Virgin Olive Oil By-Products: Biological Activity of Phenolic Extract of Pâté on AGS Gastric Cells
Abstract
1. Introduction
2. Results and Discussion
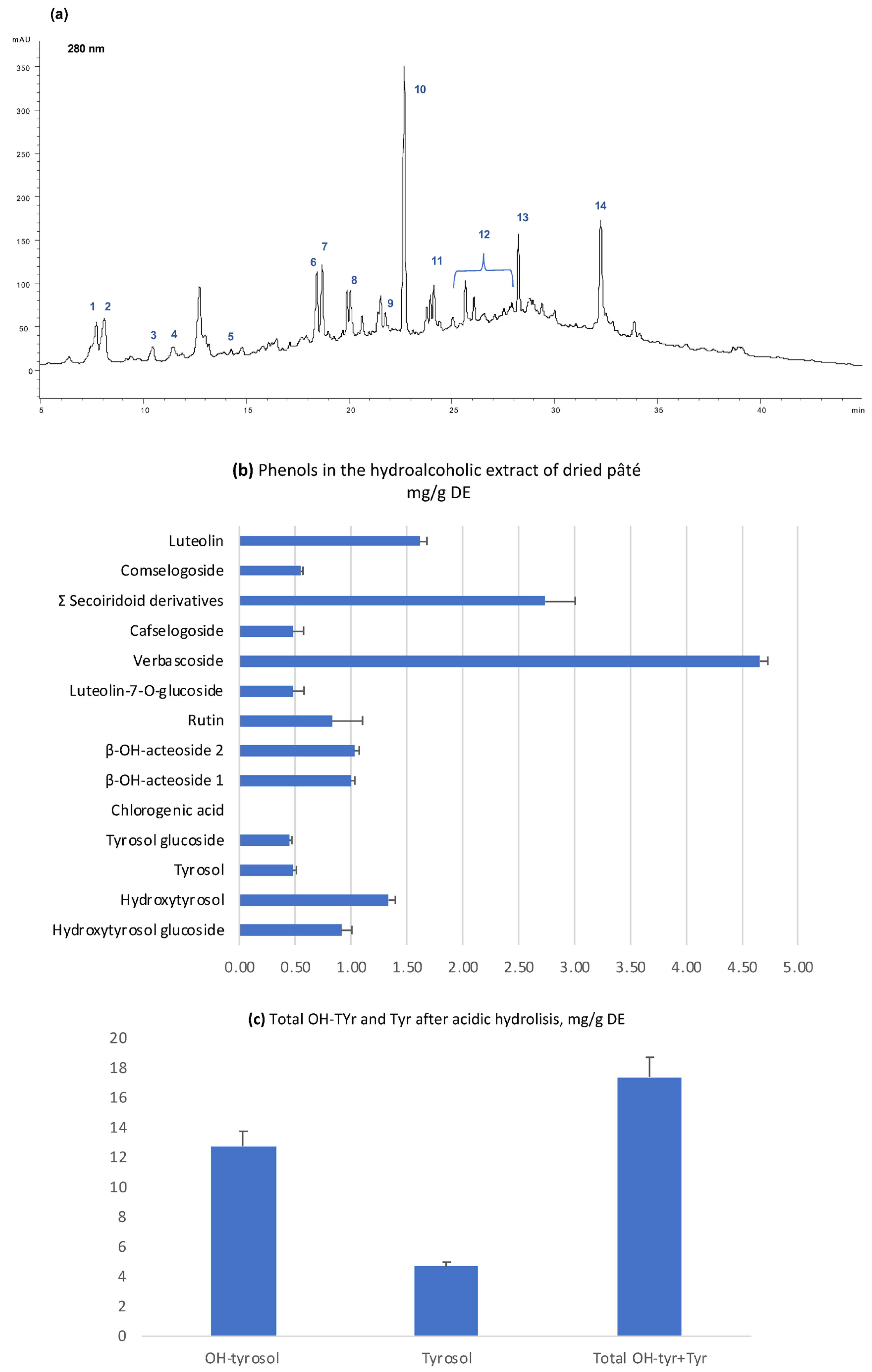
2.1. Chemical Composition of Pâté
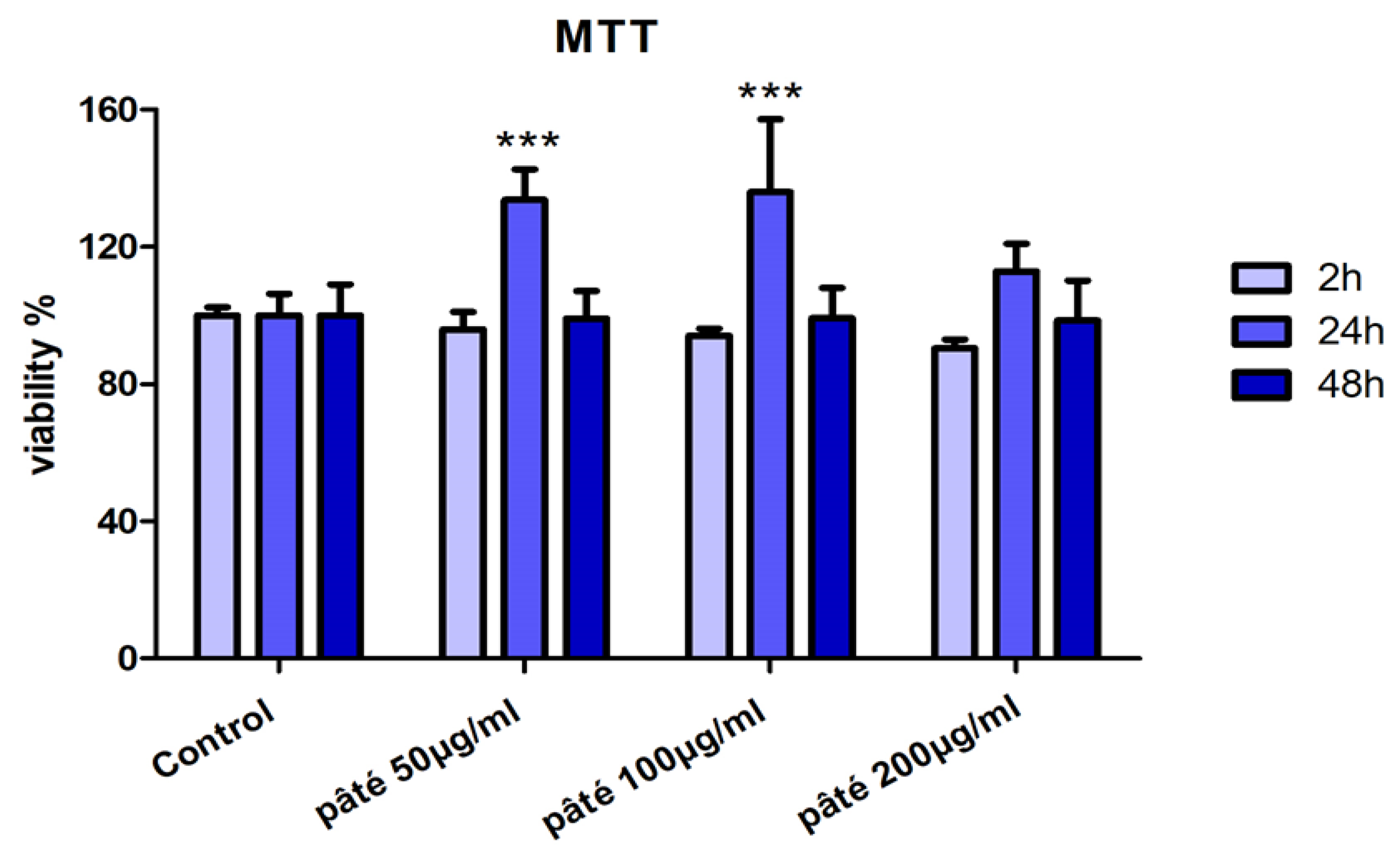
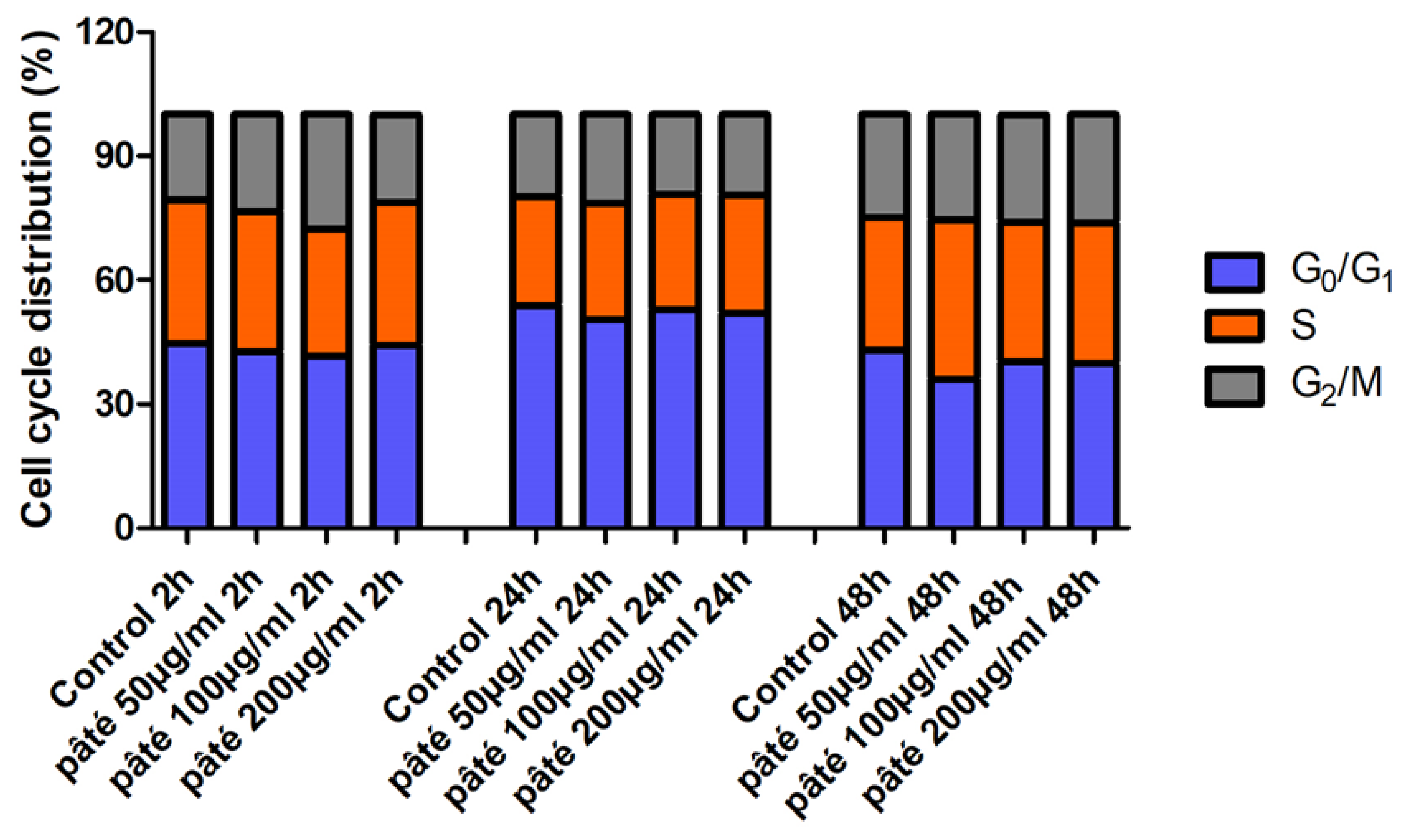
2.2. Viability Assay with MTT Test and Cell Proliferation with Cytofluorimetric Analysis
2.3. Scratch Test Results
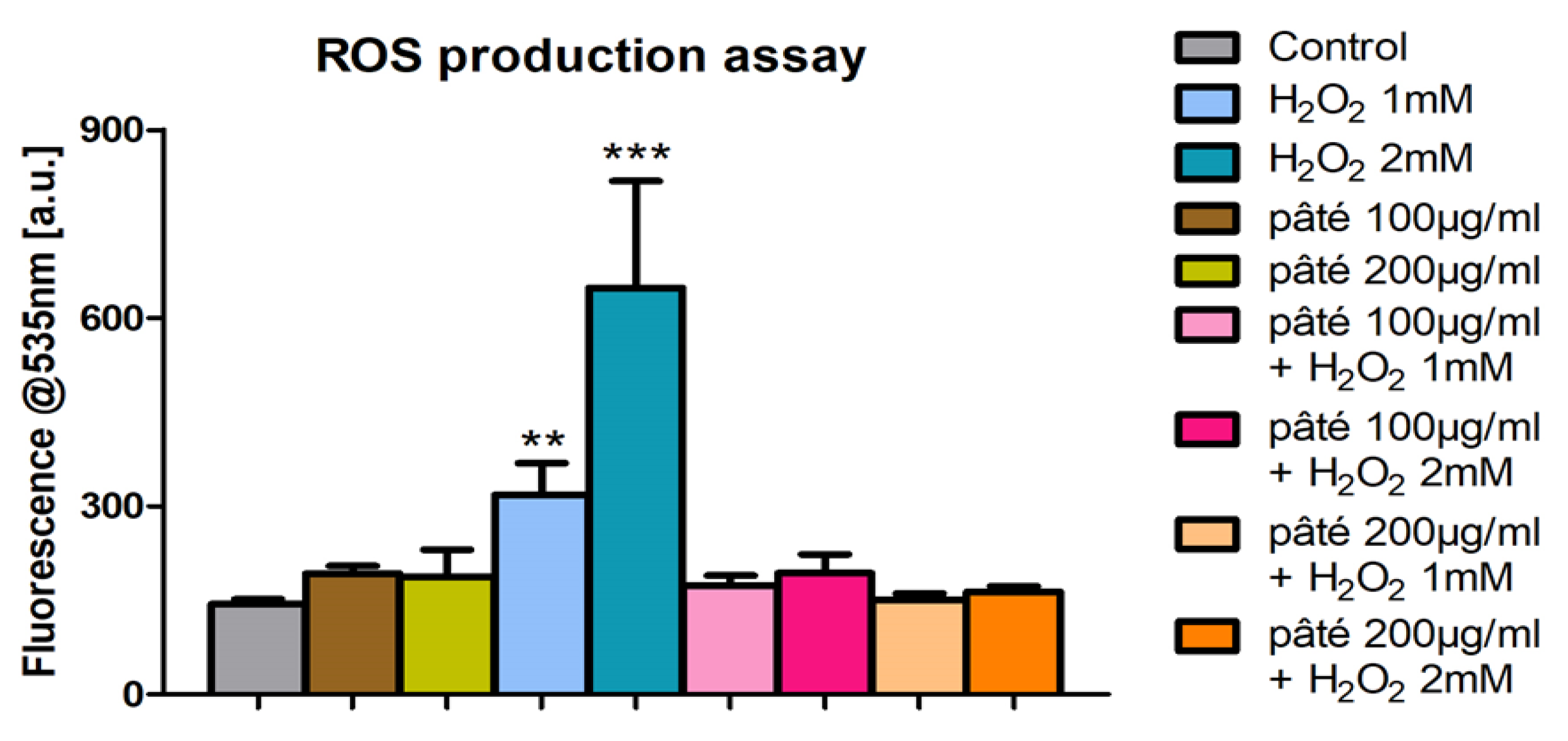
2.4. Evaluation of ROS Production
2.5. Enzymatic Assays and PCA Analysis
3. Materials and Methods
3.1. Phenolic Extract of Pâté
3.2. Analyses by HPLC-DAD-MS of the Phenolic Extract from Pâté
3.3. Cell Line
3.4. AGS Cells Viability (MTT Test)
3.5. Cytofluorimetric Analysis of Cell Cycle
3.6. Scratch Assay
3.7. Intracellular ROS Production Assay
3.8. Enzymatic Assays
3.9. Principal Component Analysis (PCA) of Enzymatic Activities
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health effects of phenolic compounds found in extra-virgin olive oil, by-products, and leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Substantiation of Health Claims Related to Polyphenols in Olive Oil and Protection of LDL Particles from Oxidative Damage. EFSA J. 2011, 9, 2033. [Google Scholar]
- Cecchi, L.; Migliorini, M.; Zanoni, B.; Breschi, C.; Mulinacci, N. An effective HPLC-based approach for the evaluation of the content of total phenolic compounds transferred from olives to virgin olive oil during the olive milling process. J. Sci. Food Agric. 2018, 98, 3636–3643. [Google Scholar] [CrossRef]
- Cecchi, L.; Migliorini, M.; Giambanelli, E.; Canuti, V.; Bellumori, M.; Mulinacci, N.; Zanoni, B. Exploitation of virgin olive oil by-products (Olea europaea L.): Phenolic and volatile compounds transformations phenomena in fresh two-phase olive pomace (‘alperujo’) under different storage conditions. J. Sci. Food Agric. 2022, 102, 2515–2525. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, L.; Bellumori, M.; Cipriani, C.; Mocali, A.; Mulinacci, N.; Giovannelli, L. A two-phase olive mill by-product (pâté) as a convenient source of phenolic compounds: Content, stability, and antiaging properties in cultured human fibroblasts. J. Funct. Foods 2018, 40, 751–759. [Google Scholar] [CrossRef]
- Mulinacci, N.; Valletta, A.; Pasqualetti, V.; Innocenti, M.; Giuliani, C.; Bellumori, M.; De Angelis, G.; Carnevale, A.; Locato, V.; Di Venanzio, C.; et al. Effects of ionizing radiation on bioactive plant extracts useful for preventing oxidative damages. Nat. Prod. Res. 2019, 33, 1106–1114. [Google Scholar] [CrossRef]
- Bellumori, M.; De Marchi, L.; Mainente, F.; Zanoni, F.; Cecchi, L.; Innocenti, M.; Mulinacci, N.; Zoccatelli, G. A by-product from virgin olive oil production (pâté) encapsulated by fluid bed coating: Evaluation of the phenolic profile after shelf-life test and in vitro gastrointestinal digestion. Int. J. Food Sci. Technol. 2021, 56, 3773–3783. [Google Scholar] [CrossRef]
- Vitali Čepo, D.; Radić, K.; Jurmanović, S.; Jug, M.; Grdić Rajković, M.; Pedisić, S.; Moslavac, T.; Albahari, P. Valorization of Olive Pomace-Based Nutraceuticals as Antioxidants in Chemical, Food, and Biological Models. Molecules 2018, 23, 2070. [Google Scholar] [CrossRef]
- Giuliani, C.; Marzorati, M.; Daghio, M.; Franzetti, A.; Innocenti, M.; de Wiele, T.V.; Mulinacci, N. Effects of olive and pomegranate by-products on human microbiota: A study using the SHIME® in vitro simulator. Molecules 2019, 24, 3791. [Google Scholar] [CrossRef]
- Parisio, C.; Lucarini, E.; Micheli, L.; Toti, A.; Bellumori, M.; Cecchi, L.; Calosi, L.; Bani, D.; Di Cesare Mannelli, L.; Mulinacci, N.; et al. Extra virgin olive oil and related by-products (Olea europaea L.) as natural sources of phenolic compounds for abdominal pain relief in gastrointestinal disorders in rats. Food Funct. 2020, 11, 10423–10435. [Google Scholar] [CrossRef] [PubMed]
- Balli, D.; Cecchi, L.; Innocenti, M.; Bellumori, M.; Mulinacci, N. Food by-products valorisation: Grape pomace and olive pomace (pâté) as sources of phenolic compounds and fiber for enrichment of tagliatelle pasta. Food Chem. 2021, 355, 129642. [Google Scholar] [CrossRef]
- Cecchi, L.; Schuster, N.; Flynn, D.; Bechtel, R.; Bellumori, M.; Innocenti, M.; Mulinacci, N.; Guinard, J.X. Sensory profiling and consumer acceptance of pasta, bread and granola bar fortified with dried olive pomace (pâté)—A by-product from virgin olive oil production. J. Food Sci. 2019, 84, 2995–3008. [Google Scholar] [CrossRef]
- Terramoccia, S.; Bartocci, S.; Taticchi, A.; Di Giovanni, S.; Pauselli, M.; Mourvaki, E.; Urbani, S.; Servili, M. Use of dried stoned olive pomace in the feeding of lactating buffaloes: Effect on the quantity and quality of the milk produced. Asian-Australas. J. Anim. Sci. 2013, 26, 971–980. [Google Scholar] [CrossRef]
- Dinu, M.; Pagliai, G.; Scavone, F.; Bellumori, M.; Cecchi, L.; Nediani, C.; Maggini, N.; Sofi, F.; Giovannelli, L.; Mulinacci, N. Effects of an Olive By-Product Called Pâté on Cardiovascular Risk Factors. J. Am. Coll. Nutr. 2021, 40, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Faraoni, P.; Gnerucci, A.; Ranaldi, F.; Orsini, B.; Romano, G.; Fusi, F. Side effects of intra-gastric photodynamic therapy: An in vitro study. J. Photochem. Photobiol. B 2018, 186, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Rafter, J.; Jenner, A. Health promotion by flavonoids, tocopherols, tocotrienols, and other phenols: Direct or indirect effects? Antioxidant or not? Am. J. Clin. Nutr. 2005, 81 (Suppl. 1), 268S–276S. [Google Scholar] [CrossRef] [PubMed]
- Speciale, A.; Anwar, S.; Canali, R.; Chirafisi, J.; Saija, A.; Virgili, F.; Cimino, F. Cyanidin-3-O-glucoside counters the response to TNF-alpha of endothelial cells by activating Nrf2 pathway. Mol. Nutr. Food Res. 2013, 57, 1979–1987. [Google Scholar] [CrossRef]
- Cheli, F.; Baldi, A. Nutrition-based health: Cell-based bioassays for food antioxidant activity evaluation. J. Food Sci. 2011, 76, R197–R205. [Google Scholar] [CrossRef]
- Xu, B.; Chang, S.K. Phenolic substance characterization and chemical and cell-based antioxidant activities of 11 lentils grown in the northern United States. J. Agric. Food Chem. 2010, 58, 1509–1517. [Google Scholar] [CrossRef]
- Theoduloz, C.; Burgos-Edwards, A.; Schmeda-Hirschmannb, G.; Jiménez-Aspee, F. Effect of polyphenols from wild Chilean currants (Ribes spp.) on the activity of intracellular antioxidant enzymes in human gastric AGS cells. Food Biosci. 2018, 24, 80–88. [Google Scholar] [CrossRef]
- Ávila, F.; Theoduloz, C.; López-Alarcón, C.; Dorta, E.; Schmeda-Hirschmann, G. Cytoprotective Mechanisms Mediated by Polyphenols from Chilean Native Berries against Free Radical-Induced Damage on AGS Cells. Oxid. Med. Cell. Longev. 2017, 2017, 9808520. [Google Scholar] [CrossRef] [PubMed]
- Rusz, M.; Del Favero, G.; El Abiead, Y.; Gerner, C.; Keppler, B.K.; Jakupec, M.A.; Koellensperger, G. Morpho-metabotyping the oxidative stress response. Sci. Rep. 2021, 11, 15471. [Google Scholar] [CrossRef] [PubMed]
- Molavian, H.R.; Kohandel, M.; Sivaloganathan, S. High Concentrations of H2O2 Make Aerobic Glycolysis Energetically More Favorable for Cellular Respiration. Front. Physiol. 2016, 7, 362. [Google Scholar] [CrossRef]
- Grant, C.M. Metabolic reconfiguration is a regulated response to oxidative stress. J. Biol. 2008, 7, 1. [Google Scholar] [CrossRef]
- Hollman, P.C.; Cassidy, A.; Comte, B.; Heinonen, M.; Richelle, M.; Richling, E.; Serafini, M.; Scalbert, A.; Sies, H.; Vidry, S. The biological relevance of direct antioxidant effects of polyphenols for cardiovascular health in humans is not established. J. Nutr. 2011, 41, 989S–1009S. [Google Scholar] [CrossRef]
- Thakur, V.S.; Gupta, K.; Gupta, S. The chemopreventive and chemotherapeutic potentials of tea polyphenols. Curr. Pharm. Biotechnol. 2012, 13, 191–199. [Google Scholar] [CrossRef]
- Son, T.G.; Camandola, S.; Mattson, M.P. Hormetic dietary phytochemicals. Neuromol. Med. 2008, 10, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Orena, S.; Owen, J.; Jin, F.; Fabian, M.; Gillitt, N.D.; Zeisel, S.H. Extracts of Fruits and Vegetables Activate the Antioxidant Response Element in IMR-32 Cells. J. Nutr. 2015, 145, 2006–2011. [Google Scholar] [CrossRef]
- Vriend, J.; Reiter, R.J. The Keap1-Nrf2-antioxidant response element pathway: A review of its regulation by melatonin and the proteasome. Mol. Cell. Endocrinol. 2015, 401, 213–220. [Google Scholar] [CrossRef]
- Cecchi, L.; Khatib, M.; Bellumori, M.; Civa, V.; Domizio, P.; Innocenti, M.; Balli, D.; Mulinacci, N. Industrial drying for agrifood by-products re-use: Cases studies on pomegranate peel (Punica granatum L.) and stoned olive pomace (pâtè, Olea europaea L.). Food Chem. 2023, 403, 134338. [Google Scholar] [CrossRef] [PubMed]
- Bellumori, M.; Cecchi, L.; Romani, A.; Mulinacci, N.; Innocenti, M. Recovery and stability over time of phenolic fractions by an industrial filtration system of olive mill wastewaters: A three-year study. J. Sci. Food Agric. 2018, 98, 2761–2769. [Google Scholar] [CrossRef]
- Barranco, S.C.; Townsend, C.M., Jr.; Casartelli, C.; Macik, B.G.; Burger, N.L.; Boerwinkle, W.R.; Gourley, W.K. Establishment and characterization of an in vitro model system for human adenocarcinoma of the stomach. Cancer Res. 1983, 43, 1703–1709. [Google Scholar]
- Vindeløv, L.L.; Christensen, I.J. A review of techniques and results obtained in one laboratory by an integrated system of methods designed for routine clinical flow cytometric DNA analysis. Cytometry 1990, 11, 753–770. [Google Scholar] [CrossRef]
- Gnerucci, A.; Faraoni, P.; Sereni, E.; Ranaldi, F. Scratch assay microscopy: A reaction-diffusion equation approach for common instruments and data. Math. Biosci. 2020, 330, 108482. [Google Scholar] [CrossRef] [PubMed]
- Bass, D.A.; Parce, J.W.; Dechatelet, L.R.; Szejda, P.; Seeds, M.C.; Thomas, M. Flow cytometric studies of oxidative product formation by neutrophils: A graded response to membrane stimulation. J. Immunol. 1983, 130, 1910–1917. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Bergmeyer, H.U. Methods of Enzymatic Analysis, 2nd ed.; Verlag Chemie: Weinheim, Germany; Academic Press: New York, NY, USA, 1974; Volume 1, pp. 443–444, 449, 458–459, 481–482, 473–474, 509–510. [Google Scholar]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef]
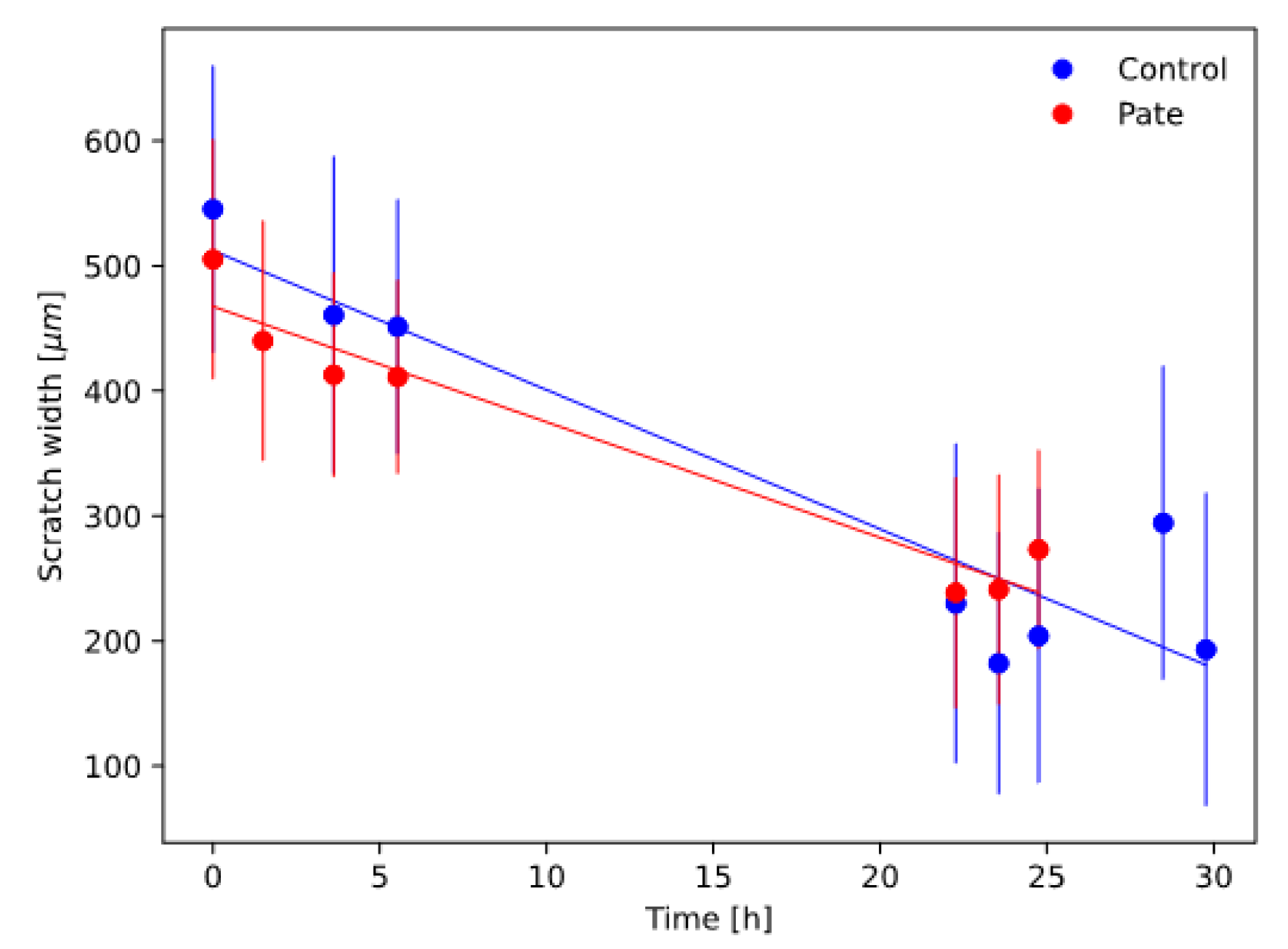


| Scratch Closure Velocity [μm/h] | Scratch Closure Time [h] | |
|---|---|---|
| Control | 11.1 ± 1.7 | 46.0 ± 7.8 |
| pâté | 9.2 ± 1.0 | 50.6 ± 5.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faraoni, P.; Cecchi, L.; Bellumori, M.; Gnerucci, A.; Ranaldi, F.; Mulinacci, N. Virgin Olive Oil By-Products: Biological Activity of Phenolic Extract of Pâté on AGS Gastric Cells. Int. J. Mol. Sci. 2023, 24, 7959. https://doi.org/10.3390/ijms24097959
Faraoni P, Cecchi L, Bellumori M, Gnerucci A, Ranaldi F, Mulinacci N. Virgin Olive Oil By-Products: Biological Activity of Phenolic Extract of Pâté on AGS Gastric Cells. International Journal of Molecular Sciences. 2023; 24(9):7959. https://doi.org/10.3390/ijms24097959
Chicago/Turabian StyleFaraoni, Paola, Lorenzo Cecchi, Maria Bellumori, Alessio Gnerucci, Francesco Ranaldi, and Nadia Mulinacci. 2023. "Virgin Olive Oil By-Products: Biological Activity of Phenolic Extract of Pâté on AGS Gastric Cells" International Journal of Molecular Sciences 24, no. 9: 7959. https://doi.org/10.3390/ijms24097959
APA StyleFaraoni, P., Cecchi, L., Bellumori, M., Gnerucci, A., Ranaldi, F., & Mulinacci, N. (2023). Virgin Olive Oil By-Products: Biological Activity of Phenolic Extract of Pâté on AGS Gastric Cells. International Journal of Molecular Sciences, 24(9), 7959. https://doi.org/10.3390/ijms24097959









