Altered Serum Alpha1-Antitrypsin Protease Inhibition before and after Clinical Hematopoietic Stem Cell Transplantation: Association with Risk for Non-Relapse Mortality
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
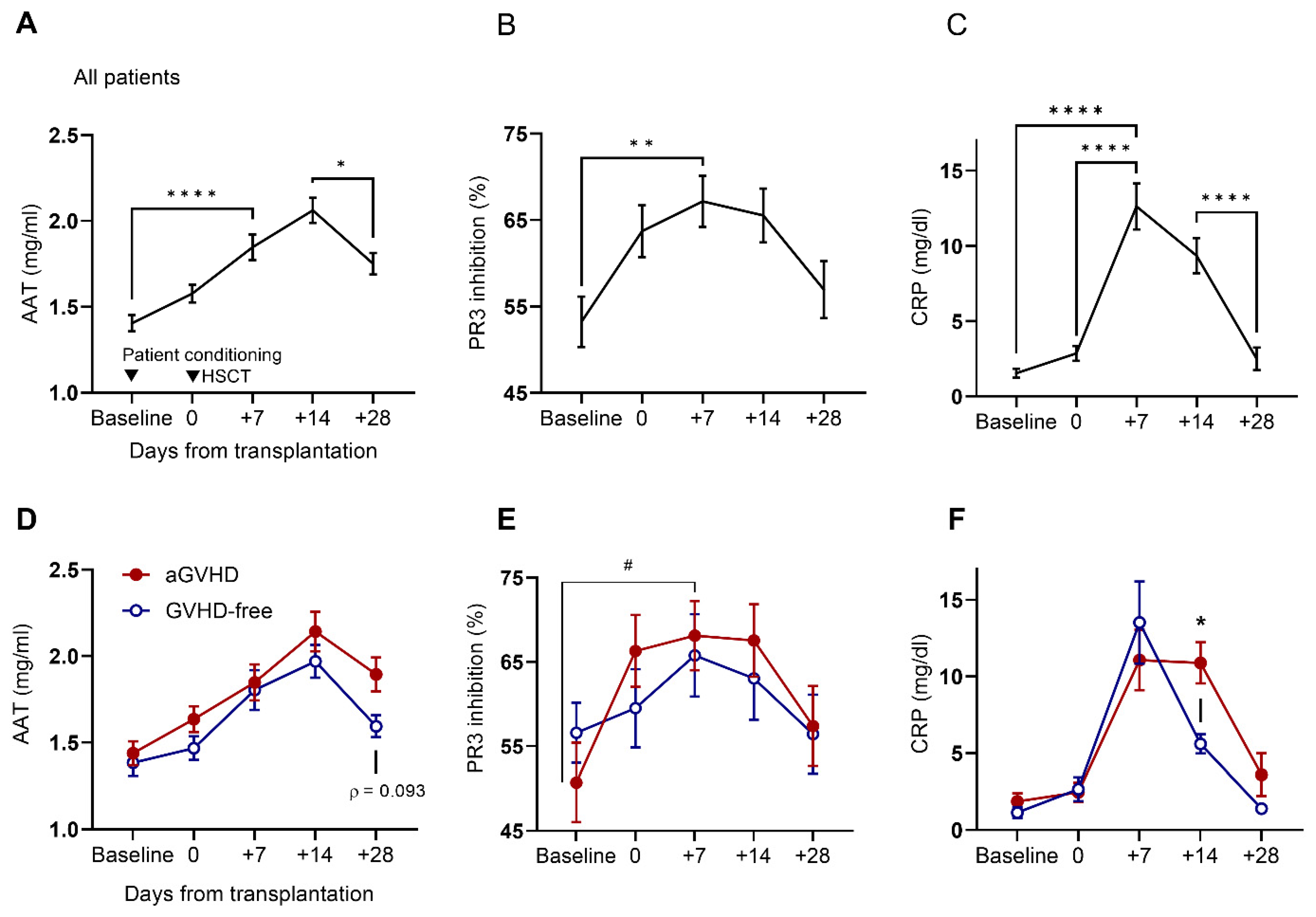
2.2. Circulating Levels of AAT and CRP and Serum Anti-PR3 Inhibitory Capacity before Conditioning and throughout the First 28 Days Post-HSCT
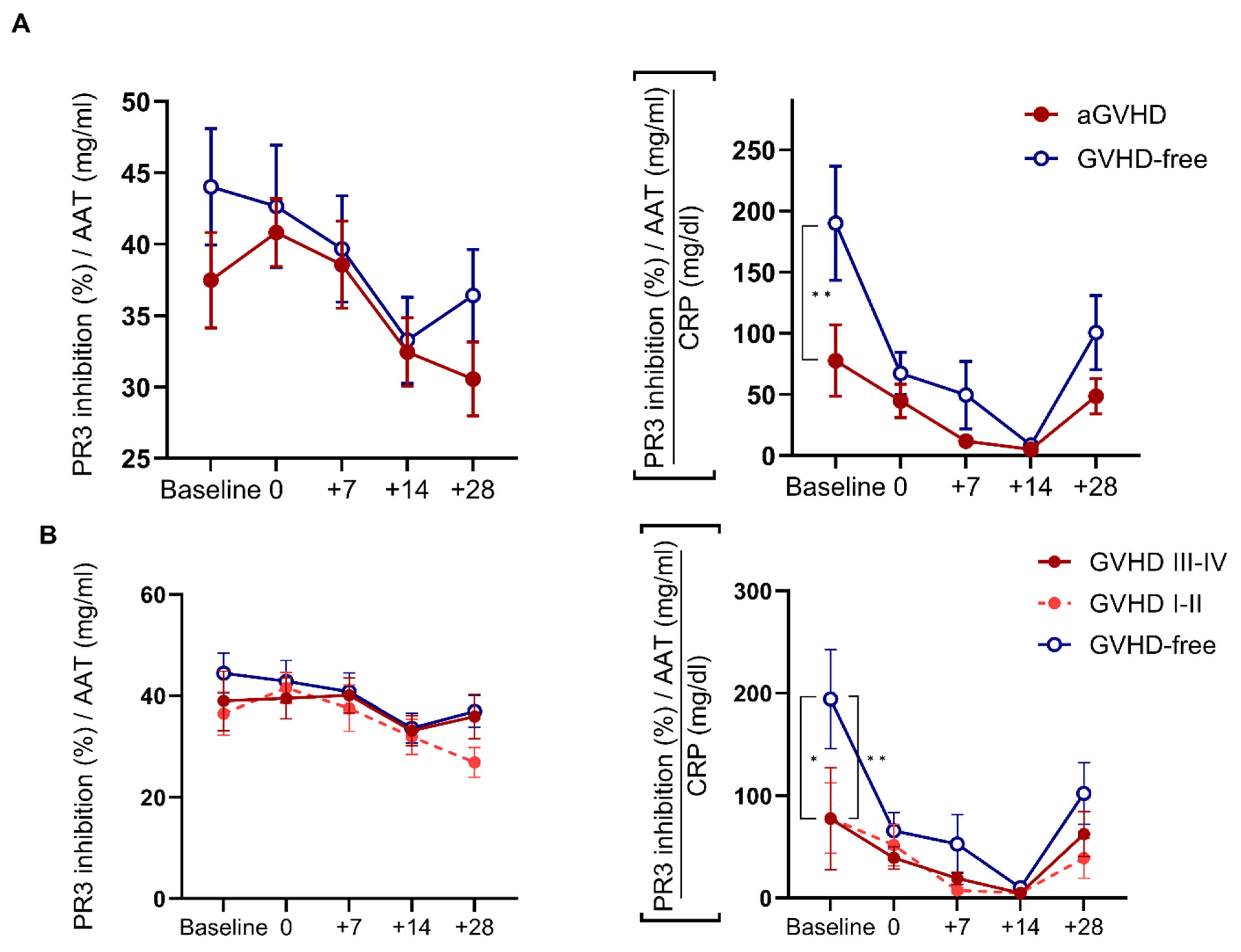
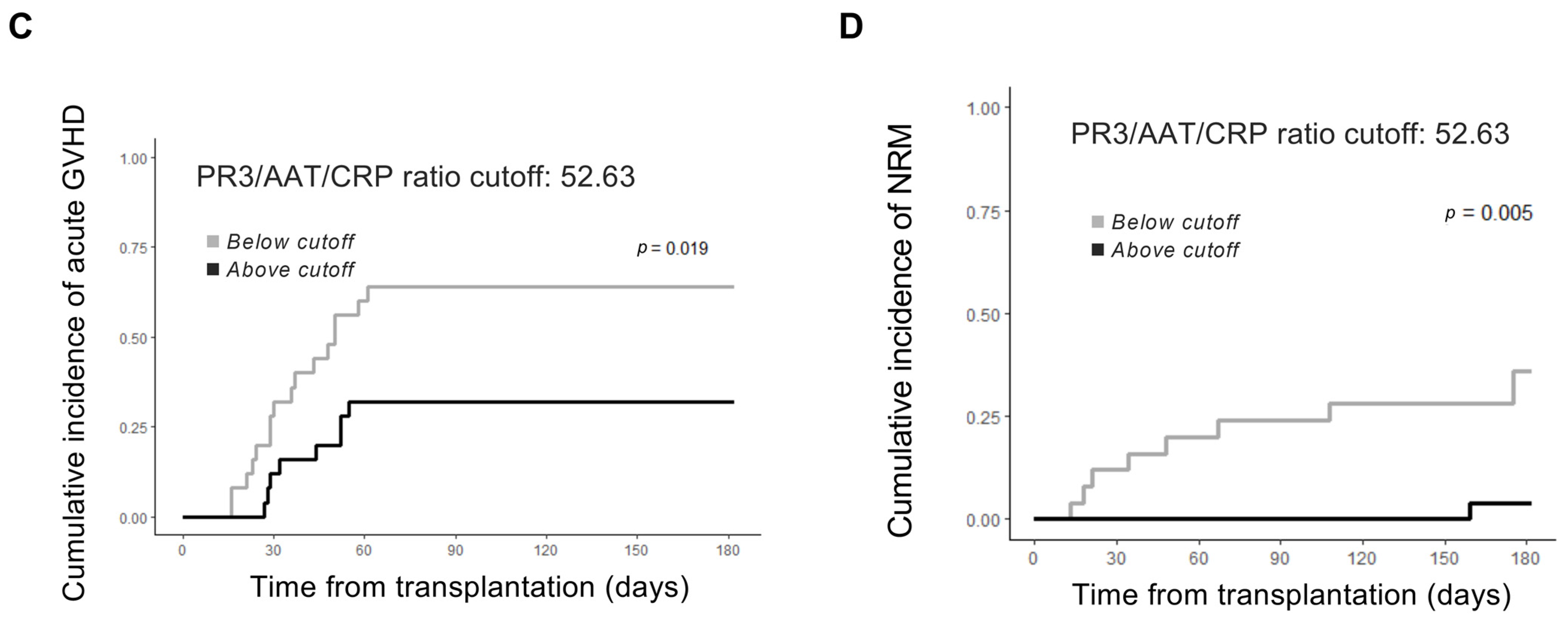
2.3. AAT Anti-PR3 Functionality Corrected to Inflammatory Status by CRP Pre- and Post-HSCT
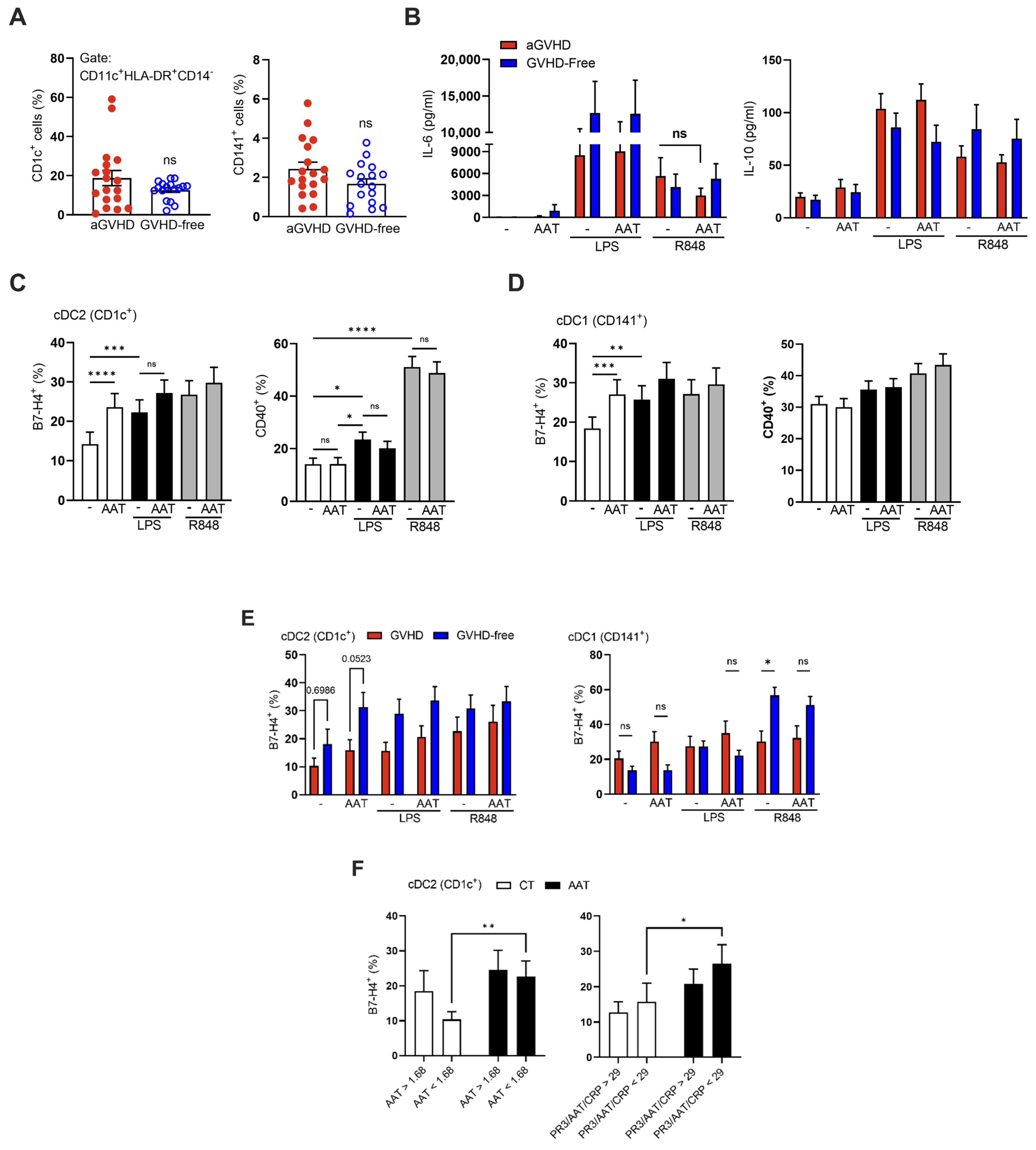
2.4. Immunomodulation of Circulating Myeloid Dendritic Cells by Exogenous AAT
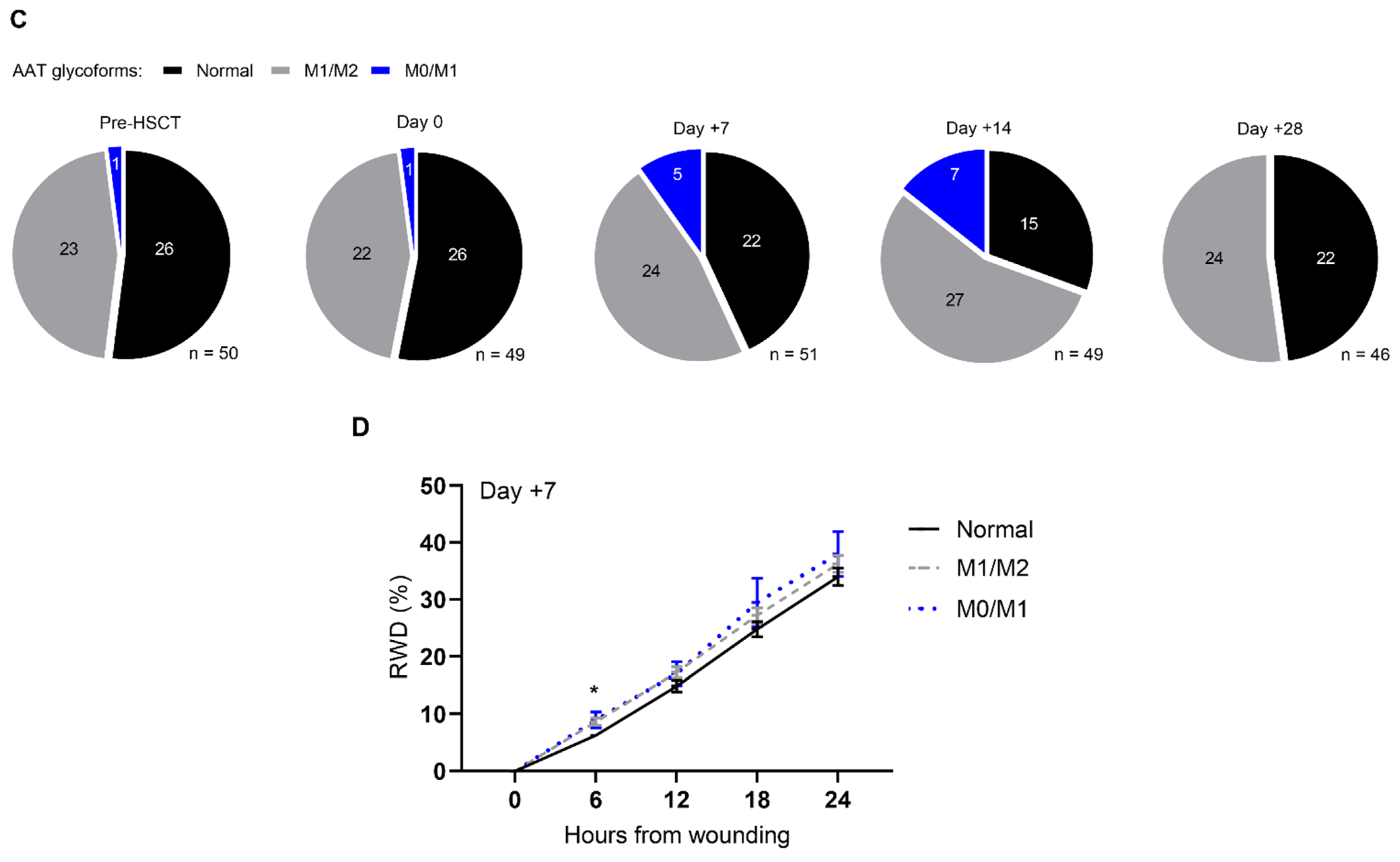
2.5. Colonic Epithelial Gap Closure and Anti-PR3 Serum Activity
3. Discussion
4. Materials and Methods
4.1. Study Approval and Design
4.2. Quantification and Enzymatic Assays
4.3. Whole Blood and Peripheral Blood Mononuclear Cell Activation Assay and Flow Cytometry Analysis
4.4. Epithelial Gap Closure Assay
4.5. Isoelectric Focusing and Phenotyping
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAT | α1-Antitrypsin |
| allo-HSCT | Allogeneic hematopoietic stem cell transplantation |
| DC | Dendritic cell |
| GI | Gastrointestinal |
| GVHD | Graft-versus-host disease |
| NRM | Non-relapse mortality |
| MMF | Mycophenolate mofetil |
| MTX | Methotrexate |
| PR3 | Proteinase-3 |
| SR | Steroid refractory |
References
- Zeiser, R.; Blazar, B.R. Acute Graft-versus-Host Disease—Biologic Process, Prevention, and Therapy. N. Engl. J. Med. 2017, 377, 2167–2179. [Google Scholar] [CrossRef] [PubMed]
- Koyama, M.; Hill, G.R. The primacy of gastrointestinal tract antigen-presenting cells in lethal graft-versus-host disease. Blood 2019, 134, 2139–2148. [Google Scholar] [CrossRef] [PubMed]
- Hülsdünker, J.; Ottmüller, K.J.; Neeff, H.P.; Koyama, M.; Gao, Z.; Thomas, O.S.; Follo, M.; Al-Ahmad, A.; Prinz, G.; Duquesne, S.; et al. Neutrophils provide cellular communication between ileum and mesenteric lymph nodes at graft-versus-host disease onset. Blood 2018, 131, 1858–1869. [Google Scholar] [CrossRef] [PubMed]
- Chakraverty, R.; Teshima, T. Graft-versus-host disease: A disorder of tissue regeneration and repair. Blood 2021, 138, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Murugaiyan, G.; Martin, S.; Saha, B. Levels of CD40 expression on dendritic cells dictate tumour growth or regression. Clin. Exp. Immunol. 2007, 149, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Ara, A.; Ahmed, K.A.; Xiang, J. Multiple effects of CD40–CD40L axis in immunity against infection and cancer. ImmunoTargets Ther. 2018, 7, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Podojil, J.R.; Miller, S.D. Potential targeting of B7-H4 for the treatment of cancer. Immunol. Rev. 2017, 276, 40–51. [Google Scholar] [CrossRef]
- Saha, A.; Taylor, P.A.; Lees, C.J.; Panoskaltsis-Mortari, A.; Osborn, M.J.; Feser, C.J.; Thangavelu, G.; Melchinger, W.; Refaeli, Y.; Hill, G.R.; et al. Donor and host B7-H4 expression negatively regulates acute graft-versus-host disease lethality. JCI Insight 2019, 4, e127716. [Google Scholar] [CrossRef]
- Hill, G.R.; Koyama, M. Cytokines and costimulation in acute graft-versus-host disease. Blood 2020, 136, 418–428. [Google Scholar] [CrossRef]
- Granot, T.; Senda, T.; Carpenter, D.J.; Matsuoka, N.; Weiner, J.; Gordon, C.L.; Miron, M.; Kumar, B.V.; Griesemer, A.; Ho, S.-H.; et al. Dendritic Cells Display Subset and Tissue-Specific Maturation Dynamics over Human Life. Immunity 2017, 46, 504–515. [Google Scholar] [CrossRef]
- Collin, M.; McGovern, N.; Haniffa, M. Human dendritic cell subsets. Immunology 2013, 140, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Ozeri, E.; Mizrahi, M.; Shahaf, G.; Lewis, E.C. α-1 antitrypsin promotes semimature, IL-10-producing and readily migrating tolerogenic dendritic cells. J. Immunol. 2012, 189, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Kaner, Z.; Ochayon, D.E.; Shahaf, G.; Baranovski, B.M.; Bahar, N.; Mizrahi, M.; Lewis, E.C. Acute phase protein α1-antitrypsin reduces the bacterial burden in mice by selective modulation of innate cell responses. J. Infect. Dis. 2015, 211, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Guttman, O.; Freixo-Lima, G.S.; Kaner, Z.; Lior, Y.; Rider, P.; Lewis, E.C. Context-Specific and Immune Cell-Dependent Antitumor Activities of α1-Antitrypsin. Front. Immunol. 2016, 7, 559. [Google Scholar] [CrossRef] [PubMed]
- Strnad, P.; McElvaney, N.G.; Lomas, D.A. Alpha-Antitrypsin Deficiency. N. Engl. J. Med. 2020, 382, 1443–1455. [Google Scholar] [CrossRef]
- McCarthy, C.; Dunlea, D.M.; Saldova, R.; Henry, M.; Meleady, P.; McElvaney, O.J.; Marsh, B.; Rudd, P.M.; Reeves, E.P.; McElvaney, N.G. Glycosylation repurposes alpha-1 antitrypsin for resolution of community-acquired pneumonia. Am. J. Respir. Crit. Care Med. Am. Thorac. 2018, 197, 1346–1349. [Google Scholar] [CrossRef]
- McCarthy, C.; Saldova, R.; Wormald, M.R.; Rudd, P.M.; McElvaney, N.G.; Reeves, E.P. The role and importance of glycosylation of acute phase proteins with focus on alpha-1 antitrypsin in acute and chronic inflammatory conditions. J. Proteome Res. 2014, 13, 3131–3143. [Google Scholar] [CrossRef]
- Sanders, C.L.; Ponte, A.; Kueppers, F. The Effects of Inflammation on Alpha 1 Antitrypsin Levels in a National Screening Cohort. COPD J. Chronic Obstr. Pulm. Dis. 2018, 15, 10–16. [Google Scholar] [CrossRef]
- Stone, H.; Pye, A.; Stockley, R.A. Disease associations in alpha-1-antitrypsin deficiency. Respir. Med. 2014, 108, 338–343. [Google Scholar] [CrossRef]
- Korkmaz, B.; Lesner, A.; Guarino, C.; Wysocka, M.; Kellenberger, C.; Watier, H.; Specks, U.; Gauthier, F.; Jenne, D.E. Inhibitors and Antibody Fragments as Potential Anti-Inflammatory Therapeutics Targeting Neutrophil Proteinase 3 in Human Disease. Pharmacol. Rev. 2016, 68, 603–630. [Google Scholar] [CrossRef]
- Bae, S.; Kang, T.; Hong, J.; Lee, S.; Choi, J.; Jhun, H.; Kwak, A.; Hong, K.; Kim, E.; Jo, S.; et al. Contradictory Functions (Activation/Termination) of Neutrophil Proteinase 3 Enzyme (PR3) in Interleukin-33 Biological Activity. J. Biol. Chem. 2012, 287, 8205–8213. [Google Scholar] [CrossRef] [PubMed]
- Karatepe, K.; Zhu, H.; Zhang, X.; Guo, R.; Kambara, H.; Loison, F.; Liu, P.; Yu, H.; Ren, Q.; Luo, X.; et al. Proteinase 3 Limits the Number of Hematopoietic Stem and Progenitor Cells in Murine Bone Marrow. Stem Cell Rep. 2018, 11, 1092–1105. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Otero, P.; Porcher, R.; De Latour, R.P.; Contreras, M.; Bouhnik, Y.; Xhaard, A.; Andreoli, A.; Ribaud, P.; Kapel, N.; Janin, A.; et al. Fecal calprotectin and alpha-1 antitrypsin predict severity and response to corticosteroids in gastrointestinal graft-versus-host disease. Blood 2012, 119, 5909–5917. [Google Scholar] [CrossRef]
- Marcondes, A.M.; Hockenbery, D.; Lesnikova, M.; Dinarello, C.A.; Woolfrey, A.; Gernsheimer, T.; Loghman-Adham, M.; Gelmont, D.; Storer, B.; Hansen, J.A.; et al. Response of Steroid-Refractory Acute GVHD to α1-Antitrypsin. Biol. Blood Marrow Transplant. 2016, 22, 1596–1601. [Google Scholar] [CrossRef] [PubMed]
- Giannoni, L.; Morin, F.; Robin, M.; Peyneau, M.; Schlageter, M.H.; Desmier, D.; Pagliuca, S.; Del Galy, A.S.; de Fontbrune, F.S.; Xhaard, A.; et al. Human-Derived α1-Antitrypsin is Still Efficacious in Heavily Pretreated Patients with Steroid-Resistant Gastrointestinal Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2020, 26, 1620–1626. [Google Scholar] [CrossRef] [PubMed]
- Magenau, J.M.; Goldstein, S.C.; Peltier, D.; Soiffer, R.J.; Braun, T.; Pawarode, A.; Riwes, M.M.; Kennel, M.; Antin, J.H.; Cutler, C.S.; et al. α1-Antitrypsin infusion for treatment of steroid-resistant acute graft-versus-host disease. Blood 2018, 131, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- Jerkins, J.H.; Hamadani, M.; Zook, F.; Epperla, N.; Shaw, B.E.; Saber, W.; Rizzo, J.D.; Pasquini, M.; Chhabra, S.; Drobyski, W.R.; et al. Alpha-1-antitrypsin for the treatment of steroid-refractory acute gastrointestinal graft-versus-host disease. Am. J. Hematol. 2017, 92, E610–E611. [Google Scholar] [CrossRef] [PubMed]
- Minculescu, L.; Kornblit, B.T.; Friis, L.S.; Schiødt, I.; Petersen, S.L.; Andersen, N.S.; Sengeloev, H. C-Reactive Protein Levels at Diagnosis of Acute Graft-versus-Host Disease Predict Steroid-Refractory Disease, Treatment-Related Mortality, and Overall Survival after Allogeneic Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2018, 24, 600–607. [Google Scholar] [CrossRef]
- Fuji, S.; Kim, S.W.; Fukuda, T.; Mori, S.I.; Yamasaki, S.; Morita-Hoshi, Y.; Ohara-Waki, F.; Heike, Y.; Tobinai, K.; Tanosaki, R.; et al. Preengraftment Serum C-Reactive Protein (CRP) Value May Predict Acute Graft-versus-Host Disease and Nonrelapse Mortality after Allogeneic Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2008, 14, 510–517. [Google Scholar] [CrossRef][Green Version]
- Mullins, R.E.; Bennett, B.; Hunter, R.L., Jr. Electrophoretic mobility, concentration, and activity of alpha 1-antitrypsin in serum of patients undergoing bone-marrow transplantation. Clin. Chem. 1987, 33, 193–195. [Google Scholar] [CrossRef]
- Marcondes, A.M.; Karoopongse, E.; Lesnikova, M.; Margineantu, D.; Welte, T.; Dinarello, C.A.; Hockenbery, D.; Janciauskiene, S.; Deeg, H.J. α-1-Antitrypsin (AAT)-modified donor cells suppress GVHD but enhance the GVL effect: A role for mitochondrial bioenergetics. Blood 2014, 124, 2881–2891. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Choi, D.-K.; Kwak, A.; Kim, S.; Nguyen, T.T.; Gil, G.; Kim, E.; Yoo, K.H.; Kim, I.A.; Lee, Y.; et al. IL-32-induced Inflammatory Cytokines Are Selectively Suppressed by α1-antitrypsin in Mouse Bone Marrow Cells. Immune Netw. 2017, 17, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; McEnery, T.; McQuillan, K.; McElvaney, O.F.; McElvaney, O.J.; Landers, S.; Coleman, O.; Bussayajirapong, A.; Hawkins, P.; Henry, M.; et al. α1 Antitrypsin therapy modulates the neutrophil membrane proteome and secretome. Eur. Respir. J. 2020, 55, 1901678. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, P.A.; Alkanani, A.K.; Michels, A.W.; Lewis, E.C.; Shapiro, L.; Dinarello, C.A.; Zipris, D. α1-Antitrypsin therapy downregulates toll-like receptor-induced IL-1β responses in monocytes and myeloid dendritic cells and may improve islet function in recently diagnosed patients with type 1 diabetes. J. Clin. Endocrinol. Metab. 2014, 99, E1418–E1426. [Google Scholar] [CrossRef] [PubMed]
- Pepys, M.B.; Hirschfield, G.M. C-reactive protein: A critical update. J. Clin. Investig. 2003, 111, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Vion, A.-C.; Rautou, P.-E.; Durand, F.; Boulanger, C.M.; Valla, D.C. Interplay of Inflammation and Endothelial Dysfunction in Bone Marrow Transplantation: Focus on Hepatic Veno-Occlusive Disease. Semin. Thromb. Hemost. 2015, 41, 629–643. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Hu, L.; Xu, Q.; Yuan, H.; Ba, L.; He, Y.; Che, H. Cytoprotective Role of Alpha-1 Antitrypsin in Vascular Endothelial Cell Under Hypoxia/Reoxygenation Condition. J. Cardiovasc. Pharmacol. 2015, 66, 96–107. [Google Scholar] [CrossRef]
- Tawara, I.; Sun, Y.; Lewis, E.C.; Toubai, T.; Evers, R.; Nieves, E.; Azam, T.; Dinarello, C.A.; Reddy, P. Alpha-1-antitrypsin monotherapy reduces graft-versus-host disease after experimental allogeneic bone marrow transplantation. Proc. Natl. Acad. Sci. USA 2012, 109, 564–569. [Google Scholar] [CrossRef]
- McElvaney, O.J.; McEvoy, N.L.; McElvaney, O.F.; Carroll, T.P.; Murphy, M.P.; Dunlea, D.M.; Choileáin, O.N.; Clarke, J.; O’connor, E.; Hogan, G.; et al. Characterization of the Inflammatory Response to Severe COVID-19 Illness. Am. J. Respir. Crit. Care Med. 2020, 202, 812–821. [Google Scholar] [CrossRef]
- Shimi, G.; Sohrab, G.; Pourvali, K.; Ghorbani, A.; Balam, F.H.; Rostami, K.; Zand, H. Correlation of Low Levels of α-1 Antitrypsin and Elevation of Neutrophil to Lymphocyte Ratio with Higher Mortality in Severe COVID-19 Patients. Mediat. Inflamm. 2021, 2021, 5555619. [Google Scholar] [CrossRef]
- Guttman, O.; Baranovski, B.M.; Schuster, R.; Kaner, Z.; Freixo-Lima, G.S.; Bahar, N.; Kalay, N.; Mizrahi, M.I.; Brami, I.; Ochayon, D.E.; et al. Acute-phase protein α1-anti-trypsin: Diverting injurious innate and adaptive immune responses from non-authentic threats. Clin. Exp. Immunol. 2015, 179, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, A.; Khan, A.Y.; Malik, S.U.; Faridi, W.; Fraz, M.A.; Usman, M.; Tariq, M.J.; Durer, S.; Durer, C.; Russ, A.; et al. Significant Risk of Graft-versus-Host Disease with Exposure to Checkpoint Inhibitors before and after Allogeneic Transplantation. Biol. Blood Marrow Transplant. 2019, 25, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Kordelas, L.; Buttkereit, U.; Heinemann, F.M.; Horn, P.A.; Giebel, B.; Beelen, D.W.; Reinhardt, H.C.; Rebmann, V. Low Soluble Programmed Cell Death Protein 1 Levels after Allogeneic Stem Cell Transplantation Predict Moderate or Severe Chronic GvHD and Inferior Overall Survival. Front. Immunol. 2021, 12, 694843. [Google Scholar] [CrossRef] [PubMed]
- Ruhaak, L.R.; Koeleman, C.A.M.; Uh, H.-W.; Stam, J.C.; van Heemst, D.; Maier, A.B.; Houwing-Duistermaat, J.J.; Hensbergen, P.J.; Slagboom, P.E.; Deelder, A.M.; et al. Targeted biomarker discovery by high throughput glycosylation profiling of human plasma alpha1-antitrypsin and immunoglobulin A. PLoS ONE 2013, 8, e73082. [Google Scholar] [CrossRef] [PubMed]
- Gergoudis, S.C.; DeFilipp, Z.; Özbek, U.; Sandhu, K.S.; Etra, A.M.; Choe, H.K.; Kitko, C.L.; Ayuk, F.; Aziz, M.; Baez, J.; et al. Biomarker-guided preemption of steroid-refractory graft-versus-host disease with α-1-antitrypsin. Blood Adv. 2020, 4, 6098–6105. [Google Scholar] [CrossRef]
- Hinkofer, L.C.; Seidel, S.A.I.; Korkmaz, B.; Silva, F.; Hummel, A.M.; Braun, D.; Jenne, D.E.; Specks, U. A monoclonal antibody (MCPR3-7) interfering with the activity of proteinase 3 by an allosteric mechanism. J. Biol. Chem. 2013, 288, 26635–26648. [Google Scholar] [CrossRef]
- Collin, M.; Bigley, V. Human dendritic cell subsets: An update. Immunology 2018, 154, 3–20. [Google Scholar] [CrossRef]
- Wooldridge, J.M. Econometric Analysis of Cross Section and Panel Data; MIT Press Books; MIT Press: Cambridge, MA, USA, 2010; Volume 1, Available online: https://ideas.repec.org/b/mtp/titles/0262232588.html (accessed on 1 January 2020).

| Cohort Characteristics (N = 53) | Median (Range) |
|---|---|
| Age (yrs) | 54 (24–74) |
| Male sex (%) | 30 (56.6) |
| Indication for HSCT | N (percent) |
| AML | 25 (13.2) |
| ALL | 8 (47.2) |
| Non-Hodgkin’s lymphoma | 7 (15.1) |
| MDS | 6 (13.2) |
| Other * | 7 (11.3) |
| Donor characteristics | Median (range) |
| Age (yrs) | 27 (13–71) |
| Male sex | 42 (79.2%) |
| Related | 24 (45.3%) |
| Unrelated | 28 (52.8%) |
| Haploidentical | 1 (1.9%) |
| Female to male | 5 (9.4%) |
| HLA-match | N (percent) |
| Matched | 49 (92.4) |
| Mismatched | 3 (5.7) |
| Haploidentical | 1 (1.9) |
| Conditioning regimen intensity | N (percent) |
| Myeloablative conditioning | 19 (35.8) |
| Reduced-intensity conditioning | 34 (64.2) |
| GVHD prophylaxis | N (percent) |
| MTX + CNI | 32 (60.4) |
| MMF + CNI | 17 (32) |
| MTX + CNI + MMF | 3 (5.7) |
| RAP + CNI | 1 (1.9) |
| ATG | 33 (62.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brami, I.; Zuckerman, T.; Ram, R.; Avni, B.; Peretz, G.; Ostrovsky, D.; Lior, Y.; Faour, C.; McElvaney, O.; McElvaney, N.G.; et al. Altered Serum Alpha1-Antitrypsin Protease Inhibition before and after Clinical Hematopoietic Stem Cell Transplantation: Association with Risk for Non-Relapse Mortality. Int. J. Mol. Sci. 2024, 25, 422. https://doi.org/10.3390/ijms25010422
Brami I, Zuckerman T, Ram R, Avni B, Peretz G, Ostrovsky D, Lior Y, Faour C, McElvaney O, McElvaney NG, et al. Altered Serum Alpha1-Antitrypsin Protease Inhibition before and after Clinical Hematopoietic Stem Cell Transplantation: Association with Risk for Non-Relapse Mortality. International Journal of Molecular Sciences. 2024; 25(1):422. https://doi.org/10.3390/ijms25010422
Chicago/Turabian StyleBrami, Ido, Tsila Zuckerman, Ron Ram, Batia Avni, Galit Peretz, Daniel Ostrovsky, Yotam Lior, Caroline Faour, Oisin McElvaney, Noel G. McElvaney, and et al. 2024. "Altered Serum Alpha1-Antitrypsin Protease Inhibition before and after Clinical Hematopoietic Stem Cell Transplantation: Association with Risk for Non-Relapse Mortality" International Journal of Molecular Sciences 25, no. 1: 422. https://doi.org/10.3390/ijms25010422
APA StyleBrami, I., Zuckerman, T., Ram, R., Avni, B., Peretz, G., Ostrovsky, D., Lior, Y., Faour, C., McElvaney, O., McElvaney, N. G., & Lewis, E. C. (2024). Altered Serum Alpha1-Antitrypsin Protease Inhibition before and after Clinical Hematopoietic Stem Cell Transplantation: Association with Risk for Non-Relapse Mortality. International Journal of Molecular Sciences, 25(1), 422. https://doi.org/10.3390/ijms25010422






