Study of the Magnesium Comenate Structure, Its Neuroprotective and Stress-Protective Activity
Abstract
1. Introduction
2. Results
2.1. Chemical Part
2.1.1. IR Spectroscopy
2.1.2. Thermal Analysis
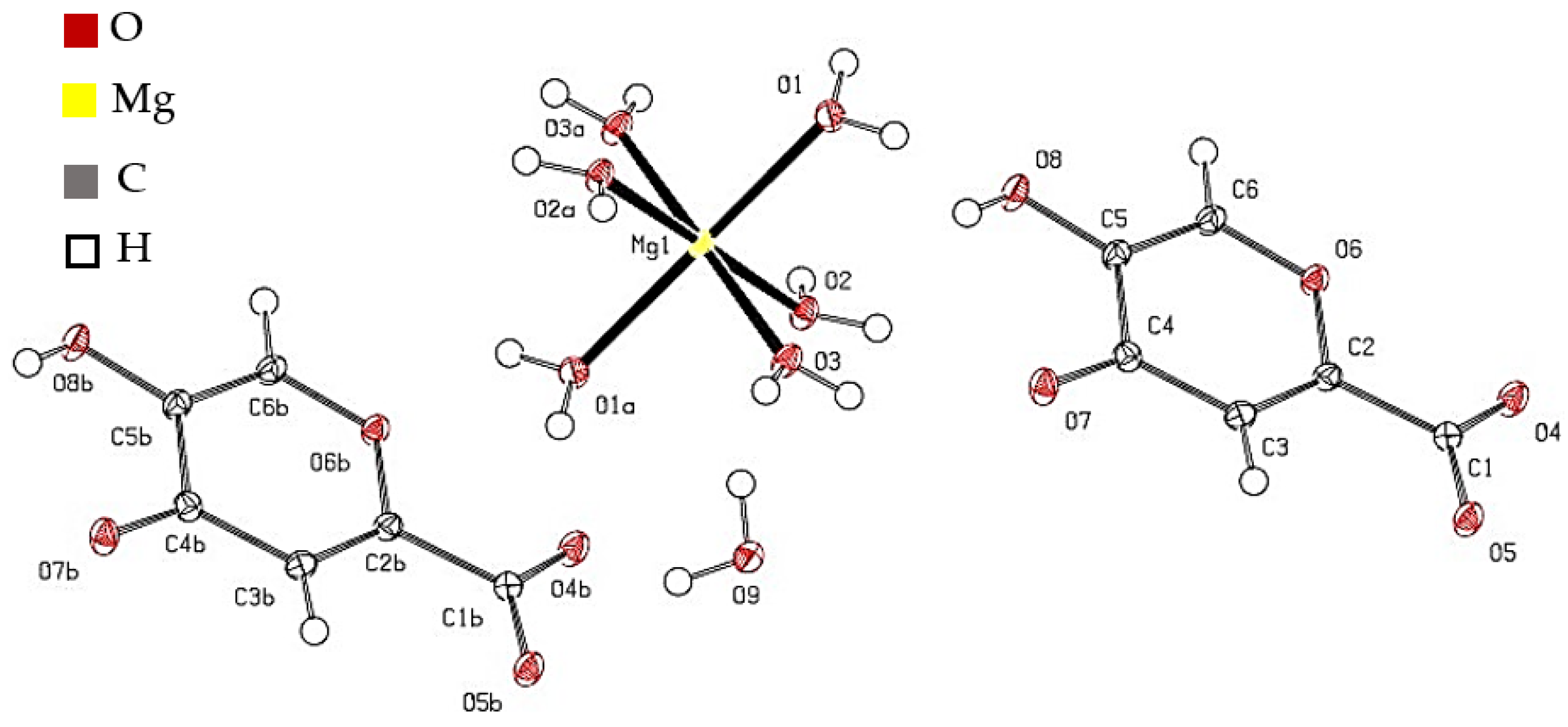
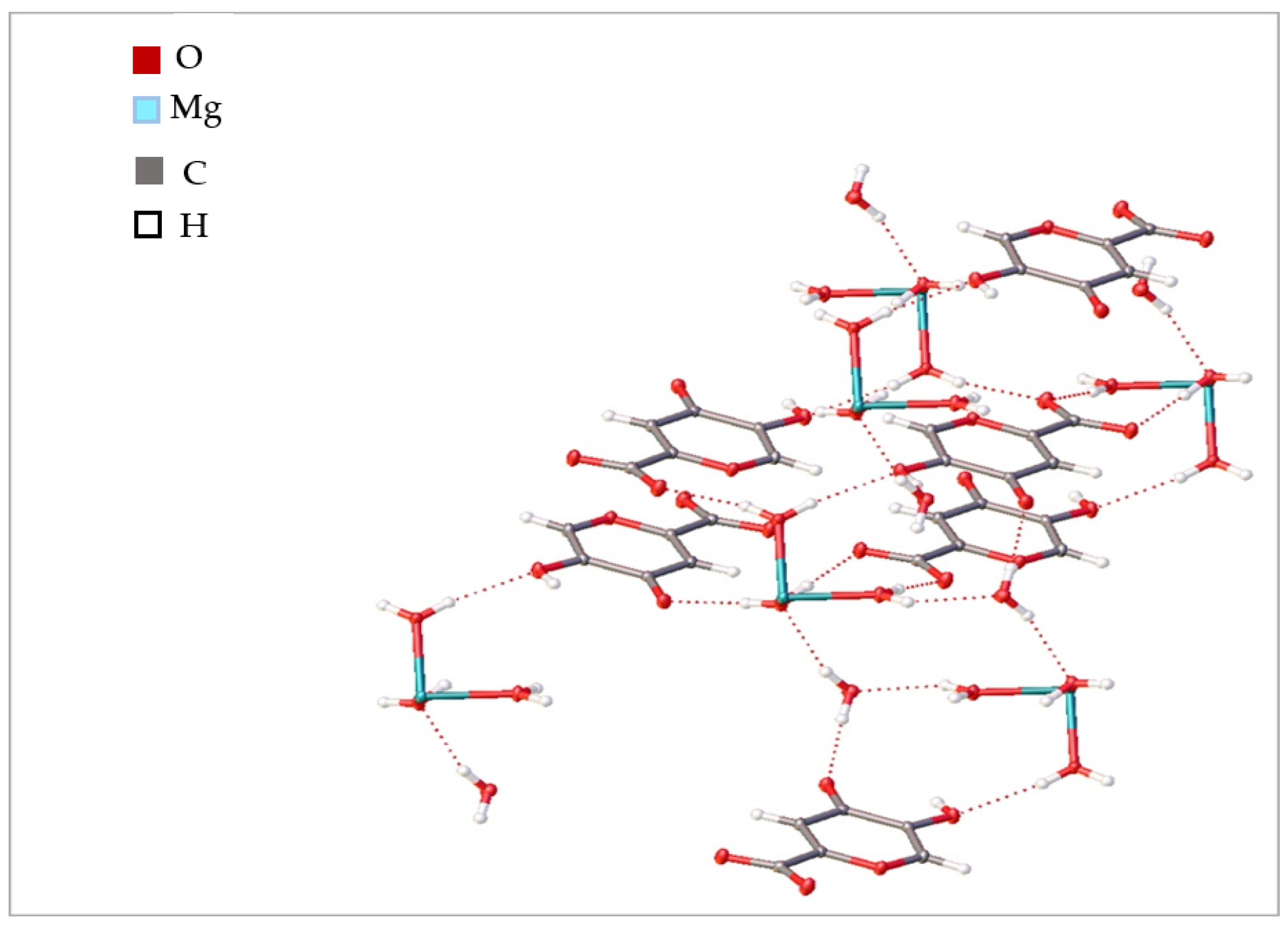
2.1.3. X-ray Diffraction Analysis
2.2. Biological Part
2.2.1. Antioxidant Activity in the “Citrate-Phosphate-Luminol” CPL Model System
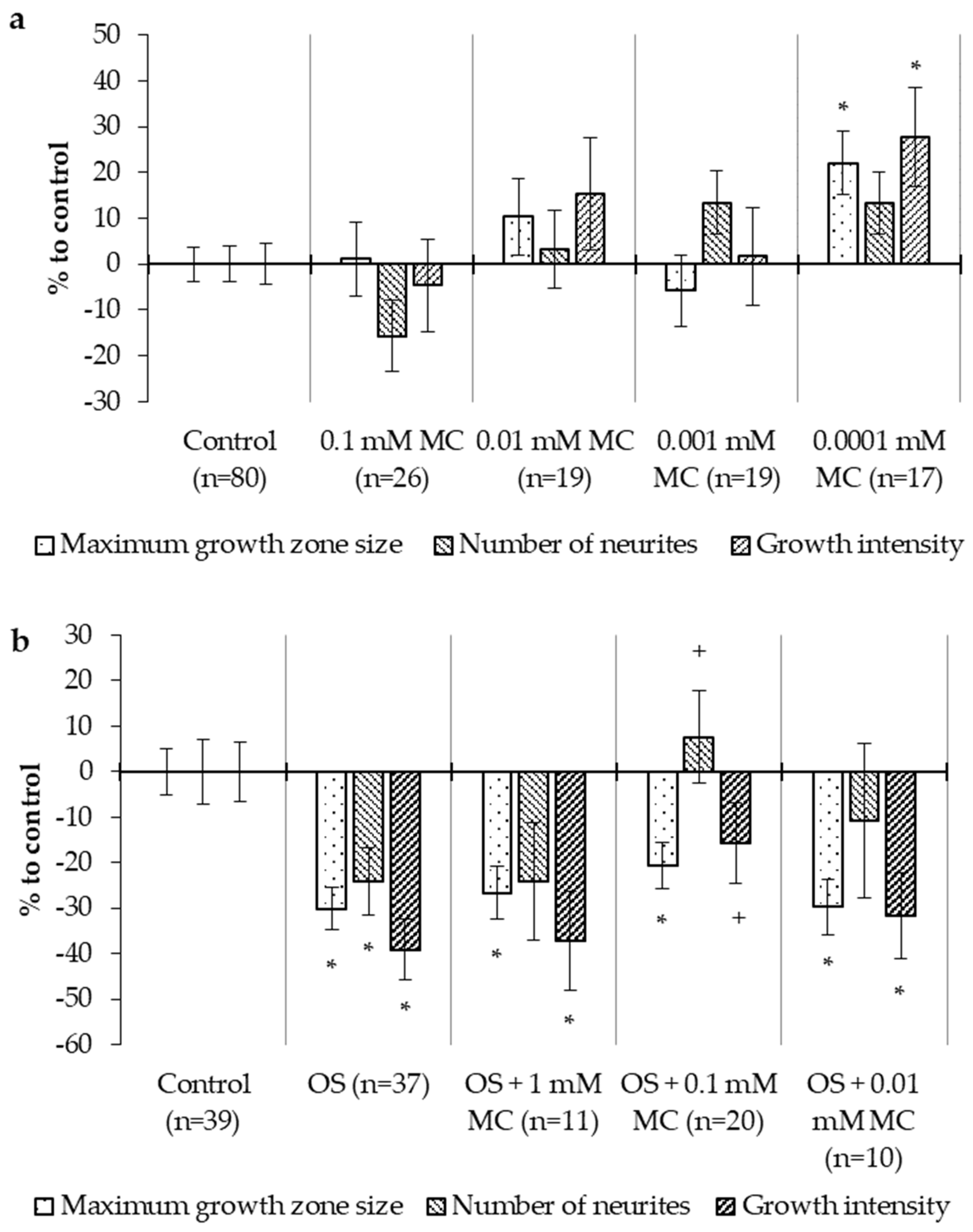
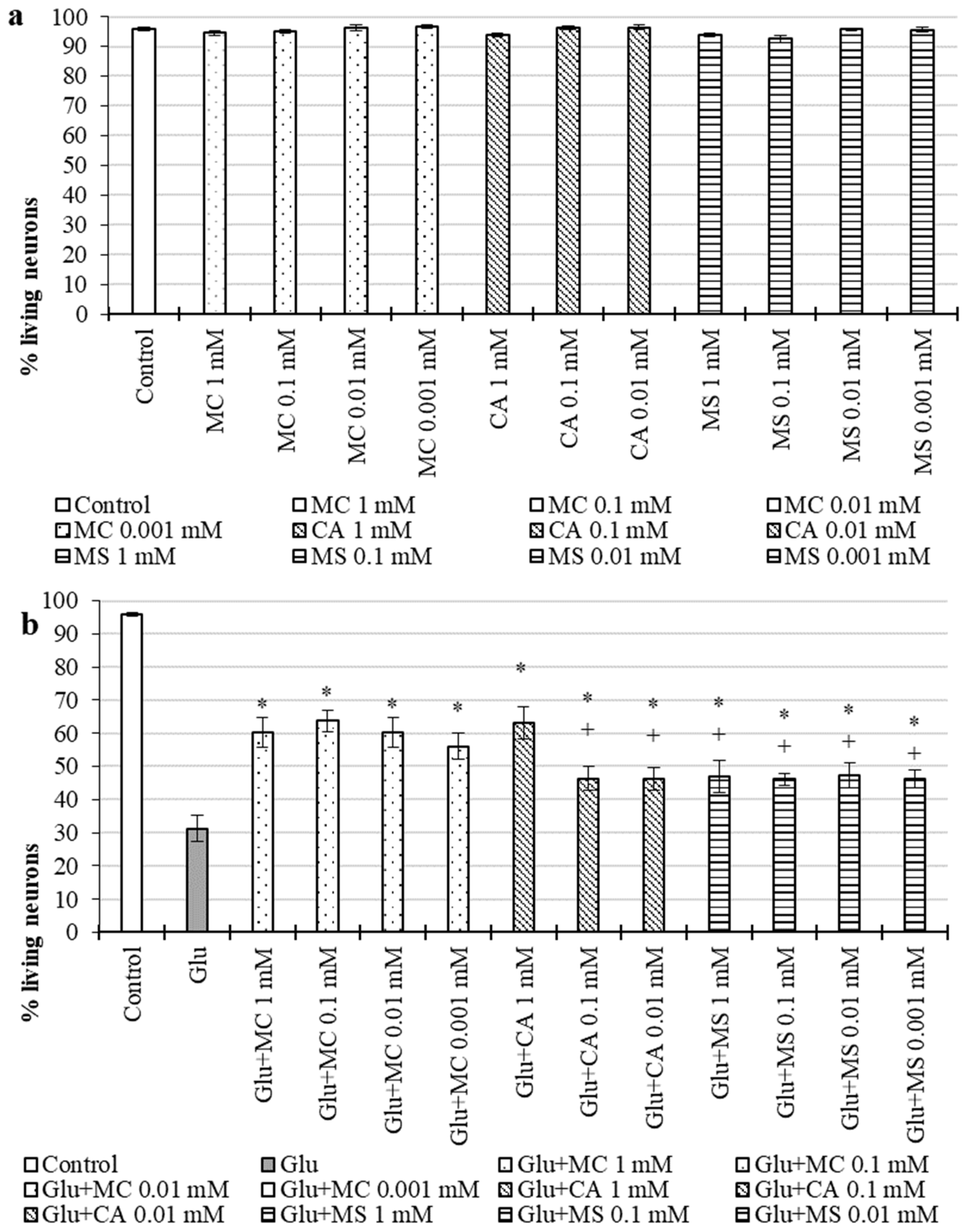
2.2.2. Neurotrophic Action in OS
2.2.3. Neuroprotective Action during Excitotoxic Exposure
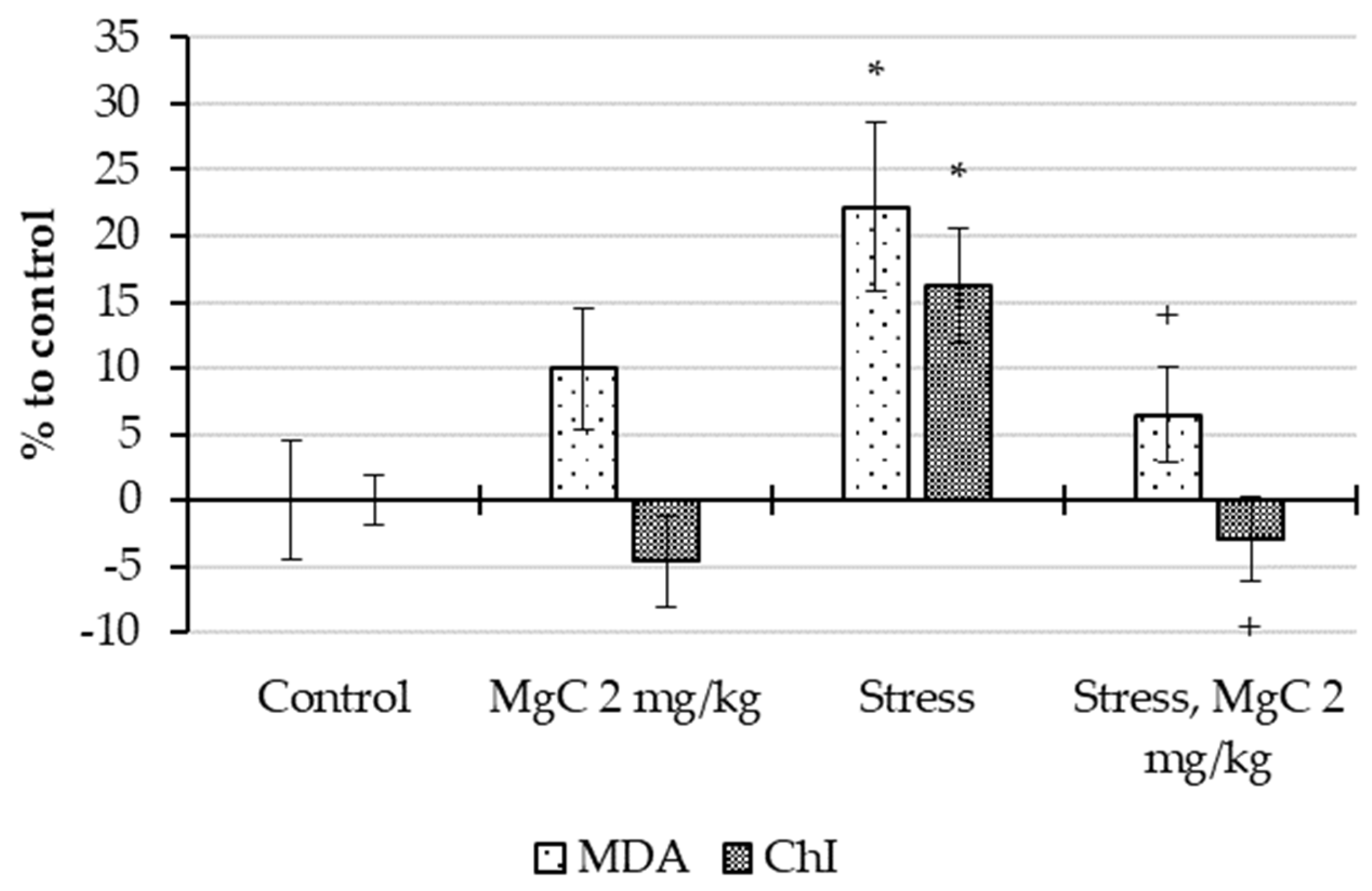
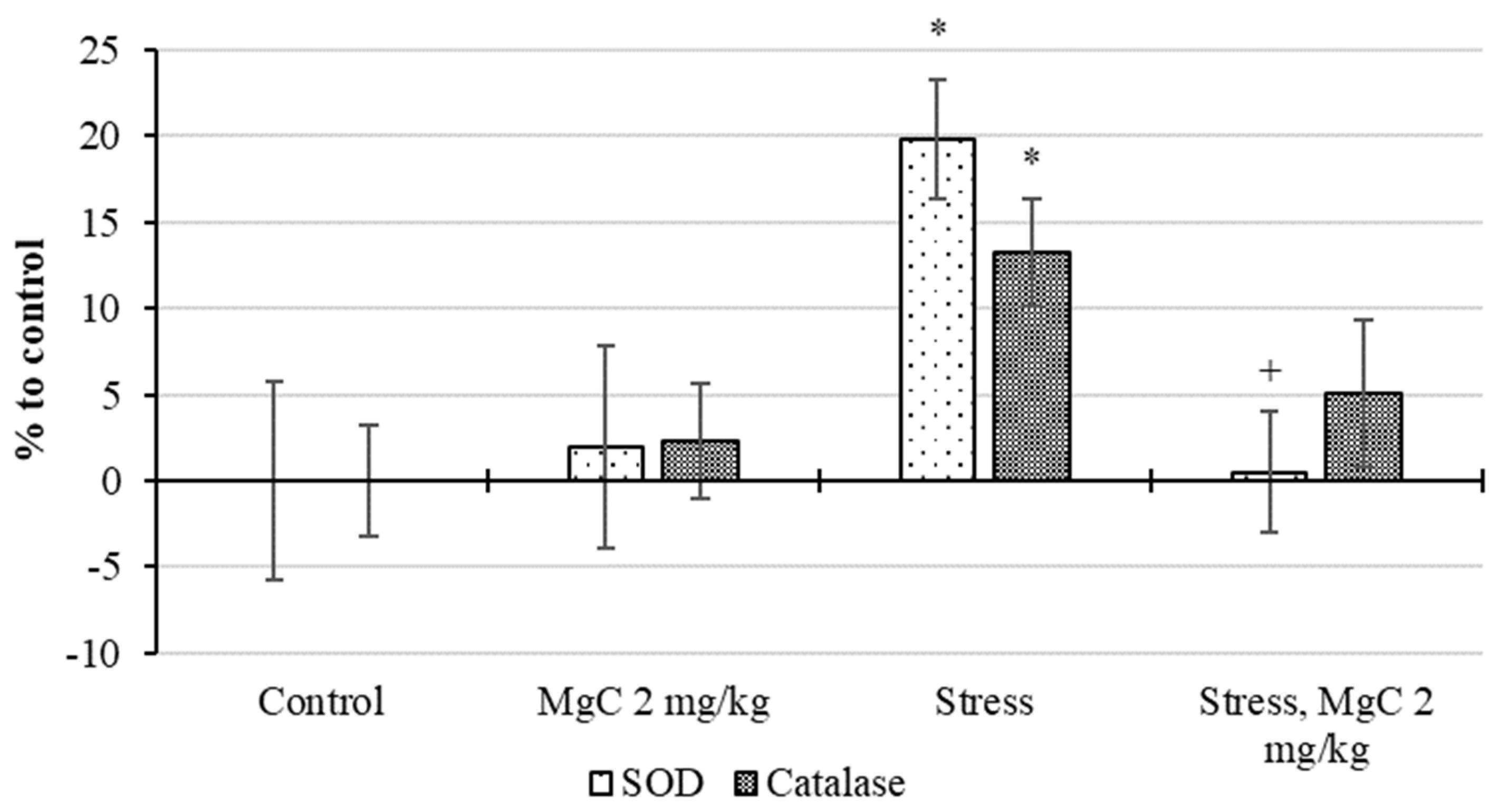
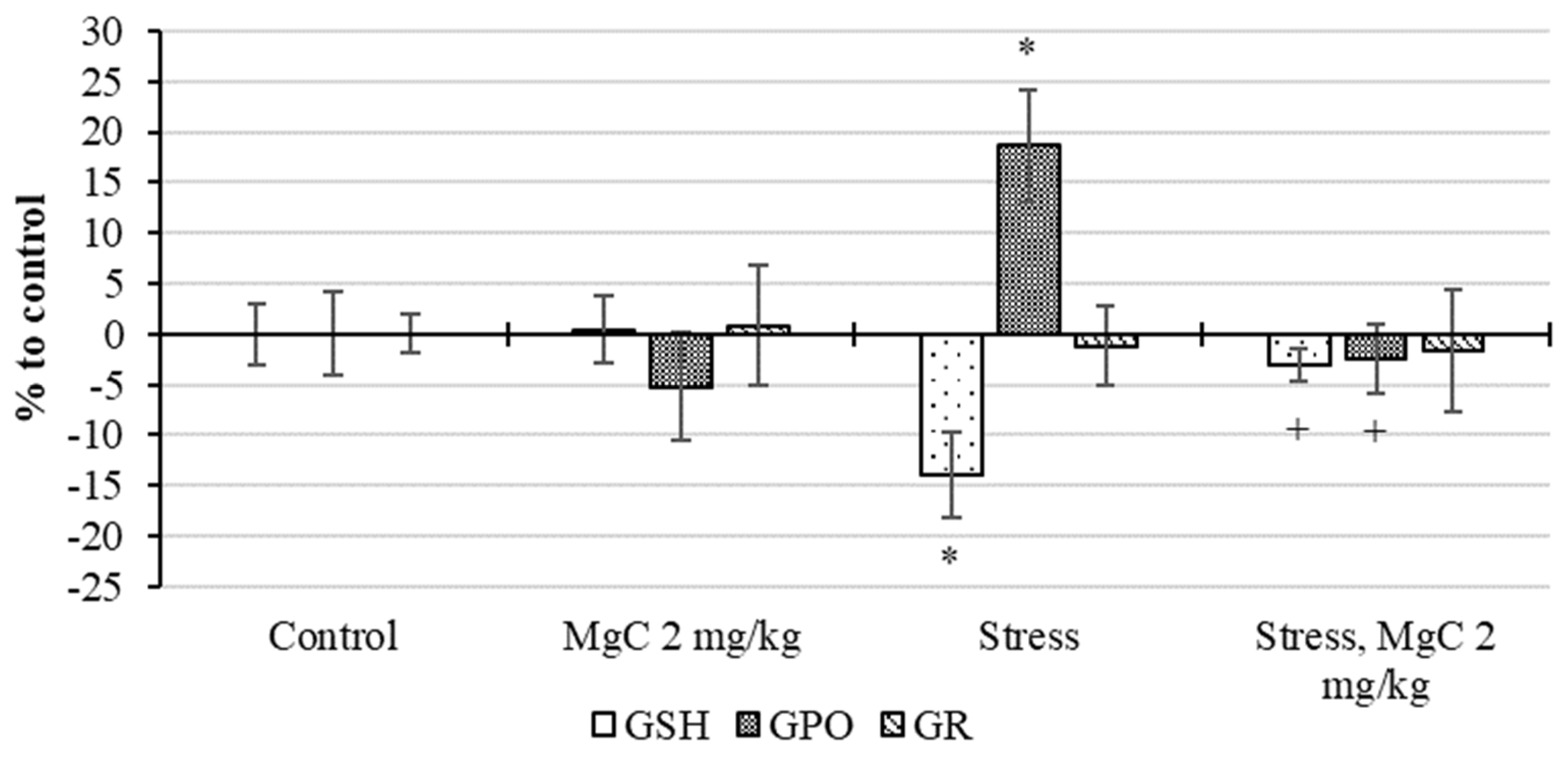
2.2.4. Stress Protective Action In Vivo
3. Discussion
4. Materials and Methods
4.1. Chemical Part
4.1.1. 5-Hydroxy-4-oxo-4H-pyran-2-carboxylic (comenoic) Acid
4.1.2. Magnesium 5-Hydroxy-4-oxo-4H-pyran-2-carboxylate (comenate)
4.2. Biological Part
4.2.1. Chemicals and Supplements
4.2.2. Obtaining Cultures of the Spinal Ganglia
4.2.3. Modeling Oxidative Stress
4.2.4. Accounting for Neurite Growth
- -
- The maximum value of the growth zone (MGZS), equal to the distance from the edge of the explant to the tip of the longest neurite;
- -
- The number of neurites (NN), which was determined by counting the number of processes in a segment of 200 μm at a distance of 250 μm from the edge of the ganglion;
- -
- Growth intensity (GI), defined as the product of MGZS and the density of neurites, which was determined by grading NN on a scale: less than 10 neurites—1; 10–30—2; 30–50—3; more than 50—4.
4.2.5. Obtaining Cultures of Cerebellar Neurons
4.2.6. Modeling of Excitotoxic Effects
4.2.7. Determining the Level of Neuron Death
4.2.8. Experiments on Laboratory Animals
- Control;
- Magnesium comenate;
- Stress;
- Stress + magnesium supplement.
4.2.9. Determination of the Pro-Oxidant and Antioxidant Status of the Brain
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garre-Olmo, J. Epidemiologia de la enfermedad de Alzheimer y otras demencias. Rev. Neurol. 2018, 66, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Hugo, J.; Ganguli, M. Dementia and cognitive impairment: Epidemiology, diagnosis, and treatment. Clin. Geriatr. Med. 2014, 30, 421–442. [Google Scholar] [CrossRef]
- Graham, N.S.; Sharp, D.J. Understanding neurodegeneration after traumatic brain injury: From mechanisms to clinical trials in dementia. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Kravtsov, A.; Kozin, S.; Basov, A.; Butina, E.; Baryshev, M.; Malyshko, V.; Moiseev, A.; Elkina, A.; Dzhimak, S. Reduction of deuterium level supports resistance of neurons to glucose deprivation and hypoxia: Study in cultures of neurons and on animals. Molecules 2022, 27, 243. [Google Scholar] [CrossRef]
- Kozin, S.; Skrebitsky, V.; Kondratenko, R.; Kravtsov, A.; Butina, E.; Moiseev, A.; Malyshko, V.; Baryshev, M.; Elkina, A.; Dzhimak, S. Electrophysiological Activity and Survival Rate of Rats Nervous Tissue Cells Depends on D/H Isotopic Composition of Medium. Molecules 2021, 26, 2036. [Google Scholar] [CrossRef]
- Morén, C.; de Souza, R.M.; Giraldo, D.M.; Uff, C. Antioxidant Therapeutic Strategies in Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 9328. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Zurlo, A.; Solmi, M.; Luchini, C.; Trevisan, C.; Bano, G.; Manzato, E.; Sergi, G.; Rylander, R. Magnesium Status in Alzheimer’s Disease: A Systematic Review. Am. J. Alzheimer’s Dis. Other Dement. 2016, 31, 208–213. [Google Scholar] [CrossRef]
- Maier, J.A.; Castiglioni, S.; Locatelli, L.; Zocchi, M.; Mazur, A. Magnesium and inflammation: Advances and perspectives. Semin. Cell Dev. Biol. 2021, 115, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, R.; Shindo, Y.; Oka, K. Magnesium Is a Key Player in Neuronal Maturation and Neuropathology. Int. J. Mol. Sci. 2019, 20, 3439. [Google Scholar] [CrossRef] [PubMed]
- Romeo, V.; Cazzaniga, A.; Maier, J.A.M. Magnesium and the blood-brain barrier in vitro: Effects on permeability and magnesium transport. Magnes. Res. 2019, 32, 16–24. [Google Scholar] [PubMed]
- Maier, J.A.M.; Locatelli, L.; Fedele, G.; Cazzaniga, A.; Mazur, A. Magnesium and the Brain: A Focus on Neuroinflammation and Neurodegeneration. Int. J. Mol. Sci. 2022, 24, 223. [Google Scholar] [CrossRef]
- Pickering, G.; Mazur, A.; Trousselard, M.; Bienkowski, P.; Yaltsewa, N.; Amessou, M.; Noah, L.; Pouteau, E. Magnesium Status and Stress: The Vicious Circle Concept Revisited. Nutrients 2020, 12, 3672. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.A.; Panonnummal, R. ‘Magnesium’-the master cation-as a drug-possibilities and evidences. Biometals 2021, 34, 955–986. [Google Scholar] [CrossRef]
- Xu, Z.P.; Li, L.; Bao, J.; Wang, Z.H.; Zeng, J.; Liu, E.J.; Li, X.G.; Huang, R.X.; Gao, D.; Li, M.Z.; et al. Magnesium protects cognitive functions and synaptic plasticity in streptozotocin-induced sporadic Alzheimer’s model. PLoS ONE 2014, 9, e108645. [Google Scholar] [CrossRef]
- Brookfield, K.F.; Vinson, A. Magnesium sulfate use for fetal neuroprotection. Curr. Opin. Obstet. Gynecol. 2019, 31, 110–115. [Google Scholar] [CrossRef]
- Ortiz, J.F.; Ruxmohan, S.; Saxena, A.; Morillo Cox, Á.; Bashir, F.; Tambo, W.; Ghani, M.R.; Moya, G.; Córdova, I. Minocycline and Magnesium As Neuroprotective Agents for Ischemic Stroke: A Systematic Review. Cureus 2020, 12, e12339. [Google Scholar] [CrossRef]
- Kravtsov, A.A.; Shurygin, A.Y.; Skorokhod, N.S.; Khaspekov, L.G. Neuroprotector effect of comenic acid against cytotoxic action of glutamate in vitro in cultured neurons of lead-poisoned rat pups. Bull. Exp. Biol. Med. 2011, 150, 436–439. [Google Scholar] [CrossRef]
- Kozin, S.V.; Kravtsov, A.A.; Kravchenko, S.V.; Ivashchenko, L.I. Cytoprotective and Antioxidant Effects of Meconic Acid in Model Systems. Bull. Exp. Biol. Med. 2021, 171, 619–622. [Google Scholar] [CrossRef]
- Shurygina, L.V.; Zlishcheva, E.I.; Khablyuk, V.V.; Kravtsova, A.N.; Abramova, N.O.; Zlishcheva, L.I.; Kravtsov, A.A. Comparative Analysis of Antioxidant Properties of Comenic Acid and Potassium Comenate in Modeled Immobilization Stress. Bull. Exp. Biol. Med. 2015, 159, 466–468. [Google Scholar] [CrossRef] [PubMed]
- Demina, N.I.; Zlishcheva, L.I.; Shurygin, A. Exposure to baliz-2 and some of its components on structural changes in the epidermis during healing of a full thickness ear wound in the rabbit. Dokl. Akad. Nauk. 1998, 363, 274–277. [Google Scholar] [PubMed]
- Chumasov, E.I.; Shurygin, A.I.; Soldatova, S.; Svetikova, K.M.; Drobyshevski, A.I. Nerve regeneration under the influence of baliz-2 and laktovit. Morfologiia 1993, 104, 25–33. [Google Scholar] [PubMed]
- Rogachevskii, I.V.; Plakhova, V.B.; Penniyaynen, V.A.; Terekhin, S.G.; Podzorova, S.A.; Krylov, B.V. New approaches to the design of analgesic medicinal substances. Can. J. Physiol. Pharmacol. 2022, 100, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Shurygina, L.V.; Zlishcheva, E.I.; Kravtsov, A.A. Neurotrophic Action of Comenic Acid and Its Derivatives Potassium Comenate and Calcium Comenate. Bull. Exp. Biol. Med. 2018, 165, 465–469. [Google Scholar] [CrossRef]
- Shurygina, L.V.; Zlishcheva, E.I.; Kravtsova, A.N.; Kravtsov, A.A. Antioxidant and Antiamnestic Effects of Potassium Comenate and Comenic Acid under Conditions of Normobaric Hypoxia with Hypercapnia. Bull. Exp. Biol. Med. 2017, 163, 344–348. [Google Scholar] [CrossRef]
- Kondratenko, R.V.; Chepkova, A.N.; Shurygin, A.Y.; Skrebitskii, V.G. Comenic acid prevents post-stress enhancement of long-term potentiation in rat hippocampus. Bull. Exp. Biol. Med. 2003, 136, 464–466. [Google Scholar] [CrossRef]
- Ismailova, G.M.; Iminova, I.M.; Tulaganov, A.A. Synthesis of Mg(II) Complexes with Nicotinic Acid and Nicodine. Pharm. Chem. J. 2002, 36, 327–328. [Google Scholar] [CrossRef]
- Case, D.R.; Zubieta, J.; Gonzalez, R.; Doyle, R.P. Synthesis and Chemical and Biological Evaluation of a Glycine Tripeptide Chelate of Magnesium. Molecules 2021, 26, 2419. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Feelisch, M.; Cortese-Krott, M.M.; Santolini, J.; Wootton, S.A.; Jackson, A.A. Systems redox biology in health and disease. EXCLI J. 2022, 21, 623–646. [Google Scholar] [PubMed]
- Basov, A.A.; Kozin, S.V.; Bikov, I.M.; Popov, K.A.; Moiseev, A.V.; Elkina, A.A.; Dzhimak, S.S. Changes in Prooxidant-Antioxidant System Indices in the Blood and Brain of Rats with Modelled Acute Hypoxia which Consumed a Deuterium-Depleted Drinking Diet. Biol. Bull. 2019, 46, 531–535. [Google Scholar] [CrossRef]
- Basov, A.; Fedulova, L.; Baryshev, M.; Dzhimak, S. Deuterium-depleted water influence on the isotope 2H/1H regulation in body and individual adaptation. Nutrients 2019, 11, 1903. [Google Scholar] [CrossRef] [PubMed]
- Tekutskaya, E.E.; Ryabova, I.S.; Kozin, S.V.; Popov, K.A.; Malyshko, V.V. Changes in Free Radical Processes under the Influence of Low-Frequency Electromagnetic Field in Rats. Bull. Exp. Biol. Med. 2022, 172, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Foyer, C.H. Intracellular Redox Compartmentation and ROS-Related Communication in Regulation and Signaling. Plant Physiol. 2016, 171, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Pan, C.; Feng, C.; Yan, C.; Yu, Y.; Chen, Z.; Guo, C.; Wang, X. Role of mitochondrial reactive oxygen species in homeostasis regulation. Redox Rep. 2022, 27, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Gevezova, M.; Sarafian, V.; Maes, M. Redox regulation of the immune response. Cell. Mol. Immunol. 2022, 19, 1079–1101. [Google Scholar] [CrossRef]
- Resende, R.; Fernandes, T.; Pereira, A.C.; Marques, A.P.; Pereira, C.F. Endoplasmic Reticulum-Mitochondria Contacts Modulate Reactive Oxygen Species-Mediated Signaling and Oxidative Stress in Brain Disorders: The Key Role of Sigma-1 Receptor. Antioxid. Redox Signal. 2022, 37, 758–780. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, G.; Zou, L.; Peana, M.; Chasapis, C.T.; Hangan, T.; Lu, J.; Maes, M. The Role of the Thioredoxin System in Brain Diseases. Antioxidants 2022, 11, 2161. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxid. Med. Cell. Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Henty-Ridilla, J.L. Multiple roles for the cytoskeleton in ALS. Exp. Neurol. 2022, 355, 114143. [Google Scholar] [CrossRef] [PubMed]
- Alberti, P.; Semperboni, S.; Cavaletti, G.; Scuteri, A. Neurons: The Interplay between Cytoskeleton, Ion Channels/Transporters and Mitochondria. Cells 2022, 11, 2499. [Google Scholar] [CrossRef] [PubMed]
- Oswald, M.C.W.; Garnham, N.; Sweeney, S.T.; Landgraf, M. Regulation of neuronal development and function by ROS. FEBS Lett. 2018, 592, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Allani, P.K.; Sum, T.; Bhansali, S.G.; Mukherjee, S.K.; Sonee, M. A comparative study of the effect of oxidative stress on the cytoskeleton in human cortical neurons. Toxicol. Appl. Pharmacol. 2004, 196, 29–36. [Google Scholar] [CrossRef]
- Chalimeswamy, A.; Yogananda Thanuja, M.; Ranganath, S.H.; Pandya, K.; Kompella, U.B.; Srinivas, S.P. Oxidative Stress Induces a Breakdown of the Cytoskeleton and Tight Junctions of the Corneal Endothelial Cells. J. Ocul. Pharmacol. Ther. 2022, 38, 74–84. [Google Scholar] [CrossRef]
- Landino, L.M.; Moynihan, K.L.; Todd, J.V.; Kennett, K.L. Modulation of the redox state of tubulin by the glutathione/glutaredoxin reductase system. Biochem. Biophys. Res. Commun. 2004, 314, 555–560. [Google Scholar] [CrossRef]
- Wilson, C.; González-Billault, C. Regulation of cytoskeletal dynamics by redox signaling and oxidative stress: Implications for neuronal development and trafficking. Front. Cell. Neurosci. 2015, 9, 381. [Google Scholar] [CrossRef]
- Lopatina, E.V.; Karetsky, A.V.; Krylov, B.V. Regenerative Anti-Inflammatory Agent and Methods of Treatment Using This Agent. RU Patent No. 2362554, 27 July 2009. Available online: https://patents.google.com/patent/RU2362554C2/ru?oq=2362554 (accessed on 20 November 2022).
- Boldyrev, A.; Bulygina, E.; Yuneva, M.; Schoner, W. Na/K-ATPase regulates intracellular ROS level in cerebellum neurons. Ann. N. Y. Acad. Sci. 2003, 986, 519–521. [Google Scholar] [CrossRef]
- Lewerenz, J.; Maher, P. Chronic Glutamate Toxicity in Neurodegenerative Diseases-What is the Evidence? Front. Neurosci. 2015, 9, 469. [Google Scholar] [CrossRef]
- Verma, M.; Lizama, B.N.; Chu, C.T. Excitotoxicity, calcium and mitochondria: A triad in synaptic neurodegeneration. Transl. Neurodegener. 2022, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Pregnolato, S.; Chakkarapani, E.; Isles, A.R.; Luyt, K. Glutamate Transport and Preterm Brain Injury. Front. Physiol. 2019, 10, 417. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, M.V.; Magazanik, L.G.; Tikhonov, D.B. Influence of external magnesium ions on the NMDA receptor channel block by different types of organic cations. Neuropharmacology 2012, 62, 2078–2085. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, N.A.; Boyer, T.M.; Burd, I. Fetal Neuroprotective Strategies: Therapeutic Agents and Their Underlying Synaptic Pathways. Front. Synaptic Neurosci. 2021, 13, 680899. [Google Scholar] [CrossRef] [PubMed]
- Bachnas, M.A.; Akbar, M.I.A.; Dachlan, E.G.; Dekker, G. The role of magnesium sulfate (MgSO4) in fetal neuroprotection. J. Matern. Fetal Neonatal Med. 2021, 34, 966–978. [Google Scholar] [CrossRef]
- Shurygin, A.Y.; Shurygina, L.V.; Lobova, N.N. Method for Producing Comenic Acid. RU Patent No. 2459623C1, 27 August 2012. Available online: https://patents.google.com/patent/RU2459623C1/ru (accessed on 15 October 2022).
- Shurygin, A.Y. The Bacterial Strain Gluconobacter Oxydans-03 Is a Balise Producer and a Method for Producing Balise. RU Patent No. 2287583 C1, 20 November 2006. Available online: https://patents.google.com/patent/RU2287583C1/ru?oq=2287583 (accessed on 15 October 2022).
- Kravtsov, A.A.; Shurygin, A.Y.; Shurygina, L.V.; Zlishcheva, L.I.; Abramova, N.O.; Khaspekov, L.G. Prenatal action of lead acetate on the antioxidant glutathione system of the brain of newborn rats in vivo and on neurite growth in vitro. Neurochem. J. 2009, 3, 196–201. [Google Scholar] [CrossRef]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Kozin, S.V.; Kravtsov, A.A.; Elkina, A.A.; Zlishcheva, E.I.; Shurygina, L.V.; Baryshev, M.G.; Barysheva, E.V.; Moiseev, A.V. Isotope Exchange of Deuterium for Protium in Rat Brain Tissues Changes Brain Tolerance to Hypoxia. Biophysics 2019, 64, 272–278. [Google Scholar] [CrossRef]
- Kravtsov, A.A.; Kozin, S.V.; Elkina, A.A.; Shashkov, D.I.; Baryshev, M.G.; Vasilevskaya, E.R.; Fedulova, L.V.; Popov, K.A.; Malyshko, V.V.; Moiseev, A.V. Effect of drinking ration with reduced deuterium content on brain tissue prooxidant-antioxidant balance in rats with acute hypoxia model. J. Pharm. Nutr. Sci. 2018, 8, 42–51. [Google Scholar] [CrossRef]
- Kozin, S.V.; Kravtsov, A.A.; Kravchenko, S.V.; Ivashchenko, L.I. Antioxidant and anxiolytic effect of Bifidobacterium adolescentis and Lactobacillus acidophilus under conditions of normobaric hypoxia with hypercapnia. Vopr. Pitan 2021, 90, 63–72. [Google Scholar] [CrossRef]
- Shurygina, L.V.; Kravtsov, A.A.; Zlishcheva, E.I.; Khaspekov, L.G. The in vitro and in vivo neuroprotective activity of sodium comenate in stress. Neurochem. J. 2017, 11, 250–254. [Google Scholar] [CrossRef]


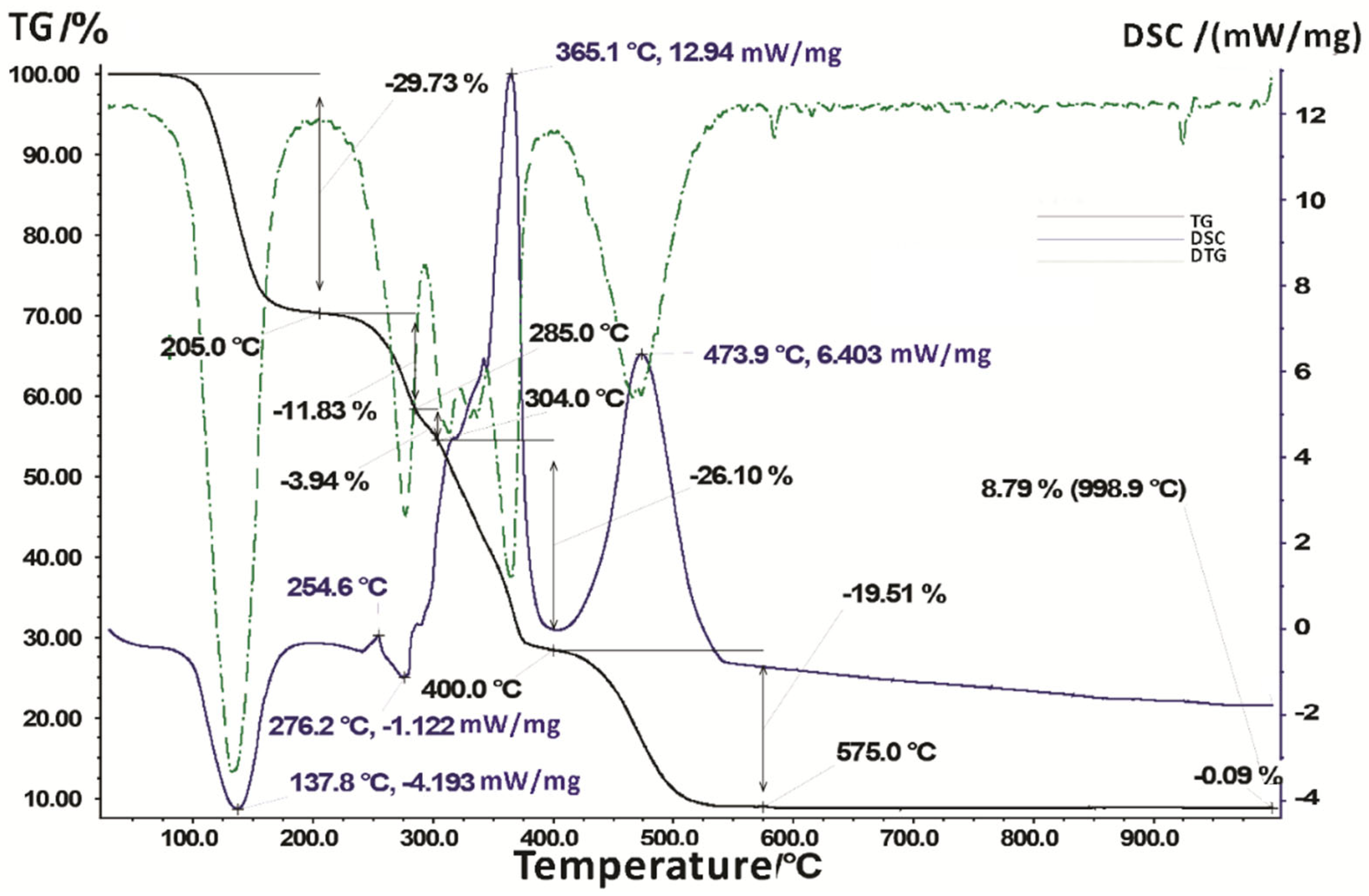

| Temperature Range, °C | Thermal Effect, °C | Effect Type | Mass Loss, % | The Amount Consumed/ Released Energy, mW/mg | Processes that Occur in a Substance When Heated |
|---|---|---|---|---|---|
| 25–205 | 137.8 | endo | 29.73 | −4.193 | Dehydration and the beginning of the ligand decomposition process |
| 205–285 | 254.6 | exo | 11.83 | – | Removal of hydroxyl and carboxyl groups of the ligand |
| 285–304 | 276.2 | endo | 3.94 | −1.122 | |
| 304–400 | 365.1 | exo | 26.10 | +12.94 | Continued decomposition of two ligand molecules and combustion of decomposition products |
| 400–575 | 473.9 | exo | 19.51 | +6.403 | |
| 575–998.9 | – | – | 0.09 | – | End of ligand decomposition and combustion of decay products |
| Residual mass—8.79% (998.9 °C) | |||||
| Experimentally Calculated Mass Fraction, % | Estimated Mass Fraction, % | |||
|---|---|---|---|---|
| Thermogravimetry | XRF Elemental Analysis | Complexometric Titration | ||
| Mg | 5.30 ± 0.05 | 5.14 ± 0.02 | 5.25 ± 0.03 | 5.08 |
| HCom− | 61.4 ± 0.6 | 63.52 ± 0.25 | – | 64.81 |
| H2O | 29.7 ± 0.3 | – | – | 30.11 |
| Substance | Substance Concentration, mg/mL | |
|---|---|---|
| 0.01 | 0.1 | |
| Magnesium comenate | 30.67 ± 1.20 * | 67.03 ± 0.51 *# |
| Comenic acid | 33.29 ± 1.36 * | 69.39 ± 1.16 *# |
| C | H | O | Mg | |
|---|---|---|---|---|
| Found, % | 28.11 | 4.45 | 57.81 | 5.14 |
| Calculated, % | 30.11 | 4.63 | 60.17 | 5.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozin, S.; Kravtsov, A.; Ivashchenko, L.; Dotsenko, V.; Vasilyeva, L.; Vasilyev, A.; Tekutskaya, E.; Aksenov, N.; Baryshev, M.; Dorohova, A.; et al. Study of the Magnesium Comenate Structure, Its Neuroprotective and Stress-Protective Activity. Int. J. Mol. Sci. 2023, 24, 8046. https://doi.org/10.3390/ijms24098046
Kozin S, Kravtsov A, Ivashchenko L, Dotsenko V, Vasilyeva L, Vasilyev A, Tekutskaya E, Aksenov N, Baryshev M, Dorohova A, et al. Study of the Magnesium Comenate Structure, Its Neuroprotective and Stress-Protective Activity. International Journal of Molecular Sciences. 2023; 24(9):8046. https://doi.org/10.3390/ijms24098046
Chicago/Turabian StyleKozin, Stanislav, Alexandr Kravtsov, Lev Ivashchenko, Victor Dotsenko, Lada Vasilyeva, Alexander Vasilyev, Elena Tekutskaya, Nicolai Aksenov, Mikhail Baryshev, Anna Dorohova, and et al. 2023. "Study of the Magnesium Comenate Structure, Its Neuroprotective and Stress-Protective Activity" International Journal of Molecular Sciences 24, no. 9: 8046. https://doi.org/10.3390/ijms24098046
APA StyleKozin, S., Kravtsov, A., Ivashchenko, L., Dotsenko, V., Vasilyeva, L., Vasilyev, A., Tekutskaya, E., Aksenov, N., Baryshev, M., Dorohova, A., Fedulova, L., & Dzhimak, S. (2023). Study of the Magnesium Comenate Structure, Its Neuroprotective and Stress-Protective Activity. International Journal of Molecular Sciences, 24(9), 8046. https://doi.org/10.3390/ijms24098046







