Evaluation of Photobiomodulation and Boldine as Alternative Treatment Options in Two Diabetic Retinopathy Models
Abstract
1. Introduction
2. Results
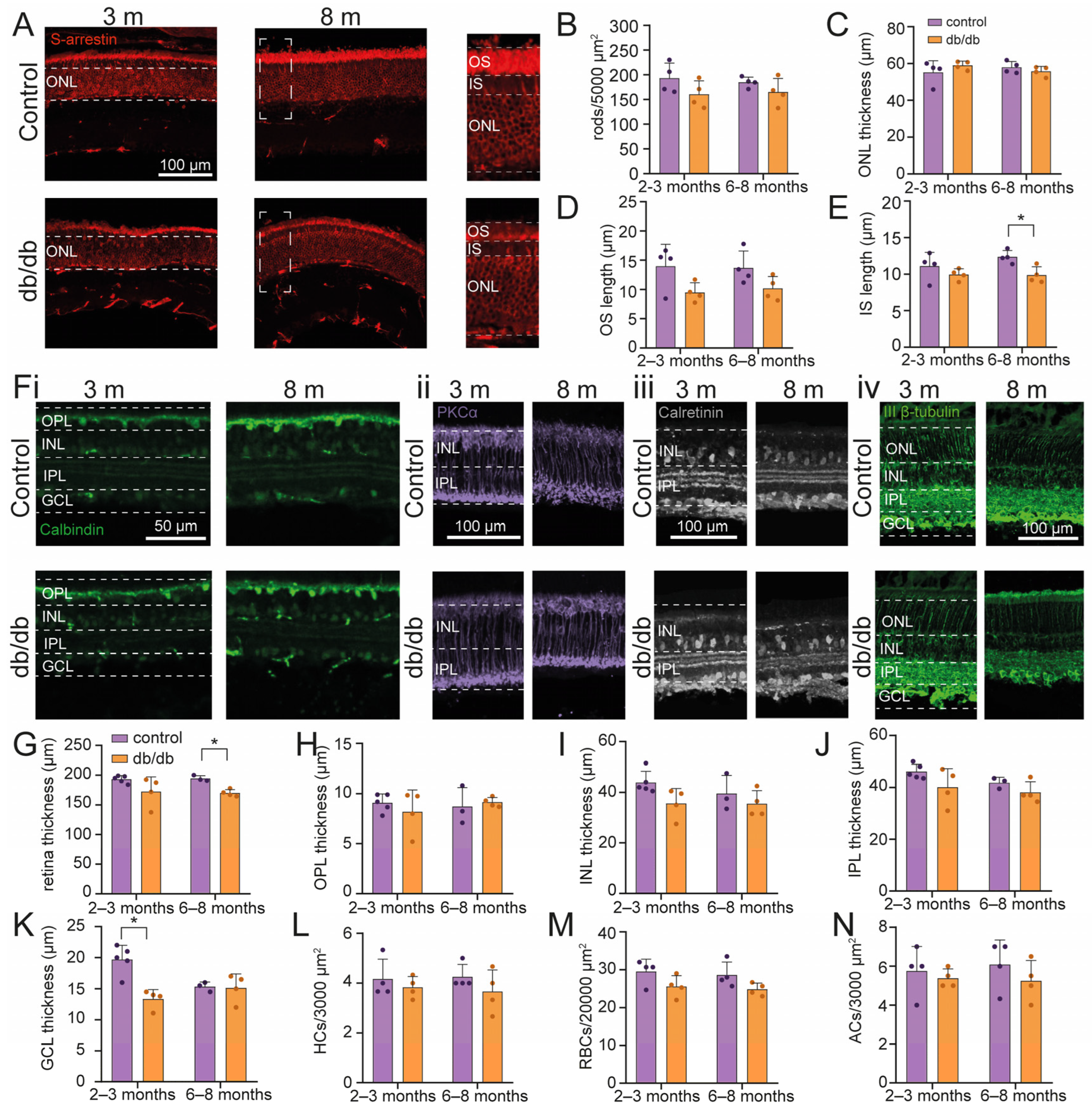
2.1. Retinal Cell Survival in a Diabetic Model Mouse
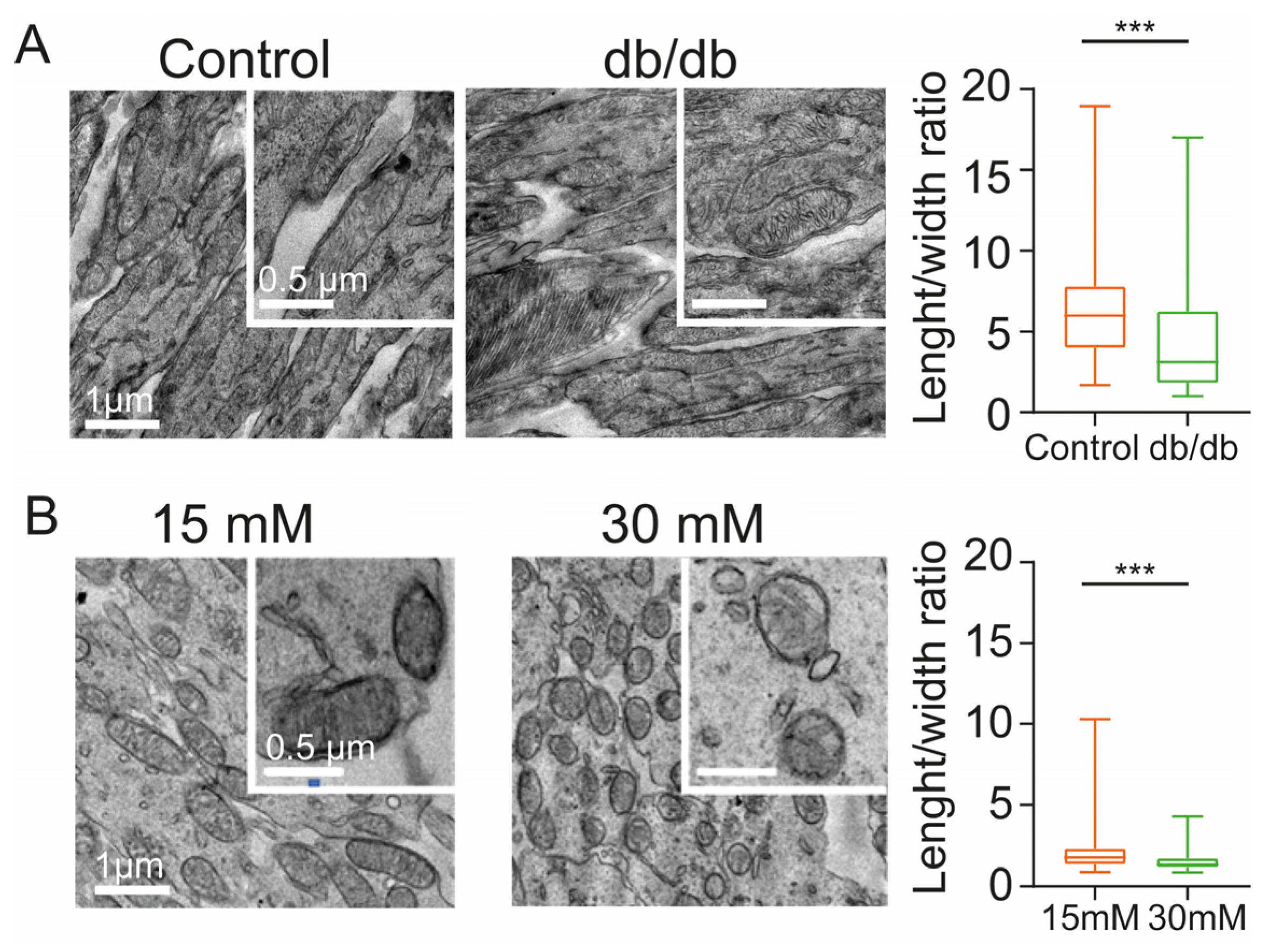
2.2. Diabetic Conditions Produce Morphological Changes of Mitochondria in the Inner Segment of Photoreceptors
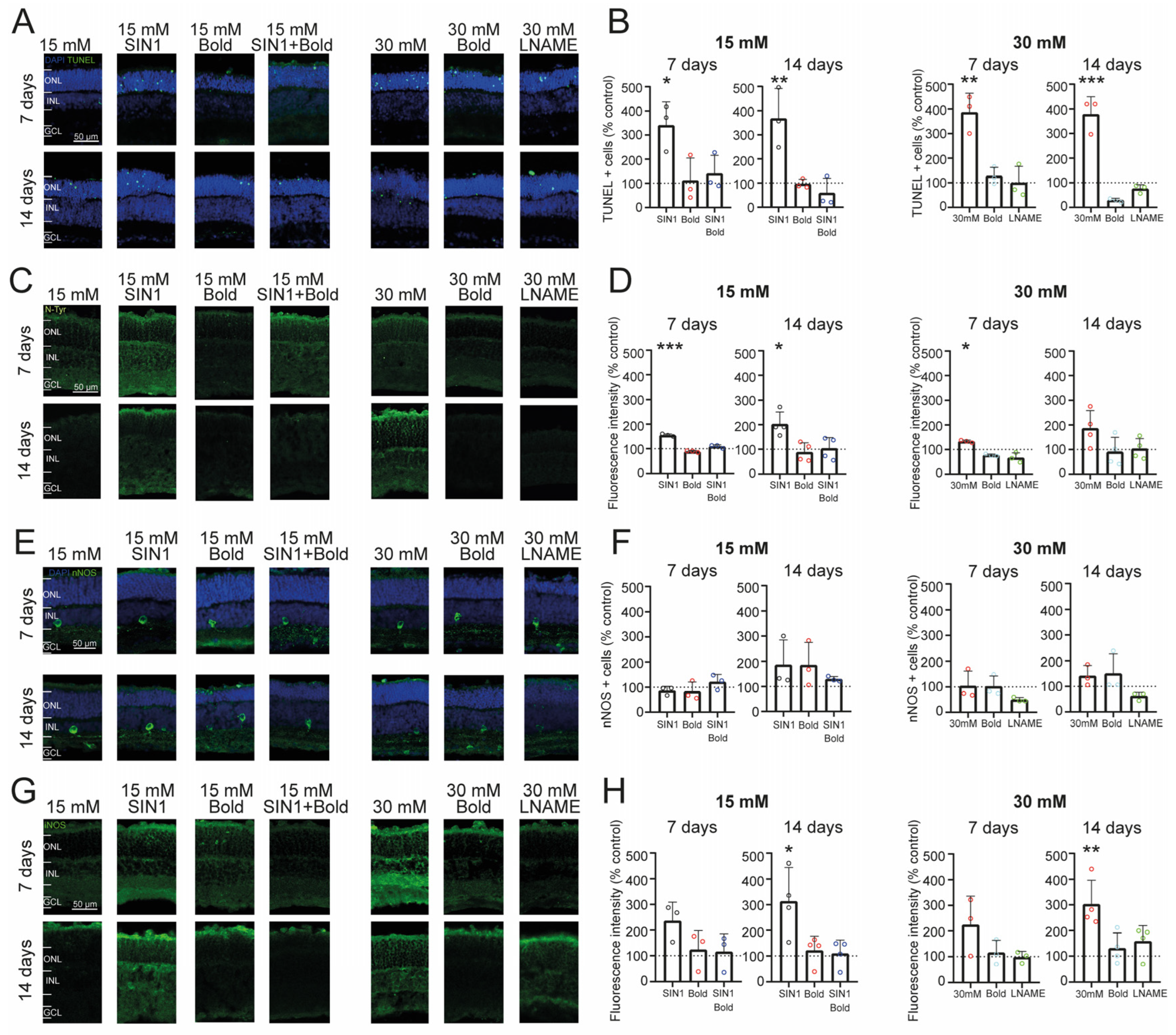
2.3. Retinal Cell Death Rate Is Increased by Exposure to High Glucose and Nitrosative Stress
2.4. Prolonged Exposure to High Glucose Leads to an Inflammatory Environment and Increased Nitric Oxide from iNOS in Retinal Explants
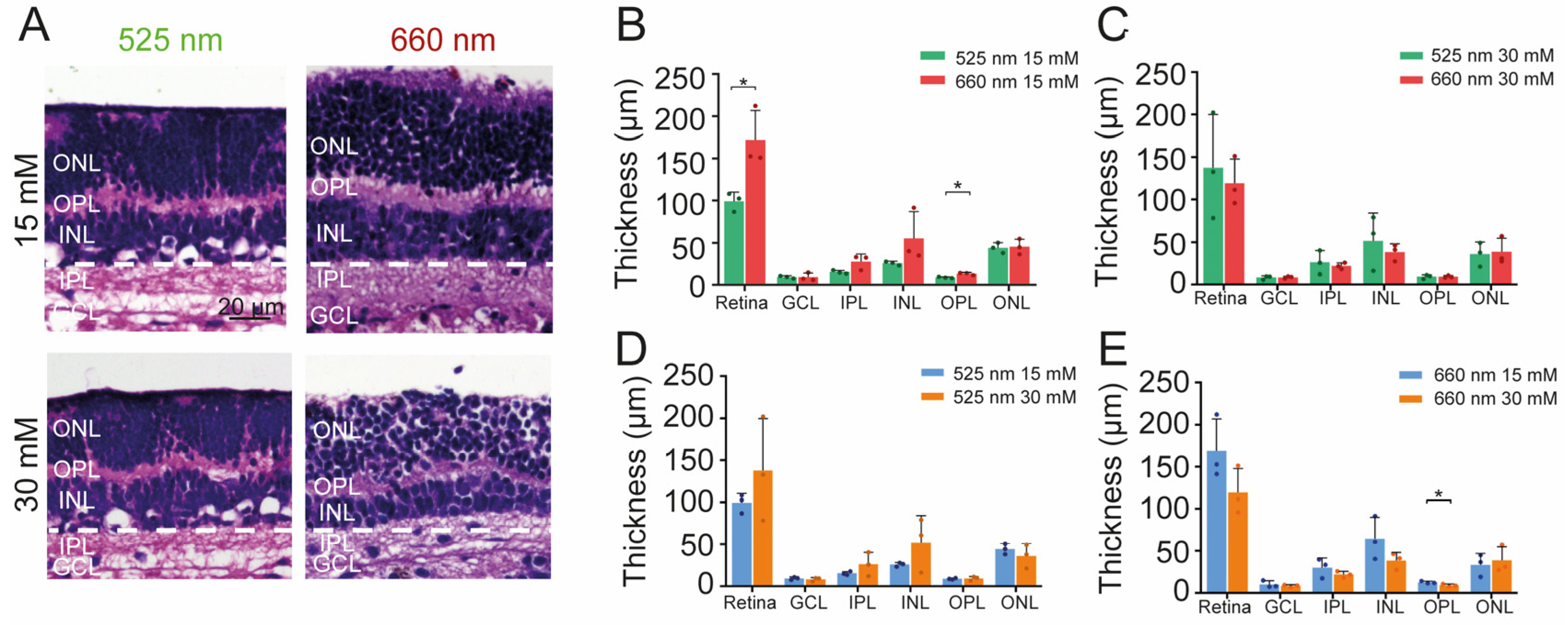
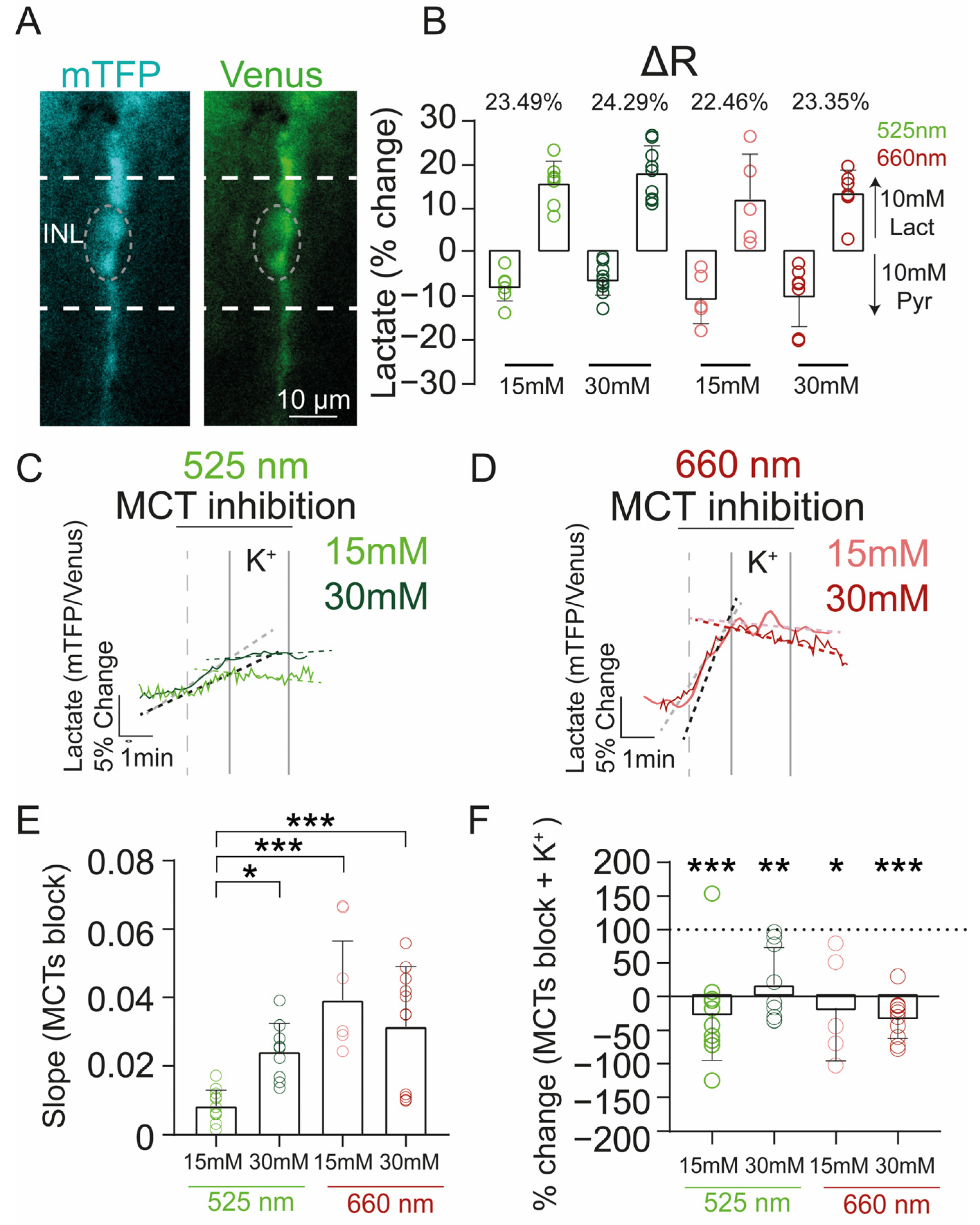
2.5. Effect of Photobiomodulation Treatment on Retinal Structure and Metabolism under High Glucose Conditions
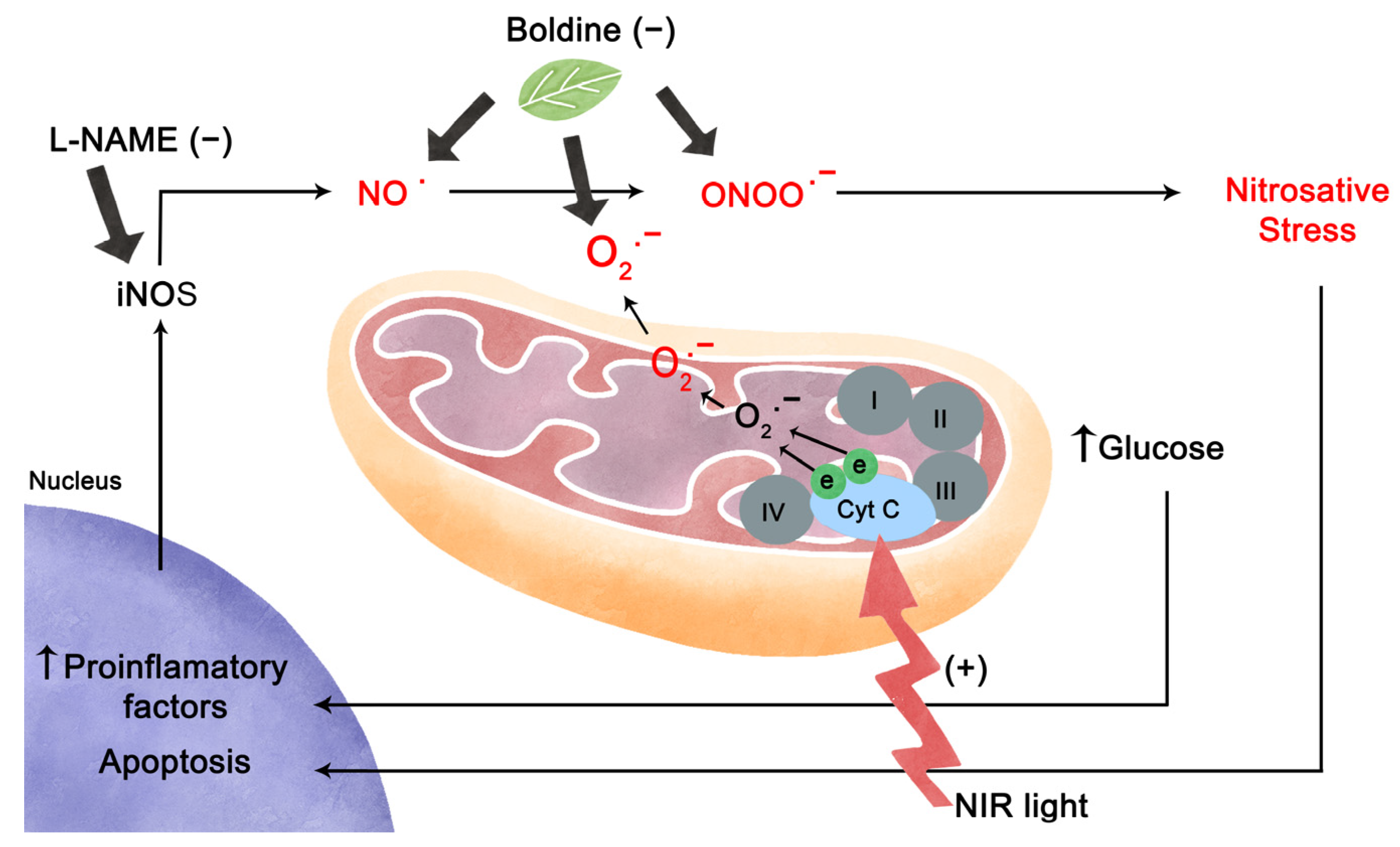
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Organotypic Retinal Explant Culture
4.3. Immunostainings
4.4. TUNEL Assay
4.5. Transmission Electron Microscopy (TEM)
4.6. FRET Measurements
4.7. Retinal Slice Preparation for FRET Experiments
4.8. Photobiomodulation
4.9. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruta, L.M.; Magliano, D.J.; Lemesurier, R.; Taylor, H.R.; Zimmet, P.Z.; Shaw, J.E. Review Article Prevalence of Diabetic Retinopathy in Type 2 Diabetes in Developing and Developed Countries. Diabet. Med. 2013, 30, 387–398. [Google Scholar] [CrossRef]
- Lasker/IRRF. Diabetic Retinopathy—Where We Are and a Path to Progress. Lasker/IRRF Initiat. Innov. Vis. Sci. 2014. [Google Scholar]
- Cunha-Vaz, J. Diabetic Retinopathy; World Scientific Publishing: Singapore, 2011; ISBN 9814304433. [Google Scholar]
- Tzekov, R.; Arden, G.B. The Electroretinogram in Diabetic Retinopathy. Surv. Ophthalmol. 1999, 44, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Kohzaki, K.; Vingrys, A.J.; Bui, B.V. Early Inner Retinal Dysfunction in Streptozotocin-Induced Diabetic Rats. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3595–3604. [Google Scholar] [CrossRef] [PubMed]
- Luu, C.D.; Szental, J.A.; Lee, S.Y.; Lavanya, R.; Wong, T.Y. Correlation between Retinal Oscillatory Potentials and Retinal Vascular Caliber in Type 2 Diabetes. Investig. Ophthalmol. Vis. Sci. 2010, 51, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Aung, M.H.; Kim, M.K.; Olson, D.E.; Thule, P.M.; Pardue, M.T. Early Visual Deficits in Streptozotocin-Induced Diabetic Long Evans Rats. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1370–1377. [Google Scholar] [CrossRef]
- Woodruff, L.D.; Bounkeo, J.M.; Brannon, W.M.; Dawes, K.S.; Barham, C.D.; Waddell, D.L.; Enwemeka, C.S. The Efficacy of Laser Therapy in Wound Repair: A Meta-Analysis of the Literature. Photomed. Laser Surg. 2004, 22, 241–247. [Google Scholar] [CrossRef]
- Chung, H.; Dai, T.; Sharma, S.K.; Huang, Y.Y.; Carroll, J.D.; Hamblin, M.R. The Nuts and Bolts of Low-Level Laser (Light) Therapy. Ann. Biomed. Eng. 2012, 40, 516–533. [Google Scholar] [CrossRef]
- Jagdeo, J.; Austin, E.; Mamalis, A.; Wong, C.; Ho, D.; Siegel, D.M. Light-Emitting Diodes in Dermatology: A Systematic Review of Randomized Controlled Trials. Lasers Surg. Med. 2018, 50, 613–628. [Google Scholar] [CrossRef]
- Escudero, J.S.B.; Perez, M.G.B.; de Oliveira Rosso, M.P.; Buchaim, D.V.; Pomini, K.T.; Campos, L.M.G.; Audi, M.; Buchaim, R.L. Photobiomodulation Therapy (PBMT) in Bone Repair: A Systematic Review. Injury 2019, 50, 1853–1867. [Google Scholar] [CrossRef]
- Albarracin, R.; Eells, J.; Valter, K. Photobiomodulation Protects the Retina from Light-Induced Photoreceptor Degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3582–3592. [Google Scholar] [CrossRef]
- Albarracin, R.; Natoli, R.; Rutar, M.; Valter, K.; Provis, J. Degeneration in C57BL/6J Mouse Retina. BMC Neurosci. 2013, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Geneva, I.I. Photobiomodulation for the Treatment of Retinal Diseases: A Review. Int. J. Ophthalmol. 2016, 9, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Heinig, N.; Schumann, U.; Calzia, D.; Panfoli, I.; Ader, M.; Schmidt, M.H.H.; Funk, R.H.W.; Roehlecke, C. Photobiomodulation Mediates Neuroprotection against Blue Light Induced Retinal Photoreceptor Degeneration. Int. J. Mol. Sci. 2020, 21, 2370. [Google Scholar] [CrossRef] [PubMed]
- Natoli, R.; Valter, K.; Barbosa, M.; Dahlstrom, J.; Rutar, M.; Kent, A.; Provis, J. 670 nm Photobiomodulation as a Novel Protection against Retinopathy of Prematurity: Evidence from Oxygen Induced Retinopathy Models. PLoS ONE 2013, 8, e72135. [Google Scholar] [CrossRef]
- Kent, A.L.; Abdel-Latif, M.E.; Cochrane, T.; Broom, M.; Dahlstrom, J.E.; Essex, R.W.; Shadbolt, B.; Natoli, R. A Pilot Randomised Clinical Trial of 670 Nm Red Light for Reducing Retinopathy of Prematurity. Pediatr. Res. 2020, 87, 131–136. [Google Scholar] [CrossRef]
- Grewal, M.K.; Sivapathasuntharam, C.; Chandra, S.; Gurudas, S.; Chong, V.; Bird, A.; Jeffery, G.; Sivaprasad, S. A Pilot Study Evaluating the Effects of 670 Nm Photobiomodulation in Healthy Ageing and Age-Related Macular Degeneration. J. Clin. Med. 2020, 9, 1001. [Google Scholar] [CrossRef]
- Markowitz, S.N.; Devenyi, R.G.; Munk, M.R.; Croissant, C.L.; Tedford, S.E.; Rückert, R.; Walker, M.G.; Patino, B.E.; Chen, L.; Nido, M.; et al. A Double-Masked, Randomized, Sham-Controlled, Single-Center Study With Photobiomodulation for the Treatment of Dry Age-Related Macular Degeneration. Retina 2020, 40, 1471–1482. [Google Scholar] [CrossRef]
- Tang, J.; Du, Y.; Lee, C.A.; Talahalli, R.; Eells, J.T.; Kern, T.S. Low-Intensity Far-Red Light Inhibits Early Lesions That Contribute to Diabetic Retinopathy: In Vivo and in Vitro. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3681–3690. [Google Scholar] [CrossRef]
- Yang, M.; Chen, Y.; Vagionitis, S.; Körtvely, E.; Ueffing, M.; Schmachtenberg, O.; Hu, Z.; Jiao, K.; Paquet-Durand, F. Expression of Glucose Transporter-2 in Murine Retina: Evidence for Glucose Transport from Horizontal Cells to Photoreceptor Synapses. J. Neurochem. 2022, 160, 283–296. [Google Scholar] [CrossRef]
- Saliba, A.; Du, Y.; Liu, H.; Patel, S.; Roberts, R.; Berkowitz, B.A.; Kern, T.S. Photobiomodulation Mitigates Diabetes-Induced Retinopathy by Direct and Indirect Mechanisms: Evidence from Intervention Studies in Pigmented Mice. PLoS ONE 2015, 10, e0139003. [Google Scholar] [CrossRef]
- Cheng, Y.; Du, Y.; Liu, H.; Tang, J.; Veenstra, A.; Kern, T.S. Photobiomodulation Inhibits Long-Term Structural and Functional Lesions of Diabetic Retinopathy. Diabetes 2018, 67, 291–298. [Google Scholar] [CrossRef]
- Shen, W.; Teo, K.Y.C.; Wood, J.P.M.; Vaze, A.; Chidlow, G.; Ao, J.; Lee, S.R.; Yam, M.X.; Cornish, E.E.; Fraser-Bell, S.; et al. Preclinical and Clinical Studies of Photobiomodulation Therapy for Macular Oedema. Diabetologia 2020, 63, 1900–1915. [Google Scholar] [CrossRef]
- Kim, J.E.; Glassman, A.R.; Josic, K.; Melia, M.; Aiello, L.P.; Baker, C.; Eells, J.T.; Jampol, L.M.; Kern, T.S.; Marcus, D.; et al. A Randomized Trial of Photobiomodulation Therapy for Center-Involved Diabetic Macular Edema with Good Visual Acuity (Protocol AE). Ophthalmol. Retin. 2022, 6, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Muste, J.C.; Russell, M.W.; Singh, R.P. Photobiomodulation Therapy for Age-Related Macular Degeneration and Diabetic Retinopathy: A Review. Clin. Ophthalmol. 2021, 15, 3709–3720. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, P.; Corraliza, L.; Villena, J.A.; Carvalho, A.R.; Garcia-Arumí, J.; Ramos, D.; Ruberte, J.; Simó, R.; Hernández, C. The Db/Db Mouse: A Useful Model for the Study of Diabetic Retinal Neurodegeneration. PLoS ONE 2014, 9, e97302. [Google Scholar] [CrossRef]
- Speisky, H.; Cassels, B.K. Boldo and Boldine: An Emerging Case of Natural Drug Development. Pharmacol. Res. 1994, 29, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rowe, A.A.; Patel, P.D.; Gordillo, R.; Wert, K.J. Replenishment of TCA Cycle Intermediates Provides Photoreceptor Resilience against Neurodegeneration during Progression of Retinitis Pigmentosa. JCI Insight 2021, 6, e150898. [Google Scholar] [CrossRef]
- Barot, M.; Gokulgandhi, M.R.; Mitra, A.K. Mitochondrial Dysfunction in Retinal Diseases. Curr. Eye Res. 2011, 36, 1069–1077. [Google Scholar] [CrossRef]
- Xie, L.L.; Shi, F.; Tan, Z.; Li, Y.; Bode, A.M.; Cao, Y. Mitochondrial Network Structure Homeostasis and Cell Death. Cancer Sci. 2018, 109, 3686–3694. [Google Scholar] [CrossRef]
- Valdés, J.; Trachsel-Moncho, L.; Sahaboglu, A.; Trifunović, D.; Miranda, M.; Ueffing, M.; Paquet-Durand, F.; Schmachtenberg, O. Organotypic Retinal Explant Cultures as in Vitro Alternative for Diabetic Retinopathy Studies. ALTEX 2016, 33, 459–464. [Google Scholar] [CrossRef]
- Calbiague, V.M.; Vielma, A.H.; Cadiz, B.; Paquet-Durand, F.; Schmachtenberg, O. Physiological Assessment of High Glucose Neurotoxicity in Mouse and Rat Retinal Explants. J. Comp. Neurol. 2020, 528, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, D.R.; Gardiner, N.J. Glucose Neurotoxicity. Nat. Rev. Neurosci. 2008, 9, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ruiz, A.; Cadenas, S.; Lamas, S. Nitric Oxide Signaling: Classical, Less Classical, and Nonclassical Mechanisms. Free Radic. Biol. Med. 2011, 51, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Opatrilova, R.; Kubatka, P.; Caprnda, M.; Büsselberg, D.; Krasnik, V.; Vesely, P.; Saxena, S.; Ruia, S.; Mozos, I.; Rodrigo, L.; et al. Nitric Oxide in the Pathophysiology of Retinopathy: Evidences from Preclinical and Clinical Researches. Acta Ophthalmol. 2018, 96, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.J.; Hogg, N.; Joseph, J.; Konorev, E.; Kalyanaraman, B. The Peroxynitrite Generator, SIN-1, Becomes a Nitric Oxide Donor in the Presence-of Electron Acceptors. Arch. Biochem. Biophys. 1999, 361, 331–339. [Google Scholar] [CrossRef]
- Yi, C.; Ezan, P.; Fernández, P.; Schmitt, J.; Sáez, J.C.; Giaume, C.; Koulakoff, A. Inhibition of Glial Hemichannels by Boldine Treatment Reduces Neuronal Suffering in a Murine Model of Alzheimer’s Disease. Glia 2017, 65, 1607–1625. [Google Scholar] [CrossRef]
- Otero, C.; Miranda-Rojas, S.; Llancalahuén, F.M.; Fuentes, J.A.; Atala, C.; González-Silva, G.; Verdugo, D.; Sierra-Rosales, P.; Moreno, A.; Gordillo-Fuenzalida, F. Biochemical Characterization of Peumus Boldus Fruits: Insights of Its Antioxidant Properties through a Theoretical Approach. Food Chem. 2022, 370, 131012. [Google Scholar] [CrossRef]
- Bandookwala, M.; Sengupta, P. 3-Nitrotyrosine: A Versatile Oxidative Stress Biomarker for Major Neurodegenerative Diseases. Int. J. Neurosci. 2020, 130, 1047–1062. [Google Scholar] [CrossRef]
- Vielma, A.H.; Retamal, M.A.; Schmachtenberg, O. Nitric Oxide Signaling in the Retina: What Have We Learned in Two Decades? Brain Res. 2012, 1430, 112–125. [Google Scholar] [CrossRef]
- Boughaleb, H.; Lobysheva, I.; Dei Zotti, F.; Balligand, J.L.; Montiel, V. Biological Assessment of the NO-Dependent Endothelial Function. Molecules 2022, 27, 7921. [Google Scholar] [CrossRef]
- Tang, J.; Kern, T. Inflammation in Diabetic Retinopathy. Prog. Retin. Eye Res. 2011, 30, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.X.; Lou, Y.; Chi, J.; Bao, X.L.; Fan, B.; Li, G.Y. Considerations for the Use of Photobiomodulation in the Treatment of Retinal Diseases. Biomolecules 2022, 12, 1811. [Google Scholar] [CrossRef] [PubMed]
- Silveira, P.C.L.; Ferreira, G.K.; Zaccaron, R.P.; Glaser, V.; Remor, A.P.; Mendes, C.; Pinho, R.A.; Latini, A. Effects of Photobiomodulation on Mitochondria of Brain, Muscle, and C6 Astroglioma Cells. Med. Eng. Phys. 2019, 71, 108–113. [Google Scholar] [CrossRef]
- Lunova, M.; Smolková, B.; Uzhytchak, M.; Janoušková, K.Ž.; Jirsa, M.; Egorova, D.; Kulikov, A.; Kubinová, Š.; Dejneka, A.; Lunov, O. Light-Induced Modulation of the Mitochondrial Respiratory Chain Activity: Possibilities and Limitations. Cell. Mol. Life Sci. 2020, 77, 2815–2838. [Google Scholar] [CrossRef]
- Hurley, J.B.; Lindsay, K.J.; Du, J. Mini-Review Glucose, Lactate, and Shuttling of Metabolites in Vertebrate Retinas. J. Neurosci. Res. 2015, 1092, 1079–1092. [Google Scholar] [CrossRef]
- Vecino, E.; Rodriguez, F.D.; Ruzafa, N.; Pereiro, X.; Sharma, S.C. Glia-Neuron Interactions in the Mammalian Retina. Prog. Retin. Eye Res. 2016, 51, 1–40. [Google Scholar] [CrossRef]
- Vohra, R.; Gurubaran, I.S.; Henriksen, U.; Bergersen, L.H.; Rasmussen, L.J.; Desler, C.; Skytt, D.M.; Kolko, M. Disturbed Mitochondrial Function Restricts Glutamate Uptake in the Human Müller Glia Cell Line, MIO-M1. Mitochondrion 2017, 36, 52–59. [Google Scholar] [CrossRef]
- Calbiague Garcia, V.; Chen, Y.; Cádiz, B.; Paquet-durand, F. Imaging of Lactate Metabolism in Retinal Müller Cells with a FRET Nanosensor. Exp. Eye Res. 2023, 226, 109352. [Google Scholar] [CrossRef] [PubMed]
- Poole, R.C.; Halestrap, A.P. Transport of Lactate and Other Monocarboxylates across Mammalian Plasma Membranes. Am. J. Physiol.-Cell Physiol. 1993, 264, 761–782. [Google Scholar] [CrossRef]
- Bittner, C.X. High Resolution Measurement of the Glycolytic Rate. Front. Neuroenergetics 2010, 2, 26. [Google Scholar] [CrossRef] [PubMed]
- San Martín, A.; Ceballo, S.; Ruminot, I.; Lerchundi, R.; Frommer, W.B.; Barros, L.F. A Genetically Encoded FRET Lactate Sensor and Its Use To Detect the Warburg Effect in Single Cancer Cells. PLoS ONE 2013, 8, e57712. [Google Scholar] [CrossRef] [PubMed]
- Salehpour, F.; Mahmoudi, J.; Kamari, F.; Sadigh-Eteghad, S.; Rasta, S.H.; Hamblin, M.R. Brain Photobiomodulation Therapy: A Narrative Review. Mol. Neurobiol. 2018, 55, 6601–6636. [Google Scholar] [CrossRef] [PubMed]
- Shanks, S.; Leisman, G. Perspective on Broad-Acting Clinical Physiological Effects of Photobiomodulation. Adv. Exp. Med. Biol. 2018, 1096, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Basta, D.; Gröschel, M.; Strübing, I.; Boyle, P.; Fröhlich, F.; Ernst, A.; Seidl, R. Near-Infrared-Light Pre-Treatment Attenuates Noise-Induced Hearing Loss in Mice. PeerJ 2020, 2020, 1–18. [Google Scholar] [CrossRef]
- Foo, A.S.C.; Soong, T.W.; Yeo, T.T.; Lim, K.L. Mitochondrial Dysfunction and Parkinson’s Disease—Near-Infrared Photobiomodulation as a Potential Therapeutic Strategy. Front. Aging Neurosci. 2020, 12, 89. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Dutta, A. Computational Modeling of the Photon Transport, Tissue Heating, and Cytochrome c Oxidase Absorption during Transcranial near-Infrared Stimulation. Brain Sci. 2019, 9, 179. [Google Scholar] [CrossRef]
- Beauvoit, B.; Kitai, T.; Chance, B. Contribution of the Mitochondrial Compartment to the Optical Properties of the Rat Liver: A Theoretical and Practical Approach. Biophys. J. 1994, 67, 2501–2510. [Google Scholar] [CrossRef]
- Karu, T. Primary and Secondary Mechanisms of Action of Visible to Near-IR Radiation on Cells. J. Photochem. Photobiol. B Biol. 1999, 49, 1–17. [Google Scholar] [CrossRef]
- Karu, T.I.; Pyatibrat, L.V.; Kalendo, G.S. Photobiological Modulation of Cell Attachment via Cytochrome c Oxidase. Photochem. Photobiol. Sci. 2004, 3, 211–216. [Google Scholar] [CrossRef]
- Mason, M.G.; Nicholls, P.; Cooper, C.E. Re-Evaluation of the near Infrared Spectra of Mitochondrial Cytochrome c Oxidase: Implications for Non Invasive in Vivo Monitoring of Tissues. Biochim. Biophys. Acta-Bioenerg. 2014, 1837, 1882–1891. [Google Scholar] [CrossRef] [PubMed]
- Beirne, K.; Rozanowska, M.; Votruba, M. Photostimulation of Mitochondria as a Treatment for Retinal Neurodegeneration. Mitochondrion 2017, 36, 85–95. [Google Scholar] [CrossRef]
- Wong-Riley, M.T.T.; Liang, H.L.; Eells, J.T.; Chance, B.; Henry, M.M.; Buchmann, E.; Kane, M.; Whelan, H.T. Photobiomodulation Directly Benefits Primary Neurons Functionally Inactivated by Toxins: Role of Cytochrome c Oxidase. J. Biol. Chem. 2005, 280, 4761–4771. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Mechanisms and Mitochondrial Redox Signaling in Photobiomodulation. Photochem. Photobiol. 2018, 94, 199–212. [Google Scholar] [CrossRef]
- Gonzalez-Lima, F. Rojas Low-Level Light Therapy of the Eye and Brain. Eye Brain 2011, 3, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Genova, M.L.; Lenaz, G. Functional Role of Mitochondrial Respiratory Supercomplexes. Biochim. Biophys. Acta-Bioenerg. 2014, 1837, 427–443. [Google Scholar] [CrossRef]
- Guerra-Castellano, A.; Díaz-Quintana, A.; Pérez-Mejías, G.; Elena-Real, C.A.; González-Arzola, K.; García-Mauriño, S.M.; De la Rosa, M.A.; Díaz-Moreno, I. Oxidative Stress Is Tightly Regulated by Cytochrome c Phosphorylation and Respirasome Factors in Mitochondria. Proc. Natl. Acad. Sci. USA 2018, 115, 7955–7960. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.C.; Park, J.A.; Kim, D.K.; Lee, J.H. Photobiomodulation (660 nm) Therapy Reduces Oxidative Stress and Induces BDNF Expression in the Hippocampus. Sci. Rep. 2019, 9, 10114. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Bolaños, J.P. Nitric Oxide, Cell Bioenergetics and Neurodegeneration. J. Neurochem. 2006, 97, 1676–1689. [Google Scholar] [CrossRef]
- Bellamy, T.C.; Griffiths, C.; Garthwaite, J. Differential Sensitivity of Guanylyl Cyclase and Mitochondrial Respiration to Nitric Oxide Measured Using Clamped Concentrations. J. Biol. Chem. 2002, 277, 31801–31807. [Google Scholar] [CrossRef]
- Yu, W.; Naim, J.O.; McGowan, M.; Ippolito, K.; Lanzafame, R.J. Photomodulation of Oxidative Metabolism and Electron Chain Enzymes in Rat Liver Mitochondria. Photochem. Photobiol. 1997, 66, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Passarella, S.; Casamassima, E.; Molinari, S.; Pastore, D.; Quagliariello, E.; Catalano, I.M.; Cingolani, A. Increase of Proton Electrochemical Potential and ATP Synthesis in Rat Liver Mitochondria Irradiated in Vitro by Helium-Neon Laser. FEBS Lett. 1984, 175, 95–99. [Google Scholar] [CrossRef]
- Morimoto, Y.; Aral, T.; Kikuchi, M.; Nakajima, S.; Nakamura, H. Effect of Low-intensity Argon Laser Irradiation on Mitochondrial Respiration. Lasers Surg. Med. 1994, 15, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Oron, U.; Yaakobi, T.; Oron, A.; Mordechovitz, D.; Shofti, R.; Hayam, G.; Dror, U.; Gepstein, L.; Wolf, T.; Haudenschild, C.; et al. Low-Energy Laser Irradiation Reduces Formation of Scar Tissue after Myocardial Infarction in Rats and Dogs. Circulation 2001, 103, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, S.; Fong, C.C.; Tsang, C.H.; Yang, Z.; Yang, M. CDNA Microarray Analysis of Gene Expression Profiles in Human Fibroblast Cells Irradiated with Red Light. J. Investig. Dermatol. 2003, 120, 849–857. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.Y.; Wang, Y.; Lyu, P.; Hamblin, M.R. Red (660 nm) or near-Infrared (810 nm) Photobiomodulation Stimulates, While Blue (415 nm), Green (540 nm) Light Inhibits Proliferation in Human Adipose-Derived Stem Cells. Sci. Rep. 2017, 7, 7781. [Google Scholar] [CrossRef]
- Feng, R.; Morine, Y.; Tetsuya, I.; Imura, S.; Iwahashi, S.; Saito, Y.; Shimada, M. Photobiomodulation with Red Light-emitting Diodes Accelerates Hepatocyte Proliferation.Pdf. Hepatol. Res. 2018, 48, 926–936. [Google Scholar] [CrossRef]
- Chang, B.; Qiu, H.; Zhao, H.; Yang, X.; Wang, Y.; Ji, T.; Zhang, Y.; Quan, Q.; Li, Y.; Zeng, J.; et al. The Effects of Photobiomodulation on MC3T3-E1 Cells via 630 Nm and 810 Nm Light-Emitting Diode. Med. Sci. Monit. 2019, 25, 8744–8752. [Google Scholar] [CrossRef]
- Yang, L.; Tucker, D.; Dong, Y.; Wu, C.; Lu, Y.; Li, Y.; Zhang, J.; Liu, T.C.Y.; Zhang, Q. Photobiomodulation Therapy Promotes Neurogenesis by Improving Post-Stroke Local Microenvironment and Stimulating Neuroprogenitor Cells. Exp. Neurol. 2018, 299, 86–96. [Google Scholar] [CrossRef]
- Quirk, B.J.; Whelan, H.T. Effect of Red-to-near Infrared Light on the Reaction of Isolated Cytochrome c Oxidase with Cytochrome C. Photomed. Laser Surg. 2016, 34, 631–637. [Google Scholar] [CrossRef]
- Rajendran, N.K.; George, B.P.; Chandran, R.; Tynga, I.M.; Houreld, N.; Abrahamse, H. The Influence of Light on Reactive Oxygen Species and NF-KB in Disease Progression. Antioxidants 2019, 8, 640. [Google Scholar] [CrossRef] [PubMed]
- Tonade, D.; Liu, H.; Kern, T.S. Photoreceptor Cells Produce Inflammatory Mediators That Contribute to Endothelial Cell Death in Diabetes. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4264–4271. [Google Scholar] [CrossRef]
- Zheng, L.; Kern, T. Role of Nitric Oxide, Superoxide, Peroxynitrate and PARP in Diabetic Retinopathy. Front. Biosci. 2009, 14, 3974–3987. [Google Scholar] [CrossRef]
- Yu, T.; Robotham, J.L.; Yoon, Y. Increased Production of Reactive Oxygen Species in Hyperglycemic Conditions Requires Dynamic Change of Mitochondrial Morphology. Proc. Natl. Acad. Sci. USA 2006, 103, 2653–2658. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Park, J.W.; Park, S.J.; Kim, K.Y.; Chung, J.W.; Chun, M.H.; Oh, S.J. Apoptotic Death of Photoreceptors in the Streptozotocin-Induced Diabetic Rat Retina. Diabetologia 2003, 46, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Énzsöly, A.; Szabó, A.; Kántor, O.; Dávid, C.; Szalay, P.; Szabó, K.; Szél, Á.; Németh, J.; Lukáts, Á. Pathologic Alterations of the Outer Retina in Streptozotocin-Induced Diabetes. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3686–3699. [Google Scholar] [CrossRef]
- Du, Y.; Veenstra, A.; Palczewski, K.; Kern, T.S. Photoreceptor Cells Are Major Contributors to Diabetes-Induced Oxidative Stress and Local Inflammation in the Retina. Proc. Natl. Acad. Sci. USA 2013, 110, 16586–16591. [Google Scholar] [CrossRef]
- Kern, T.S.; Berkowitz, B.A. Photoreceptors in Diabetic Retinopathy. J. Diabetes Investig. 2015, 6, 371–380. [Google Scholar] [CrossRef]
- Franco, M.C.; Estévez, A.G. Tyrosine Nitration as Mediator of Cell Death. Cell. Mol. Life Sci. 2014, 71, 3939–3950. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Ramdial, K.; Franco, M.C.; Estevez, A.G. Cellular Mechanisms of Peroxynitrite-Induced Neuronal Death. Brain Res. Bull. 2017, 133, 4–11. [Google Scholar] [CrossRef]
- Rodriguez-Carrizalez, A.; Castellanos-González, J.; Martinez-Romero, E.; Miller-Arrevillaga, G.; Villa-Hernández, D.; Hernández-Godínez, P.; Ortiz, G.; Pachero-Moisés, F.; Cardona-Muñoz, E.; Miranda-Díaz, A. Oxidants Antioxidants and Mitochondrial Function in Non-proliferative.Pdf. J. Diabetes 2014, 6, 167–175. [Google Scholar] [CrossRef]
- Schaefer, S.; Kajimura, M.; Tsuyama, S.; Uchida, K.; Sato, E.; Inoue, M.; Suematsu, M.; Watanabe, K. Aberrant Utilization of Nitric Oxide and Regulation of Soluble Guanylate Cyclase in Rat Diabetic Retinopathy. Antioxid. Redox Signal. 2003, 5, 457–465. [Google Scholar] [CrossRef]
- Du, Y.; Smith, M.A.; Miller, C.M.; Kern, T.S. Diabetes-Induced Nitrative Stress in the Retina, and Correction by Aminoguanidine. J. Neurochem. 2002, 80, 771–779. [Google Scholar] [CrossRef]
- Mandal, L.K.; Choudhuri, S.; Dutta, D.; Mitra, B.; Kundu, S.; Chowdhury, I.H.; Sen, A.; Chatterjee, M.; Bhattacharya, B. Oxidative Stress-Associated Neuroretinal Dysfunction and Nitrosative Stress in Diabetic Retinopathy. Can. J. Diabetes 2013, 37, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.F.; Casellini, C.M.; Parson, H.K.; Obrosova, I.G.; Yorek, M.; Vinik, A.I. Role of Peroxynitrite in the Development of Diabetic Peripheral Neuropathy. Diabetes Care 2015, 38, e100–e101. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.S.; Tian, X.Y.; Mustafa, M.R.; Murugan, D.; Liu, J.; Zhang, Y.; Lau, C.W.; Huang, Y. Boldine Improves Endothelial Function in Diabetic Db/Db Mice through Inhibition of Angiotensin II-Mediated BMP4-Oxidative Stress Cascade. Br. J. Pharmacol. 2013, 170, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.S.; Ling, W.C.; Murugan, D.; Mustafa, M.R. Boldine Ameliorates Vascular Oxidative Stress and Endothelial Dysfunction: Therapeutic Implication for Hypertension and Diabetes. J. Cardiovasc. Pharmacol. 2015, 65, 522–531. [Google Scholar] [CrossRef]
- Jang, Y.Y.; Song, J.H.; Shin, Y.K.; Han, E.S.; Lee, C.S. Protective Effect of Boldine on Oxidative Mitochondrial Damage in Streptozotocin-Induced Diabetic Rats. Pharmacol. Res. 2000, 42, 361–371. [Google Scholar] [CrossRef]
- Shahraki, Z.S.; Karbalaei, N.; Nemati, M. Improving Effect of Combined Inorganic Nitrate and Nitric Oxide Synthase Inhibitor on Pancreatic Oxidative Stress and Impaired Insulin Secretion in Streptozotocin Induced-Diabetic Rats. J. Diabetes Metab. Disord. 2020, 19, 353–362. [Google Scholar] [CrossRef]
- Ahlawat, A.; Sharma, S. A New Promising Simultaneous Approach for Attenuating Type II Diabetes Mellitus Induced Neuropathic Pain in Rats: INOS Inhibition and Neuroregeneration. Eur. J. Pharmacol. 2018, 818, 419–428. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calbiague García, V.; Cadiz, B.; Herrera, P.; Díaz, A.; Schmachtenberg, O. Evaluation of Photobiomodulation and Boldine as Alternative Treatment Options in Two Diabetic Retinopathy Models. Int. J. Mol. Sci. 2023, 24, 7918. https://doi.org/10.3390/ijms24097918
Calbiague García V, Cadiz B, Herrera P, Díaz A, Schmachtenberg O. Evaluation of Photobiomodulation and Boldine as Alternative Treatment Options in Two Diabetic Retinopathy Models. International Journal of Molecular Sciences. 2023; 24(9):7918. https://doi.org/10.3390/ijms24097918
Chicago/Turabian StyleCalbiague García, Víctor, Bárbara Cadiz, Pablo Herrera, Alejandra Díaz, and Oliver Schmachtenberg. 2023. "Evaluation of Photobiomodulation and Boldine as Alternative Treatment Options in Two Diabetic Retinopathy Models" International Journal of Molecular Sciences 24, no. 9: 7918. https://doi.org/10.3390/ijms24097918
APA StyleCalbiague García, V., Cadiz, B., Herrera, P., Díaz, A., & Schmachtenberg, O. (2023). Evaluation of Photobiomodulation and Boldine as Alternative Treatment Options in Two Diabetic Retinopathy Models. International Journal of Molecular Sciences, 24(9), 7918. https://doi.org/10.3390/ijms24097918






