Fractalkine Improves the Expression of Endometrium Receptivity-Related Genes and Proteins at Desferrioxamine-Induced Iron Deficiency in HEC-1A Cells
Abstract
1. Introduction
2. Results
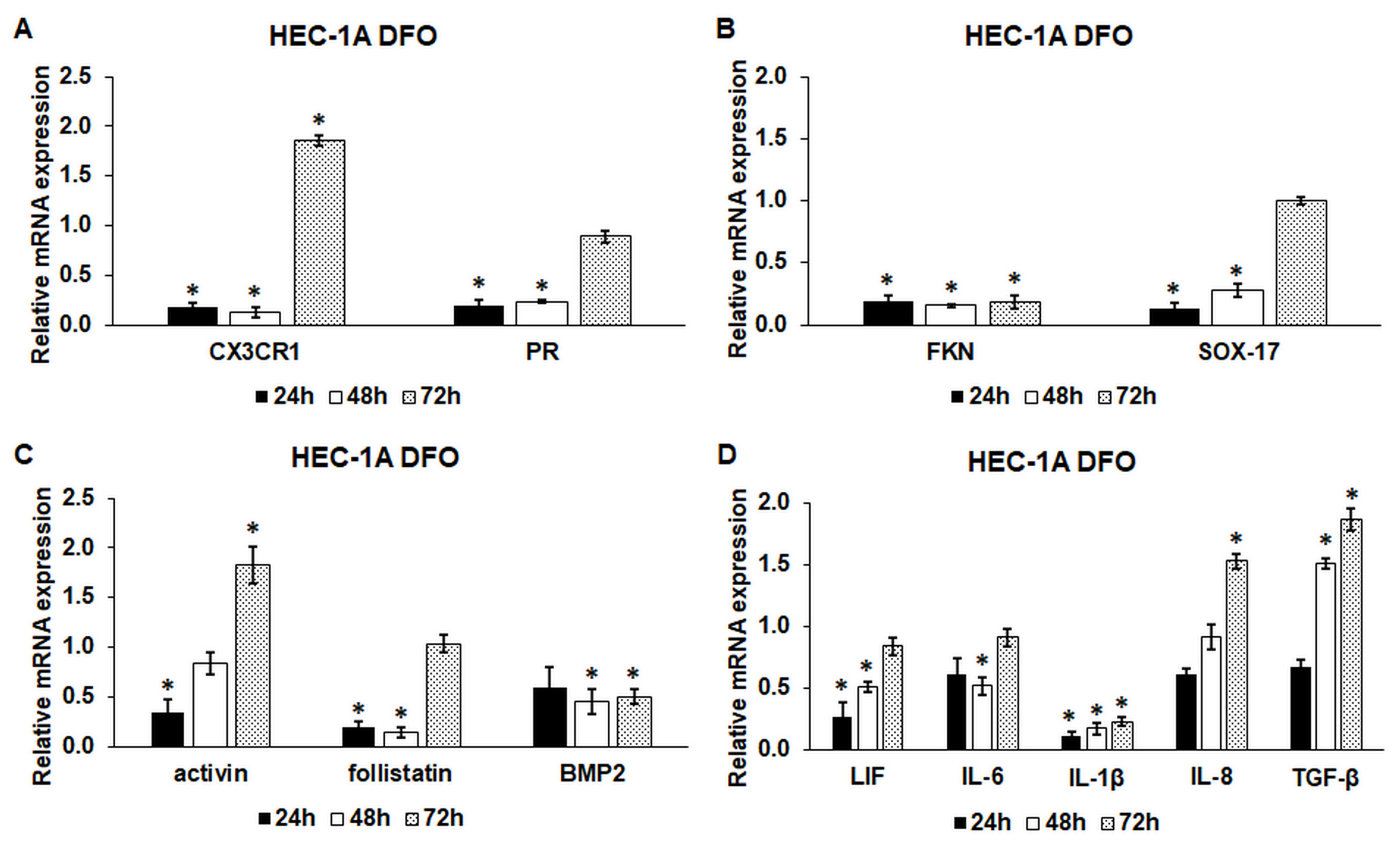
2.1. Iron Deficiency Significantly Decreases the mRNA Expression Levels of the Endometrium Receptivity-Related Genes
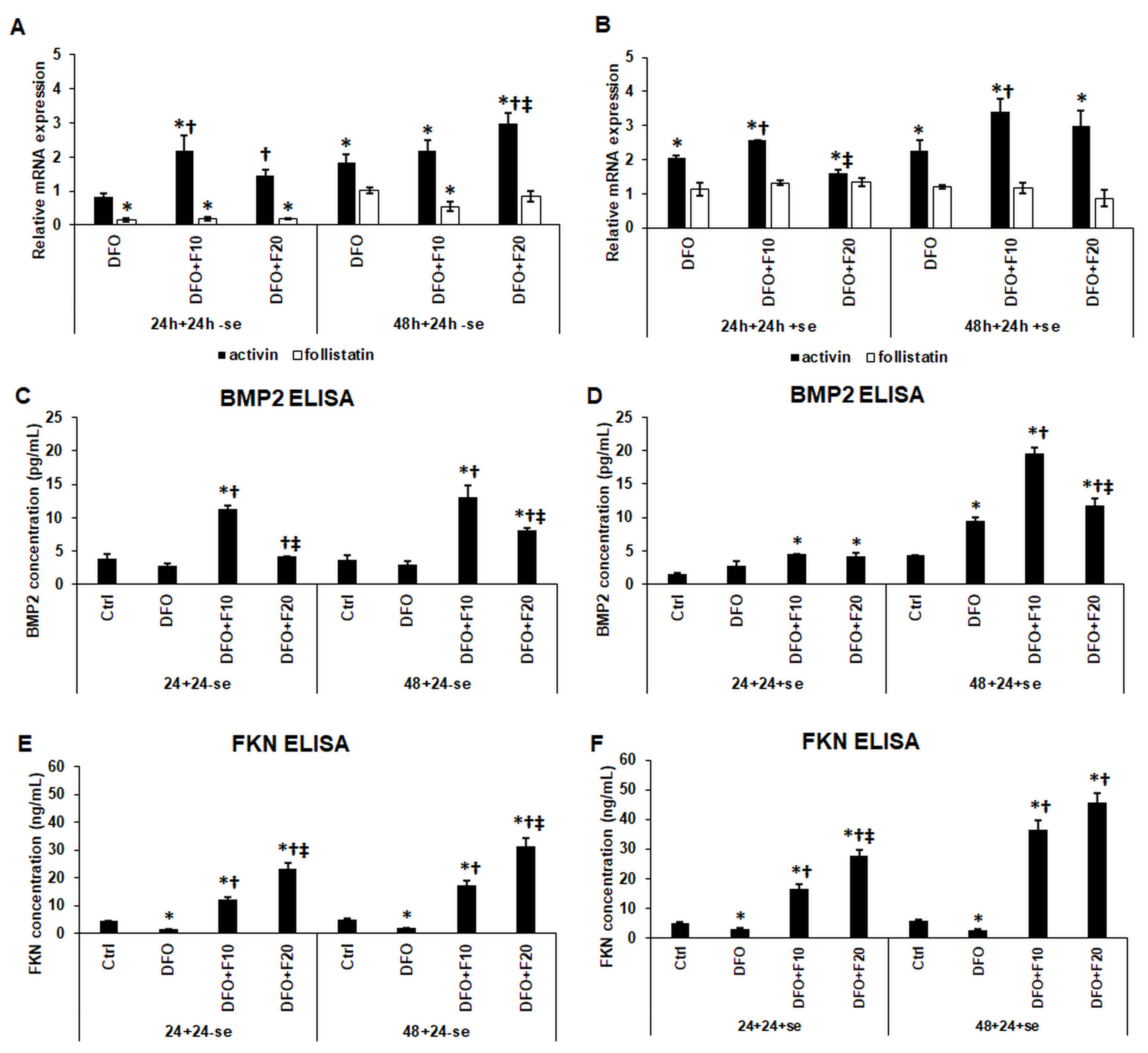
2.2. Fractalkine Ameliorates the Effect of Iron Deficiency on the mRNA Expression of Activin and Follistatin, and the Secretions of FKN and BMP2 of the HEC-1A Cells
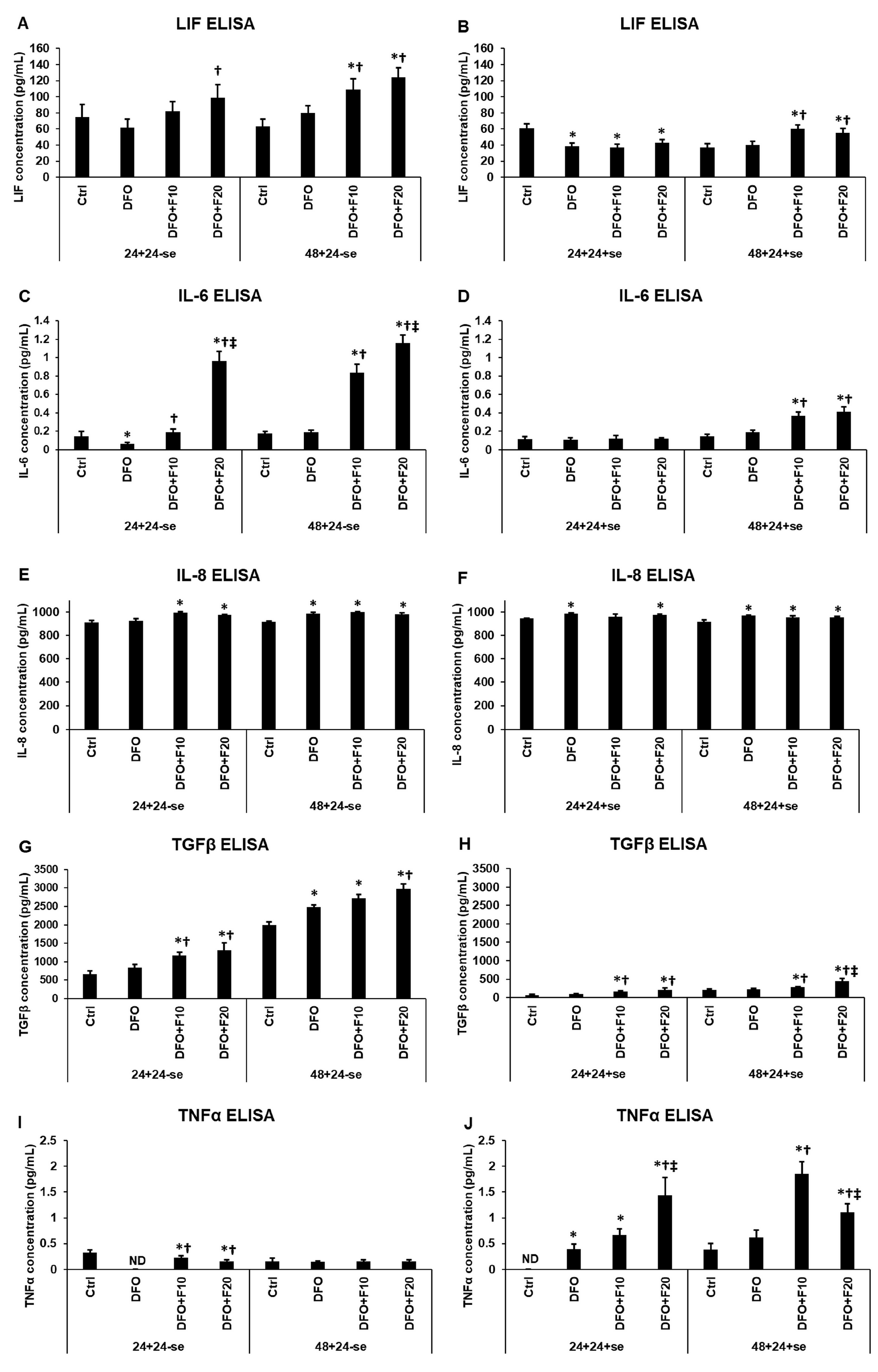
2.3. Fractalkine Alters the Effect of Iron Deficiency on the Secreted Cytokines Related to Endometrium Receptivity
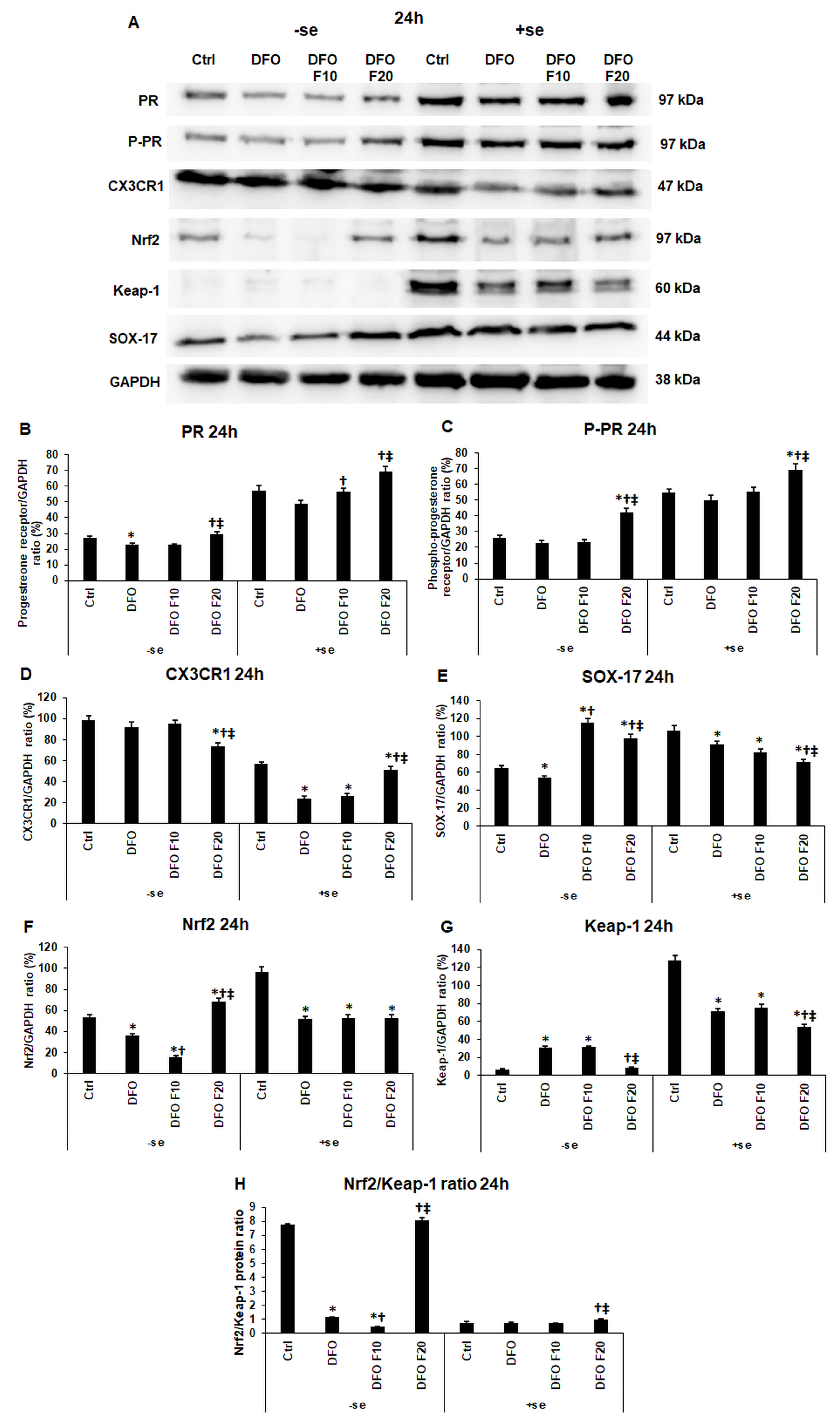
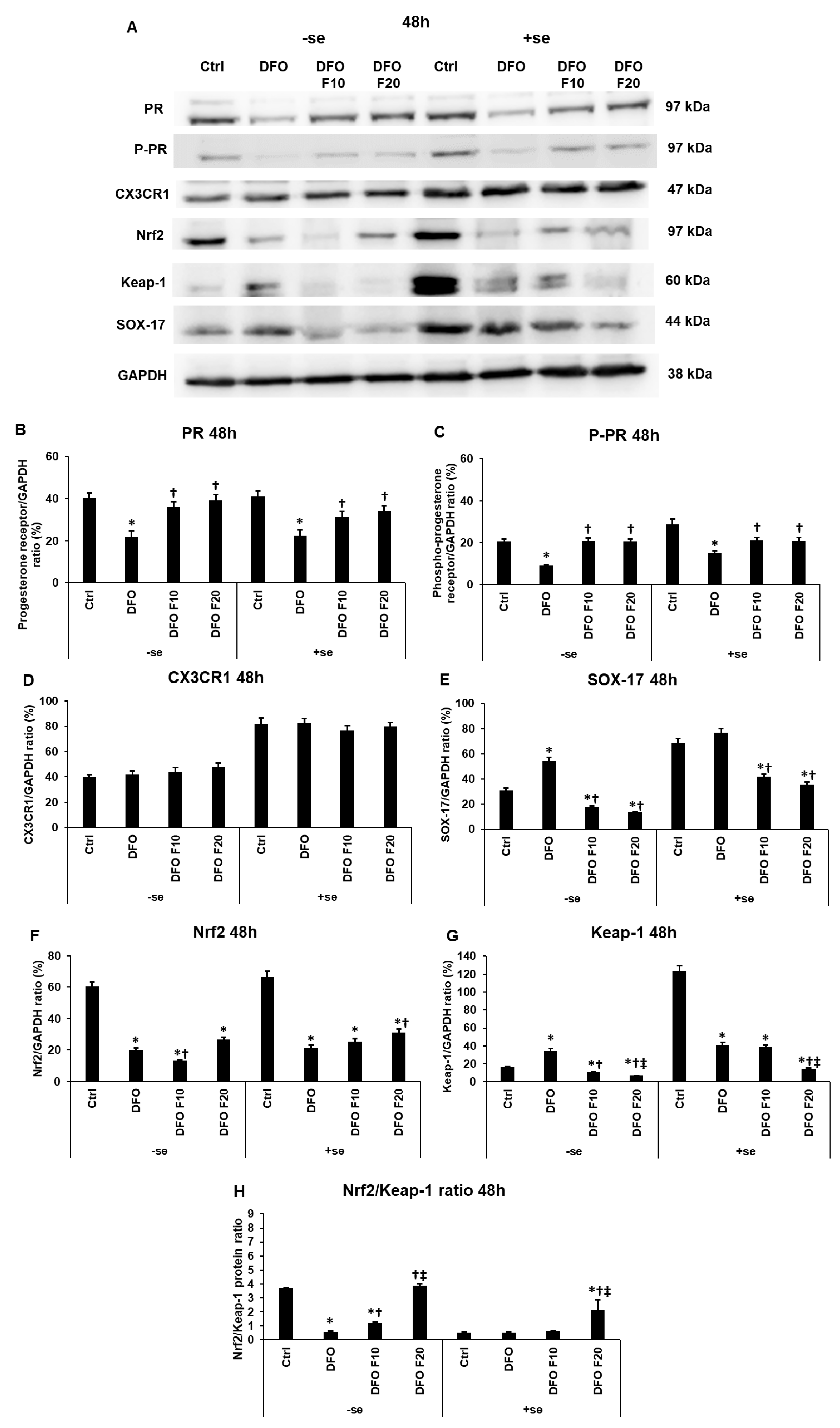
2.4. Fractalkine Modifies the Levels of the Endometrium Receptivity-Related Proteins at Iron Deficiency
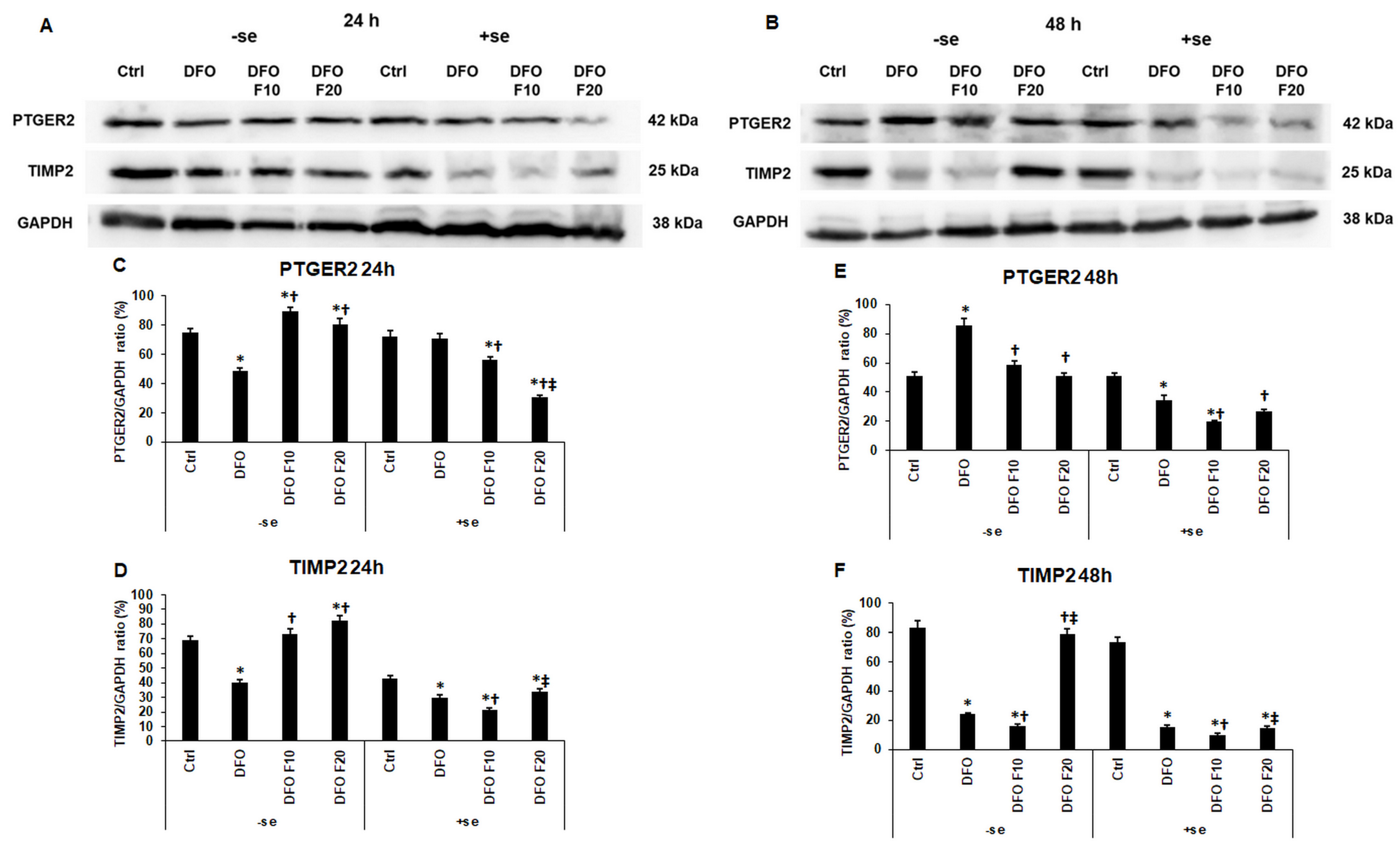
2.5. The Effect of Fractalkine on the Expression of PTGER2 and TIMP2 Depends on the Iron Availability of HEC-1A Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatments
4.2. Real-Time PCR
4.3. Enzyme-Linked Immunosorbent Assay (ELISA) Measurements
4.4. Western Blot Analysis
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, R.W.; Fazleabas, A.T. Implantation and Establishment of Pregnancy in Human and Nonhuman Primates. In Regulation of Implantation and Establishment of Pregnancy in Mammals. Advances in Anatomy, Embryology and Cell Biology; Geisert, R., Bazer, F., Eds.; Springer: Cham, Switzerland, 2015; Volume 216, pp. 189–213. [Google Scholar]
- Lessey, B.A.; Young, S.L. What Exactly Is Endometrial Receptivity? Fertil. Steril. 2019, 111, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-M.; Kim, J.-S. A Review of Mechanisms of Implantation. Dev. Reprod. 2017, 21, 351–359. [Google Scholar] [CrossRef]
- Sehring, J.; Jeelani, R. Human Implantation: The Complex Interplay between Endometrial Receptivity, Inflammation, and the Microbiome. Placenta 2022, 117, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Bui, A.H.; Timmons, D.B.; Young, S.L. Evaluation of Endometrial Receptivity and Implantation Failure. Curr. Opin. Obstet. Gynecol. 2022, 34, 107–113. [Google Scholar] [CrossRef]
- Makrigiannakis, A.; Makrygiannakis, F.; Vrekoussis, T. Approaches to Improve Endometrial Receptivity in Case of Repeated Implantation Failures. Front. Cell Dev. Biol. 2021, 9, e613277. [Google Scholar] [CrossRef]
- Chavarro, J.E.; Rich-Edwards, J.W.; Rosner, B.A.; Willett, W.C. Iron Intake and Risk of Ovulatory Infertility. Obstet. Gynecol. 2006, 108, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Cao, X.X.; Bai, B.; Zhang, J.N.; Wang, M.Q.; Zhang, Y.H. Severe Iron Deficiency Is Associated with a Reduced Conception Rate in Female Rats. Gynecol. Obstet. Investig. 2014, 77, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Sangkhae, V.; Fisher, A.L.; Wong, S.; Koenig, M.D.; Tussing-Humphreys, L.; Chu, A.; Lelić, M.; Ganz, T.; Nemeth, E. Effects of Maternal Iron Status on Placental and Fetal Iron Homeostasis. J. Clin. Investig. 2020, 130, 625–640. [Google Scholar] [CrossRef]
- Lee, D.L.; Strathmann, F.G.; Gelein, R.; Walton, J.; Mayer-Pröschel, M. Iron Deficiency Disrupts Axon Maturation of the Developing Auditory Nerve. J. Neurosci. 2012, 32, 5010–5015. [Google Scholar] [CrossRef] [PubMed]
- Seligman, P.; Siriwardana, G. Intracellular Iron Concentration and Distribution Have Multiple Effects on Cell Cycle Events. In Heavy Metal Toxicity in Public Health; IntechOpen: London, UK, 2020; p. 160. [Google Scholar]
- Kopeć, Z.; Starzyński, R.R.; Jończy, A.; Mazgaj, R.; Lipiński, P. Role of Iron Metabolism-Related Genes in Prenatal Development: Insights from Mouse Transgenic Models. Genes 2021, 12, 1382. [Google Scholar] [CrossRef]
- Gambling, L.; Danzeisen, R.; Gair, S.; Lea, R.G.; Charania, Z.; Solanky, N.; Joory, K.D.; Srai, S.K.S.; McArdle, H.J. Effect of Iron Deficiency on Placental Transfer of Iron and Expression of Iron Transport Proteins In Vivo and In Vitro. Biochem. J. 2001, 356, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Cerami, C. Iron Nutriture of the Fetus, Neonate, Infant, and Child. Ann. Nutr. Metab. 2017, 71, 8–14. [Google Scholar] [CrossRef]
- Achache, H.; Revel, A. Endometrial Receptivity Markers, the Journey to Successful Embryo Implantation. Hum. Reprod. Update 2006, 12, 731–746. [Google Scholar] [CrossRef]
- Wang, H.U.I.; Shi, G.; Li, M.; Fan, H.; Ma, H.; Sheng, L.I. Correlation of IL-1 and HB-EGF with Endometrial Receptivity. Exp. Ther. Med. 2018, 16, 5130–5136. [Google Scholar] [CrossRef] [PubMed]
- Margioula-Siarkou, C.; Prapas, Y.; Petousis, S.; Milias, S.; Ravanos, K.; Kalogiannidis, I.; Mavromatidis, G.; Haitoglou, C.; Prapas, N.; Rousso, D. LIF and LIF-R Expression in the Endometrium of Fertile and Infertile Women: A Prospective Observational Case-Control Study. Mol. Med. Rep. 2016, 13, 4721–4728. [Google Scholar] [CrossRef] [PubMed]
- Arici, A.; Engin, O.; Attar, E.; Olive, D.L. Modulation of Leukemia Inhibitory Factor Gene Expression and Protein Biosynthesis in Human Endometrium. J. Clin. Endocrinol. Metab. 1995, 80, 1908–1915. [Google Scholar] [CrossRef]
- D’Hauterive, S.P.; Charlet-Renard, C.; Dubois, M.; Berndt, S.; Goffin, F.; Foidart, J.M.; Geenen, V. Human Endometrial Leukemia Inhibitory Factor and Interleukin-6: Control of Secretion by Transforming Growth Factor-β-Related Members. Neuroimmunomodulation 2005, 12, 157–163. [Google Scholar] [CrossRef]
- Horita, H.; Kuroda, E.; Hachisuga, T.; Kashimura, M.; Yamashita, U. Induction of Prostaglandin E2 Production by Leukemia Inhibitory Factor Promotes Migration of First Trimester Extravillous Trophoblast Cell Line, HTR-8/SVneo. Hum. Reprod. 2007, 22, 1801–1809. [Google Scholar] [CrossRef]
- Ye, X.; Hama, K.; Contos, J.J.A.; Anliker, B.; Inoue, A.; Skinner, M.K.; Suzuki, H.; Amano, T.; Kennedy, G.; Arai, H.; et al. LPA3-Mediated Lysophosphatidic Acid Signalling in Embryo Implantation and Spacing. Nature 2005, 435, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Dey, S.K. Prostaglandin E2 Receptor Subtype EP2 Gene Expression in the Mouse Uterus Coincides with Differentiation of the Luminal Epithelium for Implantation. Endocrinology 1997, 138, 4599–4606. [Google Scholar] [CrossRef]
- Szóstek-Mioduchowska, A.Z.; Baclawska, A.; Rebordão, M.R.; Ferreira-Dias, G.; Skarzynski, D.J. Prostaglandins Effect on Matrix Metallopeptidases and Collagen in Mare Endometrial Fibroblasts: Effects of Prostaglandins on Endometrial MMP Expression. Theriogenology 2020, 153, 74–84. [Google Scholar] [CrossRef]
- Massimiani, M.; Lacconi, V.; La Civita, F.; Ticconi, C.; Rago, R.; Campagnolo, L. Molecular Signaling Regulating Endometrium–Blastocyst Crosstalk. Int. J. Mol. Sci. 2020, 21, 23. [Google Scholar] [CrossRef]
- Otun, H.A.; Lash, G.E.; Innes, B.A.; Bulmer, J.N.; Naruse, K.; Hannon, T.; Searle, R.F.; Robson, S.C. Effect of Tumour Necrosis Factor-α in Combination with Interferon-γ on First Trimester Extravillous Trophoblast Invasion. J. Reprod. Immunol. 2011, 88, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Basu, J.; Agamasu, E.; Bendek, B.; Salafia, C.M.; Mishra, A.; Benfield, N.; Prasad, P.; Mikhail, M. Placental Tumor Necrosis Factor-α Protein Expression during Normal Human Gestation. J. Matern. Neonatal Med. 2016, 29, 3934–3938. [Google Scholar] [CrossRef] [PubMed]
- Ryu, B.J.; Han, J.W.; Kim, R.H.; Yun, S.; Kim, T.H.; Hur, S.E.; Kim, C.J.; Lee, S.K. Activation of NOD-1/JNK/IL-8 Signal Axis in Decidual Stromal Cells Facilitates Trophoblast Invasion. Am. J. Reprod. Immunol. 2017, 78, e12672. [Google Scholar] [CrossRef] [PubMed]
- Sieg, W.; Kiewisz, J.; Podolak, A.; Jakiel, G.; Woclawek-Potocka, I.; Lukaszuk, J.; Lukaszuk, K. Inflammation-Related Molecules at the Maternal–Fetal Interface during Pregnancy and in Pathologically Altered Endometrium. Curr. Issues Mol. Biol. 2022, 44, 3792–3808. [Google Scholar] [CrossRef]
- Złotkowska, A.; Andronowska, A. Chemokines as the Modulators of Endometrial Epithelial Cells Remodelling. Sci. Rep. 2019, 9, 12968. [Google Scholar] [CrossRef] [PubMed]
- Gokce, S.; Herkïloglu, D.; Cevïk, O.; Turan, V. Role of Chemokines in Early Pregnancy Loss. Exp. Ther. Med. 2022, 23, 397. [Google Scholar] [CrossRef] [PubMed]
- Hannan, N.J.; Jones, R.L.; Critchley, H.O.D.; Kovacs, G.J.; Rogers, P.A.W.; Affandi, B.; Salamonsen, L.A. Coexpression of Fractalkine and Its Receptor in Normal Human Endometrium and in Endometrium from Users of Progestin-Only Contraception Supports a Role for Fractalkine in Leukocyte Recruitment and Endometrial Remodeling. J. Clin. Endocrinol. Metab. 2004, 89, 6119–6129. [Google Scholar] [CrossRef] [PubMed]
- Hannan, N.J.; Jones, R.L.; White, C.A.; Salamonsen, L.A. The Chemokines, CX3CL1, CCL14, and CCL4, Promote Human Trophoblast Migration at the Feto-Maternal Interface. Biol. Reprod. 2006, 74, 896–904. [Google Scholar] [CrossRef]
- Pap, R.; Montskó, G.; Jánosa, G.; Sipos, K.; Kovács, G.L.; Pandur, E. Fractalkine Regulates HEC-1A/JEG-3 Interaction by Influencing the Expression of Implantation-Related Genes in an in Vitro Co-Culture Model. Int. J. Mol. Sci. 2020, 21, 3175. [Google Scholar] [CrossRef]
- Pandur, E.; Pap, R.; Montskó, G.; Jánosa, G.; Sipos, K.; Kovács, G.L. Fractalkine Enhances Endometrial Receptivity and Activates Iron Transport towards Trophoblast Cells in an in Vitro Co-Culture System of HEC-1A and JEG-3 Cells. Exp. Cell Res. 2021, 403, 112583. [Google Scholar] [CrossRef]
- D’Haese, J.G.; Demir, I.E.; Friess, H.; Ceyhan, G.O. Fractalkine/CX3CR1: Why a Single Chemokine-Receptor Duo Bears a Major and Unique Therapeutic Potential. Expert Opin. Ther. Targets 2010, 14, 207–219. [Google Scholar] [CrossRef]
- Zhuang, Q.; Ou, J.; Zhang, S.; Ming, Y. Crosstalk between the CX3CL1/CX3CR1 Axis and Inflammatory Signaling Pathways in Tissue Injury. Curr. Protein Pept. Sci. 2019, 20, 844–854. [Google Scholar] [CrossRef]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting Molecular Cross-Talk between Nrf2 and NF-ΚB Response Pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Czogalla, B.; Kahaly, M.; Mayr, D.; Schmoeckel, E.; Niesler, B.; Hester, A.; Zeder-Göß, C.; Kolben, T.; Burges, A.; Mahner, S.; et al. Correlation of NRF2 and Progesterone Receptor and Its Effects on Ovarian Cancer Biology. Cancer Manag. Res. 2019, 11, 7673–7684. [Google Scholar] [CrossRef]
- Kim, J.E.; You, D.J.; Lee, C.; Ahn, C.; Seong, J.Y.; Hwang, J.I. Suppression of NF-ΚB Signaling by KEAP1 Regulation of IKKβ Activity through Autophagic Degradation and Inhibition of Phosphorylation. Cell. Signal. 2010, 22, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Kinnear, S.; Salamonsen, L.A.; Francois, M.; Harley, V.; Evans, J. Uterine SOX17: A Key Player in Human Endometrial Receptivity and Embryo Implantation. Sci. Rep. 2019, 9, 15495. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.J.; Chen, C.P.; Buchwalder, L.; Yu, Y.C.; Piao, L.; Huang, C.Y.; Schatz, F.; Lockwood, C.J. Regulation of CX3CL1 Expression in Human First-Trimester Decidual Cells: Implications for Preeclampsia. Reprod. Sci. 2019, 26, 1256–1265. [Google Scholar] [CrossRef]
- Szewczyk, G.; Pyzlak, M.; Pankiewicz, K.; Szczerba, E.; Stangret, A.; Szukiewicz, D.; Skoda, M.; Bierła, J.; Cukrowska, B.; Fijałkowska, A. The Potential Association between a New Angiogenic Marker Fractalkine and a Placental Vascularization in Preeclampsia. Arch. Gynecol. Obstet. 2021, 304, 365–376. [Google Scholar] [CrossRef]
- Toprak Sager, E.B.; Kale, I.; Sager, H.; Ozel, A.; Muhcu, M. Maternal Serum Fractalkine Concentrations in Pregnancies Complicated by Fetal Growth Restriction. Gynecol. Obstet. Reprod. Med. 2022, 28, 1–7. [Google Scholar] [CrossRef]
- Raei Sadigh, A.; Darabi, M.; Salmassi, A.; Hamdi, K.; Farzadi, L.; Ghasemzadeh, A.; Fattahi, A.; Nouri, M. Fractalkine and Apoptotic/Anti-Apoptotic Markers in Granulosa Cells of Women with Polycystic Ovarian Syndrome. Mol. Biol. Rep. 2020, 47, 3593–3603. [Google Scholar] [CrossRef]
- Machairiotis, N.; Vasilakaki, S.; Thomakos, N. Inflammatory Mediators and Pain in Endometriosis: A Systematic Review. Biomedicines 2021, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.E.; Nibbs, R.J.B. A Guide to Chemokines and Their Receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef] [PubMed]
- White, G.E.; Greaves, D.R. Fractalkine: A Survivor’s Guide Chemokines as Antiapoptotic Mediators. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 589–594. [Google Scholar] [CrossRef]
- Watanabe, M.; Shimoya, K.; Zhang, Q.; Temma-Asano, K.; Kimura, T.; Murata, Y. The Expression of Fractalkine in the Endometrium during the Menstrual Cycle. Int. J. Gynecol. Obstet. 2006, 92, 242–247. [Google Scholar] [CrossRef]
- Kervancioglu Demirci, E.; Salamonsen, L.A.; Gauster, M. The Role of CX3CL1 in Fetal-Maternal Interaction during Human Gestation. Cell Adhes. Migr. 2016, 10, 189–196. [Google Scholar] [CrossRef]
- Hatori, K.; Nagai, A.; Heisel, R.; Ryu, J.K.; Kim, S.U. Fractalkine and Fractalkine Receptors in Human Neurons and Glial Cells. J. Neurosci. Res. 2002, 69, 418–426. [Google Scholar] [CrossRef]
- Jones, B.A.; Beamer, M.; Ahmed, S. Fractalkine/CX3CL1: A Potential New Target for Inflammatory Diseases. Mol. Interv. 2010, 10, 263–270. [Google Scholar] [CrossRef]
- Garton, K.J.; Gough, P.J.; Blobel, C.P.; Murphy, G.; Greaves, D.R.; Dempsey, P.J.; Raines, E.W. Tumor Necrosis Factor-α-Converting Enzyme (ADAM17) Mediates the Cleavage and Shedding of Fractalkine (CX3CL1). J. Biol. Chem. 2001, 276, 37993–38001. [Google Scholar] [CrossRef]
- Panek, C.A.; Ramos, M.V.; Mejias, M.P.; Abrey-Recalde, M.J.; Fernandez-Brando, R.J.; Gori, M.S.; Salamone, G.V.; Palermo, M.S. Differential Expression of the Fractalkine Chemokine Receptor (CX3CR1) in Human Monocytes during Differentiation. Cell. Mol. Immunol. 2015, 12, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lee, Y.; Song, J.; Lee, J.; Chang, S.Y. Tissue-Specific Role of CX3CR1 Expressing Immune Cells and Their Relationships with Human Disease. Immune Netw. 2018, 18, e5. [Google Scholar] [CrossRef] [PubMed]
- Subbarayan, M.S.; Joly-Amado, A.; Bickford, P.C.; Nash, K.R. CX3CL1/CX3CR1 Signaling Targets for the Treatment of Neurodegenerative Diseases. Pharmacol. Ther. 2022, 231, 107989. [Google Scholar] [CrossRef] [PubMed]
- Tonai, S.; Kawabata, A.; Nakanishi, T.; Lee, J.Y.; Okamoto, A.; Shimada, M.; Yamashita, Y. Iron Deficiency Induces Female Infertile in Order to Failure of Follicular Development in Mice. J. Reprod. Dev. 2020, 66, 475–483. [Google Scholar] [CrossRef]
- Salama, K.M.; Alloush, M.K.; Al Hussini, R.M. Are the Cytokines TNF Alpha and IL-1Beta Early Predictors of Embryo Implantation? Cross Sectional Study. J. Reprod. Immunol. 2020, 137, 102618. [Google Scholar] [CrossRef]
- Dekel, N.; Gnainsky, Y.; Granot, I.; Mor, G. Inflammation and Implantation. Am. J. Reprod. Immunol. 2010, 63, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 Signaling Pathway: Pivotal Roles in Inflammation. Biochim. Biophys. Acta-Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, E.; Dittrich-Breiholz, O.; Holtmann, H.; Kracht, M. Multiple Control of Interleukin-8 Gene Expression. J. Leukoc. Biol. 2002, 72, 847–855. [Google Scholar] [CrossRef]
- Wijayarathna, R.; de Kretser, D.M. Activins in Reproductive Biology and Beyond. Hum. Reprod. Update 2016, 22, 342–357. [Google Scholar] [CrossRef]
- Jones, R.L.; Kaitu’u-Lino, T.J.; Nie, G.; Sanchez-Partida, L.G.; Findlay, J.K.; Salamonsen, L.A. Complex Expression Patterns Support Potential Roles for Maternally Derived Activins in the Establisment of Pregnancy in Mouse. Reproduction 2006, 132, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Thompson, T.B.; Lerch, T.F.; Cook, R.W.; Woodruff, T.K.; Jardetzky, T.S. The Structure of the Follistatin: Activin Complex Reveals Antagonism of Both Type I and Type II Receptor Binding. Dev. Cell 2005, 9, 535–543. [Google Scholar] [CrossRef]
- Jones, R.L.; Salamonsen, L.A.; Zhao, Y.C.; Ethier, J.F.; Drummond, A.E.; Findlay, J.K. Expression of Activin Receptors, Follistatin and Betaglycan by Human Endometrial Stromal Cells; Consistent with a Role for Activins during Decidualization. Mol. Hum. Reprod. 2002, 8, 363–374. [Google Scholar] [CrossRef]
- Li, Q.; Kannan, A.; Das, A.; DeMayo, F.J.; Hornsby, P.J.; Young, S.L.; Taylor, R.N.; Bagchi, M.K.; Bagchi, I.C. WNT4 Acts Downstream of BMP2 and Functions via β-Catenin Signaling Pathway to Regulate Human Endometrial Stromal Cell Differentiation. Endocrinology 2013, 154, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Clementi, C.; Tripurani, S.K.; Large, M.J.; Edson, M.A.; Creighton, C.J.; Hawkins, S.M.; Kovanci, E.; Kaartinen, V.; Lydon, J.P.; Pangas, S.A.; et al. Activin-Like Kinase 2 Functions in Peri-Implantation Uterine Signaling in Mice and Humans. PLoS Genet. 2013, 9, e1003863. [Google Scholar] [CrossRef] [PubMed]
- Monsivais, D.; Nagashima, T.; Prunskaite-Hyyryläinen, R.; Nozawa, K.; Shimada, K.; Tang, S.; Hamor, C.; Agno, J.E.; Chen, F.; Masand, R.P.; et al. Endometrial Receptivity and Implantation Require Uterine BMP Signaling through an ACVR2A-SMAD1/SMAD5 Axis. Nat. Commun. 2021, 12, 3386. [Google Scholar] [CrossRef]
- Hagan, C.R.; Daniel, A.R.; Dressing, G.E.; Lange, C.A. Role of Phosphorylation in Progesterone Receptor Signaling and Specificity. Mol. Cell. Endocrinol. 2012, 357, 43–49. [Google Scholar] [CrossRef]
- Franco, H.L.; Jeong, J.W.; Tsai, S.Y.; Lydon, J.P.; DeMayo, F.J. In Vivo Analysis of Progesterone Receptor Action in the Uterus during Embryo Implantation. Semin. Cell Dev. Biol. 2008, 19, 178–186. [Google Scholar] [CrossRef]
- Rubel, C.A.; Lanz, R.B.; Kommagani, R.; Franco, H.L.; Lydon, J.P.; Demayo, F.J. Research Resource: Genome-Wide Profiling of Progesterone Receptor Binding in the Mouse Uterus. Mol. Endocrinol. 2012, 26, 1428–1442. [Google Scholar] [CrossRef]
- King, A.E.; Critchley, H.O.D.; Kelly, R.W. The NF-ΚB Pathway in Human Endometrium and First Trimester Decidua. Mol. Hum. Reprod. 2001, 7, 175–183. [Google Scholar] [CrossRef]
- Kimber, S.J. Leukaemia Inhibitory Factor in Implantation and Uterine Biology. Reproduction 2005, 130, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Primer-BLAST Primer Designing Tool. Available online: http://www.ncbi.nlm.nih.gov/tools/primer-blast/ (accessed on 14 November 2022).
- ImageJ. Available online: https://imagej.nih.gov/ij/index.html (accessed on 14 November 2022).

| Primer | Sequence 5′→3′ |
|---|---|
| activin A forward | GAACTTATGGAGCAGACCTC |
| activin A reverse | GGACTTTTAGGAAGAGCCAG |
| BMP2 forward | TAAGTTCTATCCCCACGGAG |
| BMP2 reverse | AGCATCTTGCATCTGTTCTC |
| CX3CR1 forward | CCATTAGTCTGGGCGTCTGG |
| CX3CR1 reverse | GTCACCCAGACACTCGTTGT |
| FKN forward | TACCTGTAGCTTTGCTCATC |
| FKN reverse | GTCTCGTCTCCAAGATGATT |
| follistatin forward | CAAAGCAAAGTCCTGTGAAG |
| follistatin reverse | CCTCTCCCAACCTTGAAATC |
| GAPDH forward | TGTTCCAATATGATTCCACCC |
| GAPDH reverse | CCACTTGATTTTGGAGGGAT |
| IL-1β forward | GAAATGATGGCTTATTACAGTGG |
| IL-1β reverse | GGTGGTCGGAGATTCGTA |
| IL-6 forward | CTGAGAAAGGAGACATGTAACAAGA |
| IL-6 reverse | GGCAAGTCTCCTCATTGAATC |
| IL-8 forward | CAGTGCATAAAGACATACTCC |
| IL-8 reverse | CACTCTCAATCACTCTCAGT |
| LIF forward | CTCGGGTAAGGATGTCTTC |
| LIF reverse | GGCGATGATCTGCTTATACT |
| Progesterone receptor A/B forward | CCAAAGGCCGCAAATTCT |
| Progesterone receptor A/B reverse | TGAGGTCAGAAAGGTCATCG |
| SOX-17 forward | CAGTATCTGCACTTCGTGTG |
| SOX-17 reverse | AGTAATATACCGCGGAGCTG |
| TGF-β forward | GCCAGAGTGGTTATCTTTTG |
| TGF-β reverse | GTAGTGAACCCGTTGATGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandur, E.; Pap, R.; Jánosa, G.; Horváth, A.; Sipos, K. Fractalkine Improves the Expression of Endometrium Receptivity-Related Genes and Proteins at Desferrioxamine-Induced Iron Deficiency in HEC-1A Cells. Int. J. Mol. Sci. 2023, 24, 7924. https://doi.org/10.3390/ijms24097924
Pandur E, Pap R, Jánosa G, Horváth A, Sipos K. Fractalkine Improves the Expression of Endometrium Receptivity-Related Genes and Proteins at Desferrioxamine-Induced Iron Deficiency in HEC-1A Cells. International Journal of Molecular Sciences. 2023; 24(9):7924. https://doi.org/10.3390/ijms24097924
Chicago/Turabian StylePandur, Edina, Ramóna Pap, Gergely Jánosa, Adrienn Horváth, and Katalin Sipos. 2023. "Fractalkine Improves the Expression of Endometrium Receptivity-Related Genes and Proteins at Desferrioxamine-Induced Iron Deficiency in HEC-1A Cells" International Journal of Molecular Sciences 24, no. 9: 7924. https://doi.org/10.3390/ijms24097924
APA StylePandur, E., Pap, R., Jánosa, G., Horváth, A., & Sipos, K. (2023). Fractalkine Improves the Expression of Endometrium Receptivity-Related Genes and Proteins at Desferrioxamine-Induced Iron Deficiency in HEC-1A Cells. International Journal of Molecular Sciences, 24(9), 7924. https://doi.org/10.3390/ijms24097924







