A Descriptive Whole-Genome Transcriptomics Study in a Stem Cell-Based Tool Predicts Multiple Tissue-Specific Beneficial Potential and Molecular Targets of Carnosic Acid
Abstract
1. Introduction
2. Results
2.1. Carnosic Acid Significantly Regulated Gene Expression in hAESCs
2.2. Carnosic Acid Biased hAESCs toward Mesodermal Lineage Progression
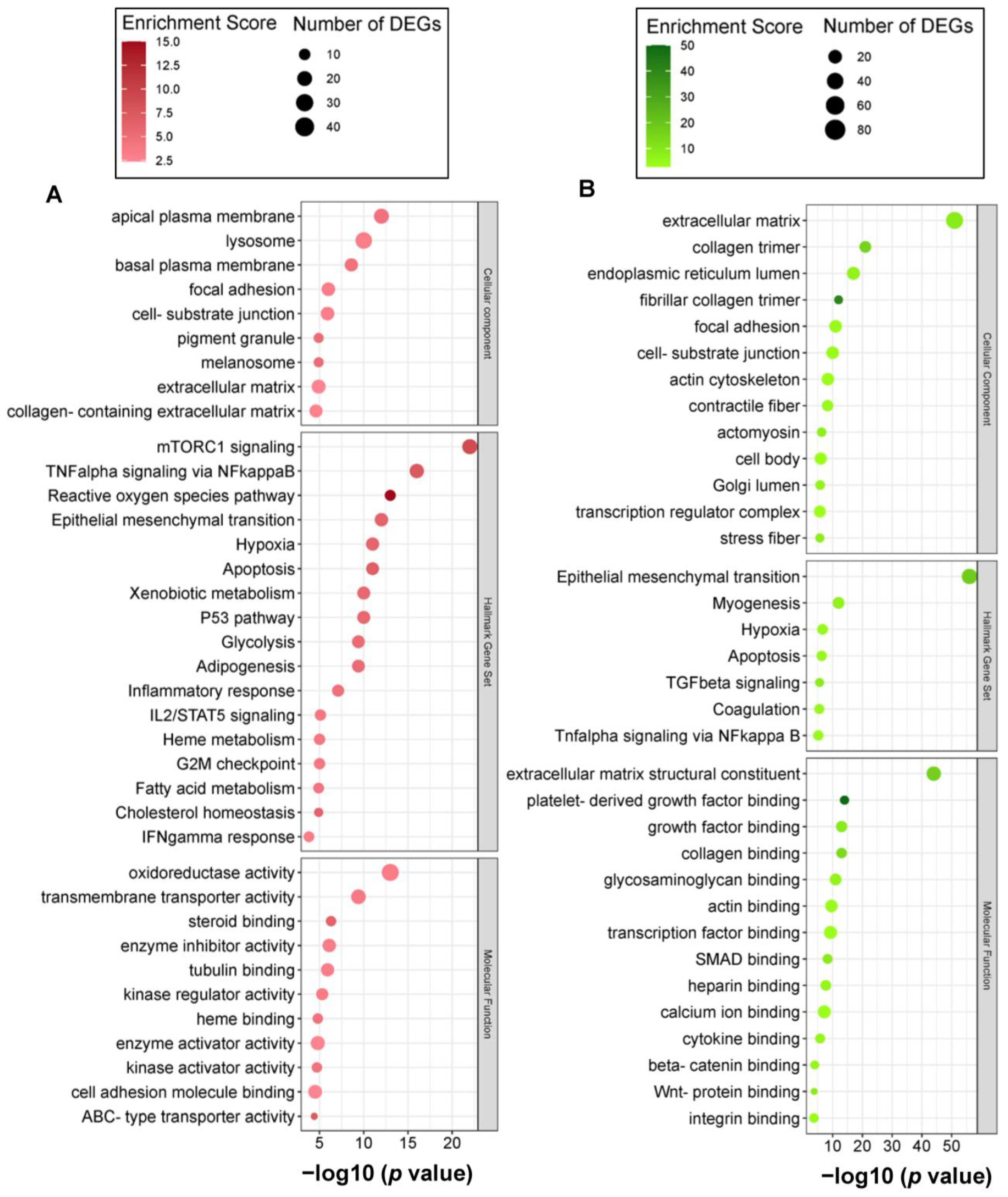
2.3. Carnosic Acid Regulated a Wide Range of Biological and Molecular Events in hAESCs
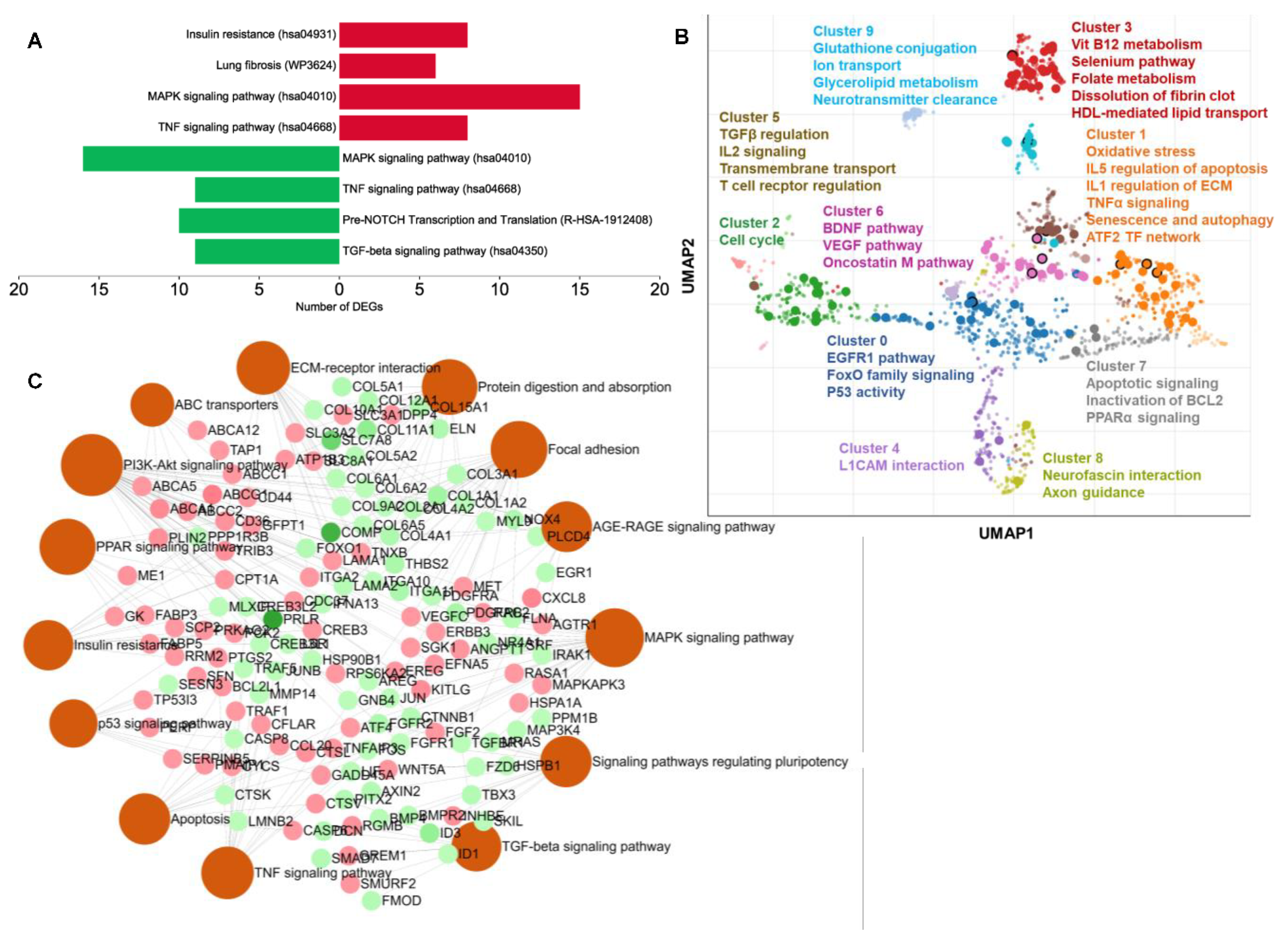
2.4. Carnosic-Acid-Regulated Germ-Layer-Specific Pathways in hAESCs
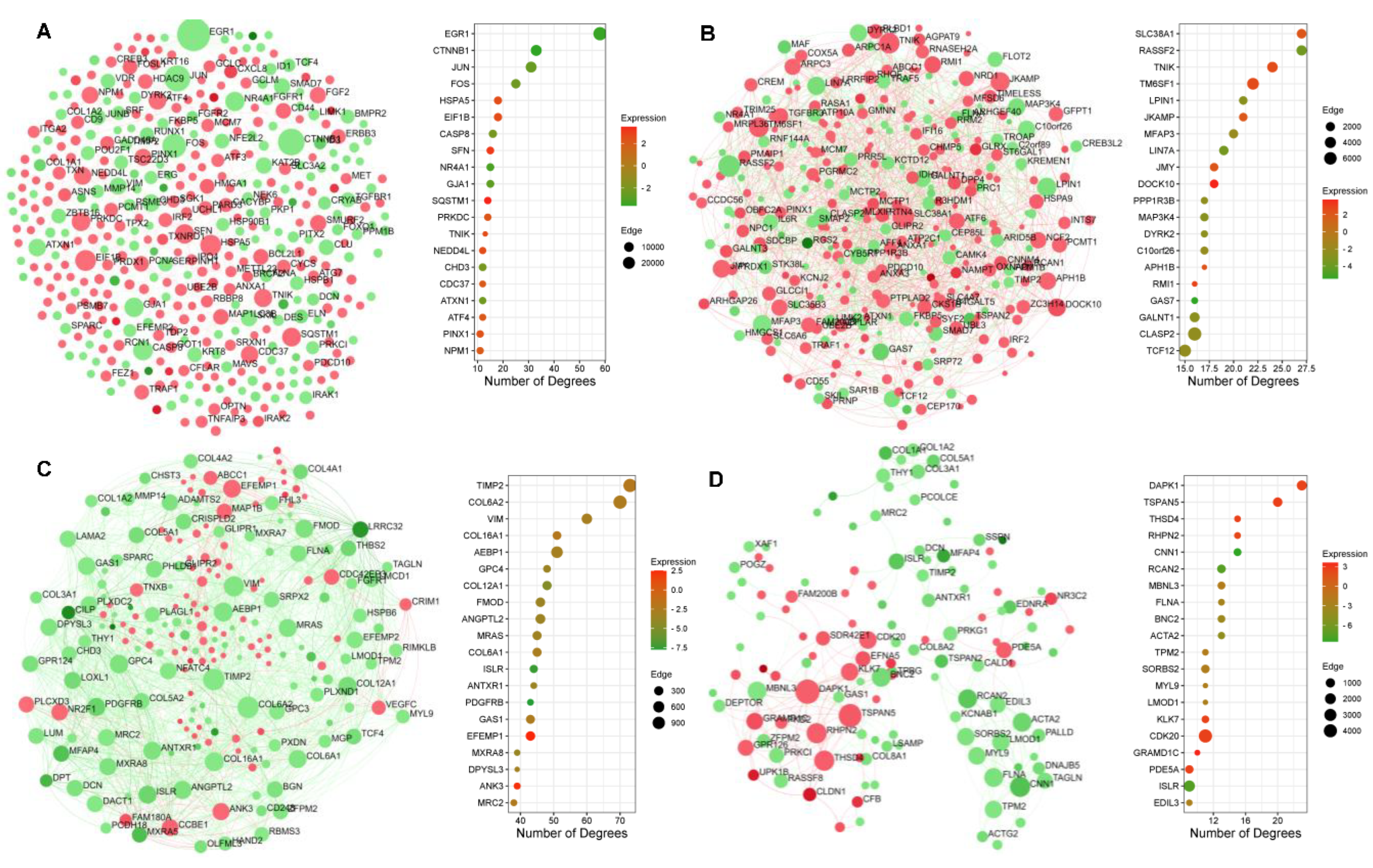
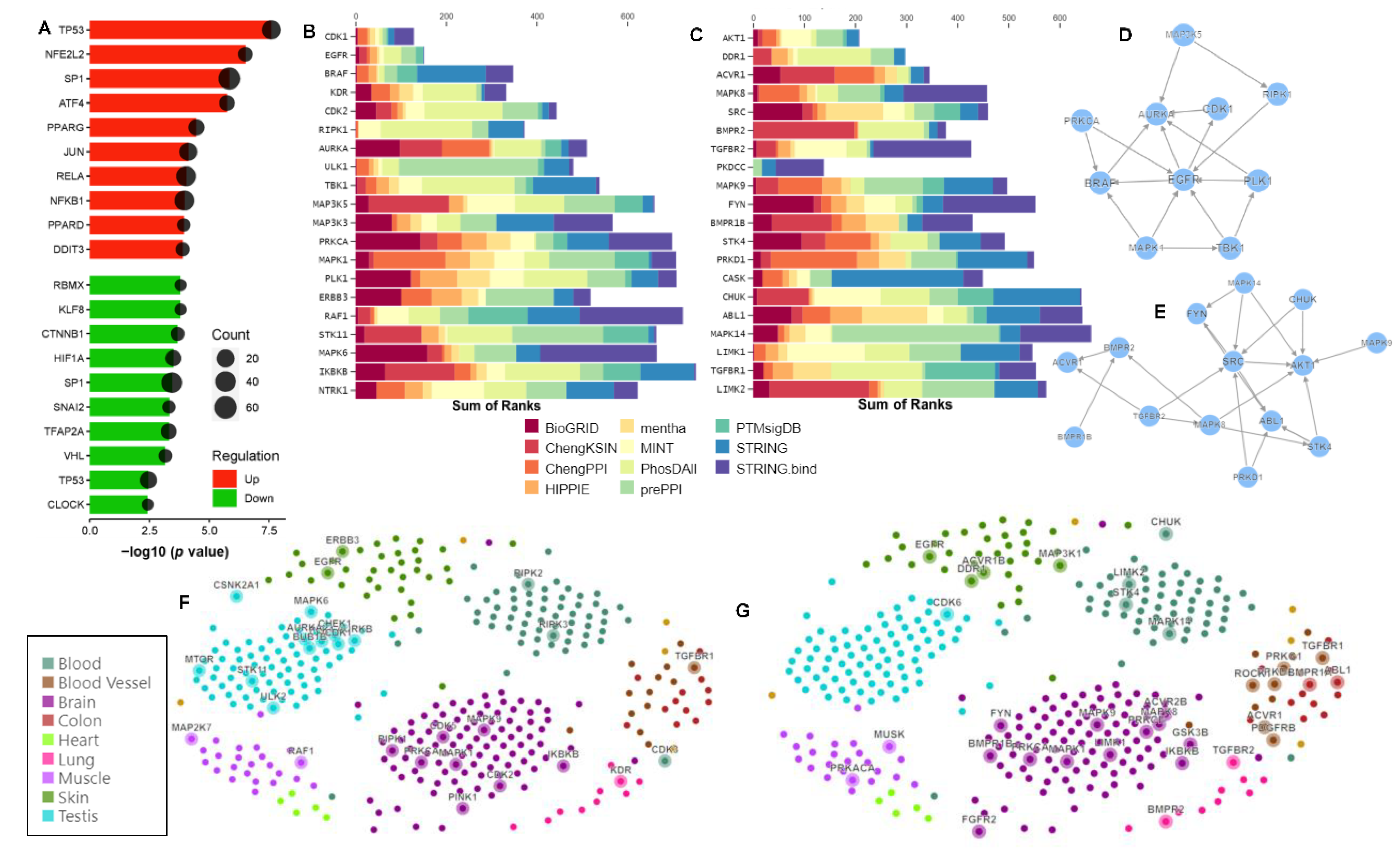
2.5. Protein–Protein Interaction Network Topography in Carnosic Acid-Treated hAESCs
2.6. Significantly Enriched Transcription Factors and Kinases in Carnosic-Acid-Treated hAESCs
2.7. Chemical and Disease Perturbation Enrichment Analyses by the DEGs in Carnosic Acid-Treated hAESCs
3. Discussion
4. Materials and Methods
4.1. hAESCs Isolation and Cell Culture Maintenance
4.2. Preparation of 3D Spheroid Formation and Cell Treatment
4.3. RNA Extraction and Quantification
4.4. DNA Microarray Analysis
4.5. Microarray Data Processing and Quality Control
4.6. Microarray Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ebert, A.D.; Svendsen, C.N. Human stem cells and drug screening: Opportunities and challenges. Nat. Rev. Drug Discov. 2010, 9, 367. [Google Scholar] [CrossRef]
- Laustriat, D.; Gide, J.; Peschanski, M. Human Pluripotent Stem Cells in Drug Discovery and Predictive Toxicology; Portland Press Ltd.: London, UK, 2010. [Google Scholar]
- McNeish, J. Embryonic stem cells in drug discovery. Nat. Rev. Drug Discov. 2004, 3, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Miki, T. Amnion-derived stem cells: In quest of clinical applications. Stem Cell Res. Ther. 2011, 2, 25. [Google Scholar] [CrossRef]
- Miki, T.; Lehmann, T.; Cai, H.; Stolz, D.B.; Strom, S.C. Stem cell characteristics of amniotic epithelial cells. Stem Cells 2005, 23, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.; Rosli, S.; Acharya, R.; Mathias, L.; Lim, R.; Wallace, E.; Jenkin, G. Amnion epithelial cell isolation and characterization for clinical use. Curr. Protoc. Stem Cell Biol. 2010, 13, 1E.6.1–1E.6.25. [Google Scholar] [CrossRef] [PubMed]
- Niknejad, H.; Peirovi, H.; Ahmadiani, A.; Ghanavi, J.; Jorjani, M. Differentiation factors that influence neuronal markers expression in vitro from human amniotic epithelial cells. Eur. Cell Mater. 2010, 19, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Shu, J.; Cai, Z. The morphologic study of the characteristics of neurobiology of the amniotic membrane. Chin. J. Rehabil. Med. 2006, 21, 46–49. [Google Scholar]
- Sakuragawa, N.; Thangavel, R.; Mizuguchi, M.; Hirasawa, M.; Kamo, I. Expression of markers for both neuronal and glial cells in human amniotic epithelial cells. Neurosci. Lett. 1996, 209, 9–12. [Google Scholar] [CrossRef]
- Toda, A.; Okabe, M.; Yoshida, T.; Nikaido, T. The potential of amniotic membrane/amnion-derived cells for regeneration of various tissues. J. Pharmacol. Sci. 2007, 105, 215–228. [Google Scholar] [CrossRef]
- Aonuma, K.; Ferdousi, F.; Xu, D.; Tominaga, K.; Isoda, H. Effects of isorhamnetin in human amniotic epithelial stem cells in vitro and its cardioprotective effects in vivo. Front. Cell Dev. Biol. 2020, 8, 578197. [Google Scholar] [CrossRef]
- Bejaoui, M.; Ferdousi, F.; Zheng, Y.-W.; Oda, T.; Isoda, H. Regulating cell fate of human amnion epithelial cells using natural compounds: An example of enhanced neural and pigment differentiation by 3,4,5-tri-O-caffeoylquinic acid. Cell Commun. Signal. 2021, 19, 26. [Google Scholar] [CrossRef] [PubMed]
- Ferdousi, F.; Furuya, K.; Sasaki, K.; Zheng, Y.-W.; Oda, T.; Isoda, H. DNA Microarray-Based Global Gene Expression Profiling in Human Amniotic Epithelial Cells Predicts the Potential of Microalgae-Derived Squalene for the Nervous System and Metabolic Health. Biomedicines 2022, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Ferdousi, F.; Kondo, S.; Sasaki, K.; Uchida, Y.; Ohkohchi, N.; Zheng, Y.-W.; Isoda, H. Microarray analysis of verbenalin-treated human amniotic epithelial cells reveals therapeutic potential for Alzheimer’s Disease. Aging 2020, 12, 5516. [Google Scholar] [CrossRef]
- Ferdousi, F.; Sasaki, K.; Uchida, Y.; Ohkohchi, N.; Zheng, Y.-W.; Isoda, H. Exploring the Potential Role of Rosmarinic Acid in Neuronal Differentiation of Human Amnion Epithelial Cells by Microarray Gene Expression Profiling. Front. Neurosci. 2019, 13, 779. [Google Scholar] [CrossRef] [PubMed]
- Ferdousi, F.; Isoda, H. Regulating Early Biological Events in Human Amniotic Epithelial Stem Cells Using Natural Bioactive Compounds: Extendable Multidirectional Research Avenues. Front. Cell Dev. Biol. 2022, 10, 865810. [Google Scholar] [CrossRef]
- Uchida, Y.; Ferdousi, F.; Zheng, Y.-W.; Oda, T.; Isoda, H. Global Gene Expression Profiling Reveals Isorhamnetin Induces Hepatic-Lineage Specific Differentiation in Human Amniotic Epithelial Cells. Front. Cell Dev. Biol. 2020, 8, 578036. [Google Scholar] [CrossRef]
- Takahashi, S.; Ferdousi, F.; Zheng, Y.-W.; Oda, T.; Isoda, H. Human Amniotic Epithelial Cells as a Tool to Investigate the Effects of Cyanidin 3-O-Glucoside on Cell Differentiation. Int. J. Mol. Sci. 2021, 22, 3768. [Google Scholar] [CrossRef]
- Ganbold, M.; Owada, Y.; Ozawa, Y.; Shimamoto, Y.; Ferdousi, F.; Tominaga, K.; Zheng, Y.-W.; Ohkohchi, N.; Isoda, H. Isorhamnetin alleviates steatosis and fibrosis in mice with nonalcoholic steatohepatitis. Sci. Rep. 2019, 9, 16210. [Google Scholar] [CrossRef]
- Lee, M.-K.; Yang, H.-K.; Ha, N.-R.; Sung, S.-H.; Kim, Y.-C. Isorhamnetin from Oenanthe javanica attenuates fibrosis in rat hepatic stellate cells via inhibition of ERK signaling pathway. Nat. Prod. Sci. 2008, 14, 81–85. [Google Scholar]
- Matsukawa, T.; Inaguma, T.; Han, J.; Villareal, M.O.; Isoda, H. Cyanidin-3-glucoside derived from black soybeans ameliorate type 2 diabetes through the induction of differentiation of preadipocytes into smaller and insulin-sensitive adipocytes. J. Nutr. Biochem. 2015, 26, 860–867. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.J.; Rodrigo-García, J.; Martínez-Ruiz, N.d.R.; Cárdenas-Robles, A.I.; Mendoza-Díaz, S.O.; Alvarez-Parrilla, E.; González-Aguilar, G.A.; De la Rosa, L.A.; Ramos-Jiménez, A.; Wall-Medrano, A. Cyanidin-3-O-glucoside: Physical-chemistry, foodomics and health effects. Molecules 2016, 21, 1264. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Yabe, T.; Gamo, Y.; Nagai, T.; Oikawa, T.; Yamada, H.; Hanawa, T. Rosmarinic acid from Perillae Herba produces an antidepressant-like effect in mice through cell proliferation in the hippocampus. Biol. Pharm. Bull. 2008, 31, 1376–1380. [Google Scholar] [CrossRef]
- Sasaki, K.; El Omri, A.; Kondo, S.; Han, J.; Isoda, H. Rosmarinus officinalis polyphenols produce anti-depressant like effect through monoaminergic and cholinergic functions modulation. Behav. Brain Res. 2013, 238, 86–94. [Google Scholar] [CrossRef]
- Takeda, H.; Tsuji, M.; Miyamoto, J.; Matsumiya, T. Rosmarinic acid and caffeic acid reduce the defensive freezing behavior of mice exposed to conditioned fear stress. Psychopharmacology 2002, 164, 233–235. [Google Scholar] [CrossRef]
- Aruoma, O.; Halliwell, B.; Aeschbach, R.; Löligers, J. Antioxidant and pro-oxidant properties of active rosemary constituents: Carnosol and carnosic acid. Xenobiotica 1992, 22, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Birtić, S.; Dussort, P.; Pierre, F.-X.; Bily, A.C.; Roller, M. Carnosic acid. Phytochemistry 2015, 115, 9–19. [Google Scholar] [CrossRef]
- de Oliveira, M.R. The dietary components carnosic acid and carnosol as neuroprotective agents: A mechanistic view. Mol. Neurobiol. 2016, 53, 6155–6168. [Google Scholar] [CrossRef]
- Masuda, T.; Inaba, Y.; Takeda, Y. Antioxidant mechanism of carnosic acid: Structural identification of two oxidation products. J. Agric. Food Chem. 2001, 49, 5560–5565. [Google Scholar] [CrossRef]
- Anwar, F.; Qadir, R. Carnosic acid and carnosol. In A Centum of Valuable Plant Bioactives; Elsevier: Amsterdam, The Netherlands, 2021; pp. 261–274. [Google Scholar]
- White, A.I.; Jenkins, G.L. Salvia carnosa (dougl.). I—A phytochemical study. J. Am. Pharm. Assoc. 1942, 31, 33–37. [Google Scholar] [CrossRef]
- Dougherty, J.D.; Schmidt, E.F.; Nakajima, M.; Heintz, N. Analytical approaches to RNA profiling data for the identification of genes enriched in specific cells. Nucleic Acids Res. 2010, 38, 4218–4230. [Google Scholar] [CrossRef] [PubMed]
- Kuleshov, M.V.; Xie, Z.; London, A.B.; Yang, J.; Evangelista, J.E.; Lachmann, A.; Shu, I.; Torre, D.; Ma’ayan, A. KEA3: Improved kinase enrichment analysis via data integration. Nucleic Acids Res. 2021, 49, W304–W316. [Google Scholar] [CrossRef]
- Lonsdale, J.; Thomas, J.; Salvatore, M.; Phillips, R.; Lo, E.; Shad, S.; Hasz, R.; Walters, G.; Garcia, F.; Young, N. The genotype-tissue expression (GTEx) project. Nat. Genet. 2013, 45, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-P.; Hsieh, Y.-Y.; Chou, C.-J.; Yang, P.-M. Systematic polypharmacology and drug repurposing via an integrated L1000-based Connectivity Map database mining. R. Soc. Open Sci. 2018, 5, 181321. [Google Scholar] [CrossRef]
- Piñero, J.; Queralt-Rosinach, N.; Bravo, A.; Deu-Pons, J.; Bauer-Mehren, A.; Baron, M.; Sanz, F.; Furlong, L.I. DisGeNET: A discovery platform for the dynamical exploration of human diseases and their genes. Database 2015, 2015, bav028. [Google Scholar] [CrossRef]
- Davis, A.P.; Wiegers, T.C.; Johnson, R.J.; Sciaky, D.; Wiegers, J.; Mattingly, C.J. Comparative Toxicogenomics database (CTD): Update 2023. Nucleic Acids Res. 2023, 51, D1257–D1262. [Google Scholar] [CrossRef]
- Sinha, S.; Iyer, D.; Granata, A. Embryonic origins of human vascular smooth muscle cells: Implications for in vitro modeling and clinical application. Cell. Mol. Life Sci. 2014, 71, 2271–2288. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Tang, Z.; Li, X.; Jiang, Y.; Tsou, D.A.; Li, S. Derivation of smooth muscle cells with neural crest origin from human induced pluripotent stem cells. Cells Tissues Organs 2012, 195, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-P.; Lei, Q.-Y. Metabolite sensing and signaling in cell metabolism. Signal Transduct. Target. Ther. 2018, 3, 30. [Google Scholar] [CrossRef]
- Ward, P.S.; Thompson, C.B. Signaling in control of cell growth and metabolism. Cold Spring Harb. Perspect. Biol. 2012, 4, a006783. [Google Scholar] [CrossRef]
- Meng, X.-m.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-β: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef]
- Zbodakova, O.; Chalupsky, K.; Tureckova, J.; Sedlacek, R. Metalloproteinases in liver fibrosis: Current insights. Met. Med. 2017, 4, 25–35. [Google Scholar] [CrossRef]
- Williams, L.; Layton, T.; Yang, N.; Feldmann, M.; Nanchahal, J. Collagen VI as a driver and disease biomarker in human fibrosis. FEBS J. 2022, 289, 3603–3629. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.D.; Galbraith, M.D.; Andrysik, Z.; Espinosa, J.M. Mechanisms of transcriptional regulation by p53. Cell Death Differ. 2018, 25, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Laptenko, O.; Prives, C. Transcriptional regulation by p53: One protein, many possibilities. Cell Death Differ. 2006, 13, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, I.-g.; Kwak, M.-K. Regulatory crosstalk between the oxidative stress-related transcription factor Nfe2l2/Nrf2 and mitochondria. Toxicol. Appl. Pharmacol. 2018, 359, 24–33. [Google Scholar] [CrossRef]
- Cartwright, T.; Perkins, N.D.; Wilson, L.C. NFKB1: A suppressor of inflammation, ageing and cancer. FEBS J. 2016, 283, 1812–1822. [Google Scholar] [CrossRef]
- Aprile, M.; Ambrosio, M.; D’Esposito, V.; Beguinot, F.; Formisano, P.; Costa, V.; Ciccodicola, A. PPARG in human adipogenesis: Differential contribution of canonical transcripts and dominant negative isoforms. PPAR Res. 2014, 2014, 537865. [Google Scholar] [CrossRef]
- Fusakio, M.E.; Willy, J.A.; Wang, Y.; Mirek, E.T.; Al Baghdadi, R.J.; Adams, C.M.; Anthony, T.G.; Wek, R.C. Transcription factor ATF4 directs basal and stress-induced gene expression in the unfolded protein response and cholesterol metabolism in the liver. Mol. Biol. Cell 2016, 27, 1536–1551. [Google Scholar] [CrossRef]
- Ho, Y.-S.; Tsai, W.-H.; Lin, F.-C.; Huang, W.-P.; Lin, L.-C.; Wu, S.M.; Liu, Y.-R.; Chen, W.-P. Cardioprotective actions of TGFβRI inhibition through stimulating autocrine/paracrine of survivin and inhibiting Wnt in cardiac progenitors. Stem Cells 2016, 34, 445–455. [Google Scholar] [CrossRef]
- Lebrin, F.; Deckers, M.; Bertolino, P.; Ten Dijke, P. TGF-β receptor function in the endothelium. Cardiovasc. Res. 2005, 65, 599–608. [Google Scholar] [CrossRef]
- Zonneville, J.; Safina, A.; Truskinovsky, A.M.; Arteaga, C.L.; Bakin, A.V. TGF-β signaling promotes tumor vasculature by enhancing the pericyte-endothelium association. BMC Cancer 2018, 18, 670. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.K.; Seto, M.; Noma, K. Rho kinase (ROCK) inhibitors. J. Cardiovasc. Pharmacol. 2007, 50, 17. [Google Scholar] [CrossRef] [PubMed]
- Raica, M.; Cimpean, A.M. Platelet-derived growth factor (PDGF)/PDGF receptors (PDGFR) axis as target for antitumor and antiangiogenic therapy. Pharmaceuticals 2010, 3, 572–599. [Google Scholar] [CrossRef]
- Buitrago, C.G.; Pardo, V.G.; de Boland, A.R.; Boland, R. Activation of RAF-1 through Ras and protein kinase Cα mediates 1α, 25 (OH) 2-vitamin D3 regulation of the mitogen-activated protein kinase pathway in muscle cells. J. Biol. Chem. 2003, 278, 2199–2205. [Google Scholar] [CrossRef] [PubMed]
- Dahchour, A. Anxiolytic and antidepressive potentials of rosmarinic acid: A review with focus on antioxidant and anti-inflammatory effects. Pharmacol. Res. 2022, 184, 106421. [Google Scholar] [CrossRef]
- Kondo, S.; El Omri, A.; Han, J.; Isoda, H. Antidepressant-like effects of rosmarinic acid through mitogen-activated protein kinase phosphatase-1 and brain-derived neurotrophic factor modulation. J. Funct. Foods 2015, 14, 758–766. [Google Scholar] [CrossRef]
- Sasaki, K.; Ferdousi, F.; Fukumitsu, S.; Kuwata, H.; Isoda, H. Antidepressant-and anxiolytic-like activities of Rosmarinus officinalis extract in rodent models: Involvement of oxytocinergic system. Biomed. Pharmacother. 2021, 144, 112291. [Google Scholar] [CrossRef]
- Azad, N.; Rasoolijazi, H.; Joghataie, M.T.; Soleimani, S. Neuroprotective effects of carnosic acid in an experimental model of Alzheimer’s disease in rats. Cell J. 2011, 13, 39. [Google Scholar]
- Zhao, Z.; Feng, L.; Wang, J.; Cheng, D.; Liu, M.; Ling, M.; Xu, W.; Sun, K. NPC-26 kills human colorectal cancer cells via activating AMPK signaling. Oncotarget 2017, 8, 18312. [Google Scholar] [CrossRef]
- Sasaki, T.; Hwang, H.; Nguyen, C.; Kloner, R.A.; Kahn, M. The small molecule Wnt signaling modulator ICG-001 improves contractile function in chronically infarcted rat myocardium. PLoS ONE 2013, 8, e75010. [Google Scholar] [CrossRef]
- Potenza, R.L.; Lodeserto, P.; Orienti, I. Fenretinide in Cancer and Neurological Disease: A Two-Face Janus Molecule. Int. J. Mol. Sci. 2022, 23, 7426. [Google Scholar] [CrossRef] [PubMed]
- Omura, S. The antibiotic cerulenin, a novel tool for biochemistry as an inhibitor of fatty acid synthesis. Bacteriol. Rev. 1976, 40, 681–697. [Google Scholar] [CrossRef]
- Sahu, A.; Prabhash, K.; Noronha, V.; Joshi, A.; Desai, S. Crizotinib: A comprehensive review. South Asian J. Cancer 2013, 2, 91–97. [Google Scholar] [PubMed]
- Zhang, Y.-s.; Li, J.-d.; Yan, C. An update on vinpocetine: New discoveries and clinical implications. Eur. J. Pharmacol. 2018, 819, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Lassus, A. Clinical comparison of alclometasone dipropionate cream 0·05% with hydrocortisone butyrate cream 0·1% in the treatment of atopic dermatitis in children. J. Int. Med. Res. 1983, 11, 315–319. [Google Scholar] [CrossRef]
- Wang, T.; Takikawa, Y.; Satoh, T.; Yoshioka, Y.; Kosaka, K.; Tatemichi, Y.; Suzuki, K. Carnosic acid prevents obesity and hepatic steatosis in ob/ob mice. Hepatol. Res. 2011, 41, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Song, H.-M.; Li, X.; Liu, Y.-Y.; Lu, W.-P.; Cui, Z.-H.; Zhou, L.; Yao, D.; Zhang, H.-M. Carnosic acid protects mice from high-fat diet-induced NAFLD by regulating MARCKS. Int. J. Mol. Med. 2018, 42, 193–207. [Google Scholar] [CrossRef]
- Greenhill, C. Carnosic acid could be a new treatment option for patients with NAFLD or the metabolic syndrome. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 122. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Tang, Y.-H.; Zeng, G.-R.; Wu, L.-F.; Zhou, Y.-J.; Cheng, Z.-N.; Jiang, D.-J. Carnosic acid alleviates depression-like behaviors on chronic mild stressed mice via PPAR-γ-dependent regulation of ADPN/FGF9 pathway. Psychopharmacology 2021, 238, 501–516. [Google Scholar] [CrossRef]
- Azhar, M.; Zeng, G.; Ahmed, A.; Dar Farooq, A.; Choudhary, M.I.; De-Jiang, J.; Liu, X. Carnosic acid ameliorates depressive-like symptoms along with the modulation of FGF9 in the hippocampus of middle carotid artery occlusion-induced Sprague Dawley rats. Phytother. Res. 2021, 35, 384–391. [Google Scholar] [CrossRef]
- Mirza, F.J.; Zahid, S.; Holsinger, R.D. Neuroprotective Effects of Carnosic Acid: Insight into Its Mechanisms of Action. Molecules 2023, 28, 2306. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sedighi, R.; Wang, P.; Chen, H.; Zhu, Y.; Sang, S. Carnosic acid as a major bioactive component in rosemary extract ameliorates high-fat-diet-induced obesity and metabolic syndrome in mice. J. Agric. Food Chem. 2015, 63, 4843–4852. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.S.; Niharika, D.; Madhavan, A.; Sharma, S.; Joshi, A.K.R. Recent updates on antidiabetic and antiobesity potential of carnosic acid. EXCLI J. 2021, 20, 1476–1481. [Google Scholar] [PubMed]
- Den Hartogh, D.J.; Vlavcheski, F.; Giacca, A.; MacPherson, R.E.; Tsiani, E. Carnosic acid attenuates the free fatty acid-induced insulin resistance in muscle cells and adipocytes. Cells 2022, 11, 167. [Google Scholar] [CrossRef]
- Takeuchi, T.; Noguchi, M.; Kawakami, Y.; Ohkohchi, N. Use of Human Biospecimen Resources for Drug Discovery—Approach of Tsukuba Human Tissue Biobank Center. Regul. Sci. Med. Prod. 2016, 6, 57–63. [Google Scholar]
- Furuya, K.; Zheng, Y.-W.; Sako, D.; Iwasaki, K.; Zheng, D.-X.; Ge, J.-Y.; Liu, L.-P.; Furuta, T.; Akimoto, K.; Yagi, H. Enhanced hepatic differentiation in the subpopulation of human amniotic stem cells under 3D multicellular microenvironment. World J. Stem Cells 2019, 11, 705. [Google Scholar] [CrossRef]
- Goedhart, J.; Luijsterburg, M.S. VolcaNoseR is a web app for creating, exploring, labeling and sharing volcano plots. Sci. Rep. 2020, 10, 20560. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Supek, F.; Bošnjak, M.; Škunca, N.; Šmuc, T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS ONE 2011, 6, e21800. [Google Scholar] [CrossRef]
- Schlicker, A.; Domingues, F.S.; Rahnenführer, J.; Lengauer, T. A new measure for functional similarity of gene products based on Gene Ontology. BMC Bioinform. 2006, 7, 302. [Google Scholar] [CrossRef]
- Xie, Z.; Bailey, A.; Kuleshov, M.V.; Clarke, D.J.; Evangelista, J.E.; Jenkins, S.L.; Lachmann, A.; Wojciechowicz, M.L.; Kropiwnicki, E.; Jagodnik, K.M. Gene set knowledge discovery with Enrichr. Curr. Protoc. 2021, 1, e90. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Grishagin, I.; Wang, Y.; Zhao, T.; Greene, J.; Obenauer, J.C.; Ngan, D.; Nguyen, D.-T.; Guha, R.; Jadhav, A. The NCATS BioPlanet–an integrated platform for exploring the universe of cellular signaling pathways for toxicology, systems biology, and chemical genomics. Front. Pharmacol. 2019, 10, 445. [Google Scholar] [CrossRef]
- Xia, J.; Benner, M.J.; Hancock, R.E. NetworkAnalyst-integrative approaches for protein–protein interaction network analysis and visual exploration. Nucleic Acids Res. 2014, 42, W167–W174. [Google Scholar] [CrossRef] [PubMed]
- Breuer, K.; Foroushani, A.K.; Laird, M.R.; Chen, C.; Sribnaia, A.; Lo, R.; Winsor, G.L.; Hancock, R.E.; Brinkman, F.S.; Lynn, D.J. InnateDB: Systems biology of innate immunity and beyond—Recent updates and continuing curation. Nucleic Acids Res. 2013, 41, D1228–D1233. [Google Scholar] [CrossRef]
- Lee, S.; Zhang, C.; Arif, M.; Liu, Z.; Benfeitas, R.; Bidkhori, G.; Deshmukh, S.; Al Shobky, M.; Lovric, A.; Boren, J. TCSBN: A database of tissue and cancer specific biological networks. Nucleic Acids Res. 2018, 46, D595–D600. [Google Scholar] [CrossRef]
- Han, H.; Cho, J.-W.; Lee, S.; Yun, A.; Kim, H.; Bae, D.; Yang, S.; Kim, C.Y.; Lee, M.; Kim, E. TRRUST v2: An expanded reference database of human and mouse transcriptional regulatory interactions. Nucleic Acids Res. 2018, 46, D380–D386. [Google Scholar] [CrossRef] [PubMed]
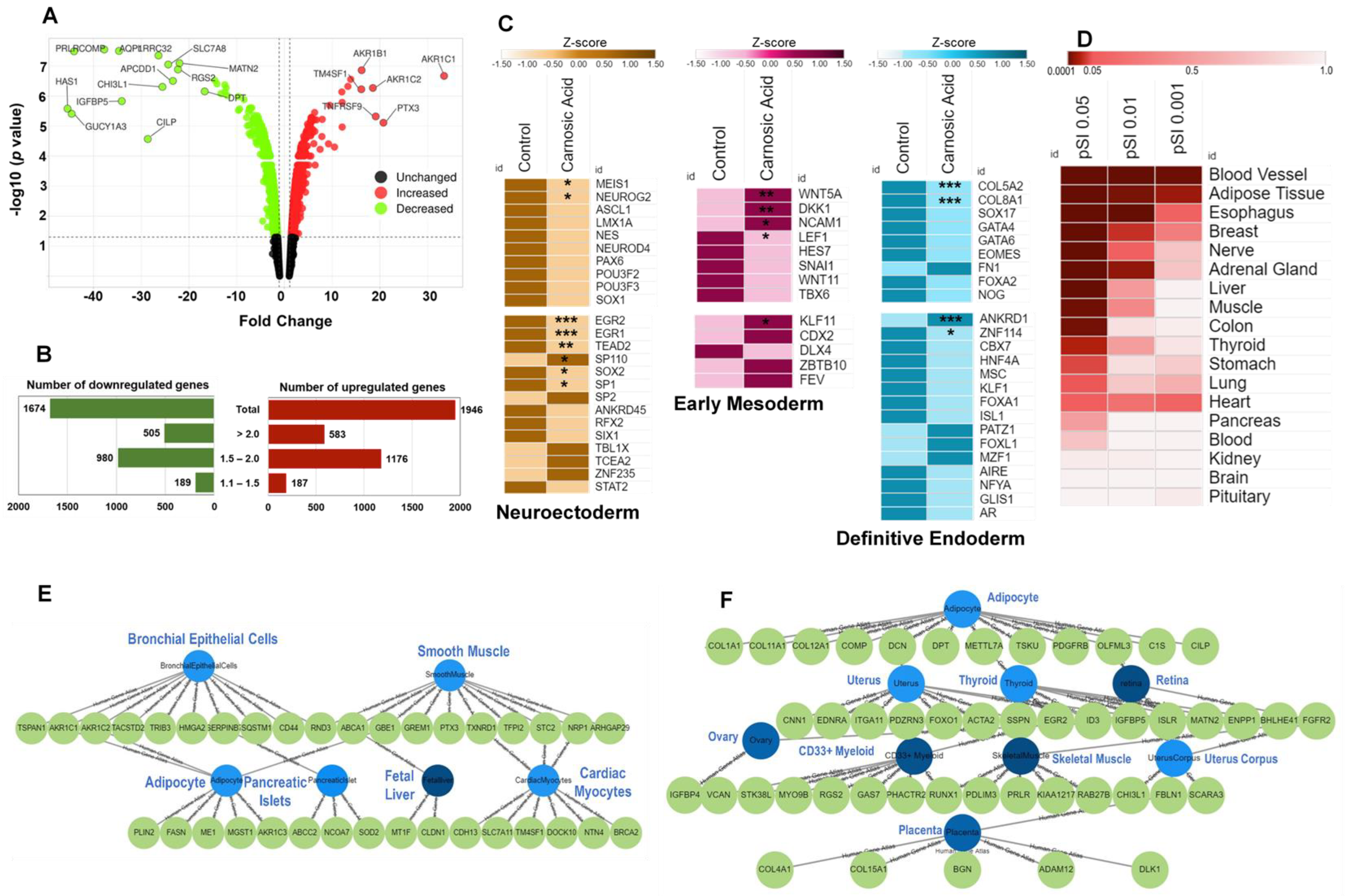


| Gene Symbol | Description | Fold Change | p Value | Biological Functions |
|---|---|---|---|---|
| AKR1C1 | aldo-keto reductase family 1, member C1 | 33.45 | 2.14 × 10−7 | bile acid and bile salt transport and metabolic process, cholesterol homeostasis, Metabolism of fat-soluble vitamins, lipids and lipoproteins, and xenobiotics by cytochrome P450 |
| PTX3 | pentraxin 3, long | 20.74 | 7.75 × 10−6 | extracellular matrix organization, innate immune response, negative regulation by host of viral exo-alpha-sialidase activity, |
| TNFRSF9 | tumor necrosis factor receptor superfamily, member 9 | 19.12 | 4.78 × 10−6 | negative regulation of IL10 and IL12 production, TNFR2 non-canonical NFkB pathway |
| AKR1C2 | aldo-keto reductase family 1, member C2 | 18.53 | 5.38 × 10−7 | G protein-coupled receptor signaling pathway, positive regulation of protein kinase B signaling, Bile acid and bile salt metabolism |
| AKR1B1 | aldo-keto reductase family 1, member B1 (aldose reductase) | 16.18 | 1.38 × 10−7 | C21-steroid hormone biosynthetic process, negative regulation of apoptotic process, positive regulation of JAK–STAT cascade, positive regulation of smooth muscle cell proliferation |
| TM4SF1 | transmembrane 4 L six family member 1 | 16.11 | 5.96 × 10−7 | plasma membrane component |
| SLC7A11 | solute carrier family 7 (anionic amino acid transporter light chain, xc- system), member 11 | 13.89 | 2.78 × 10−7 | brain development, adult locomotory behavior, cellular response to oxidative stress |
| ABI3BP | ABI family, member 3 (NESH) binding protein | 13.29 | 4.78 × 10−7 | defense response to tumor cell, extracellular matrix organization, negative regulation of cell proliferation, negative regulation of connective tissue replacement involved in inflammatory response wound healing, positive regulation of cardiocyte differentiation, gene expression decreases in aging skin |
| ANKRD1 | ankyrin repeat domain 1 (cardiac muscle) | 12.1 | 1.63 × 10−6 | cardiac muscle tissue morphogenesis, myoblast differentiation, cellular response to IL1, TGFβ, and TNF stimulus, Regulation of lipid metabolism by PPARα |
| ABCG1 | ATP binding cassette subfamily G member 1 | 12.1 | 1.08 × 10−5 | amyloid precursor protein catabolic process, positive regulation of Aβ formation (suppress), cholesterol efflux, cellular response to HDL particle stimulus, negative regulation of macrophage-derived foam cell differentiation |
| Gene Symbol | Description | Fold Change | p Value | Biological Functions |
|---|---|---|---|---|
| HAS1S1 | hyaluronan synthase 1 | −45.45 | 2.64 × 10−6 | A major component of extracellular matrix that regulates cell adhesion, migration, and differentiation. It is expressed in mesenchymal cells, such as astrocytes in the CNS and fibroblasts in heart, negative regulation of fibroblast migration, cellular response to platelet-derived growth factor stimulus. Stroke induces the expression of HAS1 |
| GUCY1A3 | guanylate cyclase 1, soluble, alpha 3 | −44.58 | 3.9 × 10−6 | a key enzyme in the nitric oxide/cGMP signaling pathway, inhibits platelet aggregation upon stimulation with nitric oxide |
| PRLR | prolactin receptor | −44.08 | 3.24 × 10−8 | Prolactin signaling pathway, activation of JAK–STAT cascade, PI3K–Akt signaling pathway, leukemia inhibitory factor signaling pathway, highly expressed in depression (rat model, brain), in breast and cervical cancer cells |
| COMP | cartilage oligomeric matrix protein | −37.75 | 2.81 × 10−8 | blood coagulation, Focal adhesion, collagen fibril organization, chondrocyte proliferation, BMP signaling pathway, PI3K–Akt signaling pathway, upregulates in cardiac fibrosis and osteoarthritis |
| AQP1 | aquaporin 1 (Colton blood group) | −34.66 | 3.15 × 10−8 | cellular response to cAMP and to hypoxia, O2/CO2 exchange in erythrocytes, induces angiotensin II-induced cardiac hypertrophy, increases the risk of myocardial infarction |
| IGFBP5 | Insulin-like growth factor binding protein 5 | −34.08 | 1.48 × 10−6 | Promotes fibrosis and increases the risk of cardiovascular disease, causes abnormal curvature and thinning of the hair shaft, induces IL6-mediated ROS production, and causes premature cell senescence |
| CILP | cartilage intermediate layer protein, nucleotide pyrophosphohydrolase | −28.63 | 2.7 × 10−5 | cellular response to TGFβ, negative regulation of insulin-like growth factor receptor signaling pathway, CILP combines with TGFβ or IGF1 to regulate the ECM synthesis in cartilage and promotes degeneration and aging progress in intervertebral discs (IVDs) (but CILP inhibits cardiac fibrosis). |
| LRRC32 | Leucine-rich repeat containing 32 | −26.39 | 4.46 × 10−8 | TGFβ receptor signaling pathway, negative regulation of activated T cell proliferation |
| CHI3L1 | chitinase 3-like 1 (cartilage glycoprotein-39) | −25.56 | 4.97 × 10−7 | It plays a major role in tissue injury, inflammation, tissue repair, and remodeling responses and has been strongly associated with diseases including asthma, arthritis, sepsis, diabetes, liver fibrosis, coronary artery disease, cancer invasion, and metastasis. |
| SLC7A8 | solute carrier family 7 (amino acid transporter light chain, L system), member 8 | −24.35 | 8.9 × 10−8 | amino acid transmembrane transport |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferdousi, F.; Sasaki, K.; Fukumitsu, S.; Kuwata, H.; Nakajima, M.; Isoda, H. A Descriptive Whole-Genome Transcriptomics Study in a Stem Cell-Based Tool Predicts Multiple Tissue-Specific Beneficial Potential and Molecular Targets of Carnosic Acid. Int. J. Mol. Sci. 2023, 24, 8077. https://doi.org/10.3390/ijms24098077
Ferdousi F, Sasaki K, Fukumitsu S, Kuwata H, Nakajima M, Isoda H. A Descriptive Whole-Genome Transcriptomics Study in a Stem Cell-Based Tool Predicts Multiple Tissue-Specific Beneficial Potential and Molecular Targets of Carnosic Acid. International Journal of Molecular Sciences. 2023; 24(9):8077. https://doi.org/10.3390/ijms24098077
Chicago/Turabian StyleFerdousi, Farhana, Kazunori Sasaki, Satoshi Fukumitsu, Hidetoshi Kuwata, Mitsutoshi Nakajima, and Hiroko Isoda. 2023. "A Descriptive Whole-Genome Transcriptomics Study in a Stem Cell-Based Tool Predicts Multiple Tissue-Specific Beneficial Potential and Molecular Targets of Carnosic Acid" International Journal of Molecular Sciences 24, no. 9: 8077. https://doi.org/10.3390/ijms24098077
APA StyleFerdousi, F., Sasaki, K., Fukumitsu, S., Kuwata, H., Nakajima, M., & Isoda, H. (2023). A Descriptive Whole-Genome Transcriptomics Study in a Stem Cell-Based Tool Predicts Multiple Tissue-Specific Beneficial Potential and Molecular Targets of Carnosic Acid. International Journal of Molecular Sciences, 24(9), 8077. https://doi.org/10.3390/ijms24098077







