Angiotensin-Converting Enzyme 2 (ACE2) Signaling in Pulmonary Arterial Hypertension: Underpinning Mechanisms and Potential Targeting Strategies
Abstract
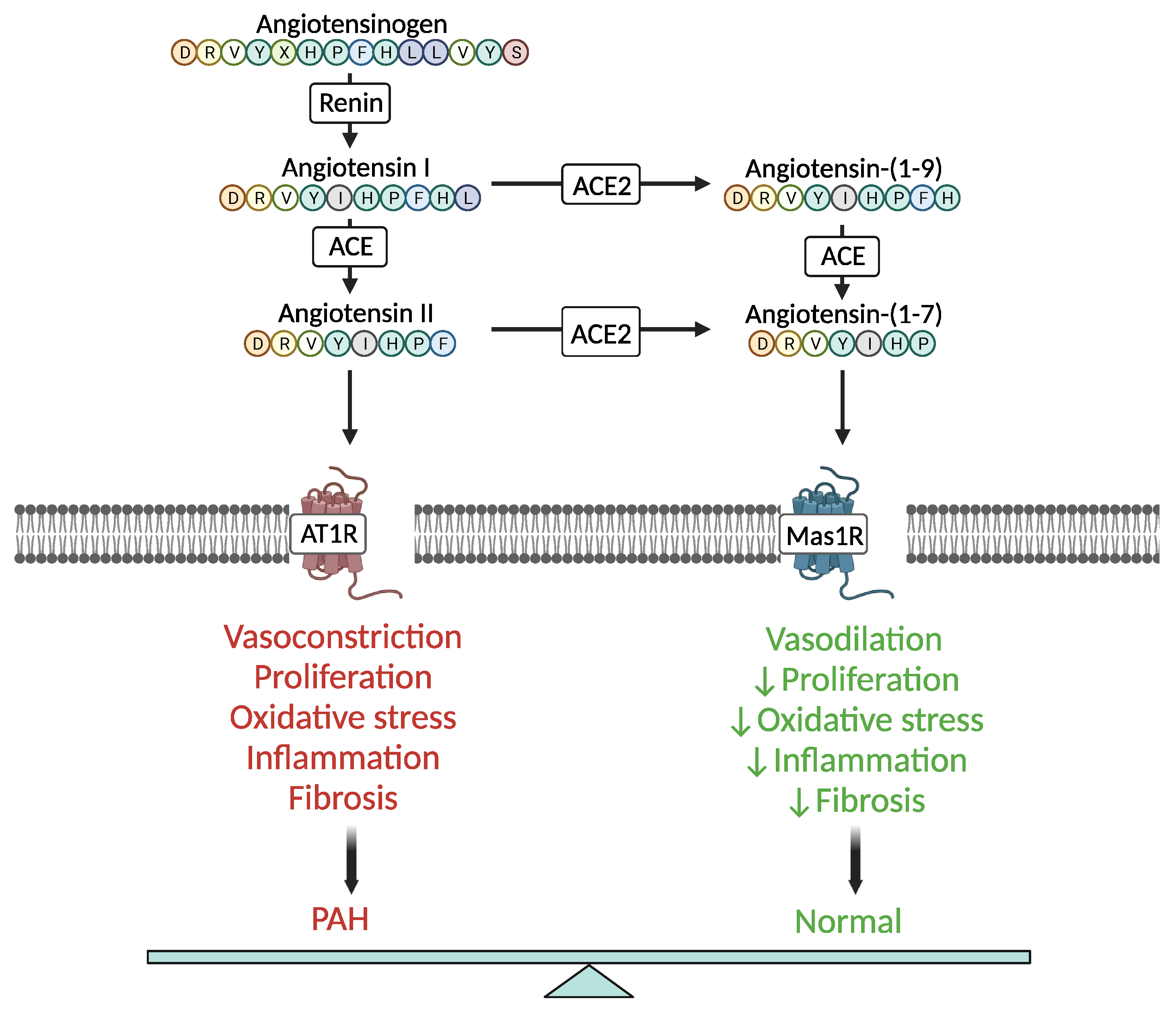
:1. Introduction
2. ACE2 Signaling in PAH Pathophysiology—Molecular Mechanisms
3. Clinical Relevance of ACE2 Signaling in PAH—Potential as Therapeutic Target
4. Concluding Remarks—Challenges and Opportunities
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Ruopp, N.F.; Cockrill, B.A. Diagnosis and Treatment of Pulmonary Arterial Hypertension: A Review. JAMA 2022, 327, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Palazzini, M.; Manes, A. Pulmonary arterial hypertension: From the kingdom of the near-dead to multiple clinical trial meta-analyses. Eur. Heart J. 2010, 31, 2080–2086. [Google Scholar] [CrossRef] [PubMed]
- Thenappan, T.; Ormiston, M.L.; Ryan, J.J.; Archer, S.L. Pulmonary arterial hypertension: Pathogenesis and clinical management. BMJ 2018, 360, j5492. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.J.; Vorla, M.; Kalra, D.K. Molecular Pathways in Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2022, 23, 10001. [Google Scholar] [CrossRef] [PubMed]
- de Man, F.S.; Tu, L.; Handoko, M.L.; Rain, S.; Ruiter, G.; François, C.; Schalij, I.; Dorfmüller, P.; Simonneau, G.; Fadel, E.; et al. Dysregulated renin-angiotensin-aldosterone system contributes to pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2012, 186, 780–789. [Google Scholar] [CrossRef]
- de Man, F.S.; Handoko, M.L.; Guignabert, C.; Bogaard, H.J.; Vonk-Noordegraaf, A. Neurohormonal axis in patients with pulmonary arterial hypertension: Friend or foe? Am. J. Respir. Crit. Care Med. 2013, 187, 14–19. [Google Scholar] [CrossRef]
- Maron, B.A.; Leopold, J.A. Emerging Concepts in the Molecular Basis of Pulmonary Arterial Hypertension: Part II: Neurohormonal Signaling Contributes to the Pulmonary Vascular and Right Ventricular Pathophenotype of Pulmonary Arterial Hypertension. Circulation 2015, 131, 2079–2091. [Google Scholar] [CrossRef]
- Donoghue, M.; Hsieh, F.; Baronas, E.; Godbout, K.; Gosselin, M.; Stagliano, N.; Donovan, M.; Woolf, B.; Robison, K.; Jeyaseelan, R.; et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ. Res. 2000, 87, E1–E9. [Google Scholar] [CrossRef]
- Bader, M. ACE2, angiotensin-(1-7), and Mas: The other side of the coin. Pflugers Arch. 2013, 465, 79–85. [Google Scholar] [CrossRef]
- Mehta, P.K.; Griendling, K.K. Angiotensin II cell signaling: Physiological and pathological effects in the cardiovascular system. Am. J. Physiol. Cell Physiol. 2007, 292, C82–C97. [Google Scholar] [CrossRef] [PubMed]
- Morrell, N.W.; Danilov, S.M.; Satyan, K.B.; Morris, K.G.; Stenmark, K.R. Right ventricular angiotensin converting enzyme activity and expression is increased during hypoxic pulmonary hypertension. Cardiovasc. Res. 1997, 34, 393–403. [Google Scholar] [CrossRef]
- Orte, C.; Polak, J.M.; Haworth, S.G.; Yacoub, M.H.; Morrell, N.W. Expression of pulmonary vascular angiotensin-converting enzyme in primary and secondary plexiform pulmonary hypertension. J. Pathol. 2000, 192, 379–384. [Google Scholar] [CrossRef]
- Patten, D.A.; Lafleur, V.N.; Robitaille, G.A.; Chan, D.A.; Giaccia, A.J.; Richard, D.E. Hypoxia-inducible factor-1 activation in nonhypoxic conditions: The essential role of mitochondrial-derived reactive oxygen species. Mol. Biol. Cell. 2010, 21, 3247–3257. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G. Role of reactive oxygen species in angiotensin II-mediated renal growth, differentiation, and apoptosis. Antioxid. Redox Signal. 2005, 7, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ramires, F.J.; Weber, K.T. Fibrosis of atria and great vessels in response to angiotensin II or aldosterone infusion. Cardiovasc. Res. 1997, 35, 138–147. [Google Scholar] [CrossRef]
- Friedberg, M.K.; Cho, M.Y.; Li, J.; Assad, R.S.; Sun, M.; Rohailla, S.; Honjo, O.; Apitz, C.; Redington, A.N. Adverse biventricular remodeling in isolated right ventricular hypertension is mediated by increased transforming growth factor-beta1 signaling and is abrogated by angiotensin receptor blockade. Am. J. Respir. Cell Mol. Biol. 2013, 49, 1019–1028. [Google Scholar] [CrossRef]
- Santos, R.A.; Ferreira, A.J.; Simões E Silva, A.C. Recent advances in the angiotensin-converting enzyme 2-angiotensin(1-7)-Mas axis. Exp. Physiol. 2008, 93, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Ocaranza, M.P.; Jalil, J.E. Protective role of the ACE2/Ang-(1-9) Axis in cardiovascular remodeling. Int. J. Hypertens. 2012, 2012, 594361. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.A.; Simoes e Silva, A.C.; Maric, C.; Silva, D.M.; Machado, R.P.; de Buhr, I.; Heringer-Walther, S.; Pinheiro, S.V.; Lopes, M.T.; Bader, M.; et al. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc. Natl. Acad. Sci. USA 2003, 100, 8258–8263. [Google Scholar] [CrossRef]
- Shenoy, V.; Ferreira, A.J.; Qi, Y.; Fraga-Silva, R.A.; Diez-Freire, C.; Dooies, A.; Jun, J.Y.; Sriramula, S.; Mariappan, N.; Pourang, D.; et al. The angiotensin-converting enzyme 2/angiogenesis-(1-7)/Mas axis confers cardiopulmonary protection against lung fibrosis and pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2010, 182, 1065–1072. [Google Scholar] [CrossRef]
- McKinney, C.A.; Fattah, C.; Loughrey, C.M.; Milligan, G.; Nicklin, S.A. Angiotensin-(1-7) and angiotensin-(1-9): Function in cardiac and vascular remodelling. Clin. Sci. 2014, 126, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, J.; Xiao, M.; Wang, J.; Yao, F.; Zeng, W.; Yu, L.; Guan, Y.; Wei, W.; Peng, Z.; et al. Mesenchymal stem cell-derived microvesicles alleviate pulmonary arterial hypertension by regulating renin-angiotensin system. J. Am. Soc. Hypertens. 2018, 12, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, H.; Zhao, L.; Zhang, Y.; Yan, D.; Liu, Y. Angiotensin-converting enzyme 2 activation ameliorates pulmonary endothelial dysfunction in rats with pulmonary arterial hypertension through mediating phosphorylation of endothelial nitric oxide synthase. J. Am. Soc. Hypertens. 2017, 11, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Gomes, E.R.; Lara, A.A.; Almeida, P.W.; Guimarães, D.; Resende, R.R.; Campagnole-Santos, M.J.; Bader, M.; Santos, R.A.; Guatimosim, S. Angiotensin-(1-7) prevents cardiomyocyte pathological remodeling through a nitric oxide/guanosine 3′,5′-cyclic monophosphate-dependent pathway. Hypertension 2010, 55, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Li, G.; Zhang, Y.; Liu, Y. Angiotensin-converting enzyme 2 activation suppresses pulmonary vascular remodeling by inducing apoptosis through the Hippo signaling pathway in rats with pulmonary arterial hypertension. Clin. Exp. Hypertens. 2019, 41, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.H.; Zhang, R.F.; Dong, L.L.; Chen, E.G.; Ying, K.J. Overexpression of ACE2 prevents hypoxia-induced pulmonary hypertension in rats by inhibiting proliferation and immigration of PASMCs. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 3968–3980. [Google Scholar]
- Li, G.; Liu, Y.; Zhu, Y.; Liu, A.; Xu, Y.; Li, X.; Li, Z.; Su, J.; Sun, L. ACE2 activation confers endothelial protection and attenuates neointimal lesions in prevention of severe pulmonary arterial hypertension in rats. Lung 2013, 191, 327–336. [Google Scholar] [CrossRef]
- Zhang, R.; Su, H.; Ma, X.; Xu, X.; Liang, L.; Ma, G.; Shi, L. MiRNA let-7b promotes the development of hypoxic pulmonary hypertension by targeting ACE2. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 316, L547–L557. [Google Scholar] [CrossRef]
- Wang, R.; Xu, J.; Wu, J.; Gao, S.; Wang, Z. Angiotensin-converting enzyme 2 alleviates pulmonary artery hypertension through inhibition of focal adhesion kinase expression. Exp. Ther. Med. 2021, 22, 1165. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, J.; Martin, M.; He, M.; Gongol, B.; Marin, T.L.; Chen, L.; Shi, X.; Yin, Y.; Shang, F.; et al. AMP-activated Protein Kinase Phosphorylation of Angiotensin-Converting Enzyme 2 in Endothelium Mitigates Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2018, 198, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zhang, J.; Wang, C.; Jain, P.P.; Xiong, M.; Shi, X.; Lei, Y.; Chen, S.; Yin, Q.; Thistlethwaite, P.A.; et al. MDM2-Mediated Ubiquitination of Angiotensin-Converting Enzyme 2 Contributes to the Development of Pulmonary Arterial Hypertension. Circulation 2020, 142, 1190–1204. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Haga, S.; Ishizaka, Y.; Mimori, A. Autoantibodies to angiotensin-converting enzyme 2 in patients with connective tissue diseases. Arthritis Res. Ther. 2010, 12, R85. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, J.; Del Valle-Mondragón, L.; Masso, F.; Zayas, N.; Pulido, T.; Teijeiro, R.; Gonzalez-Pacheco, H.; Olmedo-Ocampo, R.; Sisniega, C.; Paez-Arenas, A.; et al. Angiotensin converting enzyme 2 and angiotensin (1-7) axis in pulmonary arterial hypertension. Eur. Respir. J. 2020, 56, 1902416. [Google Scholar] [CrossRef]
- Yuan, Y.M.; Luo, L.; Guo, Z.; Yang, M.; Ye, R.S.; Luo, C. Activation of renin-angiotensin-aldosterone system (RAAS) in the lung of smoking-induced pulmonary arterial hypertension (PAH) rats. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 249–253. [Google Scholar] [CrossRef]
- Shenoy, V.; Gjymishka, A.; Jarajapu, Y.P.; Qi, Y.; Afzal, A.; Rigatto, K.; Ferreira, A.J.; Fraga-Silva, R.A.; Kearns, P.; Douglas, J.Y.; et al. Diminazene attenuates pulmonary hypertension and improves angiogenic progenitor cell functions in experimental models. Am. J. Respir. Crit. Care Med. 2013, 187, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.C.; Lin, J.Y.; Liu, Y.C.; Chai, C.Y.; Yeh, J.L.; Hsu, J.H.; Wu, B.N.; Dai, Z.K. Angiotensin-Converting Enzyme 2 Activator Ameliorates Severe Pulmonary Hypertension in a Rat Model of Left Pneumonectomy Combined With VEGF Inhibition. Front. Med. 2021, 8, 619133. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xu, Y.L.; Ling, F.; Liu, A.J.; Wang, D.; Wang, Q.; Liu, Y.L. Angiotensin-converting enzyme 2 activation protects against pulmonary arterial hypertension through improving early endothelial function and mediating cytokines levels. Chin. Med. J. 2012, 125, 1381–1388. [Google Scholar]
- Dang, Z.; Su, S.; Jin, G.; Nan, X.; Ma, L.; Li, Z.; Lu, D.; Ge, R. Tsantan Sumtang attenuated chronic hypoxia-induced right ventricular structure remodeling and fibrosis by equilibrating local ACE-AngII-AT1R/ACE2-Ang1-7-Mas axis in rat. J. Ethnopharmacol. 2020, 250, 112470. [Google Scholar] [CrossRef]
- Chang, H.; Chang, C.Y.; Lee, H.J.; Chou, C.Y.; Chou, T.C. Magnolol ameliorates pneumonectomy and monocrotaline-induced pulmonary arterial hypertension in rats through inhibition of angiotensin II and endothelin-1 expression. Phytomedicine 2018, 51, 205–213. [Google Scholar] [CrossRef]
- Shenoy, V.; Kwon, K.C.; Rathinasabapathy, A.; Lin, S.; Jin, G.; Song, C.; Shil, P.; Nair, A.; Qi, Y.; Li, Q.; et al. Oral delivery of Angiotensin-converting enzyme 2 and Angiotensin-(1-7) bioencapsulated in plant cells attenuates pulmonary hypertension. Hypertension 2014, 64, 1248–1259. [Google Scholar] [CrossRef]
- Grobe, J.L.; Sigmund, C.D. Another reason to eat your greens: Cardiopulmonary protection by dietary delivery of angiotensin-converting enzyme-2 and angiotensin-(1-7) made in plants. Hypertension 2014, 64, 1182–1183. [Google Scholar] [CrossRef]
- Daniell, H.; Mangu, V.; Yakubov, B.; Park, J.; Habibi, P.; Shi, Y.; Gonnella, P.A.; Fisher, A.; Cook, T.; Zeng, L.; et al. Investigational new drug enabling angiotensin oral-delivery studies to attenuate pulmonary hypertension. Biomaterials 2020, 233, 119750. [Google Scholar] [CrossRef]
- Yamazato, Y.; Ferreira, A.J.; Hong, K.H.; Sriramula, S.; Francis, J.; Yamazato, M.; Yuan, L.; Bradford, C.N.; Shenoy, V.; Oh, S.P.; et al. Prevention of pulmonary hypertension by Angiotensin-converting enzyme 2 gene transfer. Hypertension 2009, 54, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Breitling, S.; Krauszman, A.; Parihar, R.; Walther, T.; Friedberg, M.K.; Kuebler, W.M. Dose-dependent, therapeutic potential of angiotensin-(1-7) for the treatment of pulmonary arterial hypertension. Pulm. Circ. 2015, 5, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.J.; Shenoy, V.; Yamazato, Y.; Sriramula, S.; Francis, J.; Yuan, L.; Castellano, R.K.; Ostrov, D.A.; Oh, S.P.; Katovich, M.J.; et al. Evidence for angiotensin-converting enzyme 2 as a therapeutic target for the prevention of pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2009, 179, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Haga, S.; Tsuchiya, H.; Hirai, T.; Hamano, T.; Mimori, A.; Ishizaka, Y. A novel ACE2 activator reduces monocrotaline-induced pulmonary hypertension by suppressing the JAK/STAT and TGF-β cascades with restored caveolin-1 expression. Exp. Lung Res. 2015, 41, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Hemnes, A.R.; Rathinasabapathy, A.; Austin, E.A.; Brittain, E.L.; Carrier, E.J.; Chen, X.; Fessel, J.P.; Fike, C.D.; Fong, P.; Fortune, N.; et al. A potential therapeutic role for angiotensin-converting enzyme 2 in human pulmonary arterial hypertension. Eur. Respir. J. 2018, 51, 1702638. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.A.; Hanrott, K.; Budd, D.C.; Torres, F.; Grünig, E.; Escribano-Subias, P.; Meseguer, M.L.; Halank, M.; Opitz, C.; Hall, D.A.; et al. An open-label, dose-escalation study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of single doses of GSK2586881 in participants with pulmonary arterial hypertension. Pulm. Circ. 2022, 12, e12024. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, C.P.; Wohlford-Lenane, C.; Yamaguchi, Y.; Prindle, T.; Fulton, W.B.; Wang, S.; McCray, P.B., Jr.; Chappell, M.; Hackam, D.J.; Jia, H. Attenuation of pulmonary ACE2 activity impairs inactivation of des-Arg9 bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration. Am. J. Physiol. Lung. Cell Mol. Physiol. 2018, 314, L17–L31. [Google Scholar] [CrossRef]
- Lambert, D.W.; Yarski, M.; Warner, F.J.; Thornhill, P.; Parkin, E.T.; Smith, A.I.; Hooper, N.M.; Turner, A.J. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J. Biol. Chem. 2005, 280, 30113–30119. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.C.; Karas, M.M.; Alves, M.; He, J.; de Kloet, A.D.; Krause, E.G.; Richards, E.M.; Bryant, A.J.; Raizada, M.K. ACE2 overexpression in corticotropin-releasing-hormone cells offers protection against pulmonary hypertension. Front. Neurosci. 2023, 17, 1223733. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.C.; Yang, T.; Li, J.; Sharma, R.K.; Karas, M.K.; Bryant, A.J.; de Kloet, A.D.; Krause, E.G.; Joe, B.; Richards, E.M.; et al. Fecal matter transplant from Ace2 overexpressing mice counteracts chronic hypoxia-induced pulmonary hypertension. Pulm. Circ. 2022, 12, e12015. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Oliveira, A.C.; Yang, T.; Karas, M.M.; Li, J.; Lobaton, G.O.; Aquino, V.P.; Robles-Vera, I.; de Kloet, A.D.; Krause, E.G.; et al. Gut Pathology and Its Rescue by ACE2 (Angiotensin-Converting Enzyme 2) in Hypoxia-Induced Pulmonary Hypertension. Hypertension 2020, 76, 206–216. [Google Scholar] [CrossRef]
- Chatterjee, P.; Gheblawi, M.; Wang, K.; Vu, J.; Kondaiah, P.; Oudit, G.Y. Interaction between the apelinergic system and ACE2 in the cardiovascular system: Therapeutic implications. Clin. Sci. 2020, 134, 2319–2336. [Google Scholar] [CrossRef]

| Effect | ACE2 Signaling-Dependent Molecular Mechanism | References |
|---|---|---|
| ↓SMC proliferation and migration in PA | ERK1/2 and JAK/STAT3 signaling inhibition | [22] |
| ↓Inflammation and oxidative stress | ↓mRNA levels of proinflammatory cytokines (IL-6, IL-1β, TNF-α) and NADPH subunit (gp91phox), ↑anti-inflammatory cytokine IL-10 | [21] |
| ↓Fibrosis | ↓mRNA levels of TGF-β and collagen fiber production | [21] |
| ↑NO | ACE2-mediated phosphorylation (Ser1177) and dephosphorylation (Thr495) of endothelial nitric oxide synthetase | [24] |
| Cardiomyocyte hypertrophy inhibition | Ang-(1-7)-induced upregulation of a nitric oxide/guanosine 3′,5′-cyclic monophosphate-dependent pathway | [25] |
| Pulmonary vascular remodeling suppression | ↑Apoptosis via Hippo signaling | [26] |
| ↑PASMC proliferation and migration | ↓ACE2 expression via HIF-1-dependent upregulation of microRNA let-7b | [29] |
| ↑PASMC apoptosis | ↓FAK signaling and ↑pro-apoptotic caspase-3 | [30] |
| ↑ACE2 protein stability and ↑Ang-(1-7) and NO levels | AMPK-related ACE2 phosphorylation at Ser680 | [31] |
| ↑ACE2 protein degradation | MDM2-mediated ACE2 ubiquitination at K788 | [32] |
| ↓ACE2 protein levels and activity | Autoantibodies against ACE2 | [33,34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papavassiliou, K.A.; Gogou, V.A.; Papavassiliou, A.G. Angiotensin-Converting Enzyme 2 (ACE2) Signaling in Pulmonary Arterial Hypertension: Underpinning Mechanisms and Potential Targeting Strategies. Int. J. Mol. Sci. 2023, 24, 17441. https://doi.org/10.3390/ijms242417441
Papavassiliou KA, Gogou VA, Papavassiliou AG. Angiotensin-Converting Enzyme 2 (ACE2) Signaling in Pulmonary Arterial Hypertension: Underpinning Mechanisms and Potential Targeting Strategies. International Journal of Molecular Sciences. 2023; 24(24):17441. https://doi.org/10.3390/ijms242417441
Chicago/Turabian StylePapavassiliou, Kostas A., Vassiliki A. Gogou, and Athanasios G. Papavassiliou. 2023. "Angiotensin-Converting Enzyme 2 (ACE2) Signaling in Pulmonary Arterial Hypertension: Underpinning Mechanisms and Potential Targeting Strategies" International Journal of Molecular Sciences 24, no. 24: 17441. https://doi.org/10.3390/ijms242417441
APA StylePapavassiliou, K. A., Gogou, V. A., & Papavassiliou, A. G. (2023). Angiotensin-Converting Enzyme 2 (ACE2) Signaling in Pulmonary Arterial Hypertension: Underpinning Mechanisms and Potential Targeting Strategies. International Journal of Molecular Sciences, 24(24), 17441. https://doi.org/10.3390/ijms242417441








