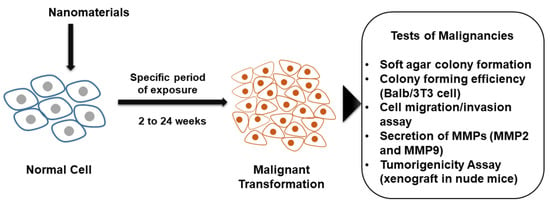
In Vitro Cell Transformation Assays: A Valuable Approach for Carcinogenic Potentiality Assessment of Nanomaterials
Abstract
1. Introduction
2. Carcinogenicity Assessment
3. Cell Transformation Assays
3.1. Essential Characteristics of Transformed Cells
3.2. CTA, Based on the Characteristics of Transformed Cell
3.3. Animal Cell Line-Based Cell Transformation Assays
3.4. Human Cell Line-Based Cell transformation Assays
4. Engineered Nanomaterials (NM) and Their Carcinogenicity Assessments
5. Application of CTA in the Carcinogenic Potentiality Assessment of Nanomaterials
5.1. Carcinogenic-Hallmark-Related Assays
5.2. Cell Lines Applied
5.3. NM Exposure to Induce Cell Transformation
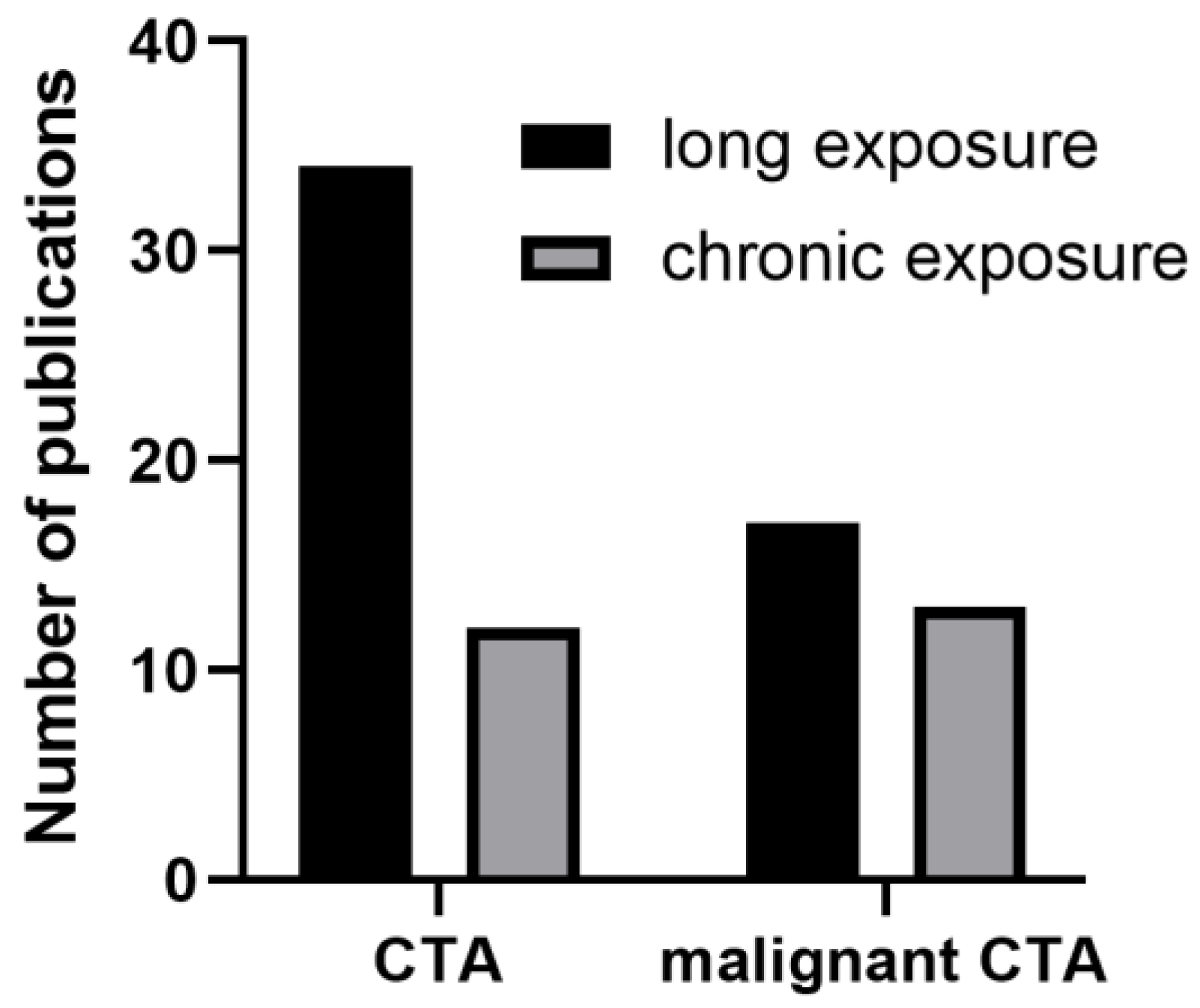
5.3.1. Time of Exposure
5.3.2. Exposure Concentrations
5.3.3. Co-Exposure with Other Environmental Pollutants
5.4. The Influence of Physicochemical Properties of NMs in Cell Transformation
5.5. Mechanism of NMNM-Induced Cell Transformation
5.5.1. Oxidative Stress and Inflammatory Biomarkers
5.5.2. Genotoxicity, DNA Damage and Repair
5.5.3. Epigenetic Modifications
5.5.4. Other Mechanisms of CTA-Induced NM
5.6. NM-Induced Cancer Stem Cells (CSCs)
5.7. NM-Induced Epithelial–Mesenchymal Transition (EMT)
6. Future Research Needs for Better Nanosafety
- The physical-chemical properties of NMs, such as their size, shape, surface modification/coating, and surface charges, are known to influence their carcinogenic potential. However, few studies have thoroughly examined the relationship between these properties and the potential for carcinogenesis, which is essential for promoting nanosafety and adopting a safe-by-design approach. Therefore, future study designs should prioritize assessing the safety of specific nanoforms in regard to their potential for carcinogenesis and evaluate them based on the ten key characteristics (10 KCs) recommended by the IARC. This will help identify which NMs pose the greatest risk for carcinogenicity and enable the development of safer nanomaterials through targeted modifications.
- Current studies on NM-induced cell transformation are primarily focused on pre-neoplastic changes, such as anchorage-independent growth in soft agar. However, these studies lack the confirmation of true malignancy through the mouse xenograft model, which is considered the gold standard for carcinogenicity evaluation. Therefore, future studies should consider finding alternative assays to replace the mouse xenograft test as a final step in assessing the true malignancy of NM-induced transformed cells.
- In vitro models, although useful for studying cellular transformation, do not fully capture the complexity of cancer formation in vivo, such as the role of the immune system and the tumor microenvironment. To bridge this gap, CTA can be combined with advanced microphysiological systems, such as organ-on-a-chip models or immune-oncology models, to better simulate in vivo situations [62]. However, single cell line-based CTA still has utility in elucidating the mechanisms of cellular transformation, which can provide insights into the formation of cancer at both the cellular and organism levels.
- While various cell transformation assays have been employed for carcinogenicity assessments of nanomaterials, there is a lack of standardization and harmonization among assays. Therefore, future studies should aim to establish standardized protocols and methods for cell transformation assays to enable better comparison of results and reproducibility.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CTA | Cell transformation assays |
| NM | Nanomaterials |
| EMT | Epithelial–mesenchymal transition |
| CSC | Cancer stem cells (CSCs) |
| KC | Key characteristics |
| IARC | International Agency for Research on Cancer |
| GJIC | Gap junctional intercellular communication |
| TiO2 | Titanium-di-oxide |
| MWCNT | Multi-walled carbon nanotubes |
| SWCNT | Single-walled carbon nanotubes |
| CeO2-NP | Cerium oxide nanoparticles |
| Fe2O3-NP | Ferric oxide |
| Ag-NP | Silver nanoparticles |
| Co-NP | Cobalt nanoparticles |
| ZnO-NP | Zinc-oxide nanoparticles |
| SAS | Synthetic amorphous silica nanoparticles |
| ROS | Reactive oxygen species |
References
- Pitot, H.C. The molecular biology of carcinogenesis. Cancer 1993, 72 (Suppl. S3), 962–970. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg Robert, A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.M.R.; Cunha-Oliveira, T.; Sobral, M.C.; Abreu, P.L.; Alpoim, M.C.; Urbano, A.M. Impact of Carcinogenic Chromium on the Cellular Response to Proteotoxic Stress. Int. J. Mol. Sci. 2019, 20, 4901. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, A.P.; Ohlsson, R.; Henikoff, S. The epigenetic progenitor origin of human cancer. Nat. Rev. Genet. 2006, 7, 21–33. [Google Scholar] [CrossRef]
- Guyton, K.Z.; Rusyn, I.; Chiu, W.A.; Corpet, D.E.; van den Berg, M.; Ross, M.K.; Christiani, D.C.; Beland, F.A.; Smith, M.T. Application of the key characteristics of carcinogens in cancer hazard identification. Carcinogenesis 2018, 39, 614–622. [Google Scholar] [CrossRef]
- Kleinstreuer, N.C.; Dix, D.J.; Houck, K.A.; Kavlock, R.J.; Knudsen, T.B.; Martin, M.T.; Paul, K.B.; Reif, D.M.; Crofton, K.M.; Hamilton, K.; et al. In vitro perturbations of targets in cancer hallmark processes predict rodent chemical carcinogenesis. Toxicol. Sci. Off. J. Soc. Toxicol. 2013, 131, 40–55. [Google Scholar] [CrossRef]
- Smith, M.T.; Guyton, K.Z.; Gibbons, C.F.; Fritz, J.M.; Portier, C.J.; Rusyn, I.; DeMarini, D.M.; Caldwell, J.C.; Kavlock, R.J.; Lambert, P.F.; et al. Key Characteristics of Carcinogens as a Basis for Organizing Data on Mechanisms of Carcinogenesis. Environ. Health Perspect. 2016, 124, 713–721. [Google Scholar] [CrossRef]
- Kohl, Y.; Rundén-Pran, E.; Mariussen, E.; Hesler, M.; El Yamani, N.; Longhin, E.M.; Dusinska, M. Genotoxicity of Nanomaterials: Advanced In Vitro Models and High Throughput Methods for Human Hazard Assessment—A Review. Nanomaterials 2020, 10, 1911. [Google Scholar] [CrossRef]
- Becker, R.A.; Dreier, D.A.; Manibusan, M.K.; Cox, L.A.T.; Simon, T.W.; Bus, J.S. How well can carcinogenicity be predicted by high throughput “characteristics of carcinogens” mechanistic data? Regul. Toxicol. Pharmacol. RTP 2017, 90, 185–196. [Google Scholar] [CrossRef]
- Corvi, R.; Madia, F.; Guyton, K.Z.; Kasper, P.; Rudel, R.; Colacci, A.; Kleinjansf, J.; Jennings, P. Moving forward in carcinogenicity assessment: Report of an EURL ECVAM/ESTIV workshop. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2017, 45 Pt 3, 278–286. [Google Scholar] [CrossRef]
- Smith, M.T.; Guyton, K.Z.; Kleinstreuer, N.; Borrel, A.; Cardenas, A.; Chiu, W.A.; Felsher, D.W.; Gibbons, C.F.; Goodson, W.H., 3rd; Houck, K.A.; et al. The Key Characteristics of Carcinogens: Relationship to the Hallmarks of Cancer, Relevant Biomarkers, and Assays to Measure Them. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1887–1903. [Google Scholar] [CrossRef]
- Jacobs, M.N.; Colacci, A.; Louekari, K.; Luijten, M.; Hakkert, B.C.; Paparella, M.; Vasseur, P. International regulatory needs for development of an IATA for non-genotoxic carcinogenic chemical substances. Altex 2016, 33, 359–392. [Google Scholar] [CrossRef]
- Jacobs, M.N.; Colacci, A.; Corvi, R.; Vaccari, M.; Aguila, M.C.; Corvaro, M.; Delrue, N.; Desaulniers, D.; Ertych, N.; Jacobs, A.; et al. Chemical carcinogen safety testing: OECD expert group international consensus on the development of an integrated approach for the testing and assessment of chemical non-genotoxic carcinogens. Arch. Toxicol. 2020, 94, 2899–2923. [Google Scholar] [CrossRef]
- Braakhuis, H.M.; Slob, W.; Olthof, E.D.; Wolterink, G.; Zwart, E.P.; Gremmer, E.R.; Rorije, E.; van Benthem, J.; Woutersen, R.; van der Laan, J.W.; et al. Is current risk assessment of non-genotoxic carcinogens protective? Crit. Rev. Toxicol. 2018, 48, 500–511. [Google Scholar] [CrossRef]
- Combes, R.; Balls, M.; Curren, R.; Fischbach, M.; Fusenig, N.; Kirkland, D.; Lasne, C.; Landolph, J.; LeBoeuf, R.; Marquardt, H.; et al. Cell transformation assays as predictors of human carcinogenicity. Altern. Lab. Anim. ATLA 1999, 27, 745–767. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Costa, M. A comprehensive review of metal-induced cellular transformation studies. Toxicol. Appl. Pharmacol. 2017, 331, 33–40. [Google Scholar] [CrossRef]
- Creton, S.; Aardema, M.J.; Carmichael, P.L.; Harvey, J.S.; Martin, F.L.; Newbold, R.F.; O’Donovan, M.R.; Pant, K.; Poth, A.; Sakai, A.; et al. Cell transformation assays for prediction of carcinogenic potential: State of the science and future research needs. Mutagenesis 2012, 27, 93–101. [Google Scholar] [CrossRef]
- Breheny, D.; Oke, O.; Faux, S.P. The use of in vitro systems to assess cancer mechanisms and the carcinogenic potential of chemicals. Altern. Lab. Anim. ATLA 2011, 39, 233–255. [Google Scholar] [CrossRef]
- Kavsan, V.M.; Iershov, A.V.; Balynska, O.V. Immortalized cells and one oncogene in malignant transformation: Old insights on new explanation. BMC Cell Biol. 2011, 12, 23. [Google Scholar] [CrossRef]
- Vasseur, P.; Lasne, C. OECD Detailed Review Paper (DRP) number 31 on “Cell Transformation Assays for Detection of Chemical Carcinogens”: Main results and conclusions. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2012, 744, 8–11. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Umeda, M.; Tanaka, N.; Sakai, A.; Nishiyama, H.; Yoshimura, I.; Ajimi, S.; Asada, S.; Asakura, M.; Baba, H.; et al. Application of the Improved BALB/c 3T3 Cell Transformation Assay to the Examination of the Initiating and Promoting Activities of Chemicals: The Second Inter-laboratory Collaborative Study by the Non-genotoxic Carcinogen Study Group of Japan. Altern. Lab. Anim. 2010, 38, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Umeda, M.; Sakai, A.; Yamazaki, S.; Tanaka, N. Transformation assay in Bhas 42 cells: A model using initiated cells to study mechanisms of carcinogenesis and predict carcinogenic potential of chemicals. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2015, 33, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Sakai, A.; Sasaki, K.; Hayashi, K.; Muramatsu, D.; Arai, S.; Endou, N.; Kuroda, S.; Poth, A.; Bohnenberger, S.; Kunkelmann, T.; et al. An international validation study of a Bhas 42 cell transformation assay for the prediction of chemical carcinogenicity. Mutat. Res. 2011, 725, 57–77. [Google Scholar] [CrossRef] [PubMed]
- Heeg, S.; Doebele, M.; von Werder, A.; Opitz, O.G. In vitro transformation models: Modeling human Cancer. Cell Cycle 2006, 5, 630–634. [Google Scholar] [PubMed]
- Harvey, J.S.; Howe, J.R.; Lynch, A.M.; Rees, R.W. The results of five coded compounds: Genistein, metaproterenol, rotenone, p-anisidine and resorcinol tested in the pH 6.7 Syrian hamster embryo cell morphological transformation assay. Mutagenesis 2005, 20, 51–56. [Google Scholar] [CrossRef]
- Park, Y.H.; Kim, D.; Dai, J.; Zhang, Z. Human bronchial epithelial BEAS-2B cells, an appropriate in vitro model to study heavy metals induced carcinogenesis. Toxicol. Appl. Pharmacol. 2015, 287, 240–245. [Google Scholar] [CrossRef]
- Liu, S.; Xia, T. Continued Efforts on Nanomaterial-Environmental Health and Safety Is Critical to Maintain Sustainable Growth of Nanoindustry. Small 2020, 16, 2000603. [Google Scholar] [CrossRef]
- Patil-Sen, Y. Advances in nano-biomaterials and their applications in biomedicine. Emerg. Top. Life Sci. 2021, 5, 169–176. [Google Scholar]
- Guerra, F.D.; Attia, M.F.; Whitehead, D.C.; Alexis, F. Nanotechnology for Environmental Remediation: Materials and Applications. Molecules 2018, 23, 1760. [Google Scholar] [CrossRef]
- Shin, S.W.; Song, I.H.; Um, S.H. Role of Physicochemical Properties in Nanoparticle Toxicity. Nanomaterials 2015, 5, 1351–1365. [Google Scholar] [CrossRef]
- Kah, M. Nanopesticides and Nanofertilizers: Emerging Contaminants or Opportunities for Risk Mitigation? Front. Chem. 2015, 3, 64. [Google Scholar] [CrossRef]
- Simkó, M.; Mattsson, M.O. Interactions between nanosized materials and the brain. Curr. Med. Chem. 2014, 21, 4200–4214. [Google Scholar] [CrossRef]
- IARC. Carbon black, titanium dioxide, and talc. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2010; Volume 93, pp. 1–413. [Google Scholar]
- Grosse, Y.; Loomis, D.; Guyton, K.Z.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Scoccianti, C.; Mattock, H.; et al. Carcinogenicity of fluoro-edenite, silicon carbide fibres and whiskers, and carbon nanotubes. Lancet Oncol. 2014, 15, 1427–1428. [Google Scholar] [CrossRef]
- Nymark, P.; Karlsson, H.; Halappanavar, S.; Vogel, U. Adverse Outcome Pathway Development for Assessment of Lung Carcinogenicity by Nanoparticles. Front. Toxicol. 2021, 3, 653386. [Google Scholar] [CrossRef]
- Huang, X.; Tian, Y.; Shi, W.; Chen, J.; Yan, L.; Ren, L.; Zhang, X.; Zhu, J. Role of inflammation in the malignant transformation of pleural mesothelial cells induced by multi-walled carbon nanotubes. Nanotoxicology 2020, 14, 947–967. [Google Scholar] [CrossRef]
- Annangi, B.; Rubio, L.; Alaraby, M.; Bach, J.; Marcos, R.; Hernández, A. Acute and long-term in vitro effects of zinc oxide nanoparticles. Arch. Toxicol. 2016, 90, 2201–2213. [Google Scholar] [CrossRef]
- Meng, J.; Zhou, X.; Yang, J.; Qu, X.; Cui, S. Exposure to low dose ZnO nanoparticles induces hyperproliferation and malignant transformation through activating the CXCR2/NF-κB/STAT3/ERK and AKT pathways in colonic mucosal cells. Environ. Pollut. 2020, 263, 114578. [Google Scholar] [CrossRef]
- Choo, W.H.; Park, C.H.; Jung, S.E.; Moon, B.; Ahn, H.; Ryu, J.S.; Kim, K.S.; Lee, Y.H.; Yu, I.J.; Oh, S.M. Long-term exposures to low doses of silver nanoparticles enhanced in vitro malignant cell transformation in non-tumorigenic BEAS-2B cells. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2016, 37, 41–49. [Google Scholar] [CrossRef]
- Huang, S.; Chueh, P.J.; Lin, Y.W.; Shih, T.S.; Chuang, S.M. Disturbed mitotic progression and genome segregation are involved in cell transformation mediated by nano-TiO2 long-term exposure. Toxicol. Appl. Pharmacol. 2009, 241, 182–194. [Google Scholar] [CrossRef]
- Fontana, C.; Kirsch, A.; Seidel, C.; Marpeaux, L.; Darne, C.; Gaté, L.; Remy, A.; Guichard, Y. In vitro cell transformation induced by synthetic amorphous silica nanoparticles. Mutat. Res. 2017, 823, 22–27. [Google Scholar] [CrossRef]
- Vila, L.; Marcos, R.; Hernández, A. Long-term effects of silver nanoparticles in caco-2 cells. Nanotoxicology 2017, 11, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Rubio, L.; Bach, J.; Marcos, R.; Hernández, A. Synergistic role of nanoceria on the ability of tobacco smoke to induce carcinogenic hallmarks in lung epithelial cells. Nanomedicine 2017, 12, 2623–2635. [Google Scholar] [CrossRef] [PubMed]
- Combes, R.D. Cell transformation assays: Are we barking up the wrong tree? Altern. Lab. Anim. ATLA 2012, 40, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, A.; Dubois-Pot-Schneider, H.; Fontana, C.; Schohn, H.; Gaté, L.; Guichard, Y. Predictive early gene signature during mouse Bhas 42 cell transformation induced by synthetic amorphous silica nanoparticles. Chem.-Biol. Interact. 2020, 315, 108900. [Google Scholar] [CrossRef]
- Vales, G.; Rubio, L.; Marcos, R. Long-term exposures to low doses of titanium dioxide nanoparticles induce cell transformation, but not genotoxic damage in BEAS-2B cells. Nanotoxicology 2015, 9, 568–578. [Google Scholar] [CrossRef]
- Ju, L.; Zhu, L.; Wu, H.; Yu, M.; Yin, X.; Jia, Z.; Feng, L.; Ying, S.; Xia, H.; Zhang, S.; et al. miR221 regulates cell migration by targeting annexin a1 expression in human mesothelial MeT-5A cells neoplastic-like transformed by multi-walled carbon nanotube. Genes Environ. Off. J. Jpn. Environ. Mutagen Soc. 2021, 43, 34. [Google Scholar] [CrossRef]
- Chappell, G.; Pogribny, I.P.; Guyton, K.Z.; Rusyn, I. Epigenetic alterations induced by genotoxic occupational and environmental human chemical carcinogens: A systematic literature review. Mutat. Res. Rev. Mutat. Res. 2016, 768, 27–45. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Choo, W.; Moon, B.; Song, S.; Oh, S.M. Morphological transformation induced by silver nanoparticles in a Balb/c 3T3 A31-1-1 mouse cell model to evaluate in vitro carcinogenic potential. Environ. Health Toxicol. 2017, 32, e2017016. [Google Scholar] [CrossRef]
- Wang, J.; Tian, X.; Zhang, J.; Tan, L.; Ouyang, N.; Jia, B.; Chen, C.; Ge, C.; Li, J. Postchronic Single-Walled Carbon Nanotube Exposure Causes Irreversible Malignant Transformation of Human Bronchial Epithelial Cells through DNA Methylation Changes. ACS Nano 2021, 15, 7094–7104. [Google Scholar] [CrossRef]
- Barguilla, I.; Barszczewska, G.; Annangi, B.; Domenech, J.; Velázquez, A.; Marcos, R.; Hernández, A. MTH1 is involved in the toxic and carcinogenic long-term effects induced by zinc oxide and cobalt nanoparticles. Arch. Toxicol. 2020, 94, 1973–1984. [Google Scholar] [CrossRef]
- Demir, E.; Akça, H.; Kaya, B.; Burgucu, D.; Tokgün, O.; Turna, F.; Aksakal, S.; Vales, G.; Creus, A.; Marcos, R. Zinc oxide nanoparticles: Genotoxicity, interactions with UV-light and cell-transforming potential. J. Hazard. Mater. 2014, 264, 420–429. [Google Scholar] [CrossRef]
- Stoccoro, A.; Di Bucchianico, S.; Uboldi, C.; Coppedè, F.; Ponti, J.; Placidi, C.; Blosi, M.; Ortelli, S.; Costa, A.L.; Migliore, L. A panel of in vitro tests to evaluate genotoxic and morphological neoplastic transformation potential on Balb/3T3 cells by pristine and remediated titania and zirconia nanoparticles. Mutagenesis 2016, 31, 511–529. [Google Scholar] [CrossRef]
- Lohcharoenkal, W.; Wang, L.; Stueckle, T.A.; Park, J.; Tse, W.; Dinu, C.Z.; Rojanasakul, Y. Role of H-Ras/ERK signaling in carbon nanotube-induced neoplastic-like transformation of human mesothelial cells. Front. Physiol. 2014, 5, 222. [Google Scholar] [CrossRef]
- Chang, G.; Xie, D.; Hu, J.; Wu, T.; Cao, K.; Luo, X. Identification of Candidate lncRNA and Pseudogene Biomarkers Associated with Carbon-Nanotube-Induced Malignant Transformation of Lung Cells and Prediction of Potential Preventive Drugs. Int. J. Environ. Res. Public Health 2022, 19, 2936. [Google Scholar] [CrossRef]
- Kornberg, T.G.; Stueckle, T.A.; Coyle, J.; Derk, R.; Demokritou, P.; Rojanasakul, Y.; Rojanasakul, L.W. Iron Oxide Nanoparticle-Induced Neoplastic-Like Cell Transformation in Vitro Is Reduced with a Protective Amorphous Silica Coating. Chem. Res. Toxicol. 2019, 32, 2382–2397. [Google Scholar] [CrossRef]
- Ponti, J.; Broggi, F.; Mariani, V.; De Marzi, L.; Colognato, R.; Marmorato, P.; Gioria, S.; Gilliland, D.; Pascual Garcìa, C.; Meschini, S.; et al. Morphological transformation induced by multiwall carbon nanotubes on Balb/3T3 cell model as an in vitro end point of carcinogenic potential. Nanotoxicology 2013, 7, 221–233. [Google Scholar] [CrossRef]
- Stueckle, T.A.; Davidson, D.C.; Derk, R.; Wang, P.; Friend, S.; Schwegler-Berry, D.; Zheng, P.; Wu, N.; Castranova, V.; Rojanasakul, Y.; et al. Effect of surface functionalizations of multi-walled carbon nanotubes on neoplastic transformation potential in primary human lung epithelial cells. Nanotoxicology 2017, 11, 613–624. [Google Scholar] [CrossRef]
- Lou, D.; Wei, X.; Xiao, P.; Huo, Q.; Hong, X.; Sun, J.; Shuai, Y.; Tao, G. Demethylation of the NRF2 Promoter Protects Against Carcinogenesis Induced by Nano-SiO(2). Front. Genet. 2020, 11, 818. [Google Scholar] [CrossRef]
- Barguilla, I.; Domenech, J.; Rubio, L.; Marcos, R.; Hernandez, A. Nanoplastics and Arsenic Co-Exposures Exacerbate Oncogenic Biomarkers under an In Vitro Long-Term Exposure Scenario. Int. J. Mol. Sci. 2022, 23, 2958. [Google Scholar] [CrossRef]
- Gliga, A.R.; Di Bucchianico, S.; Åkerlund, E.; Karlsson, H.L. Transcriptome Profiling and Toxicity Following Long-Term, Low Dose Exposure of Human Lung Cells to Ni and NiO Nanoparticles-Comparison with NiCl(2). Nanomaterials 2020, 10, 649. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Stueckle, T.A.; Mishra, A.; Derk, R.; Meighan, T.; Castranova, V.; Rojanasakul, Y. Neoplastic-like transformation effect of single-walled and multi-walled carbon nanotubes compared to asbestos on human lung small airway epithelial cells. Nanotoxicology 2014, 8, 485–507. [Google Scholar] [CrossRef] [PubMed]
- Vales, G.; Rubio, L.; Marcos, R. Genotoxic and cell-transformation effects of multi-walled carbon nanotubes (MWCNT) following in vitro sub-chronic exposures. J. Hazard. Mater. 2016, 306, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Annangi, B.; Bach, J.; Vales, G.; Rubio, L.; Marcos, R.; Hernandez, A. Long-term exposures to low doses of cobalt nanoparticles induce cell transformation enhanced by oxidative damage. Nanotoxicology 2014, 9, 138–147. [Google Scholar] [CrossRef]
- Ballesteros, S.; Vales, G.; Velázquez, A.; Pastor, S.; Alaraby, M.; Marcos, R.; Hernández, A. MicroRNAs as a Suitable Biomarker to Detect the Effects of Long-Term Exposures to Nanomaterials. Studies on TiO(2) NP and MWCNT. Nanomaterials 2021, 11, 3458. [Google Scholar] [CrossRef]
- Kisin, E.R.; Yanamala, N.; Rodin, D.; Menas, A.; Farcas, M.; Russo, M.; Guppi, S.; Khaliullin, T.O.; Iavicoli, I.; Harper, M.; et al. Enhanced morphological transformation of human lung epithelial cells by continuous exposure to cellulose nanocrystals. Chemosphere 2020, 250, 126170. [Google Scholar] [CrossRef]
- Guo, C.; Wang, J.; Yang, M.; Li, Y.; Cui, S.; Zhou, X.; Li, Y.; Sun, Z. Amorphous silica nanoparticles induce malignant transformation and tumorigenesis of human lung epithelial cells via P53 signaling. Nanotoxicology 2017, 11, 1176–1194. [Google Scholar] [CrossRef]
- Phuyal, S.; Kasem, M.; Rubio, L.; Karlsson, H.L.; Marcos, R.; Skaug, V.; Zienolddiny, S. Effects on human bronchial epithelial cells following low-dose chronic exposure to nanomaterials: A 6-month transformation study. Toxicol. Vitr. 2017, 44, 230–240. [Google Scholar] [CrossRef]
- Lohcharoenkal, W.; Wang, L.; Stueckle, T.A.; Dinu, C.Z.; Castranova, V.; Liu, Y.; Rojanasakul, Y. Chronic exposure to carbon nanotubes induces invasion of human mesothelial cells through matrix metalloproteinase-2. ACS Nano 2013, 7, 7711–7723. [Google Scholar] [CrossRef]
- Kiratipaiboon, C.; Stueckle, T.A.; Ghosh, R.; Rojanasakul, L.W.; Chen, Y.C.; Dinu, C.Z.; Rojanasakul, Y. Acquisition of Cancer Stem Cell-like Properties in Human Small Airway Epithelial Cells after a Long-term Exposure to Carbon Nanomaterials. Environ. Sci. Nano 2019, 6, 2152–2170. [Google Scholar] [CrossRef]
- Sridharan, S.; Taylor-Just, A.; Bonner, J.C. Osteopontin mRNA expression by rat mesothelial cells exposed to multi-walled carbon nanotubes as a potential biomarker of chronic neoplastic transformation in vitro. Toxicol. Vitr. 2021, 73, 105126. [Google Scholar] [CrossRef]
- Ballesteros, S.; Barguilla, I.; Marcos, R.; Hernandez, A. Nanoceria, alone or in combination with cigarette-smoke condensate, induce transforming and epigenetic cancer-like features in vitro. Nanomedicine 2021, 16, 293–305. [Google Scholar] [CrossRef]
- Stueckle, T.A.; Davidson, D.C.; Derk, R.; Kornberg, T.G.; Schwegler-Berry, D.; Pirela, S.V.; Deloid, G.; Demokritou, P.; Luanpitpong, S.; Rojanasakul, Y.; et al. Evaluation of tumorigenic potential of CeO(2) and Fe(2)O(3) engineered nanoparticles by a human cell in vitro screening model. NanoImpact 2017, 6, 39–54. [Google Scholar] [CrossRef]
- Setyawati, M.I.; Sevencan, C.; Bay, B.H.; Xie, J.; Zhang, Y.; Demokritou, P.; Leong, D.T. Nano-TiO(2) Drives Epithelial-Mesenchymal Transition in Intestinal Epithelial Cancer Cells. Small 2018, 14, e1800922. [Google Scholar] [CrossRef]
- Luanpitpong, S.; Wang, L.; Castranova, V.; Rojanasakul, Y. Induction of stem-like cells with malignant properties by chronic exposure of human lung epithelial cells to single-walled carbon nanotubes. Part. Fibre Toxicol. 2014, 11, 22. [Google Scholar] [CrossRef]
- Wang, P.; Voronkova, M.; Luanpitpong, S.; He, X.; Riedel, H.; Dinu, C.Z.; Wang, L.; Rojanasakul, Y. Induction of Slug by Chronic Exposure to Single-Walled Carbon Nanotubes Promotes Tumor Formation and Metastasis. Chem. Res. Toxicol. 2017, 30, 1396–1405. [Google Scholar] [CrossRef]
- Uboldi, C.; Giudetti, G.; Broggi, F.; Gilliland, D.; Ponti, J.; Rossi, F. Amorphous silica nanoparticles do not induce cytotoxicity, cell transformation or genotoxicity in Balb/3T3 mouse fibroblasts. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2012, 745, 11–20. [Google Scholar] [CrossRef]
- Dusinska, M.; Tulinska, J.; El Yamani, N.; Kuricova, M.; Liskova, A.; Rollerova, E.; Rundén-Pran, E.; Smolkova, B. Immunotoxicity, genotoxicity and epigenetic toxicity of nanomaterials: New strategies for toxicity testing? Food Chem. Toxicol. 2017, 109 (Pt 1), 797–811. [Google Scholar] [CrossRef]
- Dusinska, M.; Mariussen, E.; Rundén-Pran, E.; Hudecova, A.M.; Elje, E.; Kazimirova, A.; El Yamani, N.; Dommershausen, N.; Tharmann, J.; Fieblinger, D.; et al. In Vitro Approaches for Assessing the Genotoxicity of Nanomaterials. Methods Mol. Biol. 2019, 1894, 83–122. [Google Scholar]
- Wu, P.; Yuan, S.S.; Ho, C.C.; Hsieh, W.Y.; Hong, Q.S.; Yu, S.L.; Chen, W.; Chen, H.Y.; Wang, C.D.; Li, K.C.; et al. Focal amplification of HOXD-harboring chromosome region is implicated in multiple-walled carbon nanotubes-induced carcinogenicity. Nano Lett. 2013, 13, 4632–4641. [Google Scholar] [CrossRef]
- Seidel, C.; Kirsch, A.; Fontana, C.; Visvikis, A.; Remy, A.; Gaté, L.; Darne, C.; Guichard, Y. Epigenetic changes in the early stage of silica-induced cell transformation. Nanotoxicology 2017, 11, 923–935. [Google Scholar] [CrossRef] [PubMed]
- Shivapurkar, N.; Reddy, J.; Chaudhary, P.M.; Gazdar, A.F. Apoptosis and lung cancer: A review. J. Cell. Biochem. 2003, 88, 885–898. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Luanpitpong, S.; Castranova, V.; Tse, W.; Lu, Y.; Pongrakhananon, V.; Rojanasakul, Y. Carbon nanotubes induce malignant transformation and tumorigenesis of human lung epithelial cells. Nano Lett. 2011, 11, 2796–2803. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Despeaux, E.; Stueckle, T.A.; Chi, A.; Castranova, V.; Dinu, C.Z.; Wang, L.; Rojanasakul, Y. Role of mesothelin in carbon nanotube-induced carcinogenic transformation of human bronchial epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 311, L538–L549. [Google Scholar] [CrossRef]
- Chen, D.; Stueckle, T.A.; Luanpitpong, S.; Rojanasakul, Y.; Lu, Y.; Wang, L. Gene expression profile of human lung epithelial cells chronically exposed to single-walled carbon nanotubes. Nanoscale Res. Lett. 2015, 10, 12. [Google Scholar] [CrossRef]
- Beck, B.; Blanpain, C. Unravelling Cancer stem cell potential. Nat. Rev. Cancer 2013, 13, 727–738. [Google Scholar] [CrossRef]
- Xu, Y.; Tokar, E.J.; Sun, Y.; Waalkes, M.P. Arsenic-transformed malignant prostate epithelia can convert noncontiguous normal stem cells into an oncogenic phenotype. Environ. Health Perspect. 2012, 120, 865–871. [Google Scholar] [CrossRef]
- Voronkova, M.A.; Luanpitpong, S.; Rojanasakul, L.W.; Castranova, V.; Dinu, C.Z.; Riedel, H.; Rojanasakul, Y. SOX9 Regulates Cancer Stem-Like Properties and Metastatic Potential of Single-Walled Carbon Nanotube-Exposed Cells. Sci. Rep. 2017, 7, 11653. [Google Scholar] [CrossRef]
- Guo, C.; You, D.Y.; Li, H.; Tuo, X.Y.; Liu, Z.J. Spherical silica nanoparticles promote malignant transformation of BEAS-2B cells by stromal cell-derived factor-1α (SDF-1α). J. Int. Med. Res. 2019, 47, 1264–1278. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Duan, Y.; Cai, X.; You, D.; Zhou, F.; Yang, C.; Tuo, X.; Liu, Z. Blockage of TGF-α Induced by Spherical Silica Nanoparticles Inhibits Epithelial-Mesenchymal Transition and Proliferation of Human Lung Epithelial Cells. BioMed Res. Int. 2019, 2019, 8231267. [Google Scholar] [CrossRef]
- Polimeni, M.; Gulino, G.R.; Gazzano, E.; Kopecka, J.; Marucco, A.; Fenoglio, I.; Cesano, F.; Campagnolo, L.; Magrini, A.; Pietroiusti, A.; et al. Multi-walled carbon nanotubes directly induce epithelial-mesenchymal transition in human bronchial epithelial cells via the TGF-β-mediated Akt/GSK-3β/SNAIL-1 signalling pathway. Part. Fibre Toxicol. 2016, 13, 27. [Google Scholar] [CrossRef]
- Luanpitpong, S.; Wang, L.; Castranova, V.; Dinu, C.Z.; Issaragrisil, S.; Chen, Y.C.; Rojanasakul, Y. Induction of cancer-associated fibroblast-like cells by carbon nanotubes dictates its tumorigenicity. Sci. Rep. 2016, 6, 39558. [Google Scholar] [CrossRef]
- Gliga, A.R.; Di Bucchianico, S.; Lindvall, J.; Fadeel, B.; Karlsson, H.L. RNA-sequencing reveals long-term effects of silver nanoparticles on human lung cells. Sci. Rep. 2018, 8, 6668. [Google Scholar] [CrossRef]
- Ellinger-Ziegelbauer, H.; Aubrecht, J.; Kleinjans, J.C.; Ahr, H.J. Application of toxicogenomics to study mechanisms of genotoxicity and carcinogenicity. Toxicol. Lett. 2009, 186, 36–44. [Google Scholar] [CrossRef]
- Waters, M.D.; Jackson, M.; Lea, I. Characterizing and predicting carcinogenicity and mode of action using conventional and toxicogenomics methods. Mutat. Res. 2010, 705, 184–200. [Google Scholar] [CrossRef]
- Parfett, C.L.; Desaulniers, D. A Tox21 Approach to Altered Epigenetic Landscapes: Assessing Epigenetic Toxicity Pathways Leading to Altered Gene Expression and Oncogenic Transformation In Vitro. Int. J. Mol. Sci. 2017, 18, 1179. [Google Scholar] [CrossRef]
- Akagi, T. Oncogenic transformation of human cells: Shortcomings of rodent model systems. Trends Mol. Med. 2004, 10, 542–548. [Google Scholar] [CrossRef]
- Mo, Y.; Zhang, Y.; Zhang, Y.; Yuan, J.; Mo, L.; Zhang, Q. Nickel nanoparticle-induced cell transformation: Involvement of DNA damage and DNA repair defect through HIF-1α/miR-210/Rad52 pathway. J. Nanobiotechnol. 2021, 19, 370. [Google Scholar] [CrossRef]
| Nanomaterials (Name, Size, Surface Modification, Shape etc.) | Cell Model | Exposure | Assays | Main Findings | References | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dose | Time | Cancer Phenotypic Hall Mark | Genotoxicity | Epigenetic Markers | Other Related Assays | |||||
| Cobalt Nanoparticles (CoNP) | Size (<50 nm), density (8.9 g/mL), surface area (>15 m2/g) * | Mouse embryonic fibroblasts (MEF Ogg1+/+) and MEF Ogg1−/−) | 0.05 and 0.1 µg/mL | 12 weeks | Anchorage-independent cell growth (soft-agar assay), morphology, MMP 2 & MMP 9 secretion, | Comet assay (DNA damage and oxidative DNA damage with FPG comet) | N/A | Cellular uptake, cell viability, ROS formation, gene expressions | CoNPs may pose a carcinogenic risk by inducing oxidative DNA damage, as suggested by increased sensitivity of MEF Ogg1−/− | [37] |
| Size (<50 nm), density (8.9 g/mL), surface area (>15 m2/g) * | Mouse embryonic fibroblasts (MEF Ogg1+/+) and MEF Ogg1−/−) | 0.1 µg/mL | 12 weeks for MEF Ogg1−/−) & 6 weeks after MTH1 knockdown (KD) (shRNA) | Anchorage-independent cell growth (soft-agar assay), cell migration and invasion assays | N/A | N/A | Cell viability, MTH1 gene expressions | MTH1 (decreased phenotype in KD cell lines) is a significant contributor to NP-induced carcinogenicity | [38] | |
| Zinc oxide Nanoparticles (ZnO-NP) | Size (<100 nm), surface area (>15–25 m2/g) | Mouse embryonic fibroblasts (MEF Ogg1+/+) and MEF Ogg1−/−) | 1 µg/mL | 12 weeks | Anchorage-independent cell growth (soft-agar assay), cell migration and invasion assays | N/A | N/A | Cell viability, MTH1 gene expressions | MTH1 elicits as a relevant player in the NP-induced toxicity and carcinogenicity | [38] |
| Size (<100 nm), surface area (>15–25 m2/g) | Mouse embryonic fibroblasts (MEF Ogg1+/+) and MEF Ogg1−/−) | 1 μg/mL | 12 weeks | Anchorage-independent cell growth (soft-agar assay), MMP2 and MMP9 secretion | Comet assay (DNA damage and oxidative DNA damage with FPG comet) | N/A | Cellular uptake, cell viability and internalization, ROS formation, gene expressions | Both cell types did not show any cellular transformation | [39] | |
| size distribution 35.6 ± 32.0 nm. | Mouse colon epithelial cells (IMEC) | 1 μg/mL | 30 passages | Anchorage-independent cell growth (soft-agar assay), wound-healing assay, xenograft tumorigenesis (in nude mice) | N/A | N/A | Cellular uptake, ROS formation, protein expression, knockdown of CXCR2 | The CXCR2/NF-kB/STAT3/ERK and AKT pathways may be responsible for malignant transformation | [40] | |
| Silver Nanoparticles (AgNP) | 1–80 nm in size, diameter of <100 nm (average—80.0 ± 6.0 nm) | Balb/c 3T3 A31-1-1 mouse cell | 0.17, 0.66, 2.65, 5.30, and 10.60 μg/mL | 72 h | Cell transformation assay | A cytokinesis-block micronucleus (CBMN) assay | N/A | Cytoxicity assay (colony formation) | The frequency of morphological malignant transformation increased significantly in a dose-dependent manner | [41] |
| 1–80 nm in size, diameter of <100 nm (average—80.0 ± 6.0 nm) | BEAS-2B cells | 0.13 and 1.33 µg/mL | 4 months (40 passages) | Anchorage-independent cell growth (soft-agar assay), cell migration and invasion assays | N/A | N/A | Cell viability assay, EMT/MAPK proteins expressions, anti-apoptotic-related gene/protein expressions | The complex regulation of JNK, p38, p53, and ERK1/2 signalling pathways and activation of MMP-9/TIMP-1 were found to mediate malignant cell transformation | [42] | |
| 8.52 ± 1.82 nm in size and 83.52 ± 0.70 nm in diameter |
Caco-2 cells | 0.5 and 1 µg/mL | 6 weeks (assessment on 2nd, 4th, and 6th week) | Anchorage-independent cell growth (soft-agar assay), cell migration and invasion assays, secretion MMP2 and MMP9, ability to promote the growth of another tumor cell line (HCT116) with conditioned medium from 72 h exposed Caco-2 cells | N/A | N/A | Cellular uptake, measurement of release of Ag ion | Potential carcinogenic risk associated with long-term exposure | [43] | |
| Citrate coated Silver Nanoparticles size: 10 nm and 75 nm | BEAS-2B cells | 1 µg/mL or approx. 0.2 µg/cm2 | 6 weeks (assessment on 2rd, 4th, and 6th week | Anchorage-independent cell growth (soft-agar assay), cell migration and invasion assays, E- and N-cadherin expression (EMT assays), collagen analysis | Comet and micronucleus assays | Genome-wide DNA methylation analysis | Intracellular uptake, transcriptomics analysis (RNA-seq) | Induce fibrosis (pro-fibrotic), EMT, and cell transformation | [44] | |
| Silica nanoparticles | Synthetic amorphous silica nanoparticles (SAS) NM-200 and NM-201, NM-202 and NM-203 * | Bhas 42 cells | 2 μg/cm2 to 80 μg/cm2 | 21 days | Bhas 42 cell transformation assay (by following OECD guidelines) | N/A | N/A | N/A | SAS may act as tumor promoters | [45] |
| Synthetic amorphous silica nanoparticles (SAS) NM-203 | Bhas 42 cells | 1 μg/cm2 to 40 μg/cm2 | 21 days | Bhas 42 cell transformation assay (by following OECD guidelines) | N/A | N/A | Cell proliferation, transcriptomics (microarray) | A 12-gene signature could potentially serve as an early “bio-marker” of cell transformation | [46] | |
| Amorphous silica nanoparticle (NM-203) and crystalline silica particle (Min-U-SilVR 5) | Bhas 42 cells | NM-203: 0 to 5 μg/cm2; Min-U-SilVR 5: 0 to 25 μg/cm2 | 21 days Cell pellets were collected on Day 6 (D6) for epigenetic modification analysis. | No phenotype of cell transformation assays | N/A | Global DNA methylation, global histone acetylation (ELISA) with DNMTs and HDACs protein expressions, gene specific epigenetic analysis for c-Myc promoter (ChIP-qPCR) | c-Myc expression | Min-U-SilVR 5 reduced global DNA methylation and increased expression of DNMT3a, DNMT3b, histone H4 acetylation, and HDAC protein levels. NM-203 treatment showed no changes in epigenetic modification. Modulated parameters at D6 were restored in transformed cells at D21 | [47] | |
| Amorphous silica nanoparticles (SiNPs) Diameter: 57.66 ± 7.30 nm | Human lung epithelial (BEAS-2B) cells | 5 µg/mL | 18 weeks (40 passages) | Enhanced cellular proliferation (MTT), anchorage-independent cell growth (soft-agar assay), and increased cell migration (wound-healing assay), xenograft tumorigenesis (in nude mice) | N/A | N/A | Morphology and proliferation assay, Cell-cycle assessment, genome-wide transcriptional analysis (microarray), gene and protein expressions (qRT-PCR and Western blotting) | Induced malignant transformation via p-53 signalling | [48] | |
| Amorphous silica nanoparticles (aSiO2NPs) NM-200, NM-203, NRT-808, NRT-817, NRT-820, NRT-944 | Balb/3T3 mouse fibroblasts | 1, 10, and 100 µg/mL | 72 h | Balb/3T3 cell transformation assay, colony forming efficiency | Cytokinesis-block micronucleus assay | N/A | Cell viability | No cyto-genotoxic effect and no induction of morphological transformation | [49] | |
| Amorphous silica nanoparticles (SiO2-NP) size: 19 nm, surface area: 147 m2/g | Human lung epithelial (BEAS-2B) cells | ~0.24 μg/cm2 delivered dose (0.6 μg/cm2 administered dose and) | 6.5 months | Attachment-independent colony formation (soft-agar colony formation assay) (55, 83, 111, 138, 174, 202 days measurements) | Induction of double-stranded DNA damage (γ-H2AX immunostaining assay) | N/A | Particle uptake (TEM), cell proliferation (WST-1) and ROS production, intracellular iron and lysosome counts (LysoTracker) | No significant changes in attachment-independent colony formation throughout the exposure period | [50] | |
| Nano silicon dioxide (Nano-SiO2) | Human lung epithelial (16HBE and BEAS-2B cells and) | 10.0 µg/mL for 16HBE cells and 40.0 mg/mL for BEAS-2B cells | 32 passages for 16HBE and 45 passages for BEAS-2B cells | Anchorage-independent cell growth (soft-agar assay), wound-healing assay, enhanced cellular proliferation (MTT), xenograft tumorigenesis (in nude mice) | N/A | 5mC content detection, DNMT enzyme activity, promoter methylation analysis (for NRF2 with MSP-PCR | Selected gene and protein expressions, cell transfection for NRF2 gene knockdown and overexpression | Induces malignant cellular transformation through global DNA hypomethylation. Demethylation of the NRF2 promoter activates NRF2 expression, which is essential in protecting against carcinogenesis induced by Nano-SiO2 | [51] | |
| Nickel nanoparticles (NiNPs) | Size: <100 nm, BET-area of 6.41 m2/g), nickel(II) oxide NPs (NiO, <50 nm, BET-area of 102 m2/g) | Human lung epithelial (BEAS-2B) cells | 0.5 µg/mL | 6 weeks | Anchorage-independent cell growth (soft-agar assay), cell migration and invasion assays | DNA strand breaks (comet assay), micronucleus (Flow Cytometric), cell cycle | N/A | Cytotoxicity, whole-genome gene expression analysis (RNA-seq), intracellular Ni level | No significant changes were observed in cell transformation or cell motility. DNA strand breaks were observed, but no induction of micronuclei was seen. Gene expression changes included calcium-binding proteins (S100A14 and S100A2) and genes such as TIMP3, CCND2, EPCAM, IL4R, and DDIT4 | [52] |
| Size: 20 nm, composed of anatase (90%) and rutile (10%), specific surface area is 43.8 m2/g | Human lung epithelial (BEAS-2B) cells | 0.25 and 0.5 µg/mL | ~150 days (21 cycles) | Anchorage-independent cell growth (soft-agar assay) | DNA damage response (DDR)-associated proteins expression | miRNA (miR-210) expression (qPCR) | HIF-1α/miR-210/Rad52 pathway gene expression | Exposure-induced DNA damage and DNA repair defects through HIF-1α/miR-210/Rad52 pathway likely contribute to genomic instability and ultimately cell transformation | [52] | |
| TiO2 nanoparticles (TiO2-NP) | diameter <100 nm | Mouse embryonic (NIH 3T3) | 10 μg/mL | 12 weeks | Anchorage-independent growth assay (soft-agar colony formation assay), enhanced cellular proliferation (MTT and colony formation), | Micronucleus formation, cell-cycle analysis (flow cytometry), perturbed mitosis and cytokinesis | N/A | Cell viability, ROS, apoptosis, intracellular TiO2 level, MEK/ERK signalling pathway | Disrupts cell-cycle progression, causing chromosomal instability and cell transformation. PLK1 has been identified as the target for nano-TiO2 in the regulation of mitotic progression | [53] |
| NM102: 21.7 ± 0.6 nm | Human lung epithelial (BEAS-2B) cells | 0 to 20 μg/mL | 4 weeks | Anchorage-independent growth assay (soft-agar colony formation assay) | Comet and micronucleus (MN) assays | N/A | Cell uptake, ROS | No ROS formation or genotoxic effects were observed, but there was a significant increase in transformed cell colonies, indicating a potential carcinogenic risk associated with nano-TiO2 exposure, which does not involve a genotoxic mechanism | [54] | |
| NM-102 size: 20.1 ± 7.4 nm * | Human lung epithelial (BEAS-2B) cells | 10 µg/mL equivalent to 1.34 µg/cm2 | 6 weeks (endpoints analyzed at 3rd week and 6th week) | Anchorage-independent growth assay (soft-agar colony formation assay) in previously reported studies [54,55] | N/A | miRNA expression with qPCR (selected 33) | Cell viability, uptake | A set of five miRNAs (miR-23a, miR-25, miR-96, miR-210, and miR-502) were identified as informative biomarkers of NM-induced transformed cells | [56] | |
| NM62002 and KC7000 size: NM62002 1026 ± 895 nm, KC7000 4422 ± 1644 nm (FP7- NANoREG project) | Human bronchial epithelial cell line (HBEC-3KT) | 1.92 and 0.96 μg/cm2 | 6 months (26 weeks) | Anchorage-independent growth assay (soft-agar colony formation assay) at 4, 8, 12, 16, 20, 24, and 26 weeks expansion of colonies picked from soft agar | N/A | N/A | Cytotoxicity | Exposure to NM62002, but not KC70000, led to cell transformation at week 12. However, the potential for colony formation was significantly reduced from weeks 12 to 16 | [57] | |
| Aeroxide TiO2 (80:20 anatase/rutile structure) size: 23 nm | Human lung epithelial (BEAS-2B) cells | ~0.57 μg/cm2 delivered dose (0.6 μg/cm2 administered dose and) | 6.5 months (55, 83, 111, 138, 174, 202 days measurements) | Anchorage-independent growth assay (soft-agar colony formation assay) | Induction of double-stranded DNA damage (γ-H2AX immunostaining assay) | N/A | Intracellular uptake, cell proliferation, ROS production, intracellular iron and lysosome counts | Colony formation showed a significant 1.3-fold increase at 111 and 138 days but returned to levels similar to the non-treated control cells by 174 days and remained at baseline levels for the rest of the exposure period | [50] | |
| pristine (uncoated), surface modification with citrate and/or silica * | Balb/3T3 mouse fibroblasts | 0 to 40 μg/cm2 | 72 h (followed by 31–35 days continued culture in clean media) | Balb/3T3 cell transformation assay, colony forming efficiency | DNA damage (comet assay), Cytokinesis-block micronucleus cytome assay | N/A | Cell viability, cell death (apoptosis/necrosis) | No cell transformation was evident | [58] | |
| Zirconia nanoparticle (ZrO2-NP) | pristine (uncoated), surface modification with citrate and/or silica * | Balb/3T3 mouse fibroblasts | 0 to 40 μg/cm2 | 72 h (followed by 31–35 days continued culture in clean media) | Balb/3T3 cell transformation assay, colony forming efficiency | DNA damage (comet assay), Cytokinesis-block micronucleus cytome assay | N/A | Cell viability, cell death (apoptosis/necrosis) | Induce cell transformation, except silica coated one | [58] |
| Iron Oxide Nanoparticle (Fe2O3-NP) | (i) no coating (nFe2O3) size: 19.6 nm, surface area: 42 m2/g) (ii) amorphous silica coating (SiO2-nFe2O3) size: 21.3 nm surface area: 49 m2/g (iii) gas metal arc mild steel welding fumes (GMA-MS) size: 15–45 nm * | Human lung epithelial (BEAS-2B) cells | nFe2O3 ~0.58 μg/cm2, SiO2-nFe2O3 ~0.55 μg/cm2 (delivered dose) (0.6 μg/cm2 administered dose) | 6.5 months | Anchorage-independent growth assay (soft-agar colony formation assay) | Induction of double-stranded DNA damage (γ-H2AX immunostaining assay) | N/A | Intracellular uptake, cell proliferation, ROS production, intracellular iron and lysosome Counts | nFe2O3, but not SiO2-nFe2O3, induced a neoplastic-like phenotype, as evidenced by a significant increase in colony formation at 83 days and 138 days, which was maintained through the exposure period. No significant colony formation was observed at 55 days. GMA-MS-exposed cells had a significant increase in colony number | [50] |
| ferric oxide (nFe2O3) nanoparticles | Human primary small airway epithelial cells (pSAECs) | 0.6 μg/cm2 | 10 weeks (detection at 6th and 10th week) | Anchorage-independent cell growth (soft-agar assay), cell migration and invasion assays, increased proiferation | N/A | N/A | Intracellular uptake, ROS formation, CD71, DMT1, SLC40A1, FTH1expressions, | nFe2O3-exposed cells exhibited immortalization and retention of the malignant phenotype | [59] | |
| Cerium oxide | cerium oxide (nCeO2) nanoparticles | Human primary small airway epithelial cells (pSAECs) | 0.6 μg/cm2 | 10 weeks (detection at 6th and 10th week) | Anchorage-independent cell growth (soft-agar assay), cell migration and invasion assays, cell proliferation | N/A | N/A | Intracellular uptake, ROS formation, CD71, DMT1, SLC40A1, FTH1expressions | Increased proliferative capacity but no cell transformation ability | [59] |
| size: 9.52 ± 0.66 nm with/without Cigarette smoke condensate (CSC) | Human lung epithelial (BEAS-2B) cells | 1 and 5 μg/mL of CSC (CSC1 and CSC5), 2.5 μg/mL of CeO2NP alone or the Ce + CSC1 and Ce + CSC5 | 6 weeks | Anchorage-independent cell growth (soft-agar assay), cell proliferation, cell morphology, cell proliferation, wound-healing assay, secretion of MMP-9, FRA-1 as a biomarker of carcinogenesis | N/A | N/A | Cell viability, uptake (TEM), selected gene expressions | Although CeO2NP did not demonstrate any transforming ability, it was found to have a synergistic effect with CSC, enhancing the transforming effects of CSC and exacerbating the expression of FRA-1 | [60] | |
| Size: <25 nm and density 7.13 g/mL) with/without Cigarette smoke | Human lung epithelial (BEAS-2B) cells | 5 μg/mL of CSC, 2.5 μg/mL of nanoceria, and the combinations of both compounds (CeO2NPs plus CSC) | 6 weeks | Invasion assay, tumorsphere formation assay | N/A | miRNA expression with qPCR (selected 33) | Cell viability, uptake (TEM) | Induces cell transformation and exhibits a positive interaction with the cell-transforming effects of cigarette smoke condensate | [61] | |
| Nanocellulose | Cellulose nanocrystals (CNC) (powder and gel (10% wt.)) | Human lung epithelial (BEAS-2B) cells | 30 μg/cm2 | 4 weeks | Anchorage-independent cell growth (soft-agar assay), cell migration and invasion assays, cell proliferation, cell morphology | DNA damage (OxiSelect™ Comet assay) | N/A | Intracellular uptakes, oxidative stress assays, generation, inflammation marker assessment, apoptosis assay | Cellular transformation, enhanced invasion/migration, triggered oxidative stress and inflammatory response, and induced DNA damage were evident | [62] |
| Nano plastics | polystyrene nanoplastics (PSNPLs) size: 45.91 nm with/without Arsenic (ASIII) * | Mouse embryonic fibroblasts (MEF Ogg1+/+) and MEF Ogg1−/−) | 25 µg/mL PSNPLs; 2 µM AsIII, and combination of both (25 µg/mL PSNPLs + 2 µM AsIII) | 12 weeks | Anchorage-independent cell growth (soft-agar assay), cell migration and invasion assays, cell proliferation, cell morphology, tumorsphere formation | DNA damage (comet assay) | N/A | Physical interaction of PSNPLs and ASIII, intracellular uptake | Under co-exposed conditions, the PSNPLs showed the highest level of increased DNA damage and aggravated cellular transformation, followed by ASIII. The general order of the tested endpoints was PSNPLs ≤ ASIII < co-exposure of (PSNPLs + ASIII). | [63] |
| Carbon-based nanomaterials Multiwalled carbon nanotubes (MWCNTs) Carbon-based nanomaterials (CNMs) carbon nanotubes (CNTs) single-walled (SWCNT) | MWCNTs, diameter: 9.5 nm; length: <1–1.5 µm Pristine and Functionalised (-NH2, -OH, -COOH) | Balb/3T3 | 1, 10, and 100 µg/mL | 72 h | Colony forming efficiency and cell morphological transformation (31 days) | Micronucleus assay | N/A | Cell uptake, cytotoxicity | Clear evidence of morphological transformation without cytotoxic and genotoxic effects | [64] |
| tangled (tMWCNT) and rigid (rMWCNT) | Normal rat mesothelial (NRM2) cells | 0.1 μg/mL | 45 weeks (>85 passages) | Cell morphology, cell invasion | N/A | N/A | Osteopontin mRNA expressions (biomarker) | An invasive phenotype and increased OPN mRNA expression were observed in rMWCNTs, but not tMWCNT-exposed condition | [65] | |
| MWCNT diameter: 110 nm–170 nm; length: 5 μm−9 μm | Human pleural mesothelial (MeT-5A) cells | 10 μg/cm2 | 1 year | Anchorage-independent growth (soft-agar assay), wound-healing assay | N/A | MicroRNA profiling | Application of miR221 mimics, ANNEXIN A1 expressions | The miR221-annexin a1 axis regulates cell migration in the induced transformed cells | [66] | |
| CNMs: MWCNTs SWCNTs UFCB ASB * | Primary human SAECs (immortalised with hTERT) cells | 0.02 μg/cm2, equivalent to 0.1 μg/mL | 6 months | Anchorage-independent growth (soft-agar assay), spheroid formation, Anoikis and apoptosis assays | DNA-strand breaks (γ-H2AX), DNA damage response (p53) | Stem cells marker assessment | Genotoxicity and CSC-like properties were evident in all CNM-exposed conditions. Gene signalling networks suggest involvement of SOX2 and SNAI1 signalling in cell transformation | [67] | ||
| MWCNT (i) NM-400 diameter: 351 ± 140 nm and (ii) NM-401 Diameter: 710 ± 20 nm | human bronchial epithelial cell line (HBEC-3KT) | 1.92 and 0.96 μg/cm2 | 6 months (26 weeks) (4th, 8th, 12th, 16th, 20th, 24th, and 26th week for assessments) | Anchorage-independent growth assay (soft-agar colony formation assay), expanding single colonies selected from soft agar | N/A | N/A | Cytotoxicity | NM-400, but not the agglomerated NM-401, showed cell transformation | [57] | |
| MWCNT, NM403 diameter: 12.0 ± 7.0 nm | Human lung epithelial (BEAS-2B) cells | 1, 10 or 20 µg/mL | 4 weeks | Anchorage-independent growth assay (soft-agar colony formation assay) | Comet assay and micronucleus (MN) assays | N/A | Detection of ROS and different interleukins (IL) such as IL-1B, IL-6 and IL-8, as well as HO-1 | Increase in transformed cell colonies and decreased cytokine expression; no primary DNA damage but chromosome damage were observed | [55] | |
| MWCNTs Inner diameter: 2–10 nm Outer diameter: 10–30 nm Length: 1–30 µm | Human lung epithelial (BEAS-2B) cells | 1 μg/mL (0.16 μg/cm2) | 40 passages | Anchorage-independent growth assay (soft-agar colony formation assay), in vivo tumorigenicity assay | Cytokinesis-block micronucleus (CBMN) assay | N/A | Chromosomal instability (aCGH analysis), microarray, HOXD9 and HOXD13 gene function analysis (siRNA transfection) | Induction of irreversible oncogenic transformation and chromosomal aberration (in chromosome 2q31-32) may be attributed to HOXD9 and HOXD13, which are located in the same region | [68] | |
| Functionlised MWCNTs (fMWCNTs) (i) three-month aged as-prepared-(pMWCNT), (ii) carboxylated-(MW-COOH), and (iii) aminated-MWCNTs (MW-NHx) * | Human primary small airway epithelial cells (SAEC) | 0.06 µg/cm2 | 12 weeks (8th and 12th weeks) | Anchorage-independent cell growth (soft-agar assay), cell migration and invasion assays, cell proliferation, cell morphology | N/A | N/A | Intracellular uptake | The surface properties of aged fMWCNTs can induce cell transformation, while exposure to pMWCNTs and MW-COOH also result in significant invasion behaviour | [69] | |
| MWCNTs diameter: 30–40 nm length: 10–20 µm | Mono as well as co-culturing macrophages (THP-1) and mesothelial (MeT5A) cells | 0.1 mg/mL (in the co-cultured system, MeT5A cells in the upper chamber were exposed to MWCNTs only.) | 3 months | Cell proliferation (every 24 h until 6 days), cell migration and invasion assay, colony formation assay (2 weeks) | Chromosome aberration assay | N/A | inflammatory cytokines (IL-1βIL-8, TNF-a, and IL-6) assay, NF-κB/IL-6/STAT3 pathway gene and protein expressions, transcriptomics | The NF-κB (p65)/IL-6/STAT3 pathway, induced by MWCNT-induced inflammation, played a crucial role in the malignant transformation | [70] | |
| MWCNT NM-401 size: 5.9 ± 4.6 nm) * | Human lung epithelial (BEAS-2B) cells | 20 µg/mL of MWCNT, equivalent to 2.67 µg/cm2 | 6 weeks (endpoints analyzed at 3rd week and 6th week | Anchorage-independent growth assay (soft-agar colony formation assay) in previously reported studies [54,55] | N/A | miRNA expression with qPCR (selected 33) | Cell viability, uptake | A set of five miRNAs (miR-23a, miR-25, miR-96, miR-210, and miR-502) were identified as informative biomarkers of NM-induced transformed cells | [56] | |
| MWCNT Diameter: 81 ± 5 nm Length: 8.19 ± 1.7 µm and SWCNT Diameter: 1–4 nm Length: 1–4 µm * | Human primary small airway epithelial cells (SAEC) | 0.02 μg/cm2 equivalent to 0.1 μg/mL | 6 months | Anchorage-independent cell growth (soft-agar assay), cell migration and invasion assays, cell proliferation, cell morphology, angiogenesis assays | N/A | N/A | Intracellular uptake, whole-genome expressions (microarray) | MWCNTs and SWCNTs share similar gene signalling signatures that result in a neoplastic-like transformation phenotype | [71] | |
| MWCNT and SWCNT | Human pleural mesothelial (MeT5A) | 0.02 μg/cm2 (sub cytotoxic) | 4 months | Cell proliferation, cell migration and invasion, MMP-2 expressions | N/A | N/A | Whole-genome expression (microarray), expressions of MMP-2 and knockdown (shRNA) | Role of MMP-2 in CNT-induced cell transformation with cancer-like Properties, such as rapid growth and increased cell invasion and migration | [72] | |
| SWCNT | Human pleural mesothelial (MeT5A) | 0.02, 0.06, and 0.2 μg/cm2 | 2 months | Anchorage-independent growth (soft-agar colony formation), cell invasion | N/A | N/A | H-Ras expressions and siRNA transfection, ERK1/2 expressions and inhibition | induced neoplastic transformation linked to H-Ras and ERK1/2 signaling | [73] | |
| SWCNTs outer diameter: <2 nm and length: 5–30 μm | Human lung epithelial (BEAS-2B) cells | 10 μg/mL | 60 days | Anchorage-independent cell growth (soft-agar assay), cell migration and invasion assays, wound-healing assay, in vivo tumorigenicity assay | N/A | Genome-wide DNA methylation arrays | Cell viability, ROS, cell apoptosis, cell cycle, MMP analysis | DNA methylation and transcriptome dysregulation, with enrichment in cancer-related pathways resulting in ‘irreversible’ transformation | [74] | |
| SWCNT Diameter: 0.8–1.2 nm Length: 0.1–1 μm * | Human small airway epithelial cells (SAECs) | 0.02 μg/cm2 (physiologically relevant conc.) | 6 months | Anchorage-independent cell growth (soft-agar assay), cell migration and invasion assays, apoptosis assay, tumor sphere assay, in vivo tumorigenicity assay | N/A | N/A | p53 (GFP) expressions, human stem cell proteome array, stem cell surface markers expressions | Irreversible malignant transformation and self-renewal, with in vivo tumorigenesis phenotypes and aberrant expression of stem cell markers (Nanog, SOX-2, SOX-17, and E-cadherin) and surface markers (CD24low and CD133high), indicating the presence of SWCNT-induced cancer stem cells | [75] | |
| SWCNT Diameter: 0.8–1.2 nm Length: 0.1–1 μm * | Human lung epithelial (BEAS-2B) cells | 0.02 μg/cm2 equivalent to 0.1 μg/mL | 6 months | Anchorage-independent cell growth (soft-agar assay), cell migration and invasion assays, tumor sphere assay, in vivo tumorigenicity assay | N/A | N/A | SOX9 expressions, knockdown, ALDH activity | SOX9 plays a role in the formation of SWCNT-induced cancer-stem-like cells, tumor metastasis, and the expression of stem cell marker ALDH1A1 | [76] | |
| SWCNT Diameter: 0.8–1.2 nm Length: 0.1–1 μm | Human lung epithelial (BEAS-2B) cells | 0.02 μg/cm2 equivalent to 0.1 μg/mL | 6 months (24 weeks) | Anchorage-independent cell growth (soft-agar assay), cell migration and invasion assays, apoptosis assay, tumor sphere assay, angiogenesis assays, in vivo tumorigenicity assay | N/A | N/A | Protein array to evaluate apoptosis resistance mechanisms in transformed cells | p53-mediated apoptosis resistant in transformed cells | [77] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatterjee, N.; Alfaro-Moreno, E. In Vitro Cell Transformation Assays: A Valuable Approach for Carcinogenic Potentiality Assessment of Nanomaterials. Int. J. Mol. Sci. 2023, 24, 8219. https://doi.org/10.3390/ijms24098219
Chatterjee N, Alfaro-Moreno E. In Vitro Cell Transformation Assays: A Valuable Approach for Carcinogenic Potentiality Assessment of Nanomaterials. International Journal of Molecular Sciences. 2023; 24(9):8219. https://doi.org/10.3390/ijms24098219
Chicago/Turabian StyleChatterjee, Nivedita, and Ernesto Alfaro-Moreno. 2023. "In Vitro Cell Transformation Assays: A Valuable Approach for Carcinogenic Potentiality Assessment of Nanomaterials" International Journal of Molecular Sciences 24, no. 9: 8219. https://doi.org/10.3390/ijms24098219
APA StyleChatterjee, N., & Alfaro-Moreno, E. (2023). In Vitro Cell Transformation Assays: A Valuable Approach for Carcinogenic Potentiality Assessment of Nanomaterials. International Journal of Molecular Sciences, 24(9), 8219. https://doi.org/10.3390/ijms24098219