Applications of the Whole-Cell System in the Efficient Biosynthesis of Heme
Abstract
1. Introduction
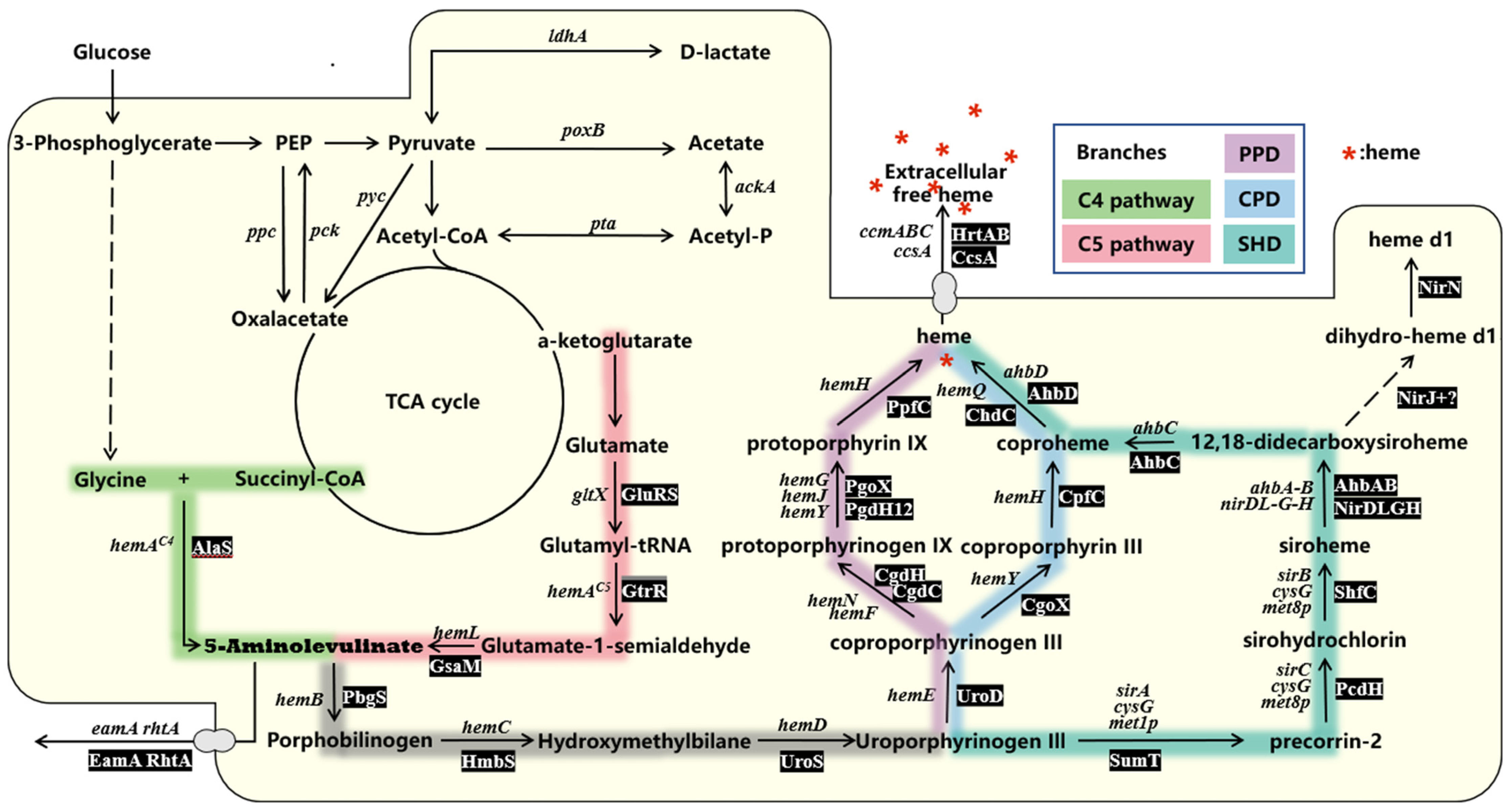
2. Natural Biosynthetic Pathway of Heme
2.1. Synthesis of ALA
| Abbreviation | Definition | E. coil | Pichia pastoris | Saccharomyces cerevisiae |
|---|---|---|---|---|
| AlaS | ALA synthase | HemAC4 | HEM1 | HEM1 |
| CgdC | Coproporphyrinogen decarboxylase | HemF | HEM13 | HEM13 |
| CgdH | Coproporphyrinogen dehydrogenase | HemN | / | / |
| CgoX | Coproporphyrinogen oxidase | HemY | / | / |
| ChdC | Coproheme decarboxylase | HemQ | / | / |
| ChdH | Coproheme dehydrogenase | / | / | / |
| CpfC | Coproporphyrin ferrochelatase | HemH | / | / |
| GluRS | Glutamyl-tRNA synthetase | GltX | / | / |
| GsaM | Glutamate-1-semialdehyde-2,1-aminomutase | HemL | / | / |
| GtrR | Glutamyl-tRNA reductase | HemAC5 | / | / |
| HmbS | Hydroxymethylbilane synthase | HemC | HEM3 | HEM3 |
| PbgS | Porphobilinogen synthase | HemB | HEM2 | HEM2 |
| PcdH | Precorrin-2 dehydrogenase | CysG | Met8p | SirC |
| PgdH1 | Protoporphyrinogen dehydrogenase | HemJ | / | / |
| PgdH2 | Protoporphyrinogen dehydrogenase | HemY | / | / |
| PgoX | Protoporphyrinogen IX oxidase | HemG | HEM14 | HEM14 |
| PpfC | Protoporphyrin ferrochelatase | HemH | HEM15 | HEM15 |
| ShfC | Sirohydrochlorin ferrochelatase | CysG | Met8p | SirB |
| SumT | S-adenosyl-L-methionine dependent uroporphyrinogen methyltransferase | CysG | Met1p | SirA |
| UroD | Uroporphyrinogen decarboxylase | HemE | HEM12 | HEM12 |
| UroS | Uroporphyrinogen synthase | HemD | HEM4 | HEM4 |
2.2. Formation of Uroporphyrinogen III
2.3. Three Downstream Heme Biosynthesis Pathways
3. Selection of Chassis Cells and Culture Methods
3.1. Selection of Strains for Metabolic Regulation
3.1.1. Escherichia coli
3.1.2. Other Common Strains
3.2. Optimization of Cultivation Conditions
3.2.1. Cultivation Conditions for ALA Production
3.2.2. Cultivation Conditions for Heme Production
4. Synthetic Biology Strategies to Enhance Heme Production
4.1. Enhances the Ability to Produce the Important Precursor ALA
4.1.1. Supplementation of the Substrate Succinyl CoA
4.1.2. Reduced Secretion of ALA to the Extracellular
4.1.3. Regulation in the Heme Biosynthesis Pathway
4.1.4. Other Regulations in TCA Cycle
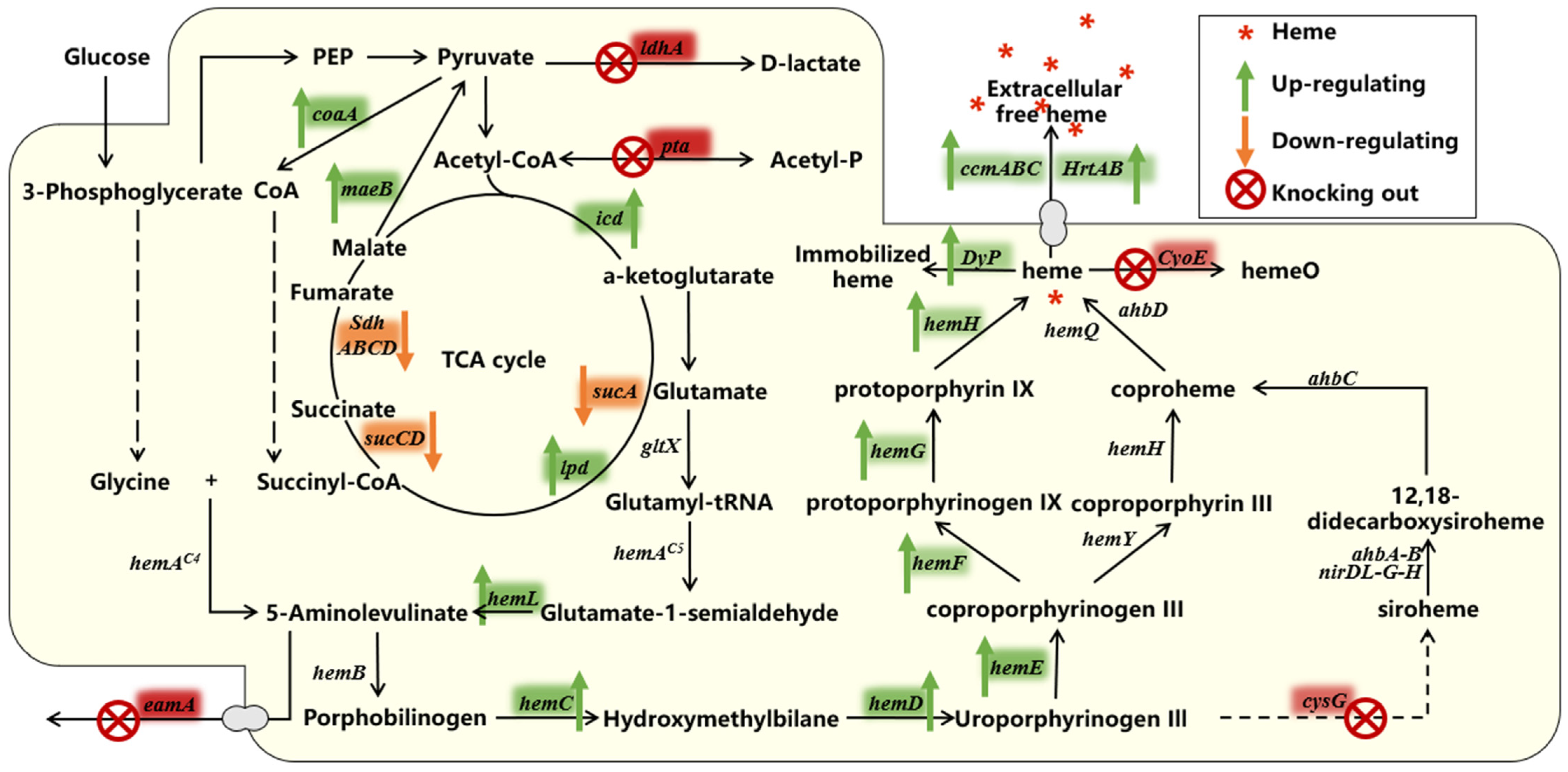
4.2. Removing Competitive Pathways
4.3. Enhance the Secretion of Intracellular Free Heme
| Categories | Microorganisms | Strategies | Ref | |
|---|---|---|---|---|
| Increase the titer of ALA | ||||
| Supplementation of the substrate succinyl CoA | E. coli | Overexpressing the maeB encoding MAE and the coaA encoding PANK to provide the precursor pyruvate for CoA and additional CoA for ALA synthesis, respectively | [75] | |
| Knocking out sucCD to block the conversion of succinyl CoA to other downstream metabolites. | [49] | |||
| Bacillus glutamicus | Overexpressing odhA-sucB-lpd encoding 2-oxo-tartaric acid dehydrogenase to catalyze the synthesis reaction of succinyl CoA. | [57] | ||
| C. glutamicum | Upregulating the expression of the upstream genes icd and pda of succinyl CoA while down-regulating the expression of the downstream genes sucCD and sdhABCD to amplify the source of succinyl CoA, reduce its consumption | [21] | ||
| Reduced secretion of ALA to the extracellular | E. coli | Knocking down eamA to reduce ALA secretion | [72] | |
| Regulation in the heme biosynthesis pathway | E. coli | Heterologously expressing DyP to immobilize heme and alleviate the feedback inhibition of ALA synthesis | [72] | |
| Enhancing expression of hemC, hemD, hemE in the downstream synthesis pathway of heme | [14,75] | |||
| Upregulating hemAs and hemL to promote ALA synthesis while reducing the feedback inhibition of heme | [12] | |||
| Other regulations in TCA cycle | E. coli | Eliminating sucA to block the TCA cycle result in accumulating α-ketoglutarate and thus allowing more carbon flux to flow to ALA production, | [58] | |
| C. glutamicum | Knocking down aceA and deleting gdhA to alleviate competition between the glyoxylate and TCA cycles and reduce the carbon flux to glutamate. | [21] | ||
| Removing competitive pathways | E. coli | Knocking out CysG and hemX to block the synthesis of Siroheme and knocking out CyoE to prevent conversion of heme to hemeO | [77] | |
| Knocking out of pta catalyzing acetate synthesis and ldhA, that catalyzing lactate synthesis to ensure a metabolic flux from glucose to l-glutamate | [78] | |||
| Deleting yfeX to prevent disruption of heme homeostasis | [79] | |||
| Enhance the secretion of intracellular free Heme | E. coli | Upregulating ccmABC encoding a potential heme exporter | [79] | |
| C. glutamicum | Overexpressing the HrtA and HrtB transporter | [54] | ||
| Disrupt the HtaA, HmuT and HrrS by the CRISPR-cas12a system to reduce the binding of heme to membrane proteins | [54] | |||
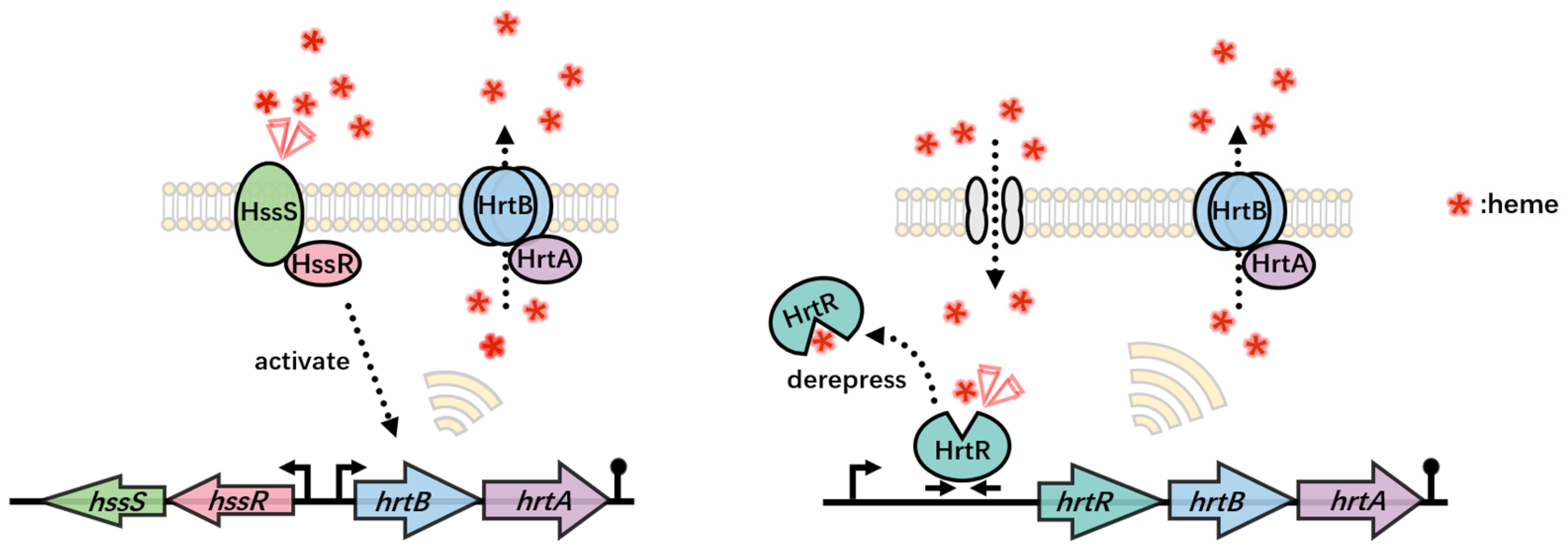
5. Whole-Cell Biosensor in Heme Production
5.1. Biosensor for ALA Detection
5.2. Common Heme Detection Systems in Bacteria
5.2.1. One-Component Systems
5.2.2. Two-Component Systems
5.3. Application of Heme Biosensor in Heme Production
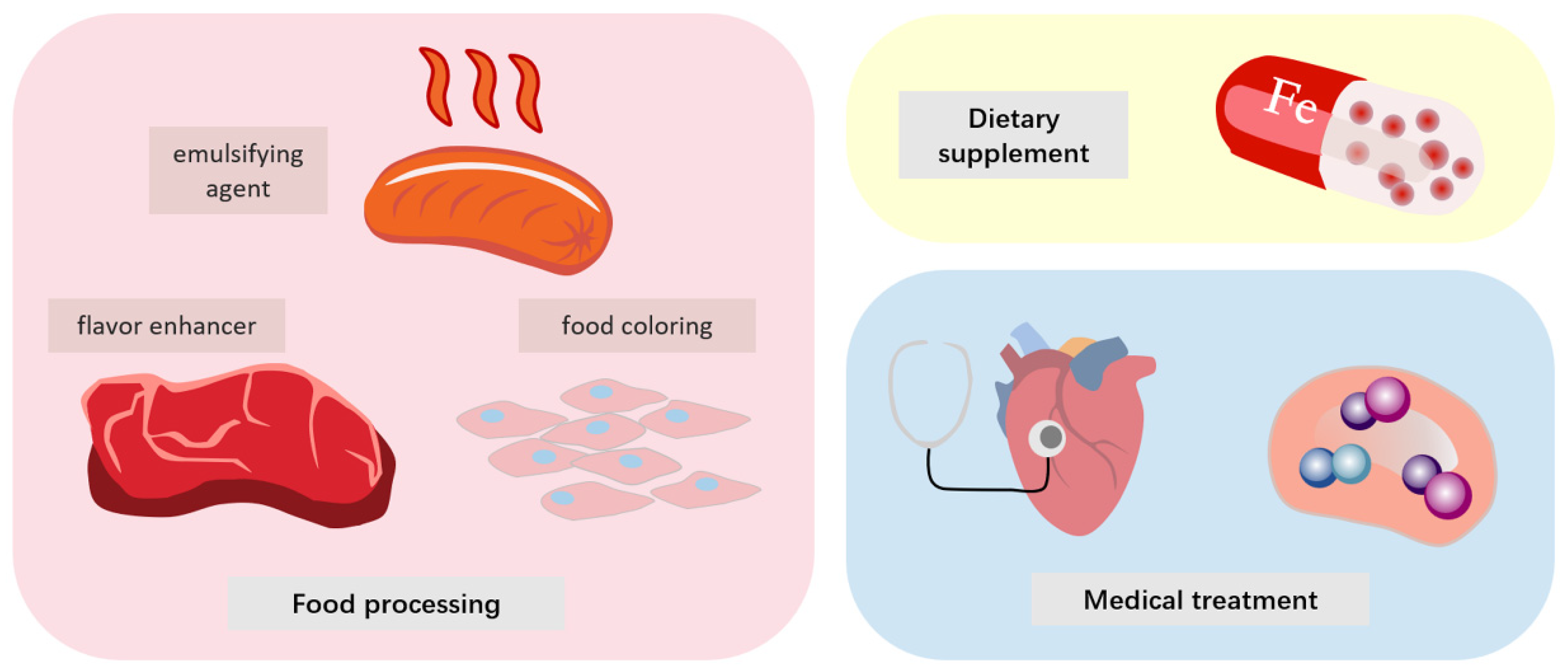
6. Application of Heme Protein and Its Derivatives in Various Fields
6.1. Medical
6.2. Dietary Supplements
6.3. Food Processing
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| AlaS | ALA synthase |
| ALA | 5-aminolevulinic acid |
| CBM | cell-based meat |
| CgdC | Coproporphyrinogen decarboxylase |
| CgdH | Coproporphyrinogen dehydrogenase |
| CgoX | Coproporphyrinogen oxidase |
| ChdC | Coproheme decarboxylase |
| ChdH | Coproheme dehydrogenase |
| CPIII | Coproporphyrinogen III |
| CPD | Coproporphyrin-dependent |
| CpfC | Coproporphyrin ferrochelatase |
| CoA | Coenzyme A |
| DBTL | Design-build-test-learning |
| DtxR | Diphtheria toxin repressor |
| DyP | Dye-decolorizing peroxidase |
| FACS | Fluorescence-activated cell sorting |
| GluRS | Glutamyl-tRNA synthetase |
| GsaM | Glutamate-1-semialdehyde-2,1-aminomutase |
| GtrR | Glutamyl-tRNA reductase |
| HmbS | Hydroxymethylbilane synthase |
| HO | Heme oxygenase |
| HPLC | High-Performance Liquid Chromatography |
| IDA | Iron deficiency anemia |
| MAE | Malic enzyme |
| NGS | Next-generation sequencing |
| PANK | Pantothenate kinase |
| PBF | Phytochrome-based fluorophore |
| PbgS | Porphobilinogen synthase |
| PBM | Plant-based meat |
| SfGFP | Super folder green fluorescent protein |
| TCA | Tricarboxylic acid |
| PAS | Per-Arnt-Sim |
| PcdH | Precorrin-2 dehydrogenase |
| PcyA | Phycocyanobilin: ferredoxin oxidoreductase |
| PgdH1 | Protoporphyrinogen dehydrogenase |
| PgdH2 | Protoporphyrinogen dehydrogenase |
| PgoX | Protoporphyrinogen IX oxidase |
| PPD | Protoporphyrin-dependent |
| PpfC | Protoporphyrin ferrochelatase |
| PPIX | Protoporphyrin IX |
| SHD | Siroheme-dependent |
| ShfC | Sirohydrochlorin ferrochelatase |
| SumT | S-adenosyl-L-methionine dependent uroporphyrinogen methyltransferase |
| TRNA | Transfer RNA |
| UroD | Uroporphyrinogen decarboxylase |
| UroS | Uroporphyrinogen synthase |
References
- Dailey, H.A. A Primer for Heme Biosynthesis. Biochim. Biophys. Acta (BBA) Bioenerg. 2022, 403, 1863. [Google Scholar]
- Hanna, D.A.; Martinez-Guzman, O.; Reddi, A.R. Heme Gazing: Illuminating Eukaryotic Heme Trafficking, Dynamics, and Signaling with Fluorescent Heme Sensors. Biochemistry 2017, 56, 1815–1823. [Google Scholar] [CrossRef] [PubMed]
- Reddi, A.R.; Hamza, I. Heme Mobilization in Animals: A Metallolipid’s Journey. Acc. Chem. Res. 2016, 49, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P.; Yoshimoto, F.K. Formation and Cleavage of C-C Bonds by Enzymatic Oxidation-Reduction Reactions. Chem. Rev. 2018, 118, 6573–6655. [Google Scholar] [CrossRef]
- Girvan, H.M.; Munro, A.W. Applications of Microbial Cytochrome P450 Enzymes in Biotechnology and Synthetic Biology. Curr. Opin. Chem. Biol. 2016, 31, 136–145. [Google Scholar] [CrossRef]
- Donegan, R.K.; Moore, C.M.; Hanna, D.A.; Reddi, A.R. Handling Heme: The Mechanisms Underlying the Movement of Heme within and between Cells. Free Radic. Biol. Med. 2019, 133, 88–100. [Google Scholar] [CrossRef]
- Shimizu, T.; Lengalova, A.; Martinek, V.; Martinkova, M. Heme: Emergent Roles of Heme in Signal Transduction, Functional Regulation and as Catalytic Centres. Chem. Soc. Rev. 2019, 48, 5624–5657. [Google Scholar] [CrossRef]
- Abdelazim, I.A.; Abu-Faza, M.; Shikanova, S.; Zhurabekova, G.; Maghrabi, M.M. Heme-Bound Iron in Treatment of Pregnancy-Associated Iron Deficiency Anemia. J. Family Med. Prim. Care 2018, 7, 1434–1438. [Google Scholar] [CrossRef]
- Mayfield, J.A.; Dehner, C.A.; DuBois, J.L. Recent Advances in Bacterial Heme Protein Biochemistry. Curr. Opin. Chem. Biol. 2011, 15, 260–266. [Google Scholar] [CrossRef]
- Fraser, R.Z.; Shitut, M.; Agrawal, P.; Mendes, O.; Klapholz, S. Safety Evaluation of Soy Leghemoglobin Protein Preparation Derived from Pichia Pastoris, Intended for Use as a Flavor Catalyst in Plant-Based Meat. Int. J. Toxicol. 2018, 37, 241–262. [Google Scholar] [CrossRef]
- Krintiras, G.A.; Gadea Diaz, J.; van der Goot, A.J.; Stankiewicz, A.I.; Stefanidis, G.D. On the Use of the Couette Cell Technology for Large Scale Production of Textured Soy-Based Meat Replacers. J. Food Eng. 2016, 169, 205–213. [Google Scholar] [CrossRef]
- Simsa, R.; Yuen, J.; Stout, A.; Rubio, N.; Fogelstrand, P.; Kaplan, D.L. Extracellular Heme Proteins Influence Bovine Myosatellite Cell Proliferation and the Color of Cell-Based Meat. Foods 2019, 8, 521. [Google Scholar] [CrossRef]
- Espinas, N.A.; Kobayashi, K.; Takahashi, S.; Mochizuki, N.; Masuda, T. Evaluation of Unbound Free Heme in Plant Cells by Differential Acetone Extraction. Plant Cell Physiol. 2012, 53, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- In, M.J.; Kim, D.C.; Chae, H.J.; Oh, N.S. Effects of Degree of Hydrolysis and Ph on the Solubility of Heme-Iron Enriched Peptide in Hemoglobin Hydrolysate. Biosci. Biotechnol. Biochem. 2003, 67, 365–367. [Google Scholar] [CrossRef]
- Geng, Z.; Ge, J.; Cui, W.; Zhou, H.; Deng, J.; Xu, B. Efficient De Novo Biosynthesis of Heme by Membrane Engineering in Escherichia Coli. Int. J. Mol. Sci. 2022, 23, 15524. [Google Scholar] [CrossRef] [PubMed]
- Jouhten, P. Metabolic Modelling in the Development of Cell Factories by Synthetic Biology. Comput. Struct. Biotechnol. J. 2012, 3, e201210009. [Google Scholar] [CrossRef] [PubMed]
- Filote, C.; Santos, S.C.R.; Popa, V.I.; Botelho, C.M.S.; Volf, I. Biorefinery of Marine Macroalgae into High-Tech Bioproducts: A Review. Environ. Chem. Lett. 2020, 19, 969–1000. [Google Scholar] [CrossRef]
- Singh, R.; Ryu, J.; Kim, S.W. An Overview on Methanotrophs and the Role of Methylosinus Trichosporium Ob3b for Biotechnological Applications. Biotechnol. Bioprocess Eng. 2022, 27, 468–481. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Lv, X.; Li, J.; Du, G.; Liu, L. Applications of Crispr in a Microbial Cell Factory: From Genome Reconstruction to Metabolic Network Reprogramming. ACS Synth. Biol. 2020, 9, 2228–2238. [Google Scholar] [CrossRef]
- Ge, B.; Chen, Y.; Yu, Q.; Lin, X.; Li, J.; Qin, S. Regulation of the Heme Biosynthetic Pathway for Combinational Biosynthesis of Phycocyanobilin in Escherichia Coli. Process Biochem. 2018, 71, 23–30. [Google Scholar] [CrossRef]
- Liu, L.; Martinez, J.L.; Liu, Z.; Petranovic, D.; Nielsen, J. Balanced Globin Protein Expression and Heme Biosynthesis Improve Production of Human Hemoglobin in Saccharomyces Cerevisiae. Metab. Eng. 2014, 21, 9–16. [Google Scholar] [CrossRef]
- Shao, Y.; Xue, C.; Liu, W.; Zuo, S.; Wei, P.; Huang, L.; Lian, J.; Xu, Z. High-Level Secretory Production of Leghemoglobin in Pichia Pastoris through Enhanced Globin Expression and Heme Biosynthesis. Bioresour. Technol. 2022, 363, 127884–127894. [Google Scholar] [CrossRef] [PubMed]
- Dailey, H.A.; Septer, A.N.; Daugherty, L.; Thames, D.; Gerdes, S.; Stabb, E.V.; Dunn, A.K.; Dailey, T.A.; Phillips, J.D. The Escherichia Coli Protein Yfex Functions as a Porphyrinogen Oxidase, Not a Heme Dechelatase. mBio 2011, 2, e00248-11. [Google Scholar] [CrossRef]
- Zhao, X.R.; Choi, K.R.; Lee, S.Y. Metabolic Engineering of Escherichia Coli for Secretory Production of Free Haem. Nat. Catal. 2018, 1, 720–728. [Google Scholar] [CrossRef]
- Igarashi, J.; Murase, M.; Iizuka, A.; Pichierri, F.; Martinkova, M.; Shimizu, T. Elucidation of the Heme Binding Site of Heme-Regulated Eukaryotic Initiation Factor 2alpha Kinase and the Role of the Regulatory Motif in Heme Sensing by Spectroscopic and Catalytic Studies of Mutant Proteins. J. Biol. Chem. 2008, 283, 18782–18791. [Google Scholar] [CrossRef]
- Shimizu, T.; Huang, D.; Yan, F.; Stranava, M.; Bartosova, M.; Fojtikova, V.; Martinkova, M. Gaseous O2, No, and Co in Signal Transduction: Structure and Function Relationships of Heme-Based Gas Sensors and Heme-Redox Sensors. Chem. Rev. 2015, 115, 6491–6533. [Google Scholar] [CrossRef]
- Kitanishi, K.; Shimonaka, M.; Unno, M. Characterization of a Cobalt-Substituted Globin-Coupled Oxygen Sensor Histidine Kinase from Anaeromyxobacter Sp. Fw109-5: Insights into Catalytic Regulation by Its Heme Coordination Structure. ACS Omega 2021, 6, 34912–34919. [Google Scholar] [CrossRef] [PubMed]
- Glanville, D.G.; Mullineaux-Sanders, C.; Corcoran, C.J.; Burger, B.T.; Imam, S.; Donohue, T.J.; Ulijasz, A.T. A High-Throughput Method for Identifying Novel Genes That Influence Metabolic Pathways Reveals New Iron and Heme Regulation in Pseudomonas Aeruginosa. Msystems 2021, 6, 1392–1408. [Google Scholar] [CrossRef]
- Martinkova, M.; Kitanishi, K.; Shimizu, T. Heme-Based Globin-Coupled Oxygen Sensors: Linking Oxygen Binding to Functional Regulation of Diguanylate Cyclase, Histidine Kinase, and Methyl-Accepting Chemotaxis. J. Biol. Chem. 2013, 288, 27702–27711. [Google Scholar] [CrossRef]
- Koreny, L.; Obornik, M.; Horakova, E.; Waller, R.F.; Lukes, J. The Convoluted History of Haem Biosynthesis. Biol. Rev. Camb. Philos. Soc. 2022, 97, 141–162. [Google Scholar] [CrossRef]
- Layer, G. Heme Biosynthesis in Prokaryotes. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118861–118874. [Google Scholar] [CrossRef] [PubMed]
- Kaufholz, A.L.; Hunter, G.A.; Ferreira, G.C.; Lendrihas, T.; Hering, V.; Layer, G.; Jahn, M.; Jahn, D. Aminolaevulinic Acid Synthase of Rhodobacter Capsulatus: High-Resolution Kinetic Investigation of the Structural Basis for Substrate Binding and Catalysis. Biochem. J. 2013, 451, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Zamarreño Beas, J.; Videira, M.A.M.; Saraiva, L.M. Regulation of Bacterial Haem Biosynthesis. Coord. Chem. Rev. 2022, 452, 241286–241302. [Google Scholar] [CrossRef]
- Ko, Y.J.; Joo, Y.C.; Hyeon, J.E.; Lee, E.; Lee, M.E.; Seok, J.; Kim, S.W.; Park, C.; Han, S.O. Biosynthesis of Organic Photosensitizer Zn-Porphyrin by Diphtheria Toxin Repressor (Dtxr)-Mediated Global Upregulation of Engineered Heme Biosynthesis Pathway in Corynebacterium Glutamicum. Sci. Rep. 2018, 8, 14460–14473. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhou, J.; Du, G.; Chen, J. Recent Advances in the Microbial Synthesis of Hemoglobin. Trends Biotechnol. 2021, 39, 286–297. [Google Scholar] [CrossRef]
- Richtova, J.; Sheiner, L.; Gruber, A.; Yang, S.M.; Koreny, L.; Striepen, B.; Obornik, M. Using Diatom and Apicomplexan Models to Study the Heme Pathway of Chromera Velia. Int. J. Mol. Sci. 2021, 22, 6495. [Google Scholar] [CrossRef]
- Jaffe, E.K. The Porphobilinogen Synthase Family of Metalloenzymes. Acta Crystallogr. Sect. D-Biol. Crystallogr. 2000, 56, 115–128. [Google Scholar] [CrossRef]
- Silva, P.J.; Ramos, M.J. Comparative Density Functional Study of Models for the Reaction Mechanism of Uroporphyrinogen Iii Synthase. J. Phys. Chem. B 2008, 112, 3144–3148. [Google Scholar] [CrossRef]
- Schubert, H.L.; Phillips, J.D.; Heroux, A.; Hill, C.P. Structure and Mechanistic Implications of a Uroporphyrinogen Iii Synthase-Product Complex. Biochemistry 2008, 47, 8648–8655. [Google Scholar] [CrossRef]
- Dailey, H.A.; Dailey, T.A.; Gerdes, S.; Jahn, D.; Jahn, M.; O’Brian, M.R.; Warren, M.J. Prokaryotic Heme Biosynthesis: Multiple Pathways to a Common Essential Product. Microbiol. Mol. Biol. Rev. 2017, 81, e00048-16. [Google Scholar] [CrossRef]
- Phillips, J.D. Heme Biosynthesis and the Porphyrias. Mol. Genet Metab. 2019, 128, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Masuda, T.; Tajima, N.; Wada, H.; Sato, N. Molecular Phylogeny and Intricate Evolutionary History of the Three Isofunctional Enzymes Involved in the Oxidation of Protoporphyrinogen Ix. Genome Biol. Evol. 2014, 6, 2141–2155. [Google Scholar] [CrossRef]
- Hoggins, M.; Dailey, H.A.; Hunter, C.N.; Reid, J.D. Direct Measurement of Metal Ion Chelation in the Active Site of Human Ferrochelatase. Biochemistry 2007, 46, 8121–8127. [Google Scholar] [CrossRef] [PubMed]
- Hansson, M.; Hederstedt, L. Bacillus Subtilis Hemy Is a Peripheral Membrane Protein Essential for Protoheme Ix Synthesis Which Can Oxidize Coproporphyrinogen Iii and Protoporphyrinogen Ix. J. Bacteriol. 1994, 176, 5962–5970. [Google Scholar] [CrossRef] [PubMed]
- Kuhner, M.; Haufschildt, K.; Neumann, A.; Storbeck, S.; Streif, J.; Layer, G. The Alternative Route to Heme in the Methanogenic Archaeon Methanosarcina Barkeri. Archaea 2014, 2014, 327637. [Google Scholar] [CrossRef]
- Celis, A.I.; DuBois, J.L. Substrate, Product, and Cofactor: The Extraordinarily Flexible Relationship between the Cde Superfamily and Heme. Arch. Biochem. Biophys. 2015, 574, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Lobo, S.A.; Lawrence, A.D.; Romao, C.V.; Warren, M.J.; Teixeira, M.; Saraiva, L.M. Characterisation of Desulfovibrio Vulgaris Haem B Synthase, a Radical Sam Family Member. Biochim. Biophys. Acta 2014, 1844, 1238–1247. [Google Scholar] [CrossRef]
- Lee, M.J.; Kim, H.J.; Lee, J.Y.; Kwon, A.S.; Jun, S.Y.; Kang, S.H.; Kim, P. Effect of Gene Amplifications in Porphyrin Pathway on Heme Biosynthesis in a Recombinant Escherichia Coli. J. Microbiol. Biotechnol. 2013, 23, 668–673. [Google Scholar] [CrossRef]
- Xue, J.; Zhou, J.; Li, J.; Du, G.; Chen, J.; Wang, M.; Zhao, X. Systematic Engineering of Saccharomyces Cerevisiae for Efficient Synthesis of Hemoglobins and Myoglobins. Bioresour. Technol. 2023, 370, 128556–128565. [Google Scholar] [CrossRef]
- Dietz, J.V.; Willoughby, M.M.; Piel, R.B., 3rd; Ross, T.A.; Bohovych, I.; Addis, H.G.; Fox, J.L.; Lanzilotta, W.N.; Dailey, H.A.; Wohlschlegel, J.A.; et al. Mitochondrial Contact Site and Cristae Organizing System (Micos) Machinery Supports Heme Biosynthesis by Enabling Optimal Performance of Ferrochelatase. Redox. Biol. 2021, 46, 102125–102137. [Google Scholar] [CrossRef]
- Ko, Y.J.; Kim, M.; You, S.K.; Shin, S.K.; Chang, J.; Choi, H.J.; Jeong, W.Y.; Lee, M.E.; Hwang, D.H.; Han, S.O. Animal-Free Heme Production for Artificial Meat in Corynebacterium Glutamicum Via Systems Metabolic and Membrane Engineering. Metab. Eng. 2021, 66, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, G.; Li, J.; Li, X.; Zhang, J. Effects of Metal Ions on Biomass and 5-Aminolevulinic Acid Production in Rhodopseudomonas Palustris Wastewater Treatment. Water Sci. Technol. 2016, 73, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Erskine, P.T.; Norton, E.; Cooper, J.B.; Lambert, R.; Coker, A.; Lewis, G.; Spencer, P.; Sarwar, M.; Wood, S.P.; Warren, M.J.; et al. X-ray Structure of 5-Aminolevulinic Acid Dehydratase from Escherichia Coli Complexed with the Inhibitor Levulinic Acid at 2.0 Angstrom Resolution. Biochemistry 1999, 38, 4266–4276. [Google Scholar] [CrossRef]
- Cui, Z.; Jiang, Z.; Zhang, J.; Zheng, H.; Jiang, X.; Gong, K.; Liang, Q.; Wang, Q.; Qi, Q. Stable and Efficient Biosynthesis of 5-Aminolevulinic Acid Using Plasmid-Free Escherichia Coli. J. Agric. Food Chem. 2019, 67, 1478–1483. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Liu, W.; Cheng, X.; Wang, J.; Wang, Q.; Qi, Q. A New Strategy for Production of 5-Aminolevulinic Acid in Recombinant Corynebacterium Glutamicum with High Yield. Appl. Environ. Microbiol. 2016, 82, 2709–2717. [Google Scholar] [CrossRef]
- Lee, M.J.; Chun, S.J.; Kim, H.J.; Kwon, A.S.; Jun, S.Y.; Kang, S.H.; Kim, P. Porphyrin Derivatives from a Recombinant Escherichia Coli Grown on Chemically Defined Medium. J. Microbiol. Biotechnol. 2012, 22, 1653–1658. [Google Scholar] [CrossRef]
- Li, F.; Wang, Y.; Gong, K.; Wang, Q.; Liang, Q.; Qi, Q. Constitutive Expression of Ryhb Regulates the Heme Biosynthesis Pathway and Increases the 5-Aminolevulinic Acid Accumulation in Escherichia Coli. FEMS Microbiol. Lett. 2014, 350, 209–215. [Google Scholar] [CrossRef]
- Pranawidjaja, S.; Choi, S.I.; Lay, B.W.; Kim, P. Analysis of Heme Biosynthetic Pathways in a Recombinant Escherichia Coli. J. Microbiol. Biotechnol. 2015, 25, 880–886. [Google Scholar] [CrossRef]
- Davy, A.M.; Kildegaard, H.F.; Andersen, M.R. Cell Factory Engineering. Cell Syst. 2017, 4, 262–275. [Google Scholar] [CrossRef]
- Han, Y.H.; Kim, G.; Seo, S.W. Programmable Synthetic Biology Tools for Developing Microbial Cell Factories. Curr. Opin. Biotechnol. 2023, 79, 102874–102885. [Google Scholar] [CrossRef]
- Ge, F.; Li, X.; Ge, Q.; Zhu, D.; Li, W.; Shi, F.; Chen, H. Modular Control of Multiple Pathways of Corynebacterium Glutamicum for 5-Aminolevulinic Acid Production. AMB Express 2021, 11, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Weng, H.; Du, G.; Chen, J.; Kang, Z. 5-Aminolevulinic Acid Production from Inexpensive Glucose by Engineering the C4 Pathway in Escherichia Coli. J. Ind. Microbiol. Biotechnol. 2017, 44, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Mundhada, H.; Schneider, K.; Christensen, H.B.; Nielsen, A.T. Engineering of High Yield Production of L-Serine in Escherichia Coli. Biotechnol. Bioeng. 2016, 113, 807–816. [Google Scholar] [CrossRef]
- Feng, C.; Pan, M.; Tang, L. 5-Aminolevulinic Acid Level and Dye-Decolorizing Peroxidase Expression Regulate Heme Synthesis in Escherichia Coli. Biotechnol. Lett. 2022, 44, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Wang, Y.; Gu, P.; Wang, Q.; Qi, Q. Engineering Escherichia Coli for Efficient Production of 5-Aminolevulinic Acid from Glucose. Metab. Eng. 2011, 13, 492–498. [Google Scholar] [CrossRef]
- Shih, I.T.; Yi, Y.C.; Ng, I.S. Plasmid-Free System and Modular Design for Efficient 5-Aminolevulinic Acid Production by Engineered Escherichia Coli. Appl. Biochem. Biotechnol. 2021, 193, 2858–2871. [Google Scholar] [CrossRef]
- Ko, Y.J.; You, S.K.; Kim, M.; Lee, E.; Shin, S.K.; Park, H.M.; Oh, Y.; Han, S.O. Enhanced Production of 5-Aminolevulinic Acid Via Flux Redistribution of Tca Cycle toward L-Glutamate in Corynebacterium Glutamicum. Biotechnol. Bioprocess Eng. 2019, 24, 915–923. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Y.; Fu, J.; Mao, Y.; Chen, T.; Zhao, X.; Wang, Z. Metabolic Engineering of Corynebacterium Glutamicum for Efficient Production of 5-Aminolevulinic Acid. Biotechnol. Bioeng. 2016, 113, 1284–1293. [Google Scholar] [CrossRef]
- Zhang, J.; Kang, Z.; Ding, W.; Chen, J.; Du, G. Integrated Optimization of the in Vivo Heme Biosynthesis Pathway and the in Vitro Iron Concentration for 5-Aminolevulinate Production. Appl. Biochem. Biotechnol. 2016, 178, 1252–1262. [Google Scholar] [CrossRef]
- Su, T.; Guo, Q.; Zheng, Y.; Liang, Q.; Wang, Q.; Qi, Q. Fine-Tuning of Hemb Using Crispri for Increasing 5-Aminolevulinic Acid Production in Escherichia Coli. Front. Microbiol. 2019, 10, 1731–1738. [Google Scholar] [CrossRef]
- Noh, M.H.; Lim, H.G.; Park, S.; Seo, S.W.; Jung, G.Y. Precise Flux Redistribution to Glyoxylate Cycle for 5-Aminolevulinic Acid Production in Escherichia Coli. Metab. Eng. 2017, 43, 1–8. [Google Scholar] [CrossRef]
- Vuoristo, K.S.; Mars, A.E.; Sangra, J.V.; Springer, J.; Eggink, G.; Sanders, J.P.; Weusthuis, R.A. Metabolic Engineering of the Mixed-Acid Fermentation Pathway of Escherichia Coli for Anaerobic Production of Glutamate and Itaconate. AMB Express 2015, 5, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Turlin, E.; Heuck, G.; Simoes Brandao, M.I.; Szili, N.; Mellin, J.R.; Lange, N.; Wandersman, C. Protoporphyrin (Ppix) Efflux by the Macab-Tolc Pump in Escherichia Coli. Microbiologyopen 2014, 3, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Frunzke, J.; Gatgens, C.; Brocker, M.; Bott, M. Control of Heme Homeostasis in Corynebacterium Glutamicum by the Two-Component System Hrrsa. J. Bacteriol. 2011, 193, 1212–1221. [Google Scholar] [CrossRef]
- Muraki, N.; Kitatsuji, C.; Ogura, M.; Uchida, T.; Ishimori, K.; Aono, S. Structural Characterization of Heme Environmental Mutants of Cghmut That Shuttles Heme Molecules to Heme Transporters. Int. J. Mol. Sci. 2016, 17, 829. [Google Scholar] [CrossRef]
- Keppel, M.; Davoudi, E.; Gatgens, C.; Frunzke, J. Membrane Topology and Heme Binding of the Histidine Kinases Hrrs and Chrs in Corynebacterium Glutamicum. Front. Microbiol. 2018, 9, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Longeville, S.; Stingaciu, L.R. Hemoglobin Diffusion and the Dynamics of Oxygen Capture by Red Blood Cells. Sci. Rep. 2017, 7, 10448–100461. [Google Scholar] [CrossRef]
- Jeney, V.; Balla, J.; Yachie, A.; Varga, Z.; Vercellotti, G.M.; Eaton, J.W.; Balla, G. Pro-Oxidant and Cytotoxic Effects of Circulating Heme. Blood 2002, 100, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Kristinsson, H.G.; Rasco, B.A. Fish Protein Hydrolysates: Production, Biochemical, and Functional Properties. Crit. Rev. Food Sci. Nutr. 2000, 40, 43–81. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, H.; Lin, Z.; Ye, T.; Xu, D.; Zeng, Q. Heme in Cardiovascular Diseases: A Ubiquitous Dangerous Molecule Worthy of Vigilance. Front. Cell Dev. Biol. 2021, 9, 781839–781851. [Google Scholar] [CrossRef]
- Moraskie, M.; Roshid, M.H.O.; O’Connor, G.; Dikici, E.; Zingg, J.M.; Deo, S.; Daunert, S. Microbial Whole-Cell Biosensors: Current Applications, Challenges, and Future Perspectives. Biosens. Bioelectron. 2021, 191, 113359–113371. [Google Scholar] [CrossRef]
- Chung, S.-Y.; Seo, K.-H.; Rhee, J.I. Influence of Culture Conditions on the Production of Extra-Cellular 5-Aminolevulinic Acid (Ala) by Recombinant E. Coli. Process Biochem. 2005, 40, 385–394. [Google Scholar] [CrossRef]
- Tan, S.I.; You, S.C.; Shih, I.T.; Ng, I.S. Quantification, Regulation and Production of 5-Aminolevulinic Acid by Green Fluorescent Protein in Recombinant Escherichia Coli. J. Biosci. Bioeng. 2020, 129, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, J.Z.; Rao, Z.M.; Zhang, W.G. An Enzymatic Colorimetric Whole-Cell Biosensor for High-Throughput Identification of Lysine Overproducers. Biosens. Bioelectron. 2022, 216, 114681–114693. [Google Scholar] [CrossRef]
- Saillant, V.; Lipuma, D.; Ostyn, E.; Joubert, L.; Boussac, A.; Guerin, H.; Brandelet, G.; Arnoux, P.; Lechardeur, D. A Novel Enterococcus Faecalis Heme Transport Regulator (Fhtr) Senses Host Heme to Control Its Intracellular Homeostasis. mBio 2021, 12, 1–16. [Google Scholar] [CrossRef]
- Sawai, H.; Yamanaka, M.; Sugimoto, H.; Shiro, Y.; Aono, S. Structural Basis for the Transcriptional Regulation of Heme Homeostasis in Lactococcus Lactis. J. Biol. Chem. 2012, 287, 30755–30768. [Google Scholar] [CrossRef] [PubMed]
- Lechardeur, D.; Cesselin, B.; Liebl, U.; Vos, M.H.; Fernandez, A.; Brun, C.; Gruss, A.; Gaudu, P. Discovery of Intracellular Heme-Binding Protein Hrtr, Which Controls Heme Efflux by the Conserved Hrtb-Hrta Transporter in Lactococcus Lactis. J. Biol. Chem. 2012, 287, 4752–4758. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.J.; Stauff, D.L.; Pishchany, G.; Bezbradica, J.S.; Gordy, L.E.; Iturregui, J.; Anderson, K.L.; Dunman, P.M.; Joyce, S.; Skaar, E.P. A Staphylococcus Aureus Regulatory System That Responds to Host Heme and Modulates Virulence. Cell Host. Microbe 2007, 1, 109–119. [Google Scholar] [CrossRef]
- Stauff, D.L.; Torres, V.J.; Skaar, E.P. Signaling and DNA-Binding Activities of the Staphylococcus Aureus Hssr-Hsss Two-Component System Required for Heme Sensing. J. Biol. Chem. 2007, 282, 26111–26121. [Google Scholar] [CrossRef]
- Stauff, D.L.; Skaar, E.P. The Heme Sensor System of Staphylococcus Aureus. Contrib. Microbiol. 2009, 16, 120–135. [Google Scholar]
- Skalova, T.; Lengalova, A.; Dohnalek, J.; Harlos, K.; Mihalcin, P.; Kolenko, P.; Stranava, M.; Blaha, J.; Shimizu, T.; Martínková, M. Disruption of the Dimerization Interface of the Sensing Domain in the Dimeric Heme-Based Oxygen Sensor Afgchk Abolishes Bacterial Signal Transduction. J. Biol. Chem. 2020, 295, 1587–1597. [Google Scholar] [CrossRef]
- Stauff, D.L.; Skaar, E.P. Bacillus Anthracis Hssrs Signalling to Hrtab Regulates Haem Resistance During Infection. Mol. Microbiol. 2009, 72, 763–778. [Google Scholar] [CrossRef]
- Schmidt, R.M.; Carter, M.M.; Chu, M.L.; Latario, C.J.; Stadler, S.K.; Stauff, D.L. Heme Sensing in Bacillus Thuringiensis: A Supplementary Hssrs-Regulated Heme Resistance System. FEMS Microbiol. Lett. 2016, 363, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Leung, G.C.; Fung, S.S.; Gallio, A.E.; Blore, R.; Alibhai, D.; Raven, E.L.; Hudson, A.J. Unravelling the Mechanisms Controlling Heme Supply and Demand. Proc. Natl. Acad. Sci. USA 2021, 118, e2104008118. [Google Scholar] [CrossRef]
- Hu, B.; Yu, H.; Zhou, J.; Li, J.; Chen, J.; Du, G.; Lee, S.Y.; Zhao, X. Whole-Cell P450 Biocatalysis Using Engineered Escherichia Coli with Fine-Tuned Heme Biosynthesis. Adv. Sci. 2022, 10, e2205580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Z.; Su, T.; Sun, H.; Zhu, Y.; Qi, Q.; Wang, Q. Tuning the Binding Affinity of Heme-Responsive Biosensor for Precise and Dynamic Pathway Regulation. iScience 2020, 23, 101067–101075. [Google Scholar] [CrossRef]
- Mike, L.A.; Dutter, B.F.; Stauff, D.L.; Moore, J.L.; Vitko, N.P.; Aranmolate, O.; Kehl-Fie, T.E.; Sullivan, S.; Reid, P.R.; DuBois, J.L.; et al. Activation of Heme Biosynthesis by a Small Molecule That Is Toxic to Fermenting Staphylococcus Aureus. Proc. Natl. Acad. Sci. USA 2013, 110, 8206–8211. [Google Scholar] [CrossRef] [PubMed]
- Ishchuk, O.P.; Domenzain, I.; Sanchez, B.J.; Muniz-Paredes, F.; Martinez, J.L.; Nielsen, J.; Petranovic, D. Genome-Scale Modeling Drives 70-Fold Improvement of Intracellular Heme Production in Saccharomyces Cerevisiae. Proc. Natl. Acad. Sci. USA 2022, 119, e2108245119. [Google Scholar] [CrossRef]
- Mans, R.; van Rossum, H.M.; Wijsman, M.; Backx, A.; Kuijpers, N.G.; van den Broek, M.; Daran-Lapujade, P.; Pronk, J.T.; van Maris, A.J.; Daran, J.M. Crispr/Cas9: A Molecular Swiss Army Knife for Simultaneous Introduction of Multiple Genetic Modifications in Saccharomyces Cerevisiae. FEMS Yeast Res. 2015, 15, fov004. [Google Scholar] [CrossRef]
- Gamage, S.M.K.; Dissabandara, L.; Lam, A.K.; Gopalan, V. The Role of Heme Iron Molecules Derived from Red and Processed Meat in the Pathogenesis of Colorectal Carcinoma. Crit. Rev. Oncol. Hematol. 2018, 126, 121–128. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Zhao, L.; Yin, Y.; Wang, Q.; Zhou, H. Addition of Haptoglobin to Rbcs Storage, a New Strategy to Improve Quality of Stored Rbcs and Transfusion. Med. Hypotheses 2014, 82, 125–128. [Google Scholar] [CrossRef]
- Dei Zotti, F.; Lobysheva, I.I.; Balligand, J.L. Nitrosyl-Hemoglobin Formation in Rodent and Human Venous Erythrocytes Reflects No Formation from the Vasculature in Vivo. PLoS ONE 2018, 13, e0200352. [Google Scholar] [CrossRef]
- Zhang, R.; Hess, D.T.; Reynolds, J.D.; Stamler, J.S. Hemoglobin S-Nitrosylation Plays an Essential Role in Cardioprotection. J. Clin. Investig. 2016, 126, 4654–4658. [Google Scholar] [CrossRef]
- Tang, N.; Zhu, Y.; Zhuang, H. Antioxidant and Anti-Anemia Activity of Heme Iron Obtained from Bovine Hemoglobin. Food Sci. Biotechnol. 2015, 24, 635–642. [Google Scholar] [CrossRef]
- Fontes, P.R.; Gomide, L.A.; Fontes, E.A.; Ramos, E.M.; Ramos, A.L. Composition and Color Stability of Carbon Monoxide Treated Dried Porcine Blood. Meat. Sci. 2010, 85, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Xiaoqing, M.; Yang, L.; Yangying, S.; Daodong, P.; Jinxuan, C. Effect of Phosphorylated Nitroso Porcine Hemoglobin as Partial Nitrite Substitute on the Quality of Emulsified Sausage. Food Sci. 2022, 43, 46–52. [Google Scholar]
- Jin, Y.; He, X.; Andoh-Kumi, K.; Fraser, R.Z.; Lu, M.; Goodman, R.E. Evaluating Potential Risks of Food Allergy and Toxicity of Soy Leghemoglobin Expressed in Pichia Pastoris. Mol. Nutr. Food Res. 2018, 62, 1700297. [Google Scholar] [CrossRef]
- Viana, F.R.; Silva, V.D.; Delvivo, F.M.; Bizzotto, C.S.; Silvestre, M.P. Quality of Ham Pate Containing Bovine Globin and Plasma as Fat Replacers. Meat. Sci. 2005, 70, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Matsumoto, K.; Maruyama, T.; Otagiri, M. Strategy of Drug Development Based on the Bioactive Gas-Carrying Capacity of Hemoglobin. Yakugaku Zasshi-J. Pharm. Soc. Jpn. 2020, 140, 141–146. [Google Scholar] [CrossRef]
- Shayeghi, M.; Latunde-Dada, G.O.; Oakhill, J.S.; Laftah, A.H.; Takeuchi, K.; Halliday, N.; Khan, Y.; Warley, A.; McCann, F.E.; Hider, R.C.; et al. Identification of an Intestinal Heme Transporter. Cell 2005, 122, 789–801. [Google Scholar] [CrossRef]
- McKie, A.T.; Barrow, D.; Latunde-Dada, G.O.; Rolfs, A.; Sager, G.; Mudaly, E.; Mudaly, M.; Richardson, C.; Barlow, D.; Bomford, A.; et al. An Iron-Regulated Ferric Reductase Associated with the Absorption of Dietary Iron. Science 2001, 291, 1755–1759. [Google Scholar] [CrossRef]
- Hoppe, M.; Brun, B.; Larsson, M.P.; Moraeus, L.; Hulthen, L. Heme Iron-Based Dietary Intervention for Improvement of Iron Status in Young Women. Nutrition 2013, 29, 89–95. [Google Scholar] [CrossRef] [PubMed]
- West, A.R.; Oates, P.S. Mechanisms of Heme Iron Absorption: Current Questions and Controversies. World J. Gastroenterol. 2008, 14, 4101–4110. [Google Scholar] [CrossRef] [PubMed]
- Fontes, P.R.; Gomide, L.A.; Ramos, E.M.; Stringheta, P.C.; Parreiras, J.F. Color Evaluation of Carbon Monoxide Treated Porcine Blood. Meat. Sci. 2004, 68, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Terns, M.J.; Milkowski, A.L.; Claus, J.R.; Sindelar, J.J. Investigating the Effect of Incubation Time and Starter Culture Addition Level on Quality Attributes of Indirectly Cured, Emulsified Cooked Sausages. Meat. Sci. 2011, 88, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.T.; Li, P.J.; Kong, B.H.; Liu, Q.; Chang, Z.; Yang, H.H.; Jiang, Y.N. Application of Glycosylated Nitrosohemoglobin in Meat Batters: Color Formation and Antimicrobial Activity. Adv. Mater. Res. 2012, 554–556, 1057–1063. [Google Scholar] [CrossRef]

| Field | Categories | Application | Ref |
|---|---|---|---|
| Medical | Heme | Catalyzes the formation of nitroso-compounds | [100] |
| Hemoglobin | Biomimetic CO delivery system based on hemoglobin | [101] | |
| Quantitative monitoring of nitric oxide | [102] | ||
| Hemoglobin βCys93 | Cardioprotection | [103] | |
| Globin | Red blood cells storage | [101] | |
| Dietary supplements | Heme iron | Iron supplement | [104] |
| Food processing | Hemoglobin | Natural color enhancer | [105] |
| Coloration of cell-based meat. | [12] | ||
| Nitrosohemoglobin | Coloration of meat | [106] | |
| Glycosylated nitrosohemoglobin | Coloration of meat | [106] | |
| Soy leghemoglobin | Increase meat-like flavor of plant-based meat | [107] | |
| Globin | Emulsifier | [79] | |
| Fat substitute | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, H.; Chen, X.; Chen, S.; Guo, M.; Liu, H. Applications of the Whole-Cell System in the Efficient Biosynthesis of Heme. Int. J. Mol. Sci. 2023, 24, 8384. https://doi.org/10.3390/ijms24098384
Su H, Chen X, Chen S, Guo M, Liu H. Applications of the Whole-Cell System in the Efficient Biosynthesis of Heme. International Journal of Molecular Sciences. 2023; 24(9):8384. https://doi.org/10.3390/ijms24098384
Chicago/Turabian StyleSu, Hongfei, Xiaolin Chen, Shijing Chen, Mingzhang Guo, and Huilin Liu. 2023. "Applications of the Whole-Cell System in the Efficient Biosynthesis of Heme" International Journal of Molecular Sciences 24, no. 9: 8384. https://doi.org/10.3390/ijms24098384
APA StyleSu, H., Chen, X., Chen, S., Guo, M., & Liu, H. (2023). Applications of the Whole-Cell System in the Efficient Biosynthesis of Heme. International Journal of Molecular Sciences, 24(9), 8384. https://doi.org/10.3390/ijms24098384






