Increased Chymase-Positive Mast Cells in High-Grade Mucoepidermoid Carcinoma of the Parotid Gland
Abstract
1. Introduction
2. Results
2.1. Subject Profile
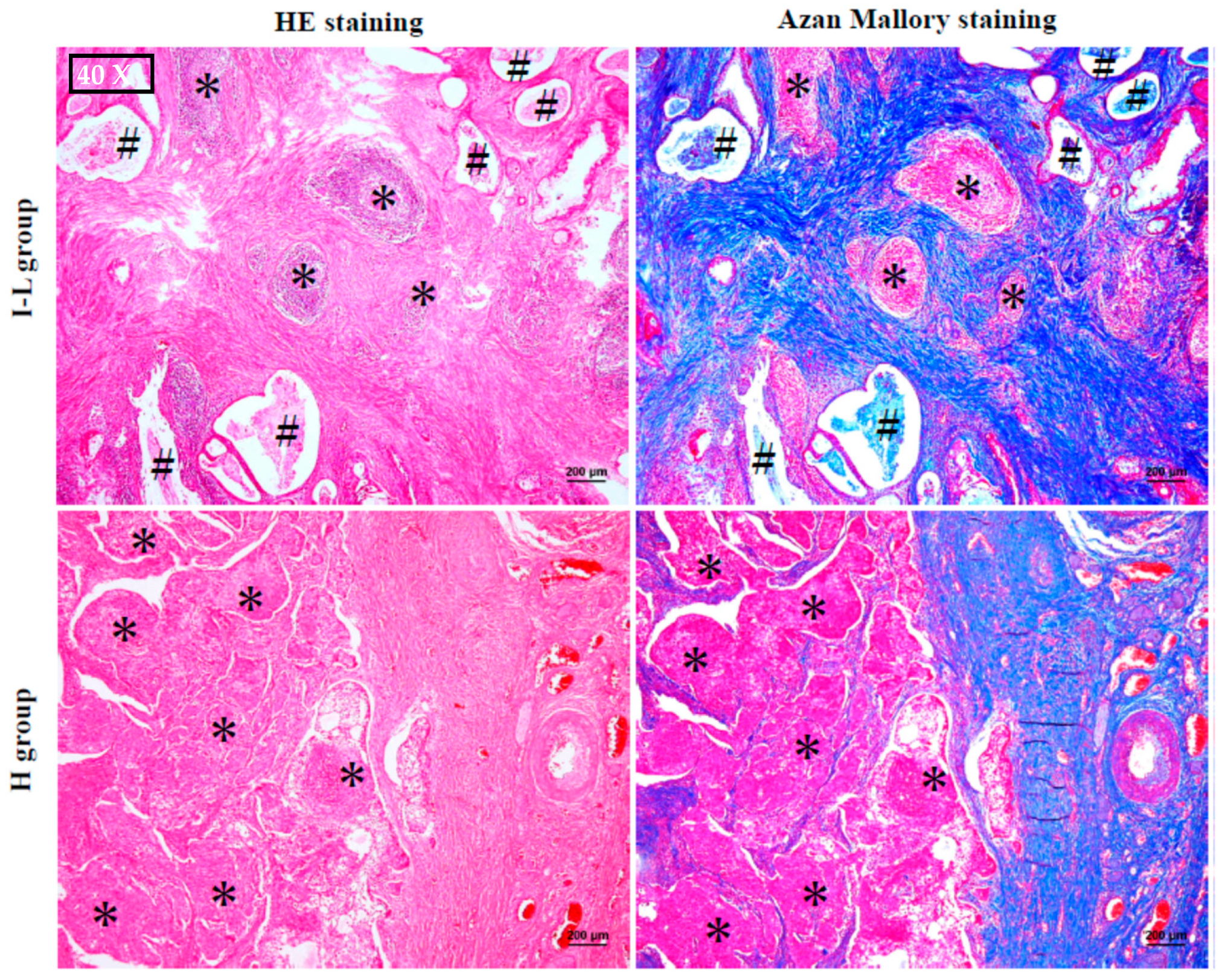
2.2. The Histological Features of MEC
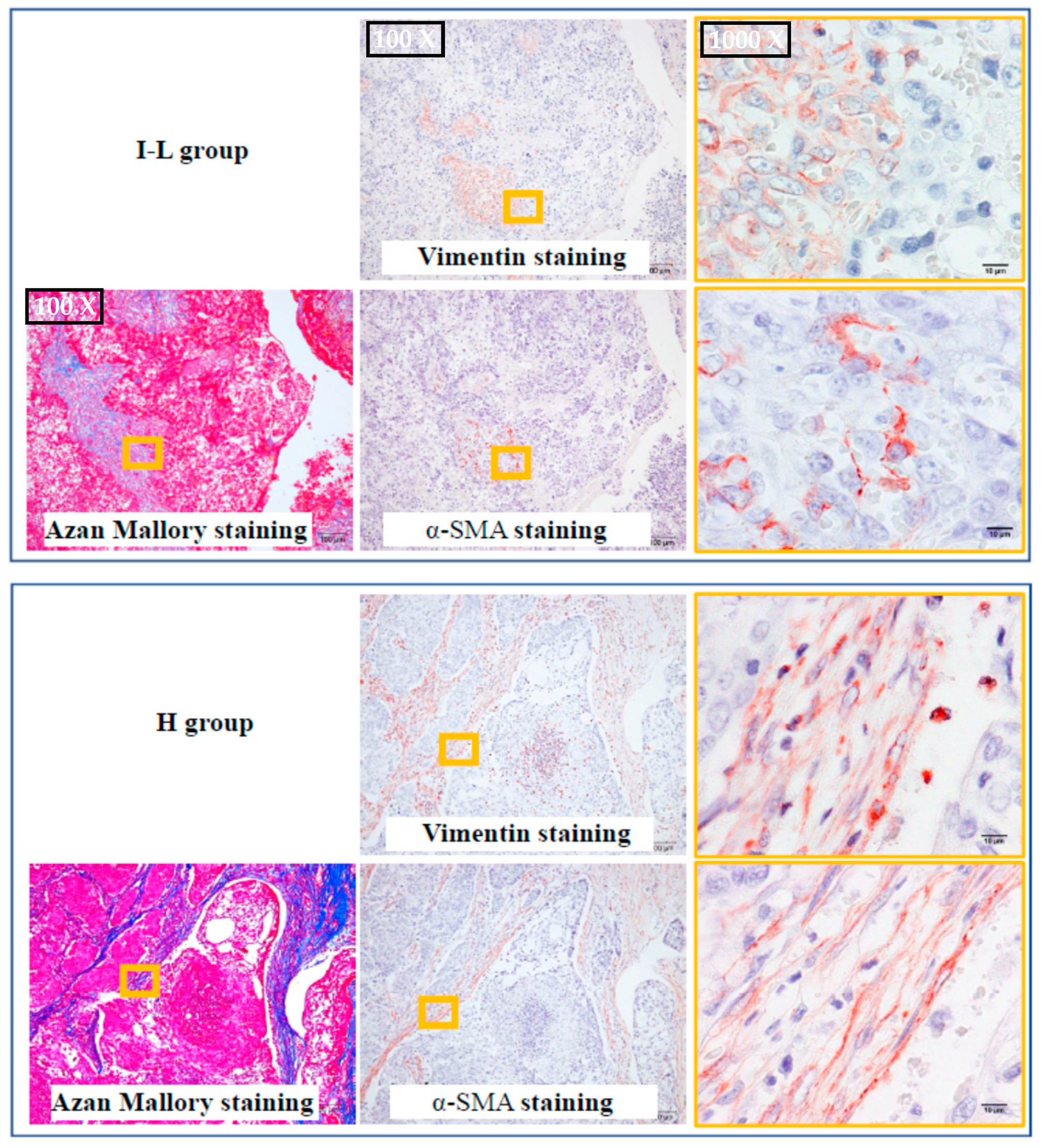
2.3. The Identification of Fibroblasts and Cancer-Associated Fibroblasts
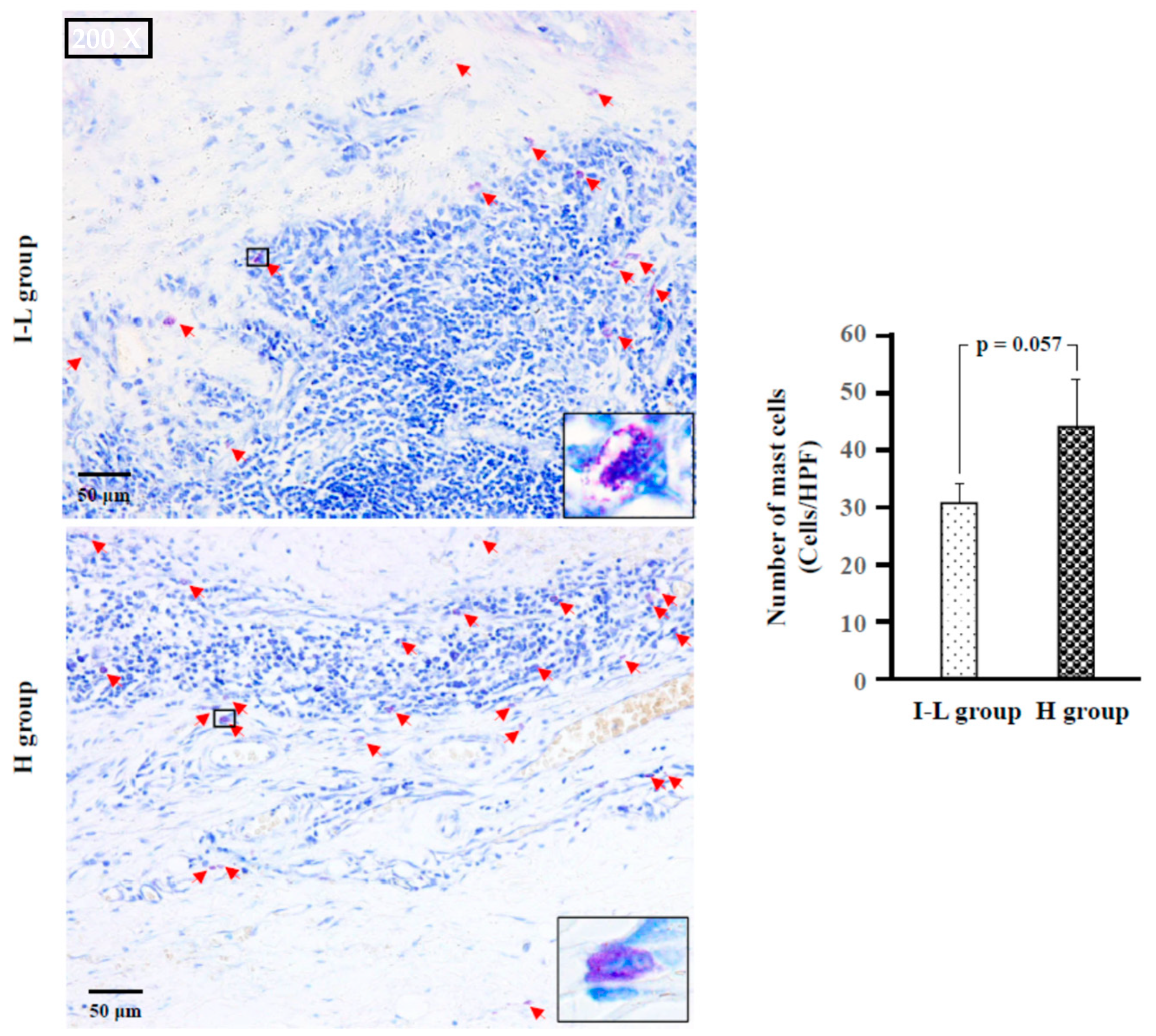
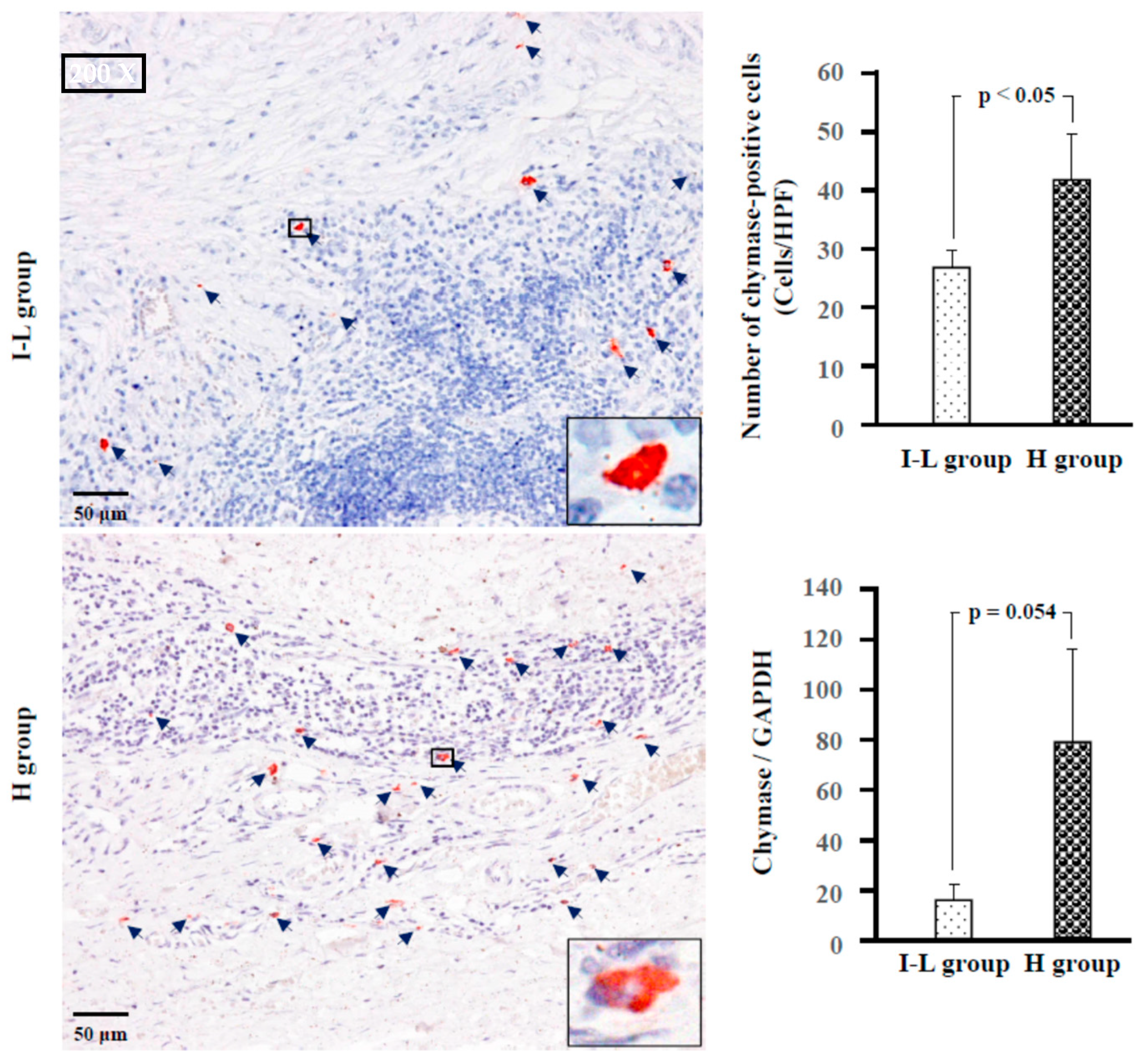
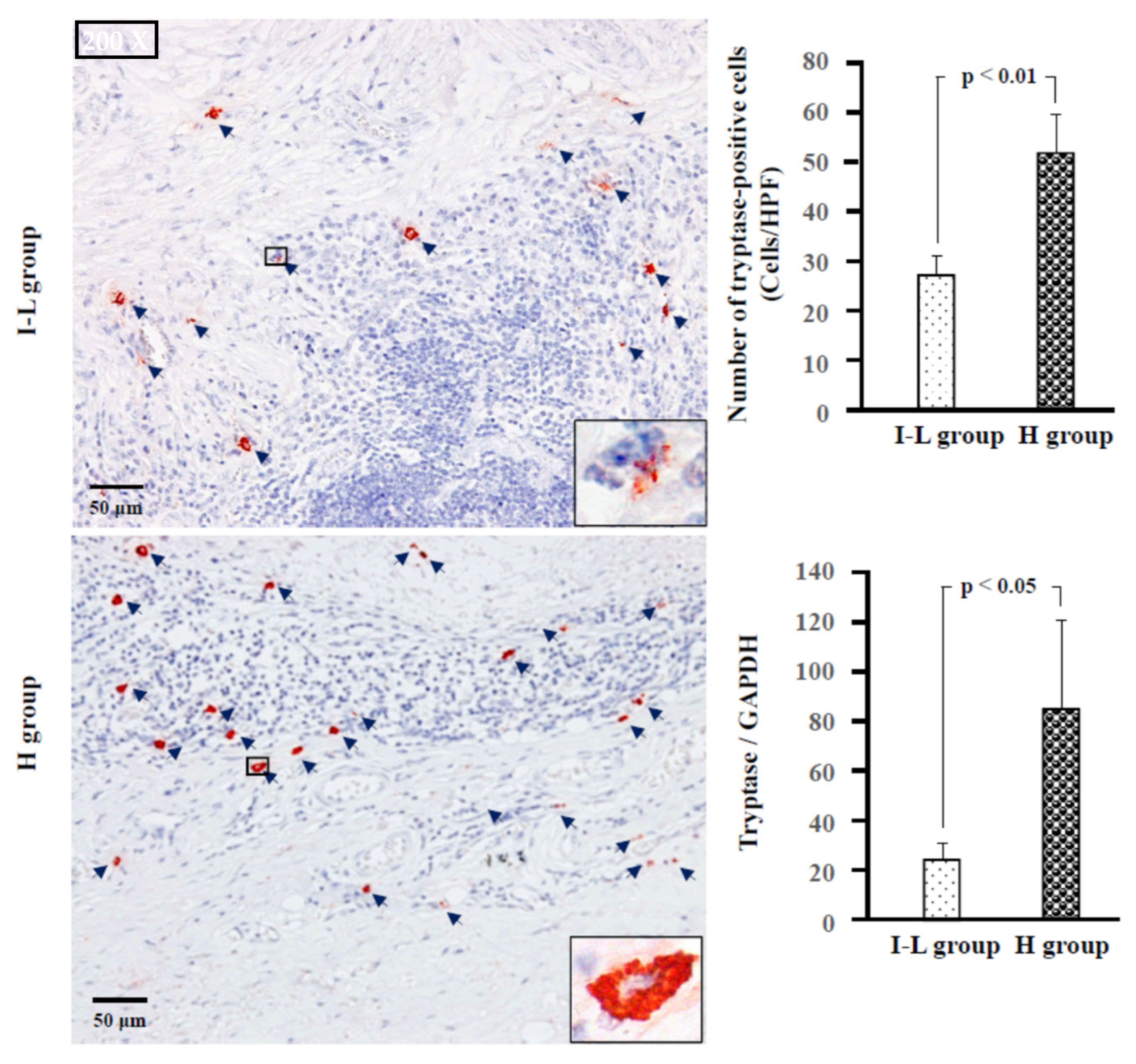
2.4. Identification of Type of Mast Cells
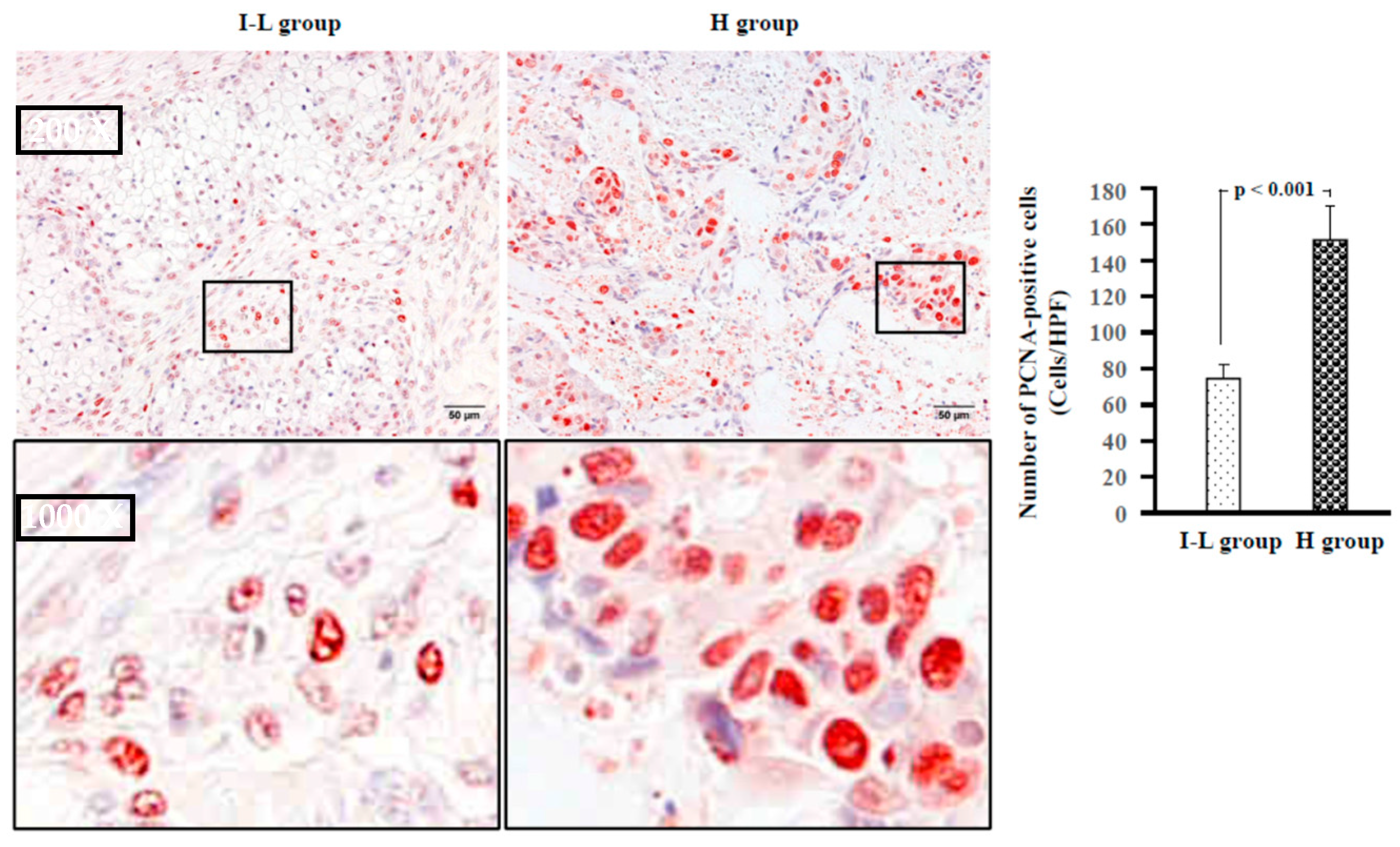
2.5. Examination of Proliferative Cells, Neovascularization, and Lymphangiogenesis
2.6. Identification of MMP-9, MMP-2, TGFβ-1 and SCF-Positive Cells
3. Discussion
Limitations
4. Materials and Methods
4.1. Sample Collection and Grouping
4.2. General Histological and Immunohistological Studies
4.3. Real-Time Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vander Poorten, V.; Bradley, P.J.; Takes, R.P.; Rinaldo, A.; Woolgar, J.A.; Ferlito, A. Diagnosis and management of parotid carcinoma with a special focus on recent advances in molecular biology. Head Neck. 2012, 34, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Auclair, P.L.; Goode, R.K.; Ellis, G.L. Mucoepidermoid carcinoma of intraoral salivary glands. Evaluation and application of grading criteria in 143 cases. Cancer 1992, 69, 2021–2030. [Google Scholar] [CrossRef] [PubMed]
- Goode, R.K.; Auclair, P.L.; Ellis, G.L. Mucoepidermoid carcinoma of the major salivary glands: Clinical and histopathologic analysis of 234 cases with evaluation of grading criteria. Cancer 1998, 82, 1217–1224. [Google Scholar] [CrossRef]
- McHugh, C.H.; Roberts, D.B.; El-Naggar, A.K.; Hanna, E.Y.; Garden, A.S.; Kies, M.S.; Weber, R.S.; Kupferman, M.E. Prognostic factors in mucoepidermoid carcinoma of the salivary glands. Cancer 2012, 118, 3928–3936. [Google Scholar] [CrossRef]
- Nance, M.A.; Seethala, R.R.; Wang, Y.; Chiosea, S.I.; Myers, E.N.; Johnson, J.T.; Lai, S.Y. Treatment and survival outcomes based on histologic grading in patients with head and neck mucoepidermoid carcinoma. Cancer 2008, 113, 2082–2089. [Google Scholar] [CrossRef]
- Kinoshita, I.; Jin, D.; Higashino, M.; Terada, T.; Kurisu, Y.; Takai, S.; Kawata, R. Increase in Chymase-Positive Mast Cells in Recurrent Pleomorphic Adenoma and Carcinoma Ex Pleomorphic Adenoma of the Parotid Gland. Int. J. Mol. Sci. 2021, 22, 12613. [Google Scholar] [CrossRef]
- Kondo, K.; Muramatsu, M.; Okamoto, Y.; Jin, D.; Takai, S.; Tanigawa, N.; Miyazaki, M. Expression of chymase-positive cells in gastric cancer and its correlation with the angiogenesis. J. Surg. Oncol. 2006, 93, 36–42. [Google Scholar] [CrossRef]
- Ibaraki, T.; Muramatsu, M.; Takai, S.; Jin, D.; Maruyama, H.; Orino, T.; Katsumata, T.; Miyazaki, M. The relationship of tryptase- and chymase-positive mast cells to angiogenesis in stage I non-small cell lung cancer. Eur. J. Cardio-Thorac. Surg. 2005, 28, 617–621. [Google Scholar] [CrossRef]
- Okunishi, H.; Miyazaki, M.; Toda, N. Evidence for a putatively new angiotensin II-generating enzyme in the vascular wall. J. Hypertens. 1984, 2, 277–284. [Google Scholar] [CrossRef]
- Takai, S.; Shiota, N.; Yamamoto, D.; Okunishi, H.; Miyazaki, M. Purification and characterization of angiotensin II-generating chymase from hamster cheek pouch. Life Sci. 1996, 58, 591–597. [Google Scholar] [CrossRef]
- Lindstedt, K.A.; Wang, Y.; Shiota, N.; Saarinen, J.; Hyytiäinen, M.; Kokkonen, J.O.; Keski-Oja, J.; Kovanen, P.T. Activation of paracrine TGF-beta1 signaling upon stimulation and degranulation of rat serosal mast cells: A novel function for chymase. FASEB J. 2001, 15, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.C.; Raymond, W.W.; Lazarus, S.C.; Caughey, G.H. Dog mastocytoma cells secrete a 92-kD gelatinase activated extracellularly by mast cell chymase. J. Clin. Investig. 1996, 97, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Batlle, E.; Massague, J. Transforming Growth Factor-beta Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Xu, S.; Luo, Z.; Li, D.; Wang, R.; Wang, T. Epidemiological Evidence Between Variants in Matrix Metalloproteinases-2, -7, and -9 and Cancer Risk. Front. Oncol. 2022, 12, 856831. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, M.; Yamada, M.; Takai, S.; Miyazaki, M. Suppression of basic fibroblast growth factor-induced angiogenesis by a specific chymase inhibitor, BCEAB, through the chymase-angiotensin-dependent pathway in hamster sponge granulomas. Br. J. Pharmacol. 2002, 137, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Stamenovic, D. Mechanics of vimentin intermediate filaments. J. Muscle. Res. Cell. Motil. 2002, 23, 535–540. [Google Scholar] [CrossRef]
- Tomasek, J.J.; McRae, J.; Owens, G.K.; Haaksma, C.J. Regulation of alpha-smooth muscle actin expression in granulation tissue myofibroblasts is dependent on the intronic CArG element and the transforming growth factorbeta1 control element. Am. J. Pathol. 2005, 166, 1343–1351. [Google Scholar] [CrossRef]
- Irvine, A.F.; Waise, S.; Green, E.W.; Stuart, B.; Thomas, G.J. Characterising cancer-associated fibroblast heterogeneity in non-small cell lung cancer: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 3727. [Google Scholar] [CrossRef]
- Irani, A.M.; Bradford, T.R.; Kepley, C.L.; Schechter, N.M.; Schwartz, L.B. Detection of MCT and MCTC types of human mast cells by immunohistochemistry using new monoclonal anti-tryptase and anti-chymase antibodies. J. Histochem. Cytochem. 1989, 37, 1509–1515. [Google Scholar] [CrossRef]
- Juríková, M.; Danihel, Ľ.; Polák, Š.; Varga, I. Ki67, PCNA, and MCM proteins: Markers of proliferation in the diagnosis of breast cancer. Acta Histochem. 2016, 118, 544–552. [Google Scholar] [CrossRef]
- Tahan, S.R.; Neuberg, D.S.; Dieffenbach, A.; Yacoub, L. Prediction of Early Relapse and Shortened Survival in Patients with Breast Cancer by Proliferating Cell Nuclear Antigen Score. Cancer 1993, 71, 3552–3559. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Rha, K.S.; Shim, G.A.; Kim, J.H.; Kim, J.M.; Huang, S.M.; Koo, B.S. Podoplanin is involved in the prognosis of head and neck squamous cell carcinoma through interaction with VEGF-C. Oncol. Rep. 2015, 34, 833–842. [Google Scholar] [CrossRef]
- Kahn, H.J.; Marks, A. A new monoclonal antibody, D2-40, for detection of lymphatic invasion in primary tumors. Lab Investig. 2002, 82, 1255–1257. [Google Scholar] [CrossRef] [PubMed]
- Patmore, S.; Dhami, S.P.S.; O’Sullivan, J.M. Von Willebrand factor and cancer; metastasis and coagulopathies. J. Thromb. Haemost. 2020, 18, 2444–2456. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y. VEGF-targeted cancer therapeutics-paradoxical effects in endocrine organs. Nat. Rev. Endocrinol. 2014, 10, 530–539. [Google Scholar] [CrossRef]
- Hirakawa, S.; Kodama, S.; Kunstfeld, R.; Kajiya, K.; Brown, L.F.; Detmar, M.J. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. Exp. Med. 2005, 201, 1089–1099. [Google Scholar] [CrossRef]
- Ho, K.; Lin, H.; Ann, D.K.; Chu, P.G.; Yen, Y. An overview of the rare parotid gland cancer. Head Neck Oncol. 2011, 14, 40. [Google Scholar] [CrossRef]
- Taniuchi, M.; Kawata, R.; Terada, T.; Higashino, M.; Nishimura, H.; Kurisu, Y.; Kuwabara, H.; Hirose, Y. Management and outcome of parotid mucoepidermoid carcinoma by histological grade: A 21-year review. Laryngoscope Investig. Otolaryngol. 2022, 7, 766–773. [Google Scholar] [CrossRef]
- Qian, K.; Sun, W.; Guo, K.; Zheng, X.; Sun, T.; Chen, L.; Xiang, J.; Li, D.; Wu, Y.; Ji, Q.; et al. The number and ratio of positive lymph nodes are independent prognostic factors for patients with major salivary gland cancer: Results from the surveillance, epidemiology, and End Results dataset. Eur. J. Surg. Oncol. 2019, 45, 1025–1032. [Google Scholar] [CrossRef]
- Duivenvoorden, W.C.; Hirte, H.W.; Singh, G. Transforming growth factor beta1 acts as an inducer of matrix metalloproteinase expression and activity in human bone-metastasizing cancer cells. Clin. Exp. Metastasis 1999, 17, 27–34. [Google Scholar] [CrossRef]
- Groschwitz, K.R.; Wu, D.; Osterfeld, H.; Ahrens, R.; Hogan, S.P. Chymase-mediated intestinal epithelial permeability is regulated by a protease-activating receptor/matrix metalloproteinase-2-dependent mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G479–G489. [Google Scholar] [CrossRef] [PubMed]
- Visse, R.; Nagase, H. Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases: Structure, Function, and Biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Okumura, K.; Takai, S.; Muramatsu, M.; Katayama, S.; Sakaguchi, M.; Kishi, K.; Jin, D.; Miyazaki, M. Human chymase degrades human fibronectin. Clin. Chim. Acta 2004, 347, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Komi, D.E.A.; Wöhrl, S.; Bielory, L. Mast Cell Biology at Molecular Level: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2020, 58, 342–365. [Google Scholar] [CrossRef]
- Longley, B.J.; Tyrrell, L.; Ma, Y.; Williams, D.A.; Halaban, R.; Langley, K.; Lu, H.S.; Schechter, N.M. Chymase cleavage of stem cell factor yields a bioactive, soluble product. Proc. Natl. Acad. Sci. USA 1997, 94, 9017–9021. [Google Scholar] [CrossRef]
- Vienot, A.; Pallandre, J.R.; Renaude, E.; Viot, J.; Bouard, A.; Spehner, L.; Kroemer, M.; Abdeljaoued, S.; van der Woning, B.; de Haard, H.; et al. Chemokine switch regulated by TGF-β1 in cancer-associated fibroblast subsets determines the efficacy of chemo-immunotherapy. Oncoimmunology 2022, 11, 2144669. [Google Scholar] [CrossRef]
- Gaggioli, C.; Hooper, S.; Hidalgo-Carcedo, C.; Grosse, R.; Marshall, J.F.; Harrington, K.; Sahai, E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat. Cell Biol. 2007, 9, 1392–1400. [Google Scholar] [CrossRef]
- Moore-Smith, L.D.; Isayeva, T.; Lee, J.H.; Frost, A.; Ponnazhagan, S. Silencing of TGF-beta1 in tumor cells impacts MMP-9 in tumor microenvironment. Sci. Rep. 2017, 7, 8678. [Google Scholar] [CrossRef]
- Tzachanis, D.; Freeman, G.J.; Hirano, N.; van Puijenbroek, A.A.; Delfs, M.W.; Berezovskaya, A.; Nadler, L.M.; Boussiotis, V.A. Tob is a negative regulator of activation that is expressed in anergic and quiescent T cells. Nat. Immunol. 2001, 2, 1174–1182. [Google Scholar] [CrossRef]
- Uemura, H.; Nakaigawa, N.; Ishiguro, H.; Kubota, Y. Antiproliferative efficacy of angiotensin II receptor blockers in prostate cancer. Curr. Cancer Drug Targets 2005, 5, 307–323. [Google Scholar] [CrossRef]
- Luo, Y.; Ohmori, H.; Shimomoto, T.; Fujii, K.; Sasahira, T.; Chihara, Y.; Kuniyasu, H. Anti-angiotensin and hypoglycemic treatments suppress liver metastasis of colon cancer cells. Pathobiology 2011, 78, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Ueda, H.; Takai, S.; Okamoto, Y.; Muramatsu, M.; Sakaguchi, M.; Shibahara, N.; Katsuoka, Y.; Miyazaki, M. Effect of chymase inhibition on the arteriovenous fistula stenosis in dogs. J. Am. Soc. Nephrol. 2005, 16, 1024–1034. [Google Scholar] [CrossRef] [PubMed]



| High-Grade (n = 18) | Intermediate-Grade (n = 9) | Low-Grade (n = 17) | p Value | ||
|---|---|---|---|---|---|
| Sex | Male (Age range) | 12 (42–85) | 4 (22–72) | 5 (19–59) | p < 0.05 |
| Female (Age range) | 6 (42–82) | 5 (23–79) | 12 (19–69) | ||
| Anatomic location of parotid tumor | Superficial | 14 | 8 | 10 | p = 0.83 |
| Others | 4 | 1 | 7 | ||
| Symptom of pain | Yes | 11 | 3 | 8 | p = 0.22 |
| No | 7 | 6 | 9 | ||
| Facial paralysis | Yes | 3 | 0 | 0 | p < 0.05 |
| No | 15 | 9 | 17 | ||
| Lymph node metastasis | Yes | 11 | 2 | 1 | p < 0.001 |
| No | 7 | 7 | 16 | ||
| Recurrence | Yes | 8 | 0 | 2 | p < 0.01 |
| No | 10 | 9 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimura, H.; Jin, D.; Kinoshita, I.; Taniuchi, M.; Higashino, M.; Terada, T.; Takai, S.; Kawata, R. Increased Chymase-Positive Mast Cells in High-Grade Mucoepidermoid Carcinoma of the Parotid Gland. Int. J. Mol. Sci. 2023, 24, 8267. https://doi.org/10.3390/ijms24098267
Nishimura H, Jin D, Kinoshita I, Taniuchi M, Higashino M, Terada T, Takai S, Kawata R. Increased Chymase-Positive Mast Cells in High-Grade Mucoepidermoid Carcinoma of the Parotid Gland. International Journal of Molecular Sciences. 2023; 24(9):8267. https://doi.org/10.3390/ijms24098267
Chicago/Turabian StyleNishimura, Hiromi, Denan Jin, Ichita Kinoshita, Masataka Taniuchi, Masaaki Higashino, Tetsuya Terada, Shinji Takai, and Ryo Kawata. 2023. "Increased Chymase-Positive Mast Cells in High-Grade Mucoepidermoid Carcinoma of the Parotid Gland" International Journal of Molecular Sciences 24, no. 9: 8267. https://doi.org/10.3390/ijms24098267
APA StyleNishimura, H., Jin, D., Kinoshita, I., Taniuchi, M., Higashino, M., Terada, T., Takai, S., & Kawata, R. (2023). Increased Chymase-Positive Mast Cells in High-Grade Mucoepidermoid Carcinoma of the Parotid Gland. International Journal of Molecular Sciences, 24(9), 8267. https://doi.org/10.3390/ijms24098267






