of Rosuvastatin in Patients with Chronic Heart Failure (the GISSI-HF Trial): A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2008, 372, 1231–1239. [CrossRef]
- Filppula, A.M.; Hirvensalo, P.; Parviainen, H.; Ivaska, V.E.; Lönnberg, K.I.; Deng, F.; Viinamäki, J.; Kurkela, M.; Neuvonen, M.; Niemi, M. Comparative Hepatic and Intestinal Metabolism and Pharmacodynamics of Statins. Drug Metab. Dispos. 2021, 49, 658–667. [Google Scholar] [CrossRef]
- Fong, C. Statins in Therapy: Cellular Transport, Side Effects, Drug-Drug Interactions and Cytotoxicity—The Unrecognized Role of Lactones; Eigen Energy: Adelaide, Australia, 2014. [Google Scholar]
- Wagner, J.; Abdel-Rahman, S.M. Pediatric Statin Administration: Navigating a Frontier with Limited Data. J. Pediatr. Pharmacol. Ther. 2016, 21, 380–403. [Google Scholar] [CrossRef]
- Cid-Conde, L.; López-Castro, J. Pharmacokinetic Aspects of Statins. In Cardiovascular Risk Factors in Pathology; Abukabda, A., Suciu, M., Andor, M., Eds.; IntechOpen: London, UK, 2021; ISBN 978-1-83881-949-1. [Google Scholar]
- Cybulska, B.; Kłosiewicz-Latoszek, L. How to Safely Use Statins? Chor. Serca I Naczyń 2012, 9, 199–207. [Google Scholar]
- Kalliokoski, A.; Niemi, M. Impact of OATP Transporters on Pharmacokinetics. Br. J. Pharmacol. 2009, 158, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Causevic-Ramosevac, A.; Semiz, S. Drug Interactions with Statins. Acta Pharm. 2013, 63, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Vinci, P.; Panizon, E.; Tosoni, L.M.; Cerrato, C.; Pellicori, F.; Mearelli, F.; Biasinutto, C.; Fiotti, N.; Di Girolamo, F.G.; Biolo, G. Statin-Associated Myopathy: Emphasis on Mechanisms and Targeted Therapy. Int. J. Mol. Sci. 2021, 22, 11687. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.K.M.; Walker, S.W. Statins and Their Interactions with Other Lipid-Modifying Medications: Safety Issues in the Elderly. Ther. Adv. Drug Saf. 2012, 3, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, R.Y.A.; Reid, J.; Reckless, J.P.D. Pitavastatin: Pitavastatin. Int. J. Clin. Pract. 2005, 59, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Luvai, A.; Mbagaya, W.; Hall, A.S.; Barth, J.H. Rosuvastatin: A Review of the Pharmacology and Clinical Effectiveness in Cardiovascular Disease. Clin. Med. Insights Cardiol. 2012, 6, CMC.S4324. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Paulose-Ram, R.; Burt, V.L.; Kit, B.K. Prescription Cholesterol-Lowering Medication Use in Adults Aged 40 and over: United States, 2003–2012. NCHS Data Brief 2014, 177, 1–8. [Google Scholar]
- Bellosta, S.; Corsini, A. Statin Drug Interactions and Related Adverse Reactions: An Update. Expert. Opin. Drug Saf. 2018, 17, 25–37. [Google Scholar] [CrossRef]
- Bellosta, S.; Paoletti, R.; Corsini, A. Safety of Statins: Focus on Clinical Pharmacokinetics and Drug Interactions. Circulation 2004, 109, III50-7. [Google Scholar] [CrossRef]
- Wiggins, B.S.; Saseen, J.J.; Page, R.L.; Reed, B.N.; Sneed, K.; Kostis, J.B.; Lanfear, D.; Virani, S.; Morris, P.B. Recommendations for Management of Clinically Significant Drug-Drug Interactions With Statins and Select Agents Used in Patients With Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e468–e495. [Google Scholar] [CrossRef]
- Lilja, J.J.; Kivistö, K.T.; Neuvonen, P.J. Grapefruit Juice—Simvastatin Interaction: Effect on Serum Concentrations of Simvastatin, Simvastatin Acid, and HMG-CoA Reductase Inhibitors. Clin. Pharmacol. Ther. 1998, 64, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Skrypnik, D.; Skrypnik, K.; Suliburska, J.; Bogdański, P.; Pupek-Musialik, D. Drug Interactions with Food in Metabolic Diseases. Forum Zaburzeń Metab. 2013, 4, 192–198. [Google Scholar]
- Ward, N.C.; Watts, G.F.; Eckel, R.H. Statin Toxicity: Mechanistic Insights and Clinical Implications. Circ. Res. 2019, 124, 328–350. [Google Scholar] [CrossRef] [PubMed]
- Tobert, J.A. Efficacy and Long-Term Adverse Effect Pattern of Lovastatin. Am. J. Cardiol. 1988, 62, J28–J34. [Google Scholar] [CrossRef]
- Schaefer, W.H.; Lawrence, J.W.; Loughlin, A.F.; Stoffregen, D.A.; Mixson, L.A.; Dean, D.C.; Raab, C.E.; Yu, N.X.; Lankas, G.R.; Frederick, C.B. Evaluation of Ubiquinone Concentration and Mitochondrial Function Relative to Cerivastatin-Induced Skeletal Myopathy in Rats. Toxicol. Appl. Pharmacol. 2004, 194, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Hanai, J.; Cao, P.; Tanksale, P.; Imamura, S.; Koshimizu, E.; Zhao, J.; Kishi, S.; Yamashita, M.; Phillips, P.S.; Sukhatme, V.P.; et al. The Muscle-Specific Ubiquitin Ligase Atrogin-1/MAFbx Mediates Statin-Induced Muscle Toxicity. J. Clin. Investig. 2007, 117, JCI32741. [Google Scholar] [CrossRef] [PubMed]
- Backman, J.T.; Kyrklund, C.; Neuvonen, M.; Neuvonen, P.J. Gemfibrozil Greatly Increases Plasma Concentrations of Cerivastatin. Clin. Pharmacol. Ther. 2002, 72, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Muntean, D.M.; Thompson, P.D.; Catapano, A.L.; Stasiolek, M.; Fabis, J.; Muntner, P.; Serban, M.-C.; Banach, M. Statin-Associated Myopathy and the Quest for Biomarkers: Can We Effectively Predict Statin-Associated Muscle Symptoms? Drug Discov. Today 2017, 22, 85–96. [Google Scholar] [CrossRef]
- Główczyńska, R.; Paluch, W.; Filipiak, K. Działania Niepożądane Statyn. Chor. Serca I Naczyń 2007, 4, 18–34. [Google Scholar]
- Mancini, G.B.J.; Baker, S.; Bergeron, J.; Fitchett, D.; Frohlich, J.; Genest, J.; Gupta, M.; Hegele, R.A.; Ng, D.; Pearson, G.J.; et al. Diagnosis, Prevention, and Management of Statin Adverse Effects and Intolerance: Canadian Consensus Working Group Update (2016). Can. J. Cardiol. 2016, 32, S35–S65. [Google Scholar] [CrossRef]
- Gluba-Brzozka, A.; Franczyk, B.; Toth, P.P.; Rysz, J.; Banach, M. Molecular Mechanisms of Statin Intolerance. Arch. Med. Sci. 2016, 3, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Q. Cholesterol Metabolism and Homeostasis in the Brain. Protein Cell 2015, 6, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, J.; Liu, Q. Brain Cell Type-Specific Cholesterol Metabolism and Implications for Learning and Memory. Trends Neurosci. 2022, 45, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Lütjohann, D. Brain Cholesterol and Suicidal Behaviour. Int. J. Neuropsychopharmacol. 2007, 10, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Vance, J.E. Dysregulation of Cholesterol Balance in the Brain: Contribution to Neurodegenerative Diseases. Dis. Model. Mech. 2012, 5, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Yasar, S.; Whitmer, R. Statin Use and Risk of Alzheimer Disease: A New View on an Old Relationship. Neurology 2018, 90, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.A.; Golomb, B.A. Statin-Associated Adverse Cognitive Effects: Survey Results from 171 Patients. Pharmacotherapy 2009, 29, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Cham, S.; Koslik, H.J.; Golomb, B.A. Mood, Personality, and Behavior Changes during Treatment with Statins: A Case Series. Drug Saf.-Case Rep. 2016, 3, 1. [Google Scholar] [CrossRef]
- Pinal-Fernandez, I.; Casal-Dominguez, M.; Mammen, A.L. Statins: Pros and Cons. Med. Clínica 2018, 150, 398–402. [Google Scholar] [CrossRef]
- Jukema, J.W.; Cannon, C.P.; De Craen, A.J.M.; Westendorp, R.G.J.; Trompet, S. The Controversies of Statin Therapy. J. Am. Coll. Cardiol. 2012, 60, 875–881. [Google Scholar] [CrossRef]
- Agostini, J.V.; Tinetti, M.E.; Han, L.; McAvay, G.; Foody, J.M.; Concato, J. Effects of Statin Use on Muscle Strength, Cognition, and Depressive Symptoms in Older Adults. J. Am. Geriatr. Soc. 2007, 55, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Alberton, M.; Wu, P.; Druyts, E.; Briel, M.; Mills, E.J. Adverse Events Associated with Individual Statin Treatments for Cardiovascular Disease: An Indirect Comparison Meta-Analysis. QJM Int. J. Med. 2012, 105, 145–157. [Google Scholar] [CrossRef] [PubMed]
- McFarland, A.; Anoopkumar-Dukie, S.; Arora, D.; Grant, G.; McDermott, C.; Perkins, A.; Davey, A. Molecular Mechanisms Underlying the Effects of Statins in the Central Nervous System. Int. J. Mol. Sci. 2014, 15, 20607–20637. [Google Scholar] [CrossRef] [PubMed]
- Thelen, K.M.; Rentsch, K.M.; Gutteck, U.; Heverin, M.; Olin, M.; Andersson, U.; Von Eckardstein, A.; Björkhem, I.; Lütjohann, D. Brain Cholesterol Synthesis in Mice Is Affected by High Dose of Simvastatin but Not of Pravastatin. J. Pharmacol. Exp. Ther. 2006, 316, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Engelberg, H. Low Serum Cholesterol and Suicide. Lancet 1992, 339, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Tatley, M.; Savage, R. Psychiatric Adverse Reactions with Statins, Fibrates and Ezetimibe: Implications for the Use of Lipid-Lowering Agents. Drug Saf. 2007, 30, 195–201. [Google Scholar] [CrossRef]
- Pop, G.; Farcaș, A.; Butucă, A.; Morgovan, C.; Arseniu, A.M.; Pumnea, M.; Teodoru, M.; Gligor, F.G. Post-Marketing Surveillance of Statins—A Descriptive Analysis of Psychiatric Adverse Reactions in EudraVigilance. Pharmaceuticals 2022, 15, 1536. [Google Scholar] [CrossRef]
- Olson, M.B.; Kelsey, S.F.; Matthews, K.A.; Bairey Merz, C.N.; Eteiba, W.; McGorray, S.P.; Cornell, C.E.; Vido, D.A.; Muldoon, M.F. Lipid-Lowering Medication Use and Aggression Scores in Women: A Report from the NHLBI-Sponsored WISE Study. J. Women’s Health 2008, 17, 187–194. [Google Scholar] [CrossRef]
- Glueck, C.J.; Tieger, M.; Kunkel, R.; Hamer, T.; Tracy, T.; Speirs, J. Hypocholesterolemia and Affective Disorders. Am. J. Med. Sci. 1994, 308, 218–225. [Google Scholar] [CrossRef]
- Horsten, M.; Wamala, S.P.; Vingerhoets, A.; Orth-Gomer, K. Depressive Symptoms, Social Support, and Lipid Profile in Healthy Middle-Aged Women: Psychosom. Med. 1997, 59, 521–528. [Google Scholar] [CrossRef]
- Morgan, R.E.; Palinkas, L.A.; Barrett-Connor, E.L.; Wingard, D.L. Plasma Cholesterol and Depressive Symptoms in Older Men. Lancet 1993, 341, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Gallerani, M.; Manfredini, R.; Caracciolo, S.; Scapoli, C.; Molinari, S.; Fersini, C. Serum Cholesterol Concentrations in Parasuicide. BMJ 1995, 310, 1632–1636. [Google Scholar] [CrossRef] [PubMed]
- Golier, J.A.; Marzuk, P.M.; Leon, A.C.; Weiner, C.; Tardiff, K. Low Serum Cholesterol Level and Attempted Suicide. Am. J. Psychiatry 1995, 152, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Papassotiropoulos, A.; Hawellek, B.; Frahnert, C.; Rao, G.S.; Rao, M.L. The Risk of Acute Suicidality in Psychiatric Inpatients Increases with Low Plasma Cholesterol. Pharmacopsychiatry 1999, 32, 1–4. [Google Scholar] [CrossRef] [PubMed]
- New, A.S.; Sevin, E.M.; Mitropoulou, V.; Reynolds, D.; Novotny, S.L.; Callahan, A.; Trestman, R.L.; Siever, L.J. Serum Cholesterol and Impulsivity in Personality Disorders. Psychiatry Res. 1999, 85, 145–150. [Google Scholar] [CrossRef] [PubMed]
- King, D.S.; Wilburn, A.J.; Wofford, M.R.; Harrell, T.K.; Lindley, B.J.; Jones, D.W. Cognitive Impairment Associated with Atorvastatin and Simvastatin. Pharmacotherapy 2003, 23, 1663–1667. [Google Scholar] [CrossRef]
- Ali, S.; Khan, S.A.; Iram, S. Hypocholesterolemia Secondary to Atrovastatin Therapy. J. Ayub Med. Coll. Abbottabad 2010, 22, 225–227. [Google Scholar]
- Wagstaff, L.R.; Mitton, M.W.; Arvik, B.M.; Doraiswamy, P.M. Statin-Associated Memory Loss: Analysis of 60 Case Reports and Review of the Literature. Pharmacotherapy 2003, 23, 871–880. [Google Scholar] [CrossRef]
- Golomb, B.A.; Kane, T.; Dimsdale, J.E. Severe Irritability Associated with Statin Cholesterol-Lowering Drugs. QJM 2004, 97, 229–235. [Google Scholar] [CrossRef]

| Drug | LDL-C | HDL-C | TG | Adverse Effects and Dosage Characteristics |
|---|---|---|---|---|
| Type I—fungal derived statins | ||||
| lovastatin | 20 mg/day—Low intensity dosage 40–80 mg/day—Moderate intensity dosage [65] ↓ 17%—10 mg/day [72] ↓ <30%—20 mg/day [73] ↓ 24%—20 mg/day [72] ↓25%—20 mg/day [74] ↓ 25%—40 mg/day [75] ↓ 27%—40 mg/day [72] ↓ 30–50%—40–80 mg/day [73] | ↑ 8.3%—20 mg/day [74] | ↓10.9%—20 mg/day [74] ↓ 30%—40 mg [75] | It is advisable to take the medication in the evening with a meal. Safety and effectiveness in children have not been established [76]. |
| pravastatin | 10–20 mg/day—Low intensity dosage 40–80 mg/day—Moderate intensity dosage [65] ↓ 23%—5 mg/day and 10 mg/day [77] ↓ 33%—20 mg/day [77] ↓ 25.4%—40 mg/day [78] | Not observed significant changes—40 mg/day [78] Not observed significant changes—5 mg/day and 10 mg/day [77] ↑ 11%—20 mg/day [77] | Not observed significant changes—40 mg/day [78] Not observed significant changes—5 mg/day, 10 mg/day, and 20 mg/day [77] | Recommended intake in the evening. To reduce hyperlipidemia in organ transplant patients receiving immunosuppressive therapy [79]. |
| simvastatin | 10 mg/day—Low intensity dosage 20–40 mg/day—Moderate intensity dosage [65] ↓ 20–30%—10 mg/day [27] ↓ 30–40%—20 mg/day [27] ↓ 40–45%—40 mg/day [27] ↓ 46–50%—80 mg/day [27] ↓ 49%—80 mg/day [80] | ↑ 4.2%—10 mg [81] ↑ 5.3%—80 mg [81] ↑ 6%—80 mg/day [80] | Falling to 35%—dose 80 mg/day [80] | Recommended intake in the evening [82]. If the activity of hepatic organic anion transport proteins is reduced, there may be an increased risk of myopathy and rhabdomyolysis. These result from an increase in simvastatin acid exposure. Those who take interacting drugs or who carry the SLCO1B1 c.521T > C genotype are most at risk [83]. |
| Type II—synthetically derived statins | ||||
| fluvastatin | 20–40 mg/day—Low intensity dosage 40 mg 2x/day; 80 mg XL—Moderate intensity dosage [65] ↓ 15% to 33%—10 mg/day to 80 mg/day [84] ↓ 22–36%—20–80 mg/day [85] | ↑ 3.3–5.6%—20–80 mg/day [85] | ↓ 3%–7.5%—10 mg/day–0 mg/day [84] ↓12–18%—20–80 mg/day [85] | In the case of 20 mg and 40 mg doses, it is recommended to take the tablet in the evening; in the case of 80 mg, any time of day [86]. |
| atorvastatin | 10–20 mg/day—Moderate intensity dosage 40–80 mg/day—High intensity dosage [65] ↓ 35%—10 mg/day [87] ↓ 30–40%—10 mg/day [27] ↓ 40–45%—20 mg/day [27] ↓ 46–50%—40 mg/day [27] ↓ 50–55%—80 mg/day [27] ↓ 37.1%—51.7%—10–80 mg/day [88] ↓ 36% to 53%—10–80 mg/day [89] | ↑ 8%—10 mg/day [87] ↑ 4.5%—10 mg [81] Falling to 2.3%—80 mg [81] | ↓ 20%—10 mg/day [90] ↓ 22.6%—20 mg/day [90] ↓ 26.8%—40 mg/day [90] | Taking a statin at any time of the day [91]. A dose of 80 mg increases the risk of hamorrhagic stroke after a stroke, or TIA [92]. |
| cerivastatin | ↓ 11%—40.8%;—0.025–0.8 mg/day [93] ↓ 37.3%—0.4 mg/day [94] ↓ 42.2%—0.8 mg/day [94] | ↑ 5%— dose-independent [93] | ↓ 9%—21.4%—0.025–0.8 mg/day [93] | Due to the high risk of rhabdomyolysis, it is presently not used [29]. |
| pitavastatin | 1–4 mg/day—Moderate intensity dosage [65] ↓ 33.3%–4.7%—1 mg/day–6 mg/day [95] | There was no evidence of a dose-dependent effect of pitavastatin on blood HDL. cholesterol levels. An average increase of 4% (for all doses) [95] ↑ 8.2%—2 mg/day [96] ↑ 20.1%—2–4 mg/day [96] ↑ 6.3%—4 mg/day [96] | ↓ 13.0%–8.1%—1 mg/day–6 mg/day [95] | Taking a statin at any time of the day. Not for use under age 18 [97] It causes a higher increase in HDL-C than other statins. This level is maintained over a longer period of time. Its characteristic feature is its metabolism, which avoids interactions with other drugs (e.g., no interaction with warfarin) [31]. Due to its pleiotropic effects, it contributes to the stabilization of the coronary plaque, reduces the migration of monocytes, and promotes the formation of foam cells [98]. The only statin capable of plasma adiponectin elevation is [96]. |
| rosuvastatin | 5–10 mg/day—Moderate intensity dosage 20–40 mg/day—High intensity dosage [65] ↓ 40%—5 mg/day [87] ↓ 30–40%—5 mg/day [27] ↓ 43%—10 mg/day [87] ↓ 40–45%—5–10 mg/day [27] ↓ 46–50%—10–20 mg/day [27] ↓ 50–55%—20 mg/day [27] ↓ 53%—40 mg/day [99] ↓ 56–60%—40 mg/day [27] ↓ 46%—55%;—10–40 mg/day [100] | From 5.5% to 7.9%; dose 5–40 mg/day [81] ↑ 13%—5 mg/day [87] ↑ 12%—10 mg/day [87] | ↓19.8%—10 mg/day [15] ↓23.7%—20 mg/day [15] ↓26.1%—40 mg/day [15] | Taking a statin at any time of the day, Statins can be used from the age of 6 [101]. It has the strongest hypolipemic effects; for example, a dose of rosuvastain of 5–10 mg has the same effect as 20–30 mg of atorvastain. There is no significant effect on the risk of renal failure in people without renal impairment [99]. In renal impairment in patients with creatinine clearance (CrCl) < 30 mL/min, it is recommended to start with the lowest possible dose of 5 mg/day with a max dosage of 10 mg/day; if the CrCl ≥ 30 mL/min, no dosage adjustment is necessary [102]. |
| Drug | Clinical Effects/ the Clinical Trial Number | Pleiotropic Effects/ the Clinical Trial Number |
|---|---|---|
| Type I—fungal derived statins | ||
| lovastatin | reduction of LDL cholesterol level/NCT03242499 [111], NCT04359823 [112] | the effect on synaptic plasticity, cognitive function, and attention in patients with RASopathies/NCT03504501 [113] |
| improvement in the treatment of diffuse superficial actinic porokeratosis/NCT04359823 [112] | ||
| modulation of cortical inhibition in neurofibromatosis type 1 (NF1)/NCT03826940 [114] | ||
| reduction of motor symptoms in patients with early-stage Parkinson’s/NCT03242499 [111] | ||
| pravastatin | reduction of LDL cholesterol level/ACTRN12616000535471 [115] | prevention of pre-eclampsia in pregnant women/NCT01717586 [116] |
| biomarker for monitoring the clinical benefit of statin treatment in secondary prevention/ ACTRN12616000535471 [115] | ||
| prevention of the occurrence of atherothrombotic brain infarction in noncardioembolic infarction patients/NCT00361530 [117] | ||
| reducing the incidence of lacunar stroke/NCT00221104 [118] | ||
| reduction in levels of lipoprotein subclasses/NCT03073018 [119] | ||
| reduction of vascular inflammation in non-cardiogenic ischemic stroke/NCT00361699 [120] | ||
| simvastatin | inhibition of 3-hydroxy-3-methyl-glutaryl- CoA reductase/ NCT00529139 [121] reduction in serum LDL concentration/NCT00939822 [122], NCT01061671 [123] | activation of PI3K-kinase/NCT00676897 [121] |
| positive effect on emotions/NCT04652089 [124] | ||
| improved survival in ever-smokers with extensive disease (ED)–small cell lung cancer (SCLC)/NCT01441349 [125] | ||
| reductions in total cholesterol, lipoprotein, and triglycerides observed in children with type 1 diabetes mellitus/NCT03660293 [126] | ||
| anti- inflammatory and neuroprotective effects/NCT01999309 [127] | ||
| prevention of decompensation in patients with compensated cirrhosis/NCT03654053 [128] | ||
| Type II—synthetically derived statins | ||
| fluvastatin | reduction in blood lipid levels/ ChiCTR-TRC-12002642 [129] decrease in LDL-level/NCT00421005 [130] | decrease of inflammatory index, ultrasonic index and electrocardiographic measurement results in atrial fibrillation/ChiCTR-TRC-12002642 [129] |
| normalization of bilirubin levels and reduced blood lipids in patients with nephrotic syndrome/ChiCTR-TQR-12002602 [131] | ||
| preventing pathologic changes in graft coronary arteries/NCT00421005 [130] | ||
| atorvastatin | reduction of levels of inflammatory markers, such as CD62-L-selectin, matrix metalloproteinases-2, and TNF-α/NCT04072601 [132] | |
| acceleration wound healing and alleviating pain from laparotomy surgical wounds/IRCT20190810044500N3 [133] | ||
| reduction of the coronary plaque/NCT00965185 [134] reduction of LDL cholesterol level/NCT02579499 [135] | prevention of adverse cardiovascular outcomes, particularly carotid intimal thickening, in HIV-infected patients on viral suppression/NCT04101136 [136] | |
| reduction of the incidence of cardiac dysfunction among patients with lymphoma treated with anthracycline-based chemotherapy/NCT02943590 [137] | ||
| prevention of contrast-induced nephropathy in patients with chronic kidney disease undergoing angiography/IRCT20190810044500N3 [138] | ||
| pitavastatin | reduction of LDL-C level/NCT04289649 [139], NCT00846118 [140], NCT04584736 [141] | lower risk of major adverse cardiovascular articipants with HIV infection/NCT02344290 [142] |
| increase of PCOLCE (enzymatic cleavage of type I procollagen) and decrease of PLA2G7 (systemic marker of arterial inflammation) in patients with HIV/NCT01301066 [143], anti-inflammatory effects in people with HIV/NCT02442700 [144] | ||
| Reduced mortality in dyslipidaemic patients on chronic haemodialysis/NCT00846118 [140] | ||
| reduce insulin hepatotoxicity/NCT02290106 [145] | ||
| rosuvastatin | reduction of LDL-C level/NCT04826354 [146], NCT03044665 [147], NCT03951207 [148] | improved lipid profiles and reduction of vascular inflammation/NCT02749994 [149] |
| Enzyme | CYP2C9 | CYP3A4 |
|---|---|---|
| Statin substrates | Fluvastatin, rosuvastatin | Atorvastatin, lovastatin, and simvastatin |
| Inducers | Carbamazepine, phenobarbital, phenytoin, and rifampin | Aprepitant, carbamazepine, cyclophosphamide, corticosteroids, efavirenz, nevirapine, phenytoin, pioglitazone, phenobarbital, and St. John’s wort |
| Inhibitors | Amiodarone, capecitabine, fluconazole, fluvoxamine, ketoconazole, metronidazole, miconazole, sulfamethoxazole/trimethoprim, voriconazole, zafirlukast | Amiodarone, clarithromycin, cyclosporine A, diltiazem, erythromycin, fluconazole, fluoxetine, fluvoxamine, grapefruit juice, isoniazid, itraconazole, ketoconazole, mibefradil, midazolam, nefazodone, protease inhibitors, sertraline, tacrolimus, ticagrelor, tricyclic antidepressants, verapamil |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadowska, A.; Osiński, P.; Roztocka, A.; Kaczmarz-Chojnacka, K.; Zapora, E.; Sawicka, D.; Car, H. Statins—From Fungi to Pharmacy. Int. J. Mol. Sci. 2024, 25, 466. https://doi.org/10.3390/ijms25010466
Sadowska A, Osiński P, Roztocka A, Kaczmarz-Chojnacka K, Zapora E, Sawicka D, Car H. Statins—From Fungi to Pharmacy. International Journal of Molecular Sciences. 2024; 25(1):466. https://doi.org/10.3390/ijms25010466
Chicago/Turabian StyleSadowska, Anna, Patryk Osiński, Alicja Roztocka, Karolina Kaczmarz-Chojnacka, Ewa Zapora, Diana Sawicka, and Halina Car. 2024. "Statins—From Fungi to Pharmacy" International Journal of Molecular Sciences 25, no. 1: 466. https://doi.org/10.3390/ijms25010466
APA StyleSadowska, A., Osiński, P., Roztocka, A., Kaczmarz-Chojnacka, K., Zapora, E., Sawicka, D., & Car, H. (2024). Statins—From Fungi to Pharmacy. International Journal of Molecular Sciences, 25(1), 466. https://doi.org/10.3390/ijms25010466






