Metabolic Disturbance of High-Saturated Fatty Acid Diet in Cognitive Preservation
Abstract
1. Introduction
2. Results
2.1. Body Weight, Triglycerides Levels and Liver Weight
2.2. Glucose Levels and Pancreatic Mass Weight
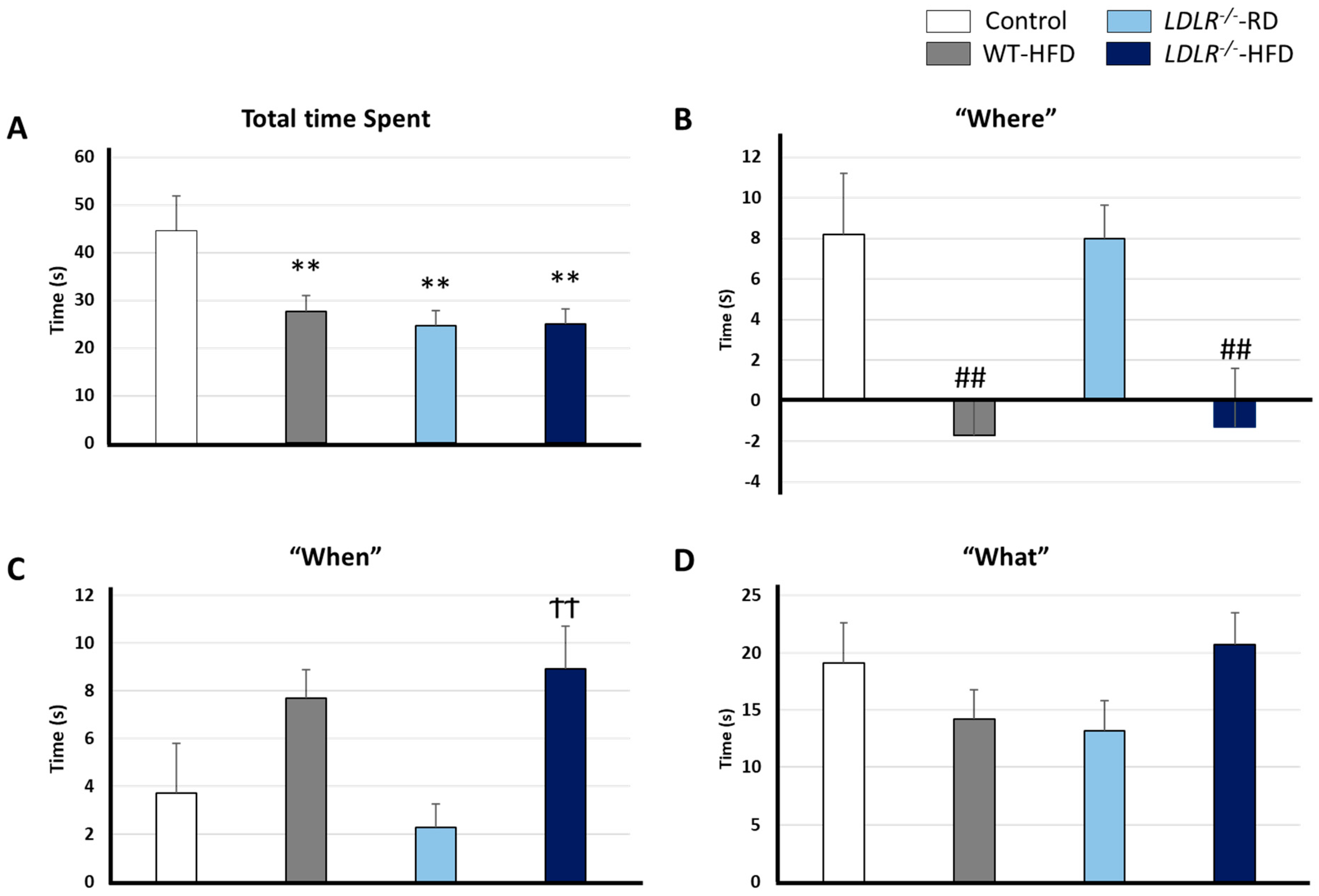
2.3. Cognitive Task: NOD Test
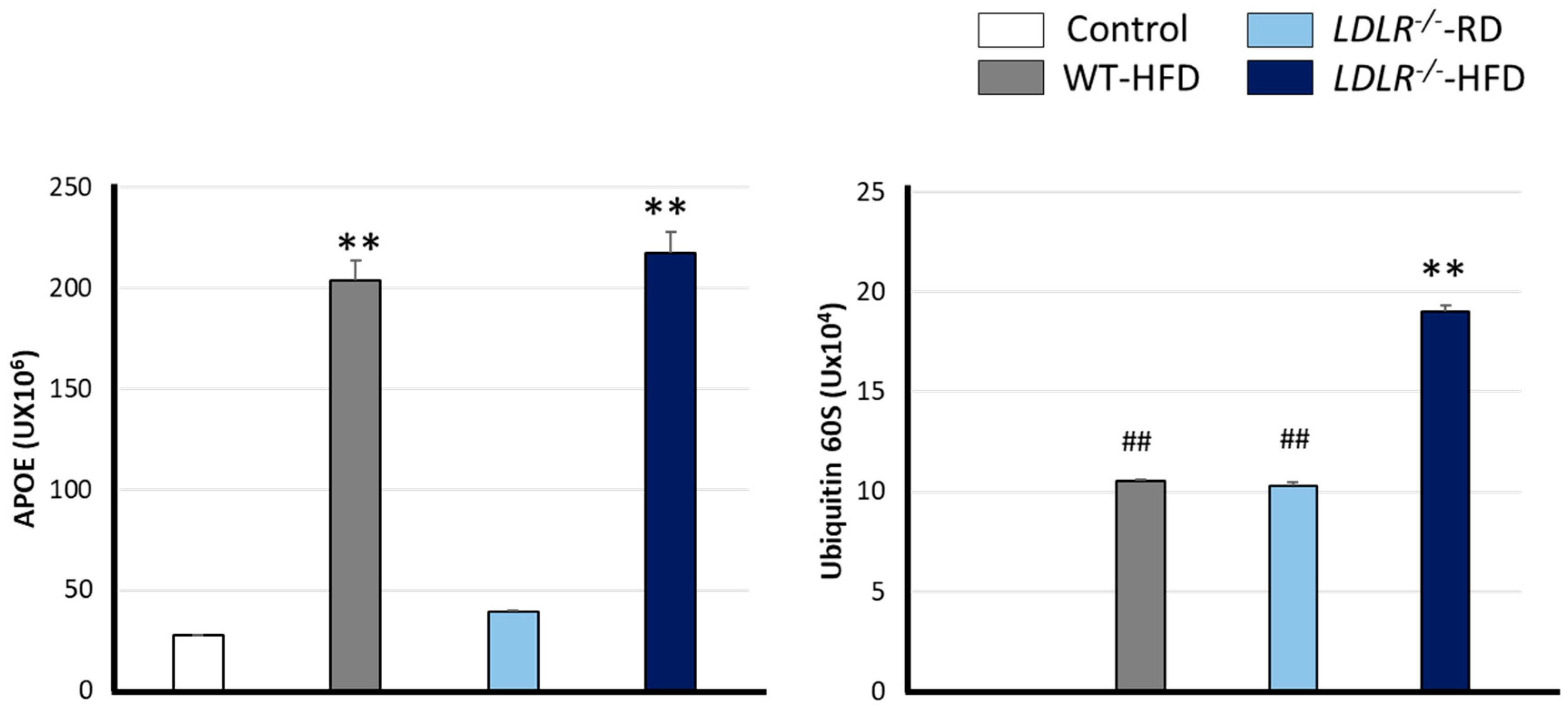
2.4. Plasmatic Levels of APOE and Ubiquitin 60S
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Metabolic Determinations
4.3. Locomotor Activity and Cognitive Test
4.4. Tissue Processing
4.5. Triglycerides Measurements
4.6. APOE and Ubiquitin 60S
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fontbonne, A.; Currie, A.; Tounian, P.; Picot, M.C.; Foulatier, O.; Nedelcu, M.; Nocca, D. Prevalence of Overweight and Obesity in France: The 2020 Obepi-Roche Study by the “Ligue Contre l’Obesite”. J. Clin. Med. 2023, 12, 925. [Google Scholar] [CrossRef] [PubMed]
- Lasarte-Velillas, J.J.; Lamiquiz-Moneo, I.; Lasarte-Sanz, I.; Sala-Fernandez, L.; Marin-Andres, M.; Rubio-Sanchez, P.; Moneo-Hernandez, M.I.; Hernandez-Aguilar, M.T. Prevalence of overweight and obesity in Aragon and variations according to health determinants. An. Pediatr. 2023, 98, 157–164. [Google Scholar] [CrossRef]
- Schramm, S.; Sorensen, T.I.A.; Davidsen, M.; Tolstrup, J.S. Changes in adult obesity prevalence in Denmark, 1987–2021: Age-period-cohort analysis of nationally representative data. Eur. J. Public Health 2023, ckad024. [Google Scholar] [CrossRef] [PubMed]
- Apovian, C.M. Obesity: Definition, comorbidities, causes, and burden. Am. J. Manag. Care 2016, 22, s176–s185. [Google Scholar]
- Bray, G.A.; Kim, K.K.; Wilding, J.P.H. Obesity: A chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2017, 18, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Xu, S.; Huang, L.; Chen, C. Obesity and insulin resistance: Pathophysiology and treatment. Drug Discov. Today 2022, 27, 822–830. [Google Scholar] [CrossRef]
- de Oliveira, F.F.; Bertolucci, P.H.; Chen, E.S.; Smith, M.C. Risk factors for age at onset of dementia due to Alzheimer’s disease in a sample of patients with low mean schooling from Sao Paulo, Brazil. Int. J. Geriatr. Psychiatry 2014, 29, 1033–1039. [Google Scholar] [CrossRef]
- de Oliveira, F.F.; Pivi, G.A.; Chen, E.S.; Smith, M.C.; Bertolucci, P.H. Risk factors for cognitive and functional change in one year in patients with Alzheimer’s disease dementia from Sao Paulo, Brazil. J. Neurol. Sci. 2015, 359, 127–132. [Google Scholar] [CrossRef]
- Pardo-Moreno, T.; Mohamed-Mohamed, H.; Rivas-Dominguez, A.; Garcia-Morales, V.; Garcia-Lara, R.A.; Suleiman-Martos, S.; Bermudez-Pulgarin, B.; Ramos-Rodriguez, J.J. Poor Cognitive Agility Conservation in Obese Aging People. Biomedicines 2023, 11, 138. [Google Scholar] [CrossRef]
- Guillemot-Legris, O.; Muccioli, G.G. Obesity-Induced Neuroinflammation: Beyond the Hypothalamus. Trends Neurosci. 2017, 40, 237–253. [Google Scholar] [CrossRef]
- O’Brien, P.D.; Hinder, L.M.; Callaghan, B.C.; Feldman, E.L. Neurological consequences of obesity. Lancet. Neurol. 2017, 16, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Amen, D.G.; Wu, J.; George, N.; Newberg, A. Patterns of Regional Cerebral Blood Flow as a Function of Obesity in Adults. J. Alzheimer’s Dis. JAD 2020, 77, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Woo, A.; Botta, A.; Shi, S.S.W.; Paus, T.; Pausova, Z. Obesity-Related Neuroinflammation: Magnetic Resonance and Microscopy Imaging of the Brain. Int. J. Mol. Sci. 2022, 23, 8790. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, Y.J. Effect of Obesity on Cognitive Impairment in Vascular Dementia Rat Model via BDNF-ERK-CREB Pathway. Biol. Res. Nurs. 2021, 23, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.L.; Zhao, S.P.; Hu, J.R. Cholesterol imbalance in adipocytes: A possible mechanism of adipocytes dysfunction in obesity. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2010, 11, 560–567. [Google Scholar] [CrossRef]
- Petek, B.; Villa-Lopez, M.; Loera-Valencia, R.; Gerenu, G.; Winblad, B.; Kramberger, M.G.; Ismail, M.A.; Eriksdotter, M.; Garcia-Ptacek, S. Connecting the brain cholesterol and renin-angiotensin systems: Potential role of statins and RAS-modifying medications in dementia. J. Intern. Med. 2018, 284, 620–642. [Google Scholar] [CrossRef]
- Wang, D.; Zheng, W. Dietary cholesterol concentration affects synaptic plasticity and dendrite spine morphology of rabbit hippocampal neurons. Brain Res. 2015, 1622, 350–360. [Google Scholar] [CrossRef]
- Querfurth, H.W.; LaFerla, F.M. Alzheimer’s disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.F.; Chen, E.S.; Smith, M.C.; Bertolucci, P.H. Associations of cerebrovascular metabolism genotypes with neuropsychiatric symptoms and age at onset of Alzheimer’s disease dementia. Rev. Bras. Psiquiatr. 2017, 39, 95–103. [Google Scholar] [CrossRef]
- Defesche, J.C.; Gidding, S.S.; Harada-Shiba, M.; Hegele, R.A.; Santos, R.D.; Wierzbicki, A.S. Familial hypercholesterolaemia. Nat. Rev. Dis. Prim. 2017, 3, 17093. [Google Scholar] [CrossRef]
- Evangelho, J.S.; Casali, K.R.; Campos, C.; De Angelis, K.; Veiga, A.B.; Rigatto, K. Hypercholesterolemia magnitude increases sympathetic modulation and coagulation in LDLr knockout mice. Auton. Neurosci. Basic Clin. 2011, 159, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Saher, G.; Stumpf, S.K. Cholesterol in myelin biogenesis and hypomyelinating disorders. Biochim. Biophys. Acta 2015, 1851, 1083–1094. [Google Scholar] [CrossRef]
- Mulder, M.; Koopmans, G.; Wassink, G.; Al Mansouri, G.; Simard, M.L.; Havekes, L.M.; Prickaerts, J.; Blokland, A. LDL receptor deficiency results in decreased cell proliferation and presynaptic bouton density in the murine hippocampus. Neurosci. Res. 2007, 59, 251–256. [Google Scholar] [CrossRef]
- Paciullo, F.; Fallarino, F.; Bianconi, V.; Mannarino, M.R.; Sahebkar, A.; Pirro, M. PCSK9 at the crossroad of cholesterol metabolism and immune function during infections. J. Cell. Physiol. 2017, 232, 2330–2338. [Google Scholar] [CrossRef] [PubMed]
- Katsouri, L.; Georgopoulos, S. Lack of LDL receptor enhances amyloid deposition and decreases glial response in an Alzheimer’s disease mouse model. PLoS ONE 2011, 6, e21880. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, P.; Hajnal, A. Short-term high-fat diet consumption increases body weight and body adiposity and alters brain stem taste information processing in rats. Chem. Senses 2022, 47, bjac020. [Google Scholar] [CrossRef] [PubMed]
- Bieghs, V.; Verheyen, F.; van Gorp, P.J.; Hendrikx, T.; Wouters, K.; Lutjohann, D.; Gijbels, M.J.; Febbraio, M.; Binder, C.J.; Hofker, M.H.; et al. Internalization of modified lipids by CD36 and SR-A leads to hepatic inflammation and lysosomal cholesterol storage in Kupffer cells. PLoS ONE 2012, 7, e34378. [Google Scholar] [CrossRef]
- Subramanian, S.; Goodspeed, L.; Wang, S.; Kim, J.; Zeng, L.; Ioannou, G.N.; Haigh, W.G.; Yeh, M.M.; Kowdley, K.V.; O’Brien, K.D.; et al. Dietary cholesterol exacerbates hepatic steatosis and inflammation in obese LDL receptor-deficient mice. J. Lipid Res. 2011, 52, 1626–1635. [Google Scholar] [CrossRef]
- Gobato, A.O.; Vasques, A.C.; Zambon, M.P.; Barros Filho Ade, A.; Hessel, G. Metabolic syndrome and insulin resistance in obese adolescents. Rev. Paul. Pediatr. Orgao Of. Da Soc. Pediatr. Sao Paulo 2014, 32, 55–62. [Google Scholar] [CrossRef]
- Deusdará, R.; de Moura Souza, A.; Szklo, M. Association between Obesity, Overweight, Elevated Waist Circumference, and Insulin Resistance Markers among Brazilian Adolescent Students. Nutrients 2022, 14, 3487. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Rodriguez, J.J.; Ortiz, O.; Jimenez-Palomares, M.; Kay, K.R.; Berrocoso, E.; Murillo-Carretero, M.I.; Perdomo, G.; Spires-Jones, T.; Cozar-Castellano, I.; Lechuga-Sancho, A.M.; et al. Differential central pathology and cognitive impairment in pre-diabetic and diabetic mice. Psychoneuroendocrinology 2013, 38, 2462–2475. [Google Scholar] [CrossRef] [PubMed]
- Nagy, C.; Einwallner, E. Study of In Vivo Glucose Metabolism in High-fat Diet-fed Mice Using Oral Glucose Tolerance Test (OGTT) and Insulin Tolerance Test (ITT). J. Vis. Exp. JoVE 2018, 131, e56672. [Google Scholar] [CrossRef]
- Dere, E.; Huston, J.P.; De Souza Silva, M.A. Episodic-like memory in mice: Simultaneous assessment of object, place and temporal order memory. Brain Res. Brain Res. Protoc. 2005, 16, 10–19. [Google Scholar] [CrossRef]
- Yoshizaki, K.; Asai, M.; Hara, T. High-Fat Diet Enhances Working Memory in the Y-Maze Test in Male C57BL/6J Mice with Less Anxiety in the Elevated Plus Maze Test. Nutrients 2020, 12, 2036. [Google Scholar] [CrossRef]
- de Mendonça, A.; Felgueiras, H.; Verdelho, A.; Câmara, S.; Grilo, C.; Maroco, J.; Pereira, A.; Guerreiro, M. Memory complaints in amnestic Mild Cognitive Impairment: More prospective or retrospective? Int. J. Geriatr. Psychiatry 2018, 33, 1011–1018. [Google Scholar] [CrossRef]
- Matthews, B.R. Memory dysfunction. Contin. (Minneap. Minn.) 2015, 21, 613–626. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Das, S.; Hyman, B.T. APOE and Alzheimer’s disease: Advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 2021, 20, 68–80. [Google Scholar] [CrossRef]
- Fang, E.F.; Hou, Y.; Palikaras, K.; Adriaanse, B.A.; Kerr, J.S.; Yang, B.; Lautrup, S.; Hasan-Olive, M.M.; Caponio, D.; Dan, X.; et al. Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat. Neurosci. 2019, 22, 401–412. [Google Scholar] [CrossRef]
- Peters, R.; Xu, Y.; Antikainen, R.; Beckett, N.; Gussekloo, J.; Jagger, C.; Jukema, J.W.; Keinanen-Kiukaanniemi, S.; Rydén, L.; Skoog, I.; et al. Evaluation of High Cholesterol and Risk of Dementia and Cognitive Decline in Older Adults Using Individual Patient Meta-Analysis. Dement. Geriatr. Cogn. Disord. 2021, 50, 318–325. [Google Scholar] [CrossRef]
- Hosseini, M.; Poljak, A.; Braidy, N.; Crawford, J.; Sachdev, P. Blood fatty acids in Alzheimer’s disease and mild cognitive impairment: A meta-analysis and systematic review. Ageing Res. Rev. 2020, 60, 101043. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, S.C.; Schneider, J.A.; Tangney, C.; Tremblay-Mercier, J.; Fortier, M.; Bennett, D.A.; Morris, M.C. Plasma and brain fatty acid profiles in mild cognitive impairment and Alzheimer’s disease. J. Alzheimer’s Dis. JAD 2012, 29, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Rodriguez, J.J.; Ortiz-Barajas, O.; Gamero-Carrasco, C.; de la Rosa, P.R.; Infante-Garcia, C.; Zopeque-Garcia, N.; Lechuga-Sancho, A.M.; Garcia-Alloza, M. Prediabetes-induced vascular alterations exacerbate central pathology in APPswe/PS1dE9 mice. Psychoneuroendocrinology 2014, 48, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Thomas, M.J.; Wu, D.; Carman, C.V.; Ordovas, J.M.; Meydani, M. Edible Mushrooms Reduce Atherosclerosis in Ldlr−/− Mice Fed a High-Fat Diet. J. Nutr. 2019, 149, 1377–1384. [Google Scholar] [CrossRef]
- Ko, J.; Skudder-Hill, L.; Tarrant, C.; Kimita, W.; Bharmal, S.H.; Petrov, M.S. Intra-pancreatic fat deposition as a modifier of the relationship between habitual dietary fat intake and insulin resistance. Clin. Nutr. 2021, 40, 4730–4737. [Google Scholar] [CrossRef] [PubMed]
- Gierach, M.; Junik, R. Insulin resistance in metabolic syndrome depending on the occurrence of its components. Endokrynol. Pol. 2021, 72, 243–248. [Google Scholar] [CrossRef]
- Jiménez-Palomares, M.; Ramos-Rodríguez, J.J.; López-Acosta, J.F.; Pacheco-Herrero, M.; Lechuga-Sancho, A.M.; Perdomo, G.; García-Alloza, M.; Cózar-Castellano, I. Increased Aβ production prompts the onset of glucose intolerance and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1373–E1380. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Urbina, A.; Benitez, S.; Perez, A.; Sanchez-Quesada, J.L. Modified low-density lipoproteins as biomarkers in diabetes and metabolic syndrome. Front. Biosci. (Landmark Ed.) 2018, 23, 1220–1240. [Google Scholar] [CrossRef]
- Smith, G.I.; Shankaran, M.; Yoshino, M.; Schweitzer, G.G.; Chondronikola, M.; Beals, J.W.; Okunade, A.L.; Patterson, B.W.; Nyangau, E.; Field, T.; et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J. Clin. Investig. 2020, 130, 1453–1460. [Google Scholar] [CrossRef]
- Ono, H.; Shimano, H.; Katagiri, H.; Yahagi, N.; Sakoda, H.; Onishi, Y.; Anai, M.; Ogihara, T.; Fujishiro, M.; Viana, A.Y.; et al. Hepatic Akt activation induces marked hypoglycemia, hepatomegaly, and hypertriglyceridemia with sterol regulatory element binding protein involvement. Diabetes 2003, 52, 2905–2913. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef]
- Cignarelli, A.; Genchi, V.A.; Perrini, S.; Natalicchio, A.; Laviola, L.; Giorgino, F. Insulin and Insulin Receptors in Adipose Tissue Development. Int. J. Mol. Sci. 2019, 20, 759. [Google Scholar] [CrossRef]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose tissue and insulin resistance in obese. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef] [PubMed]
- Lisi, S.; Gamucci, O.; Vottari, T.; Scabia, G.; Funicello, M.; Marchi, M.; Galli, G.; Arisi, I.; Brandi, R.; D’Onofrio, M.; et al. Obesity-associated hepatosteatosis and impairment of glucose homeostasis are attenuated by haptoglobin deficiency. Diabetes 2011, 60, 2496–2505. [Google Scholar] [CrossRef]
- Silva, L.; Fernandes, M.S.S.; Lima, E.A.; Stefano, J.T.; Oliveira, C.P.; Jukemura, J. Fatty Pancreas: Disease or Finding? Clinics 2021, 76, e2439. [Google Scholar] [CrossRef] [PubMed]
- Kouvari, M.; D’Cunha, N.M.; Travica, N.; Sergi, D.; Zec, M.; Marx, W.; Naumovski, N. Metabolic Syndrome, Cognitive Impairment and the Role of Diet: A Narrative Review. Nutrients 2022, 14, 333. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.; Mukadam, N.; Petersen, I.; Cooper, C. Mild cognitive impairment and progression to dementia in people with diabetes, prediabetes and metabolic syndrome: A systematic review and meta-analysis. Soc. Psychiatry Psychiatr. Epidemiol. 2018, 53, 1149–1160. [Google Scholar] [CrossRef]
- Hassing, L.B.; Grant, M.D.; Hofer, S.M.; Pedersen, N.L.; Nilsson, S.E.; Berg, S.; McClearn, G.; Johansson, B. Type 2 diabetes mellitus contributes to cognitive decline in old age: A longitudinal population-based study. J. Int. Neuropsychol. Soc. JINS 2004, 10, 599–607. [Google Scholar] [CrossRef]
- Marseglia, A.; Fratiglioni, L.; Laukka, E.J.; Santoni, G.; Pedersen, N.L.; Bäckman, L.; Xu, W. Early Cognitive Deficits in Type 2 Diabetes: A Population-Based Study. J. Alzheimer’s Dis. JAD 2016, 53, 1069–1078. [Google Scholar] [CrossRef]
- Nasiri, M.; Moayedfar, S.; Purmohammad, M.; Ghasisin, L. Investigating sentence processing and working memory in patients with mild Alzheimer and elderly people. PLoS ONE 2022, 17, e0266552. [Google Scholar] [CrossRef]
- Chatzikostopoulos, A.; Moraitou, D.; Tsolaki, M.; Masoura, E.; Papantoniou, G.; Sofologi, M.; Papaliagkas, V.; Kougioumtzis, G.; Papatzikis, E. Episodic Memory in Amnestic Mild Cognitive Impairment (aMCI) and Alzheimer’s Disease Dementia (ADD): Using the “Doors and People” Tool to Differentiate between Early aMCI-Late aMCI-Mild ADD Diagnostic Groups. Diagnostics 2022, 12, 1768. [Google Scholar] [CrossRef] [PubMed]
- Custodio, N.; Montesinos, R.; Cruzado, L.; Alva-Díaz, C.; Failoc-Rojas, V.E.; Celis, V.; Cuenca-Alfaro, J.; Lira, D. Comparative study of the word capacity and episodic memory of patients with degenerative dementia. Rev. Colomb. Psiquiatr. (Engl. Ed.) 2022, 51, 8–16. [Google Scholar] [CrossRef]
- Rutkowsky, J.M.; Lee, L.L.; Puchowicz, M.; Golub, M.S.; Befroy, D.E.; Wilson, D.W.; Anderson, S.; Cline, G.; Bini, J.; Borkowski, K.; et al. Reduced cognitive function, increased blood-brain-barrier transport and inflammatory responses, and altered brain metabolites in LDLr−/− and C57BL/6 mice fed a western diet. PLoS ONE 2018, 13, e0191909. [Google Scholar] [CrossRef]
- Ettcheto, M.; Petrov, D.; Pedrós, I.; de Lemos, L.; Pallàs, M.; Alegret, M.; Laguna, J.C.; Folch, J.; Camins, A. Hypercholesterolemia and neurodegeneration. Comparison of hippocampal phenotypes in LDLr knockout and APPswe/PS1dE9 mice. Exp. Gerontol. 2015, 65, 69–78. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.; Engel, D.F.; de Paula, G.C.; Dos Santos, D.B.; Lopes, J.B.; Farina, M.; Moreira, E.L.G.; de Bem, A.F. High Cholesterol Diet Exacerbates Blood-Brain Barrier Disruption in LDLr−/− Mice: Impact on Cognitive Function. J. Alzheimer’s Dis. JAD 2020, 78, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Morales, V.; Gonzalez-Acedo, A.; Melguizo-Rodriguez, L.; Pardo-Moreno, T.; Costela-Ruiz, V.J.; Montiel-Troya, M.; Ramos-Rodriguez, J.J. Current Understanding of the Physiopathology, Diagnosis and Therapeutic Approach to Alzheimer’s Disease. Biomedicines 2021, 9, 1910. [Google Scholar] [CrossRef]
- Thirumangalakudi, L.; Prakasam, A.; Zhang, R.; Bimonte-Nelson, H.; Sambamurti, K.; Kindy, M.S.; Bhat, N.R. High cholesterol-induced neuroinflammation and amyloid precursor protein processing correlate with loss of working memory in mice. J. Neurochem. 2008, 106, 475–485. [Google Scholar] [CrossRef]
- Ullrich, C.; Pirchl, M.; Humpel, C. Hypercholesterolemia in rats impairs the cholinergic system and leads to memory deficits. Mol. Cell. Neurosci. 2010, 45, 408–417. [Google Scholar] [CrossRef]
- de Oliveira, J.; Moreira, E.L.; dos Santos, D.B.; Piermartiri, T.C.; Dutra, R.C.; Pinton, S.; Tasca, C.I.; Farina, M.; Prediger, R.D.; de Bem, A.F. Increased susceptibility to amyloid-β-induced neurotoxicity in mice lacking the low-density lipoprotein receptor. J. Alzheimer’s Dis. JAD 2014, 41, 43–60. [Google Scholar] [CrossRef]
- Shi, Y.; Andhey, P.S.; Ising, C.; Wang, K.; Snipes, L.L.; Boyer, K.; Lawson, S.; Yamada, K.; Qin, W.; Manis, M.; et al. Overexpressing low-density lipoprotein receptor reduces tau-associated neurodegeneration in relation to apoE-linked mechanisms. Neuron 2021, 109, 2413–2426.e2417. [Google Scholar] [CrossRef]
- Parhizkar, S.; Holtzman, D.M. APOE mediated neuroinflammation and neurodegeneration in Alzheimer’s disease. Semin. Immunol. 2022, 59, 101594. [Google Scholar] [CrossRef] [PubMed]
- Pickles, S.; Vigié, P.; Youle, R.J. Mitophagy and Quality Control Mechanisms in Mitochondrial Maintenance. Curr. Biol. CB 2018, 28, R170–R185. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.S.; Adriaanse, B.A.; Greig, N.H.; Mattson, M.P.; Cader, M.Z.; Bohr, V.A.; Fang, E.F. Mitophagy and Alzheimer’s Disease: Cellular and Molecular Mechanisms. Trends Neurosci. 2017, 40, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Menzies, F.M.; Fleming, A.; Rubinsztein, D.C. Compromised autophagy and neurodegenerative diseases. Nat. Rev. Neurosci. 2015, 16, 345–357. [Google Scholar] [CrossRef]
- Cai, Q.; Tammineni, P. Alterations in Mitochondrial Quality Control in Alzheimer’s Disease. Front. Cell. Neurosci. 2016, 10, 24. [Google Scholar] [CrossRef]
- Mattson, M.P. Lifelong brain health is a lifelong challenge: From evolutionary principles to empirical evidence. Ageing Res. Rev. 2015, 20, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Wan, R.; Yang, J.L.; Kamimura, N.; Son, T.G.; Ouyang, X.; Luo, Y.; Okun, E.; Mattson, M.P. Involvement of PGC-1alpha in the formation and maintenance of neuronal dendritic spines. Nat. Commun. 2012, 3, 1250. [Google Scholar] [CrossRef]
- Bentzon, J.F.; Falk, E. Atherosclerotic lesions in mouse and man: Is it the same disease? Curr. Opin. Lipidol. 2010, 21, 434–440. [Google Scholar] [CrossRef]
- Knowles, J.W.; Maeda, N. Genetic modifiers of atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2336–2345. [Google Scholar] [CrossRef]
- Engel, D.F.; de Oliveira, J.; Lopes, J.B.; Santos, D.B.; Moreira, E.L.G.; Farina, M.; Rodrigues, A.L.S.; de Souza Brocardo, P.; de Bem, A.F. Is there an association between hypercholesterolemia and depression? Behavioral evidence from the LDLr(−/−) mouse experimental model. Behav. Brain Res. 2016, 311, 31–38. [Google Scholar] [CrossRef]
- Zadelaar, S.; Kleemann, R.; Verschuren, L.; de Vries-Van der Weij, J.; van der Hoorn, J.; Princen, H.M.; Kooistra, T. Mouse models for atherosclerosis and pharmaceutical modifiers. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1706–1721. [Google Scholar] [CrossRef]
- Ramos-Rodriguez, J.J.; Infante-Garcia, C.; Galindo-Gonzalez, L.; Garcia-Molina, Y.; Lechuga-Sancho, A.; Garcia-Alloza, M. Increased Spontaneous Central Bleeding and Cognition Impairment in APP/PS1 Mice with Poorly Controlled Diabetes Mellitus. Mol. Neurobiol. 2016, 53, 2685–2697. [Google Scholar] [CrossRef] [PubMed]
- Dere, E.; Huston, J.P.; De Souza Silva, M.A. Integrated memory for objects, places, and temporal order: Evidence for episodic-like memory in mice. Neurobiol. Learn. Mem. 2005, 84, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Tulving, E. Episodic memory: From mind to brain. Annu. Rev. Psychol. 2002, 53, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Fossati, P.; Prencipe, L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin. Chem. 1982, 28, 2077–2080. [Google Scholar] [CrossRef] [PubMed]




| Groups | Female (n) | Male (n) | Total (n) |
|---|---|---|---|
| Control | 4 | 5 | 9 |
| Wt-RD | 5 | 4 | 9 |
| LDLR−/−-RD | 5 | 7 | 12 |
| LDLR−/−-HFD | 5 | 6 | 11 |
| Regular Diet | High Fat Diet | |||||
|---|---|---|---|---|---|---|
| Weight (%) | Kcal/g | Kcal/100 Kcal Intake | Weight (%) | Kcal/g | Kcal/100 Kcal Intake | |
| Protein | 20 | 0.8 | 24.2 | 14.8 | 0.6 | 12.1 |
| Carbohydrates | 77 | 2.38 | 72.1 | 58.85 | 1.76 | 35.4 |
| Fat | 3 | 0.12 | 3.7 | 29 | 2.61 | 52.5 |
| Paradigm | Calculation |
|---|---|
| “What” | (a + d) − (b + c) |
| “Where” | d − a |
| “When” | a − [(b + c)/2] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivas-Domínguez, A.; Mohamed-Mohamed, H.; Jimenez-Palomares, M.; García-Morales, V.; Martinez-Lopez, L.; Orta, M.L.; Ramos-Rodriguez, J.J.; Bermudez-Pulgarin, B. Metabolic Disturbance of High-Saturated Fatty Acid Diet in Cognitive Preservation. Int. J. Mol. Sci. 2023, 24, 8042. https://doi.org/10.3390/ijms24098042
Rivas-Domínguez A, Mohamed-Mohamed H, Jimenez-Palomares M, García-Morales V, Martinez-Lopez L, Orta ML, Ramos-Rodriguez JJ, Bermudez-Pulgarin B. Metabolic Disturbance of High-Saturated Fatty Acid Diet in Cognitive Preservation. International Journal of Molecular Sciences. 2023; 24(9):8042. https://doi.org/10.3390/ijms24098042
Chicago/Turabian StyleRivas-Domínguez, Antonio, Himan Mohamed-Mohamed, Margarita Jimenez-Palomares, Victoria García-Morales, Laura Martinez-Lopez, Manuel Luis Orta, Juan José Ramos-Rodriguez, and Beatriz Bermudez-Pulgarin. 2023. "Metabolic Disturbance of High-Saturated Fatty Acid Diet in Cognitive Preservation" International Journal of Molecular Sciences 24, no. 9: 8042. https://doi.org/10.3390/ijms24098042
APA StyleRivas-Domínguez, A., Mohamed-Mohamed, H., Jimenez-Palomares, M., García-Morales, V., Martinez-Lopez, L., Orta, M. L., Ramos-Rodriguez, J. J., & Bermudez-Pulgarin, B. (2023). Metabolic Disturbance of High-Saturated Fatty Acid Diet in Cognitive Preservation. International Journal of Molecular Sciences, 24(9), 8042. https://doi.org/10.3390/ijms24098042








