Silicon-Selenium Interplay Imparts Cadmium Resistance in Wheat through an Up-Regulating Antioxidant System
Abstract
:1. Introduction
2. Results
2.1. Effects of Se-Si-Cd on Wheat Physiological Growth
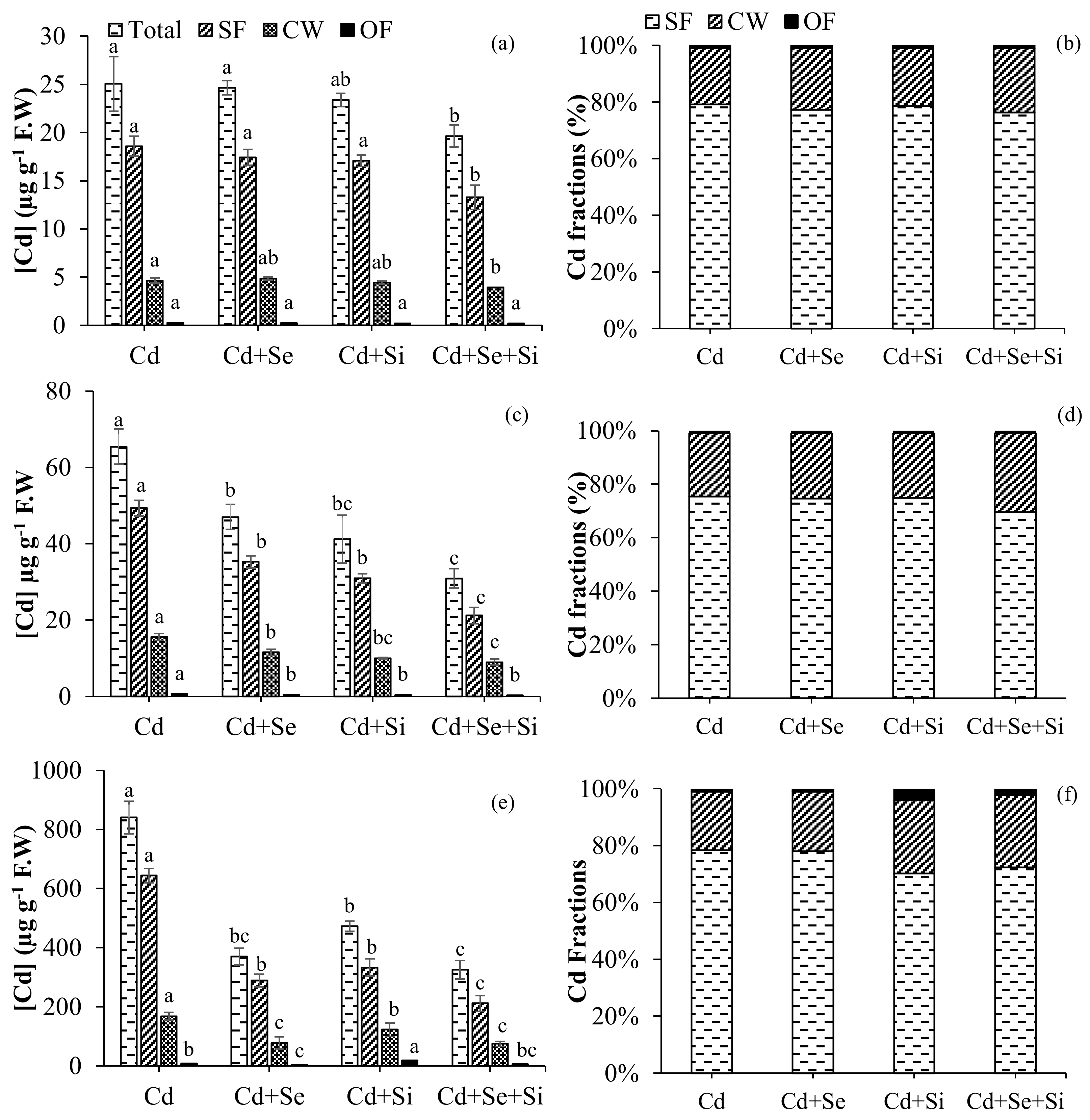
2.2. Cd Uptake Characteristics in Different Plant Components
2.2.1. Cd Concentration in Root/Shoot
2.2.2. Subcellular Distribution of Cd
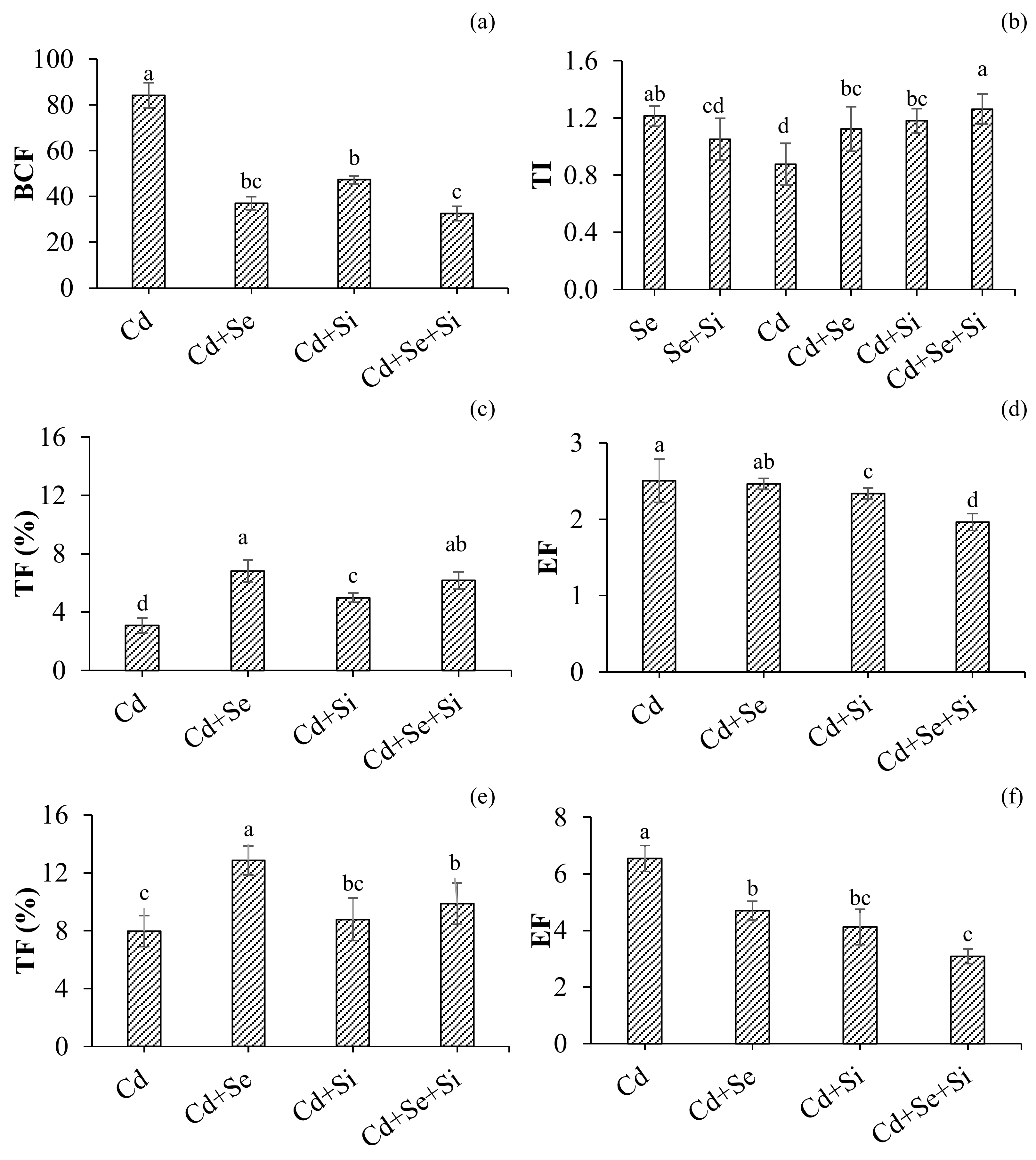
2.2.3. Cd Tolerance and Uptake Characteristics
2.3. Se-Si Interaction on Wheat Elemental and Nutritional Composition under Cd Stress
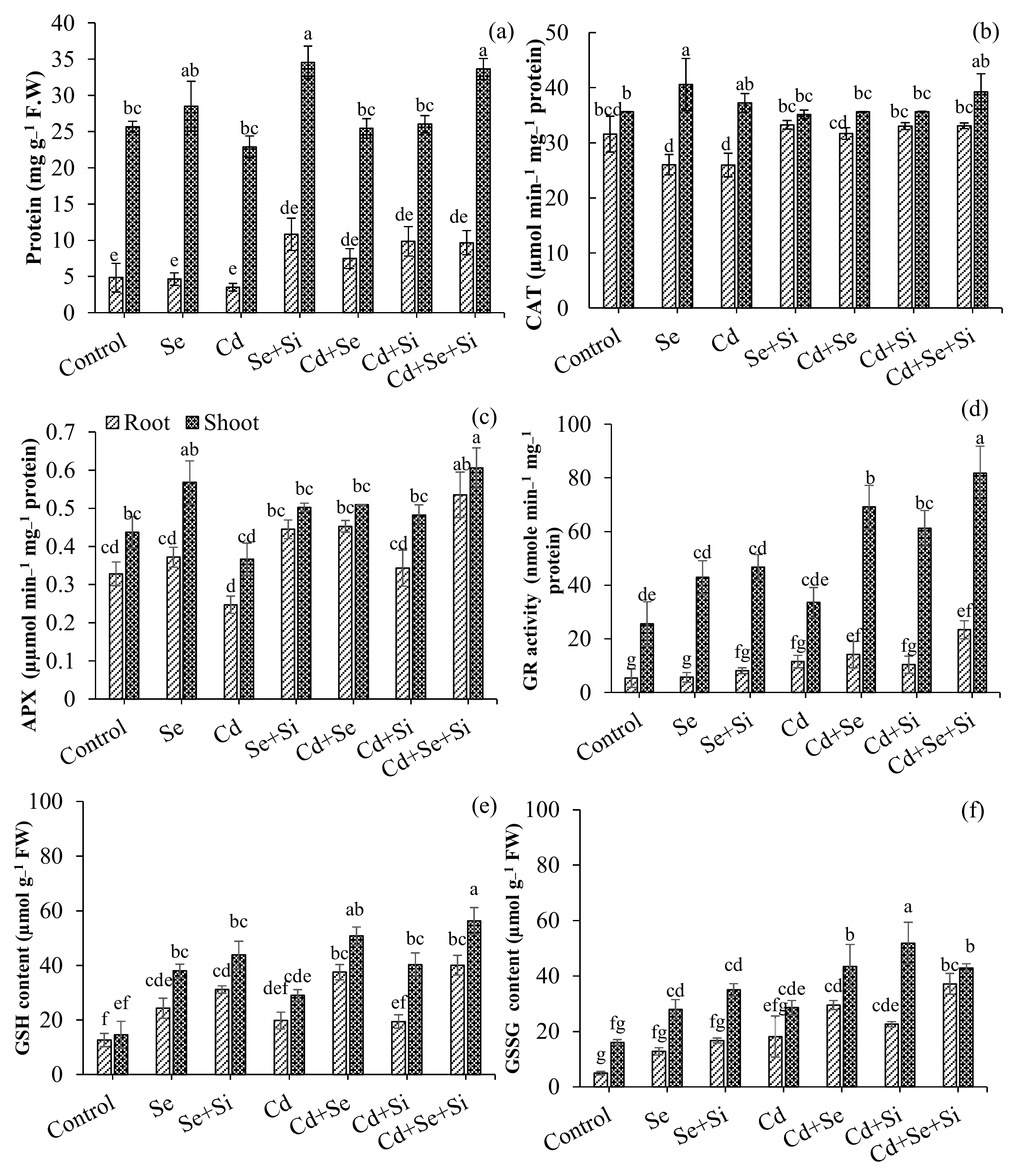
2.4. Se-Si Reduces Cd-Induced Toxicity and Regulates Antioxidant Enzyme Activities
3. Discussion
3.1. Cd Uptake Characteristics
3.2. Nutrient Composition and Isonomic Interactions
3.3. Activation of Antioxidant Resistance
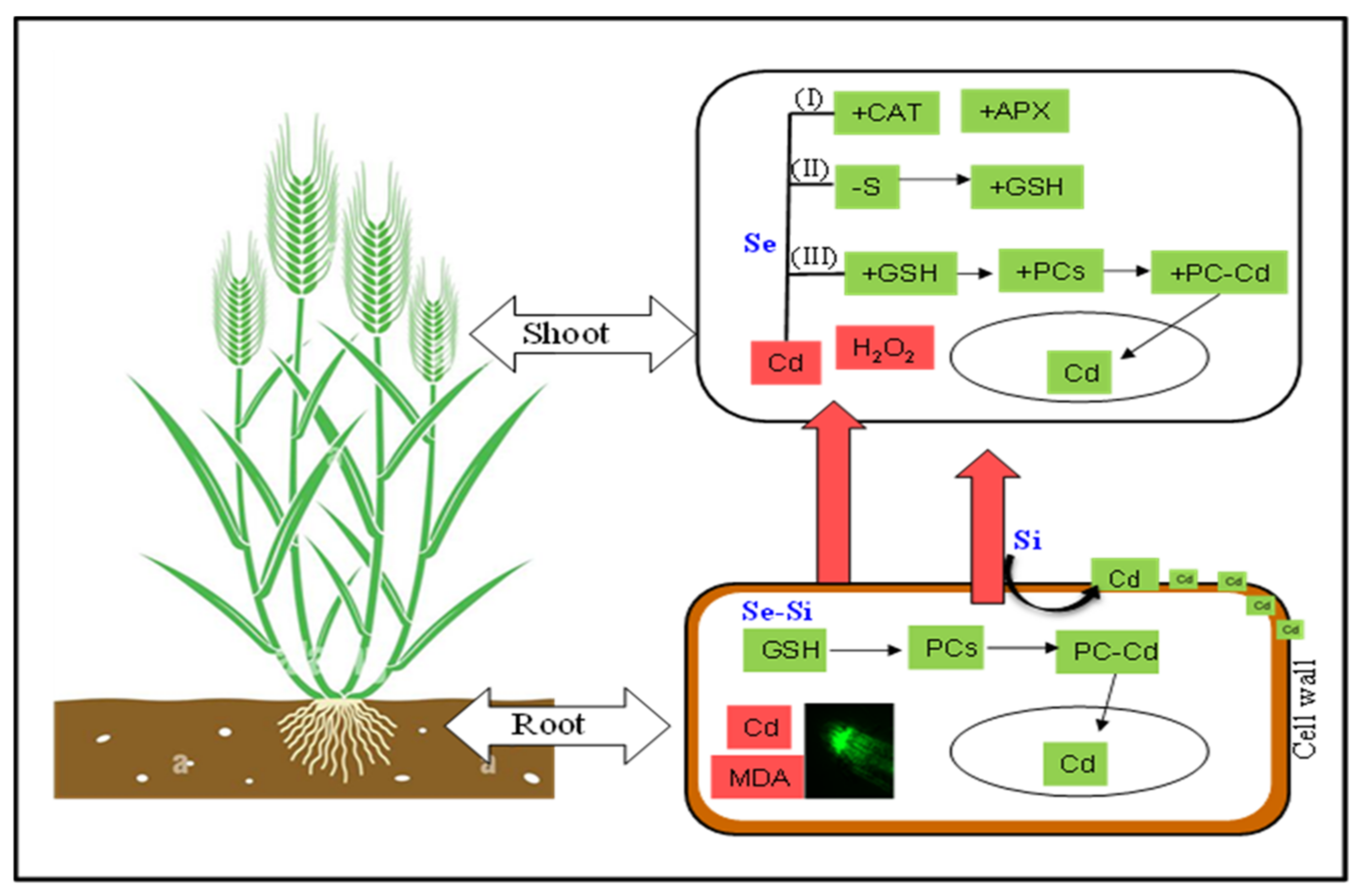
3.4. Mechanistic Insight in to the Combined Effect of Se and Si
4. Materials and Methods
4.1. Plant Culture and Experimental Design
4.2. Determination of Photosynthetic Attributes and Physiological Parameters
4.3. Subcellular Distribution of Cd
4.4. Elemental Analysis and Cd Uptake Characteristics
4.5. Localization of Cd and ROS
4.6. Estimation of ROS and Antioxidative Response
4.7. Determination of Antioxidant Enzyme Activity
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clemens, S.; Ma, J.F. Toxic heavy metal and metalloid accumulation in crop plants and foods. Annu. Rev. Plant Biol. 2016, 67, 489–512. [Google Scholar] [CrossRef] [PubMed]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in soils and groundwater: A review. Appl. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef] [PubMed]
- Gay, S.; Louwagie, G.; Sammeth, F.; Ratinger, T.; Cristoiu, A.; Marechal, B.; Prosperi, P.; Rusco, E.; Terres, J.; Adhikari, K.; et al. Addressing Soil Degradation in EU Agriculture: Relevant Processes, Practices and Policies-Report on the project’Sustainable Agriculture and Soil Conservation (SoCo)’ (No. JRC50424). Jt. Res. Cent. Seville Site 2009, 1–32. [Google Scholar]
- Khan, M.A.; Khan, S.; Khan, A.; Alam, M. Soil contamination with cadmium, consequences and remediation using organic amendments. Sci. Total Environ. 2017, 601, 1591–1605. [Google Scholar] [CrossRef] [PubMed]
- Hamid, Y.; Tang, L.; Sohail, M.I.; Cao, X.; Hussain, B.; Aziz, M.Z.; Usman, M.; He, Z.L.; Yang, X. An explanation of soil amendments to reduce cadmium phytoavailability and transfer to food chain. Sci. Total Environ. 2019, 660, 80–96. [Google Scholar] [CrossRef] [PubMed]
- Khanam, R.; Kumar, A.; Nayak, A.K.; Shahid, M.; Tripathi, R.; Vijayakumar, S.; Bhaduri, D.; Kumar, U.; Mohanty, S.; Panneerselvam, P.; et al. Metal (loid) s (As, Hg, Se, Pb and Cd) in paddy soil: Bioavailability and potential risk to human health. Sci. Total Environ. 2020, 699, 134330. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; ur Rehman, M.Z.; Rinklebe, J.; Tsang, D.C.; Bashir, A.; Maqbool, A.; Tack, F.M.G.; Ok, Y.S. Cadmium phytoremediation potential of Brassica crop species: A review. Sci. Total Environ. 2018, 631, 1175–1191. [Google Scholar] [CrossRef]
- EFSA. Cadmium dietary exposure in the European population, Scientific report of European Food Safety Authority. EFSA J. 2012, 10, 2551. [Google Scholar] [CrossRef]
- C.R. EC No 488 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Cadmium in Foodstuffs. Available online: http://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32014R0488 (accessed on 23 January 2023).
- Vergine, M.; Aprile, A.; Sabella, E.; Genga, A.; Siciliano, M.; Rampino, P.; Lenucci, M.S.; Luvisi, A.; Bellis, L.D. Cadmium concentration in grains of durum wheat (Triticum turgidum L. subsp. durum). J. Agric. Food Chem. 2017, 65, 6240–6246. [Google Scholar] [CrossRef]
- Gao, M.; Zhou, J.; Liu, H.; Zhang, W.; Hu, Y.; Liang, J.; Zhou, J. Foliar spraying with silicon and selenium reduces cadmium uptake and mitigates cadmium toxicity in rice. Sci. Total Environ. 2018, 631, 1100–1108. [Google Scholar] [CrossRef]
- Phiri, F.P.; Ander, E.L.; Bailey, E.H.; Chilima, B.; Chilimba, A.D.; Gondwe, J.; Joy, E.J.; Kalimbira, A.A.; Kumssa, D.B.; Lark, R.M.; et al. The risk of selenium deficiency in Malawi is large and varies over multiple spatial scales. Sci. Rep. 2019, 9, 6566. [Google Scholar] [CrossRef] [PubMed]
- Hawrylak-Nowak, B. Comparative effects of selenite and selenate on growth and selenium accumulation in lettuce plants under hydroponic conditions. Plant Growth Regul. 2013, 70, 149–157. [Google Scholar] [CrossRef]
- Moghaddam, A.; Heller, R.A.; Sun, Q.; Seelig, J.; Cherkezov, A.; Seibert, L.; Hackler, J.; Seemann, P.; Diegmann, J.; Pilz, M.; et al. Selenium deficiency is associated with mortality risk from COVID-19. Nutrients 2020, 12, 2098. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, R.; Awasthi, S.; Srivastava, S.; Dwivedi, S.; Pilon-Smits, E.A.; Dhankher, O.P.; Tripathi, R.D. Understanding selenium metabolism in plants and its role as a beneficial element. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1937–1958. [Google Scholar] [CrossRef]
- Alyemeni, M.N.; Ahanger, M.A.; Wijaya, L.; Alam, P.; Bhardwaj, R.; Ahmad, P. Selenium mitigates cadmium-induced oxidative stress in tomato (Solanum lycopersicum L.) plants by modulating chlorophyll fluorescence, osmolyte accumulation, and antioxidant system. Protoplasma 2018, 255, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, M.; Rizwan, M.; Dai, Z.; Yuan, Y.; Hossain, M.M.; Cao, M.; Xiong, S.; Tu, S. Synergistic effect of silicon and selenium on the alleviation of cadmium toxicity in rice plants. J. Hazard. Mater. 2021, 401, 123393. [Google Scholar] [CrossRef]
- Wu, J.; Mock, H.P.; Giehl, R.F.; Pitann, B.; Mühling, K.H. Silicon decreases cadmium concentrations by modulating root endodermal suberin development in wheat plants. J. Hazard. Mater. 2019, 364, 581–590. [Google Scholar] [CrossRef]
- Wu, J.; Geilfus, C.M.; Pitann, B.; Mühling, K.H. Silicon-enhanced oxalate exudation contributes to alleviation of cadmium toxicity in wheat. Environ. Exp. Bot. 2016, 131, 10–18. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, P.; Nie, Z.; Shi, H.; Li, C.; Wang, Y.; Qin, S.; Qin, X.; Liu, H. Selenium supply alters the subcellular distribution and chemical forms of cadmium and the expression of transporter genes involved in cadmium uptake and translocation in winter wheat (Triticum aestivum). BMC Plant Biol. 2020, 20, 550. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, C.; Du, B.; Cui, H.; Fan, X.; Zhou, D.; Zhou, J. Soil and foliar applications of silicon and selenium effects on cadmium accumulation and plant growth by modulation of antioxidant system and Cd translocation: Comparison of soft vs. durum wheat varieties. J. Hazard. Mater. 2021, 402, 123546. [Google Scholar] [CrossRef]
- Clemens, S.; Aarts, M.G.; Thomine, S.; Verbruggen, N. Plant science: The key to preventing slow cadmium poisoning. Trends Plant Sci. 2013, 18, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Z.; Chen, S.J.; Li, N.Y.; Wang, Y.Y.; Kang, G.Z. Exogenous glutathione alleviates cadmium toxicity in wheat by influencing the absorption and translocation of cadmium. Bull. Environ. Contam. Toxicol. 2021, 107, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, S.; Zhao, J.; Wang, F.; Du, Y.; Zou, S.; Li, H.; Wen, D.; Huang, Y. Comparative responses to silicon and selenium in relation to antioxidant enzyme system and the glutathione-ascorbate cycle in flowering Chinese cabbage (Brassica campestris L. ssp. chinensis var. utilis) under cadmium stress. Environ. Exp. Bot. 2017, 133, 1–11. [Google Scholar] [CrossRef]
- Abdalla, M.A.; Mühling, K.H. Selenium Exerts an Intriguing Alteration of Primary and Secondary Plant Metabolites: Advances, Challenges, and Prospects. Crit. Rev. Plant Sci. 2023, 42, 34–52. [Google Scholar] [CrossRef]
- Ramos, S.J.; Rutzke, M.A.; Hayes, R.J.; Faquin, V.; Guilherme, L.R.G.; Li, L. Selenium accumulation in lettuce germplasm. Planta 2011, 233, 649–660. [Google Scholar] [CrossRef]
- Saeed, K.; Nisa, F.K.; Abdalla, M.A.; Mühling, K.H. The Interplay of Sulfur and Selenium Enabling Variations in Micronutrient Accumulation in Red Spinach. Int. J. Mol. Sci. 2023, 24, 12766. [Google Scholar] [CrossRef]
- Manzoor, M.; Shafiq, M.; Gul, I.; Kamboh, U.R.; Guan, D.X.; Alazba, A.A.; Tomforde, S.; Arshad, M. Enhanced lead phytoextraction and soil health restoration through exogenous supply of organic ligands: Geochemical modeling. J. Environ. Manag. 2023, 348, 119435. [Google Scholar] [CrossRef]
- Li, M.Q.; Hasan, M.K.; Li, C.X.; Ahammed, G.J.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Reiter, R.J.; Yu, J.Q.; Xu, M.X.; et al. Melatonin mediates selenium-induced tolerance to cadmium stress in tomato plants. J. Pineal Res. 2016, 61, 291–302. [Google Scholar] [CrossRef]
- Réthoré, E.; Ali, N.; Yvin, J.C.; Hosseini, S.A. Silicon regulates source to sink metabolic homeostasis and promotes growth of rice plants under sulfur deficiency. Int. J. Mol. Sci. 2020, 21, 3677. [Google Scholar] [CrossRef]
- Liu, H.; Jiao, Q.; Fan, L.; Jiang, Y.; Alyemeni, M.N.; Ahmad, P.; Chen, Y.; Zhu, M.; Liu, H.; Zhao, Y.; et al. Integrated physio-biochemical and transcriptomic analysis revealed mechanism underlying of Si-mediated alleviation to cadmium toxicity in wheat. J. Hhazard. Mater. 2023, 452, 131366. [Google Scholar] [CrossRef]
- Abid, R.; Manzoor, M.; De Oliveira, L.M.; da Silva, E.; Rathinasabapathi, B.; Rensing, C.; Mahmood, S.; Liu, X.; Ma, L.Q. Interactive effects of As, Cd and Zn on their uptake and oxidative stress in As-hyperaccumulator Pteris vittata. Environ. Pollut. 2019, 248, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, S.M.; Hasanuzzaman, M.; Bhuyan, M.B.; Parvin, K.; Fujita, M. Exogenous tebuconazole and trifloxystrobin regulates reactive oxygen species metabolism toward mitigating salt-induced damages in cucumber seedling. Plants 2019, 8, 428. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Meth Enzymol. 1984, 105, 121–126. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]


| Parameters ► | Dry Biomass | Shoot Length | Tillers | Chlorophyll | Photosynthesis | Transpiration |
|---|---|---|---|---|---|---|
| Treatments ▾ | (g) | (cm) | (No.) | Units | (µmol CO2 m−2 s−1) | (µmoles H2O2 m−2 s−1) |
| Control | c 115.6 ± 5.1 | b 79.75 ± 3.6 | e 26.8 ± 0.5 | c 47.8 ± 2.3 | b 18.2 ± 1.9 | b 3.4 ± 0.1 |
| Se | a 140.3 ± 8.2 | b 77.25 ± 7.9 | bcd 31.3 ± 1.0 | a 50.8 ± 2.4 | b 19.7 ± 2.5 | a 3.7 ± 0.3 |
| Cd | d 101.3 ± 9.7 | d 67.8 ± 5.5 | de 27.3 ± 1.4 | d 40.9 ± 1.7 | c 15.7 ± 1.4 | c 2.9 ± 0.6 |
| Se + Si | bc 121.5 ± 8.8 | a 87.5 ± 4.4 | cde 31 ± 1.9 | a 50.5 ± 1.6 | a 22.3 ± 0.7 | a 3.9 ± 0.1 |
| Cd + Se | abc 129.8 ± 4.1 | cd 71.5 ± 7.9 | abc 33.8 ± 1.9 | ab 49.8 ± 1.0 | b 18.2 ± 1.7 | a 3.8 ± 0.5 |
| Cd + Si | ab 136.5 ± 8.7 | ab 80.25 ± 5.6 | ab 34.3 ± 0.9 | bc 47.7 ± 1.0 | b 18.7 ± 1.6 | a 4.1 ± 0.3 |
| Cd + Se + Si | a 146 ± 7.4 | ab 80.25 ± 3.9 | a 35 ± 2.9 | ab 50.5 ± 1.5 | a 21.0 ± 1.6 | a 4.1 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzoor, M.; Abdalla, M.A.; Hussain, M.A.; Mühling, K.H. Silicon-Selenium Interplay Imparts Cadmium Resistance in Wheat through an Up-Regulating Antioxidant System. Int. J. Mol. Sci. 2024, 25, 387. https://doi.org/10.3390/ijms25010387
Manzoor M, Abdalla MA, Hussain MA, Mühling KH. Silicon-Selenium Interplay Imparts Cadmium Resistance in Wheat through an Up-Regulating Antioxidant System. International Journal of Molecular Sciences. 2024; 25(1):387. https://doi.org/10.3390/ijms25010387
Chicago/Turabian StyleManzoor, Maria, Muna Ali Abdalla, Md Arif Hussain, and Karl Hermann Mühling. 2024. "Silicon-Selenium Interplay Imparts Cadmium Resistance in Wheat through an Up-Regulating Antioxidant System" International Journal of Molecular Sciences 25, no. 1: 387. https://doi.org/10.3390/ijms25010387
APA StyleManzoor, M., Abdalla, M. A., Hussain, M. A., & Mühling, K. H. (2024). Silicon-Selenium Interplay Imparts Cadmium Resistance in Wheat through an Up-Regulating Antioxidant System. International Journal of Molecular Sciences, 25(1), 387. https://doi.org/10.3390/ijms25010387









