Perturbation of Copper Homeostasis Sensitizes Cancer Cells to Elevated Temperature
Abstract
1. Introduction
2. Results
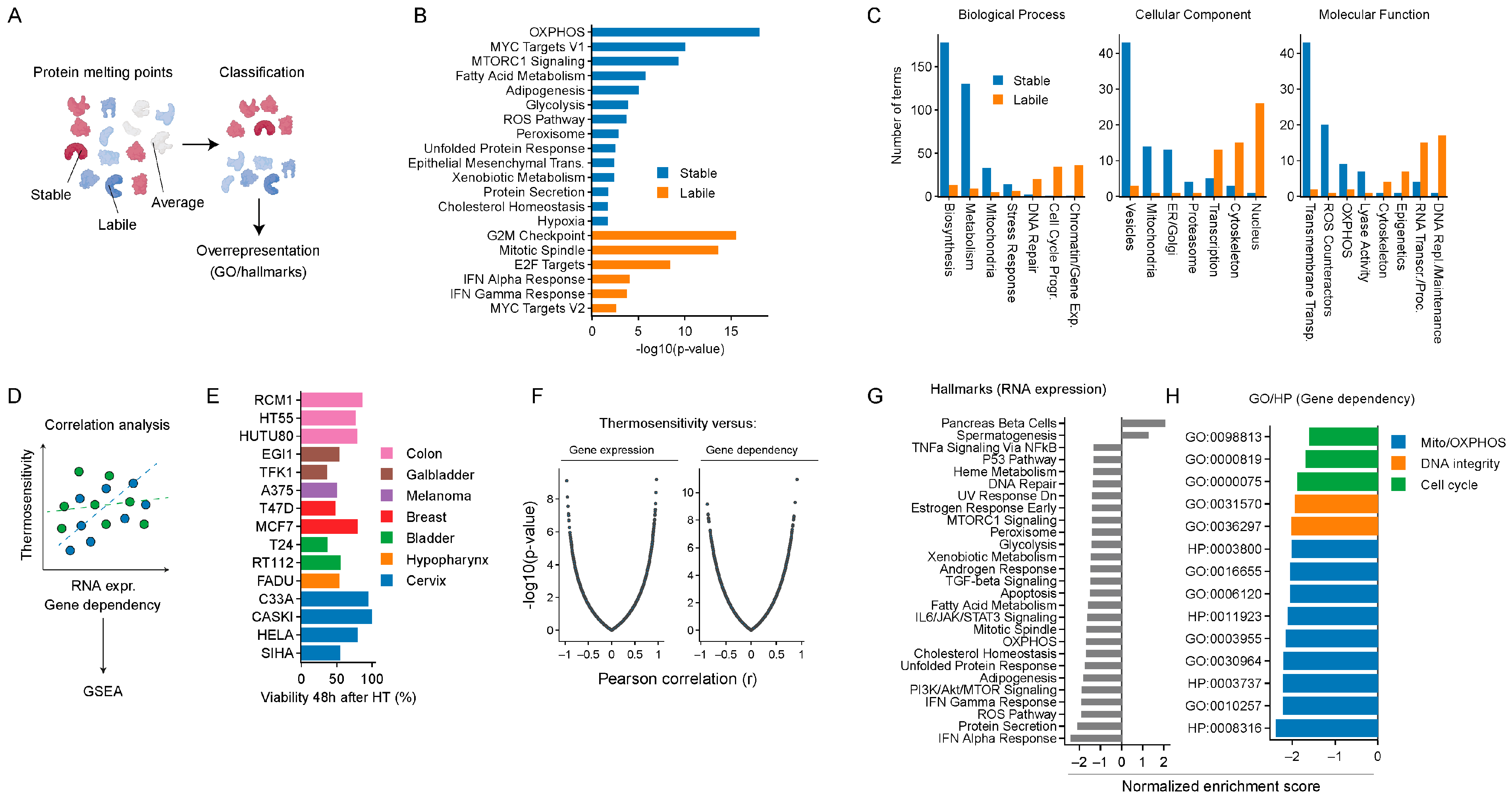
2.1. In Silico Analyses Identify Cellular Energy Metabolism and ROS Homeostasis as Potential Targets for Thermosensitization
2.2. Targeting Energy Metabolism and ROS Homeostasis as a Thermosensitization Strategy
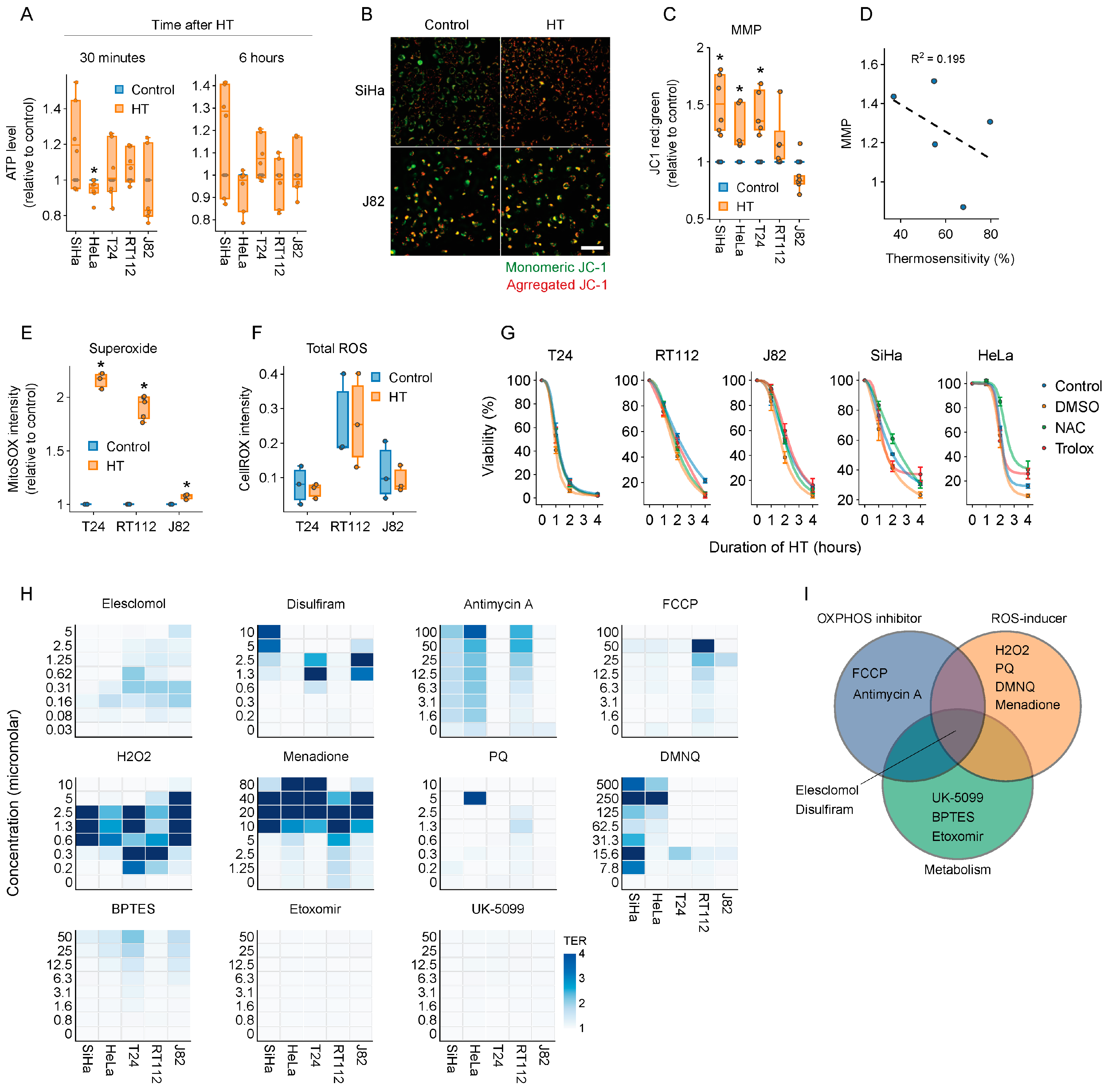
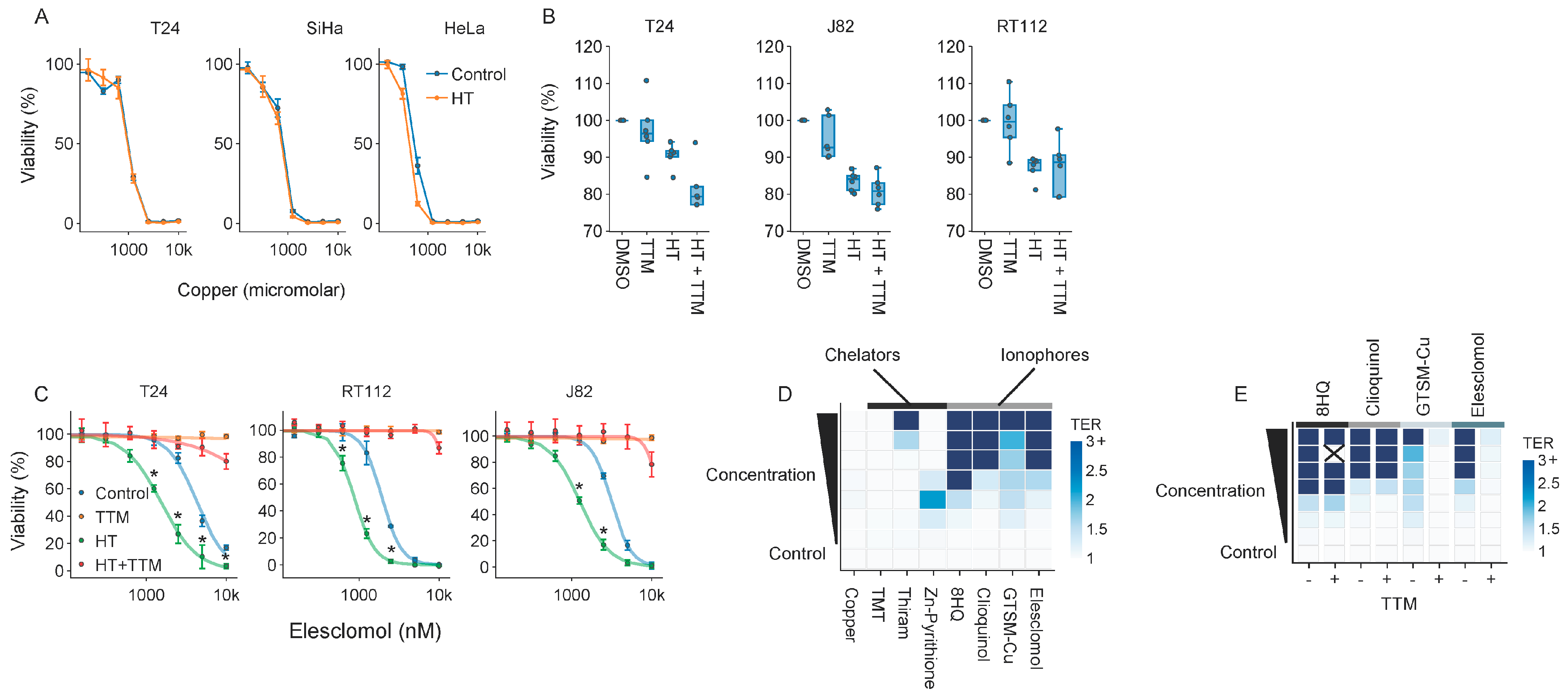
2.3. Elesclomol Is a Potent Thermosensitizer
2.4. Elesclomol and Hyperthermia Induce Alterations in Proteostasis and the Transcriptome
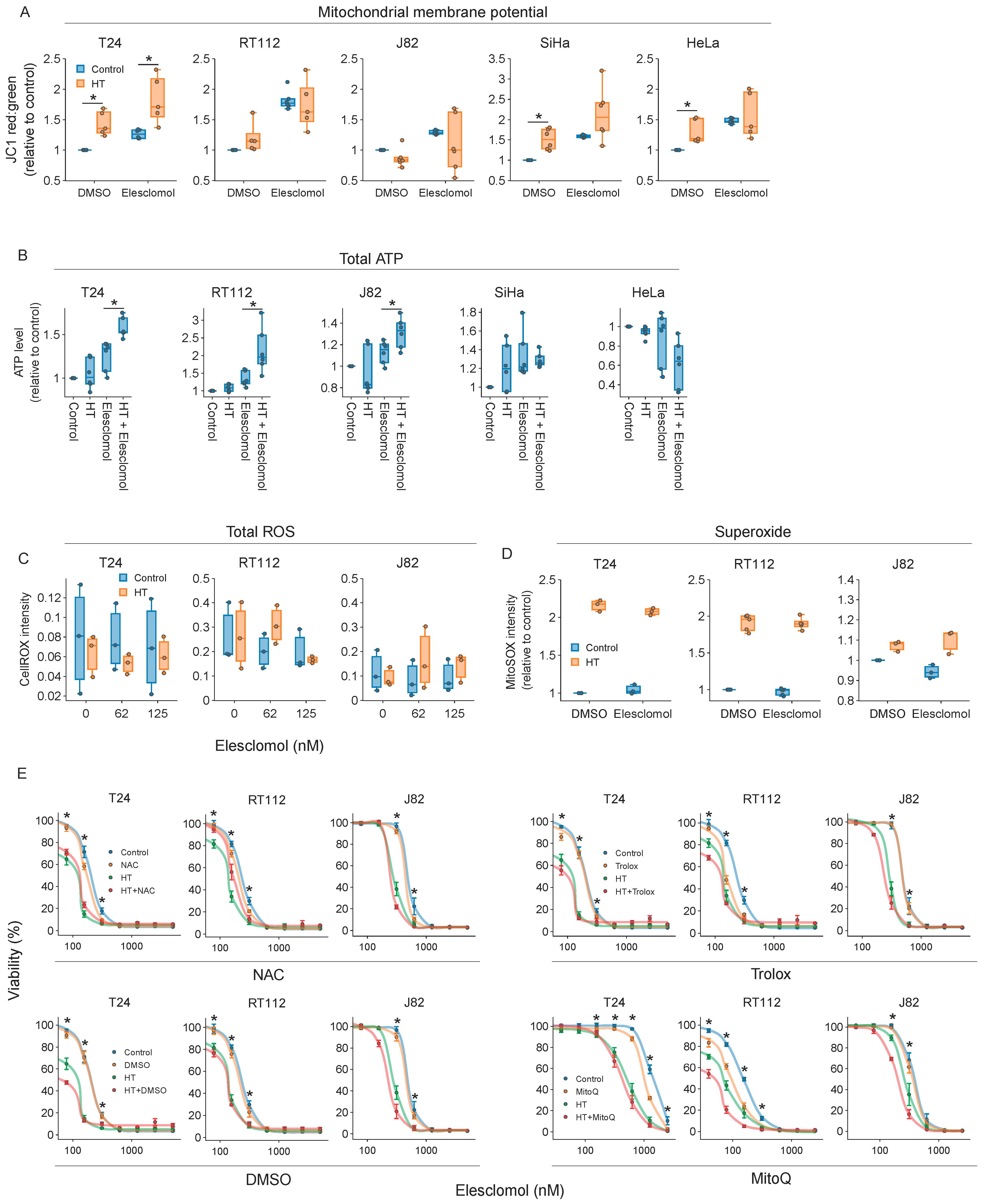
2.5. The Thermosensitizing Effects of Elesclomol Are Not Caused by Interference with Energy or ROS Production
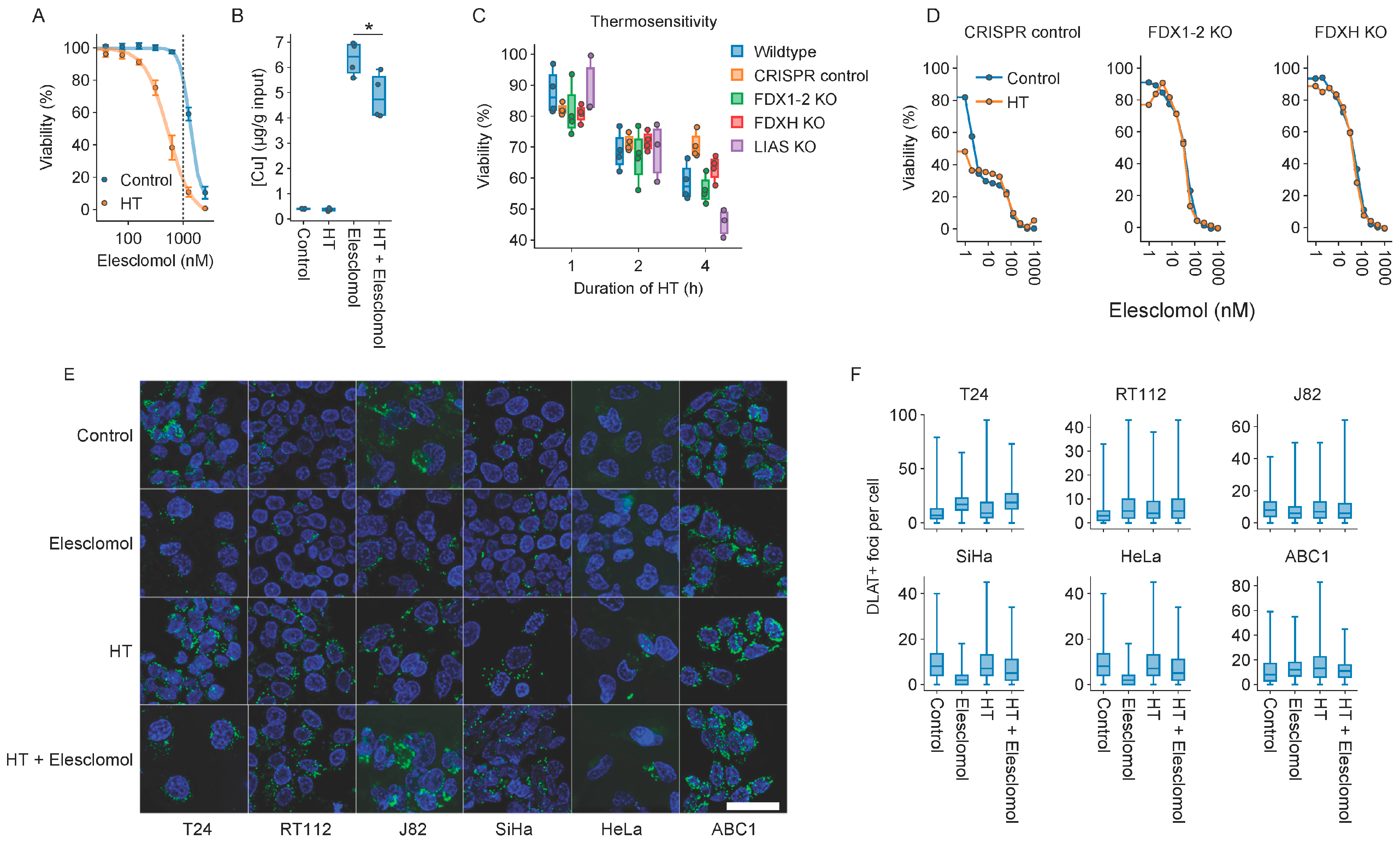
2.6. Cuproptosis Does Not Drive Thermosensitization by Elesclomol
2.7. Molecules Interfering with Copper Homeostasis Generally Thermosensitize
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Hyperthermia, Drug Treatments, and Reagents
4.3. Cell Viability Assays
4.4. In Silico Analyses
4.5. Quantification of Total ROS and Mitochondrial Superoxide
4.6. Quantification of Total ATP Levels
4.7. Determination of Intracellular Copper Concentrations
4.8. Evaluation of Mitochondrial Membrane Potential
4.9. Immunoblotting
4.10. RNA Sequencing
4.11. Fluorescence Microscopy and Image Analysis
4.12. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer Incidence and Mortality Patterns in Europe: Estimates for 40 Countries and 25 Major Cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Cihoric, N.; Tsikkinis, A.; van Rhoon, G.; Crezee, H.; Aebersold, D.M.; Bodis, S.; Beck, M.; Nadobny, J.; Budach, V.; Wust, P.; et al. Hyperthermia-Related Clinical Trials on Cancer Treatment within the ClinicalTrials.gov Registry. Int. J. Hyperth. 2015, 31, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.R.; Puric, E.; Klingbiel, D.; Gomez, S.; Bodis, S. Hyperthermia and Radiation Therapy in Locoregional Recurrent Breast Cancers: A Systematic Review and Meta-Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 1073–1087. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.R.; Rogers, S.; Ordóñez, S.G.; Puric, E.; Bodis, S. Hyperthermia and Radiotherapy in the Management of Head and Neck Cancers: A Systematic Review and Meta-Analysis. Int. J. Hyperth. 2016, 32, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.R.; Ordóñez, S.G.; Gaipl, U.S.; Paulides, M.M.; Crezee, H.; Gellermann, J.; Marder, D.; Puric, E.; Bodis, S. Local Hyperthermia Combined with Radiotherapy and-/or Chemotherapy: Recent Advances and Promises for the Future. Cancer Treat. Rev. 2015, 41, 742–753. [Google Scholar] [CrossRef] [PubMed]
- van Rhoon, G.C.; Franckena, M.; ten Hagen, T.L.M. A Moderate Thermal Dose Is Sufficient for Effective Free and TSL Based Thermochemotherapy. Adv. Drug Deliv. Rev. 2020, 163–164, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Peeken, J.C.; Vaupel, P.; Combs, S.E. Integrating Hyperthermia into Modern Radiation Oncology: What Evidence Is Necessary? Front. Oncol. 2017, 7, 132. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhu, J.; Song, Y.-X.; Wang, X.; Zhou, K.-C.; Lu, Y.; Liu, X.-Q. Thermal Intravesical Chemotherapy Reduce Recurrence Rate for Non-Muscle Invasive Bladder Cancer Patients: A Meta-Analysis. Front. Oncol. 2020, 10, 29. [Google Scholar] [CrossRef]
- Masunaga, S.; Ono, K.; Akaboshi, M.; Nishimura, Y.; Suzuki, M.; Kinashi, Y.; Takagaki, M.; Hiraoka, M.; Abe, M. Reduction of Hypoxic Cells in Solid Tumours Induced by Mild Hyperthermia: Special Reference to Differences in Changes in the Hypoxic Fraction between Total and Quiescent Cell Populations. Br. J. Cancer 1997, 76, 588–593. [Google Scholar] [CrossRef][Green Version]
- Oleson, J.R. Eugene Robertson Special Lecture Hyperthermia from the Clinic to the Laboratory: A Hypothesis. Int. J. Hyperth. 1995, 11, 315–322. [Google Scholar] [CrossRef]
- Jain, R.K.; Grantham, F.H.; Gullino, P.M. Blood Flow and Heat Transfer in Walker 256 Mammary Carcinoma. J. Natl. Cancer Inst. 1979, 62, 927–933. [Google Scholar] [PubMed]
- Vaupel, P.; Kallinowski, F. Physiological Effects of Hyperthermia. Recent Results Cancer Res. 1987, 104, 71–109. [Google Scholar] [PubMed]
- Jones, E.L.; Prosnitz, L.R.; Dewhirst, M.W.; Marcom, P.K.; Hardenbergh, P.H.; Marks, L.B.; Brizel, D.M.; Vujaskovic, Z. Thermochemoradiotherapy Improves Oxygenation in Locally Advanced Breast Cancer. Clin. Cancer Res. 2004, 10, 4287–4293. [Google Scholar] [CrossRef] [PubMed]
- Horsman, M.R.; Overgaard, J. The Impact of Hypoxia and Its Modification of the Outcome of Radiotherapy. J. Radiat. Res. 2016, 57, i90–i98. [Google Scholar] [CrossRef]
- Elming, P.B.; Sørensen, B.S.; Oei, A.L.; Franken, N.A.P.; Crezee, J.; Overgaard, J.; Horsman, M.R. Hyperthermia: The Optimal Treatment to Overcome Radiation Resistant Hypoxia. Cancers 2019, 11, 60. [Google Scholar] [CrossRef]
- Wouters, A.; Pauwels, B.; Lardon, F.; Vermorken, J.B. Review: Implications of In Vitro Research on the Effect of Radiotherapy and Chemotherapy Under Hypoxic Conditions. Oncologist 2007, 12, 690–712. [Google Scholar] [CrossRef]
- Li, L.; Ten Hagen, T.L.M.; Hossann, M.; Süss, R.; Van Rhoon, G.C.; Eggermont, A.M.M.; Haemmerich, D.; Koning, G.A. Mild Hyperthermia Triggered Doxorubicin Release from Optimized Stealth Thermosensitive Liposomes Improves Intratumoral Drug Delivery and Efficacy. J. Control. Release 2013, 168, 142–150. [Google Scholar] [CrossRef]
- Mikhail, A.S.; Negussie, A.H.; Pritchard, W.F.; Haemmerich, D.; Woods, D.; Bakhutashvili, I.; Esparza-Trujillo, J.; Brancato, S.J.; Karanian, J.; Agarwal, P.K.; et al. Lyso-Thermosensitive Liposomal Doxorubicin for Treatment of Bladder Cancer. Int. J. Hyperth. 2017, 33, 733–740. [Google Scholar] [CrossRef]
- Lyon, P.C.; Gray, M.D.; Mannaris, C.; Folkes, L.K.; Stratford, M.; Campo, L.; Chung, D.Y.F.; Scott, S.; Anderson, M.; Goldin, R.; et al. Safety and Feasibility of Ultrasound-Triggered Targeted Drug Delivery of Doxorubicin from Thermosensitive Liposomes in Liver Tumours (TARDOX): A Single-Centre, Open-Label, Phase 1 Trial. Lancet Oncol. 2018, 19, 1027–1039. [Google Scholar] [CrossRef]
- Kong, G.; Braun, R.D.; Dewhirst, M.W. Hyperthermia Enables Tumor-Specific Nanoparticle Delivery: Effect of Particle Size. Cancer Res. 2000, 60, 4440–4445. [Google Scholar]
- Calderwood, S.K. Hyperthermia, the Tumor Microenvironment and Immunity. In Tumor Ablation: Effects on Systemic and Local Anti-Tumor Immunity and on Other Tumor-Microenvironment Interactions; Springer: Dordrecht, The Netherlands, 2013; pp. 29–37. ISBN 9789400746947. [Google Scholar]
- Toraya-Brown, S.; Sheen, M.R.; Zhang, P.; Chen, L.; Baird, J.R.; Demidenko, E.; Turk, M.J.; Hoopes, P.J.; Conejo-Garcia, J.R.; Fiering, S. Local Hyperthermia Treatment of Tumors Induces CD8+T Cell-Mediated Resistance against Distal and Secondary Tumors. Nanomedicine 2014, 10, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Dai, Y. Tumor Microenvironment and Therapeutic Response. Cancer Lett. 2017, 387, 61–68. [Google Scholar] [CrossRef]
- Thews, O.; Riemann, A. Tumor pH and Metastasis: A Malignant Process beyond Hypoxia. Cancer Metastasis Rev. 2019, 38, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Repasky, E.A.; Evans, S.S.; Dewhirst, M.W. Temperature Matters! And Why It Should Matter to Tumor Immunologists. Cancer Immunol. Res. 2013, 1, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Richter, K.; Haslbeck, M.; Buchner, J. The Heat Shock Response: Life on the Verge of Death. Mol. Cell 2010, 40, 253–266. [Google Scholar] [CrossRef]
- Oei, A.L.; Vriend, L.E.M.; Crezee, J.; Franken, N.A.P.; Krawczyk, P.M. Effects of Hyperthermia on DNA Repair Pathways: One Treatment to Inhibit Them All. Radiat. Oncol. 2015, 10, 165. [Google Scholar] [CrossRef]
- Lindquist, S. The Heat-Shock Response. Annu. Rev. Biochem. 1986, 55, 1151–1191. [Google Scholar] [CrossRef]
- Overgaard, J.; Nielsen, O.S. The Importance of Thermotolerance for the Clinical Treatment with Hyperthermia. Radiother. Oncol. 1983, 1, 167–178. [Google Scholar] [CrossRef]
- Jarzab, A.; Kurzawa, N.; Hopf, T.; Moerch, M.; Zecha, J.; Leijten, N.; Bian, Y.; Musiol, E.; Maschberger, M.; Stoehr, G.; et al. Meltome Atlas—Thermal Proteome Stability across the Tree of Life. Nat. Methods 2020, 17, 495–503. [Google Scholar] [CrossRef]
- Liberzon, A.; Subramanian, A.; Pinchback, R.; Thorvaldsdóttir, H.; Tamayo, P.; Mesirov, J.P. Molecular Signatures Database (MSigDB) 3.0. Bioinformatics 2011, 27, 1739–1740. [Google Scholar] [CrossRef]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database (MSigDB) Hallmark Gene Set Collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Vihervaara, A.; Duarte, F.M.; Lis, J.T. Molecular Mechanisms Driving Transcriptional Stress Responses. Nat. Rev. Genet. 2018, 19, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Duan, S.; Zheng, Y.; Yang, Y.; Wang, L.; Li, X.; Zhang, Q.; Thorne, R.F.; Li, W.; Yang, D. Hyperthermia Inhibits Growth of Nasopharyngeal Carcinoma through Degradation of c-Myc. Int. J. Hyperth. 2022, 39, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Giulino-Roth, L.; van Besien, H.J.; Dalton, T.; Totonchy, J.E.; Rodina, A.; Taldone, T.; Bolaender, A.; Erdjument-Bromage, H.; Sadek, J.; Chadburn, A.; et al. Inhibition of Hsp90 Suppresses PI3K/AKT/mTOR Signaling and Has Antitumor Activity in Burkitt Lymphoma. Mol. Cancer Ther. 2017, 16, 1779–1790. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-L.; Liu, B.O.; Li, X.-J.; Hu, K.-P.; Zhao, K.; Ye, X.-M. Inhibition of mTOR Promotes Hyperthermia Sensitivity in SMMC-7721 Human Hepatocellular Carcinoma Cell Line. Exp. Ther. Med. 2016, 11, 961–968. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Borkamo, E.D.; Schem, B.-C.; Fluge, Ø.; Bruland, O.; Dahl, O.; Mella, O. cDNA Microarray Analysis of Serially Sampled Cervical Cancer Specimens From Patients Treated With Thermochemoradiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.; Nair, M.P.; Ambrus, J.L.; Chadha, K.C. Mild Hyperthermia Modulates Biological Activities of Interferons. Int. J. Hyperth. 2000, 16, 492–507. [Google Scholar] [CrossRef]
- Oba, M.; Yano, S.; Shuto, T.; Suico, M.A.; Eguma, A.; Kai, H. IFN-γ down-Regulates Hsp27 and Enhances Hyperthermia-Induced Tumor Cell Death in Vitro and Tumor Suppression in Vivo. Int. J. Oncol. 2008, 32, 1317–1324. [Google Scholar] [CrossRef][Green Version]
- Thorne, A.M.; Ubbink, R.; Brüggenwirth, I.M.A.; Nijsten, M.W.; Porte, R.J.; de Meijer, V.E. Hyperthermia-Induced Changes in Liver Physiology and Metabolism: A Rationale for Hyperthermic Machine Perfusion. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G43–G50. [Google Scholar] [CrossRef]
- Kanamori, T.; Miyazaki, N.; Aoki, S.; Ito, K.; Hisaka, A.; Hatakeyama, H. Investigation of Energy Metabolic Dynamism in Hyperthermia-Resistant Ovarian and Uterine Cancer Cells under Heat Stress. Sci. Rep. 2021, 11, 14726. [Google Scholar] [CrossRef]
- Roti, J.L.R. Cellular Responses to Hyperthermia (40-46°C): Cell Killing and Molecular Events. Int. J. Hyperth. 2008, 24, 3–15. [Google Scholar] [CrossRef]
- Sasaki, R.; Suzuki, Y.; Yonezawa, Y.; Ota, Y.; Okamoto, Y.; Demizu, Y.; Huang, P.; Yoshida, H.; Sugimura, K.; Mizushina, Y. DNA Polymerase Gamma Inhibition by Vitamin K3 Induces Mitochondria-Mediated Cytotoxicity in Human Cancer Cells. Cancer Sci. 2008, 99, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, E.; Rohondia, S.; Khan, R.; Dou, Q.P. Repurposing Disulfiram as An Anti-Cancer Agent: Updated Review on Literature and Patents. Recent Pat. Anticancer Drug Discov. 2019, 14, 113–132. [Google Scholar] [CrossRef] [PubMed]
- Kannappan, V.; Ali, M.; Small, B.; Rajendran, G.; Elzhenni, S.; Taj, H.; Wang, W.; Dou, Q.P. Recent Advances in Repurposing Disulfiram and Disulfiram Derivatives as Copper-Dependent Anticancer Agents. Front. Mol. Biosci. 2021, 8, 741316. [Google Scholar] [CrossRef] [PubMed]
- O’Day, S.J.; Eggermont, A.M.M.; Chiarion-Sileni, V.; Kefford, R.; Grob, J.J.; Mortier, L.; Robert, C.; Schachter, J.; Testori, A.; Mackiewicz, J.; et al. Final Results of Phase III SYMMETRY Study: Randomized, Double-Blind Trial of Elesclomol plus Paclitaxel versus Paclitaxel Alone as Treatment for Chemotherapy-naïve Patients with Advanced Melanoma. J. Clin. Oncol. 2013, 31, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Monk, B.J.; Kauderer, J.T.; Moxley, K.M.; Bonebrake, A.J.; Dewdney, S.B.; Secord, A.A.; Ueland, F.R.; Johnston, C.M.; Aghajanian, C. A Phase II Evaluation of Elesclomol Sodium and Weekly Paclitaxel in the Treatment of Recurrent or Persistent Platinum-Resistant Ovarian, Fallopian Tube or Primary Peritoneal Cancer: An NRG Oncology/gynecologic Oncology Group Study. Gynecol. Oncol. 2018, 151, 422–427. [Google Scholar] [CrossRef]
- Chen, S.; Sun, L.; Koya, K.; Tatsuta, N.; Xia, Z.; Korbut, T.; Du, Z.; Wu, J.; Liang, G.; Jiang, J.; et al. Syntheses and Antitumor Activities of N′1,N′3-Dialkyl-N′1,N′3-Di-(alkylcarbonothioyl) Malonohydrazide: The Discovery of Elesclomol. Bioorg. Med. Chem. Lett. 2013, 23, 5070–5076. [Google Scholar] [CrossRef]
- Kirshner, J.; Du, Z.; Kepros, J.; Balasubramanyam, V.; He, S.; Yang, C.-Y.; Zhang, M.; Barsoum, J.; Bertin, J. The Novel Small Molecule Elesclomol (formerly STA-4783) Induces Apoptosis in Cancer Cells through Induction of Oxidative Stress. In Proceedings of the AACR-NCI-EORTC Virtual International Conference on Molecular Targets and Cancer Therapeutics, San Francisco, CA, USA, 22–26 October 2007. [Google Scholar]
- Nagai, M.; Vo, N.H.; Shin Ogawa, L.; Chimmanamada, D.; Inoue, T.; Chu, J.; Beaudette-Zlatanova, B.C.; Lu, R.; Blackman, R.K.; Barsoum, J.; et al. The Oncology Drug Elesclomol Selectively Transports Copper to the Mitochondria to Induce Oxidative Stress in Cancer Cells. Free Radic. Biol. Med. 2012, 52, 2142–2150. [Google Scholar]
- Hasinoff, B.B.; Yadav, A.A.; Patel, D.; Wu, X. The Cytotoxicity of the Anticancer Drug Elesclomol Is due to Oxidative Stress Indirectly Mediated through Its Complex with Cu(II). J. Inorg. Biochem. 2014, 137, 22–30. [Google Scholar] [CrossRef]
- Blackman, R.K.; Cheung-Ong, K.; Gebbia, M.; Proia, D.A.; He, S.; Kepros, J.; Jonneaux, A.; Marchetti, P.; Kluza, J.; Rao, P.E.; et al. Mitochondrial Electron Transport Is the Cellular Target of the Oncology Drug Elesclomol. PLoS ONE 2012, 7, e29798. [Google Scholar] [CrossRef]
- Modica-Napolitano, J.S.; Bharath, L.P.; Hanlon, A.J.; Hurley, L.D. The Anticancer Agent Elesclomol Has Direct Effects on Mitochondrial Bioenergetic Function in Isolated Mammalian Mitochondria. Biomolecules 2019, 9, 298. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, P.; Detappe, A.; Cai, K.; Keys, H.R.; Brune, Z.; Ying, W.; Thiru, P.; Reidy, M.; Kugener, G.; Rossen, J.; et al. Mitochondrial Metabolism Promotes Adaptation to Proteotoxic Stress. Nat. Chem. Biol. 2019, 15, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper Induces Cell Death by Targeting Lipoylated TCA Cycle Proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Wang, J.; Sim, M.-S.; Liu, B.; Giuliano, A.; Barsoum, J.; Cui, X. Elesclomol, Counteracted by Akt Survival Signaling, Enhances the Apoptotic Effect of Chemotherapy Drugs in Breast Cancer Cells. Breast Cancer Res. Treat. 2010, 121, 311–321. [Google Scholar] [CrossRef]
- Maxwell, B.A.; Gwon, Y.; Mishra, A.; Peng, J.; Nakamura, H.; Zhang, K.; Kim, H.J.; Taylor, J.P. Ubiquitination Is Essential for Recovery of Cellular Activities after Heat Shock. Science 2021, 372, eabc3593. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, X.; Kroemer, G. Cuproptosis: A Copper-Triggered Modality of Mitochondrial Cell Death. Cell Res. 2022, 32, 417–418. [Google Scholar] [CrossRef]
- Zamaraeva, M.V.; Sabirov, R.Z.; Maeno, E.; Ando-Akatsuka, Y.; Bessonova, S.V.; Okada, Y. Cells Die with Increased Cytosolic ATP during Apoptosis: A Bioluminescence Study with Intracellular Luciferase. Cell Death Differ. 2005, 12, 1390–1397. [Google Scholar] [CrossRef]
- Gao, W.; Huang, Z.; Duan, J.; Nice, E.C.; Lin, J.; Huang, C. Elesclomol Induces Copper-Dependent Ferroptosis in Colorectal Cancer Cells via Degradation of ATP7A. Mol. Oncol. 2021, 15, 3527–3544. [Google Scholar] [CrossRef]
- Gabano, E.; Colangelo, D.; Ghezzi, A.R.; Osella, D. The Influence of Temperature on Antiproliferative Effects, Cellular Uptake and DNA Platination of the Clinically Employed Pt(II)-Drugs. J. Inorg. Biochem. 2008, 102, 629–635. [Google Scholar] [CrossRef]
- Ohtsubo, T.; Saito, H.; Tanaka, N.; Matsumoto, H.; Sugimoto, C.; Saito, T.; Hayashi, S.; Kano, E. Enhancement of Cisplatin Sensitivity and Platinum Uptake by 40°C Hyperthermia in Resistant Cells. Cancer Lett. 1997, 119, 47–52. [Google Scholar] [CrossRef]
- Matsubara, K.; Kayashima, T.; Mori, M.; Yoshida, H.; Mizushina, Y. Inhibitory Effects of Vitamin K3 on DNA Polymerase and Angiogenesis. Int. J. Mol. Med. 2008, 22, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Hedley, D.; Shamas-Din, A.; Chow, S.; Sanfelice, D.; Schuh, A.C.; Brandwein, J.M.; Seftel, M.D.; Gupta, V.; Yee, K.W.L.; Schimmer, A.D. A Phase I Study of Elesclomol Sodium in Patients with Acute Myeloid Leukemia. Leuk. Lymphoma 2016, 57, 2437–2440. [Google Scholar] [CrossRef]
- Tetef, M.; Margolin, K.; Ahn, C.; Akman, S.; Chow, W.; Leong, L.; Morgan, R.J., Jr.; Raschko, J.; Somlo, G.; Doroshow, J.H. Mitomycin C and Menadione for the Treatment of Lung Cancer: A Phase II Trial. Investig. New Drugs 1995, 13, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Margolin, K.A.; Akman, S.A.; Leong, L.A.; Morgan, R.J.; Somlo, G.; Raschko, J.W.; Ahn, C.; Doroshow, J.H. Phase I Study of Mitomycin C and Menadione in Advanced Solid Tumors. Cancer Chemother. Pharmacol. 1995, 36, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Zulkifli, M.; Spelbring, A.N.; Zhang, Y.; Soma, S.; Chen, S.; Li, L.; Le, T.; Shanbhag, V.; Petris, M.J.; Chen, T.-Y.; et al. FDX1-Dependent and Independent Mechanisms of Elesclomol-Mediated Intracellular Copper Delivery. Proc. Natl. Acad. Sci. USA 2023, 120, e2216722120. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, W.; Zhan, P.; Liu, X. 8-Hydroxyquinoline: A Privileged Structure with a Broad-Ranging Pharmacological Potential. Med. Chem. Commun. 2015, 6, 61–74. [Google Scholar]
- Perez, D.R.; Sklar, L.A.; Chigaev, A. Clioquinol: To Harm or Heal. Pharmacol. Ther. 2019, 199, 155–163. [Google Scholar] [CrossRef] [PubMed]
- van Stein, R.M.; Aalbers, A.G.J.; Sonke, G.S.; van Driel, W.J. Hyperthermic Intraperitoneal Chemotherapy for Ovarian and Colorectal Cancer: A Review. JAMA Oncol. 2021, 7, 1231–1238. [Google Scholar] [CrossRef]
- Kusamura, S.; Bhatt, A.; Hubner, M.; Villeneuve, L.; Deraco, M.; Bakrin, N.; Van Der Speeten, K.; Glehen, O. The 2022 PSOGI International Consensus on HIPEC Regimens for Peritoneal Malignancies: Methodology. Ann. Surg. Oncol. 2023, 30, 2508–2519. [Google Scholar] [CrossRef]
- Moreno-Ramirez, D.; de la Cruz-Merino, L.; Ferrandiz, L.; Villegas-Portero, R.; Nieto-Garcia, A. Isolated Limb Perfusion for Malignant Melanoma: Systematic Review on Effectiveness and Safety. Oncologist 2010, 15, 416–427. [Google Scholar] [CrossRef]
- Trabulsi, N.H.; Patakfalvi, L.; Nassif, M.O.; Turcotte, R.E.; Nichols, A.; Meguerditchian, A.N. Hyperthermic Isolated Limb Perfusion for Extremity Soft Tissue Sarcomas: Systematic Review of Clinical Efficacy and Quality Assessment of Reported Trials. J. Surg. Oncol. 2012, 106, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Van Hattum, J.W.; Scutigliani, E.M.; Helderman, R.F.C.P.A.; Zweije, R.; Rodermond, H.M.; Oei, A.L.; Crezee, J.; Oddens, J.R.; De Reijke, T.M.; Krawczyk, P.M. A Scalable Hyperthermic Intravesical Chemotherapy (HIVEC) Setup for Rat Models of Bladder Cancer. Sci. Rep. 2022, 12, 7017. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A Universal Enrichment Tool for Interpreting Omics Data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef] [PubMed]
- Dempster, J.M.; Boyle, I.; Vazquez, F.; Root, D.E.; Boehm, J.S.; Hahn, W.C.; Tsherniak, A.; McFarland, J.M. Chronos: A Cell Population Dynamics Model of CRISPR Experiments That Improves Inference of Gene Fitness Effects. Genome Biol. 2021, 22, 343. [Google Scholar] [CrossRef] [PubMed]
- Scutigliani, E.M.; Liang, Y.; IJff, M.; Rodermond, H.; Mei, X.; Korver, M.P.; Orie, V.S.; Hoebe, R.A.; Picavet, D.I.; Oei, A.; et al. Evaluation of the Heat Shock Protein 90 Inhibitor Ganetespib as a Sensitizer to Hyperthermia-Based Cancer Treatments. Cancers 2022, 14, 5250. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Schmidt, U.; Weigert, M.; Broaddus, C.; Myers, G. Cell Detection with Star-Convex Polygons. In Proceedings of the Medical Image Computing and Computer Assisted Intervention—MICCAI 2018, Granada, Spain, 16–20 September 2018; Springer International Publishing: Cham, Switzerland, 2018; pp. 265–273. [Google Scholar]
- Stringer, C.; Wang, T.; Michaelos, M.; Pachitariu, M. Cellpose: A Generalist Algorithm for Cellular Segmentation. Nat. Methods 2021, 18, 100–106. [Google Scholar] [CrossRef]


| Compound | Category | Mode of Action | Clinical Phase * | Disease * |
|---|---|---|---|---|
| Etoxomir | Inhibitor of fatty acid oxidation | Blocks CPT-1a | - | - |
| UK-5099 | Inhibitor of pyruvate catabolism | Inhibitor of MPC | - | - |
| BPTES | Inhibitor of glutamine catabolism | Inhibits glutaminase | - | - |
| FCCP | OXPHOS inhibitor | Complex V inhibitor | - | - |
| Antimycin A | OXPHOS inhibitor, ROS inducer | Complex III inhibitor | - | - |
| Menadione | ROS inducer | Redox cycling | I | Dermatologic neoplasms |
| DMNQ | ROS inducer | Redox cycling | - | - |
| PQ | ROS inducer | Redox cycling | - | - |
| H2O2 | ROS inducer | Reacts directly with biomolecules | I/II | Breast cancer |
| Elesclomol | OXPHOS inhibitor, ROS inducer, cuproptosis inducer | Uncoupler of OXPHOS; transports copper to mitochondria | III | Melanoma, ovarian cancer, peritoneal cancer |
| Disulfiram | I/II | Melanoma, GBM, breast, prostate cancer, myeloma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scutigliani, E.M.; van Hattum, J.; Lobo-Cerna, F.; Kruyswijk, J.; Myrcha, M.; Dekkers, F.E.G.A.; Hoebe, R.A.; Edwards, F.; Oppelaar, J.J.; Vogt, L.; et al. Perturbation of Copper Homeostasis Sensitizes Cancer Cells to Elevated Temperature. Int. J. Mol. Sci. 2024, 25, 423. https://doi.org/10.3390/ijms25010423
Scutigliani EM, van Hattum J, Lobo-Cerna F, Kruyswijk J, Myrcha M, Dekkers FEGA, Hoebe RA, Edwards F, Oppelaar JJ, Vogt L, et al. Perturbation of Copper Homeostasis Sensitizes Cancer Cells to Elevated Temperature. International Journal of Molecular Sciences. 2024; 25(1):423. https://doi.org/10.3390/ijms25010423
Chicago/Turabian StyleScutigliani, Enzo M., Jons van Hattum, Fernando Lobo-Cerna, Joanne Kruyswijk, Maja Myrcha, Frederique E. G. A. Dekkers, Ron A. Hoebe, Finn Edwards, Jetta J. Oppelaar, Liffert Vogt, and et al. 2024. "Perturbation of Copper Homeostasis Sensitizes Cancer Cells to Elevated Temperature" International Journal of Molecular Sciences 25, no. 1: 423. https://doi.org/10.3390/ijms25010423
APA StyleScutigliani, E. M., van Hattum, J., Lobo-Cerna, F., Kruyswijk, J., Myrcha, M., Dekkers, F. E. G. A., Hoebe, R. A., Edwards, F., Oppelaar, J. J., Vogt, L., Bootsma, S., Bijlsma, M. F., Picavet, D. I., Crezee, J., Oddens, J. R., de Reijke, T. M., & Krawczyk, P. M. (2024). Perturbation of Copper Homeostasis Sensitizes Cancer Cells to Elevated Temperature. International Journal of Molecular Sciences, 25(1), 423. https://doi.org/10.3390/ijms25010423








