Maize Transcription Factor ZmHsf28 Positively Regulates Plant Drought Tolerance
Abstract
1. Introduction
2. Results
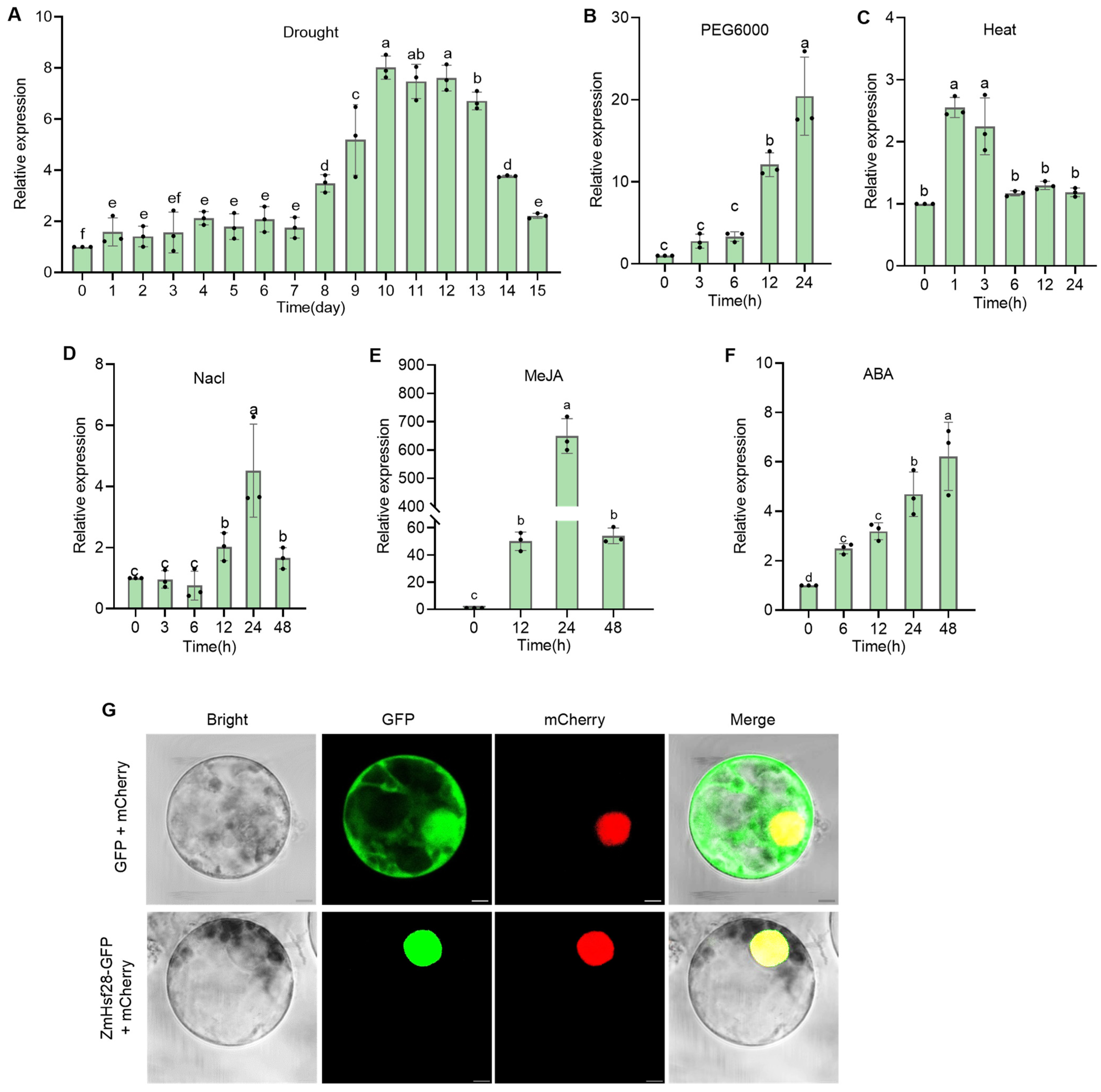
2.1. Identification of ZmHsf28 in Response to Drought Treatment
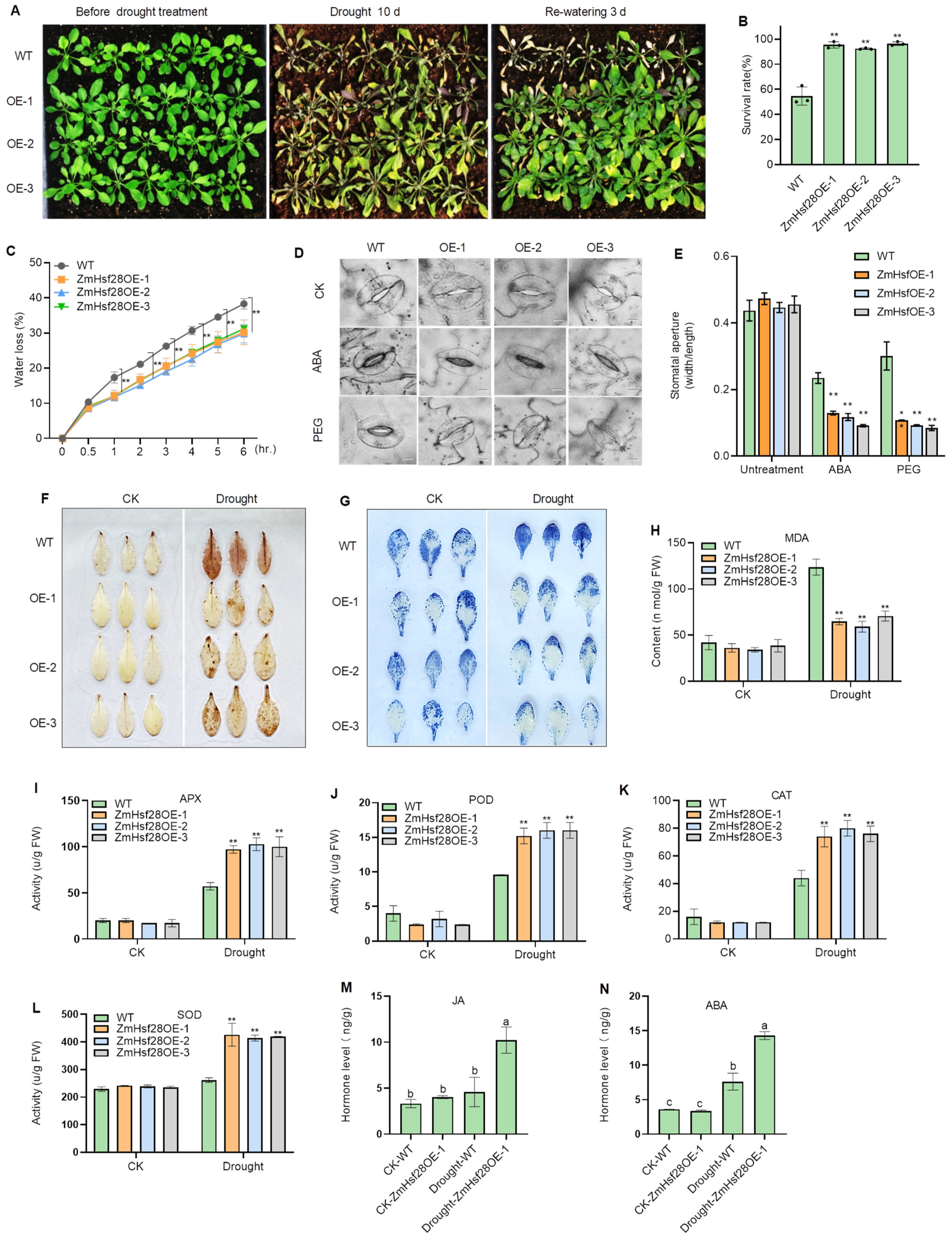
2.2. Overexpression of ZmHsf28 Elevated Drought Tolerance in Arabidopsis
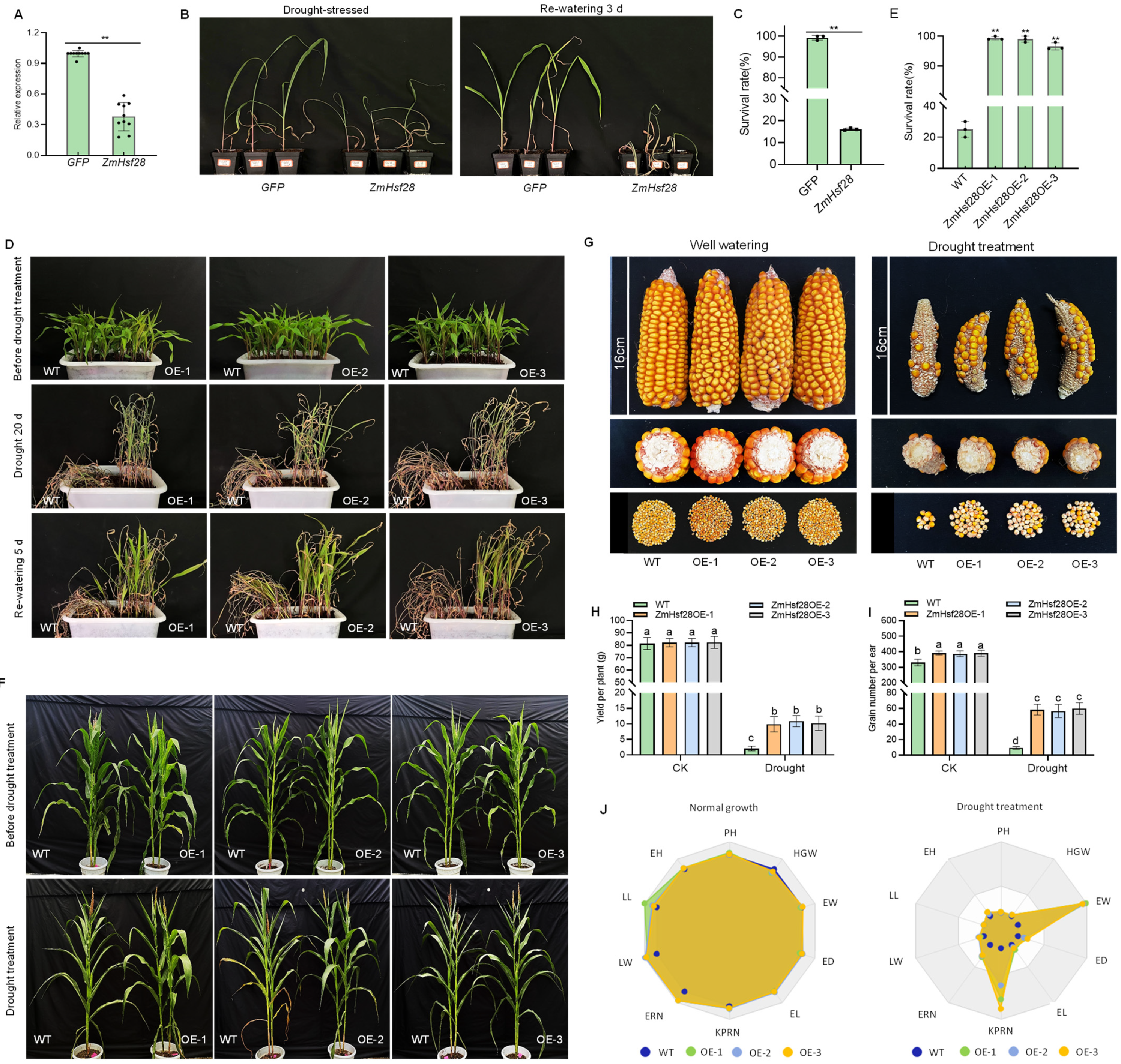
2.3. ZmHsf28 Regulated Drought Tolerance in Maize
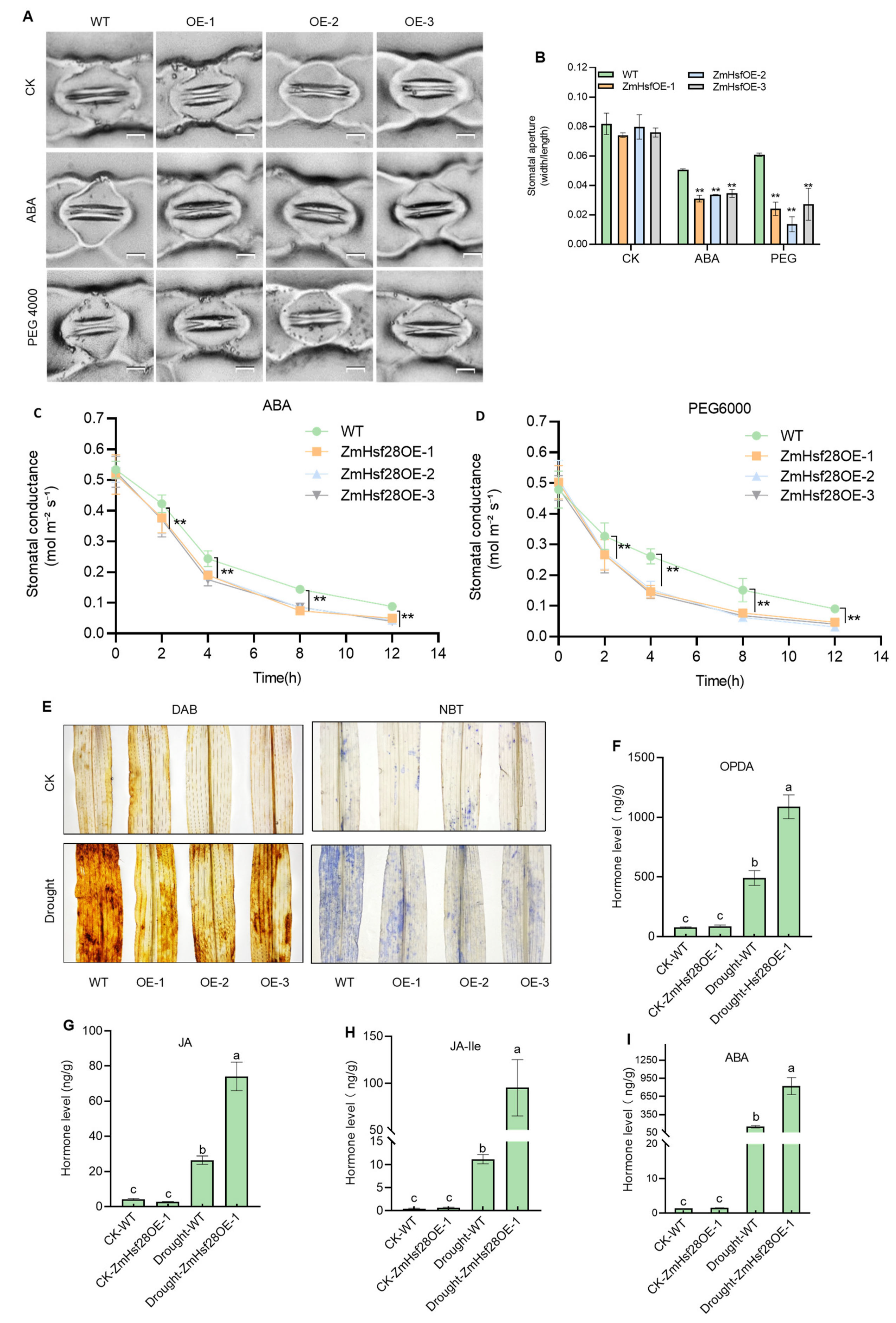
2.4. Overexpression of ZmHsf28 Promoted Physiological Adaptation to Drought
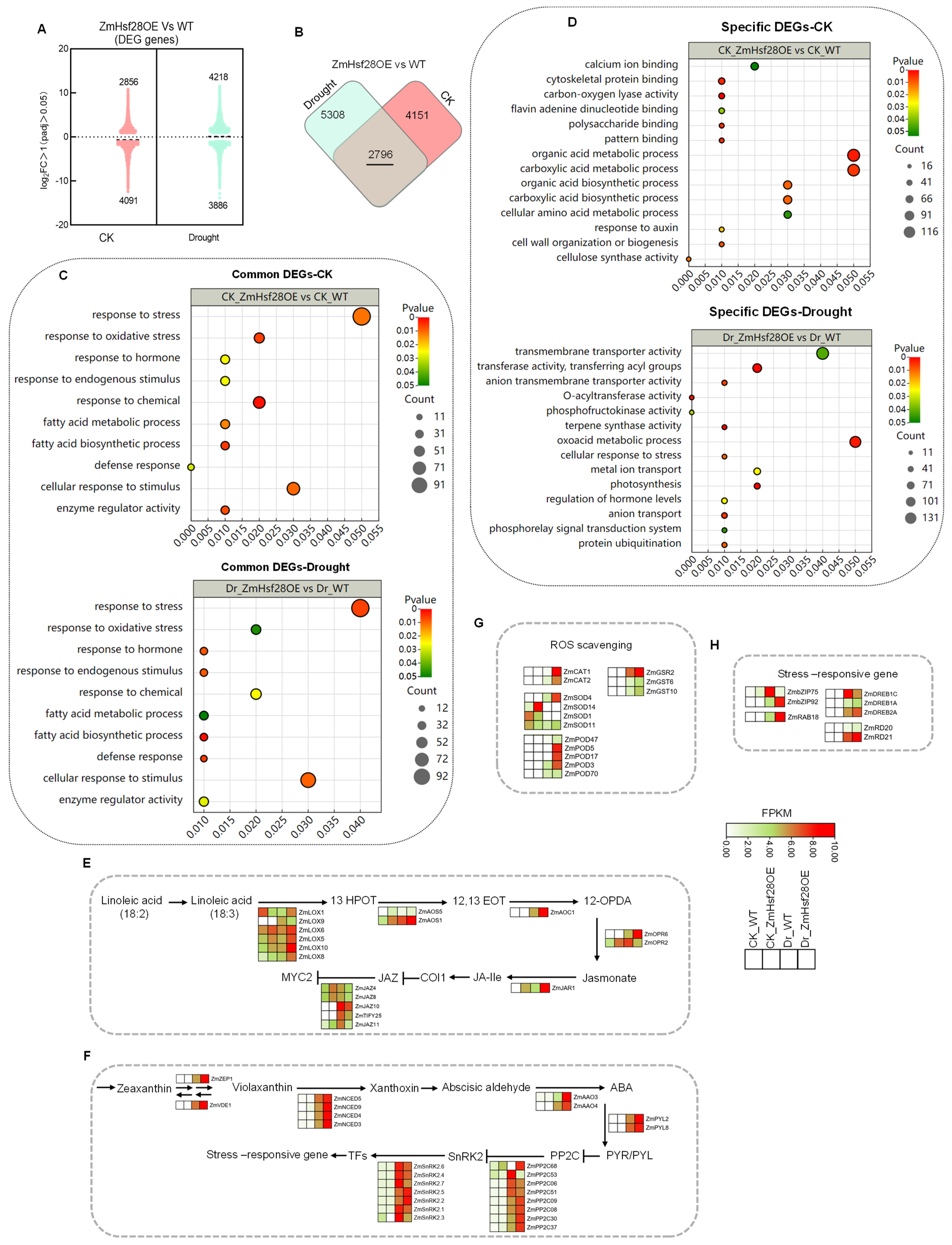
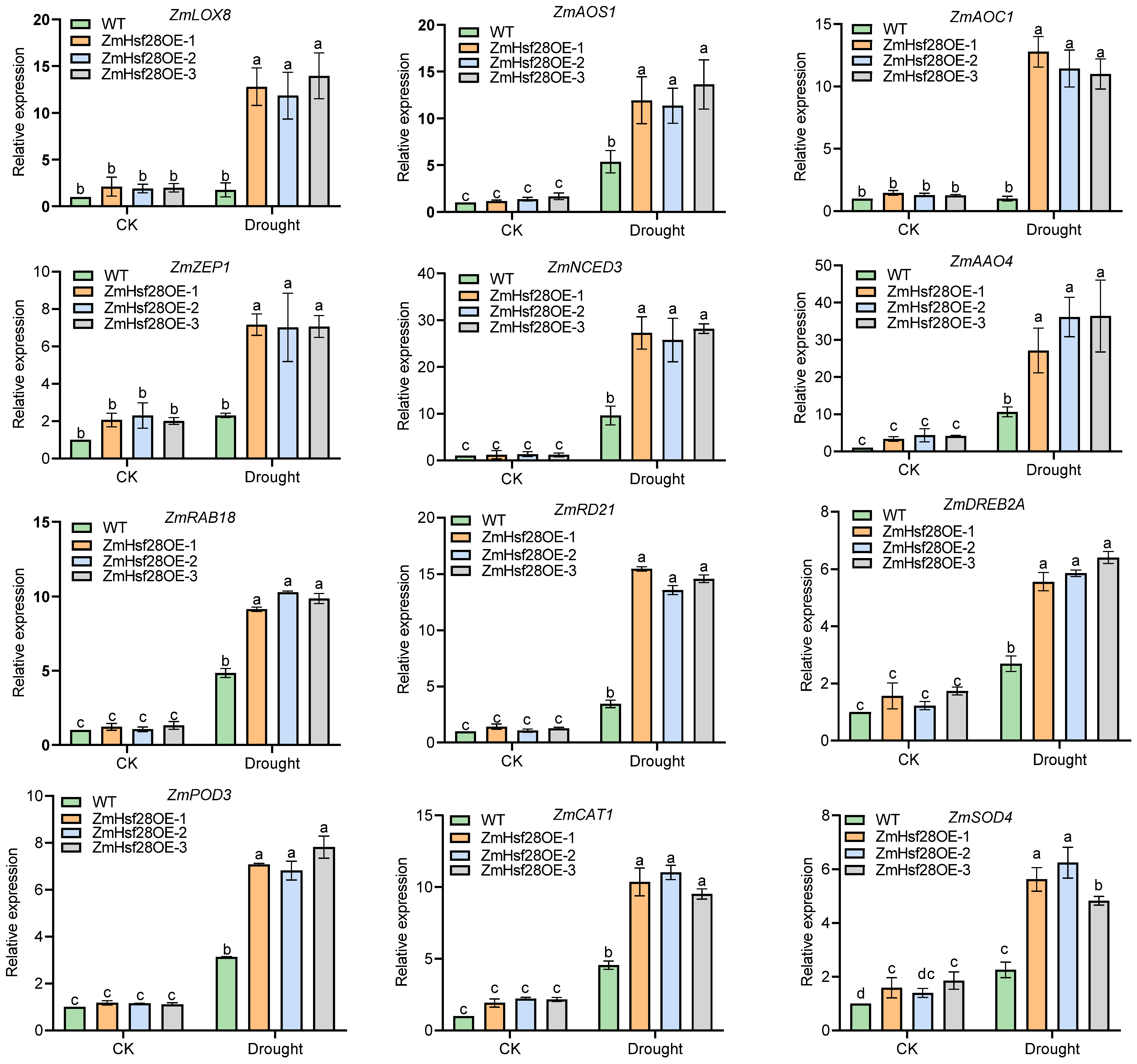
2.5. Overexpression of ZmHs28 Reprogrammed Extensive Gene Expression in Maize
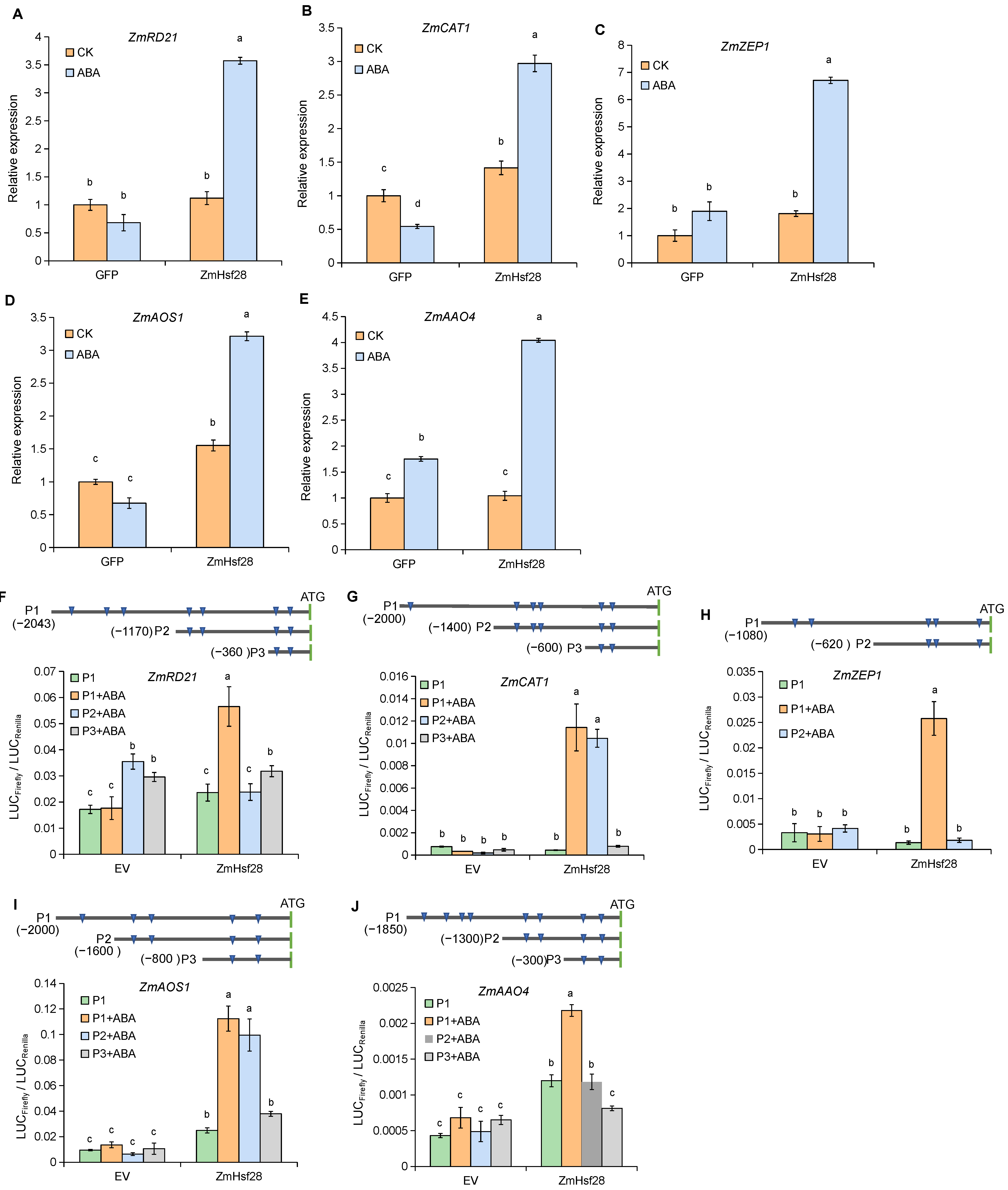
2.6. ABA Treatment Promoted ZmHsf28 Regulation on Target Genes
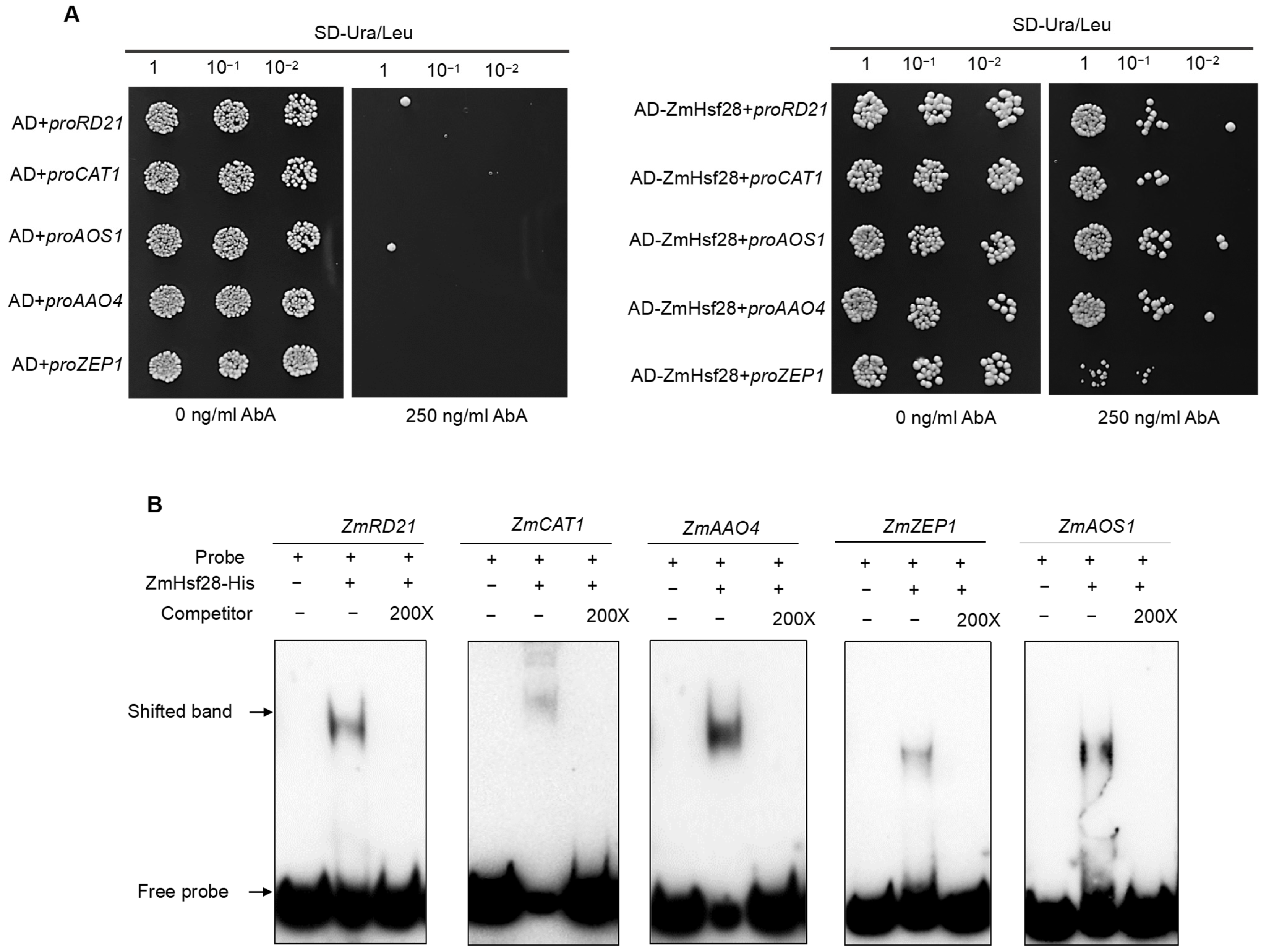
2.7. ZmHsf28 Directly Bound to Target Gene Promoters
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Treatments, Gene Expression Analysis and Cloning
4.3. Transgenic Plants
4.4. Virus-Induced Gene Silencing (VIGS)
4.5. Drought Treatments of Transgenic Plants
4.6. Water Loss Rate
4.7. ROS Detection, Anti-Oxidant Enzyme Activity and MDA Measurement
4.8. Measurement of Stomatal Aperture and Conductance
4.9. Plant Hormone Measurement
4.10. Protoplast Preparation and Transient Overexpression
4.11. Subcellular Localization
4.12. Promoter-Luciferase Assays
4.13. Yeast One-Hybrid (Y1H) Assays
4.14. Electrophoresis Mobility Shift Assay (EMSA)
4.15. RNA-Seq Analysis
4.16. Sequence Alignment, Phylogenetic Analysis and Cis-Element Prediction
4.17. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The Physiology of Plant Responses to Drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Liu, J.-H.; Ma, X.; Luo, D.-X.; Gong, Z.-H.; Lu, M.-H. The Plant Heat Stress Transcription Factors (HSFs): Structure, Regulation, and Function in Response to Abiotic Stresses. Front. Plant Sci. 2016, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-C.; Liao, H.-T.; Charng, Y.-Y. The Role of Class A1 Heat Shock Factors (HSFA1s) in Response to Heat and Other Stresses in Arabidopsis. Plant Cell Environ. 2011, 34, 738–751. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Tripp, J.; Winkelhaus, S.; Tschiersch, B.; Theres, K.; Nover, L.; Scharf, K.-D. In the Complex Family of Heat Stress Transcription Factors, HsfA1 Has a Unique Role as Master Regulator of Thermotolerance in Tomato. Genes. Dev. 2002, 16, 1555–1567. [Google Scholar] [CrossRef]
- Yoshida, T.; Ohama, N.; Nakajima, J.; Kidokoro, S.; Mizoi, J.; Nakashima, K.; Maruyama, K.; Kim, J.-M.; Seki, M.; Todaka, D.; et al. Arabidopsis HsfA1 Transcription Factors Function as the Main Positive Regulators in Heat Shock-Responsive Gene Expression. Mol. Genet. Genom. 2011, 286, 321–332. [Google Scholar] [CrossRef]
- Gai, W.-X.; Ma, X.; Li, Y.; Xiao, J.-J.; Khan, A.; Li, Q.-H.; Gong, Z.-H. CaHsfA1d Improves Plant Thermotolerance via Regulating the Expression of Stress- and Antioxidant-Related Genes. Int. J. Mol. Sci. 2020, 21, 8374. [Google Scholar] [CrossRef]
- Guo, L.; Chen, S.; Liu, K.; Liu, Y.; Ni, L.; Zhang, K.; Zhang, L. Isolation of Heat Shock Factor HsfA1a-Binding Sites in Vivo Revealed Variations of Heat Shock Elements in Arabidopsis Thaliana. Plant Cell Physiol. 2008, 49, 1306–1315. [Google Scholar] [CrossRef]
- Gu, L.; Jiang, T.; Zhang, C.; Li, X.; Wang, C.; Zhang, Y.; Li, T.; Dirk, L.M.A.; Downie, A.B.; Zhao, T. Maize HSFA2 and HSBP2 Antagonistically Modulate Raffinose Biosynthesis and Heat Tolerance in Arabidopsis. Plant J. 2019, 100, 128–142. [Google Scholar] [CrossRef]
- Wang, X.; Zhuang, L.; Shi, Y.; Huang, B. Up-Regulation of HSFA2c and HSPs by ABA Contributing to Improved Heat Tolerance in Tall Fescue and Arabidopsis. Int. J. Mol. Sci. 2017, 18, 1981. [Google Scholar] [CrossRef]
- Wang, X.; Huang, W.; Liu, J.; Yang, Z.; Huang, B. Molecular Regulation and Physiological Functions of a Novel FaHsfA2c Cloned from Tall Fescue Conferring Plant Tolerance to Heat Stress. Plant Biotechnol. J. 2017, 15, 237–248. [Google Scholar] [CrossRef]
- Zang, D.; Wang, J.; Zhang, X.; Liu, Z.; Wang, Y. Arabidopsis Heat Shock Transcription Factor HSFA7b Positively Mediates Salt Stress Tolerance by Binding to an E-Box-like Motif to Regulate Gene Expression. J. Exp. Bot. 2019, 70, 5355–5374. [Google Scholar] [CrossRef]
- Zhu, M.-D.; Zhang, M.; Gao, D.-J.; Zhou, K.; Tang, S.-J.; Zhou, B.; Lv, Y.-M. Rice OsHSFA3 Gene Improves Drought Tolerance by Modulating Polyamine Biosynthesis Depending on Abscisic Acid and ROS Levels. Int. J. Mol. Sci. 2020, 21, 1857. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Mitsuda, N.; Ohme-Takagi, M. Arabidopsis HsfB1 and HsfB2b Act as Repressors of the Expression of Heat-Inducible Hsfs but Positively Regulate the Acquired Thermotolerance. Plant Physiol. 2011, 157, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Schippers, J.H.M.; Welker, A.; Mieulet, D.; Guiderdoni, E.; Mueller-Roeber, B. Transcription Factor OsHsfC1b Regulates Salt Tolerance and Development in Oryza sativa Ssp. Japonica. AoB Plants 2012, 2012, pls011. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Busch, W.; Birke, H.; Kemmerling, B.; Nürnberger, T.; Schöffl, F. Heat Shock Factors HsfB1 and HsfB2b Are Involved in the Regulation of Pdf1.2 Expression and Pathogen Resistance in Arabidopsis. Mol. Plant 2009, 2, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ju, Y.; Zuo, L.; Shang, L.; Li, X.; Li, X.; Feng, S.; Ding, X.; Chu, Z. OsHsfB4d Binds the Promoter and Regulates the Expression of OsHsp18.0-CI to Resistant Against Xanthomonas Oryzae. Rice 2020, 13, 28. [Google Scholar] [CrossRef]
- Buckley, T.N. How Do Stomata Respond to Water Status? New Phytol. 2019, 224, 21–36. [Google Scholar] [CrossRef]
- Kuromori, T.; Seo, M.; Shinozaki, K. ABA Transport and Plant Water Stress Responses. Trends Plant Sci. 2018, 23, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic Acid Dynamics, Signaling, and Functions in Plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef]
- Nambara, E.; Marion-Poll, A. ABA Action and Interactions in Seeds. Trends Plant Sci. 2003, 8, 213–217. [Google Scholar] [CrossRef]
- Raghavendra, A.S.; Gonugunta, V.K.; Christmann, A.; Grill, E. ABA Perception and Signalling. Trends Plant Sci. 2010, 15, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Liu, C.; Li, Z.; Ran, Q.; Xie, G.; Wang, B.; Fang, S.; Chu, J.; Zhang, J. ZmbZIP4 Contributes to Stress Resistance in Maize by Regulating ABA Synthesis and Root Development. Plant Physiol. 2018, 178, 753–770. [Google Scholar] [CrossRef] [PubMed]
- Jia, K.-P.; Mi, J.; Ali, S. An Alternative, Zeaxanthin Epoxidase-Independent Abscisic Acid Biosynthetic Pathway in Plants. Mol. Plant 2022, 15, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Fujita, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. ABA-Mediated Transcriptional Regulation in Response to Osmotic Stress in Plants. J. Plant Res. 2011, 124, 509–525. [Google Scholar] [CrossRef]
- Zhu, J.-K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Xiang, Y.; Sun, X.; Gao, S. Deletion of an Endoplasmic Reticulum Stress Response Element in a ZmPP2C-A Gene Facilitates Drought Tolerance of Maize Seedlings. Mol. Plant 2017, 10, 456–469. [Google Scholar] [CrossRef]
- Wang, B.; Li, L.; Liu, M.; Peng, D.; Wei, A.; Hou, B.; Lei, Y.; Li, X. TaFDL2-1A Confers Drought Stress Tolerance by Promoting ABA Biosynthesis, ABA Responses, and ROS Scavenging in Transgenic Wheat. Plant J. 2022, 112, 722–737. [Google Scholar] [CrossRef]
- Cao, L.; Lu, X.; Wang, G.; Zhang, Q.; Zhang, X.; Fan, Z.; Cao, Y.; Wei, L.; Wang, T.; Wang, Z. Maize ZmbZIP33 Is Involved in Drought Resistance and Recovery Ability Through an Abscisic Acid-Dependent Signaling Pathway. Front. Plant Sci. 2021, 12, 629903. [Google Scholar] [CrossRef]
- Li, X.-D.; Gao, Y.-Q.; Wu, W.-H.; Chen, L.-M.; Wang, Y. Two Calcium-dependent Protein Kinases Enhance Maize Drought Tolerance by Activating Anion Channel ZmSLAC1 in Guard Cells. Plant Biotechnol. J. 2021, 20, 143–157. [Google Scholar] [CrossRef]
- Browse, J. Jasmonate Passes Muster: A Receptor and Targets for the Defense Hormone. Annu. Rev. Plant Biol. 2009, 60, 183–205. [Google Scholar] [CrossRef]
- Wasternack, C.; Strnad, M. Jasmonates Are Signals in the Biosynthesis of Secondary Metabolites Pathways, Transcription Factors and Applied Aspects. New Biotechnol. 2019, 48, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C. Jasmonates: An Update on Biosynthesis, Signal Transduction and Action in Plant Stress Response, Growth and Development. Ann. Bot. 2007, 100, 681–697. [Google Scholar] [CrossRef] [PubMed]
- Xing, Q.; Liao, J.; Cao, S.; Li, M.; Lv, T.; Qi, H. CmLOX10 Positively Regulates Drought Tolerance through Jasmonic Acid -Mediated Stomatal Closure in Oriental Melon (Cucumis melo Var. makuwa Makino). Sci. Rep. 2020, 10, 17452. [Google Scholar] [CrossRef] [PubMed]
- De Domenico, S.; Bonsegna, S.; Horres, R.; Pastor, V.; Taurino, M.; Poltronieri, P.; Imtiaz, M.; Kahl, G.; Flors, V.; Winter, P.; et al. Transcriptomic Analysis of Oxylipin Biosynthesis Genes and Chemical Profiling Reveal an Early Induction of Jasmonates in Chickpea Roots under Drought Stress. Plant Physiol. Biochem. 2012, 61, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, S.; Ullah, C.; Kortz, A.; Bhattacharyya, S.; Yu, P.; Gershenzon, J.; Vothknecht, U.C. Constitutive Expression of JASMONATE RESISTANT 1 Induces Molecular Changes That Prime the Plants to Better Withstand Drought. Plant Cell Environ. 2022, 45, 2906–2922. [Google Scholar] [CrossRef]
- Zhang, H.; Li, G.; Hu, D.; Zhang, Y.; Zhang, Y.; Shao, H.; Zhao, L.; Yang, R.; Guo, X. Functional Characterization of Maize Heat Shock Transcription Factor Gene ZmHsf01 in Thermotolerance. PeerJ 2020, 8, e8926. [Google Scholar] [CrossRef]
- Jiang, Y.; Zheng, Q.; Chen, L.; Liang, Y.; Wu, J. Ectopic Overexpression of Maize Heat Shock Transcription Factor Gene ZmHsf04 Confers Increased Thermo and Salt-Stress Tolerance in Transgenic Arabidopsis. Acta Physiol. Plant. 2018, 40, 9. [Google Scholar] [CrossRef]
- Si, W.; Liang, Q.; Chen, L.; Song, F.; Chen, Y.; Jiang, H. Ectopic Overexpression of Maize Heat Stress Transcription Factor ZmHsf05 Confers Drought Tolerance in Transgenic Rice. Genes 2021, 12, 1568. [Google Scholar] [CrossRef]
- Li, G.-L.; Zhang, H.-N.; Shao, H.; Wang, G.-Y.; Zhang, Y.-Y.; Zhang, Y.-J.; Zhao, L.-N.; Guo, X.-L.; Sheteiwy, M.S. ZmHsf05, a New Heat Shock Transcription Factor from Zea mays L. Improves Thermotolerance in Arabidopsis Thaliana and Rescues Thermotolerance Defects of the athsfa2 Mutant. Plant Sci. 2019, 283, 375–384. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Li, G.; Liu, Z.; Zhang, Y.; Zhang, H.; Guo, X. Expression of Maize Heat Shock Transcription Factor Gene ZmHsf06 Enhances the Thermotolerance and Drought-Stress Tolerance of Transgenic Arabidopsis. Funct. Plant Biol. 2015, 42, 1080. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L.; Long, Y.; Si, W.; Cheng, B.; Jiang, H. A Novel Heat Shock Transcription Factor (ZmHsf08) Negatively Regulates Salt and Drought Stress Responses in Maize. Int. J. Mol. Sci. 2021, 22, 11922. [Google Scholar] [CrossRef] [PubMed]
- Seeve, C.M.; Cho, I.; Hearne, L.B.; Srivastava, G.P.; Joshi, T.; Smith, D.O.; Sharp, R.E.; Oliver, M.J. Water-deficit-induced Changes in Transcription Factor Expression in Maize Seedlings. Plant Cell Environ. 2017, 40, 686–701. [Google Scholar] [CrossRef] [PubMed]
- Hoopes, G.M.; Hamilton, J.P.; Wood, J.C.; Esteban, E.; Pasha, A.; Vaillancourt, B.; Provart, N.J.; Buell, C.R. An Updated Gene Atlas for Maize Reveals Organ-Specific and Stress-Induced Genes. Plant J. 2019, 97, 1154–1167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Thriving under Stress: How Plants Balance Growth and the Stress Response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Qin, F. The Battle of Crops against Drought: Genetic Dissection and Improvement. J. Integr. Plant Biol. 2023, 65, 496–525. [Google Scholar] [CrossRef]
- Yang, X.; Gavya, S.L.; Zhou, Z.; Urano, D.; Lau, O.S. Abscisic Acid Regulates Stomatal Production by Imprinting a SnRK2 Kinase–Mediated Phosphocode on the Master Regulator SPEECHLESS. Sci. Adv. 2022, 8, eadd2063. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.-K. Abiotic Stress Responses in Plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Fujita, Y.; Sayama, H.; Kidokoro, S.; Maruyama, K.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AREB1, AREB2, and ABF3 Are Master Transcription Factors That Cooperatively Regulate ABRE-dependent ABA Signaling Involved in Drought Stress Tolerance and Require ABA for Full Activation. Plant J. 2010, 61, 672–685. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.; Tang, N.; Yang, J.; Peng, L.; Ma, S.; Xu, Y.; Li, G.; Xiong, L. Feedback Regulation of ABA Signaling and Biosynthesis by a BZIP Transcription Factor Targets Drought-Resistance-Related Genes. Plant Physiol. 2016, 171, 2810–2825. [Google Scholar] [CrossRef]
- Pérez-Salamó, I.; Papdi, C.; Rigó, G.; Zsigmond, L.; Vilela, B.; Lumbreras, V.; Nagy, I.; Horváth, B.; Domoki, M.; Darula, Z.; et al. The Heat Shock Factor A4A Confers Salt Tolerance and Is Regulated by Oxidative Stress and the Mitogen-Activated Protein Kinases MPK3 and MPK61. Plant Physiol. 2014, 165, 319–334. [Google Scholar] [CrossRef]
- Andrási, N.; Rigó, G.; Zsigmond, L.; Pérez-Salamó, I.; Papdi, C.; Klement, E.; Pettkó-Szandtner, A.; Baba, A.I.; Ayaydin, F.; Dasari, R.; et al. The Mitogen-Activated Protein Kinase 4-Phosphorylated Heat Shock Factor A4A Regulates Responses to Combined Salt and Heat Stresses. J. Exp. Bot. 2019, 70, 4903–4918. [Google Scholar] [CrossRef] [PubMed]
- de Ollas, C.; Dodd, I.C. Physiological Impacts of ABA–JA Interactions under Water-Limitation. Plant Mol. Biol. 2016, 91, 641–650. [Google Scholar] [CrossRef]
- Yang, J.; Duan, G.; Chunqin, L. The Crosstalks Between Jasmonic Acid and Other Plant Hormone Signaling Highlight the Involvement of Jasmonic Acid as a Core Component in Plant Response to Biotic and Abiotic Stresses. Front. Plant Sci. 2019, 10, 1349. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Huang, H.; Wang, J.; Wang, X.; Xu, B.; Yao, X.; Sun, L.; Yang, R.; Wang, J.; Sun, A.; et al. Jasmonic Acid Enhances Osmotic Stress Responses by MYC2-Mediated Inhibition of Protein Phosphatase 2C1 and Response Regulators 26 Transcription Factor in Tomato. Plant J. 2023, 113, 546–561. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, M.M.; Christensen, S.; Schmelz, E.A.; Huffaker, A.; Mcauslane, H.J.; Alborn, H.T.; Romero, M.; Allen, L.H.; Teal, P.E.A. Accumulation of Terpenoid Phytoalexins in Maize Roots Is Associated with Drought Tolerance. Plant Cell Environ. 2015, 38, 2195–2207. [Google Scholar] [CrossRef] [PubMed]
- Awan, S.A.; Khan, I.; Rizwan, M.; Zhang, X.; Brestic, M.; Khan, A.; El-Sheikh, M.A.; Alyemeni, M.N.; Ali, S.; Huang, L. Exogenous Abscisic Acid and Jasmonic Acid Restrain Polyethylene Glycol-Induced Drought by Improving the Growth and Antioxidative Enzyme Activities in Pearl Millet. Physiol. Plant. 2021, 172, 809–819. [Google Scholar] [CrossRef]
- He, R.; Zhuang, Y.; Cai, Y.; Agüero, C.B.; Liu, S.; Wu, J.; Deng, S.; Walker, M.A.; Lu, J.; Zhang, Y. Overexpression of 9-Cis-Epoxycarotenoid Dioxygenase Cisgene in Grapevine Increases Drought Tolerance and Results in Pleiotropic Effects. Front. Plant Sci. 2018, 9, 970. [Google Scholar] [CrossRef]
- de Ollas, C.; Arbona, V.; GóMez-Cadenas, A. Jasmonoyl Isoleucine Accumulation Is Needed for Abscisic Acid Build-up in Roots of Arabidopsis under Water Stress Conditions. Plant Cell Environ. 2015, 38, 2157–2170. [Google Scholar] [CrossRef]
- Xu, B.-Q.; Wang, J.-J.; Peng, Y.; Huang, H.; Sun, L.-L.; Yang, R.; Suo, L.-N.; Wang, S.-H.; Zhao, W.-C. SlMYC2 Mediates Stomatal Movement in Response to Drought Stress by Repressing SlCHS1 Expression. Front. Plant. Sci. 2022, 13, 952758. [Google Scholar] [CrossRef]
- Andrási, N.; Pettkó-Szandtner, A.; Szabados, L. Diversity of Plant Heat Shock Factors: Regulation, Interactions, and Functions. J. Exp. Bot. 2021, 72, 1558–1575. [Google Scholar] [CrossRef]
- Shao, K.-Z.; Lyu, X.-P.; Li, J.-L.; Chen, J.; Zhao, L.-Y.; Ren, W.; Zhang, J.-L. Biological characteristics of heat shock transcription factors and their roles in abiotic stress adaptation of higher plant. Ying Yong Sheng Tai Xue Bao 2022, 33, 2286–2296. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-Y.; Hoh, K.L.; Boonyaves, K.; Krishnamoorthi, S.; Urano, D. Diversification of Heat Shock Transcription Factors Expanded Thermal Stress Responses during Early Plant Evolution. Plant Cell 2022, 10, 3557–3576. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-J.; Chen, D.; Lynne Mclntyre, C.; Fernanda Dreccer, M.; Zhang, Z.-B.; Drenth, J.; Kalaipandian, S.; Chang, H.; Xue, G.-P. Heat Shock Factor C2a Serves as a Proactive Mechanism for Heat Protection in Developing Grains in Wheat via an ABA-Mediated Regulatory Pathway. Plant Cell Environ. 2018, 41, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Cao, W.; Wang, J.; Yu, J.; Yang, Z.; Huang, B. Characterization and Functional Analysis of FaHsfC1b from Festuca Arundinacea Conferring Heat Tolerance in Arabidopsis. Int. J. Mol. Sci. 2018, 19, 2702. [Google Scholar] [CrossRef]
- Xue, G.-P.; Sadat, S.; Drenth, J.; McIntyre, C.L. The Heat Shock Factor Family from Triticum Aestivum in Response to Heat and Other Major Abiotic Stresses and Their Role in Regulation of Heat Shock Protein Genes. J. Exp. Bot. 2014, 65, 539–557. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Qi, S.; Wang, X.; Dou, L.; Jia, M.; Mao, T.; Guo, Y.; Wang, X. The OPEN STOMATA1–SPIRAL1 Module Regulates Microtubule Stability during Abscisic Acid-Induced Stomatal Closure in Arabidopsis. Plant Cell 2023, 35, 260–278. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, M.; Zhao, C.; Wang, Y.; Zhang, R. Physiological and Proteomic Analyses Revealed the Response Mechanisms of Two Different Drought-Resistant Maize Varieties. BMC Plant Biol. 2021, 21, 513. [Google Scholar] [CrossRef]
- Li, J.; Guo, X.; Zhang, M.; Wang, X.; Zhao, Y.; Yin, Z.; Zhang, Z.; Wang, Y.; Xiong, H.; Zhang, H.; et al. OsERF71 Confers Drought Tolerance via Modulating ABA Signaling and Proline Biosynthesis. Plant Sci. 2018, 270, 131–139. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Sun, T.; Wang, D.; Wang, Z.; Zhang, C.; Que, Y.; Guo, J.; Xu, L.; Su, Y. Sugarcane ScDREB2B-1 Confers Drought Stress Tolerance in Transgenic Nicotiana benthamiana by Regulating the ABA Signal, ROS Level and Stress-Related Gene Expression. Int. J. Mol. Sci. 2022, 23, 9557. [Google Scholar] [CrossRef]
- Kasuga, M.; Liu, Q.; Miura, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Improving Plant Drought, Salt, and Freezing Tolerance by Gene Transfer of a Single Stress-Inducible Transcription Factor. Nat. Biotechnol. 1999, 17, 287–291. [Google Scholar] [CrossRef]
- Kim, H.; Lee, K.; Hwang, H.; Bhatnagar, N.; Kim, D.-Y.; Yoon, I.S.; Byun, M.-O.; Kim, S.T.; Jung, K.-H.; Kim, B.-G. Overexpression of PYL5 in Rice Enhances Drought Tolerance, Inhibits Growth, and Modulates Gene Expression. J. Exp. Bot. 2014, 65, 453–464. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhong, J.; Sun, X.; Wang, B.; Terzaghi, W.; Dai, M. The Maize ABA Receptors ZmPYL8, 9, and 12 Facilitate Plant Drought Resistance. Front. Plant Sci. 2018, 9, 422. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Nolan, T.; Jiang, H.; Tang, B.; Zhang, M.; Li, Z.; Yin, Y. The AP2/ERF Transcription Factor TINY Modulates Brassinosteroid-Regulated Plant Growth and Drought Responses in Arabidopsis. Plant Cell 2019, 31, 1788–1806. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, M.; Zhang, M.; Jiang, W.; Liang, E.; Zhang, D.; Zhang, C.; Xiao, N.; Chen, J. Roles of a Maize Phytochrome-Interacting Factors Protein ZmPIF3 in Regulation of Drought Stress Responses by Controlling Stomatal Closure in Transgenic Rice without Yield Penalty. Plant Mol. Biol. 2018, 97, 311–323. [Google Scholar] [CrossRef]
- Shi, J.; Habben, J.E.; Archibald, R.L.; Drummond, B.J.; Chamberlin, M.A.; Williams, R.W.; La, H.R. Overexpression of ARGOS Genes Modifies Plant Sensitivity to Ethylene, Leading to Improved Drought Tolerance in Both Arabidopsis and Maize. Plant Physiol. 2015, 169, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Liu, L.; Liu, Q.; Shen, Q.; Wang, C.; Yang, P.; Zhu, C.; Wang, Q. ZmMYC2 Exhibits Diverse Functions and Enhances JA Signaling in Transgenic Arabidopsis. Plant Cell Rep. 2020, 39, 273–288. [Google Scholar] [CrossRef]
- Zhang, X.; Henriques, R.; Lin, S.-S.; Niu, Q.-W.; Chua, N.-H. Agrobacterium-Mediated Transformation of Arabidopsis thaliana Using the Floral Dip Method. Nat. Protoc. 2006, 1, 641–646. [Google Scholar] [CrossRef]
- Wang, R.; Yang, X.; Wang, N.; Liu, X.; Nelson, R.S.; Li, W.; Fan, Z.; Zhou, T. An Efficient Virus-Induced Gene Silencing Vector for Maize Functional Genomics Research. Plant J. 2016, 86, 102–115. [Google Scholar] [CrossRef]
- Gulzar, F.; Fu, J.; Zhu, C.; Yan, J.; Li, X.; Meraj, T.A.; Shen, Q.; Hassan, B.; Wang, Q. Maize WRKY Transcription Factor ZmWRKY79 Positively Regulates Drought Tolerance through Elevating ABA Biosynthesis. Int. J. Mol. Sci. 2021, 22, 10080. [Google Scholar] [CrossRef]
- Chen, J.; Nolan, T.; Ye, H.; Zhang, M.; Tong, H.; Xin, P.; Chu, J.; Chu, C.; Li, Z.; Yin, Y. 3 Arabidopsis WRKY46, WRKY54, and WRKY70 Transcription Factors 4 Are Involved in Brassinosteroid-Regulated Plant Growth and Drought 5 Responses. Plant Cell 2017, 29, 1425–1439. [Google Scholar] [CrossRef]
- Mao, H.; Yu, L.; Han, R. ZmNAC55, a Maize Stress-Responsive NAC Transcription Factor, Confers Drought Resistance in Transgenic Arabidopsis. Plant Physiol. Biochem. 2016, 105, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xiang, Y.; Neng, H.; Liu, X. Enhanced Vitamin C Production Mediated by an ABA-Induced PTP-Like Nucleotidase Improves Drought Tolerance of Arabidopsis and Maize. Mol. Plant 2020, 13, 760–776. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Banduni, P.; Mallikarjuna, M.G.; Rao, A.R.; Jain, P.A.; Dash, P.K.; Thirunavukkarasu, N. Structural, Functional, and Evolutionary Characterization of Major Drought Transcription Factors Families in Maize. Front. Chem. 2018, 6, 177. [Google Scholar] [CrossRef] [PubMed]
- Hellens, R.P.; Allan, A.C.; Friel, E.N.; Bolitho, K.; Grafton, K.; Templeton, M.D.; Karunairetnam, S.; Gleave, A.P.; Laing, W.A. Transient Expression Vectors for Functional Genomics, Quantification of Promoter Activity and RNA Silencing in Plants. Plant Methods 2005, 1, 13. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Liu, Q.; Wang, C.; Liang, J.; Liu, L.; Wang, Q. ZmWRKY79 Positively Regulates Maize Phytoalexin Biosynthetic Gene Expression and Is Involved in Stress Response. J. Exp. Bot. 2018, 69, 497–510. [Google Scholar] [CrossRef]
- Ajayo, B.S.; Li, Y.; Wang, Y.; Dai, C.; Gao, L.; Liu, H.; Yu, G.; Zhang, J.; Huang, Y.; Hu, Y. The Novel ZmTCP7 Transcription Factor Targets AGPase-Encoding Gene ZmBt2 to Regulate Storage Starch Accumulation in Maize. Front. Plant Sci. 2022, 13, 943050. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene Ontology Analysis for RNA-Seq: Accounting for Selection Bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Zhang, Y.; Tang, C.; Shen, Q.; Fu, J.; Wang, Q. Maize Transcription Factor ZmHsf28 Positively Regulates Plant Drought Tolerance. Int. J. Mol. Sci. 2023, 24, 8079. https://doi.org/10.3390/ijms24098079
Liu L, Zhang Y, Tang C, Shen Q, Fu J, Wang Q. Maize Transcription Factor ZmHsf28 Positively Regulates Plant Drought Tolerance. International Journal of Molecular Sciences. 2023; 24(9):8079. https://doi.org/10.3390/ijms24098079
Chicago/Turabian StyleLiu, Lijun, Yuhan Zhang, Chen Tang, Qinqin Shen, Jingye Fu, and Qiang Wang. 2023. "Maize Transcription Factor ZmHsf28 Positively Regulates Plant Drought Tolerance" International Journal of Molecular Sciences 24, no. 9: 8079. https://doi.org/10.3390/ijms24098079
APA StyleLiu, L., Zhang, Y., Tang, C., Shen, Q., Fu, J., & Wang, Q. (2023). Maize Transcription Factor ZmHsf28 Positively Regulates Plant Drought Tolerance. International Journal of Molecular Sciences, 24(9), 8079. https://doi.org/10.3390/ijms24098079





