Honey’s Yeast—New Source of Valuable Species for Industrial Applications
Abstract
1. Introduction
2. Results and Discussion
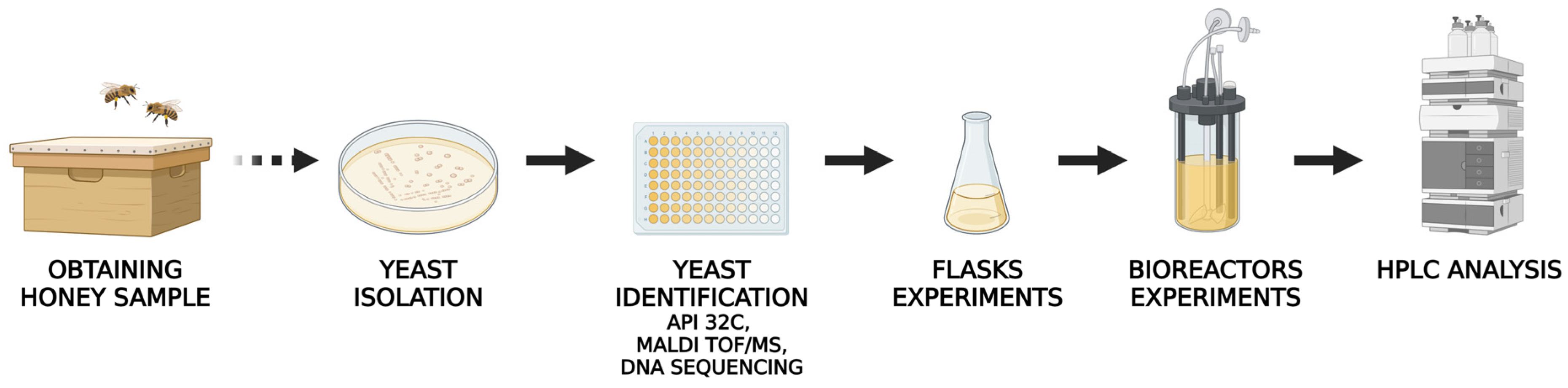
2.1. Yeast Isolation and Identification
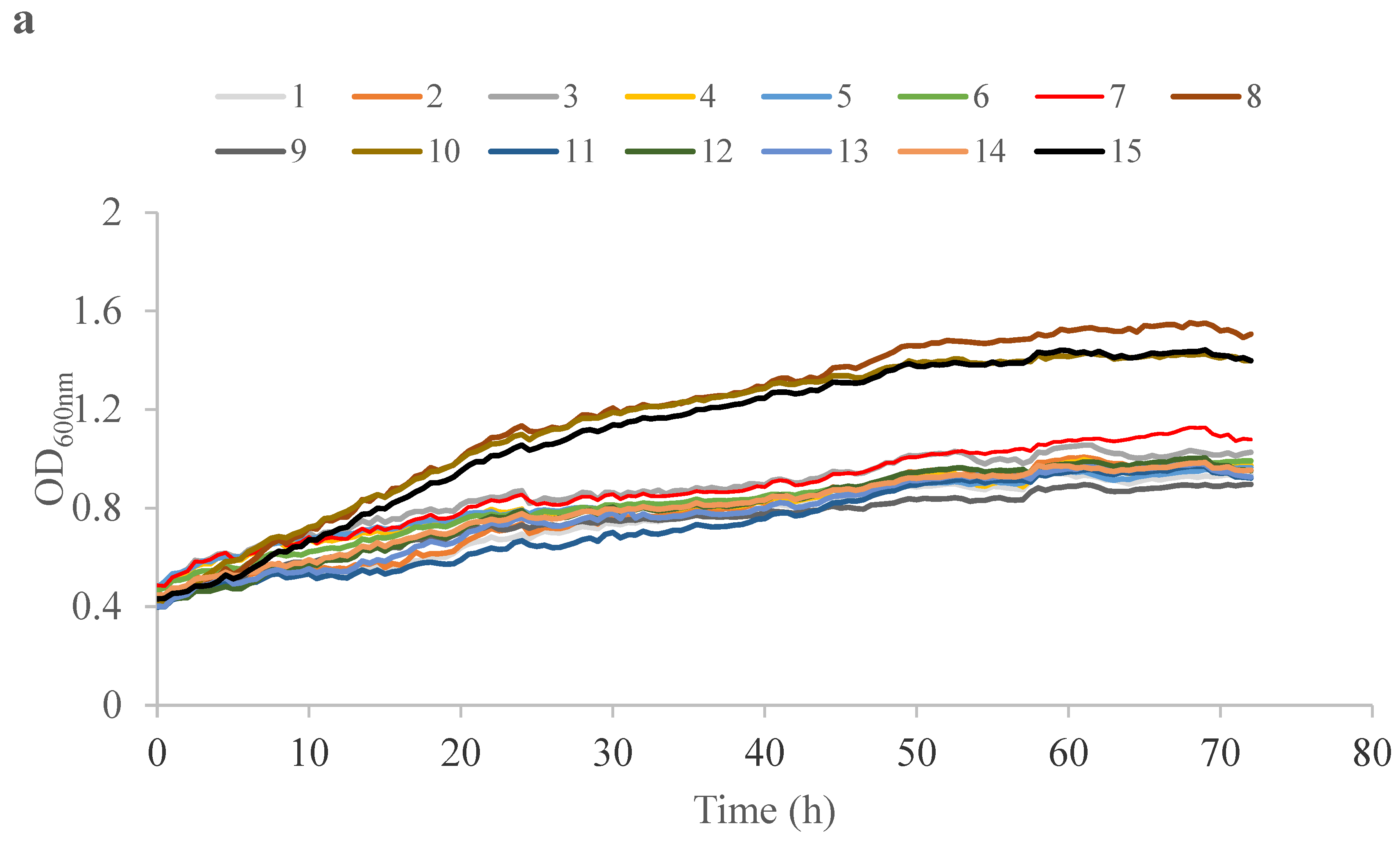
2.2. Growth of Yeasts Isolated from Honey on Different Carbon Sources
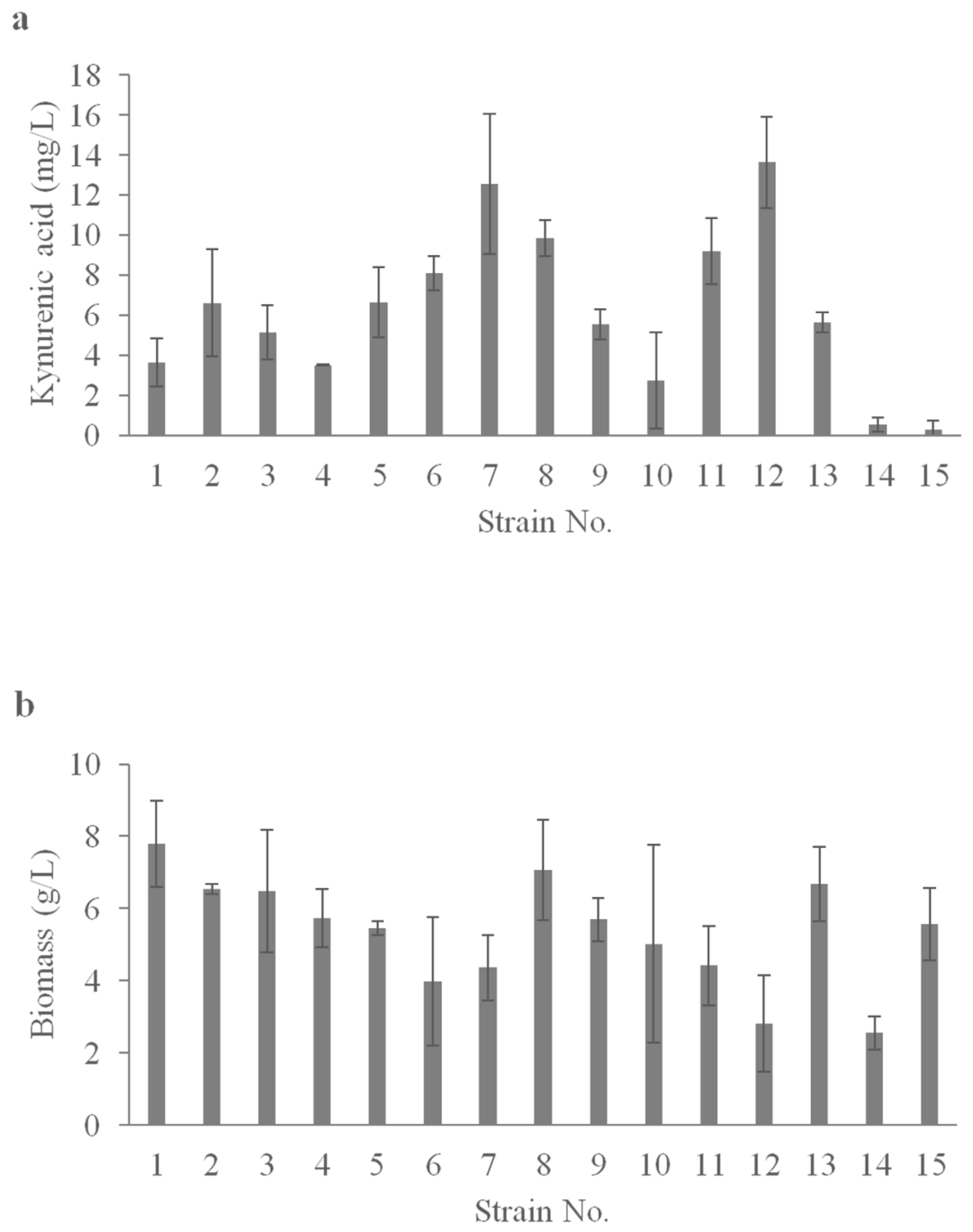
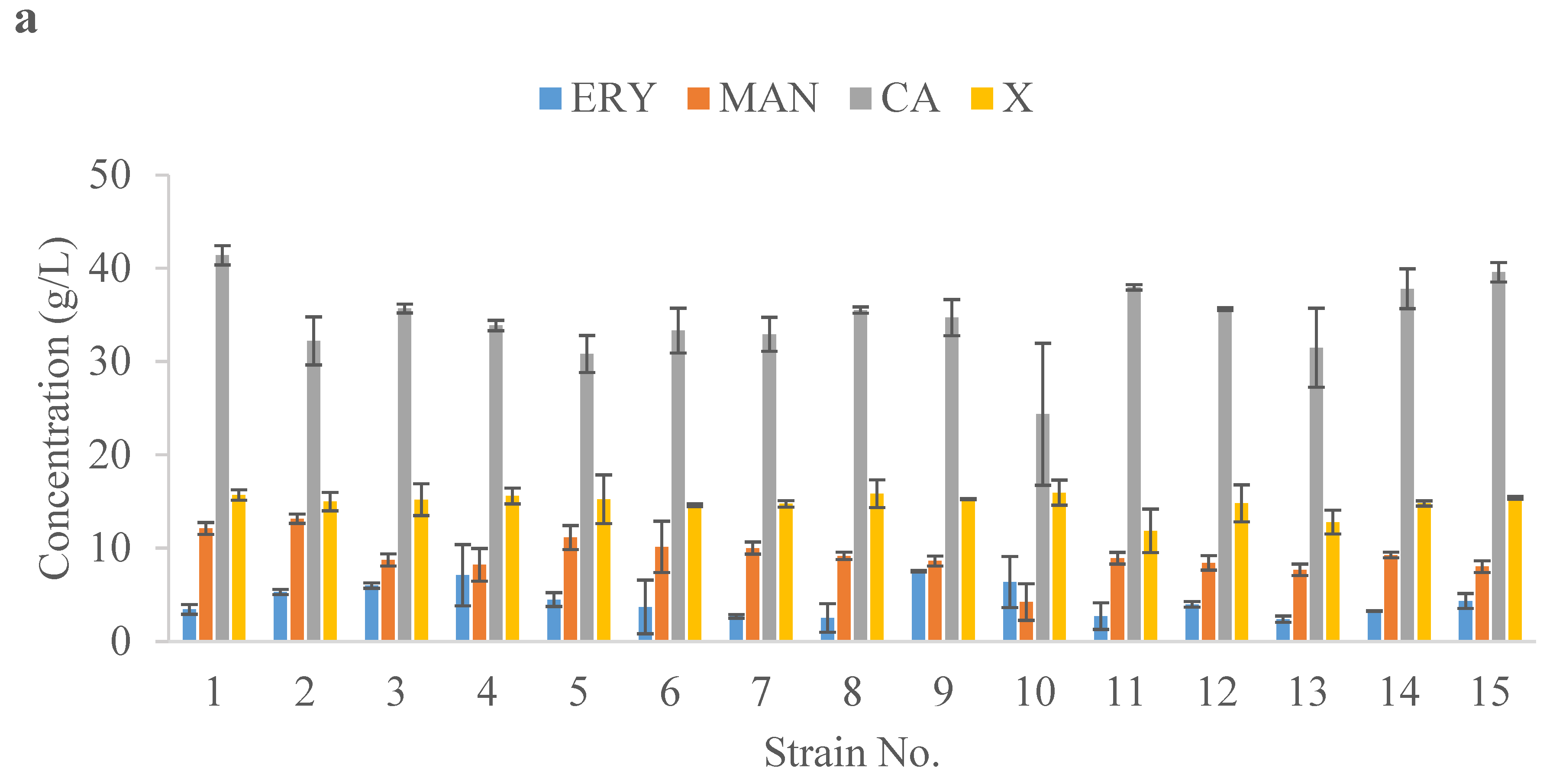
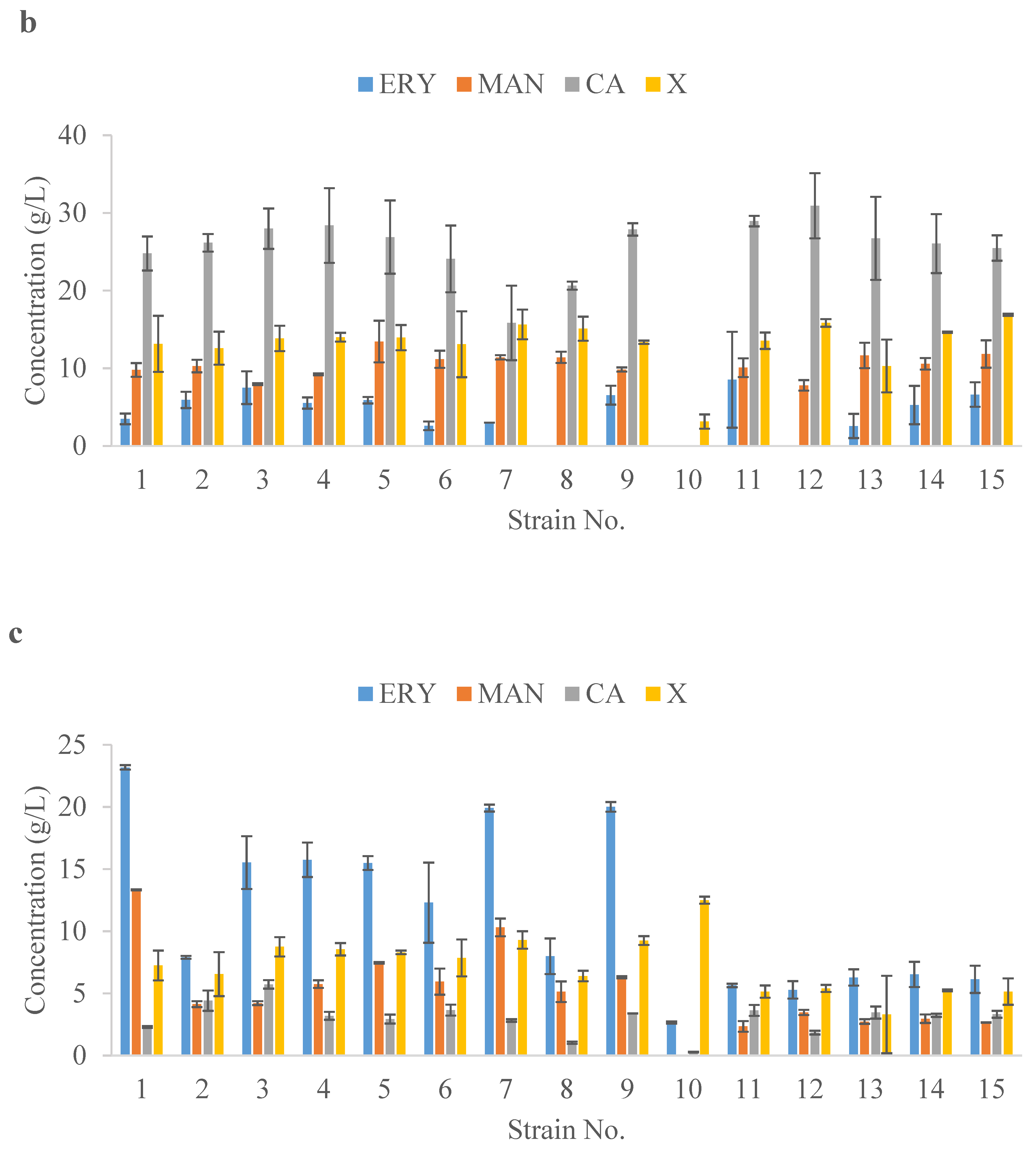
2.3. Value-Added Chemical Synthesis Using Yeast Isolated from Honey in Shake-Flask Screening
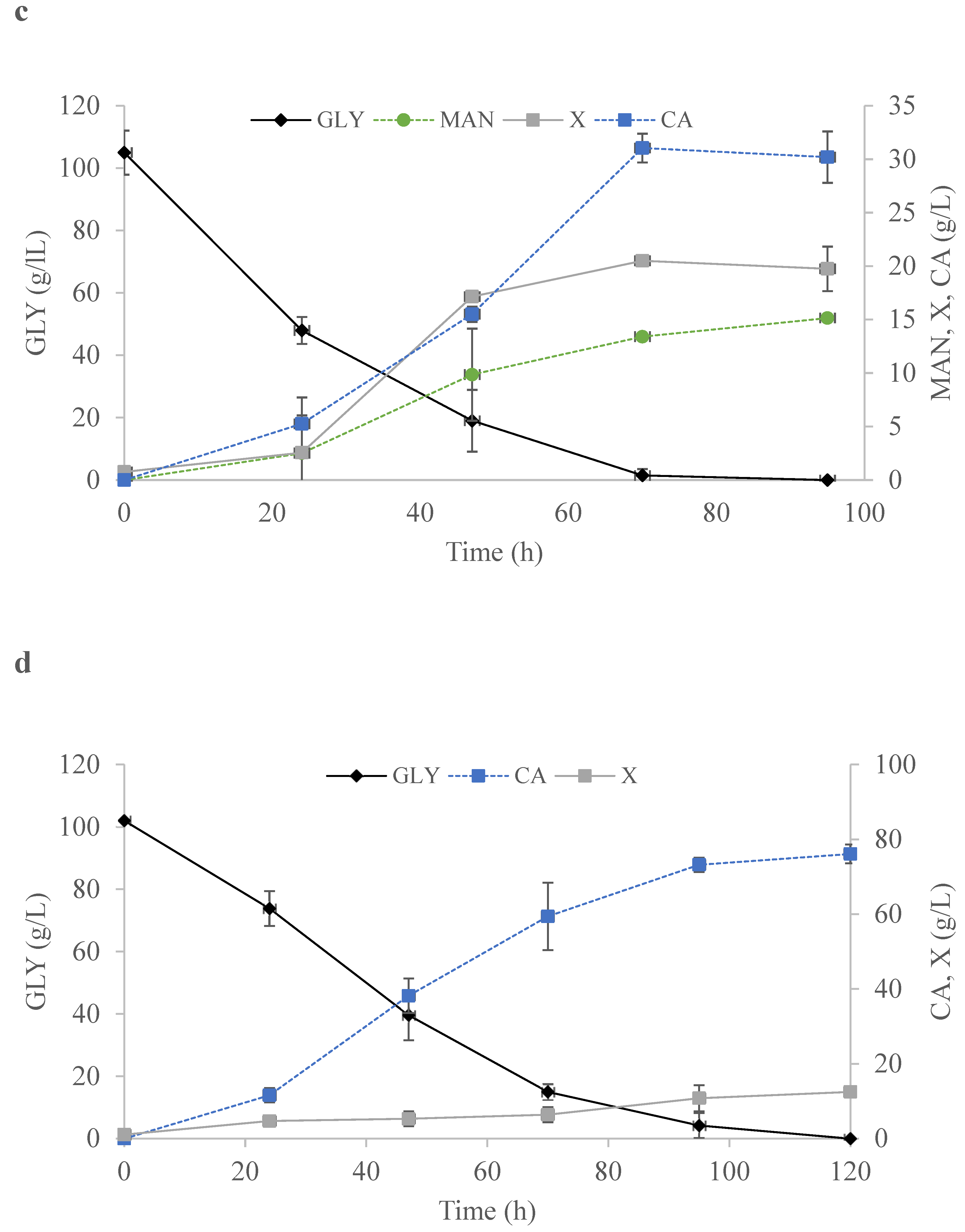
2.4. Bioreactor Studies
3. Materials and Methods
3.1. Honey Sample
3.2. Microorganism Isolation
3.3. Yeast Identification
3.4. API®/ID32
3.5. MALDI TOF/MS
3.6. rDNA Sequence-Based Identification
3.7. Microcultures
3.8. Media Compositions for Shake-Flask and Bioreactor Experiments
3.9. Culture Conditions for Inoculation, Shake-Flask and Bioreactor Experiments
3.10. Analytical Methods
3.11. Fermentation Parameter Calculations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rao, P.V.; Krishnan, K.T.; Salleh, N.; Gan, S.H. Biological and therapeutic effects of honey produced by honey bees and stingless bees: A comparative review. Rev. Bras. Farmacognosia. 2016, 26, 657–664. [Google Scholar] [CrossRef]
- Meo, S.A.; Al-Asiri, S.A.; Mahesar, A.L.; Ansari, M.J. Role of honey in modern medicine. Saudi. J. Biol. Sci. 2017, 24, 975–978. [Google Scholar]
- Silva, B.; Biluca, F.C.; Gonzaga, L.V.; Fett, R.; Dalmarco, E.M.; Caon, T.; Costa, A.C.O. In vitro anti-inflammatory properties of honey flavonoids: A review. Food Res. Int. 2021, 141, 110086. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.; Biluca, F.C.; Mohr, E.T.B.; Caon, T.; Gonzaga, L.V.; Fett, R.; Dalmarco, E.M.; Costa, A.C.O. Effect of Mimosa scabrella Bentham honeydew honey on inflammatory mediators. J. Funct. Foods 2020, 72, 104034. [Google Scholar] [CrossRef]
- Chan-Zapata, I.; Segura-Campos, M.R. Honey and its protein components: Effects in the cancer immunology. J. Food Biochem. 2021, 45, e13613. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Zamora, M.C.; Chirife, J. Determination of water activity change due to crystallization in honeys from Argentina. Food Control. 2006, 17, 59–64. [Google Scholar] [CrossRef]
- Girolamo, F.D.; D’amato, A.; Righetti, P.G. Assessment of the floral origin of honey via proteomic tools. J. Proteom. 2012, 75, 3688–3693. [Google Scholar] [CrossRef]
- Isidorow, W.; Witkowski, S.; Iwaniuk, P.; Zambrzycka, M.; Swiecicka, I. Royal Jelly Aliphatic Acids Contribute to Antimicrobial Activity of Honey. J. Apic. Sci. 2018, 62, 111–123. [Google Scholar] [CrossRef]
- Kunat-Budzyńska, M.; Rysiak, A.; Wiater, A.; Grąz, M.; Andrejko, M.; Budzyński, M.; Bryś, M.S.; Sudziński, M.; Tomczyk, M.; Gancarz, M.; et al. Chemical Composition and Antimicrobial Activity of New Honey Varietals. Int. J. Environ. Res. Public Health 2023, 20, 2458. [Google Scholar] [CrossRef]
- Olaitan, P.B.; Adeleke, O.E.; Ola, I.O. Honey: A reservoir for microorganisms and an inhibitory agent for microbes. Afr. Health Sci. 2007, 7, 159–165. [Google Scholar] [PubMed]
- Xiong, Z.R.; Sogin, J.H.; Worobo, R.W. Microbiome analysis of raw honey reveals important factors influencing the bacterial and fungal communities. Front. Microbiol. 2023, 13, 1099522. [Google Scholar] [CrossRef]
- Buzzini, P.; Turchetti, B.; Yurkov, A. Extremophilic yeasts: The toughest yeasts around? Yeast 2018, 35, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Chanchao, C. Antimicrobial activity by Trigona Laeviceps (stingless bee) honey from Thailand. Pak. J. Med. Sci. 2009, 25, 364–369. [Google Scholar]
- Grabowski, N.T.; Klein, G. Microbiology and foodborne pathogens in honey. Crit. Rev. Food Sci. Nutr. 2017, 57, 1852–1862. [Google Scholar]
- Pozo, M.I.; Jacquemyn, H. Addition of pollen increases growth of nectar-living yeasts. FEMS Microbiol. Lett. 2019, 366, fnz191. [Google Scholar] [CrossRef]
- Sinacori, M.; Francesca, N.; Alfonzo, A.; Cruciata, M.; Sannino, C.; Settanni, L.; Moschetti, G. Cultivable microorganisms associated with honeys of different geographical and botanical origin. Food Microbiol. 2014, 38, 284–294. [Google Scholar] [CrossRef]
- Snowdon, J.A.; Cliver, D.O. Microorganisms in honey. Int. J. Food Microbiol. 1996, 31, 1–26. [Google Scholar] [CrossRef]
- Ohashi, K.; Chaleckis, R.; Takaine, M.; Wheelock, C.E.; Yoshida, S. Kynurenine aminotransferase activity of Aro8/Aro9 engage tryptophan degradation by producing kynurenic acid in Saccharomyces cerevisiae. Sci. Rep. 2017, 7, 12180. [Google Scholar] [CrossRef]
- Mor, A.; Tankiewicz-Kwedlo, A.; Krupa, A.; Pawlak, D. Role of kynurenine pathway in oxidative stress during neurodegenerative disorders. Cells 2021, 10, 1603. [Google Scholar] [CrossRef]
- Walczak, K.; Dąbrowski, W.; Langner, E.; Zgrajka, W.; Piłat, J.; Kocki, T.; Rzeski, W.; Turski, W.A. Kynurenic acid synthesis and kynurenine aminotransferases expression in colon derived normal and cancer cells. Scand. J. Gastroenterol. 2011, 46, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Ha, S.E.; Park, M.Y.; Jeong, S.H.; Bhosale, P.B.; Abusaliya, A.; Kil Won, C.; Heo, J.D.; Ahn, M.; Seong, J.K.; et al. Identification of Kynurenic Acid-Induced Apoptotic Biomarkers in Gastric Cancer-Derived AGS Cells through Next-Generation Transcriptome Sequencing Analysis. Nutrients 2023, 15, 193. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Cai, T.; Tagle, D.A.; Li, J. Structure, expression, and function of kynurenine aminotransferases in human and rodent brains. Cell. Mol. Life Sci. 2010, 67, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Stone, T. Development and therapeutic potential of kynurenic acid and kynurenine derivatives for neuroprotection. Trends Pharmacol. Sci. 2000, 21, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Sorgdrager, F.J.H.; Vermeiren, Y.; Van Faassen, M.; van der Ley, C.; Nollen, E.A.A.; Kema, I.P.; De Deyn, P.P. Age- and disease-specific changes of the kynurenine pathway in Parkinson’s and Alzheimer’s disease. J. Neurochem. 2019, 151, 656–668. [Google Scholar] [CrossRef]
- Tabrizi, S.J.; Scahill, R.I.; Owen, G.; Durr, A.; Leavitt, B.R.; Roos, R.A.; Borowsky, B.; Landwehrmeyer, B.; Frost, C.; Johnson, H.; et al. Predictors of phenotypic progression and disease onset in premanifest and early-stage Huntington’s disease in the TRACK-HD study: Analysis of 36-month observational data. Lancet Neurol. 2013, 12, 637–649. [Google Scholar] [CrossRef]
- Dhakar, N.K.; Caldera, F.; Bessone, F.; Cecone, C.; Pedrazzo, A.R.; Cavall, R.; Dianzani, C.; Trotta, F. Evaluation of solubility enhancement, antioxidant activity, and cytotoxicity studies of kynurenic acid loaded cyclodextrin nanosponge. Carbohydr. Polym. 2019, 224, 115168. [Google Scholar] [CrossRef]
- Turski, M.P.; Turska, M.; Kocki, T.; Turski, W.A.; Paluszkiewicz, P. Kynurenic acid content in selected culinary herbs and spices. J. Chem. 2015, 2015, 617571. [Google Scholar] [CrossRef]
- Turska, M.; Paluszkiewicz, P.; Turski, W.A.; Parada-Turska, J. A review of the health benefits of food enriched with kynurenic acid. Nutrients 2022, 14, 4182. [Google Scholar] [CrossRef]
- Beretta, G.; Artali, R.; Caneva, E.; Orlandini, S.; Centini, M.; Facino, R.M. Quinoline Alkaloids in Honey: Further Analytical (HPLC-DAD-ESI-MS, Multidimensional Diffusion-Ordered NMR Spectroscopy), Theoretical and Chemometric Studies. J. Pharm. Biomed. Anal. 2009, 50, 432–439. [Google Scholar] [CrossRef]
- Wróbel-Kwiatkowska, M.; Turski, W.; Kocki, T.; Rakicka-Pustułka, M.; Rymowicz, W. An efficient method for production of kynurenic acid by Yarrowia lipolytica. Yeast 2020, 37, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, C.; Gökmen, V. Kinetic evaluation of the formation of tryptophan derivatives in the kynurenine pathway during wort fermentation using Saccharomyces pastorianus and Saccharomyces cerevisiae. Food Chem. 2019, 297, 124975. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, C.; Gökmen, V. Formation of amino acid derivatives in white and red wines during fermentation: Effects of non-Saccharomyces yeasts and Oenococcus Oeni. Food Chem. 2021, 343, 128415. [Google Scholar] [CrossRef]
- Rakicka-Pustułka, M.; Ziuzia, P.; Pierwoła, J.; Szymański, K.; Wróbel-Kwiatkowska, M.; Lazar, Z. The microbial production of kynurenic acid using Yarrowia lipolytica yeast growing on crude glycerol and soybean molasses. Front. Bioeng. Biotechnol. 2022, 10, 936137. [Google Scholar] [CrossRef]
- Rakicka, M.; Biegalska, A.; Dobrowolski, A.; Rymowicz, W.; Mirończuk, A.M. Polyol production from waste materials by genetically modified Yarrowia lipolytica. Bioresour. Technol. 2017, 243, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Rywińska, A.; Juszczyk, P.; Wojtatowicz, M.; Rymowicz, W. Chemostat study of citric acid production from glycerol by Yarrowia lipolytica. J. Biotechnol. 2011, 152, 54–57. [Google Scholar] [CrossRef]
- Rzechonek, D.; Dobrowolski, A.; Rymowicz, W.; Mirończuk, A. Recent advantages in biological production of erythritol. Crit. Rev. Biotechnol. 2018, 38, 620–633. [Google Scholar] [CrossRef]
- Khatape, A.B.; Dastager, S.G.; Rangaswamy, V. An overview of erythritol production by yeast strains. FEMS Microbiol. Lett. 2022, 369, fnac107. [Google Scholar] [CrossRef]
- Martău, G.A.; Coman, V.; Vodnar, D.C. Recent advances in the biotechnological production of erythritol and mannitol. Crit. Rev. Biotechnol. 2020, 40, 608–622. [Google Scholar] [CrossRef]
- Tomaszewska, L.; Rywińska, A.; Gładkowski, G. Production of erythritol and mannitol by Yarrowia lipolytica yeast in media containing glycerol. J. Ind. Microbiol. Biotechnol. 2012, 39, 1333–1343. [Google Scholar] [CrossRef]
- Soetaert, W. Synthesis of D-Mannitol and L-Sorbose by Microbial Hydrogenation and Dehydrogenation of Monosaccharides. Ph.D. Thesis, University of Gent, Gent, Belgium, 1991. [Google Scholar]
- Zhang, M.; Gu, L.; Cheng, C.; Ma, J.; Xin, F.; Liu, J.; Wu, H.; Jiang, M. Recent advantages in microbial production of mannitol: Utilization of low-cost substrate, strain development and regulation strategies. World J. Microbiol. Biotechnol. 2018, 34, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Börekçi, B.S.; Kaban, G.; Kaya, M. Citric Acid Production of Yeast: An Overview. EuroBiotech J. 2021, 5, 79–91. [Google Scholar] [CrossRef]
- Cassagne, C.; Normand, A.-C.; L’Ollivier, C.; Ranque, S.; Piarroux, R. Performance of MALDI-TOF MS platforms for fungal identification. Mycoses 2016, 59, 678–690. [Google Scholar] [CrossRef] [PubMed]
- Bankar, A.V.; Kumar, A.R.; Zinjarde, S.S. Environmental and industrial applications of Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2009, 84, 847–865. [Google Scholar] [CrossRef] [PubMed]
- Lazar, Z.; Neuvéglise, C.; Rossignol, T.; Devillers, H.; Morin, N.; Robak, M.; Nicaud, J.M.; Crutz-Le Coq, A.M. Characterization of hexose transporters in Yarrowia lipolytica reveals new groups of Sugar Porters involved in yeast growth. Fungal Genet. Biol. 2017, 100, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Echeverrigaray, S.; Scariot, F.J.; Foresti, L.; Schwarz, L.V.; Rocha, R.K.M.; da Silva, G.P.; Moreira, J.P.; Delamare, A.P.L. Yeast biodiversity in honey produced by stingless bees raised in the highlands of southern Brazil. Int. J. Food Microbiol. 2021, 347, 109200. [Google Scholar] [CrossRef]
- Dobrowolski, A.; Drzymała, K.; Rzechonek, D.; Mituła, P.; Mirończuk, M.A. Lipid Production from Waste Materials in Seawater-Based Medium by the Yeast Yarrowia lipolytica. Front. Microbiol. 2019, 10, 547. [Google Scholar] [CrossRef]
- Mirończuk, M.A.; Rakicka, M.; Biegalska, A.; Rymowicz, W.; Dobrowolski, A. A two-stage fermentation process of erythritol production by yeast Y. lipolytica from molasses and glycerol. Bioresour. Technol. 2015, 198, 445–455. [Google Scholar] [CrossRef]
- Mirończuk, A.M.; Biegalska, A.; Dobrowolski, A. Functional overexpression of genes involved in erythritol synthesis in the yeast Yarrowia lipolytica. Biotech. Biofuels. 2017, 10, 77. [Google Scholar] [CrossRef]
- Turski, M.P.; Turska, M.; Zgrajka, W.; Kuc, D.; Turski, W.A. Presence of kynurenic acid in food and honeybee products. Amino Acids. 2009, 36, 75–80. [Google Scholar] [CrossRef]
- Żubrowski, D.; Lazar, Z.; Robak, M. Preliminary choice of components concentration of sacharose medium for biosynthesis of citrate and invertase by Yarrowia lipolytica A-101-B56-5. Acta Sci. Pol. Biotechnol. 2013, 12, 31–40. [Google Scholar]
- Rywińska, A.; Marcinkiewicz, M.; Cibis, E.; Rymowicz, W. Optimization of medium composition for erythritol production from glycerol by Yarrowia lipolytica using response surface methodology. Prep. Biochem. Biotech. 2015, 45, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Rakicka, M.; Kieroń, A.; Hapeta, P.; Neuvélise, C.; Lazar, Z. Sweet and sour potential of yeast from the Yarrowia clade. Biomass Bioenergy 2016, 92, 48–54. [Google Scholar] [CrossRef]
- Tomaszewska-Hetman, L.; Rywińska, A. Erythritol biosynthesis from glycerol by Yarrowia lipolytica yeast: Effect of osmotic pressure. Chem. Pap. 2015, 70, 272–283. [Google Scholar] [CrossRef]
- Cas, D.; Vigentini, I.; Vitalini, S.; Laganaro, A.; Iriti, M.; Paroni, R.; Foschino, R. Tryptophan derivatives by Saccharomyces cerevisiae EC1118: Evaluation, optimization, and production in a soybean-based medium. Int. J. Mol. Sci. 2021, 22, 472. [Google Scholar] [CrossRef]
- Deshpande, M.S.; Kulkarni, P.P.; Kumbhar, P.S.; Ghosalkar, A.R. Erythritol production from sugar based feedstocks by Moniliella pollinis using lysate of recycled cells as nutrients source. Process. Biochem. 2022, 112, 45–52. [Google Scholar] [CrossRef]
- Li, L.; Yang, T.; Guo, W.; Ju, X.; Hu, C.; Tang, B.; Fu, J.; Gu, J.; Zhang, H. Construction of an efficient mutant strain of Trichosporonoides oedocephalis with HOG1 gene deletion for production of erythritol. J. Microbiol. Biotechnol. 2016, 26, 700–709. [Google Scholar] [CrossRef]
- Song, K.H.; Lee, J.K.; Song, J.Y.; Baek, H.; Kim, S.Y.; Hyun, H.H. Production of mannitol by a novel strain of Candida magnoliae. Biotechnol. Lett. 2002, 24, 9–12. [Google Scholar] [CrossRef]
- Yang, L.B.; Zhan, X.B.; Zheng, Z.Y.; Wu, J.R.; Gao, M.J.; Lin, C.C. A novel osmotic pressure control fed-batch fermentation strategy for improvement of erythritol production by Yarrowia lipolytica from glycerol. Bioresour. Technol. 2014, 151, 120–127. [Google Scholar] [CrossRef]
- Carsanba, E.; Papanikolaou, S.; Fickers, P.; Agirman, B.; Erten, H. Citric Acid Production by Yarrowia lipolytica. In Non-Conventional Yeasts: From Basic Research to Application, 1st ed; Sibirny, A., Ed.; Springer: Cham, Switzerland, 2019; pp. 91–117. [Google Scholar]
- Knutsen, A.K.; Robert, V.; Poot, G.A.; Epping, W.; Figge, M.; Holst-Jensen, A.; Skaar, I.; Smith, M.T. Polyphasic re-examination of Yarrowia lipolytica strains and the description of three novel Candida species: Candida osloensis sp. nov., Candida alimentaria sp. nov. and Candida hollandica sp. nov. Int. J. Syst. Evol. Micr. 2007, 57, 2426–2435. [Google Scholar]
- Hoffman, C.S.; Winston, F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene 1987, 57, 267–272. [Google Scholar] [CrossRef] [PubMed]



| Strain No. | Color | Phenotype |
|---|---|---|
| 1 | light beige | wrinkled |
| 2 | light beige | wrinkled |
| 3 | light beige | wrinkled |
| 4 | light beige | wrinkled |
| 5 | light beige | wrinkled |
| 6 | light beige | wrinkled |
| 7 | light beige | wrinkled |
| 8 | light beige | smooth |
| 9 | light beige | wrinkled |
| 10 | light beige | smooth |
| 11 | light beige | wrinkled |
| 12 | light beige | wrinkled |
| 13 | light beige | wrinkled |
| 14 | light beige | wrinkled |
| 15 | light beige | smooth |
| Strain No. | Identification Profile of the Microorganism | ||||
|---|---|---|---|---|---|
| API 32C Test | MALDI TOF/MS Identification | rDNA Sequence-Based Identification | |||
| Species | The Degree of Identification, % | Species | Identification Index Value a, % | Species | |
| 1 | Candida lipolytica | 99.9 | Yarrowia lipolytica | 1.90 | Yarrowia lipolytica |
| 2 | Candida lipolytica | 99.9 | Yarrowia lipolytica | 2.12 | Yarrowia lipolytica |
| 3 | Candida lipolytica | 99.9 | Yarrowia lipolytica | 2.00 | Yarrowia lipolytica |
| 4 | Candida lipolytica | 99.9 | Yarrowia lipolytica | 2.14 | Yarrowia lipolytica |
| 5 | Candida lipolytica | 99.9 | Yarrowia lipolytica | 1.99 | Yarrowia lipolytica |
| 6 | Candida lipolytica | 99.9 | Yarrowia lipolytica | 2.09 | Yarrowia lipolytica |
| 7 | Candida lipolytica | 99.9 | Yarrowia lipolytica | 2.18 | Yarrowia lipolytica |
| 8 | Candida magnoliae | 86.4 | Candida magnoliae | 2.13 | Candida magnoliae |
| Candida globosa | 13.4 | ||||
| 9 | Candida lipolytica | 99.9 | Yarrowia lipolytica | 2.19 | Yarrowia lipolytica |
| 10 | Candida magnoliae | 98.7 | Candida magnoliae | 2.14 | Candida magnoliae |
| 11 | Candida lipolytica | 98.8 | Yarrowia lipolytica | 2.13 | Yarrowia lipolytica |
| 12 | Candida lipolytica | 99.9 | Yarrowia lipolytica | 1.98 | Yarrowia lipolytica |
| 13 | Candida lipolytica | 99.9 | Yarrowia lipolytica | 1.91 | Yarrowia lipolytica |
| 14 | Candida lipolytica | 99.9 | Yarrowia lipolytica | 1.97 | Yarrowia lipolytica |
| 15 | Candida magnoliae | 99.3 | Candida magnoliae | 1.86 | Starmerella magnoliae |
| Metabolites | Strain No. | QX | Max. Concentration of Metabolite | QMET | YMET |
|---|---|---|---|---|---|
| g/L·h | g/L | mg/L·h a g/L h | mg/g b g/g | ||
| Kynurenic Acid Production in Bioreactor | |||||
| Kynurenic acid | 12 | 0.10 | 3.9 | 0.02 a | 0.1 |
| Erythritol production in bioreactor | |||||
| Erythritol | 9 | 0.22 | 32.6 | 0.34 | 0.33 |
| Arabitol | 1.3 | 0.01 | 0.01 | ||
| Mannitol | 4.8 | 0.05 | 0.05 | ||
| Citric acid | 1.1 | 0.01 | 0.01 | ||
| Mannitol production in bioreactor | |||||
| Mannitol | 5 | 0.21 | 15.1 | 0.16 | 0.15 |
| Arabitol | 1.6 | 0.01 | 0.02 | ||
| Citric acid | 30.2 | 0.25 | 0.30 | ||
| Citric acid production in bioreactor | |||||
| Citric acid | 3 | 0.10 | 75.7 | 0.63 | 0.76 |
| Arabitol | 2.05 | 0.17 | 0.02 | ||
| Erythritol | 4.05 | 0.03 | 0.04 | ||
| Mannitol | 7.15 | 0.06 | 0.07 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziuzia, P.; Janiec, Z.; Wróbel-Kwiatkowska, M.; Lazar, Z.; Rakicka-Pustułka, M. Honey’s Yeast—New Source of Valuable Species for Industrial Applications. Int. J. Mol. Sci. 2023, 24, 7889. https://doi.org/10.3390/ijms24097889
Ziuzia P, Janiec Z, Wróbel-Kwiatkowska M, Lazar Z, Rakicka-Pustułka M. Honey’s Yeast—New Source of Valuable Species for Industrial Applications. International Journal of Molecular Sciences. 2023; 24(9):7889. https://doi.org/10.3390/ijms24097889
Chicago/Turabian StyleZiuzia, Patrycja, Zuzanna Janiec, Magdalena Wróbel-Kwiatkowska, Zbigniew Lazar, and Magdalena Rakicka-Pustułka. 2023. "Honey’s Yeast—New Source of Valuable Species for Industrial Applications" International Journal of Molecular Sciences 24, no. 9: 7889. https://doi.org/10.3390/ijms24097889
APA StyleZiuzia, P., Janiec, Z., Wróbel-Kwiatkowska, M., Lazar, Z., & Rakicka-Pustułka, M. (2023). Honey’s Yeast—New Source of Valuable Species for Industrial Applications. International Journal of Molecular Sciences, 24(9), 7889. https://doi.org/10.3390/ijms24097889






