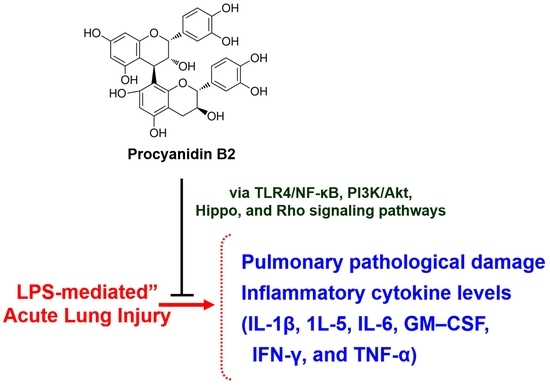
Procyanidin B2 Attenuates Sepsis-Induced Acute Lung Injury via Regulating Hippo/Rho/PI3K/NF-κB Signaling Pathway
Abstract
1. Introduction
2. Results
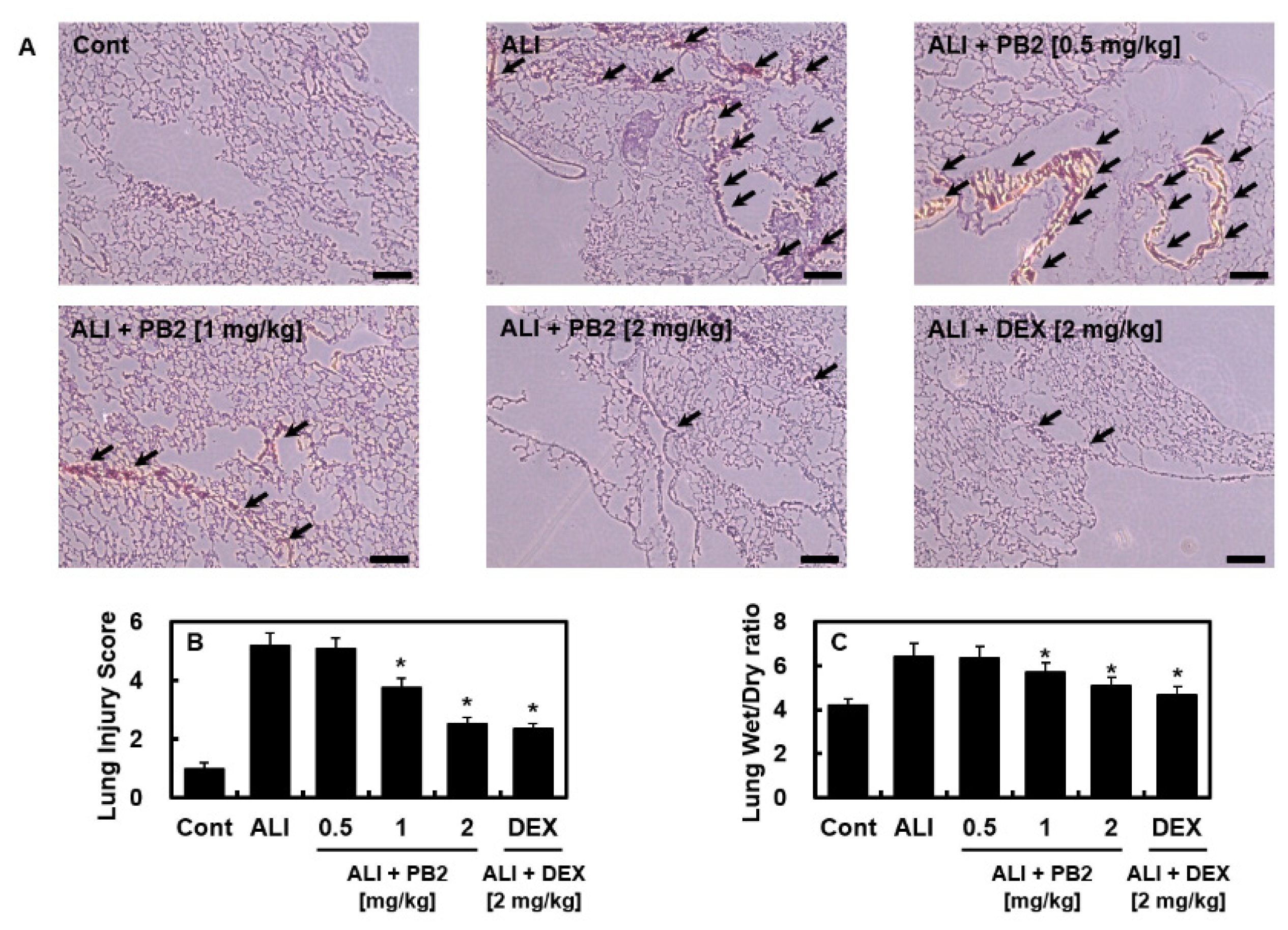
2.1. PB2 Alleviated Acute Lung Injury Induced by Intraperitoneal LPS
2.2. PB2 Reduced the Levels of Inflammatory Cytokines in Blood Serum and Lung Tissues
2.3. PB2 Inhibited ALI by Regulating the TLR4/NF-κB and PI3K/Akt Signaling Pathways
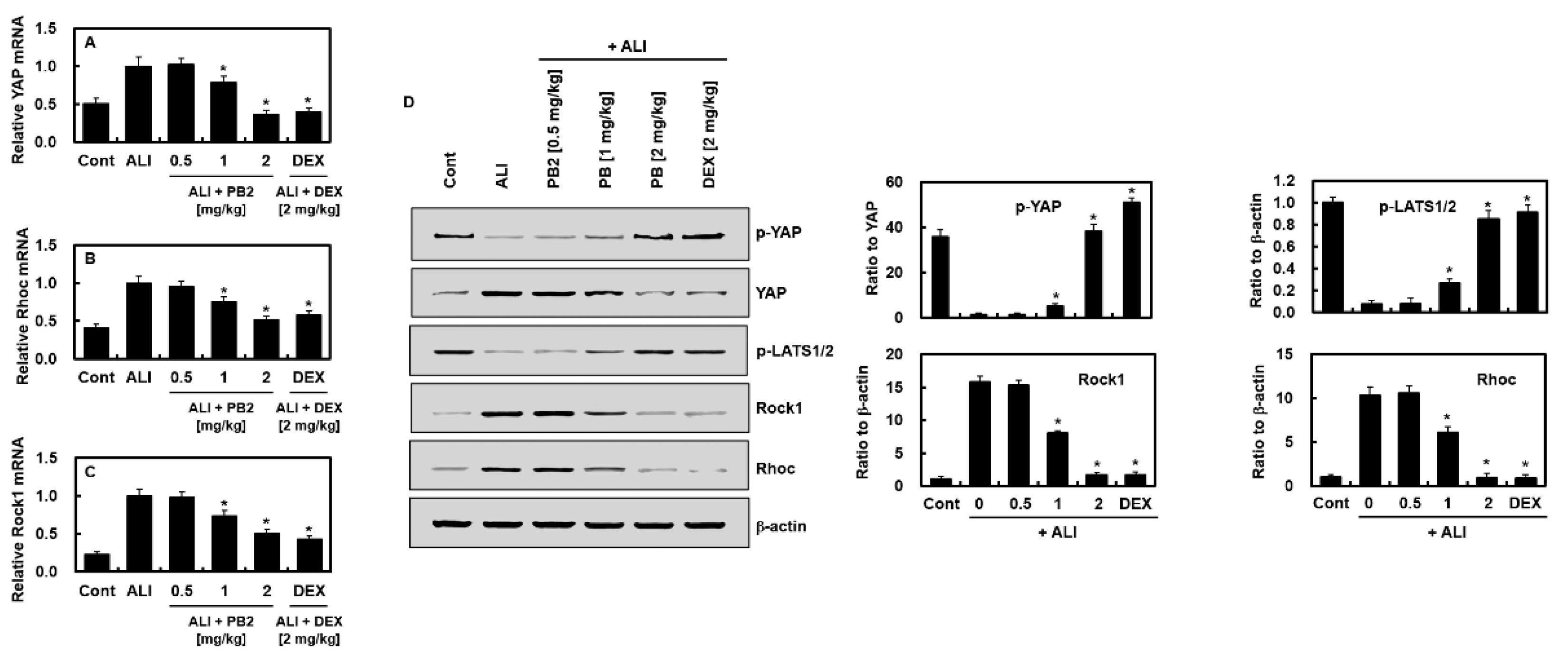
2.4. PB2 Inhibited Hippo and RHOC-ROCK1 Signaling in Sepsis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animal Care and Acute Lung Injury Model by LPS Injection
4.3. Hematoxylin and Eosin (H&E) Staining
4.4. Lung Wet/Dry (W/D) Weight Ratios
4.5. Detection of Inflammatory Cytokines
4.6. Western Blot
4.7. Total RNA Isolation and Quantitative Real-Time PCR (qPCR)
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dolmatova, E.V.; Wang, K.; Mandavilli, R.; Griendling, K.K. The effects of sepsis on endothelium and clinical implications. Cardiovasc. Res. 2021, 117, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Herrero, R.; Sanchez, G.; Lorente, J.A. New insights into the mechanisms of pulmonary edema in acute lung injury. Ann. Trans. Med. 2018, 6, 32. [Google Scholar] [CrossRef]
- Blondonnet, R.; Constantin, J.M.; Sapin, V.; Jabaudon, M. A Pathophysiologic Approach to Biomarkers in Acute Respiratory Distress Syndrome. Dis. Markers 2016, 2016, 3501373. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.K.; Khan, M.A.; Singh, S.K. Constitutive Inflammatory Cytokine Storm: A Major Threat to Human Health. J. Interferon Cytokine Res. 2020, 40, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.Y.; Hong, S.B. Sepsis and Acute Respiratory Distress Syndrome: Recent Update. Tuberc. Respir. Dis. 2016, 79, 53–57. [Google Scholar] [CrossRef]
- Wang, Y.M.; Ji, R.; Chen, W.W.; Huang, S.W.; Zheng, Y.J.; Yang, Z.T.; Qu, H.P.; Chen, H.; Mao, E.Q.; Chen, Y.; et al. Paclitaxel alleviated sepsis-induced acute lung injury by activating MUC1 and suppressing TLR-4/NF-kappaB pathway. Drug. Des. Devel. Ther. 2019, 13, 3391–3404. [Google Scholar] [CrossRef]
- Bernard, G.R.; Vincent, J.L.; Laterre, P.F.; LaRosa, S.P.; Dhainaut, J.F.; Lopez-Rodriguez, A.; Steingrub, J.S.; Garber, G.E.; Helterbrand, J.D.; Ely, E.W.; et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 2001, 344, 699–709. [Google Scholar] [CrossRef]
- Thachil, J.; Toh, C.H.; Levi, M.; Watson, H.G. The withdrawal of Activated Protein C from the use in patients with severe sepsis and DIC [Amendment to the BCSH guideline on disseminated intravascular coagulation]. Br. J. Haematol. 2012, 157, 493–494. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Thompson, B.T.; Barie, P.S.; Dhainaut, J.F.; Douglas, I.S.; Finfer, S.; Gardlund, B.; Marshall, J.C.; Rhodes, A.; Artigas, A.; et al. Drotrecogin alfa (activated) in adults with septic shock. N. Engl. J. Med. 2012, 366, 2055–2064. [Google Scholar] [CrossRef]
- Martinez-Micaelo, N.; Gonzalez-Abuin, N.; Pinent, M.; Ardevol, A.; Blay, M. Procyanidin B2 inhibits inflammasome-mediated IL-1beta production in lipopolysaccharide-stimulated macrophages. Mol. Nutr. Food Res. 2015, 59, 262–269. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Savickas, A.; Vetchy, D.; Masteikova, R.; Kasauskas, A.; Bernatoniene, J. Direct effects of (-)-epicatechin and procyanidin B2 on the respiration of rat heart mitochondria. Biomed. Res. Int. 2015, 2015, 232836. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Gao, B.W.; Wang, J.; Ren, Q.L.; Chen, J.F.; Ma, Q.; Zhang, Z.J.; Xing, B.S. Critical Role of FoxO1 in Granulosa Cell Apoptosis Caused by Oxidative Stress and Protective Effects of Grape Seed Procyanidin B2. Oxid. Med. Cell. Longev. 2016, 2016, 6147345. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Bao, L.; Ren, J.; Li, Y.; Zhang, Z. Grape seed procyanidin B2 protects podocytes from high glucose-induced mitochondrial dysfunction and apoptosis via the AMPK-SIRT1-PGC-1alpha axis in vitro. Food Funct. 2016, 7, 805–815. [Google Scholar] [CrossRef]
- Bao, L.; Cai, X.; Zhang, Z.; Li, Y. Grape seed procyanidin B2 ameliorates mitochondrial dysfunction and inhibits apoptosis via the AMP-activated protein kinase-silent mating type information regulation 2 homologue 1-PPARgamma co-activator-1alpha axis in rat mesangial cells under high-dose glucosamine. Br. J. Nutr. 2015, 113, 35–44. [Google Scholar]
- Su, H.; Li, Y.; Hu, D.; Xie, L.; Ke, H.; Zheng, X.; Chen, W. Procyanidin B2 ameliorates free fatty acids-induced hepatic steatosis through regulating TFEB-mediated lysosomal pathway and redox state. Free Radic. Biol. Med. 2018, 126, 269–286. [Google Scholar] [CrossRef] [PubMed]
- Honore, P.M.; Hoste, E.; Molnar, Z.; Jacobs, R.; Joannes-Boyau, O.; Malbrain, M.; Forni, L.G. Cytokine removal in human septic shock: Where are we and where are we going? Ann. Intensive Care 2019, 9, 56. [Google Scholar] [CrossRef]
- Salomao, R.; Martins, P.S.; Brunialti, M.K.; Fernandes Mda, L.; Martos, L.S.; Mendes, M.E.; Gomes, N.E.; Rigato, O. TLR signaling pathway in patients with sepsis. Shock 2008, 30 (Suppl. 1), 73–77. [Google Scholar] [CrossRef]
- Huang, M.; Cai, S.; Su, J. The Pathogenesis of Sepsis and Potential Therapeutic Targets. Int. J. Mol. Sci. 2019, 20, 5376. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.M. Endotoxins and other sepsis triggers. Contrib. Nephrol. 2010, 167, 14–24. [Google Scholar]
- Chen, H.; Bai, C.; Wang, X. The value of the lipopolysaccharide-induced acute lung injury model in respiratory medicine. Expert Rev. Respir. Med. 2010, 4, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Haudek, S.B.; Natmessnig, B.E.; Furst, W.; Bahrami, S.; Schlag, G.; Redl, H. Lipopolysaccharide dose response in baboons. Shock 2003, 20, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro-Vornhagen, A.; Godel, P.; Subklewe, M.; Stemmler, H.J.; Schlosser, H.A.; Schlaak, M.; Kochanek, M.; Boll, B.; von Bergwelt-Baildon, M.S. Cytokine release syndrome. J. Immunother. Cancer 2018, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Yi, M.; Guo, Q.; Wang, C.; Wang, H.; Meng, S.; Liu, C.; Fu, Y.; Ji, H.; Chen, T. Protective effects of polydatin on lipopolysaccharide-induced acute lung injury through TLR4-MyD88-NF-kappaB pathway. Int. Immunopharmacol. 2015, 29, 370–376. [Google Scholar] [CrossRef]
- Oshikawa, K.; Sugiyama, Y. Gene expression of Toll-like receptors and associated molecules induced by inflammatory stimuli in the primary alveolar macrophage. Biochem. Biophys. Res. Commun. 2003, 305, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Williams, D.L.; Li, C.; Ha, T.; Ozment-Skelton, T.; Kalbfleisch, J.H.; Preiszner, J.; Brooks, L.; Breuel, K.; Schweitzer, J.B. Modulation of the phosphoinositide 3-kinase pathway alters innate resistance to polymicrobial sepsis. J. Immunol. 2004, 172, 449–456. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef]
- Vanhaesebroeck, B.; Ali, K.; Bilancio, A.; Geering, B.; Foukas, L.C. Signalling by PI3K isoforms: Insights from gene-targeted mice. Trends Biochem. Sci. 2005, 30, 194–204. [Google Scholar] [CrossRef]
- Hu, X.; Xu, Q.; Wan, H.; Hu, Y.; Xing, S.; Yang, H.; Gao, Y.; He, Z. PI3K-Akt-mTOR/PFKFB3 pathway mediated lung fibroblast aerobic glycolysis and collagen synthesis in lipopolysaccharide-induced pulmonary fibrosis. Lab. Investig. 2020, 100, 801–811. [Google Scholar] [CrossRef]
- Troutman, T.D.; Hu, W.; Fulenchek, S.; Yamazaki, T.; Kurosaki, T.; Bazan, J.F.; Pasare, C. Role for B-cell adapter for PI3K (BCAP) as a signaling adapter linking Toll-like receptors (TLRs) to serine/threonine kinases PI3K/Akt. Proc. Natl. Acad. Sci. USA 2012, 109, 273–278. [Google Scholar] [CrossRef]
- Hazeki, K.; Nigorikawa, K.; Hazeki, O. Role of phosphoinositide 3-kinase in innate immunity. Biol. Pharm. Bull. 2007, 30, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Troutman, T.D.; Bazan, J.F.; Pasare, C. Toll-like receptors, signaling adapters and regulation of the pro-inflammatory response by PI3K. Cell Cycle 2012, 11, 3559–3567. [Google Scholar] [CrossRef]
- Vadlakonda, L.; Pasupuleti, M.; Pallu, R. Role of PI3K-AKT-mTOR and Wnt Signaling Pathways in Transition of G1-S Phase of Cell Cycle in Cancer Cells. Front. Oncol. 2013, 3, 85. [Google Scholar] [CrossRef]
- Meng, Z.; Moroishi, T.; Guan, K.L. Mechanisms of Hippo pathway regulation. Genes Dev. 2016, 30, 1–17. [Google Scholar] [CrossRef]
- Lv, Y.; Kim, K.; Sheng, Y.; Cho, J.; Qian, Z.; Zhao, Y.Y.; Hu, G.; Pan, D.; Malik, A.B.; Hu, G. YAP Controls Endothelial Activation and Vascular Inflammation Through TRAF6. Circ. Res. 2018, 123, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, A.B.; Hall, A. Rho GTPases: Biochemistry and biology. Annu. Rev. Cell Dev. Biol. 2005, 21, 247–269. [Google Scholar] [CrossRef]
- Lee, W.; Ku, S.K.; Kim, J.E.; Cho, G.E.; Song, G.Y.; Bae, J.S. Pulmonary protective functions of rare ginsenoside Rg4 on particulate matter-induced inflammatory responses. Biotechnol. Bioproc. Eng. 2019, 24, 445–453. [Google Scholar] [CrossRef]



| Gene | Primer Sequences |
|---|---|
| β-actin (F) | ACCGTGAAAAGATGACCCAG |
| β-actin (R) | GTACGACCAGAGGCATACAG |
| YAP (F) | CAGGTATTGGGAGAGTCACGG |
| YAP (R) | CAAGGGGATGACTCCAGTGAG |
| RHOC (F) | CCATGGCTGCGATCCGAA |
| RHOC (R) | GGTAGGCACGTAGACCTCTG |
| Rock-1 (F) | AAGCCGCACTGATGGATATGT |
| Rock-1 (R) | GCCATCTATTCATTCCAGCCAT |
| TLR4 (F) | TTCATGTCGTGTTCTCATGG |
| TLR4 (R) | TGCGCTCGCATCATGTTC |
| NF-κB (F) | GCAAAGGGAACATTCCGATAT |
| NF-κB (R) | GCGACATCACATGGAAATCTA |
| PI3K (F) | GTGTCAGCGCTCTCCGCC |
| PI3K (R) | AGCGACCCTGTACCAAGTT |
| AKT (F) | GTGTCCAGTGTAGAATGACTC |
| AKT (R) | ATCTGTCGGAGAACACACATG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.O.; Park, D.H.; Bae, J.-S. Procyanidin B2 Attenuates Sepsis-Induced Acute Lung Injury via Regulating Hippo/Rho/PI3K/NF-κB Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 7930. https://doi.org/10.3390/ijms24097930
Kim GO, Park DH, Bae J-S. Procyanidin B2 Attenuates Sepsis-Induced Acute Lung Injury via Regulating Hippo/Rho/PI3K/NF-κB Signaling Pathway. International Journal of Molecular Sciences. 2023; 24(9):7930. https://doi.org/10.3390/ijms24097930
Chicago/Turabian StyleKim, Go Oun, Dong Ho Park, and Jong-Sup Bae. 2023. "Procyanidin B2 Attenuates Sepsis-Induced Acute Lung Injury via Regulating Hippo/Rho/PI3K/NF-κB Signaling Pathway" International Journal of Molecular Sciences 24, no. 9: 7930. https://doi.org/10.3390/ijms24097930
APA StyleKim, G. O., Park, D. H., & Bae, J.-S. (2023). Procyanidin B2 Attenuates Sepsis-Induced Acute Lung Injury via Regulating Hippo/Rho/PI3K/NF-κB Signaling Pathway. International Journal of Molecular Sciences, 24(9), 7930. https://doi.org/10.3390/ijms24097930