Two Distinct Molecular Types of Phytochrome A in Plants: Evidence of Existence and Implications for Functioning
Abstract
1. Introduction
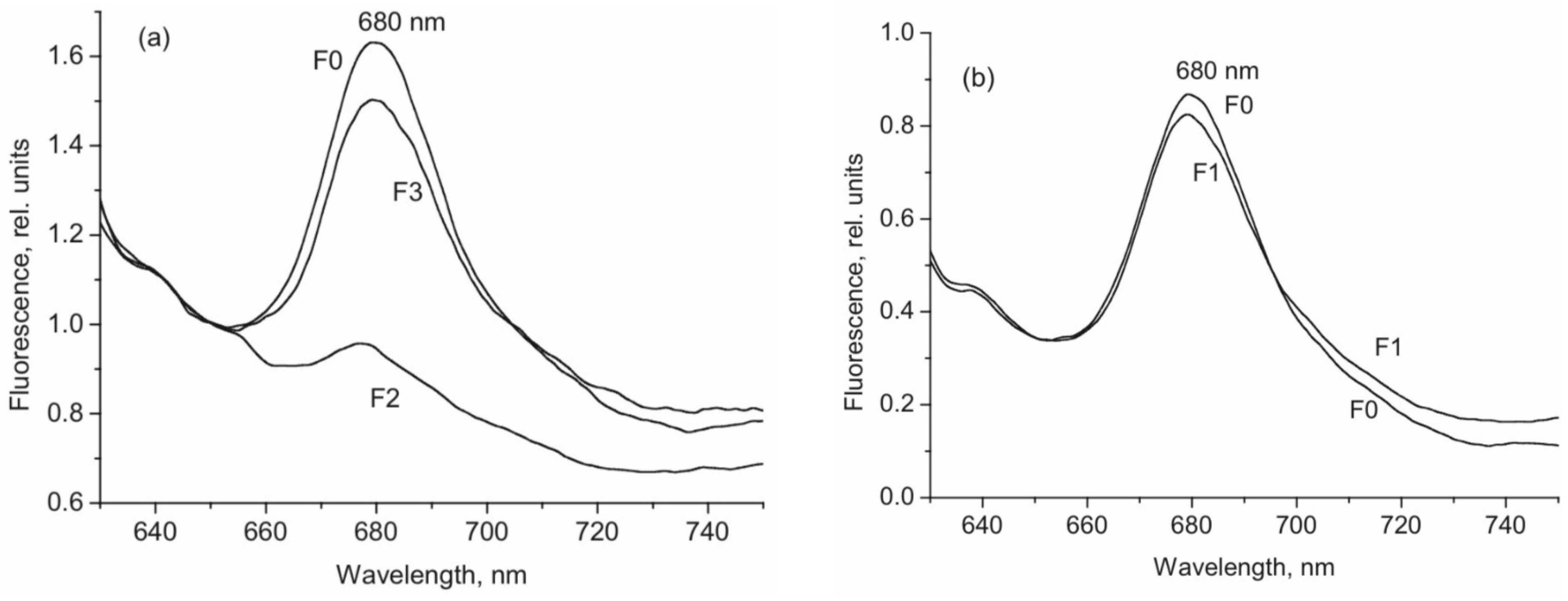
2. Phytochrome A Heterogeneity in the Cell: Chemically Distinct Species and Conformers
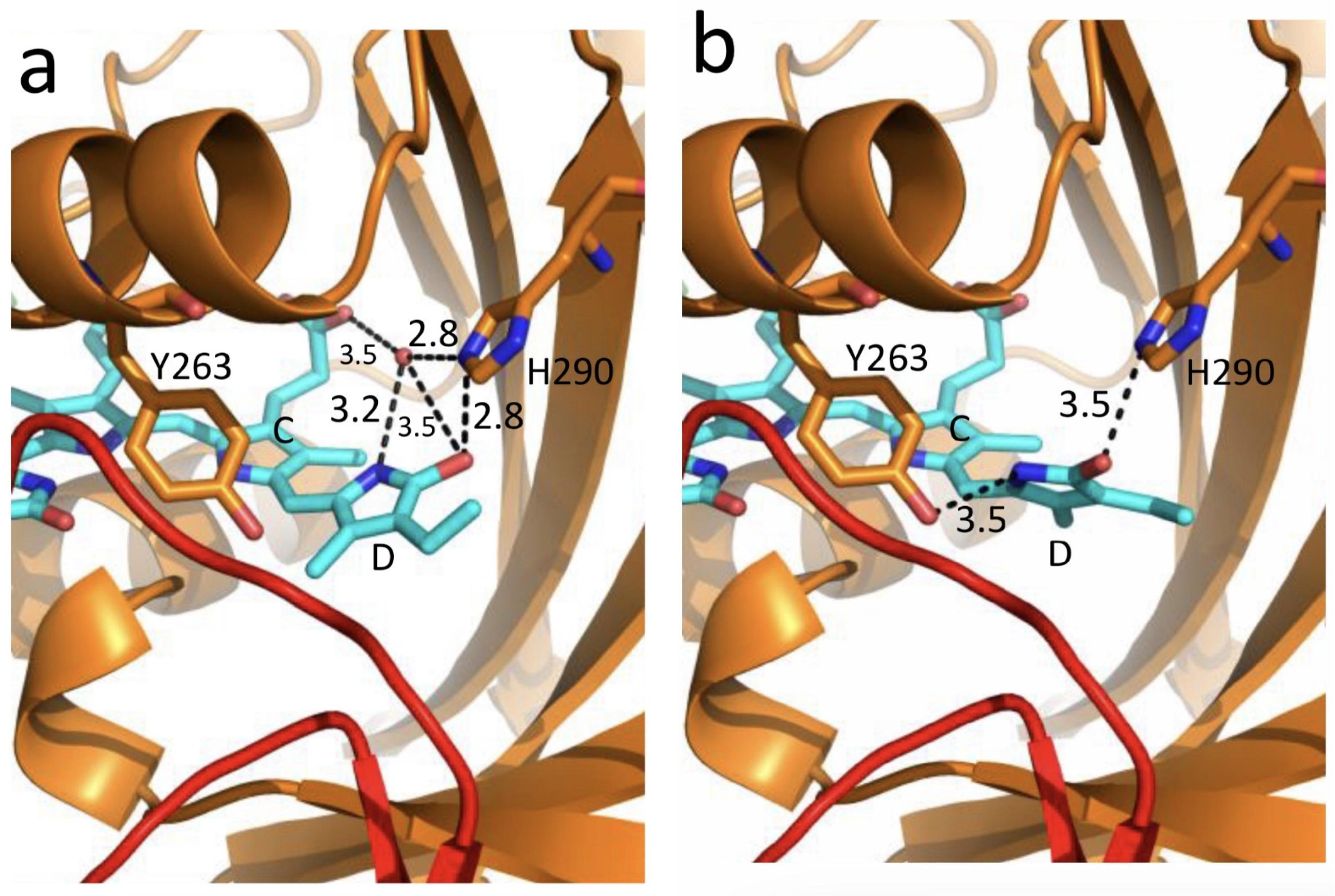
3. Photochemical and Structural Characterization of the Two phyA Types
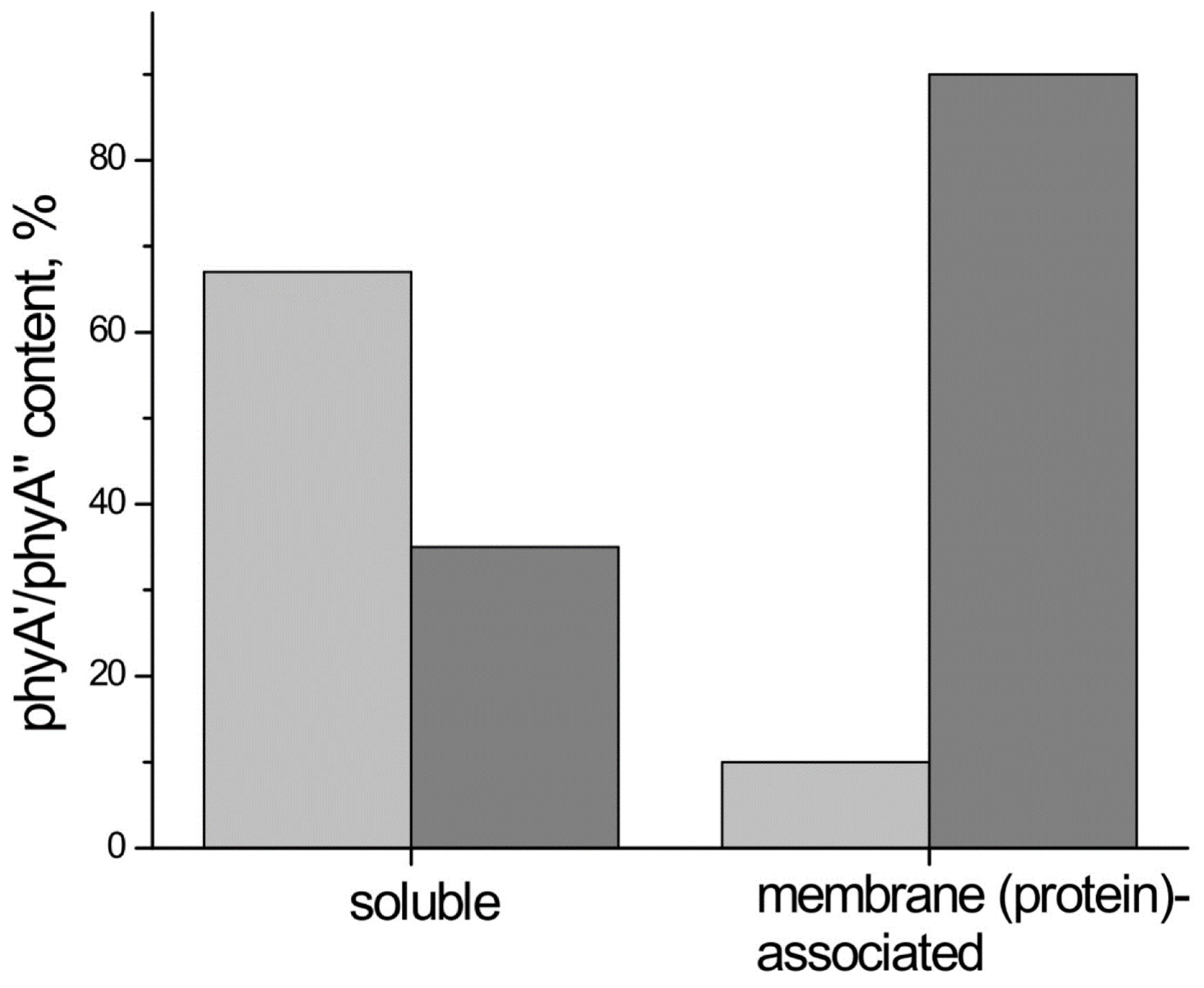
4. The phyA Pools and the Problem of the Membrane-(Protein-) Association of Phytochrome
5. The Two phyA Spices May Account for the Two Distinct Patterns of phyA Nuclear Speckle Formation
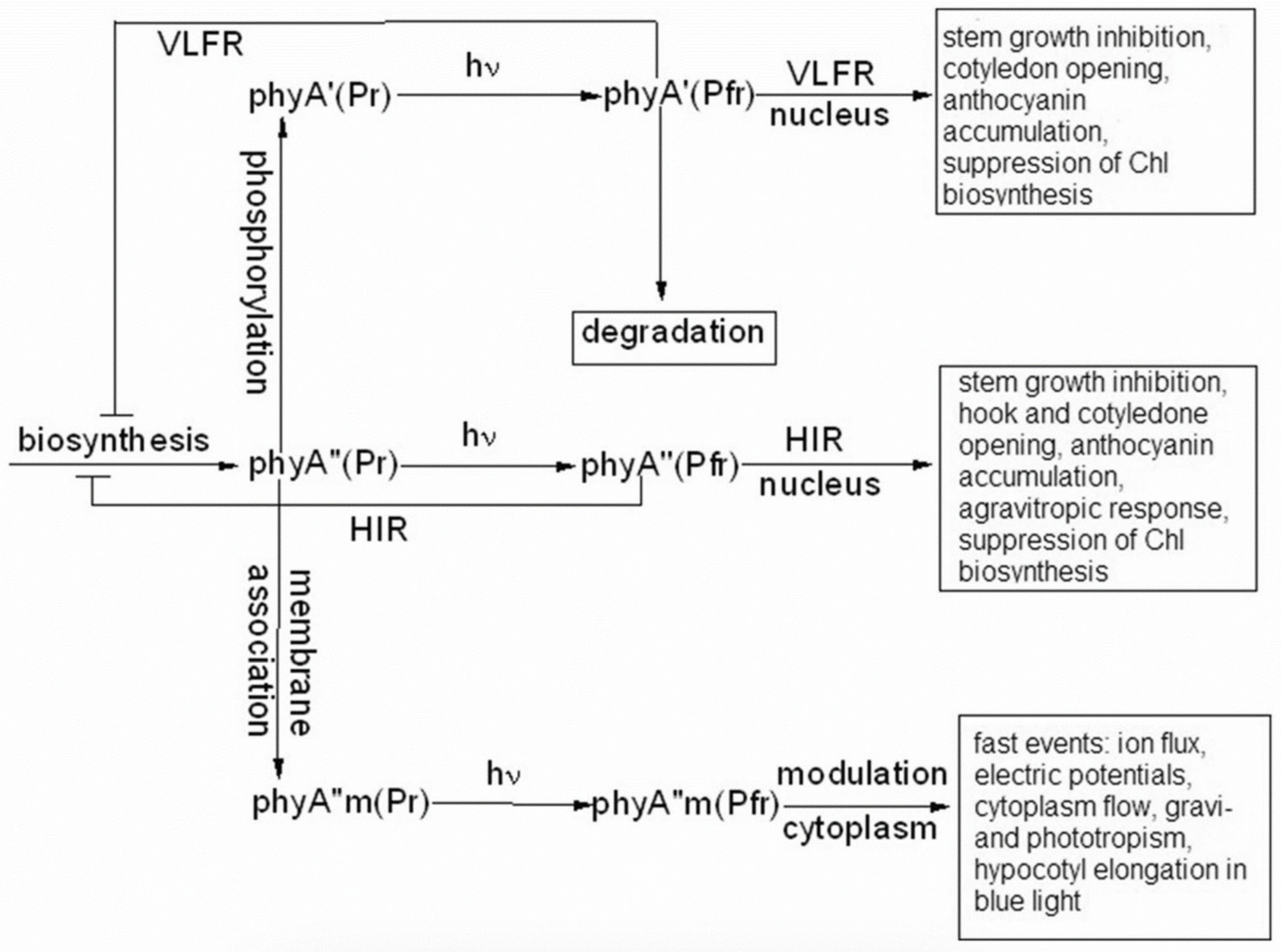
6. The phyAs Mediate Distinct Types of Photoresponses: The Major and Light-Labile phyA′—The VLFR, and the Minor and Relatively Light-Stable phyA″—The HIR
7. Action of phyA in the Cytoplasm
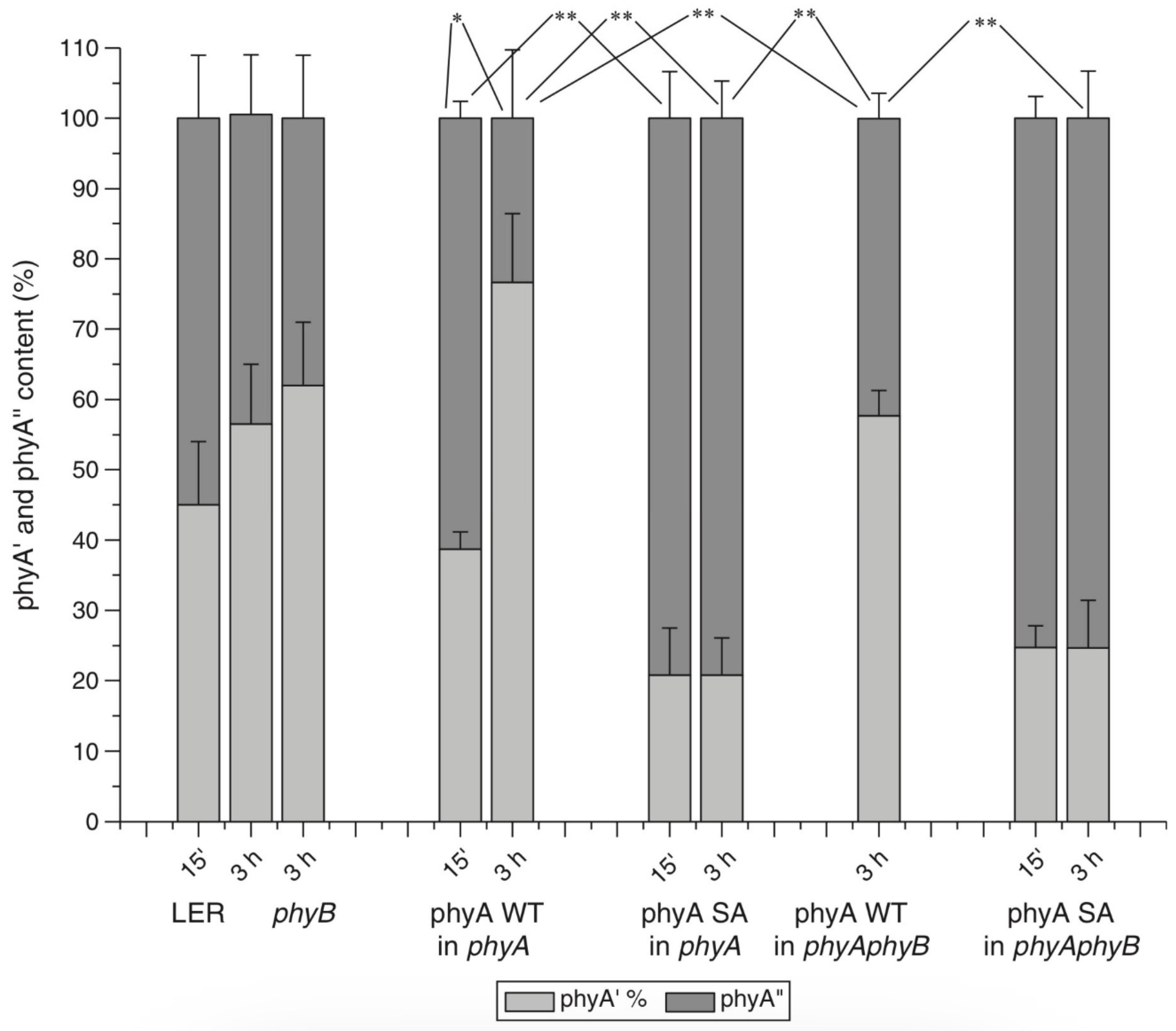
8. Regulation of the phyAs Content and Their Balance in the Dark and in the Light
9. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, H. Photomorphogenesis. Plant Cell Environ. 1997, 20, 657–844. [Google Scholar]
- Mathews, S.; Sharrock, R.A. Phytochrome gene diversity. Plant Cell Environ. 1997, 20, 666–671. [Google Scholar] [CrossRef]
- Inoue, K.; Nishihama, R.; Kohchi, T. Evolutionary origin of phytochrome responses and signaling in land plants. Plant Cell Environ. 2017, 40, 2502–2508. [Google Scholar] [CrossRef] [PubMed]
- Mawphlang, O.I.L.; Kharshiing, E.V. Photoreceptor mediated plant growth responses: Implications for photoreceptor engineering toward improved performance in crops. Front. Plant Sci. 2017, 8, 1181. [Google Scholar] [CrossRef]
- Zakurin, A.O.; Shchennikova, A.V.; Kamionskaya, A.M. Artificial-light culture in protected ground plant growing: Photosynthesis, photomorphogenesis, and prospects of LED application. Russ. J. Plant Physiol. 2020, 67, 413–424. [Google Scholar] [CrossRef]
- Chernov, K.G.; Redchuk, T.A.; Omelina, E.S.; Verkhusha, V.V. Near-infrared fluorescent proteins, biosensors, and optogenetic tools engineered from phytochromes. Chem. Rev. 2017, 117, 6423–6446. [Google Scholar] [CrossRef]
- Gourinchas, G.; Etzl, S.; Winkler, A. Bacteriophytochromes–from informative model systems of phytochrome function to powerful tools in cell biology. Curr. Opin. Struct. Biol. 2019, 57, 72–83. [Google Scholar] [CrossRef]
- Gomez, E.J.; Gerhardt, K.; Judd, J.; Tabor, J.J.; Suh, J. Light-activated nuclear translocation of adeno-associated virus nanoparticles using phytochrome B for enhanced, tunable, and spatially programmable gene delivery. ACS Nano 2016, 10, 225–237. [Google Scholar] [CrossRef]
- Sage, L.C. Pigment of the Imagination, a History of Phytochrome Research; Academic: San Diego, CA, USA, 1992. [Google Scholar]
- Furuya, M. History and insights. In Light Sensing in Plants; Springer: Berlin/Heidelberg, Germany, 2005; pp. 3–18. [Google Scholar]
- Kevei, E.; Schafer, E.; Nagy, F. Light-regulated nucleo-cytoplasmic partitioning of phytochromes. J. Exp. Bot. 2007, 58, 3113–3124. [Google Scholar] [CrossRef]
- Braslavsky, S.E.; Gärtner, W.; Schaffner, K. Phytochrome photoconversion. Plant Cell Environ. 1997, 20, 700–706. [Google Scholar] [CrossRef]
- Song, P.S.; Park, M.H.; Furuya, M. Chromophore: Apoprotein interactions in phytochrome A. Plant Cell Environ. 1997, 20, 707–712. [Google Scholar] [CrossRef]
- Sineshchekov, V.A. Photobiophysics and photobiochemistry of the heterogeneous phytochrome system. Biochim. Biophys. Acta (BBA)-Bioenerg. 1995, 1228, 125–164. [Google Scholar] [CrossRef]
- Sakamoto, K.; Nagatani, A. Nuclear localization activity of phytochrome B. Plant J. 1996, 10, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, R.; Nakamura, M.; Mochizuki, N.; Kay, S.A.; Nagatani, A. Light-dependent translocation of a phytochrome B-GFP fusion protein to the nucleus in transgenic Arabidopsis. J. Cell Biol. 1999, 145, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Kircher, S.; Kozma-Bognar, L.; Kim, L.; Adam, E.; Harter, K.; Schäfer, E.; Nagy, F. Light quality–dependent nuclear import of the plant photoreceptors phytochrome A and B. Plant Cell 1999, 11, 1445–1456. [Google Scholar] [PubMed]
- Kircher, S.; Gil, P.; Kozma-Bognár, L.; Fejes, E.; Speth, V.; Husselstein-Muller, T.; Nagy, F. Nucleocytoplasmic partitioning of the plant photoreceptors phytochrome A, B, C, D, and E is regulated differentially by light and exhibits a diurnal rhythm. Plant Cell 2002, 14, 1541–1555. [Google Scholar] [CrossRef] [PubMed]
- Hisada, A.; Hanzawa, H.; Weller, J.L.; Nagatani, A.; Reid, J.B.; Furuya, M. Light-induced nuclear translocation of endogenous pea phytochrome A visualized by immunocytochemical procedures. Plant Cell 2000, 12, 1063–1078. [Google Scholar] [CrossRef]
- Al-Sady, B.; Ni, W.; Kircher, S.; Schäfer, E.; Quail, P.H. Photoactivated phytochrome induces rapid PIF3 phosphorylation prior to proteasome-mediated degradation. Mol. Cell 2006, 23, 439–446. [Google Scholar] [CrossRef]
- Franklin, K.A.; Quail, P.H. Phytochrome functions in Arabidopsis development. J. Exp. Bot. 2010, 61, 11–24. [Google Scholar] [CrossRef]
- Viczián, A.; Klose, C.; Ádám, É.; Nagy, F. New insights of red light-induced development. Plant Cell Environ. 2017, 40, 2457–2468. [Google Scholar] [CrossRef]
- Legris, M.; Ince, Y.C.; Fankhauser, C. Molecular mechanisms underlying phytochrome-controlled morphogenesis in plants. Nat. Commun. 2019, 10, 5219. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.C.; Kathare, P.K.; Paik, I.; Huq, E. Phytochrome signaling networks. Annu. Rev. Plant Biol. 2021, 72, 217–244. [Google Scholar] [CrossRef] [PubMed]
- Hiltbrunner, A.; Tscheuschler, A.; Viczian, A.; Kunkel, T.; Kircher, S.; Schäfer, E. FHY1 and FHL act together to mediate nuclear accumulation of the phytochrome A photoreceptor. Plant Cell Physiol. 2006, 47, 1023–1034. [Google Scholar] [CrossRef]
- Rösler, J.; Klein, I.; Zeidler, M. Arabidopsis fhl/fhy1 double mutant reveals a distinct cytoplasmic action of phytochrome A. Proc. Natl. Acad. Sci. USA 2007, 104, 10737–10742. [Google Scholar] [CrossRef] [PubMed]
- Jaedicke, K.; Lichtenthäler, A.L.; Meyberg, R.; Zeidler, M.; Hughes, J. A phytochrome–phototropin light signaling complex at the plasma membrane. Proc. Natl. Acad. Sci. USA 2012, 109, 12231–12236. [Google Scholar] [CrossRef] [PubMed]
- Paik, I.; Yang, S.; Choi, G. Phytochrome regulates translation of mRNA in the cytosol. Proc. Natl. Acad. Sci. USA 2012, 109, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J. Phytochrome cytoplasmic signaling. Annu. Rev. Plant Biol. 2013, 64, 377–402. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, K.M. A general hypothesis to interpret ‘high energy phenomena′ of photomorphogenesis on the basis of phytochrome. Photochem. Photobiol. 1966, 5, 349–366. [Google Scholar] [CrossRef]
- Blaauw, O.H.; Blaauw-Jensen, G.; Van Leeuwen, W.J. An irreversible red-light-induced growth response in Avena. Planta 1968, 82, 87–104. [Google Scholar] [CrossRef]
- Mohr, H.; Schäfer, E. Photoperception and de-etiolation. Philos. Trans. R. Soc. Lond. B 1983, 303, 489–501. [Google Scholar]
- Shinomura, T.; Nagatani, A.; Hanzawa, H.; Kubota, M.; Watanabe, M.; Furuya, M. Action spectra for phytochrome A- and B-specific photoinduction of seed germination in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1996, 93, 8129–8133. [Google Scholar] [CrossRef] [PubMed]
- Shinomura, T.; Uchida, K.; Furuya, M. Elementary processes of photoperception by phytochrome A for high irradiance response of hypocotyl elongations in Arabidopsis thaliana. Plant Physiol. 2000, 122, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Casal, J.J.; Sanchez, R.A.; Yanovsky, M.J. The function of phytochrome A. Plant Cell Environ. 1997, 20, 813–819. [Google Scholar] [CrossRef]
- Casal, J.J.; Luccioni, L.G.; Oliverio, K.A.; Boccalandro, H.E. Light, phytochrome signalling and photomorphogenesis in Arabidopsis. Photochem. Photobiol. Sci. 2003, 2, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.; Whitelam, G.C. Phytochrome, a family of photoreceptors with multiple physiological roles. Plant Cell Environ. 1990, 13, 695–707. [Google Scholar] [CrossRef]
- Kendrick, R.E.; Kronenberg, G.H. (Eds.) Photomorphogenesis in Plants; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Brockmann, J.; Schaufer, E. Analysis of Pfr destruction in Amaranthus caudatus L Evidence for two pools of phytochrome. Photochem. Photobiol. 1982, 35, 555–558. [Google Scholar] [CrossRef]
- Tokuhisa, J.G.; Daniels, S.M.; Quail, P.H. Phytochrome in green tissue: Spectral and immunochemical evidence for two distinct molecular species of phytochrome in light-grown Avena sativa L. Planta 1985, 164, 321–332. [Google Scholar] [CrossRef]
- Pratt, L.H. Distribution and localization of phytochrome within the plant. In Photomorphogenesis in Plants; Springer: Berlin/Heidelberg, Germany, 1994; pp. 163–185. [Google Scholar]
- Sineshchekov, V.A.; Sineshchekov, A.V. Fluorescence of phytochrome in the cells of etiolated pea seedlings. Biophysics 1987, 32, 116–122. [Google Scholar]
- Sineshchekov, V.A.; Sineshchekov, A.V. Fluorescence of phytochrome in the cells of dark-grown plants and its connection with the phototransformations of the pigment. Photochem. Photobiol. 1989, 49, 325–330. [Google Scholar] [CrossRef]
- Furuya, M. Phytochromes: Their molecular species, gene families, and functions. Annu. Rev. Plant Biol. 1993, 44, 617–645. [Google Scholar] [CrossRef]
- Sharrock, R.A.; Quail, P.H. Novel phytochrome sequences in Arabidopsis thaliana: Structure, evolution, and differential expression of a plant regulatory photoreceptor family. Genes Dev. 1989, 3, 1745–1757. [Google Scholar] [CrossRef] [PubMed]
- Clack, T.; Mathews, S.; Sharrock, R.A. The phytochrome apoprotein family in Arabidopsis is encoded by five genes: The sequences and expression of PHYD and PHYE. Plant Mol. Biol. 1994, 25, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Hauser, B.A.; Cordonnier-Pratt, M.M.; Daniel-Vedele, F.; Pratt, L.H. The phytochrome gene family in tomato includes a novel subfamily. Plant Mol. Biol. 1995, 29, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Quail, P.H. An emerging molecular map of the phytochromes. Plant Cell Environ. 1997, 20, 657–665. [Google Scholar] [CrossRef]
- Yanovsky, M.; Casal, J.; Luppi, J. The VLF loci, polymorphic between ecotypes Landsberg erecta and Columbia, dissect two branches of phytochrome A signal transduction that correspond to very-low-fluence and high-irradiance responses. Plant J. 1997, 12, 659–667. [Google Scholar] [CrossRef]
- Kneissl, J.; Shinomura, T.; Furuya, M.; Bolle, C. A rice phytochrome A in Arabidopsis: The role of the N-terminus under red and far-red light. Mol. Plant 2008, 1, 84–102. [Google Scholar] [CrossRef]
- Long, C.; Iino, M. Light-dependent osmoregulation in pea stem protoplasts. Photoreceptors, tissue specificity, ion relationships, and physiological implications. Plant Physiol. 2001, 125, 1854–1869. [Google Scholar] [CrossRef]
- Pham, V.N.; Kathare, P.K.; Huq, E. Phytochromes and phytochrome interacting factors. Plant Physiol. 2018, 176, 1025–1038. [Google Scholar] [CrossRef]
- Stoltzfus, A. On the possibility of constructive neutral evolution. J. Mol. Evol. 1999, 49, 169–181. [Google Scholar] [CrossRef]
- Force, A.; Lynch, M.; Pickett, F.B.; Amores, A.; Yan, Y.L.; Postlethwait, J. Preservation of duplicate genes by complementary, degenerative mutations. Genetics 1999, 151, 1531–1545. [Google Scholar] [CrossRef]
- Mathews, S. Phytochrome-mediated development in land plants: Red light sensing evolves to meet the challenges of changing light environments. Mol. Ecol. 2006, 15, 3483–3503. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Harada, K.; Abe, J. Genetic and molecular bases of photoperiod responses of flowering in soybean. Breed. Sci. 2012, 61, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Schiessl, S. Regulation and subfunctionalization of flowering time genes in the allotetraploid oil crop Brassica napus. Front. Plant Sci. 2020, 11, 605155. [Google Scholar] [CrossRef]
- Lin, X.; Dong, L.; Tang, Y.; Li, H.; Cheng, Q.; Li, H.; Kong, F. Novel and multifaceted regulations of photoperiodic flowering by phytochrome A in soybean. Proc. Natl. Acad. Sci. USA 2022, 119, e2208708119. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.J.; Song, P.S.; Kim, J.L. Phytochrome-mediated photomorphogenesis in plants. J. Plant Biol. 2007, 50, 230–240. [Google Scholar] [CrossRef]
- Hughes, J. Phytochrome three-dimensional structures and functions. Biochem. Soc. Trans. 2010, 38, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.M.; Kim, S.H.; Han, Y.J.; Kim, J.I. Regulation of Plant Photoresponses by Protein Kinase Activity of Phytochrome A. Int. J. Mol. Sci. 2023, 24, 2110. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Abid, M.A.; Qanmber, G.; Askari, M.; Zhou, L.; Song, Y.; Zhang, R. Photomorphogenesis in plants: The central role of phytochrome interacting factors (PIFs). Environ. Exp. Bot. 2022, 194, 104704. [Google Scholar] [CrossRef]
- Montgomery, B.L. Spatiotemporal phytochrome signaling during photomorphogenesis: From physiology to molecular mechanisms and back. Front. Plant Sci. 2016, 7, 480. [Google Scholar] [CrossRef]
- Butler, W.L.; Norris, K.H.; Siegelman, H.W.; Hendricks, S.B. Detection, assay, and preliminary purification of the pigment controlling photoresponsive development of plants. Proc. Natl. Acad. Sci. USA 1959, 45, 1703–1708. [Google Scholar] [CrossRef]
- Butler, W.L.; Hendricks, S.B.; Siegelman, H.W. Actton spectra of phytochrome in vitro. Photochem. Photobiol. 1964, 3, 521–528. [Google Scholar] [CrossRef]
- Sineshchekov, V.A.; Sineshchekov, A.V. Procedure for combined fluorescence-absorption investigation of phytochrome in plant tissue. Sov. Plant Physiol. 1987, 34, 672–675. [Google Scholar]
- Sineshchekov, V. Two spectroscopically and photochemically distinguishable phytochromes in etiolated seedlings of monocots and dicots. Photochem. Photobiol. 1994, 59, 77–85. [Google Scholar] [CrossRef]
- Sineshchekov, V.A. Phytochrome A: Functional diversity and polymorphism. Photochem. Photobiol. Sci. 2004, 3, 596–607. [Google Scholar] [CrossRef]
- Sineshchekov, V.A. Fluorescence and photochemical investigations of phytochrome in higher plants. J. Bot. 2010, 2010, 358372. [Google Scholar] [CrossRef]
- Sineshchekov, V.A.; Sineshchekov, A.V. Different photoactive states of the red phytochrome form in the cells of etiolated pea and oat seedlings. J. Photochem. Photobiol. B Biol. 1990, 5, 197–217. [Google Scholar] [CrossRef]
- Sineshchekov, V.; Koppel, L.; Shor, E.; Kochetova, G.; Galland, P.; Zeidler, M. Protein phosphatase activity and acidic/alkaline balance as factors regulating the state of phytochrome A and its two native pools in the plant cell. Photochem. Photobiol. 2013, 89, 83–96. [Google Scholar] [CrossRef]
- Sineshchekov, V.; Ogorodnikova, O.; Thiele, A.; Gatz, C. Fluorescence and photochemical characterization of phytochromes A and B in transgenic potato expressing Arabidopsis phytochrome B. J. Photochem. Photobiol. B Biol. 2000, 59, 139–146. [Google Scholar] [CrossRef]
- Sineshchekov, V.; Koppel, L.; Okamoto, H.; Wada, M. Fern Adiantum capillus-veneris phytochrome 1 comprises two native photochemical types similar to seed plant phytochrome A. J. Photochem. Photobiol. B Biol. 2014, 130, 20–29. [Google Scholar] [CrossRef]
- Sineshchekov, V.A.; Akhobadze, V.V. Phytochrome states in etiolated pea seedlings: Fluorescence and primary photoreactions at low temperatures. Photochem. Photobiol. 1992, 56, 743–749. [Google Scholar] [CrossRef]
- Sineshchekov, V.A. Polymorphism of phytochrome A and its functional implications. In Light Sensing in Plants; Springer: Berlin/Heidelberg, Germany, 2005; pp. 95–102. [Google Scholar]
- Sineshchekov, V. Two molecular species of phytochrome A with distinct modes of action. Funct. Plant Biol. 2018, 46, 118–135. [Google Scholar] [CrossRef] [PubMed]
- Sineshchekov, V.A. Evidence for the existence of two phytochrome A populations. J. Photochem. Photobiol. B Biol. 1995, 28, 53–55. [Google Scholar] [CrossRef]
- Sineshchekov, V.A.; Ogorodnikova, O.B.; Devlin, P.F.; Whitelam, G.C. Fluorescence spectroscopy and photochemistry of phytochromes A and B in wild-type, mutant and transgenic strains of Arabidopsis thaliana. J. Photochem. Photobiol. B Biol. 1998, 42, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Sineshchekov, V.A.; Ogorodnikova, O.B.; Weller, J.L. Fluorescence and photochemical properties of phytochromes A and B in etiolated pea seedlings. J. Photochem. Photobiol. B Biol. 1999, 49, 204–211. [Google Scholar] [CrossRef]
- Sineshchekov, V.; Loskovich, A.; Inagaki, N.; Takano, M. Two native pools of phytochrome A in monocots: Evidence from fluorescence investigations of phytochrome mutants of rice. Photochem. Photobiol. 2006, 82, 1116–1122. [Google Scholar] [CrossRef]
- Sineshchekov, V.; Mailliet, J.; Psakis, G.; Feilke, K.; Kopycki, J.; Zeidler, M.; Essen, L.-O.; Hughes, J. Tyrosine 263 in cyanobacterial phytochrome Cph1 optimizes photochemistry at the prelumi-R→lumi-R step. Photochem. Photobiol. 2014, 90, 786–795. [Google Scholar] [CrossRef]
- Sineshchekov, V.A.; Bekasova, O.D. Two distinct photoprocesses in cyanobacterial bilin pigments: Energy migration in light-harvesting phycobiliproteins versus photoisomerization in phytochromes. Photochem. Photobiol. 2020, 96, 750–767. [Google Scholar] [CrossRef]
- Sineshchekov, V.A.; Clough, R.C.; Jordan-Beebe, E.T.; Vierstra, R.D. Fluorescence analysis of oat phyA deletion mutants expressed in tobacco suggests that the N-terminal domain determines the photochemical and spectroscopic distinctions between phyA′ and phyA″. Photochem. Photobiol. 1999, 69, 728–732. [Google Scholar]
- Sineshchekov, V.; Koppel’, L.; Esteban, B.; Hughes, J.; Lamparter, T. Fluorescence investigation of the recombinant cyanobacterial phytochrome (Cph1) and its C-terminally truncated monomeric species (Cph1Δ2): Implication for holoprotein assembly, chromophore-apoprotein interaction and photochemistry. J. Photochem. Photobiol. B Biol. 2002, 67, 39–50. [Google Scholar] [CrossRef]
- Sineshchekov, V.; Hennig, L.; Lamparter, T.; Hughes, J.; Gärtner, W.; Schäfer, E. Recombinant phytochrome A in yeast differs by its spectroscopic and photochemical properties from the major phyA′ and is close to the minor phyA″: Evidence for posttranslational modification of the pigment in plants. Photochem. Photobiol. 2001, 73, 692–696. [Google Scholar] [CrossRef]
- Sineshchekov, V.A.; Koppel, L.A.; Bolle, C. Two native types of phytochrome A, phyA′ and phyA″, differ by the state of phosphorylation at the N-terminus as revealed by fluorescence investigations of the Ser/Ala mutant of rice phyA expressed in transgenic Arabidopsis. Funct. Plant Biol. 2016, 45, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Sineshchekov, V.; Fankhauser, C. PKS1 and PKS2 affect the phyA state in etiolated Arabidopsis seedlings. Photochem. Photobiol. Sci. 2004, 3, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Sineshchekov, V.; Shor, E.; Koppel, L. The phosphatase/kinase balance affects phytochrome A and its native pools, phyA′ and phyA″, in etiolated maize roots: Evidence from the induction of phyA′ destruction by a protein phosphatase inhibitor sodium fluoride. Photochem. Photobiol. Sci. 2021, 20, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Haynie, D.T. Biological Thermodynamics; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Essen, L.-O.; Mailliet, J.; Hughes, J. The structure of a complete phytochrome sensory module in the Pr ground state. Proc. Natl. Acad. Sci. USA 2008, 105, 14709–14714. [Google Scholar] [CrossRef]
- Mailliet, J.; Psakis, G.; Feilke, K.; Sineshchekov, V.; Essen, L.O.; Hughes, J. Spectroscopy and a high-resolution crystal structure of Tyr263 mutants of cyanobacterial phytochrome Cph1. J. Mol. Biol. 2011, 413, 115–127. [Google Scholar] [CrossRef]
- Song, C.; Essen, L.O.; Gärtner, W.; Hughes, J.; Matysik, J. Solid-state NMR spectroscopic study of chromophore-protein interactions in the Pr ground state of plant phytochrome A. Mol. Plant 2012, 5, 698–715. [Google Scholar] [CrossRef]
- Rumfeldt, J.A.; Takala, H.; Liukkonen, A.; Ihalainen, J.A. UV-vis spectroscopy reveals a correlation between Y263 and BV protonation states in bacteriophytochromes. Photochem. Photobiol. 2019, 95, 969–979. [Google Scholar] [CrossRef]
- Kirpich, J.S.; Mix, L.T.; Martin, S.S.; Rockwell, N.C.; Lagarias, J.C.; Larsen, D.S. Protonation heterogeneity modulates the ultrafast photocycle initiation dynamics of phytochrome Cph1. J. Phys. Chem. Lett. 2018, 9, 3454–3462. [Google Scholar] [CrossRef]
- Kim, P.W.; Rockwell, N.C.; Martin, S.S.; Lagarias, J.C.; Larsen, D.S. Dynamic inhomogeneity in the photodynamics of cyanobacterial phytochrome Cph1. Biochemistry 2014, 53, 2818–2826. [Google Scholar] [CrossRef]
- Song, C.; Psakis, G.; Lang, C.; Mailliet, J.; Gärtner, W.; Hughes, J.; Matysik, J. Two ground state isoforms and a chromophore D-ring photoflip triggering extensive intramolecular changes in a canonical phytochrome. Proc. Natl. Acad. Sci. USA 2011, 108, 3842–3847. [Google Scholar] [CrossRef]
- Song, C.; Mroginski, M.A.; Lang, C.; Kopycki, J.; Gärtner, M.J.; Hughes, J. 3D structures of plant phytochrome A as Pr and Pfr from solid-state NMR: Implications for molecular function. Front. Plant Sci. 2018, 9, 498. [Google Scholar] [CrossRef] [PubMed]
- Quail, P.H.; Marmé, D.; Schäfer, E. Particle-bound phytochrome from maize and pumpkin. Nat. New Biol. 1973, 245, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Roux, S.J. Phytochrome and membranes. In Photomorphogenesis in Plants; Springer: Berlin/Heidelberg, Germany, 1986; pp. 115–134. [Google Scholar]
- Roux, S.J.; McEntire, K.; Slocum, R.D.; Cedel, T.E.; Hale, C.C. Phytochrome induces photoreversible calcium fluxes in a purified mitochondrial fraction from oats. Proc. Natl. Acad. Sci. USA 1981, 78, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, J.M., Jr.; Coleman, R.A.; Briggs, W.R.; Pratt, L.H. Reversible redistribution of phytochrome within the cell upon conversion to its physiologically active form. Proc. Natl. Acad. Sci. USA 1975, 72, 799–803. [Google Scholar] [CrossRef]
- Rubinstein, B.; Drury, K.S.; Park, R.B. Evidence for bound phytochrome in oat seedlings. Plant Physiol. 1969, 44, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Haupt, W. Über den Dichroismus von Phytochrom-660 und Phytochrom-730 bei Mougeotia. Z. Pflanzenphysiol. 1970, 62, 287–298. [Google Scholar]
- Clegg, J.S. Properties and metabolism of the aqueous cytoplasm and its boundaries. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1984, 246, R133–R151. [Google Scholar] [CrossRef]
- Sineshchekov, V.A.; MV Lomonosov Moscow State University, Moscow, Russia; Zeidler, M.; Justus-Liebig-Universität, Gießen, German. Unpublished results. 2012.
- Sineshchekov, V.A. Extreme dehydration of plant tissues irreversibly converts the major and variable phyA′ into the minor and conserved phyA″. J. Photochem. Photobiol. B Biol. 2006, 85, 85–91. [Google Scholar] [CrossRef]
- Lamparter, T.; Lutterbuese, P.; Schneider-Poetsch, H.A.W.; Hertel, R. A study of membrane-associated phytochrome: Hydrophobicity test and native size determination. Photochem. Photobiol. 1992, 56, 697–707. [Google Scholar] [CrossRef]
- Terry, M.J.; Hall, J.L.; Thomas, B. The association of type I phytochrome with wheat leaf plasma membranes. J. Plant Physiol. 1992, 140, 691–698. [Google Scholar] [CrossRef]
- Sineshchekov, V.; Lamparter, T.; Hartmann, E. Evidence for the existence of membrane-associated phytochrome in the cell. Photochem. Photobiol. 1994, 60, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.; Kircher, S.; Toth, R.; Adam, E.; SchaÈfer, E.; Nagy, F. Light-induced nuclear import of phytochrome-A: GFP fusion proteins is differentially regulated in transgenic tobacco and Arabidopsis. Plant J. 2000, 22, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Hare, P.D.; Yang, S.W.; Zeidler, M.; Huang, L.F.; Chua, N.H. FHL is required for full phytochrome A signaling and shares overlapping functions with FHY1. Plant J. 2005, 43, 356–370. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhou, Z.; Feng, S.; Li, J.; Tan-Wilson, A.; Qu, L.J.; Deng, X.W. Phytochrome A mediates rapid red light–induced phosphorylation of Arabidopsis FAR-RED ELONGATED HYPOCOTYL1 in a low fluence response. Plant Cell 2009, 21, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Genoud, T.; Schweizer, F.; Tscheuschler, A.; Debrieux, D.; Casal, J.J.; Schäfer, E.; Fankhauser, C. FHY1 mediates nuclear import of the light-activated phytochrome A photoreceptor. PLoS Genet. 2008, 4, e1000143. [Google Scholar] [CrossRef]
- Helizon, H.; Rösler-Dalton, J.; Gasch, P.; von Horsten, S.; Essen, L.O.; Zeidler, M. Arabidopsis phytochrome A nuclear translocation is mediated by a far-red elongated hypocotyl 1–importin complex. Plant J. 2018, 96, 1255–1268. [Google Scholar] [CrossRef]
- Sokolova, V.; Bindics, J.; Kircher, S.; Ádám, É.; Schäfer, E.; Nagy, F.; Viczián, A. Missense mutation in the amino terminus of phytochrome A disrupts the nuclear import of the photoreceptor. Plant Physiol. 2012, 158, 107–118. [Google Scholar] [CrossRef]
- Bauer, D.; Vicziaén, A.; Kircher, S.; Nobis, T.; Nitschke, R.; Kunkel, T.; Nagy, F. Constitutive photomorphogenesis 1 and multiple photoreceptors control degradation of phytochrome interacting factor 3, a transcription factor required for light signaling in Arabidopsis. Plant Cell 2004, 16, 1433–1445. [Google Scholar] [CrossRef] [PubMed]
- Van Buskirk, E.K.; Decker, P.V.; Chen, M. Photobodies in light signaling. Plant Physiol. 2012, 158, 52–60. [Google Scholar] [CrossRef]
- Chen, M.; Schwab, R.; Chory, J. Characterization of the requirements for localization of phytochrome B to nuclear bodies. Proc. Natl. Acad. Sci. USA 2003, 100, 14493–14498. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; McLoughlin, K.E.; Sorkin, M.L.; Burgie, E.S.; Bindbeutel, R.K.; Vierstra, R.D.; Nusinow, D.A. PCH1 regulates light, temperature, and circadian signaling as a structural component of phytochrome B-photobodies in Arabidopsis. Proc. Natl. Acad. Sci. USA 2019, 116, 8603–8608. [Google Scholar] [CrossRef] [PubMed]
- Hahm, J.; Kim, K.; Qiu, Y.; Chen, M. Increasing ambient temperature progressively disassembles Arabidopsis phytochrome B from individual photobodies with distinct thermostabilities. Nat. Commun. 2020, 11, 1660. [Google Scholar] [CrossRef] [PubMed]
- Pardi, S.A.; Nusinow, D.A. Out of the dark and into the light: A new view of phytochrome photobodies. Front. Plant Sci. 2021, 12, 732947. [Google Scholar] [CrossRef] [PubMed]
- Menon, C.; Klose, C.; Hiltbrunner, A. Arabidopsis FHY1 and FHY1-LIKE are not required for phytochrome A signal transduction in the nucleus. Plant Commun. 2020, 1, 100007. [Google Scholar] [CrossRef] [PubMed]
- Casal, J.J.; Davis, S.J.; Kirchenbauer, D.; Viczian, A.; Yanovsky, M.J.; Clough, R.C.; Vierstra, R.D. The serine-rich N-terminal domain of oat phytochrome A helps regulate light responses and subnuclear localization of the photoreceptor. Plant Physiol. 2002, 129, 1127–1137. [Google Scholar] [CrossRef]
- Sineshchekov, V.; Sudnitsin, A.; Ádám, É.; Schäfer, E.; Viczián, A. phyA-GFP is spectroscopically and photochemically similar to phyA and comprises both its native types, phyA′ and phyA″. Photochem. Photobiol. Sci. 2014, 13, 1671–1679. [Google Scholar] [CrossRef]
- Casal, J.J.; Sánchez, R.A.; Botto, J.F. Modes of action of phytochromes. J. Exp. Bot. 1998, 49, 127–138. [Google Scholar] [CrossRef]
- Cerdán, P.D.; Staneloni, R.J.; Ortega, J.; Bunge, M.M.; Rodriguez-Batiller, M.J.; Sánchez, R.A.; Casal, J.J. Sustained but not transient phytochrome A signaling targets a region of an Lhcb1* 2 promoter not necessary for phytochrome B action. Plant Cell 2000, 12, 1203–1211. [Google Scholar] [CrossRef]
- Rausenberger, J.; Tscheuschler, A.; Nordmeier, W.; Wüst, F.; Timmer, J.; Schäfer, E.; Hiltbrunner, A. Photoconversion and nuclear trafficking cycles determine phytochrome A’s response profile to far-red light. Cell 2011, 146, 813–825. [Google Scholar] [CrossRef]
- Yanovsky, M.J.; Luppi, J.P.; Kirchbauer, D.; Ogorodnikova, O.B.; Sineshchekov, V.A.; Adam, E.; Casal, J.J. Missense mutation in the PAS2 domain of phytochrome A impairs subnuclear localization and a subset of responses. Plant Cell 2002, 14, 1591–1603. [Google Scholar] [CrossRef]
- Weller, J.L.; Batge, S.L.; Smith, J.J.; Kerckhoffs, L.H.J.; Sineshchekov, V.A.; Murfet, I.C.; Reid, J.B. A dominant mutation in the pea PHYA gene confers enhanced responses to light and impairs the light-dependent degradation of phytochrome A. Plant Physiol. 2004, 135, 2186–2195. [Google Scholar] [CrossRef] [PubMed]
- Sineshchekov, V.A.; Weller, J.L. Two modes of the light-induced phytochrome A decline–with and without changes in the proportion of its isoforms (phyA′ and phyA″): Evidence from fluorescence investigations of mutant phyA-3D pea. J. Photochem. Photobiol. B Biol. 2004, 75, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Mateos, J.L.; Luppi, J.P.; Ogorodnikova, O.B.; Sineshchekov, V.A.; Yanovsky, M.J.; Braslavsky, S.E.; Casal, J.J. Functional and biochemical analysis of the N-terminal domain of phytochrome A. J. Biol. Chem. 2006, 281, 34421–34429. [Google Scholar] [CrossRef]
- Kopell, L.A.; Sineshchekov, V.A.; MV Lomonosov Moscow State University, Moscow, Russia. Unpublished results. 2010.
- Barnes, S.A.; Nishizawa, N.K.; Quaggio, R.B.; Whitelam, G.C.; Chua, N.H. Far-red light blocks greening of Arabidopsis seedlings via a phytochrome A-mediated change in plastid development. Plant Cell 1996, 8, 601–615. [Google Scholar]
- Sineshchekov, V.; Koppel, L. Phytochrome A in plants comprises two structurally and functionally distinct populations—Water-soluble phyA′ and amphiphilic phyA″. Biophys. Rev. 2022, 14, 905–921. [Google Scholar] [CrossRef] [PubMed]
- Shlumukov, L.R.; Barro, F.; Barcelo, P.; Lazzeri, P.; Smith, H. Establishment of far-red high irradiance responses in wheat through transgenic expression of an oat phytochrome A gene. Plant Cell Environ. 2001, 24, 703–712. [Google Scholar] [CrossRef]
- Heyer, A.G.; Mozley, D.; Landschutze, V.; Thomas, B.; Gatz, C. Function of phytochrome A in potato plants as revealed through the study of transgenic plants. Plant Physiol. 1995, 109, 53–61. [Google Scholar] [CrossRef]
- Sineshchekov, V.; Koppel, L.; Shlumukov, L.; Barro, F.; Barcelo, P.; Lazzeri, P.; Smith, H. Fluorescence and photochemical properties of phytochromes in wild-type wheat and a transgenic line overexpressing an oat phytochrome A (PHYA) gene: Functional implications. Plant Cell Environ. 2001, 24, 1289–1297. [Google Scholar] [CrossRef]
- Sineshchekov, V.A.; Heyer, A.G.; Gatz, C. Phytochrome states in transgenic potato plants with altered phytochrome A levels. J. Photochem. Photobiol. B Biol. 1996, 34, 137–142. [Google Scholar] [CrossRef]
- Boylan, M.T.; Quail, P.H. Oat phytochrome is biologically active in transgenic tomatoes. Plant Cell 1989, 1, 765–773. [Google Scholar] [CrossRef]
- Clough, R.C.; Vierstra, R.D. Phytochrome degradation. Plant Cell Environ. 1997, 20, 713–721. [Google Scholar] [CrossRef]
- Yeh, K.C.; Lagarias, J.C. Eukaryotic phytochromes: Light-regulated serine/threonine protein kinases with histidine kinase ancestry. Proc. Natl. Acad. Sci. USA 1998, 95, 13976–13981. [Google Scholar] [CrossRef] [PubMed]
- McMichael, R.W., Jr.; Lagarias, J.C. Phosphopeptide mapping of Avena phytochrome phosphorylated by protein kinases in vitro. Biochemistry 1990, 29, 3872–3878. [Google Scholar] [CrossRef] [PubMed]
- Lapko, V.N.; Jiang, X.Y.; Smith, D.L.; Song, P.S. Mass spectrometric characterization of oat phytochrome A: Isoforms and posttranslational modifications. Protein Sci. 1999, 8, 1032–1044. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Park, J.E.; Zarate, X.; Song, P.S. Phytochrome phosphorylation in plant light signaling. Photochem. Photobiol. Sci. 2005, 4, 681–687. [Google Scholar] [CrossRef]
- Han, Y.J.; Kim, H.S.; Kim, Y.M.; Shin, A.Y.; Lee, S.S.; Bhoo, S.H.; Kim, J.I. Functional characterization of phytochrome autophosphorylation in plant light signaling. Plant Cell Physiol. 2010, 51, 596–609. [Google Scholar] [CrossRef]
- Saijo, Y.; Zhu, D.; Li, J.; Rubio, V.; Zhou, Z.; Shen, Y.; Deng, X.W. Arabidopsis COP1/SPA1 complex and FHY1/FHY3 associate with distinct phosphorylated forms of phytochrome A in balancing light signaling. Mol. Cell 2008, 31, 607–613. [Google Scholar] [CrossRef]
- Sineshchekov, V.; Koppel, L.; Kim, J.I. The dephosphorylated S8A and S18A mutants of (oat) phytochrome A comprise its two species, phyA′ and phyA″, suggesting that autophosphorylation at these sites is not involved in the phyA differentiation. Photochem. Photobiol. Sci. 2019, 18, 1242–1248. [Google Scholar] [CrossRef]
- Shin, A.Y.; Han, Y.J.; Baek, A.; Ahn, T.; Kim, S.Y.; Nguyen, T.S.; Son, M.; Lee, K.W.; Shen, Y.; Song, P.-S.; et al. Evidence that phytochrome functions as a protein kinase in plant light signalling. Nat. Commun. 2016, 7, 11545. [Google Scholar] [CrossRef]
- Hoang, Q.T.; Tripathi, S.; Cho, J.Y.; Choi, D.M.; Shin, A.Y.; Kwon, S.Y.; Kim, J.I. Suppression of phytochrome-interacting factors enhances photoresponses of seedlings and delays flowering with increased plant height in brachypodium distachyon. Front. Plant Sci. 2021, 12, 756795. [Google Scholar] [CrossRef]
- Kim, D.H.; Kang, J.G.; Yang, S.S.; Chung, K.S.; Song, P.S.; Park, C.M. A phytochrome-associated protein phosphatase 2A modulates light signals in flowering time control in Arabidopsis. Plant Cell 2002, 14, 3043–3056. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Shen, Y.; Han, Y.J.; Park, J.E.; Kirchenbauer, D.; Soh, M.S.; Song, P.S. Phytochrome phosphorylation modulates light signaling by influencing the protein–protein interaction. Plant Cell 2004, 16, 2629–2640. [Google Scholar] [CrossRef] [PubMed]
- Lifschitz, S.; Gepstein, S.; Horwitz, B.A. Phytochrome regulation of greening in wild type and long-hypocotyl mutants of Arabidopsis thaliana. Planta 1990, 181, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Batschauer, A.; Apel, K. An inverse control by phytochrome of the expression of two nuclear genes in barley (Hordeum vulgave L.). Eur. J. Biochem. 1984, 143, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Runge, S.; Sperling, U.; Frick, G.; Apel, K.; Armstrong, G.A. Distinct roles for light-dependent NADPH: Protochlorophyllide oxidoreductases (POR) A and B during greening in higher plants. Plant J. 1996, 9, 513–523. [Google Scholar] [CrossRef]
- Sineshchekov, V.; Belyaeva, O.; Sudnitsin, A. Up-regulation by phytochrome A of the active protochlorophyllide, Pchlide655, biosynthesis in dicots under far-red light. J. Photochem. Photobiol. B Biol. 2004, 74, 47–54. [Google Scholar] [CrossRef]
- Yanovsky, M.J.; Whitelam, G.C.; Casal, J.J. fhy3-1 retains inductive responses of phytochrome A. Plant Physiol. 2000, 123, 235–242. [Google Scholar] [CrossRef]
- Schendel, R.; Dörnemann, D.; Rüdiger, W.; Sineshchekov, V. Comparative investigations of the effect of 5-aminolevulinate feeding on phytochrome and protochlorophyll (ide) content in dark-grown seedlings of barley, cucumber and cress. J. Photochem. Photobiol. B Biol. 1996, 36, 245–253. [Google Scholar] [CrossRef]
- Sineshchekov, V.A.; Belyaeva, O.B. Regulation of chlorophyll biogenesis by phytochrome A. Biochemistry 2019, 84, 491–508. [Google Scholar] [CrossRef]
- Trupkin, S.A.; Debrieux, D.; Hiltbrunner, A.; Fankhauser, C.; Casal, J.J. The serine-rich N-terminal region of Arabidopsis phytochrome A is required for protein stability. Plant Mol. Biol. 2007, 63, 669–678. [Google Scholar] [CrossRef]
- Halliday, K.J.; Fankhauser, C. Phytochrome-hormonal signaling networks. New Phytol. 2020, 157, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.L.; Okamoto, H. Molecular interaction of jasmonate and phytochrome A signalling. J. Exp. Bot. 2014, 65, 2847–2857. [Google Scholar] [CrossRef] [PubMed]
- Lau, O.S.; Deng, X.W. Plant hormone signaling lightens up: Integrators of light and hormones. Curr. Opin. Plant Biol. 2010, 13, 571–577. [Google Scholar] [CrossRef]
- Leivar, P.; Quail, P.H. PIFs: Pivotal components in a cellular signaling hub. Trends Plant Sci. 2011, 16, 19–28. [Google Scholar] [CrossRef] [PubMed]
- de Lucas, M.; Prat, S. PIF s get BR right: PHYTO- CHROME INTERACTING FACTOR s as integrators of light and hormonal signals. New Phytol. 2014, 202, 1126–1141. [Google Scholar] [CrossRef] [PubMed]
- Gavassi, M.A.; Alves, F.R.R.; Carvalho, R.F. Phytochrome and Hormone Signaling Crosstalk in Response to Abiotic Stresses in Plants. In Plant Hormones and Climate Change; Springer Nature: Singapore, 2023; pp. 145–165. [Google Scholar]
- Jeong, J.; Kim, K.; Kim, M.E.; Kim, H.G.; Heo, G.S.; Park, O.K.; Park, Y.I.; Choi, G.; Oh, E. Phytochrome and ethylene signaling integration in Arabidopsis occurs via the transcriptional regulation of genes cotargeted by PIFs and EIN3. Front. Plant Sci. 2016, 7, 1055. [Google Scholar] [CrossRef] [PubMed]
- Sineshchekov, V.A.; Loskovich, A.V.; Riemann, M.; Nick, P. The jasmonate-free rice mutant hebiba is affected in the response of phyA′/phyA″ pools and protochlorophyllide biosynthesis to far-red light. Photochem. Photobiol. Sci. 2004, 3, 1058–1062. [Google Scholar] [CrossRef] [PubMed]
- Sineshchekov, V.; Koppel, L.; Riemann, M.; Nick, P. Phytochrome A and its Functional Manifestations in Etiolated and Far-red Light-grown Seedlings of the Wild-type Rice and its Hebiba and Cpm2 Mutants Deficient in the Defense-related Phytohormone Jasmonic Acid. Photochem. Photobiol. 2021, 97, 335–342. [Google Scholar] [CrossRef]
- Robson, F.; Okamoto, H.; Patrick, E.; Harris, S.R.; Wasternack, C.; Brearley, C.; Turner, J.G. Jasmonate and phytochrome A signaling in Arabidopsis wound and shade responses are integrated through JAZ1 stability. Plant Cell 2010, 22, 1143–1160. [Google Scholar] [CrossRef]
- Svyatyna, K.; Riemann, M. Light-dependent regulation of the jasmonate pathway. Protoplasma 2012, 249, 137–145. [Google Scholar] [CrossRef]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef] [PubMed]
- Takano, M.; Inagaki, N.; Xie, X.; Yuzurihara, N.; Hihara, F.; Ishizuka, T.; Shinomura, T. Distinct and cooperative functions of phytochromes A, B, and C in the control of deetiolation and flowering in rice. Plant Cell 2005, 17, 3311–3325. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Shinomura, T.; Yamamoto, K.T. Similarities and differences between phytochrome-mediated growth inhibition of coleoptiles and seminal roots in rice seedlings. Plant Signal. Behav. 2010, 5, 134–135. [Google Scholar] [CrossRef]
- Xie, X.; Shinomura, T.; Inagaki, N.; Kiyota, S.; Takano, M. Phytochrome-mediated inhibition of coleoptile growth in rice: Age-dependency and action spectra. Photochem. Photobiol. 2007, 83, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, H. JA modulates phytochrome a signaling via repressing FHY3 activity by JAZ proteins. Plant Signal. Behav. 2020, 15, 1726636. [Google Scholar] [CrossRef] [PubMed]
- Whippo, C.W.; Hangarter, R.P. Phytochrome modulation of blue-light-induced phototropism. Plant Cell Environ. 2004, 27, 1223–1228. [Google Scholar] [CrossRef]
- Sakamoto, K.; Briggs, W.R. Cellular and subcellular localization of phototropin 1. Plant Cell 2002, 14, 1723–1735. [Google Scholar] [CrossRef]
- Kong, S.G.; Wada, M. Recent advances in understanding the molecular mechanism of chloroplast photorelocation movement. Biochim. Biophys. Acta (BBA)-Bioenerg. 2014, 1837, 522–530. [Google Scholar] [CrossRef]
- Fankhauser, C.; Yeh, K.C.; Lagarias, J.C.; Zhang, H.; Elich, T.D.; Chory, J. PKS1, a substrate phosphorylated by phytochrome that modulates light signaling in Arabidopsis. Science 1999, 284, 1539–1541. [Google Scholar] [CrossRef]
- Lariguet, P.; Schepens, I.; Hodgson, D.; Pedmale, U.V.; Trevisan, M.; Kami, C.; Fankhauser, C. PHYTOCHROME KINASE SUBSTRATE 1 is a phototropin 1 binding protein required for phototropism. Proc. Natl. Acad. Sci. USA 2006, 103, 10134–10139. [Google Scholar] [CrossRef]
- Titapiwatanakun, B.; Murphy, A.S. Post-transcriptional regulation of auxin transport proteins: Cellular trafficking, protein phosphorylation, protein maturation, ubiquitination, and membrane composition. J. Exp. Bot. 2009, 60, 1093–1107. [Google Scholar] [CrossRef] [PubMed]
- Christie, J.M.; Kaiserli, E.; Sullivan, S. Light sensing at the plasma membrane. In The Plant Plasma Membrane; Springer: Berlin/Heidelberg, Germany, 2010; pp. 423–436. [Google Scholar]
- Kim, K.B.; Park, M.H.; Chae, Q. Light Effects on the Membrane Potential in Oat Cells. BMB Rep. 1995, 28, 382–386. [Google Scholar]
- Schwenk, P.; Hiltbrunner, A. Phytochrome A Mediates the Disassembly of Processing Bodies in Far-Red Light. Front. Plant Sci. 2022, 13, 828529. [Google Scholar] [CrossRef] [PubMed]
- Jang, G.J.; Yang, J.Y.; Hsieh, H.L.; Wu, S.H. Processing bodies control the selective translation for optimal development of Arabidopsis young seedlings. Proc. Natl. Acad. Sci. USA 2019, 116, 6451–6456. [Google Scholar] [CrossRef]
- Correll, M.J.; Kiss, J.Z. The roles of phytochromes in elongation and gravitropism of roots. Plant Cell Physiol. 2005, 46, 317–323. [Google Scholar] [CrossRef]
- Correll, M.J.; Coveney, K.M.; Raines, S.V.; Mullen, J.L.; Hangarter, R.P.; Kiss, J.Z. Phytochromes play a role in phototropism and gravitropism in Arabidopsis roots. Adv. Space Res. 2003, 31, 2203–2210. [Google Scholar] [CrossRef]
- Salisbury, F.J.; Hall, A.; Grierson, C.S.; Halliday, K.J. Phytochrome coordinates Arabidopsis shoot and root development. Plant J. 2007, 50, 429–438. [Google Scholar] [CrossRef]
- Shin, D.H.; Cho, M.H.; Kim, T.L.; Yoo, J.; Kim, J.I.; Han, Y.J.; Hahn, T.R. A small GTPase activator protein interacts with cytoplasmic phytochromes in regulating root development. J. Biol. Chem. 2010, 285, 32151–32159. [Google Scholar] [CrossRef]
- Shen, Y.; Kim, J.I.; Song, P.S. NDPK2 as a signal transducer in the phytochrome-mediated light signaling. J. Biol. Chem. 2005, 280, 5740–5749. [Google Scholar] [CrossRef]
- Cantón, F.R.; Quail, P.H. Both phyA and phyB mediate light-imposed repression of PHYA gene expression in Arabidopsis. Plant Physiol. 1999, 121, 1207–1215. [Google Scholar] [CrossRef]
- Sineshchekov, V.A.; Frances, S.; White, M.J. Fluorescence and photochemical characterization of phytochrome in de-etiolated pea mutant lip. J. Photochem. Photobiol. B Biol. 1995, 28, 47–51. [Google Scholar] [CrossRef]
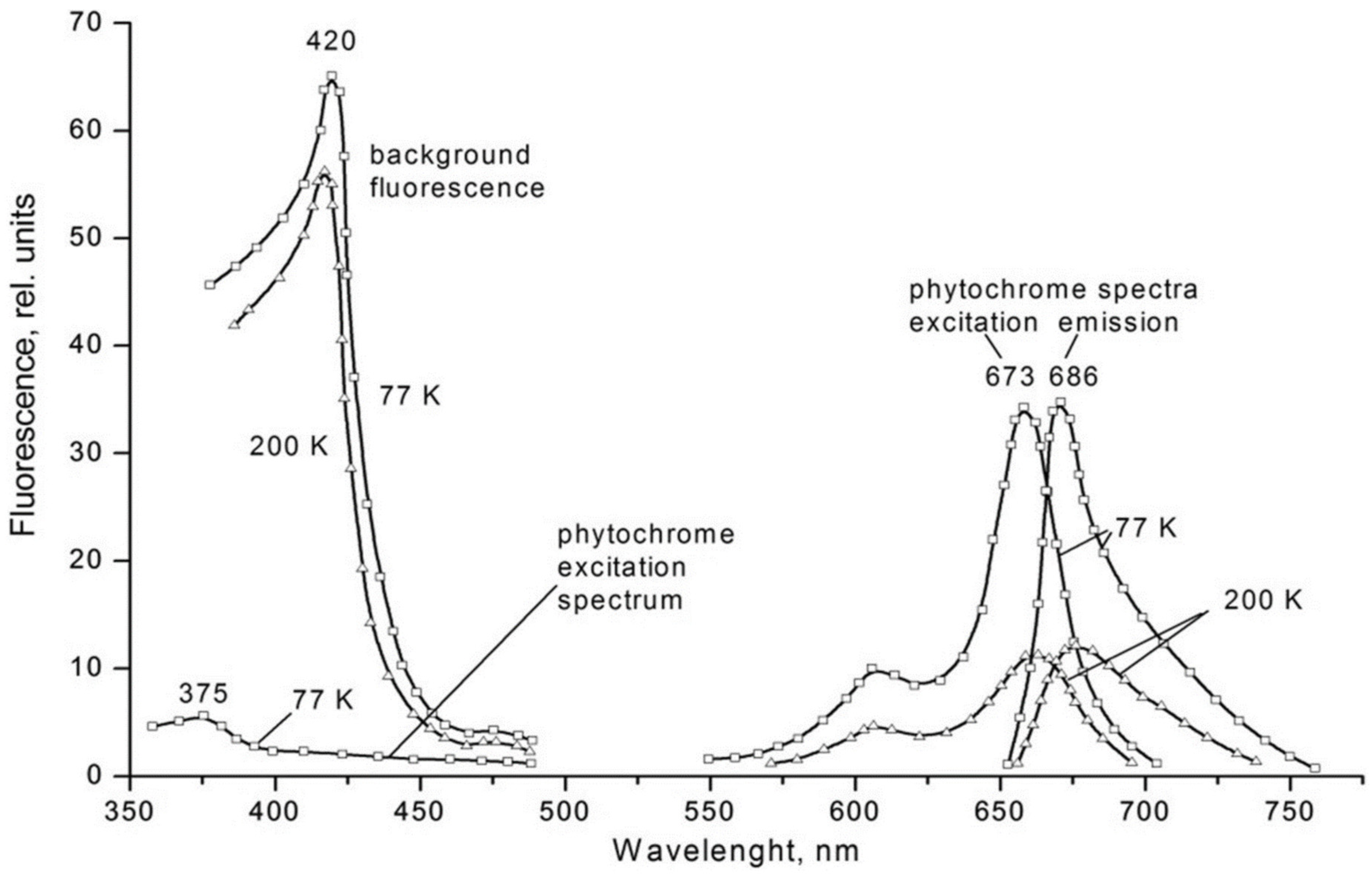

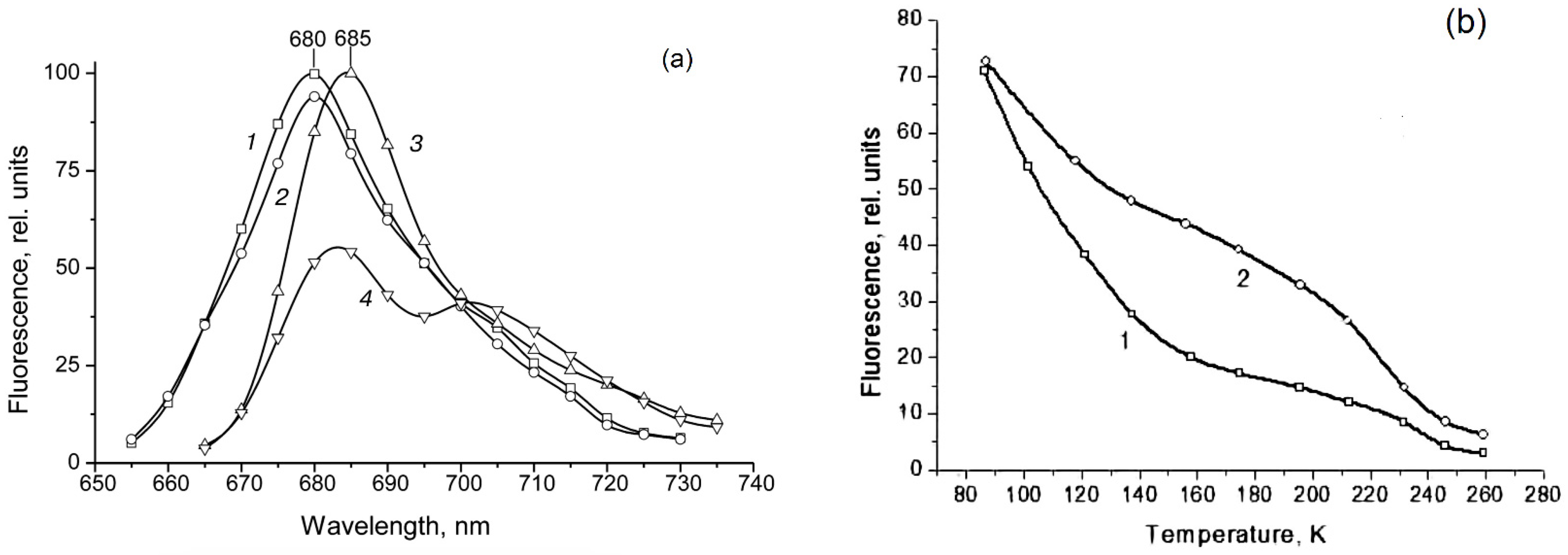

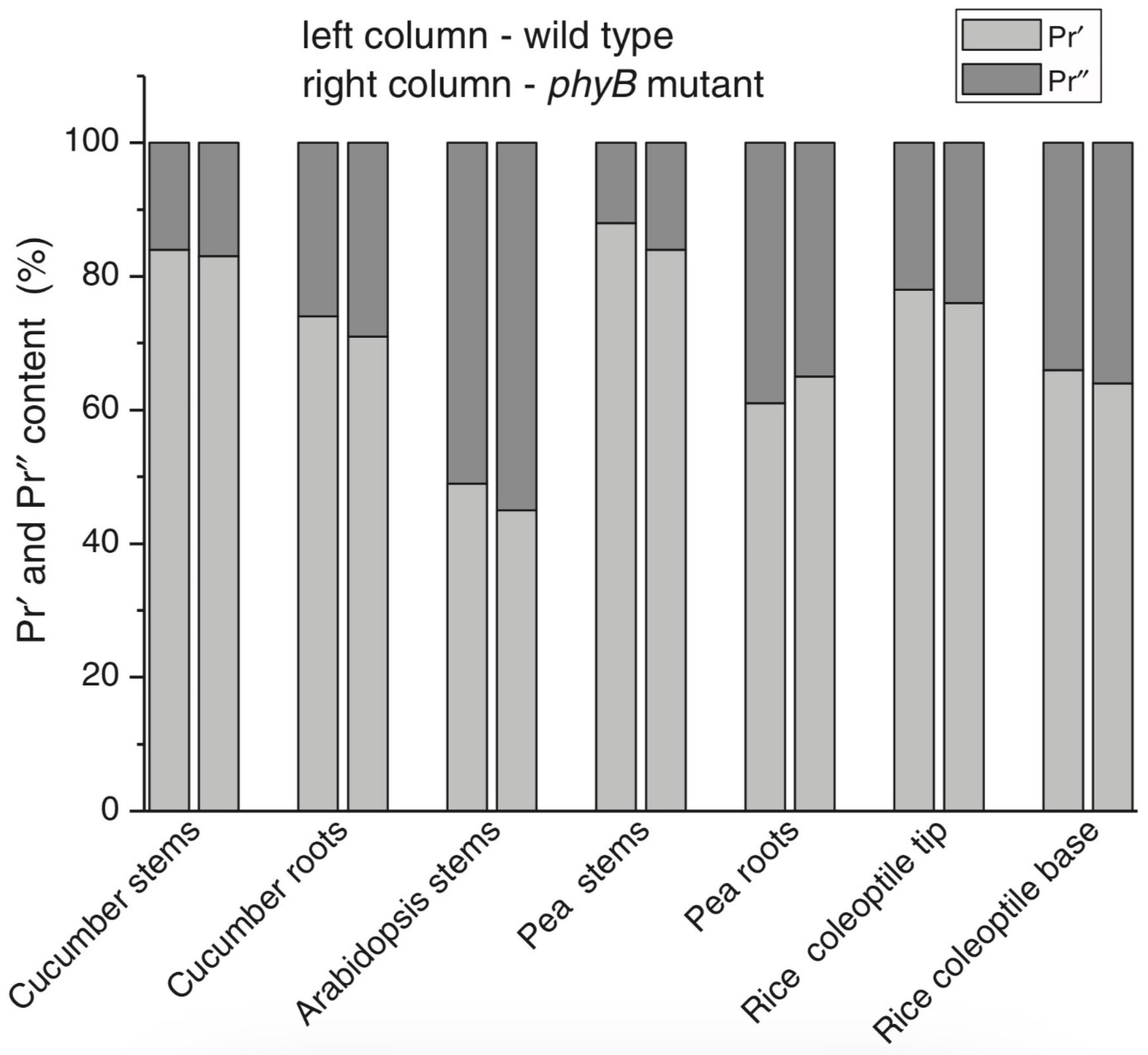
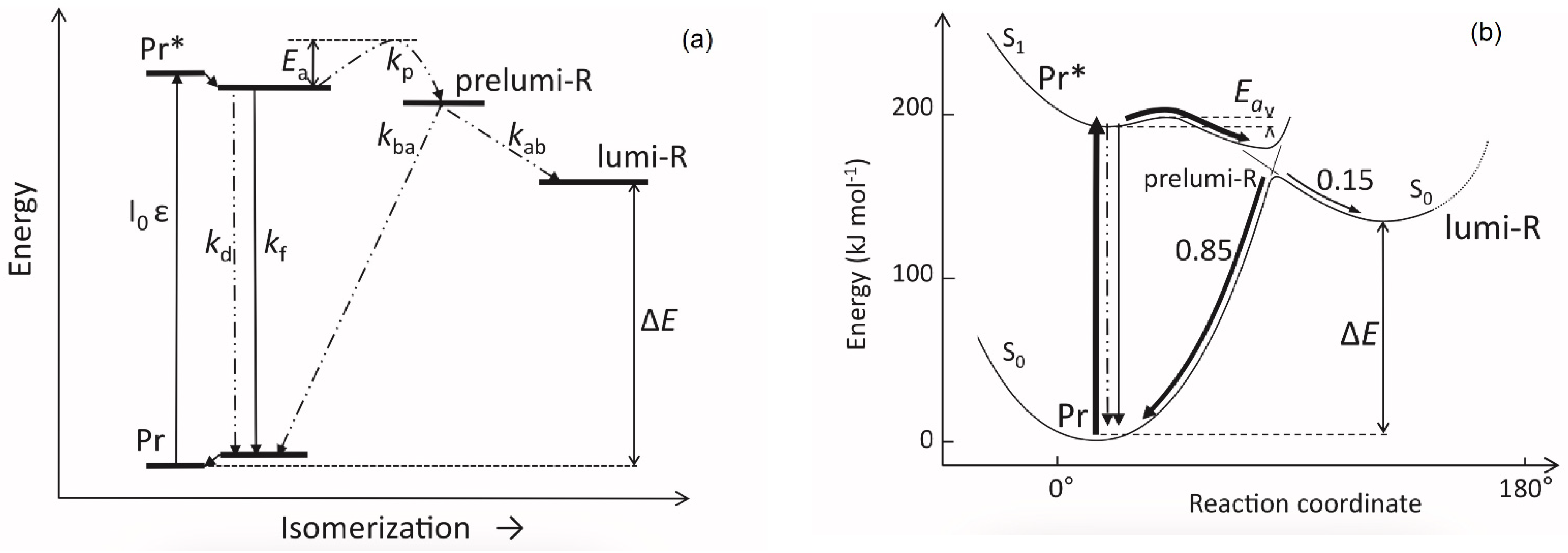

| Parameter | Phytochrome Type | |
|---|---|---|
| Pr′ (phyA′) | Pr″ (phyA″) | |
| Position of emission/absorption maxima, λmax, nm | Longer wavelength (685/672) | Shorter wavelength (680/667) |
| Half-band width, nm | 22–24 | 30 |
| Extent of Pr→lumi-R conversion at 85 K, γ1 | 0.49 ± 0.03 | ≤0.05 |
| Activation barrier in excited state, Ea, kJ/mol | ≤1 | ≥10 |
| Extent of Pr→Pfr conversion at 273 K, γ2 | 0.80–0.85 | 0.75 |
| Light lability | Light labile | Relatively light stable |
| Hydrophilicity/Hydrophobicity | Water–soluble | Ambiquitous—soluble and membrane-(protein-) associated |
| Content in etiolated tissues | Major, variable | Minor, saturated, conserved |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sineshchekov, V.A. Two Distinct Molecular Types of Phytochrome A in Plants: Evidence of Existence and Implications for Functioning. Int. J. Mol. Sci. 2023, 24, 8139. https://doi.org/10.3390/ijms24098139
Sineshchekov VA. Two Distinct Molecular Types of Phytochrome A in Plants: Evidence of Existence and Implications for Functioning. International Journal of Molecular Sciences. 2023; 24(9):8139. https://doi.org/10.3390/ijms24098139
Chicago/Turabian StyleSineshchekov, Vitaly A. 2023. "Two Distinct Molecular Types of Phytochrome A in Plants: Evidence of Existence and Implications for Functioning" International Journal of Molecular Sciences 24, no. 9: 8139. https://doi.org/10.3390/ijms24098139
APA StyleSineshchekov, V. A. (2023). Two Distinct Molecular Types of Phytochrome A in Plants: Evidence of Existence and Implications for Functioning. International Journal of Molecular Sciences, 24(9), 8139. https://doi.org/10.3390/ijms24098139





