A Novel Role for UNC119 as an Enhancer of Synaptic Transmission
Abstract
1. Introduction
2. Results
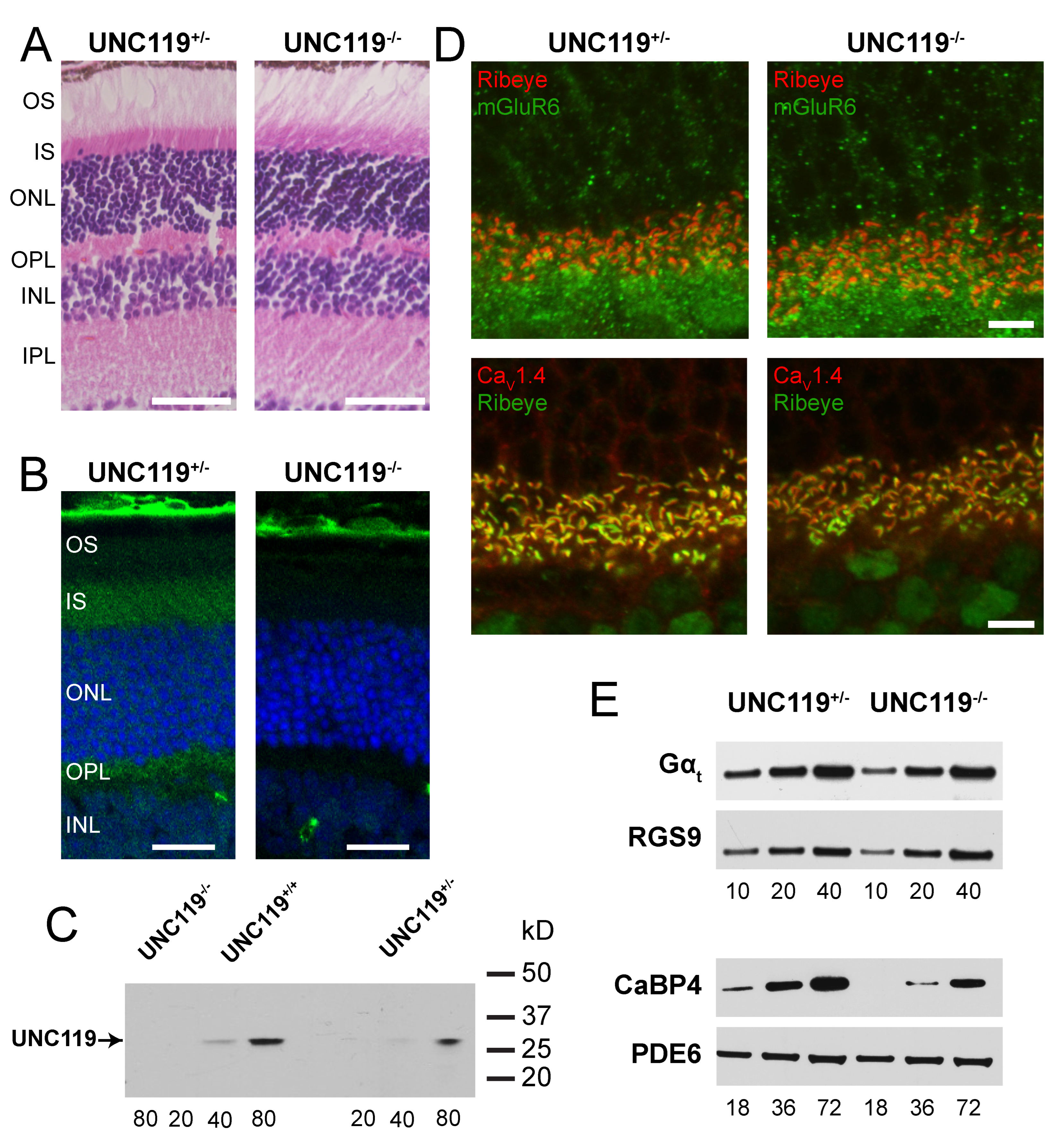
2.1. Characterization of UNC119−/− Retina
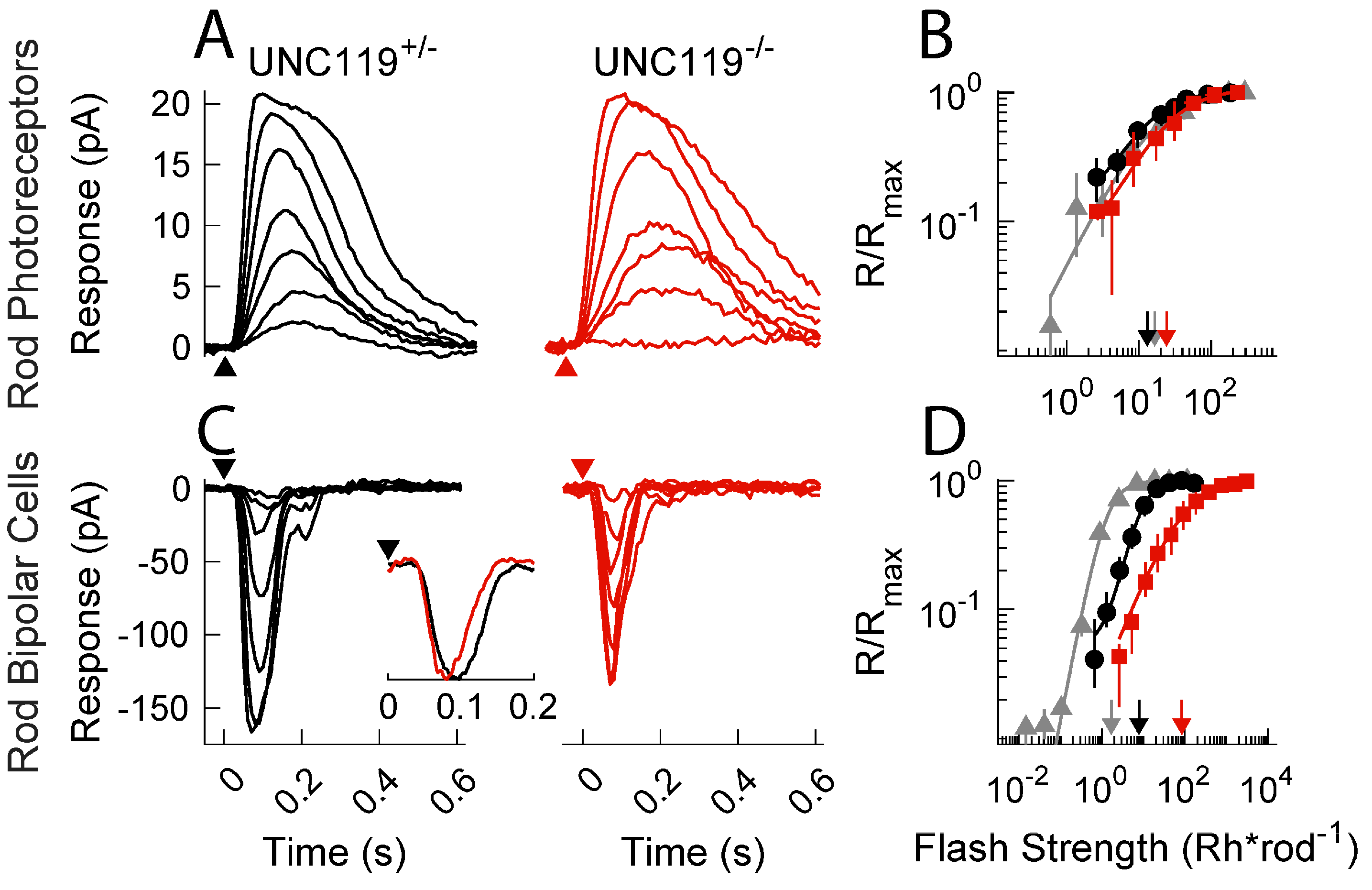
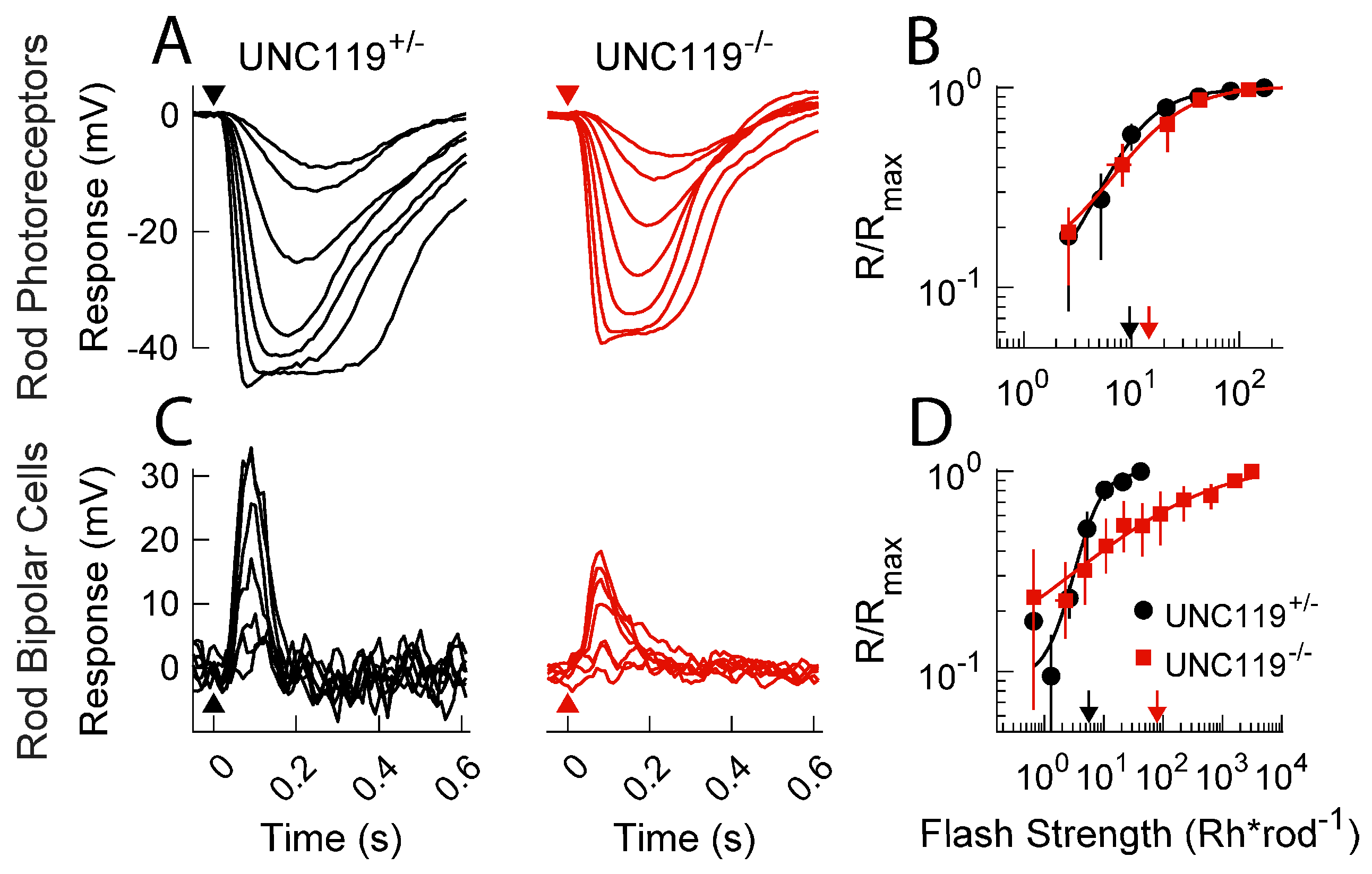
2.2. Effect of UNC119 Deletion on Dark-Adapted Current Responses of Rods and Rod Bipolar Cells
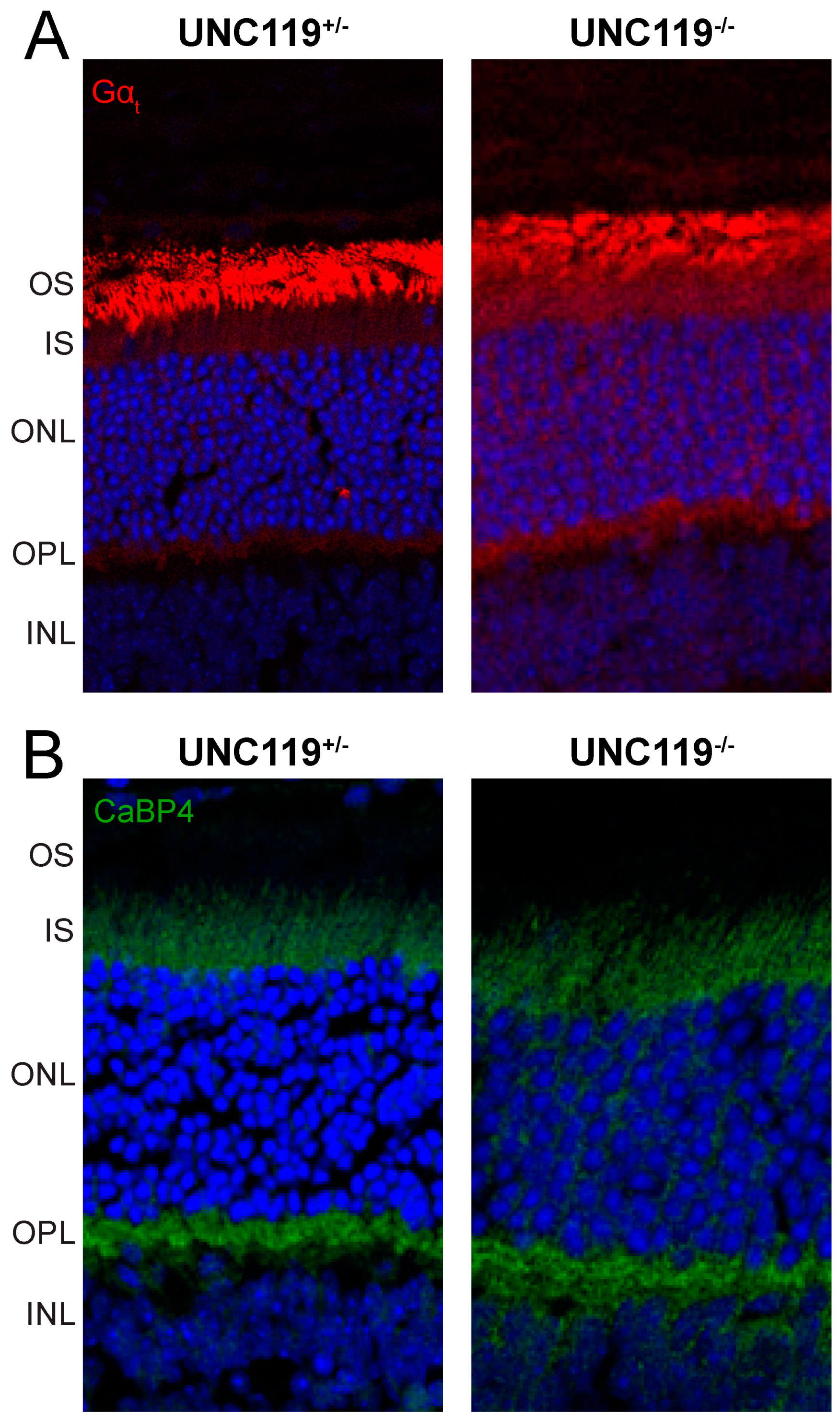
2.3. Effect of Transducin Translocation
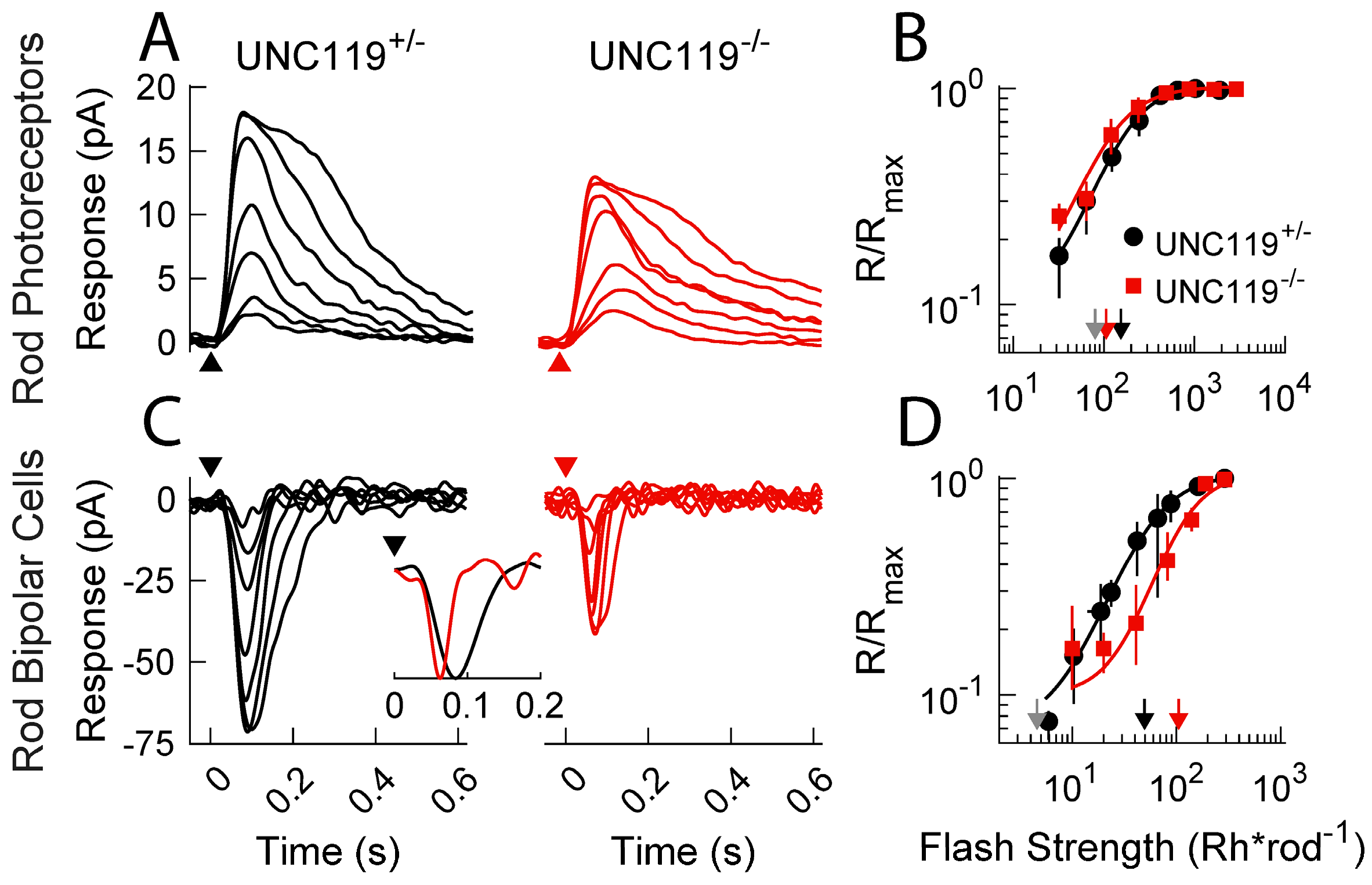
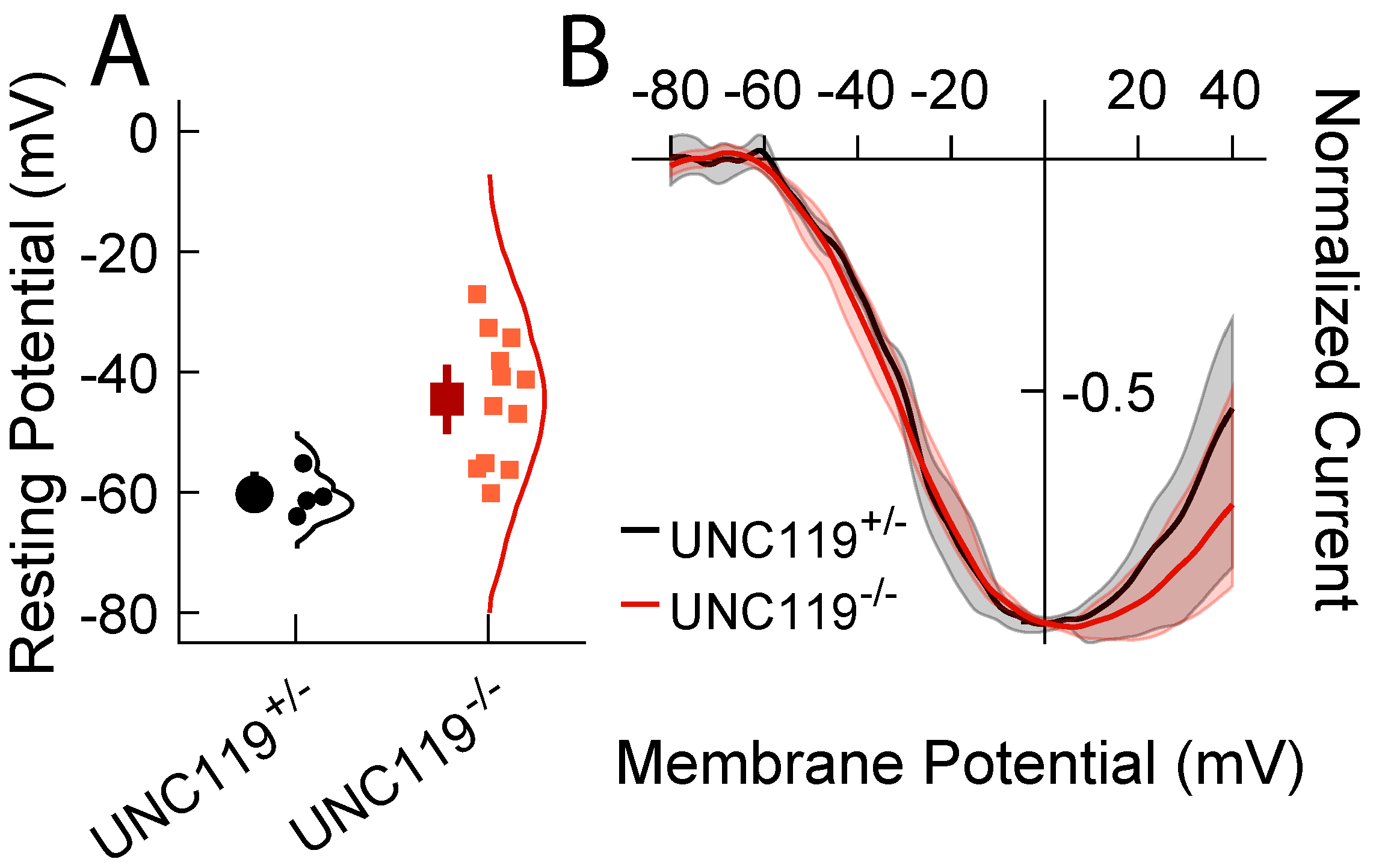
2.4. Measurement of Voltage Responses
2.5. Lack of Effect of Deletion of UNC119 on Voltage Dependence of Calcium Current
3. Discussion
3.1. Modulation of Synaptic Transmission by Transducin
3.2. Role of CaBP4 and Calcium Channels
3.3. Mechanism of Action of UNC119
4. Materials and Methods
4.1. UNC119Knockout Mice
4.2. Immunofluorescence
4.3. Immunoblotting
4.4. Retinal Morphology
4.5. Physiological Recording from Rods and Rod Bipolar Cells
4.6. Light-induced Transducin Translocation
4.7. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jean, F.; Pilgrim, D. Coordinating the uncoordinated: UNC119 trafficking in cilia. Eur. J. Cell Biol. 2017, 96, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, M.; Yadav, R.P.; Brooks, C.; Artemyev, N.O. Chaperones and retinal disorders. Adv. Protein Chem. Struct. Biol. 2019, 114, 85–117. [Google Scholar] [CrossRef] [PubMed]
- Hanke-Gogokhia, C.; Zhang, H.; Frederick, J.M.; Baehr, W. The Function of Arf-like Proteins ARL2 and ARL3 in Photoreceptors. In Retinal Degenerative Diseases; Bowes Rickman, C., LaVail, M.M., Anderson, R.E., Grimm, C., Hollyfield, J., Ash, J., Eds.; Springer International Publishing Switzerland: Cham, Switzerland, 2016; pp. 655–661. [Google Scholar]
- Sinha, S.; Majumder, A.; Belcastro, M.; Sokolov, M.; Artemyev, N.O. Expression and subcellular distribution of UNC119a, a protein partner of transducin alpha subunit in rod photoreceptors. Cell Signal. 2013, 25, 341–348. [Google Scholar] [CrossRef]
- Frederick, J.M.; Hanke-Gogokhia, C.; Ying, G.; Baehr, W. Diffuse or hitch a ride: How photoreceptor lipidated proteins get from here to there. Biol. Chem. 2020, 401, 573–584. [Google Scholar] [CrossRef]
- Sokolov, M.; Lyubarsky, A.L.; Strissel, K.J.; Savchenko, A.B.; Govardovskii, V.I.; Pugh, E.N., Jr.; Arshavsky, V.Y. Massive light-driven translocation of transducin between the two major compartments of rod cells: A novel mechanism of light adaptation. Neuron 2002, 34, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Lobanova, E.S.; Finkelstein, S.; Song, H.; Tsang, S.H.; Chen, C.K.; Sokolov, M.; Skiba, N.P.; Arshavsky, V.Y. Transducin translocation in rods is triggered by saturation of the GTPase-activating complex. J. Neurosci. 2007, 27, 1151–1160. [Google Scholar] [CrossRef]
- Zhang, H.; Constantine, R.; Vorobiev, S.; Chen, Y.; Seetharaman, J.; Huang, Y.J.; Xiao, R.; Montelione, G.T.; Gerstner, C.D.; Davis, M.W.; et al. UNC119 is required for G protein trafficking in sensory neurons. Nat. Neurosci. 2011, 14, 874–880. [Google Scholar] [CrossRef]
- Higashide, T.; McLaren, M.J.; Inana, G. Localization of HRG4, a photoreceptor protein homologous to Unc-119, in ribbon synapse. Investig. Ophthalmol. Vis. Sci. 1998, 39, 690–698. [Google Scholar]
- Alpadi, K.; Magupalli, V.G.; Kappel, S.; Koblitz, L.; Schwarz, K.; Seigel, G.M.; Sung, C.H.; Schmitz, F. RIBEYE recruits Munc119, a mammalian ortholog of the Caenorhabditis elegans protein unc119, to synaptic ribbons of photoreceptor synapses. J. Biol. Chem. 2008, 283, 26461–26467. [Google Scholar] [CrossRef]
- Schmitz, F. The making of synaptic ribbons: How they are built and what they do. Neuroscientist 2009, 15, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Zanazzi, G.; Matthews, G. The molecular architecture of ribbon presynaptic terminals. Mol. Neurobiol. 2009, 39, 130–148. [Google Scholar] [CrossRef] [PubMed]
- Mesnard, C.S.; Barta, C.L.; Sladek, A.L.; Zenisek, D.; Thoreson, W.B. Eliminating Synaptic Ribbons from Rods and Cones Halves the Releasable Vesicle Pool and Slows Down Replenishment. Int. J. Mol. Sci. 2022, 23, 6429. [Google Scholar] [CrossRef]
- Haeseleer, F. Interaction and colocalization of CaBP4 and UNC119 (MRG4) in photoreceptors. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2366–2375. [Google Scholar] [CrossRef] [PubMed]
- Haeseleer, F.; Imanishi, Y.; Maeda, T.; Possin, D.E.; Maeda, A.; Lee, A.; Rieke, F.; Palczewski, K. Essential role of Ca2+-binding protein 4, a Cav1.4 channel regulator, in photoreceptor synaptic function. Nat. Neurosci. 2004, 7, 1079–1087. [Google Scholar] [CrossRef]
- Majumder, A.; Pahlberg, J.; Boyd, K.K.; Kerov, V.; Kolandaivelu, S.; Ramamurthy, V.; Sampath, A.P.; Artemyev, N.O. Transducin translocation contributes to rod survival and enhances synaptic transmission from rods to rod bipolar cells. Proc. Natl. Acad. Sci. USA 2013, 110, 12468–12473. [Google Scholar] [CrossRef] [PubMed]
- Sampath, A.P.; Strissel, K.J.; Elias, R.; Arshavsky, V.Y.; McGinnis, J.F.; Chen, J.; Kawamura, S.; Rieke, F.; Hurley, J.B. Recoverin improves rod-mediated vision by enhancing signal transmission in the mouse retina. Neuron 2005, 46, 413–420. [Google Scholar] [CrossRef]
- Cornwall, M.C.; Fain, G.L. Bleached pigment activates transduction in isolated rods of the salamander retina. J. Physiol. (Lond.) 1994, 480, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Nymark, S.; Frederiksen, R.; Woodruff, M.L.; Cornwall, M.C.; Fain, G.L. Bleaching of mouse rods: Microspectrophotometry and suction-electrode recording. J. Physiol. 2012, 590, 2353–2364. [Google Scholar] [CrossRef]
- Gopalakrishna, K.N.; Doddapuneni, K.; Boyd, K.K.; Masuho, I.; Martemyanov, K.A.; Artemyev, N.O. Interaction of transducin with uncoordinated 119 protein (UNC119): Implications for the model of transducin trafficking in rod photoreceptors. J. Biol. Chem. 2011, 286, 28954–28962. [Google Scholar] [CrossRef]
- Sampath, A.P.; Rieke, F. Selective transmission of single photon responses by saturation at the rod-to-rod bipolar synapse. Neuron 2004, 41, 431–443. [Google Scholar] [CrossRef]
- Arman, A.C.; Sampath, A.P. Dark-adapted response threshold of OFF ganglion cells is not set by OFF bipolar cells in the mouse retina. J. Neurophysiol. 2012, 107, 2649–2659. [Google Scholar] [CrossRef] [PubMed]
- Martemyanov, K.A.; Sampath, A.P. The Transduction Cascade in Retinal ON-Bipolar Cells: Signal Processing and Disease. Annu. Rev. Vis. Sci. 2017, 3, 25–51. [Google Scholar] [CrossRef]
- Suiwal, S.; Dembla, M.; Schwarz, K.; Katiyar, R.; Jung, M.; Carius, Y.; Maxeiner, S.; Lauterbach, M.A.; Lancaster, C.R.D.; Schmitz, F. Ciliary Proteins Repurposed by the Synaptic Ribbon: Trafficking Myristoylated Proteins at Rod Photoreceptor Synapses. Int. J. Mol. Sci. 2022, 23, 7135. [Google Scholar] [CrossRef]
- Jaiswal, M.; Fansa, E.K.; Kosling, S.K.; Mejuch, T.; Waldmann, H.; Wittinghofer, A. Novel Biochemical and Structural Insights into the Interaction of Myristoylated Cargo with UNC119 Protein and Their Release by Arl2/3. J. Biol. Chem. 2016, 291, 20766–20778. [Google Scholar] [CrossRef] [PubMed]
- Campla, C.K.; Bocchero, U.; Strickland, R.; Nellissery, J.; Advani, J.; Ignatova, I.; Srivastava, D.; Aponte, A.M.; Wang, Y.; Gumerson, J.; et al. Frmpd1 Facilitates Trafficking of G-Protein Transducin and Modulates Synaptic Function in Rod Photoreceptors of Mammalian Retina. eNeuro 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Ishiba, Y.; Higashide, T.; Mori, N.; Kobayashi, A.; Kubota, S.; McLaren, M.J.; Satoh, H.; Wong, F.; Inana, G. Targeted inactivation of synaptic HRG4 (UNC119) causes dysfunction in the distal photoreceptor and slow retinal degeneration, revealing a new function. Exp. Eye Res. 2007, 84, 473–485. [Google Scholar] [CrossRef]
- Cao, Y.; Posokhova, E.; Martemyanov, K.A. TRPM1 forms complexes with nyctalopin in vivo and accumulates in postsynaptic compartment of ON-bipolar neurons in mGluR6-dependent manner. J. Neurosci. 2011, 31, 11521–11526. [Google Scholar] [CrossRef]
- Liu, X.; Kerov, V.; Haeseleer, F.; Majumder, A.; Artemyev, N.; Baker, S.A.; Lee, A. Dysregulation of Ca(v)1.4 channels disrupts the maturation of photoreceptor synaptic ribbons in congenital stationary night blindness type 2. Channels (Austin) 2013, 7, 514–523. [Google Scholar] [CrossRef]
- Armstrong-Gold, C.E.; Rieke, F. Bandpass filtering at the rod to second-order cell synapse in salamander (Ambystoma tigrinum) retina. J. Neurosci. 2003, 23, 3796–3806. [Google Scholar] [CrossRef]
- Miyagishima, K.J.; Cornwall, M.C.; Sampath, A.P. Metabolic constraints on the recovery of sensitivity after visual pigment bleaching in retinal rods. J. Gen. Physiol. 2009, 134, 165–175. [Google Scholar] [CrossRef]
- Okawa, H.; Pahlberg, J.; Rieke, F.; Birnbaumer, L.; Sampath, A.P. Coordinated control of sensitivity by two splice variants of Galpha(o) in retinal ON bipolar cells. J. Gen. Physiol. 2010, 136, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Ingram, N.T.; Sampath, A.P.; Fain, G.L. Membrane conductances of mouse cone photoreceptors. J. Gen. Physiol. 2020, 152, e201912520. [Google Scholar] [CrossRef] [PubMed]
- Efron, B. Better bootstrap confidence intervals. J. Am. Stat. Assoc. 1987, 82, 171–185. [Google Scholar] [CrossRef]
- DiCiccio, T.J.; Efron, B. Bootstrap confidence intervals. Stat. Sci. 1996, 11, 189–212. [Google Scholar] [CrossRef]
- Petráš, I.; Bednárová, D. Total least squares approach to modeling: A MATLAB toolbox. Acta Montan. Slovaca 2010, 15, 158–170. [Google Scholar]
- Efron, B.; Tibshirani, R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat. Sci. 1986, 1, 54–75. [Google Scholar] [CrossRef]
- Freedman, D.A. Bootstrapping Regression Models. Ann. Stat. 1981, 9, 1218–1228. [Google Scholar] [CrossRef]
- Welch, B.L. The significance of the difference between two means when population variances are unequal. Biometrika 1938, 29, 350–362. [Google Scholar] [CrossRef]
- Welch, B.L. The generalisation of student’s problems when several different population variances are involved. Biometrika 1947, 34, 28–35. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fehlhaber, K.E.; Majumder, A.; Boyd, K.K.; Griffis, K.G.; Artemyev, N.O.; Fain, G.L.; Sampath, A.P. A Novel Role for UNC119 as an Enhancer of Synaptic Transmission. Int. J. Mol. Sci. 2023, 24, 8106. https://doi.org/10.3390/ijms24098106
Fehlhaber KE, Majumder A, Boyd KK, Griffis KG, Artemyev NO, Fain GL, Sampath AP. A Novel Role for UNC119 as an Enhancer of Synaptic Transmission. International Journal of Molecular Sciences. 2023; 24(9):8106. https://doi.org/10.3390/ijms24098106
Chicago/Turabian StyleFehlhaber, Katherine E., Anurima Majumder, Kimberly K. Boyd, Khris G. Griffis, Nikolai O. Artemyev, Gordon L. Fain, and Alapakkam P. Sampath. 2023. "A Novel Role for UNC119 as an Enhancer of Synaptic Transmission" International Journal of Molecular Sciences 24, no. 9: 8106. https://doi.org/10.3390/ijms24098106
APA StyleFehlhaber, K. E., Majumder, A., Boyd, K. K., Griffis, K. G., Artemyev, N. O., Fain, G. L., & Sampath, A. P. (2023). A Novel Role for UNC119 as an Enhancer of Synaptic Transmission. International Journal of Molecular Sciences, 24(9), 8106. https://doi.org/10.3390/ijms24098106







