Abstract
This short review reports the surprising phenomenon of nuclear hyperpolarization occurring in chemical reactions, which is called CIDNP (chemically induced dynamic nuclear polarization) or photo-CIDNP if the chemical reaction is light-driven. The phenomenon occurs in both liquid and solid-state, and electron transfer systems, often carrying flavins as electron acceptors, are involved. Here, we explain the physical and chemical properties of flavins, their occurrence in spin-correlated radical pairs (SCRP) and the possible involvement of flavin-carrying SCRPs in animal magneto-reception at earth’s magnetic field.
1. Flavins: Electronic Properties, Reactivity, and Photochemistry
Flavin derivatives perform an outstanding role in the manifold of biological processes. These colorful compounds have unique photochemical and redox properties and, thus, possess interesting chemical and biological potential [1]. Extensive research has been conducted on flavoenzymes, which rely on a flavin cofactor. The mechanisms and structures of these enzymes have been thoroughly documented, with a particular focus on their diverse catalytic activities. The main structural feature of flavins is their reactive 7,8-dimethyl-10-alkylisoalloxazine core structure that is part of the three most important flavins in nature (-)-riboflavin (RF, 1), flavin mononucleotide (FMN, 2), and flavin adenine dinucleotide (FAD, 3) (Figure 1). RF (1), widely known as vitamin B2, performs a key role in energy metabolism [2]. Furthermore, it serves as the starting material for the in vivo synthesis of 2 and 3. They are the biologically active forms of RF (1) and act as rather non-covalently than covalently bound cofactors in most flavoenzymes [3,4,5,6].
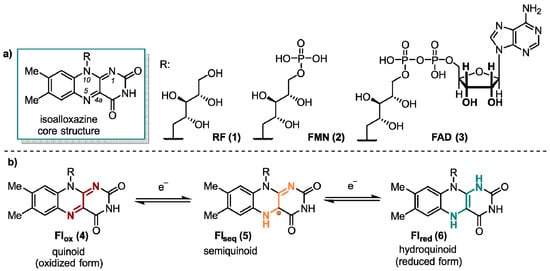
Figure 1.
(a) Structural composition of the 7,8-dimethyl-10-alkylalloxazine core with its biologically most relevant derivatives all substituted at the N(10)-position. (b) The most common redox states of flavin derivatives.
Since the first studies on enzyme class, containing FMN (2) or FAD (3) as a prosthetic group, in the middle 1930s by Warburg [7], Krebs [8], and Theorel [9], it is known that flavins perform a crucial role in numerous oxidation and reduction reactions. This includes the four primary energy metabolism systems, photosynthesis, aerobic respiration, denitrification, and sulfur respiration. Unlike other cofactors, flavins are, in principle, able to handle both one-electron (single-electron transfer, SET) and two-electron transfer processes [10]. This special ability is due to the three redox states of the isoalloxazine moiety. The quinoid form 4 (Flox), which possesses a conjugated π-system between the rings, is a powerful oxidant, which constitutes the primary reactivity towards substrates. In addition, flavins exist in their radical Flseq semiquinone (5) and the completely reduced Flred form (6, Figure 1).
The typical redox potential for free RF (1) in solution at pH 7 is Em = −200 mV. Em is the so-called two-electron midpoint reduction potential and is defined as the average value of both oxidation/reduction steps. This is due to the frequently occurring low stability of the one-electron reduced state 5, which results from the alteration of the planarity when the redox state changes [11]. However, in enzymes, the Em value of RF (1) varies drastically from −400 mV to +60 mV, because of stabilizing effects on the individual oxidation states originating from the protein’s environment [12,13,14].
One of the most important oxidizing agents in enzymatic flavin catalysis is molecular oxygen. Due to the high reduction potential of O2, it is an excellent oxidant from a thermodynamic point of view [15]. Flavin-dependent oxidases and monooxygenases utilize O2 to perform oxygen transfer reactions, such as Baeyer-Villiger oxidations [16] and hydroxylation-dehydrogenations [17]. For both enzyme classes, the transformation starts with a SET from ground-state triplet O2 to the reduced ground-state singlet flavin 6 forming a caged radical pair 7 (Figure 2). In case of oxidases, O2 is used just as an electron acceptor, yielding hydrogen peroxide and the oxidized cofactor 4 after a second SET from the flavin cofactor to the superoxide. However, monooxygenases form C(4a)-hydroperoxyflavin (8) as the key reactive intermediate from singlet-triplet interactions. After inserting an oxygen atom in the substrate hydroxyflavin (9) is generated that gives oxidized flavin 4 after the loss of water [18]. Uncontrolled reduction in dioxygen associated with a spontaneous collapse of the caged radical pair or the peroxyflavin often produces toxic reactive oxygen species (ROS) as side products that may react non-specifically with cellular components and, thus, are toxic [19]. Thus, nature developed oxygen-utilizing enzymes controlling and fine-tuning the oxidation potential of oxygen [20]. The two flavoenzyme classes mentioned above react rapidly with their small-molecule oxidizer, while dehydrogenases, a group of enzymes also categorized as O2-responsive flavoenzymes, interact in most cases very slowly with oxygen [21]. Dehydrogenases yield a mixture of H2O2 and a very reactive superoxide anion. In general, enzymes require certain tactics to improve the rate of the reaction with oxygen. Improving accessibility to O2 in the protein environment and stabilizing the trapped [O2− FlH●] are here among the most important tools [14].
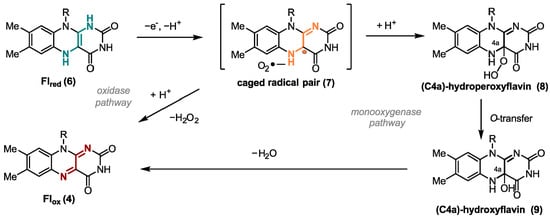
Figure 2.
Different SET reaction pathways of the flavin cofactor.
Contrary to the above-mentioned mechanisms utilizing SET processes, certain flavoenzymes possess the ability of a two-electron transfer [22]. One example of such enzymes are flavin-dependent halogenases. They catalyze the formation of carbon-halogen bonds in particular at electron rich aromatic C-atoms by oxidizing a halide ion (Figure 3a) [23,24]. Here, reduced flavin (6) is directly attacked at the C4a position generating hydroperoxide species (8), which undergoes a nucleophilic substitution at the electrophilic oxygen atom O1 by a halide Hal−, such as chloride. The formed hypohalous acid HOHal (10) is a potent electrophilic halogenating agent. The generated hydroxylated flavin 9 produces oxidized flavin 4 that is reduced by a flavine-reductase to close the catalytic cycle [25,26,27].
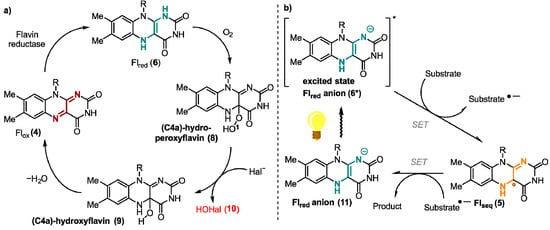
Figure 3.
Proposed mechanism of (a) two-electron transfer mechanism exemplary shown for flavin-dependent halogenase and (b) one-electron reductions of light-induced flavin catalysis.
In addition to their versatile behavior utilizing molecular oxygen, flavins also possess very interesting photophysical properties, which will be addressed in the following chapters in detail. The absorption peaks of Flox (4) in aqueous solution show four maxima at 445, 375, 265, and 220 nm. π → π* transitions are responsible for the relatively high molar absorption coefficients [28]. In nature, these photophysical properties are utilized in light-dependent flavin catalysis for one-electron reductions (Figure 3b). In these processes, upon excitation by the light a reduced flavin anion (6*) species transfers a single electron to a substrate. The now generated, highly reactive semiquinone 5 reduces the corresponding substrate through a second SET. One enzyme conducting this type of reaction is the DNA-photolyase, which reduces thymine dimers generated by UV-light and, thus, is part of the DNA-strain repair mechanism in cells [29]. The redox-potential of the thymine dimer in contrast to its one-electron reduced radical is −2.2 V vs. saturated calomel electrode (SCE), which underlines the high reducing potential of flavin in this case [30,31].
The remarkable photophysics of flavins are not only used by nature. Additionally, in synthetic organic chemistry, flavins perform a crucial role as photocatalysts [32], mostly in oxidative reactions [33]. As the chemical activity of flavins in a non-enzymatical surrounding is significantly reduced, chemically modified, tailored flavins are used very frequently. The repertoire of flavin catalyzed photoreactions spans from the oxidation of alkyl groups, cycloadditions of diens, dehalogenation of aryl halides, aromatic halogenations to cofactor regenerations in biocatalysis [34,35,36,37].
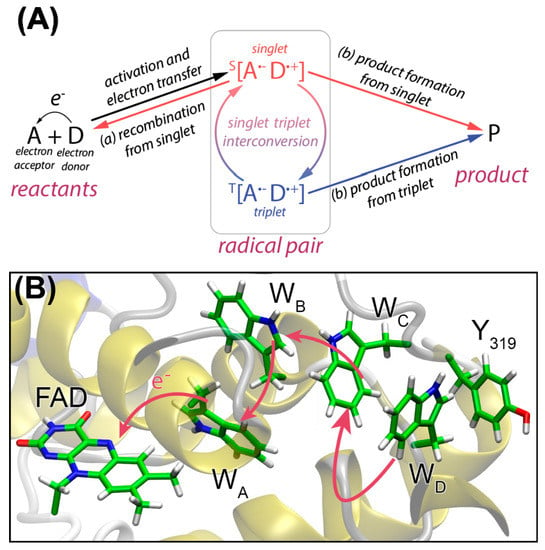
Another type of flavin-dependent enzymes performing an important role in the biology of animals and plants are cryptochromes. Cryptochromes are flavin-containing, blue-light absorbing proteins that are assumed to be crucial for the magnetic sense of migratory birds, the circadian clock of insects and mammalians, and for the movement and development of plants [38,39]. While the mechanism for magnetic perceptions in birds is still uncertain, a likely explanation is based on the electron-transfer chain between the acceptor flavin and the neighboring tryptophan donors in the protein environment. Starting with a photoexcited flavin in the cryptochrome, tryptophan residues in close proximity, then transfer electrons to the flavin, yielding a flavin-tryptophan radical pair. It is assumed that this radical pair is a magnetoreceptor due to its spin-dynamics and, thus, can interact with the weak magnetic field of the earth [40,41]. Dependent on how the cryptochromes are aligned, different physiological signals are generated [42]. Furthermore, the observed radical pair mechanism led to increased research of magnetic fields acting on anthropogenic biological systems, including flavoproteins [43]. Generation of a spin-correlated radical pair (SCRP) can be investigated by so-called CIDNP nuclear magnetic resonance spectroscopy (NMR) which will be discussed in the following chapters of this review.
2. Flavins Acting in the Classical Radical Pair Mechanism in Liquid-State NMR
The flourishing field of spin-chemistry is based on a bold assumption very much contrary to chemical intuition: Even if the recombination of two radicals is energetically highly favorable, it will not happen if it is “spin forbidden”. Hence, recombination, although thermodynamically allowed, is kinetically controlled by the electronic spin states. In this chapter, we will report on the discovery and the implications of this principle. We will see that flavins are ideal “players” in the field of spin-chemistry.
2.1. Discovery of the Principle of Photo-CIDNP in Liquid-State NMR
The discovery of the photo-CIDNP effect originated from the 1967 research of Bargon, Fischer and Johnsen [44]. It was observed during the thermal decomposition of organic peroxides and azo compounds in a series of NMR experiments. As the authors noted, intermittently, in some reactions, the proton resonance lines of the reaction products appeared in emission (i.e., negative) rather than in enhanced absorption (i.e., positive). This allowed them to suggest that the molecules were originally formed in a negative spin polarization state of their proton spin system due to the chemical reaction. The same observation has been made independently by Ward and Lawler for the reactions of alkyl halides with lithium alkyls afterward [45]. It then was discovered that nuclear polarization could also be induced by photoexcitation of a redox-competent dye in the presence of the biomolecule of interest 1968 [46] and the effect has been coined photo-CIDNP. However, the first observation of this phenomenon [47,48] appeared already in 1963, since radical chemical reactions may give rise to an analog effect in electron paramagnetic resonance (EPR) spectra, but the observation was not further discussed. Therefore, the first work cited as CIDEP (chemically induced dynamic electron polarization) was that of Paul and Fischer, who, in 1970 [49], studied the reduction and oxidation of propionic and isobutyric acids.
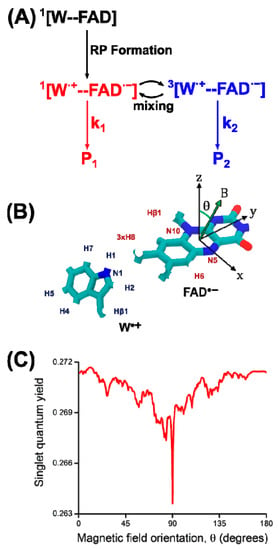
It was necessary to have a theoretical model in order to explain all the observed phenomena. The original model by Bargon and Fischer [45,50,51] proposed to explain signal amplification on the spectrum was based on an Overhauser effect and depended on electron-nuclear cross-relaxation in free radicals. However, for a variety of reasons [44,52,53,54,55], this model has now been abandoned. Kaptein and Oosterhoff [56] and Closs and Closs [54] proposed the radical pair mechanism (RPM) that satisfactorily explains large enhancements and multiplet spectra and allows for spectral simulations. To describe CIDEP spectra, this theoretical concept was adjusted, and the term “spin-correlated radical pair” was coined [57,58]. In flavoproteins, such as cryptochrome, a SCRP has also been observed (see Figure 4). For photo-CIDNP NMR experiments, SCRPs are often produced by illumination of flavin compounds, forming a molecular triplet state and, subsequently, oxidizing aromatic compounds as, e.g., an aromatic amino acid. In this case, due to spin conservation, the SCRP is born in its triplet state.
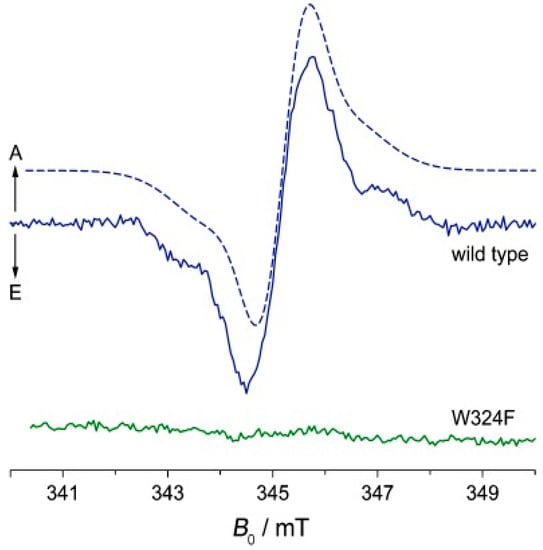
Figure 4.
Time-resolved EPR spectrum of a SCRP in WT cryptochrome (Cry-DASH from Xenopus laevis) in blue and after mutation of tryptophan 324 to phenylalanine 500 ns after pulser laser excitation [59].
According to the model, nuclear spin states can control the electronic state and therefore the chemical fate [60]. The central elements of this mechanism are two radicals (molecules with an unpaired electron) forming a SCRP, which can exist in its singlet or one of the three triplet states (Figure 5A) or in a coherent superposition (in the high-field limit) of (Figure 5B) [61]. In many cases, the recombination reaction of a singlet state SCRP occurs efficiently, while the recombination of the triplet SCRP is kinetically spin-forbidden.
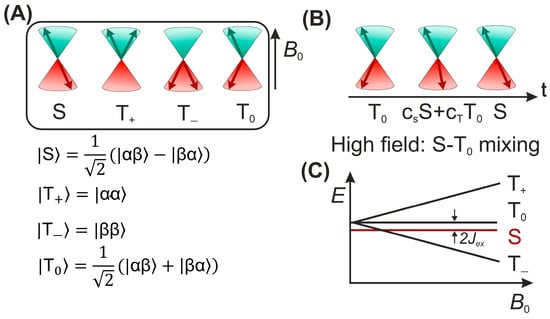
Figure 5.
(A) The vector representation of four spin states of SCRP; (B) Vector model for visualization of the intercombination conversion of a radical pair in high magnetic fields. Left: State ; center: superposition of states; right: singlet state; (C) The energy of the four spin states of a radical pair along a magnetic field; refers to the exchange coupling constant.
Since the recombination of the SCRP reaction is spin-selective, the singlet-triplet inter-conversion affects the recombination rate (Figure 5C). For a SCRP formed by the radicals 1 and 2, and a single magnetic nucleus (I = 1/2) with isotropic hyperfine coupling to one of the radicals, the Hamiltonian is written as follows:
where is the electron Larmor frequency, is the isotropic part of the hyperfine interaction, and is the exchange interaction in the SCRP. The difference in the Larmor frequencies of the electrons and the isotropic part of the hyperfine interaction leads to the mixing of the |S⟩ and |T0⟩ states. The term , in which the raising (lowering) operators are defined as , and is associated with |S⟩->|T+⟩ and |S⟩->|T−⟩ transitions, which are possible only in weak magnetic fields (fields less or about the hyperfine coupling, HFC) or in the neighborhood of the |S⟩ to |T±⟩ level-crossing point. This concept has been discussed in detail here [62,63,64].
Hence, since only the singlet SCRPs are allowed to recombine and form “recombination products”, the triplet radical pairs will diffuse apart and form so-called “escape products” when the time scale for diffusion is shorter than the reverse intersystem crossing time (in which otherwise a singlet could be obtained first). This selection process is called “spin sorting”. The distinguished reaction products appear to be enriched in certain spin states of magnetic nuclei, i.e., non-equilibrium polarized [65] leading to a signal pattern in the NMR spectrum, which is explained by Kaptein’s sign rules [66,67]. According to these rules, the phase of the enhancement , i.e., positive or negative, for a given nucleus is provided by the product of four signs:
where —the sign of the isotropic hyperfine coupling constant for that nucleus, —sign of the difference between the two g-factors of the two radicals, —an expression for the electronic spin state of the precursor state, and —the reaction product, which is positive for recombination products and negative for escape products [68]. However, it should be emphasized that the sign rules apply only when, during the diffusion convergence, the radicals of the geminate radical pair have sufficient time to undergo a singlet-triplet inter-conversion one or more times. Therefore, deviations from the Kaptein rules can be expected when the viscosity of the solution is high, or in systems in which the hyperfine interaction constants or g-factor differences are relatively large [65], as discussed by Salikhov [69]. For many of these 1H liquid-state photo-CIDNP NMR studies, flavin compounds were used for the efficient light-induced formation of the SCRP, and vice versa, photo-CIDNP NMR offered a method to detect short-lived flavin radicals in solution [70].
There also have been works on the photo-CIDNP effect beyond 1H liquid-state NMR using flavin dyes, including studies in the gas phase [71,72], on various nuclei as 19F nuclei, using various fluorescent dyes [73], and studies of organic-chemical reaction mechanisms [74,75,76]. Protein structures have been investigated using the photo-CIDNP effect [77]. Methods of time-resolved photo-CIDNP effect were developed [78], the concept of isotropic mixing by Vieth and co-workers [63,79,80] and field-cycling photo-CIDNP NMR of liquid samples [79,81,82] was developed. Very strong CIDNP effects have been found in molecular systems, such as radicals complexed with free-moving limit micelles [83,84] and biradical systems [85,86,87]. Hence, photo-CIDNP in the fluid phase with SCRPs formed by small molecules has been explored in many aspects and, in general, well rationalized in terms of the classical RPM.
2.2. Flavoproteins Studied by Liquid-State Photo-CIDNP NMR
As mentioned above, FMN and FAD are present in a great variety of biocatalytic processes. In particular, the common 7,8-dimethyl isoalloxazine ring structural motif is responsible for not only the color, but also electron-mediation processes and has been investigated intensively. Initially, continuous-wave (CW) EPR [88,89,90] and ENDOR [91] experiments were applied to study the electronic structure of flavin isoalloxazine moiety in its Flseq state in solution. The active function of the flavin in electron-transfer reactions and enzyme-catalyzed processes were also identified [92,93]. Moreover, protein-cofactor interactions in flavin radicals were unraveled and the formation of a molecular triplet state [94,95] led in some cases to a formation of a covalent bond with nearby amino acid residue [96].
Liquid-state photo-CIDNP NMR studies on these flavoproteins carrying both donor and acceptor were hampered by the impossibility to undergo diffusion of the two parts of the SCRP, i.e., forming escape products. In 2005, however, Richter et al. [97] observed strongly spin-hyperpolarized signals from the light-oxygen-voltage-sensing number 2 (LOV2) domain of the blue-light photoreceptor phototropin from oat (Avena sativa), when the 13C liquid-state NMR spectra were recorded under illumination (Figure 6). This study has been the first observation of a photo-CIDNP effect in an integral flavoprotein system, having both electron donor and acceptor incorporated within the protein. This new observation broke the pattern that the photo-CIDNP effect in electron-transfer proteins was limited in photosynthetic reaction centers (RCs) studied under magic-angle spinning (MAS) NMR conditions. In this highly relevant experiment, the FMN was uniformly labeled with 13C and the cysteine located near FMN was mutated to alanine (C450A) to allow the formation of SCRPs. Based on the observation of the strong molecular triplet populations in the system observed by optical spectroscopy and time-resolved EPR [98], Eisenreich et al. [99], in 2008, proposed a cyclic reaction scheme for the production of photo-CIDNP in LOV2-C450A of phototropin: Upon illumination of the cofactor FMN, a molecular triplet state of the FMN and, induced by electron transfer from an electron-donating aromatic amino acid, a SCRP is formed. Due to spin-conservation rules, the SCRP is formed in its triplet state.
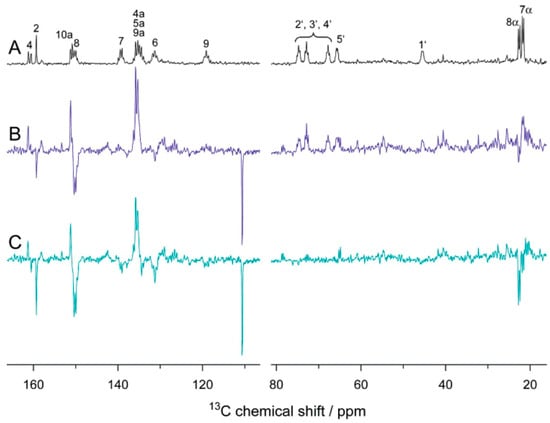
Figure 6.
13C NMR spectra of [u13C17]FMN-reconstituted LOV2-C450A domain. (A) “Dark” spectrum. (B) Scaled “light” spectrum. (C) Difference spectrum “light”-minus-“dark” [97]. Reprinted with permission from [97]. Copyright 2005 American Chemical Society.
In 2015, Eisenreich et al. [100] presented strategies for 13C labeling of tryptophan in LOV2-C450A of phototropin and obtained 13C liquid-state photo-CIDNP NMR spectra of single and double mutant LOV2 and discussed the benefit of fractional labeling method on the light-induced nuclear spin polarization quantitative analysis in terms of their strongly attenuating cross relaxation pathways. A very similar sample, phototropin LOV1-C57S, was studied by 13C MAS NMR under illumination and showed a strong solid-state photo-CIDNP effect (see below) [101,102,103].
Recently, Pompe et al. [104] investigated the electronic structure of flavin semiquinone radicals in solution in terms of their 13C hyperfine coupling constants by using the photo-CIDNP effect and discussed the spin density distribution within the radicals, the HFC constants determined in this work provide the reference for further studies on 13C hyperfine couplings in the transient radical involved flavoprotein system.
3. Flavoproteins Showing a Solid-State Photo-CIDNP Effect
While the photo-CIDNP effect in liquids relies on the classical RPM, the latter cannot be used for the explanation of the effect in the solid-state. Since diffusion of the SCRP, which is required for the classical RPM, is severely limited, spin sorting does not lead to a separation of the hyperpolarized signals. However, the observation of a photo-CIDNP effect in frozen photosynthetic RCs from Rhodobacter sphaeroides [105] demonstrated the possibility that the effect might also occur in the solid-state. While time-resolved photo-CIDNP MAS experiments showed that classical spin-sorting is active during the spin-evolution of the SCRP, the effect of the RPM vanishes after about 100 μs [106]; instead, steady-state nuclear hyperpolarization is observed, which is not caused by the RPM. Therefore, different mechanisms were proposed, namely, three-spin mixing (TSM) [107,108], differential decay (DD) [109], and differential relaxation (DR) [110,111]. The TSM relies on the coupling of two electron spins to a nuclear spin, which evolves coherently in the singlet-triplet manifold of the SCRP and converts electronic into nuclear hyperpolarization. It is driven by the electron-electron dipolar coupling and the anisotropic pseudosecular hyperfine coupling. These interactions lead to a degeneracy of three of the four electron-electron-nuclear energy levels in the S-T0 manifold and enabling mixing between the states allowing the conversion of electron-electron zero-quantum coherence into nuclear anti-phase coherence. It is maximized at the double-matching condition and vanishes if pseudosecular hyperfine coupling are zero. This generally excludes that the TSM is active in liquids; however, given that the molecule or protein is large, and tumbling is substantially slowed, anisotropic electron-nuclear interactions are not averaged allowing for photo-CIDNP contributions from TSM. The DD mechanism relies on different lifetimes of the singlet and the triplet branch in order to build up hyperpolarization. This difference breaks the symmetry of the SCRP by pseudosecular hyperfine interactions and, thus, leads to a conversion of polarization into nuclear coherence. It depends on a single-matching conditions since it does not rely on electron-electron dipolar couplings. In photosynthetic reaction centers, TSM and DD contribute simultaneously to the photo-CIDNP signals with TSM as its dominating factor leading to an emissive signal pattern. Polarization from DD; instead, it leads to a set of absorptive signal intensities. In systems, where a long-lived molecular triplet state occurs, for example, photosynthetic reaction centers without its adjacent carotenoid (R26), the electronic triplet induces paramagnetic relaxation of nuclear spins resulting in an imbalance of nuclear hyperpolarization in the electronic ground state which is described by the DR mechanism. The polarization generated in the triplet branch is removed while polarization in the singlet branch is maintained. As a result, the polarization generated by photo-CIDNP in the singlet branch, which in photosynthetic reaction centers resembles the RPM, is the only contribution towards hyperpolarized signals in the ground state. Recently, these concepts have been unified with the liquid-state photo-CIDNP effect by level-crossing (LC) and level anti-crossing (LAC) analysis [112]. When a coupling matrix element disturbs the coherence, the LC is avoided and turned into a LAC leading to a mixing of the spin states. Analysis of LACs can predict signal enhancements at different magnetic field strengths and is able to attribute it to the influence of solid-state photo-CIDNP mechanisms.
It was speculated that the solid-state photo-CIDNP effect is an intrinsic property of photosynthesis, confined to the photosynthetic apparatus [113]. However, the observation of hyperpolarized signals under illumination in the LOV1 domain of phototropin C57S in frozen solution [103] demonstrated that also other electron transfer proteins are capable to induce this effect. Field-dependent measurements of phototropin LOV1 C57S in frozen solution are shown in Figure 7 with enhanced signals from FMN and tryptophan which are involved in the electron transfer and form the SCRP. Here, similar to photosynthetic RCs [114,115], the sign of polarization strongly depends on the field.
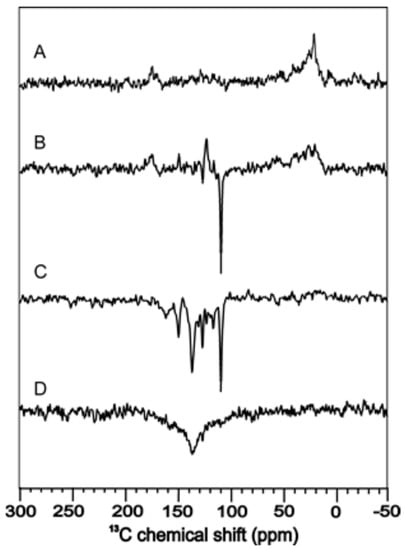
Figure 7.
13C photo-CIDNP MAS NMR of phototropin LOV1 C57S at a magnetic field of 9.4 T (A), 4.7 T (B), 2.4 T (C), and 1.4 T (D) [116].
By employing isotope enrichment, photo-CIDNP field-cycling methods became available. Figure 8 shows the field-dependent intensity of 13C and 15N enriched tryptophan inside LOV1 of phototropin and 1H of the residual water [101]. While these experiments were conducted in a liquid state, LC/LAC analysis showed that anisotropic interactions are responsible for the field-dependence shown because of the inverse dependence on the gyromagnetic constant. Therefore, solid-state photo-CIDNP mechanisms are active, even in liquids provided that sufficient orientation of the nucleus is given. In addition to LOV1 of phototropin, other flavoproteins showing the solid-state photo-CIDNP effect were reported showing an orientation dependence of the electron-donating amino acid [102]. All consist of a LOV domain with electron transfer from a conserved tryptophan to FMN, however, even without tryptophan, photo-CIDNP enhancement is observed, most likely coming from tyrosine(s) of the LOV domain as the electron donor.
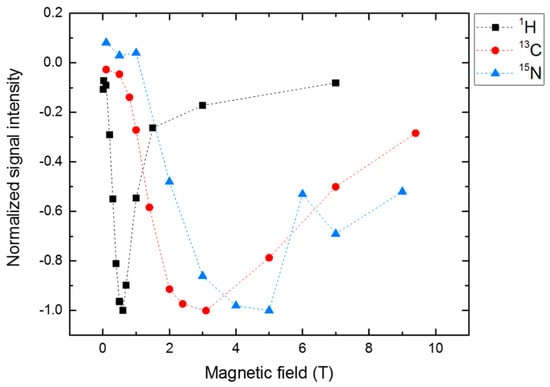
Figure 8.
Magnetic field-dependence of 13C (red) and 15N (blue) photo-CIDNP signals from labeled tryptophan from phototropin LOV1-C57S. 1H (black) photo-CIDNP is observed from residual water in deuterated water. The maxima of the light-induced signals inversely correspond to the gyromagnetic constant suggesting that even in liquid, solid-state photo-CIDNP mechanisms are responsible for the signal enhancement [101].
In Figure 9, the tentative photo-cycle for phototropin LOV1-C57S is shown. After blue-light irradiation, the FMN is in an electronically excited singlet state with subsequent intersystem-crossing (ISC) to an excited molecular triplet state. In the following, the triplet state can either relax to the electronic ground state or induces an electron transfer from the tryptophan to form the SCRP in the triplet state. The SCRP evolves under electron-nuclear spin interaction at high fields in the S-T0 manifold where it can recombine only from the singlet branch to the ground state. Herein lies the difference to the photo-cycle of photosynthetic RCs, where the SCRP is created in the singlet state allowing direct recombination. In LOV1, on the other hand, the SCRP is born in its triplet state. In case that S-T0 interconversion is affected by nuclear spin states via aiso, a certain nuclear spin state will enrich in the singlet state of the SCRP. Furthermore, nuclear hyperpolarization arising during the S-T0 interconversion might also originate from the TSM which requires solid-state conditions. In terms of spintronics, the nuclear spins stemming from the coherent spin evolution of the SCRP could be exploited as a spin-filter. The enrichment of nuclear spin polarization can influence electron spin polarization, e.g., in spintronic devices. Therefore, light-driven flavin systems implemented into such devices might provide a method to analyze spin interactions or detect electron spins via changes in nuclear hyperpolarization.
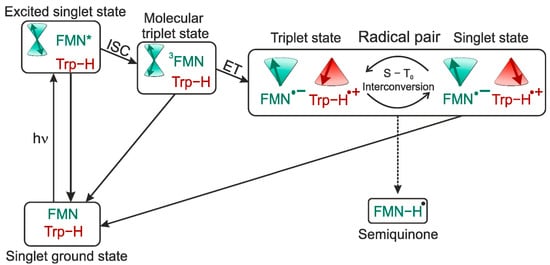
Figure 9.
Upon blue-light illumination, the FMN is in a photo-excited state followed by a subsequent ISC to the molecular triplet state. 3FMN induces an electron transfer from a nearby tryptophan to form a SCRP born in the triplet state. The radical pair coherently evolves under electron-nuclear interaction between the triplet and singlet state. Since recombination from the triplet state is spin-forbidden, decay to the electronic ground-state is only possible from the singlet state. Additionally, cyclic photo-reaction process, the formation of a semiquinone-radical (Flseq (5)) with protonation of the FMN can also occur. The star indicates an excited molecular state and is not intended as an annotation.
The recombination rate of the SCRP in the triplet state is (close to) zero, since this reaction is spin forbidden, therefore only the singlet state acts as a decay channel for the SCRP to the electronic ground state. Such scheme might be interpreted as an extreme-case DD, since the two decay branches have very different kinetics (since one branch is not at all active). Alternatively, one might consider this scheme as a special case of the DR (since one branch does not contribute at all to the nuclear spin statistics in the electronic ground state). In any case, since photo-CIDNP enhancement in LOV1 depends on anisotropic interactions [101], the involvement of DD, TSM, or both is expected. Future time-resolved photo-CIDNP MAS experiments will provide more insight into the photo-CIDNP mechanism and kinetics of flavoproteins. Furthermore, as shown in Figure 9, illumination leads to the formation of Flseq (5).
5. Outlook
Nature has chosen flavin systems for spin-chemical processes. Therefore, flavins provide an excellent starting position to develop both, artificial magneto-detection and light-pumped hyperpolarized NMR. Trusting the Einheit der Natur, as Carl Friedrich von Weizsäcker stated (Die Einheit der Natur: 1971, Carl Friedrich von Weizsäcker), the authors are convinced that there will be fundamental laws of spin-physics and spin-chemistry to provide a common basic scheme. Such a common law would provide the basis for a new spin-chemistry in soft matter.
Author Contributions
J.M., L.G., T.T., L.T., P.K.-T., G.M., R.Q., F.O., I.A.S. and T.G. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Our contribution is dedicated to the memory of the late Konstantin Ivanov. The Matysik group is grateful to the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO) and the Deutsche Forschungsgemeinschaft (DFG) for longstanding support. The Solov’yov group thank the Volkswagen Foundation (Lichtenberg Professorship to IAS), the Deutsche Forschungsgemeinschaft (DFG; GRK1885: Molecular Basis of Sensory Biology and SFB 1372: Magnetoreception and Navigation in Vertebrates), the Ministry for Science and Culture of Lower Saxony (Simulations Meet Experiments on the Nanoscale: Opening up the Quantum World to Artificial Intelligence (SMART) and Dynamik auf der Nanoskala: Von kohärenten Elementarprozessen zur Funktionalität (DyNano)). Computational resources for the simulations were provided by the CARL Cluster at the Carl-von-Ossietzky University, Oldenburg, supported by the DFG and the Ministry for Science and Culture of Lower Saxony. The authors gratefully acknowledge the computing time granted by the Resource Allocation Board and provided on the supercomputer Lise and Emmy at NHR@ZIB and NHR@Göttingen as part of the NHR infrastructure (project nip00058).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Silva, E.; Edwards, A.M. Flavins: Photochemistry and Photobiology; Royal Society of Chemistry: London, UK, 2006; ISBN 978-0-85404-331-6. [Google Scholar]
- Powers, H.J. Riboflavin (Vitamin B-2) and Health. Am. J. Clin. Nutr. 2003, 77, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Mansoorabadi, S.O.; Thibodeaux, C.J.; Liu, H.W. The Diverse Roles of Flavin Coenzymes—Nature’s Most Versatile Thespians. J. Org. Chem. 2007, 72, 6329–6342. [Google Scholar] [CrossRef] [PubMed]
- Manstein, D.J.; Pai, E.F. Purification and Characterization of FAD Synthetase from Brevibacterium Ammoniagenes. J. Biol. Chem. 1986, 261, 16169–16173. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Zhou, Q.; Mseeh, F.; Grishin, N.V.; Osterman, A.L.; Zhang, H. Crystal Structure of Human Riboflavin Kinase Reveals a β Barrel Fold and a Novel Active Site Arch. Structure 2003, 11, 265–273. [Google Scholar] [CrossRef]
- Northrop-Clewes, C.A.; Thurnham, D.I. The Discovery and Characterization of Riboflavin. Ann. Nutr. Metab. 2012, 61, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. The Yellow Enzyme and Its Functions. Biochem. Z. 1933, 266, 377–411. [Google Scholar]
- Krebs, H.A. Metabolism of Amino-Acids: Deamination of Amino-Acids. Biochem. J. 1935, 29, 1620–1644. [Google Scholar] [CrossRef]
- Theorell, H. Purification of the Active Group of the Yellow Enzyme. Biochem. Z. 1935, 275, 344–346. [Google Scholar]
- Tomiki, T.; Saitou, N. Phylogenetic Analysis of Proteins Associated in the Four Major Energy Metabolism Systems: Photosynthesis, Aerobic Respiration, Denitrification, and Sulfur Respiration. J. Mol. Evol. 2004, 59, 158–176. [Google Scholar] [CrossRef]
- Walsh, J.D.; Miller, A.F. Flavin Reduction Potential Tuning by Substitution and Bending. J. Mol. Struct. 2003, 623, 185–195. [Google Scholar] [CrossRef]
- Fraaijet, M.W.; Van Den Heuvel, R.H.H.; Van Berkel, W.J.H.; Mattevi, A. Covalent Flavinylation Is Essential for Efficient Redox Catalysis in Vanillyl-Alcohol Oxidase. J. Biol. Chem. 1999, 274, 35514–35520. [Google Scholar] [CrossRef] [PubMed]
- Dym, O.; Eisenberg, D. Sequence-Structure Analysis of FAD-Containing Proteins. Protein Sci. 2001, 10, 1712–1728. [Google Scholar] [CrossRef]
- McDonald, C.A.; Fagan, R.L.; Collard, F.; Monnier, V.M.; Palfey, B.A. Oxygen Reactivity in Flavoenzymes: Context Matters. J. Am. Chem. Soc. 2011, 133, 16809–16811. [Google Scholar] [CrossRef] [PubMed]
- Mattevi, A. To Be or Not to Be an Oxidase: Challenging the Oxygen Reactivity of Flavoenzymes. Trends Biochem. Sci. 2006, 31, 276–283. [Google Scholar] [CrossRef]
- Bučko, M.; Gemeiner, P.; Schenkmayerová, A.; Krajčovič, T.; Rudroff, F.; Mihovilovič, M.D. Baeyer-Villiger Oxidations: Biotechnological Approach. Appl. Microbiol. Biotechnol. 2016, 100, 6585–6599. [Google Scholar] [CrossRef]
- Teufel, R.; Miyanaga, A.; Michaudel, Q.; Stull, F.; Louie, G.; Noel, J.P.; Baran, P.S.; Palfey, B.; Moore, B.S. Flavin-Mediated Dual Oxidation Controls an Enzymatic Favorskii-Type Rearrangement. Nature 2013, 503, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Wongnate, T.; Surawatanawong, P.; Visitsatthawong, S.; Sucharitakul, J.; Scrutton, N.S.; Chaiyen, P. Proton-Coupled Electron Transfer and Adduct Configuration Are Important for C4a-Hydroperoxyflavin Formation and Stabilization in a Flavoenzyme. J. Am. Chem. Soc. 2013, 136, 241–253. [Google Scholar] [CrossRef]
- Malmström, B.G. Enzymology of Oxygen. Annu. Rev. Biochem. 1982, 51, 21–59. [Google Scholar] [CrossRef]
- Romero, E.; Gómez Castellanos, J.R.; Gadda, G.; Fraaije, M.W.; Mattevi, A. Same Substrate, Many Reactions: Oxygen Activation in Flavoenzymes. Chem. Rev. 2018, 118, 1742–1769. [Google Scholar] [CrossRef]
- Wang, R.; Thorpe, C. Reactivity of Medium-Chain Acyl-CoA Dehydrogenase toward Molecular Oxygen. Biochemistry 1991, 30, 7895–7901. [Google Scholar] [CrossRef]
- Traber, R.; Kramer, H.E.A.; Hemmerich, P. One and Two Electron Transfer Pathways in the Photoreduction of Flavin. Pure Appl. Chem. 1982, 54, 1651–1665. [Google Scholar] [CrossRef]
- Büchler, J.; Papadopoulou, A.; Buller, R. Recent Advances in Flavin-Dependent Halogenase Biocatalysis: Sourcing, Engineering, and Application. Catalysts 2019, 9, 1030. [Google Scholar] [CrossRef]
- Andorfer, M.C.; Lewis, J.C. Understanding and Improving the Activity of Flavin-Dependent Halogenases via Random and Targeted Mutagenesis. Annu. Rev. Biochem. 2018, 87, 159–185. [Google Scholar] [CrossRef]
- Barker, R.D.; Yu, Y.; De Maria, L.; Johannissen, L.O.; Scrutton, N.S. Mechanism of Action of Flavin-Dependent Halogenases. ACS Catal. 2022, 12, 15352–15360. [Google Scholar] [CrossRef]
- Van Pée, K.H.; Patallo, E.P. Flavin-Dependent Halogenases Involved in Secondary Metabolism in Bacteria. Appl. Microbiol. Biotechnol. 2006, 70, 631–641. [Google Scholar] [CrossRef]
- Phintha, A.; Prakinee, K.; Chaiyen, P. Structures, Mechanisms and Applications of Flavin-Dependent Halogenases. In The Enzymes; Chaiyen, P., Tamanoi, F., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 47, pp. 327–364. ISBN 9780128201374. [Google Scholar]
- Ahmad, I.; Vaid, F.H.M. Chapter 2: Photochemistry of Flavins in Aqueous and Organic Solvents. In Flavins: Photochemistry and Photobiology; Silva, E., Edwards, A.M., Eds.; Royal Society of Chemistry: London, UK, 2006; pp. 13–40. [Google Scholar]
- Hartman, T.; Cibulka, R. Photocatalytic Systems with Flavinium Salts: From Photolyase Models to Synthetic Tool for Cyclobutane Ring Opening. Org. Lett. 2016, 18, 3710–3713. [Google Scholar] [CrossRef]
- Scannell, M.P.; Fenick, D.J.; Yeh, S.R.; Falvey, D.E. Model Studies of DNA Photorepair: Reduction Potentials of Thymine and Cytosine Cyclobutane Dimers Measured by Fluorescence Quenching. J. Am. Chem. Soc. 1997, 119, 1971–1977. [Google Scholar] [CrossRef]
- Foja, R.; Walter, A.; Jandl, C.; Thyrhaug, E.; Hauer, J.; Storch, G. Reduced Molecular Flavins as Single-Electron Reductants after Photoexcitation. J. Am. Chem. Soc. 2022, 144, 4721–4726. [Google Scholar] [CrossRef]
- Rehpenn, A.; Walter, A.; Storch, G. Molecular Editing of Flavins for Catalysis. Synthesis 2021, 53, 2583–2593. [Google Scholar] [CrossRef]
- Seel, C.J.; Gulder, T. Biocatalysis Fueled by Light: On the Versatile Combination of Photocatalysis and Enzymes. ChemBioChem 2019, 20, 1871–1897. [Google Scholar] [CrossRef]
- Murray, A.T.; Matton, P.; Fairhurst, N.W.G.; John, M.P.; Carbery, D.R. Biomimetic Flavin-Catalyzed Aldehyde Oxidation. Org. Lett. 2012, 14, 3656–3659. [Google Scholar] [CrossRef]
- Graml, A.; Neveselý, T.; Jan Kutta, R.; Cibulka, R.; König, B. Deazaflavin Reductive Photocatalysis Involves Excited Semiquinone Radicals. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mojr, V.; Svobodová, E.; Straková, K.; Neveselý, T.; Chudoba, J.; Dvořáková, H.; Cibulka, R. Tailoring Flavins for Visible Light Photocatalysis: Organocatalytic [2+2] Cycloadditions Mediated by a Flavin Derivative and Visible Light. Chem. Commun. 2015, 51, 12036–12039. [Google Scholar] [CrossRef] [PubMed]
- Walter, A.; Storch, G. Synthetic C6-Functionalized Aminoflavin Catalysts Enable Aerobic Bromination of Oxidation-Prone Substrates. Angew. Chem. Int. Ed. 2020, 59, 22505–22509. [Google Scholar] [CrossRef]
- Xu, J.; Jarocha, L.E.; Zollitsch, T.; Konowalczyk, M.; Henbest, K.B.; Richert, S.; Golesworthy, M.J.; Schmidt, J.; Déjean, V.; Sowood, D.J.C.; et al. Magnetic Sensitivity of Cryptochrome 4 from a Migratory Songbird. Nature 2021, 594, 535–540. [Google Scholar] [CrossRef]
- Evans, E.W.; Dodson, C.A.; Maeda, K.; Biskup, T.; Wedge, C.J.; Timmel, C.R. Magnetic Field Effects in Flavoproteins and Related Systems. Interface Focus 2013, 3, 20130037. [Google Scholar] [CrossRef]
- Paul, S.; Meng, L.; Berger, S.; Grampp, G.; Matysik, J.; Wang, X. The Flavin–Tryptophan Dyad F10T as a Cryptochrome Model Compound: Synthesis and Photochemistry. ChemPhotoChem 2017, 1, 12–16. [Google Scholar] [CrossRef]
- Paul, S.; Kiryutin, A.S.; Guo, J.; Ivanov, K.L.; Matysik, J.; Yurkovskaya, A.V.; Wang, X. Magnetic Field Effect in Natural Cryptochrome Explored with Model Compound. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Hore, P.J.; Mouritsen, H. The Radical-Pair Mechanism of Magnetoreception. Annu. Rev. Biophys. 2016, 45, 299–344. [Google Scholar] [CrossRef]
- Messiha, H.L.; Wongnate, T.; Chaiyen, P.; Jones, A.R.; Scrutton, N.S. Magnetic Field Effects as a Result of the Radical Pair Mechanism Are Unlikely in Redox Enzymes. J. R. Soc. Interface 2015, 12, 20141155. [Google Scholar] [CrossRef]
- Bargon, J.; Fischer, H.; Johnsen, U. Kernresonanz-Emissionslinien Während Rascher Radikalreaktionen I. Aufnahmeverfahren Und Beispiele. Z. Naturforsch. A 1967, 22, 1551–1555. [Google Scholar] [CrossRef]
- Ward, F.R.; Lawler, R.G. Nuclear Magnetic Resonance Emission and Enhanced Absorption in Rapid Organometallic Reactions. J. Am. Chem. Soc. 1967, 89, 5518–5519. [Google Scholar] [CrossRef]
- Cocivera, M. Optically Induced Overhauser Effect in Solution. Nuclear Magnetic Resonance Emission. J. Am. Chem. Soc. 1968, 90, 3261–3263. [Google Scholar] [CrossRef]
- Fessenden, R.W.; Schuler, R.H. Electron Spin Resonance Studies of Transient Alkyl Radicals. J. Chem. Phys. 1963, 39, 2147–2195. [Google Scholar] [CrossRef]
- Smaller, B.; Remko, J.R.; Avery, E.C. Electron Paramagnetic Resonance Studies of Transient Free Radicals Produced by Pulse Radiolysis. J. Chem. Phys. 1968, 48, 5174–5181. [Google Scholar] [CrossRef]
- Paul, H.; Fischer, H. Emission Und Verstärkte Absorption in Den ESR-Spektren Kurzlebiger Radikale in Wäßrigen Lösungen. Z. Naturforsch. A 1970, 25, 443–445. [Google Scholar] [CrossRef]
- Fischer, H.; Bargon, J. Chemically Induced Dynamic Nuclear Polarization during Thermal Decomposition of Peroxides and Azo Compounds. Acc. Chem. Res. 1969, 2, 110–114. [Google Scholar] [CrossRef]
- Bargon, J.; Fischer, H. Kernresonanz-Emissionslinien Während Rascher Radikalreaktionen II. Chemisch Induzierte Dynamische Kernpolarisation. Z. Naturforsch. A 1967, 22, 1556–1562. [Google Scholar] [CrossRef]
- Lawler, R.G. Chemically Induced Dynamic Nuclear Polarization. J. Am. Chem. Soc. 1967, 89, 5519–5521. [Google Scholar] [CrossRef]
- Kaptein, R. Chemically Induced Dynamic Nuclear Polarization in Five Alkyl Radicals. Chem. Phys. Lett. 1968, 2, 261–267. [Google Scholar] [CrossRef]
- Closs, G.L.; Closs, L.E. Induced Dynamic Nuclear Spin Polarization in Reactions of Photochemically and Thermally Generated Triplet Diphenylmethylene. J. Am. Chem. Soc. 1969, 91, 4549–4550. [Google Scholar] [CrossRef]
- Closs, G.L.; Trifunac, A.D. Chemically Induced Nuclear Spin Polarization as a Tool for Determination of Spin Multiplicities of Radical-Pair Precursors. J. Am. Chem. Soc. 1969, 91, 4554–4555. [Google Scholar] [CrossRef]
- Kaptein, R.; Oosterhoff, J.L. Chemically Induced Dynamic Nuclear Polarization II. (Relation with Anomalous ESR Spectra). Chem. Phys. Lett. 1969, 4, 195–197. [Google Scholar] [CrossRef]
- Closs, G.L.; Forbes, M.D.E.; Norris, J.R. Spin-Polarized Electron Paramagnetic Resonance Spectra of Radical Pairs in Micelles: Observation of Electron Spin-Spin Interactions. J. Phys. Chem. 1987, 91, 3592–3599. [Google Scholar] [CrossRef]
- Hore, P.J.; Hunter, D.A.; McKie, C.D.; Hoff, A.J. Electron Paramagnetic Resonance of Spin-Correlated Radical Pairs in Photosynthetic Reactions. Chem. Phys. Lett. 1987, 137, 495–500. [Google Scholar] [CrossRef]
- Biskup, T.; Schleicher, E.; Okafuji, A.; Link, G.; Hitomi, K.; Getzoff, E.D.; Weber, S. Direct Observation of a Photoinduced Radical Pair in a Cryptochrome Blue-Light Photoreceptor. Angew. Chem. Intl. Ed. 2009, 48, 404–407. [Google Scholar] [CrossRef]
- Matysik, J.; Ding, Y.; Kim, Y.; Kurle, P.; Yurkovskaya, A.; Ivanov, K.; Alia, A. Photo-CIDNP in Solid State. Appl. Magn. Reson. 2022, 53, 521–537. [Google Scholar] [CrossRef]
- Adrian, F.J. Principles of the Radical Pair Mechanism of Chemically Induced Nuclear and Electron Spin Polarization. Rev. Chem. Intermed. 1979, 3, 3–43. [Google Scholar] [CrossRef]
- Turro, N.J.; Ramamurthy, V.; Scaiano, J.C. Principles of Molecular Photochemistry: An Introduction; University Science Books: Sausalito, CA, USA, 2009; ISBN 978-1-891389-57-3. [Google Scholar]
- Panov, M.S.; Pravdivtsev, A.N.; Ivanov, K.L.; Yurkovskaya, A.V.; Vieth, H.-M. Coherent Polarization Transfer Effects Are Crucial for Interpreting Low-Field CIDNP Data. Appl. Magn. Reson. 2014, 45, 893–900. [Google Scholar] [CrossRef]
- Ivanov, K.L.; Pravdivtsev, A.N.; Yurkovskaya, A.V.; Vieth, H.M.; Kaptein, R. The Role of Level Anti-Crossings in Nuclear Spin Hyperpolarization. Prog. Nuc. Magn. Reson. Spectrosc. 2014, 81, 1–36. [Google Scholar] [CrossRef]
- Salikhov, K.M.; Molin, Y.N.; Sagdeev, R.Z.; Buchachenko, A.L. Spin Polarization and Magnetic Effects in Radical Reactions; Elsiever: Amsterdam, The Netherlands, 1984; ISBN 0-444-99677-X. [Google Scholar]
- Kaptein, R. Simple Rules for Chemically Induced Dynamic Nuclear Polarization. J. Chem. Soc. D 1971, 732–733. [Google Scholar] [CrossRef]
- Kaptein, R. Chemically Induced Dynamic Nuclear Polarization. VIII. Spin Dynamics and Diffusion of Radical Pairs. J. Am. Chem. Soc. 1972, 94, 6251–6262. [Google Scholar] [CrossRef]
- Köckenberger, W.; Matysik, J. Hyperpolarization Methods in NMR; Topics in Current Chemistry; Kuhn, L.T., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; Volume 338, ISBN 9780128032244. [Google Scholar]
- Salikhov, K.M. Mutual Effect of Nuclei upon CIDNP in High Fields. Violation of the Kaptein Rules. Chem. Phys. 1982, 64, 371–379. [Google Scholar] [CrossRef]
- Hore, J.; Broadhurst, R.W. Photo-CIDNP of Biopolymers. Prog. Nucl. Magn. Reson. Spectrosc. 1993, 25, 345–402. [Google Scholar] [CrossRef]
- Obynochny, A.A.; Sagdeev, R.Z.; Salikhov, K.M.; Sukhenko, S.A.; Yurkovskaya, A.V. Optical Nuclear Polarization in the Gas Phase. Chem. Phys. 1986, 104, 415–419. [Google Scholar] [CrossRef]
- Yurkovskaya, A.V.; Galimov, R.R.; Obynochny, A.A.; Salikhov, K.M.; Sagdeev, R.Z. The Field Dependence of CIDNP in Gas-Phase Reactions of Biradicals. Chem. Phys. 1987, 112, 259–264. [Google Scholar] [CrossRef]
- Okuno, Y.; Cavagnero, S. Fluorescein: A Photo-CIDNP Sensitizer Enabling Hypersensitive NMR Data Collection in Liquids at Low Micromolar Concentration. J. Phys. Chem. B 2016, 120, 715–723. [Google Scholar] [CrossRef]
- Sadler, D.E.; Wendler, J.; Olbrich, G.; Schaffner, K. Photochemical Reaction Mechanisms of β,γ-Unsaturated Ketones. The 1,3-Acetyl Shift in Cyclopent-2-Enyl Methyl Ketones. J. Am. Chem. Soc. 1984, 106, 2064–2071. [Google Scholar] [CrossRef]
- Roth, H.D. Organic Radical Cations in Fluid Solution: Unusual Structures and Rearrangements. Acc. Chem. Res. 1987, 20, 343–350. [Google Scholar] [CrossRef]
- Neshchadin, D.; Gescheidt, G. CIDNP as a Tool for Rapid Rearrangements: New Insights into Cyclobutanoid 4-Center/3-Electron Radical Cations. J. Phys. Org. Chem. 2013, 26, 737–741. [Google Scholar] [CrossRef]
- Kaptein, R. Photo-CIDNP Studies of Proteins. In Biological Magnetic Resonance; Springer: Boston, MA, USA, 1982; pp. 145–191. [Google Scholar]
- Mok, K.H.; Hore, P.J. Photo-CIDNP NMR Methods for Studying Protein Folding. Methods 2004, 34, 75–87. [Google Scholar] [CrossRef]
- Ivanov, K.L.; Miesel, K.; Yurkovskaya, A.V.; Korchak, S.E.; Kiryutin, A.S.; Vieth, H.-M. Transfer of CIDNP among Coupled Spins at Low Magnetic Field. Appl. Magn. Reson. 2006, 30, 513–534. [Google Scholar] [CrossRef]
- Ivanov, K.L.; Yurkovskaya, A.V.; Vieth, H.-M. Coherent Transfer of Hyperpolarization in Coupled Spin Systems at Variable Magnetic Field. J. Chem. Phys. 2008, 128, 154701. [Google Scholar] [CrossRef] [PubMed]
- Pravdivtsev, A.N.; Yurkovskaya, A.V.; Ivanov, K.L.; Vieth, H.M. Importance of Polarization Transfer in Reaction Products for Interpreting and Analyzing CIDNP at Low Magnetic Fields. J. Magn. Reson. 2015, 254, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Yurkovskaya, A.; Grosse, S.; Dvinskikh, S.; Morozova, O.; Vieth, H.-M. Spin and Molecular Dynamics of Biradicals as Studied by Low Field Nuclear Polarization at Variable Temperature. J. Phys. Chem. A 1999, 103, 980–988. [Google Scholar] [CrossRef]
- Shkrob, I.A.; Tarasov, V.F.; Bagryanskaya, E.G. Electron Spin Exchange in Micellized Radical Pairs. I. 13C Low Field Chemically Induced Dynamic Nuclear Polarization (CIDNP) and 13C Radio Frequency Stimulated Nuclear Polarization (SNP). Chem. Phys. 1991, 153, 427–441. [Google Scholar] [CrossRef]
- Lopez, J.J.; Carter, M.A.G.; Tsentalovich, Y.P.; Morozova, O.B.; Yurkovskaya, A.V.; Hore, P.J. Effects of Surfactants on the Photosensitized Production of Tyrosine Radicals Studied by Photo-CIDNP. Photochem. Photobiol. 2002, 75, 6. [Google Scholar] [CrossRef]
- Morozova, O.B.; Yurkovskaya, A.V.; Tsentalovich, Y.P.; Vieth, H.-M. 1H and 13C Nuclear Polarization in Consecutive Biradicals during the Photolysis of 2,2,12,12-Tetramethylcyclododecanone. J. Phys. Chem. A 1997, 101, 399–406. [Google Scholar] [CrossRef]
- Morozova, O.B.; Yurkovskaya, A.V.; Tsentalovich, Y.P.; Sagdeev, R.Z.; Wu, T.; Forbes, M.D.E. Study of Consecutive Biradicals from 2-Hydroxy-2,12-Dimethylcyclododecanone by TR-CIDNP, TREPR, and Laser Flash Photolysis. J. Phys. Chem. A 1997, 101, 8803–8808. [Google Scholar] [CrossRef]
- Tsentalovich, Y.P.; Morozova, O.B.; Avdievich, N.I.; Ananchenko, G.S.; Yurkovskaya, A.V.; Ball, J.D.; Forbes, M.D.E. Influence of Molecular Structure on the Rate of Intersystem Crossing in Flexible Biradicals. J. Phys. Chem. A 1997, 101, 8809–8816. [Google Scholar] [CrossRef]
- Müller, F.; Hemmerich, P.; Ehrenberg, A.; Palmer, G.; Massey, V. The Chemical and Electronic Structure of the Neutral Flavin Radical as Revealed by Electron Spin Resonance Spectroscopy of Chemically and Isotopically Substituted Derivatives. Eur. J. Biochem. 1970, 14, 185–196. [Google Scholar] [CrossRef]
- Edmondson, D.E. Electron-Spin-Resonance Studies on Flavoenzymes. Biochem. Soc. Trans. 1985, 13, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Ehrenberg, A.; Müller, F.; Hemmerich, P. Basicity, Visible Spectra, and Electron Spin Resonance of Flavosemiquinone Anions. Eur. J. Biochem. 1967, 2, 286–293. [Google Scholar] [CrossRef]
- Bretz, N.; Mastalsky, I.; Elsner, M.; Kurreck, H. First ENDOR Investigations of Biologically Relevant Organic Radicals in Reversed Micelles. Angew. Chem. Int. Ed. 1987, 26, 345–347. [Google Scholar] [CrossRef]
- Massey, V. The Chemical and Biological Versatility of Riboflavin. Biochem. Soc. Trans. 2000, 28, 283. [Google Scholar] [CrossRef]
- Frey, P.A. Radical Mechanisms of Enzymatic Catalysis. Annu. Rev. Biochem. 2001, 70, 121–148. [Google Scholar] [CrossRef]
- Swartz, T.E.; Corchnoy, S.B.; Christie, J.M.; Lewis, J.W.; Szundi, I.; Briggs, W.R.; Bogomolni, R.A. The Photocycle of a Flavin-Binding Domain of the Blue Light Photoreceptor Phototropin. J. Biol. Chem 2001, 276, 36493–36500. [Google Scholar] [CrossRef]
- Kennis, J.T.M.; Crosson, S.; Gauden, M.; Van Stokkum, I.H.M.; Moffat, K.; Van Grondelle, R. Primary Reactions of the LOV2 Domain of Phototropin, a Plant Blue-Light Photoreceptor. Biochemistry 2003, 42, 3385–3392. [Google Scholar] [CrossRef]
- Salomon, M.; Christie, J.M.; Knieb, E.; Lempert, U.; Briggs, W.R. Photochemical and Mutational Analysis of the FMN-Binding Domains of the Plant Blue Light Receptor, Phototropin. Biochemistry 2000, 39, 9401–9410. [Google Scholar] [CrossRef]
- Richter, G.; Weber, S.; Römisch, W.; Bacher, A.; Fischer, M.; Eisenreich, W. Photochemically Induced Dynamic Nuclear Polarization in a C450A Mutant of the LOV2 Domain of the Avena Sativa Blue-Light Receptor Phototropin. J. Am. Chem. Soc. 2005, 127, 17245–17252. [Google Scholar] [CrossRef] [PubMed]
- Schleicher, E.; Kowalczyk, R.M.; Kay, C.W.M.; Hegemann, P.; Bacher, A.; Fischer, M.; Bittl, R.; Richter, G.; Weber, S. On the Reaction Mechanism of Adduct Formation in LOV Domains of the Plant Blue-Light Receptor Phototropin. J. Am. Chem. Soc. 2004, 126, 11067–11076. [Google Scholar] [CrossRef]
- Eisenreich, W.; Joshi, M.; Weber, S.; Bacher, A.; Fischer, M. Natural Abundance Solution 13C NMR Studies of a Phototropin with Photoinduced Polarization. J. Am. Chem. Soc. 2008, 130, 13544–13545. [Google Scholar] [CrossRef] [PubMed]
- Eisenreich, W.; Joshi, M.; Illarionov, B.; Kacprzak, S.; Lukaschek, M.; Kothe, G.; Budisa, N.; Fischer, M.; Bacher, A.; Weber, S. Strategy for Enhancement of 13C-Photo-CIDNP NMR Spectra by Exploiting Fractional 13C-Labeling of Tryptophan. J. Phys. Chem. B 2015, 119, 13934–13943. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Kiryutin, A.S.; Yurkovskaya, A.V.; Sosnovsky, D.V.; Sagdeev, R.Z.; Bannister, S.; Kottke, T.; Kar, R.K.; Schapiro, I.; Ivanov, K.L.; et al. Nuclear Spin-Hyperpolarization Generated in a Flavoprotein under Illumination: Experimental Field-Dependence and Theoretical Level Crossing Analysis. Sci. Rep. 2019, 9, 18436. [Google Scholar] [CrossRef]
- Ding, Y.; Kiryutin, A.S.; Zhao, Z.; Xu, Q.Z.; Zhao, K.H.; Kurle, P.; Bannister, S.; Kottke, T.; Sagdeev, R.Z.; Ivanov, K.L.; et al. Tailored Flavoproteins Acting as Light-Driven Spin Machines Pump Nuclear Hyperpolarization. Sci. Rep. 2020, 10, 18658. [Google Scholar] [CrossRef] [PubMed]
- Thamarath, S.S.; Heberle, J.; Hore, P.J.; Kottke, T.; Matysik, J. Solid-State Photo-CIDNP Effect Observed in Phototropin LOV1-C57S by 13C Magic-Angle Spinning NMR Spectroscopy. J. Am. Chem. Soc. 2010, 132, 15542–15543. [Google Scholar] [CrossRef]
- Pompe, N.; Illarionov, B.; Fischer, M.; Bacher, A.; Weber, S. Completing the Picture: Determination of 13 C Hyperfine Coupling Constants of Flavin Semiquinone Radicals by Photochemically Induced Dynamic Nuclear Polarization Spectroscopy. J. Phys.Chem. Lett. 2022, 5160–5167. [Google Scholar] [CrossRef]
- Zysmilich, M.G.; McDermott, A. Photochemically Induced Dynamic Nuclear Polarization in the Solid-State 15N Spectra of Reaction Centers from Photosynthetic Bacteria Rhodobacter Spbaeroides R-26. J. Am. Chem. Soc. 1994, 116, 8362–8363. [Google Scholar] [CrossRef]
- Daviso, E.; Alia, A.; Prakash, S.; Diller, A.; Gast, P.; Lugtenburg, J.; Matysik, J.; Jeschke, G. Electron-Nuclear Spin Dynamics in a Bacterial Photosynthetic Reaction Center. J. Phys. Chem. C 2009, 113, 10269–10278. [Google Scholar] [CrossRef]
- Jeschke, G. A New Mechanism for Chemically Induced Dynamic Nuclear Polarization in the Solid State. J. Am. Chem. Soc. 1998, 120, 4425–4429. [Google Scholar] [CrossRef]
- Jeschke, G. Electron-Electron-Nuclear Three-Spin Mixing in Spin-Correlated Radical Pairs. J. Chem. Phys. 1997, 106, 10072–10086. [Google Scholar] [CrossRef]
- Polenova, T.; McDermott, A.E. A Coherent Mixing Mechanism Explains the Photoinduced Nuclear Polarization in Photosynthetic Reaction Centers. J. Phys. Chem. B 1999, 103, 535–548. [Google Scholar] [CrossRef]
- McDermott, A.; Zysmilich, M.G.; Polenova, T. Solid State NMR Studies of Photoinduced Polarization in Photosynthetic Reaction Centers: Mechanism and Simulations. Solid State Nucl. Magn. Reson. 1998, 11, 21–47. [Google Scholar] [CrossRef] [PubMed]
- Boxer, S.G.; Chidsey, C.E.D.; Roelofs, M.G. Anisotropic Magnetic Interactions in the Primary Radical Ion-Pair of Photosynthetic Reaction Centers. Proc. Natl. Acad. Sci. USA 1982, 79, 4632–4636. [Google Scholar] [CrossRef]
- Sosnovsky, D.V.; Jeschke, G.; Matysik, J.; Vieth, H.M.; Ivanov, K.L. Level Crossing Analysis of Chemically Induced Dynamic Nuclear Polarization: Towards a Common Description of Liquid-State and Solid-State Cases. J. Chem. Phys. 2016, 144. [Google Scholar] [CrossRef] [PubMed]
- Matysik, J.; Diller, A.; Roy, E.; Alia, A. The Solid-State Photo-CIDNP Effect. Photosynth Res 2009, 102, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Thamarath, S.S.; Bode, B.E.; Prakash, S.; Sai Sankar Gupta, K.B.; Alia, A.; Jeschke, G.; Matysik, J. Electron Spin Density Distribution in the Special Pair Triplet of Rhodobacter Sphaeroides R26 Revealed by Magnetic Field Dependence of the Solid-State Photo-CIDNP Effect. J. Am. Chem. Soc. 2012, 134, 5921–5930. [Google Scholar] [CrossRef]
- Gräsing, D.; Bielytskyi, P.; Céspedes-Camacho, I.F.; Alia, A.; Marquardsen, T.; Engelke, F.; Matysik, J. Field-Cycling NMR with High-Resolution Detection under Magic-Angle Spinning: Determination of Field-Window for Nuclear Hyperpolarization in a Photosynthetic Reaction Center. Sci. Rep. 2017, 7, 12111. [Google Scholar] [CrossRef]
- Thamarath Surendran, S. Towards Photo-CIDNP MAS NMR as a Generally Applicable Enhancement Method. Ph.D. Thesis, Leiden University, Leiden, The Netherlands, 2014. [Google Scholar]
- Bouly, J.P.; Schleicher, E.; Dionisio-Sese, M.; Vandenbussche, F.; Van Der Straeten, D.; Bakrim, N.; Meier, S.; Batschauer, A.; Galland, P.; Bittl, R.; et al. Cryptochrome Blue Light Photoreceptors Are Activated through Interconversion of Flavin Redox States. J. Biol.Chem. 2007, 282, 9383–9391. [Google Scholar] [CrossRef]
- Vaidya, A.T.; Top, D.; Manahan, C.C.; Tokuda, J.M.; Zhang, S.; Pollack, L.; Young, M.W.; Crane, B.R. Flavin Reduction Activates Drosophila Cryptochrome. Proc. Natl. Acad. Sci. USA 2013, 110, 20455–20460. [Google Scholar] [CrossRef]
- Schulten, K.; Swenberg, C.E.; Weiler, A. A Biomagnetic Sensory Mechanism Based on Magnetic Field Modulated Coherent Electron Spin Motion. Z. Phys. Chem. 1978, 111, 1–5. [Google Scholar] [CrossRef]
- Maeda, K.; Henbest, K.B.; Cintolesi, F.; Kuprov, I.; Rodgers, C.T.; Liddell, P.A.; Gust, D.; Timmel, C.R.; Hore, P.J. Chemical Compass Model of Avian Magnetoreception. Nature 2008, 453, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.Y.; Frederiksen, A.; Hanić, M.; Schuhmann, F.; Grüning, G.; Hore, P.J.; Solov’yov, I.A. Navigation of Migratory Songbirds: A Quantum Magnetic Compass Sensor. Neuroforum 2021, 27, 141–150. [Google Scholar] [CrossRef]
- Solov’Yov, I.A.; Mouritsen, H.; Schulten, K. Acuity of a Cryptochrome and Vision-Based Magnetoreception System in Birds. Biophys. J. 2010, 99, 40–49. [Google Scholar] [CrossRef]
- Solov’yov, I.A.; Schulten, K. Magnetoreception through Cryptochrome May Involve Superoxide. Biophys. J. 2009, 96, 4804–4813. [Google Scholar] [CrossRef] [PubMed]
- Solov’yov, I.A.; Greiner, W. Iron-Mineral-Based Magnetoreceptor in Birds: Polarity or Inclination Compass? Eur. Phys. J. D 2009, 51, 161–172. [Google Scholar] [CrossRef]
- Ritz, T.; Adem, S.; Schulten, K. A Model for Photoreceptor-Based Magnetoreception in Birds. Biophys. J. 2000, 78, 707–718. [Google Scholar] [CrossRef]
- Ritz, T.; Thalau, P.; Phillips, J.B.; Wiltschko, R.; Wiltschko, W. Resonance Effects Indicate a Radical-Pair Mechanism for Avian Magnetic Compass. Nature 2004, 429, 177–180. [Google Scholar] [CrossRef]
- Solov’yov, I.A.; Chandler, D.E.; Schulten, K. Magnetic Field Effects in Arabidopsis Thaliana Cryptochrome-1. Biophys. J. 2007, 92, 2711–2726. [Google Scholar] [CrossRef]
- Pedersen, J.B.; Nielsen, C.; Solov’yov, I.A. Multiscale Description of Avian Migration: From Chemical Compass to Behaviour Modeling. Sci. Rep. 2016, 6, 36709. [Google Scholar] [CrossRef]
- Solov’yov, I.A.; Schulten, K. Reaction Kinetics and Mechanism of Magnetic Field Effects in Cryptochrome. J. Phys. Chem. B 2012, 116, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Grüning, G.; Wong, S.Y.; Gerhards, L.; Schuhmann, F.; Kattnig, D.R.; Hore, P.J.; Solov’yov, I.A. Effects of Dynamical Degrees of Freedom on Magnetic Compass Sensitivity: A Comparison of Plant and Avian Cryptochromes. J. Am. Chem. Soc. 2022, 144, 22902–22914. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Robinson, A.J.; Henbest, K.B.; Hogben, H.J.; Biskup, T.; Ahmad, M.; Schleicher, E.; Weber, S.; Timmel, C.R.; Hore, P.J. Magnetically Sensitive Light-Induced Reactions in Cryptochrome Are Consistent with Its Proposed Role as a Magnetoreceptor. Proc. Natl. Acad. Sci. USA 2012, 109, 4774–4779. [Google Scholar] [CrossRef]
- Kattnig, D.R.; Solov’yov, I.A.; Hore, P.J. Electron Spin Relaxation in Cryptochrome-Based Magnetoreception. Physi.Chem. Chem. Phys. 2016, 18, 12443–12456. [Google Scholar] [CrossRef] [PubMed]
- Hiscock, H.G.; Worster, S.; Kattnig, D.R.; Steers, C.; Jin, Y.; Manolopoulos, D.E.; Mouritsen, H.; Hore, P.J. The Quantum Needle of the Avian Magnetic Compass. Proc. Natl. Acad. Sci. USA 2016, 113, 4634–4639. [Google Scholar] [CrossRef]
- Nielsen, C.; Solov’yov, I.A. MolSpin—Flexible and Extensible General Spin Dynamics Software. J. Chem. Phys. 2019, 151, 194105. [Google Scholar] [CrossRef]
- Lindoy, L.P.; Fay, T.P.; Manolopoulos, D.E. Quantum Mechanical Spin Dynamics of a Molecular Magnetoreceptor. J. Chem. Phys. 2020, 152, 164107. [Google Scholar] [CrossRef]
- Worster, S.; Kattnig, D.R.; Hore, P.J. Spin Relaxation of Radicals in Cryptochrome and Its Role in Avian Magnetoreception. J. Chem. Phys. 2016, 145, 035104. [Google Scholar] [CrossRef]
- Timmel, C.R.; Till, U.; Brocklehurst, B.; Mclauchlan, K.A.; Hore, P.J. Effects of Weak Magnetic Fields on Free Radical Recombination Reactions. Mol. Phys. 1998, 95, 71–89. [Google Scholar] [CrossRef]
- Efimova, O.; Hore, P.J. Role of Exchange and Dipolar Interactions in the Radical Pair Model of the Avian Magnetic Compass. Biophys. J. 2008, 94, 1565–1574. [Google Scholar] [CrossRef]
- Babcock, N.S.; Kattnig, D.R. Radical Scavenging Could Answer the Challenge Posed by Electron–Electron Dipolar Interactions in the Cryptochrome Compass Model. J. Am. Chem. Soc. 2021, 1, 2033–2046. [Google Scholar] [CrossRef]
- Player, T.C.; Hore, P.J. Viability of Superoxide-Containing Radical Pairs as Magnetoreceptors. J. Chem. Phys. 2019, 151, 225101. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.; Kattnig, D.R.; Sjulstok, E.; Hore, P.J.; Solov’yov, I.A. Ascorbic Acid May Not Be Involved in Cryptochrome-Based Magnetoreception. J. R. Soc. Interface 2017, 14, 20170657. [Google Scholar] [CrossRef]
- Wong, S.Y.; Solov’yov, I.A.; Hore, P.J.; Kattnig, D.R. Nuclear Polarization Effects in Cryptochrome-Based Magnetoreception. J. Chem. Phys. 2021, 154, 035102. [Google Scholar] [CrossRef] [PubMed]
- Bradlaugh, A.A.; Fedele, G.; Munro, A.L.; Hansen, C.N.; Hares, J.M.; Patel, S.; Kyriacou, C.P.; Jones, A.R.; Rosato, E.; Baines, R.A. Essential Elements of Radical Pair Magnetosensitivity in Drosophila. Nature 2023, 615, 111–116. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).