Optimization of Magnetic and Paper-Based Molecularly Imprinted Polymers for Selective Extraction of Charantin in Momordica charantia
Abstract
1. Introduction
2. Results and Discussion
2.1. HPLC-DAD Optimization
2.2. Method Validation
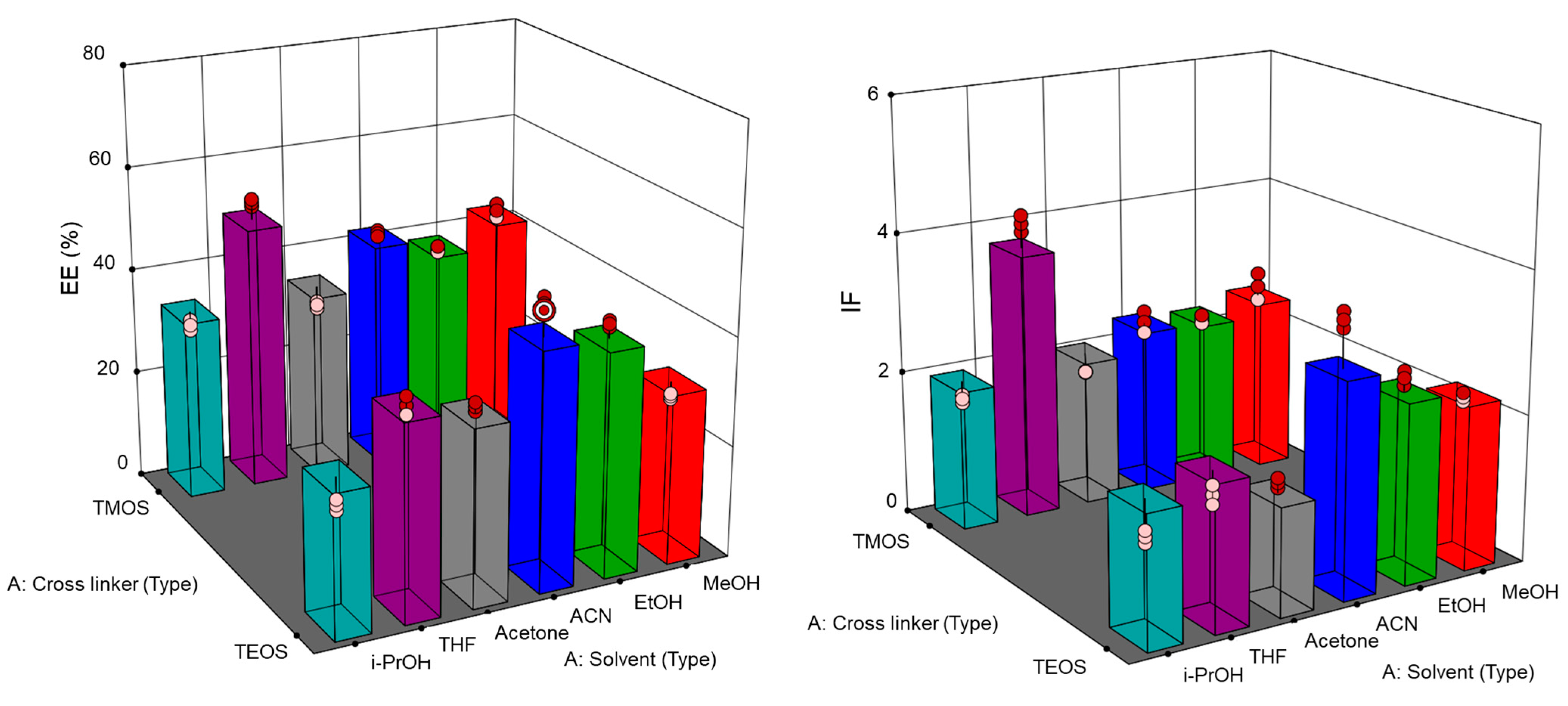
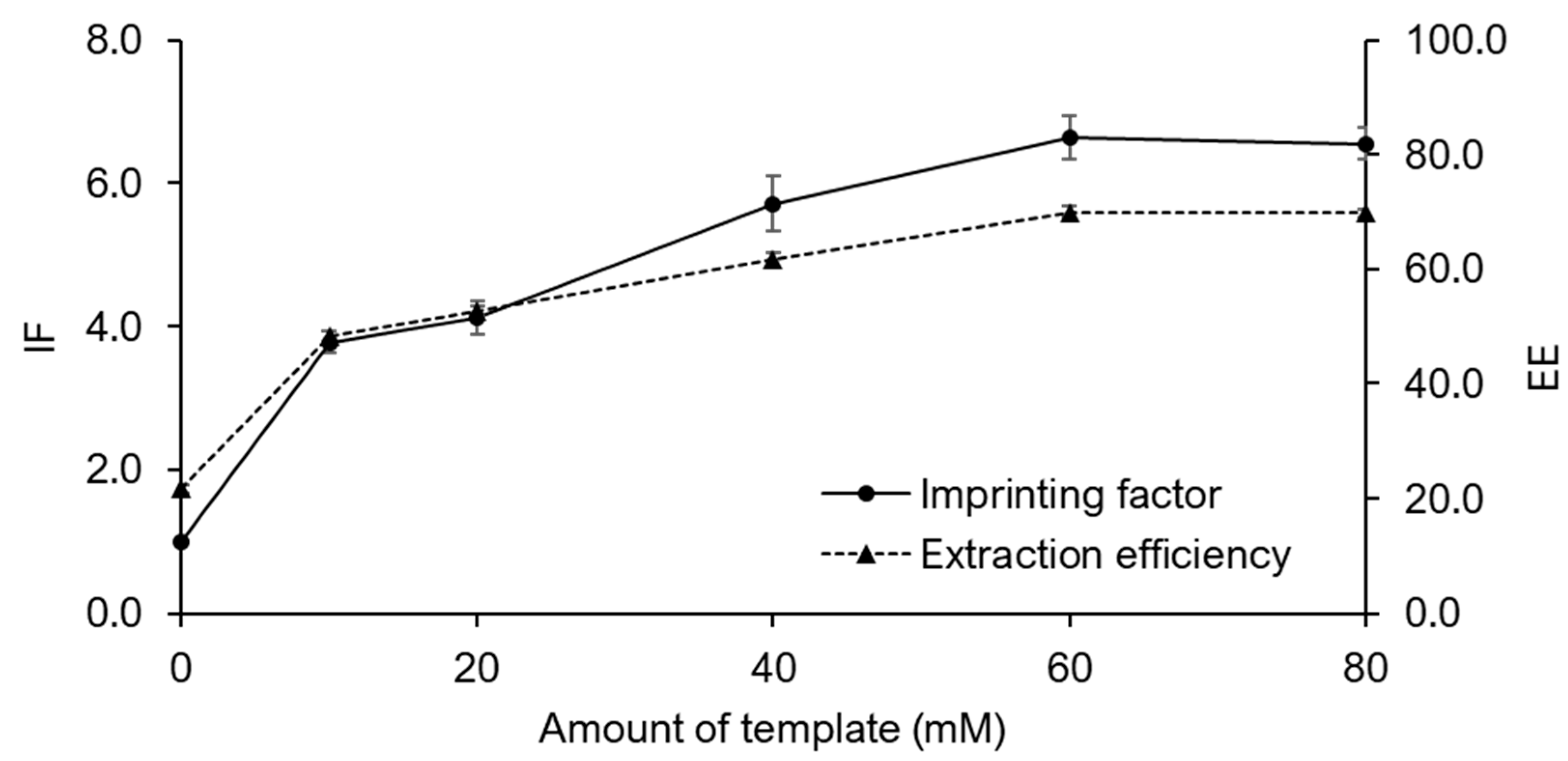
2.3. Fe3O4@MIPs and Paper@MIPs
2.4. Applications
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Standard Solution
3.3. Optimization of Fe3O4@MIPs and Paper@MIPs
3.4. HPLC-DAD Optimization
3.5. Method Validation
3.6. Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeed, F.; Afzaal, M.; Niaz, B.; Arshad, M.U.; Tufail, T.; Hussain, M.B.; Javed, A. Bitter melon (Momordica charantia): A natural healthy vegetable. Int. J. Food Prop. 2018, 21, 1270–1290. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, Y.; Xiang, F.; Li, S.; Yang, G. Antifungal activity of Momordica charantia seed extracts toward the pathogenic fungus Fusarium solani L. J. Food Drug Anal. 2016, 24, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Cuong, D.M.; Jeon, J.; Morgan, A.M.A.; Kim, C.; Kim, J.K.; Lee, S.Y.; Park, S.U. Accumulation of charantin and expression of triterpenoid biosynthesis genes in bitter melon (Momordica charantia). J. Agric. Food Chem. 2017, 65, 7240–7249. [Google Scholar] [CrossRef] [PubMed]
- Lolitkar, M.M.; Rao, M.R.R. Pharmacology of a hypoglycaemic principle isolated from the fruits of Momordica charantia Linn. Indian J. Pharm. 1966, 28, 129–133. [Google Scholar]
- Desai, S.; Tatke, P.; Mane, T.; Gabhe, S. Isolation, characterization and quantitative HPLC-DAD analysis of components of charantin from fruits of Momordica charantia. Food Chem. 2021, 345, 128717. [Google Scholar] [CrossRef]
- Çiçek, S.S. Momordica charantia L.—Diabetes-related bioactivities, quality control, and safety considerations. Front. Pharmacol. 2022, 13, 904643. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H.; Avula, B.; Liu, Y.; Khan, I.A. Determination and quantitation of five cucurbitane triterpenoids in Momordica charantia by reversed-phase high-performance liquid chromatography with evaporative light scattering detection. J. Chromatogr. Sci. 2008, 46, 133–136. [Google Scholar] [CrossRef]
- Ma, J.; Krynitsky, A.J.; Grundel, E.; Rader, J.I. Quantitative Determination of Cucurbitane-Type Triterpenes and Triterpene Glycosides in Dietary Supplements Containing Bitter Melon (Momordica charantia) by HPLC-MS/MS. J. AOAC Int. 2012, 95, 1597–1608. [Google Scholar] [CrossRef]
- Ahamad, J.; Amin, S.; Mir, S.R. Simultaneous quantification of gymnemic acid as gymnemagenin and charantin as β-sitosterol using validated HPTLC densitometric method. J. Chromatogr. Sci. 2015, 53, 1203–1209. [Google Scholar] [CrossRef]
- Pitipanapong, J.; Chitprasert, S.; Goto, M.; Jiratchariyakul, W.; Sasaki, M.; Shotipruk, A. New approach for extraction of charantin from Momordica charantia with pressurized liquid extraction. Sep. Purif. Technol. 2007, 52, 416–422. [Google Scholar] [CrossRef]
- Zaini, A.S.; Aris, N.A.; Putra, N.R.; Abd Hashib, S.; Kamaruddin, M.J.; Idham, Z.; Yunus, M.A.C. Comparison of charantin extract from Momordica charantia using modified supercritical carbon dioxide and soxhlet extraction method. Mal. J. Fund. Appl. Sci. 2018, 14, 462–466. [Google Scholar] [CrossRef]
- Ahamad, J.; Amin, S.; Mir, S. Optimization of ultrasound-assisted extraction of charantin from Momordica charantia fruits using response surface methodology. J. Pharm. Bioallied Sci. 2015, 7, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Urriza-Arsuaga, I.; Guadaño-Sánchez, M.; Urraca, J.L. Current trends in molecular imprinting: Strategies, applications and determination of target molecules in Spain. Int. J. Mol. Sci. 2023, 24, 1915. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S. Application of magnetic molecularly imprinted polymer as a versatile and highly selective tool in food and environmental analysis: Recent developments and trends. TrAC Trend Anal. Chem. 2017, 90, 89–106. [Google Scholar] [CrossRef]
- Jhu, S.C.; Wang, J.Y.; Wang, H.T.; Chen, S.F. Analysis of heterocyclic aromatic amines using selective extraction by magnetic molecularly imprinted polymers coupled with liquid chromatography—Mass spectrometry. J. Food Drug Anal. 2021, 29, 726–737. [Google Scholar] [CrossRef]
- Manmana, Y.; Chutvirasakul, B.; Suntornsuk, L.; Nuchtavorn, N. Cost effective paper-based colorimetric devices for a simple assay of dopamine in pharmaceutical formulations using 3,3′,5,5′-tetramethylbenzidine-silver nitrate as a chromogenic reagent. Pharm. Sci. Asia 2019, 46, 270–277. [Google Scholar] [CrossRef]
- Nuchtavorn, N.; Rypar, T.; Nejdl, L.; Vaculovicova, M.; Macka, M. Distance-based detection in analytical flow devices: From gas detection tubes to microfluidic chips and microfluidic paper-based analytical devices. TrAC Trend Anal. Chem. 2022, 150, 116581. [Google Scholar] [CrossRef]
- Nuchtavorn, N.; Leanpolchareanchai, J.; Suntornsuk, L.; Macka, M. Paper-based sol-gel thin films immobilized cytochrome P450 for enzyme activity measurement. Anal. Chim. Acta 2020, 1098, 86–93. [Google Scholar] [CrossRef]
- Khunkitchai, N.; Nuchtavorn, N.; Rypar, T.; Vlcnovska, M.; Nejdl, L.; Vaculovicova, M.; Macka, M. UV-light-actuated in situ preparation of paper@ZnCd quantum dots for paper-based enzymatic nanoreactors. Chem. Eng. J. 2022, 428, 132508. [Google Scholar] [CrossRef]
- Nuchtavorn, N.; Dvořák, M.; Kubáň, P. Paper-based molecularly imprinted-interpenetrating polymer network for on-spot collection and microextraction of dried blood spots for capillary electrophoresis determination of carbamazepine. Anal. Bioanal. Chem. 2020, 412, 2721–2730. [Google Scholar] [CrossRef]
- Vodova, M.; Nejdl, L.; Pavelicova, K.; Zemankova, K.; Rrypar, T.; Skopalova Sterbova, D.; Bezdekova, J.; Nuchtavorn, N.; Macka, M.; Adam, V.; et al. Detection of pesticides in food products using paper-based devices by UV-induced fluorescence spectroscopy combined with molecularly imprinted polymers. Food Chem. 2022, 380, 132141. [Google Scholar] [CrossRef]
- Qamar, S.; Torres, Y.J.M.; Parekh, H.S.; Falconer, J.R. Fractional factorial design study for the extraction of cannabinoids from CBD-dominant cannabis flowers by supercritical carbon dioxide. Processes 2022, 10, 93. [Google Scholar] [CrossRef]
- Jalilian, N.; Ebrahimzadeh, H.; Asgharinezhad, A.A.; Khodayari, P. Magnetic molecularly imprinted polymer for the selective dispersive micro solid phase extraction of phenolphthalein in urine samples and herbal slimming capsules prior to HPLC-PDA analysis. Microchim. J. 2021, 160, 105712. [Google Scholar] [CrossRef]
- Farooq, S.; Nie, J.; Cheng, Y.; Yan, Z.; Bacha, S.A.S.; Zhang, J.; Nahiyoon, R.A.; Hussain, Q. Synthesis of core–shell magnetic molecularly imprinted polymer for the selective determination of imidacloprid in apple samples. J. Sep. Sci. 2019, 42, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Luliński, P.; Janczura, M.; Sobiech, M.; Giebułtowicz, J. Magnetic molecularly imprinted nano-conjugates for effective extraction of food components—A model study of tyramine determination in craft beers. Int. J. Mol. Sci. 2021, 22, 9560. [Google Scholar] [CrossRef]
- Münger, L.H.; Boulos, S.; Nyström, L. UPLC-MS/MS based identification of dietary steryl glucosides by investigation of corresponding free sterols. Front. Chem. 2018, 6, 342. [Google Scholar] [CrossRef]
- Wewer, V.; Dombrink, I.; vom Dorp, K.; Dörmann, P. Quantification of sterol lipids in plants by quadrupole time-of-flight mass spectrometry. J. Lipid Res. 2011, 52, 1039–1054. [Google Scholar] [CrossRef]
- Jiang, K.; Gachumi, G.; Poudel, A.; Shurmer, B.; Bashi, Z.; El-Aneed, A. The establishment of tandem mass spectrometric fingerprints of phytosterols and tocopherols and the development of targeted profiling strategies in vegetable oils. J. Am. Soc. Mass Spectrom. 2019, 30, 1700–1712. [Google Scholar] [CrossRef]
- Ding, X.; Yang, J.; Dong, Y. Advancements in the preparation of high-performance liquid chromatographic organic polymer monoliths for the separation of small-molecule drugs. J. Pharm. Anal. 2018, 8, 75–85. [Google Scholar] [CrossRef]
- Pešić, M.P.; Todorov, M.D.; Becskereki, G.; Horvai, G.; Verbić, T.Ž.; Tóth, B. A novel method of molecular imprinting applied to the template cholesterol. Talanta 2020, 217, 121075. [Google Scholar] [CrossRef]
- Lo, H.-Y.; Ho, T.-Y.; Lin, C.; Li, C.-C.; Hsiang, C.-Y. Momordica charantia and its novel polypeptide regulate glucose homeostasis in mice via binding to insulin receptor. J. Agric. Food Chem. 2013, 61, 2461–2468. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Wong, W.F.; Dong, J.; Cheng, K.K. Momordica charantia suppresses inflammation and glycolysis in lipopolysaccharide-activated RAW264.7 macrophages. Molecules 2020, 25, 3783. [Google Scholar] [CrossRef] [PubMed]
- Curk, T.; Dobnikar, J.; Frenkel, D. Rational design of molecularly imprinted polymers. Soft Matter 2016, 12, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Eurachem. The Fitness for Purpose of Analytical Methods: A Laboratory Guide to Method Validation and Related Topics, 2nd ed.; Eurachem: Gembloux, Belgium, 2014. [Google Scholar]
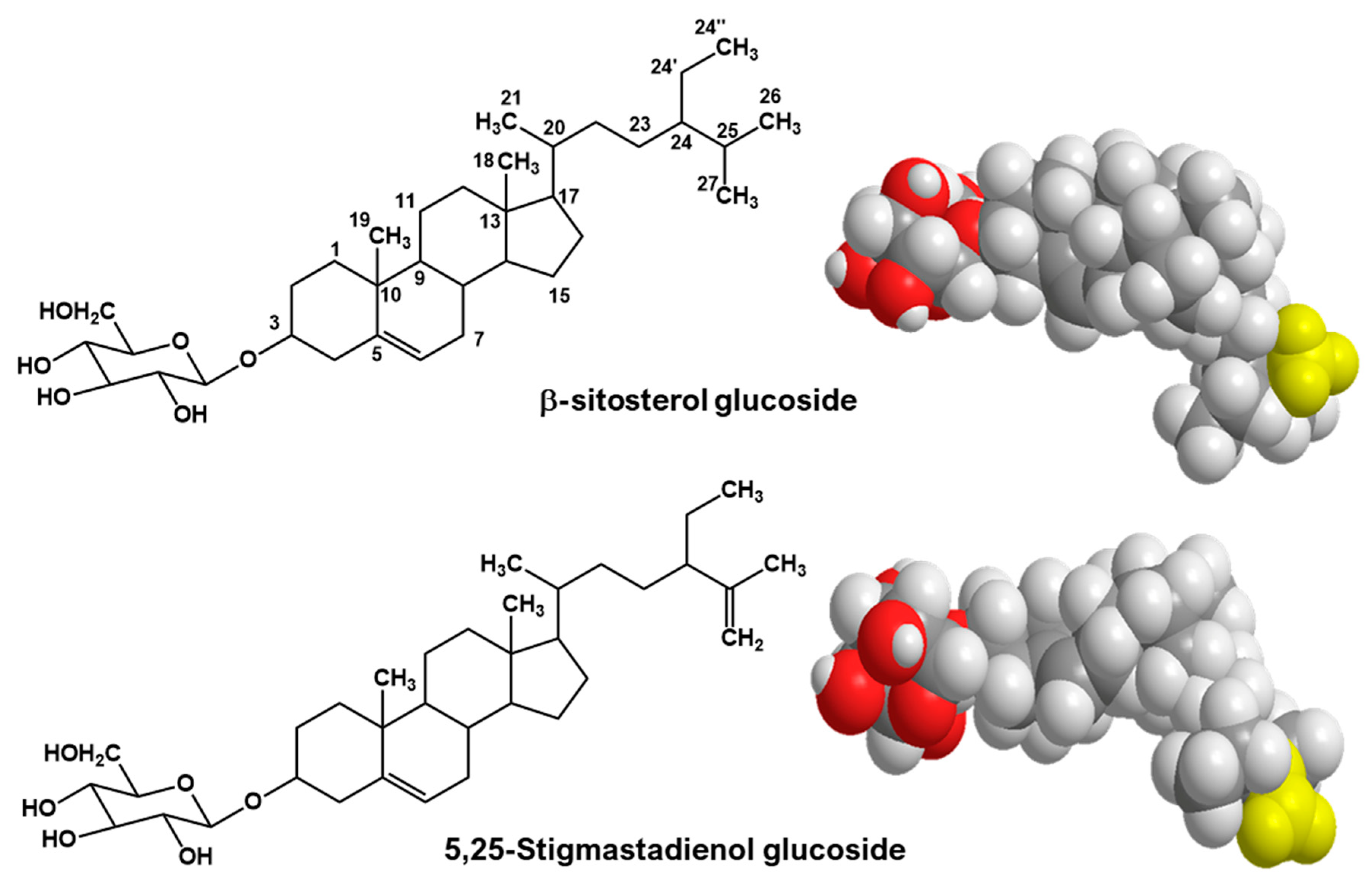

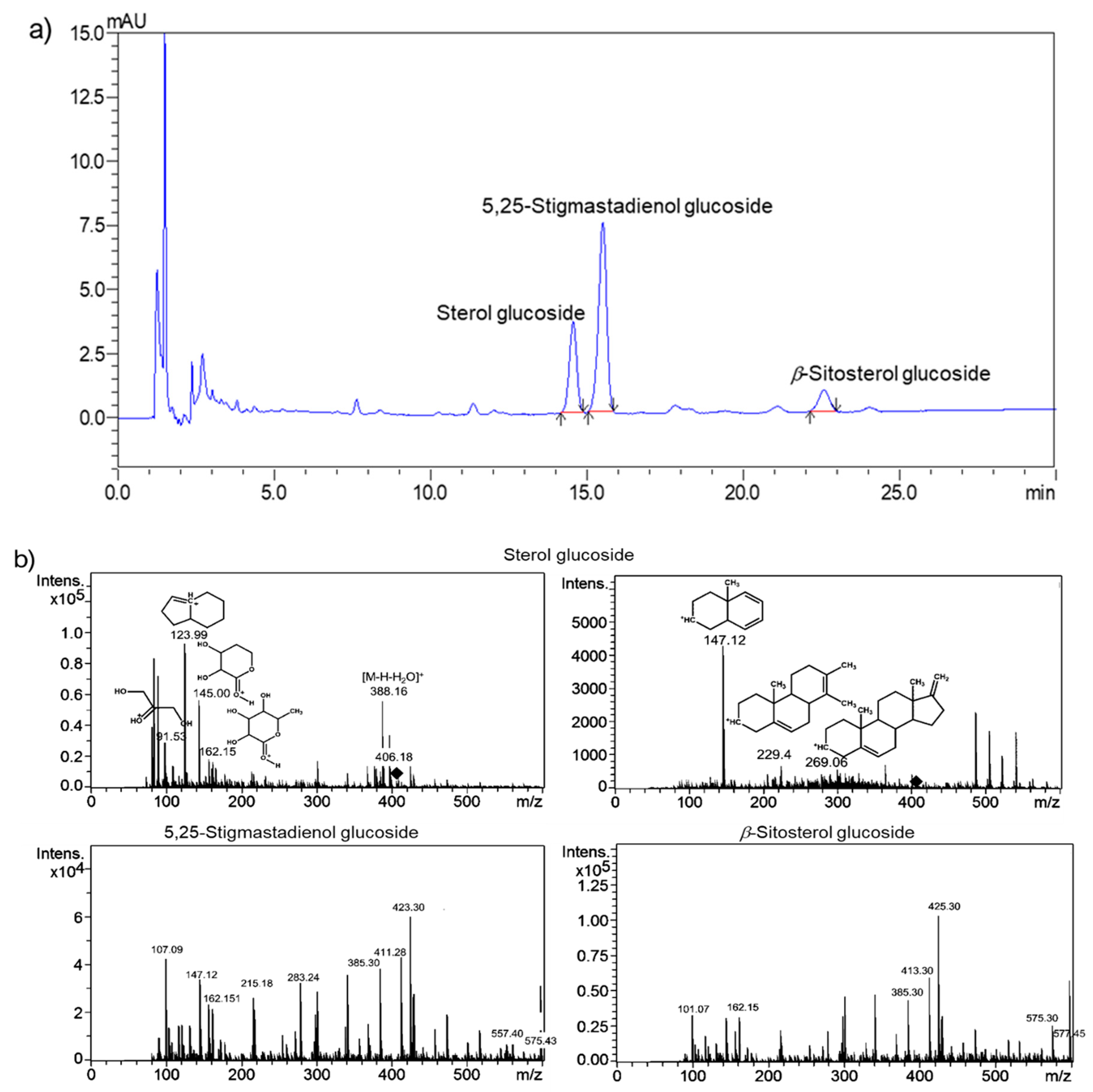
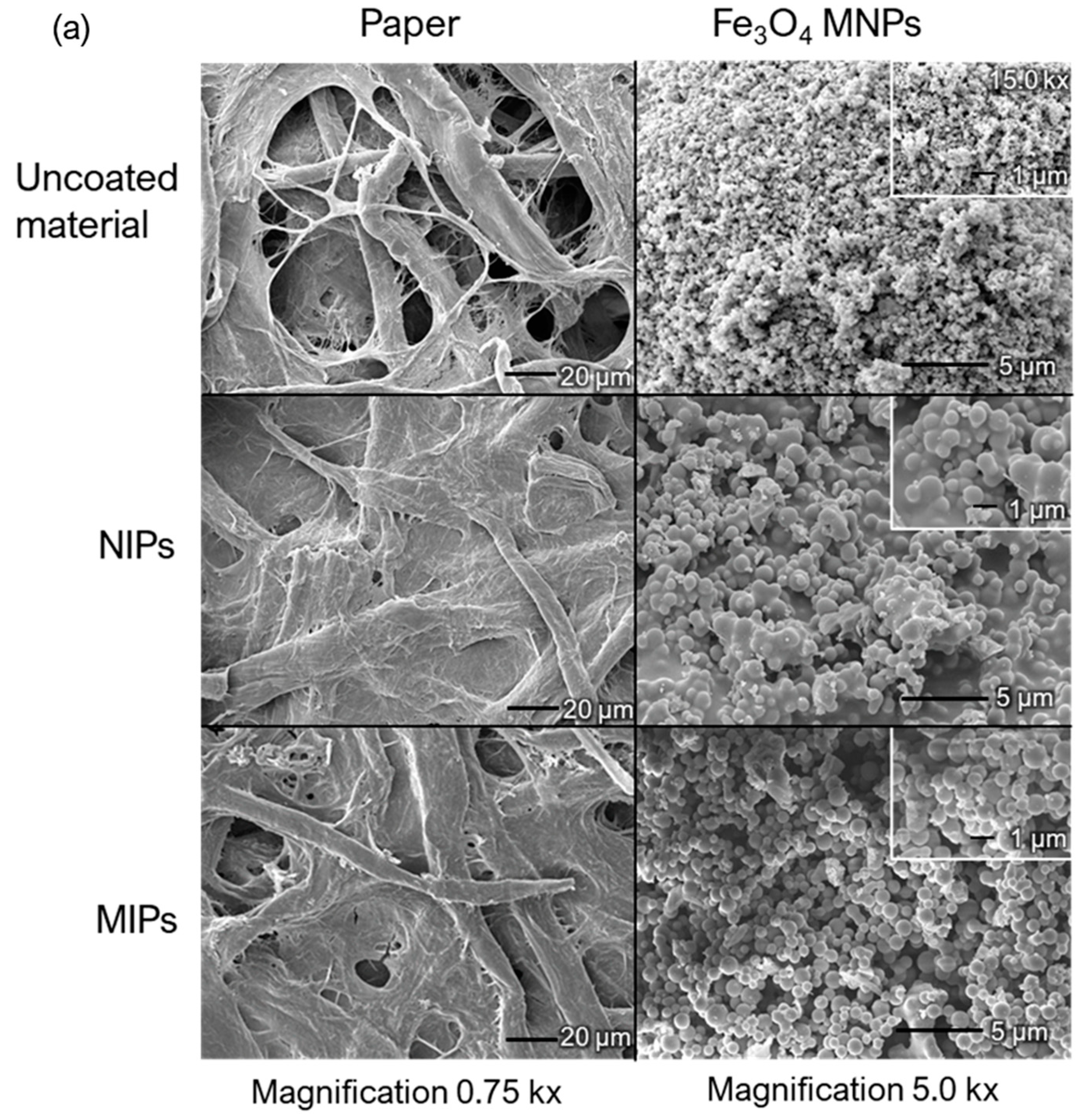
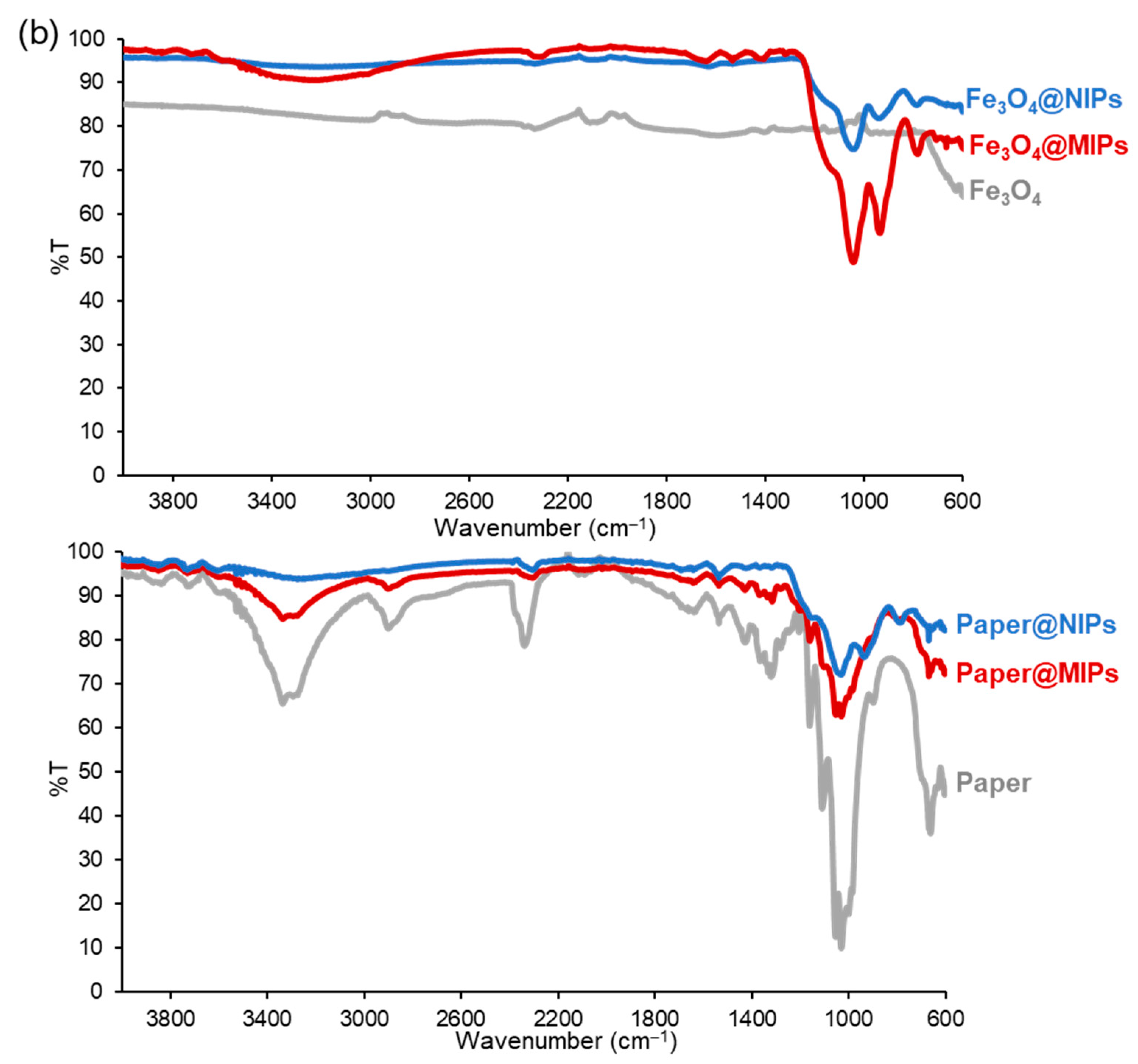
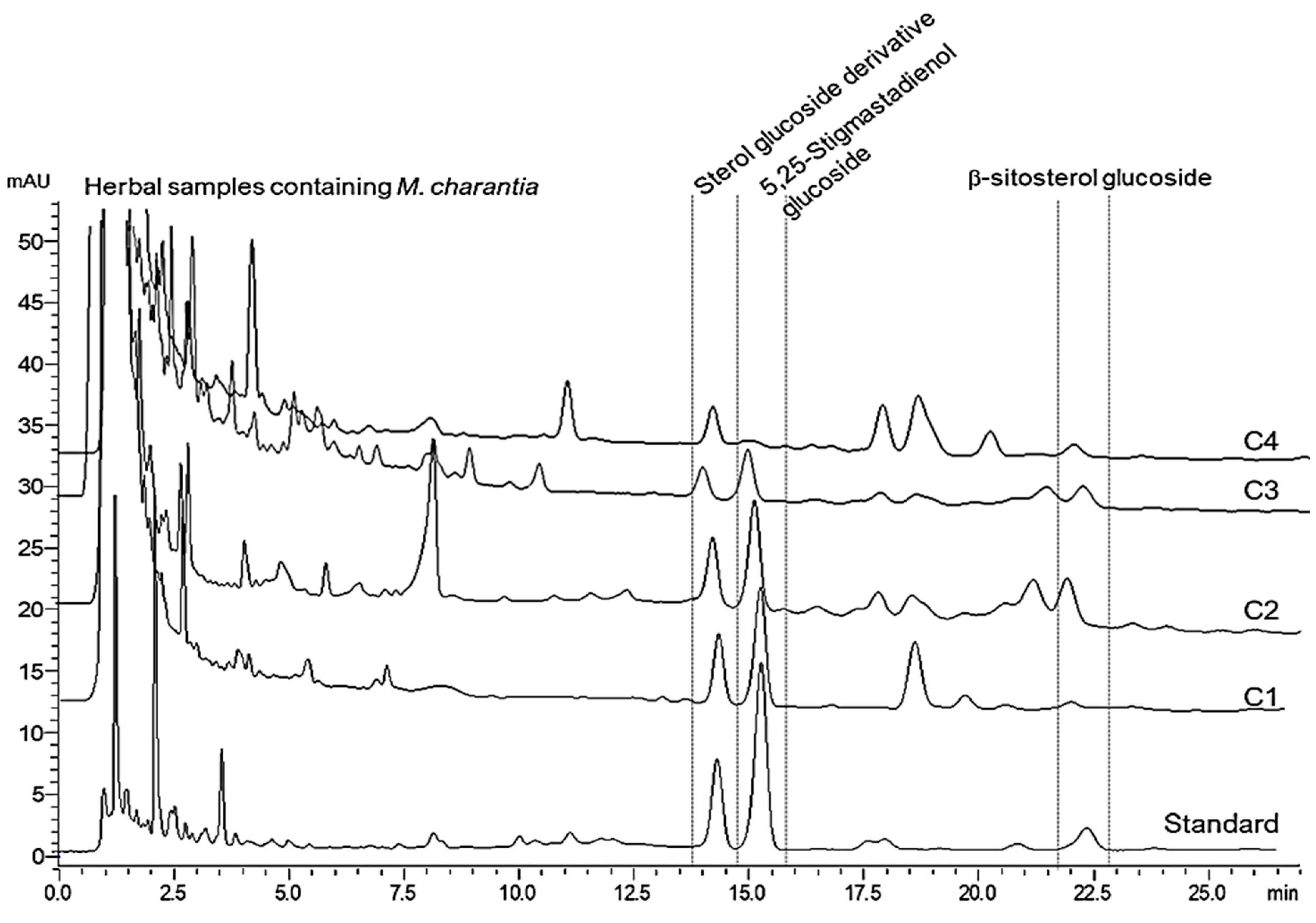
| Charantin Constituent | Content a (mg/100 g Dry Weight) | |||||||
|---|---|---|---|---|---|---|---|---|
| Fe3O4@MIPs | Paper@MIPs | |||||||
| C1 | C2 | C3 | C4 | C1 | C2 | C3 | C4 | |
| Sterol glucoside | 152.0 ± 7.1 | 48.9 ± 1.3 | 15.9 ± 2.0 | 24.5 ± 2.6 | 143.2 ± 6.4 | 46.8 ± 3.2 | 18.6 ± 2.9 | 23.3 ± 2.0 |
| 5,25-Stigmastadienol glucoside | 365.9 ± 10.2 | 83.3 ± 2.6 | 45.4 ± 2.6 | 5.1 ± 0.9 | 357.3 ± 9.9 | 80.7 ± 2.9 | 48.1 ± 3.0 | 3.9 ± 0.6 |
| β-sitosterol glucoside | 61.0 ± 3.2 | 36.1 ± 0.9 | 197.5 ± 21.6 | 25.3 ± 6.2 | 58.7 ± 2.5 | 38.1 ± 2.0 | 183.6 ± 10.5 | 21.1 ± 3.5 |
| Extraction Method | Reagent Consumption a | Extraction Time | Equipment Required | Analytical Method | Ref. |
|---|---|---|---|---|---|
| Fe3O4@MIPs and paper@MIPs as µSPE | 10 mL methanol | 10 min | Vortex, magnet separator, syringe filter holder | HPLC-DAD | The proposed method |
| Ultrasonic-assisted LLE | 5 mL 80% methanol (46 °C) | 2 h | Ultrasonicator | HPTLC densitometry | [12] |
| LLE | 20 mL hexane, 10 mL methanol | ≈1.5 h | Vortex, ultrasonicator, centrifuge | HPLC-DAD | [3] |
| Pressurized liquid extraction | 40–60 mL ethanol (100–120 °C) or 60 mL 50% ethanol (100 °C), 5 mL 50% methanol, 5 mL 70% methanol, 3 mL hexane for purification | >40 min–1 h | Ultrasonicator, centrifuge, in-house pressurized liquid extractor | HPLC-DAD | [10] |
| Soxhlet extraction | 200 mL ethanol, 30 mL methanol (78.5 °C), 5 mL 50% methanol, 5 mL 70% methanol, 3 mL hexane for purification | >2 h | Soxhlet apparatus, ultrasonicator, centrifuge | HPLC-DAD | [10] |
| Soxhlet extraction | 10 mL ethanol or water (78–100 °C) | 6 h | Soxhlet apparatus, ultrasonicator, centrifuge | HPLC-DAD | [11] |
| Shaking water bath | N/A b mL water (80 °C) | 6.5 h | Shaking water bath, ultrasonicator, centrifuge | HPLC-DAD | [11] |
| Supercritical carbon dioxide extraction | N/A mL water and ethanol (65 °C) | ≈3 h | Supercritical carbon dioxide extraction system, ultrasonicator, centrifuge | HPLC-DAD | [11] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuchtavorn, N.; Leanpolchareanchai, J.; Visansirikul, S.; Bunsupa, S. Optimization of Magnetic and Paper-Based Molecularly Imprinted Polymers for Selective Extraction of Charantin in Momordica charantia. Int. J. Mol. Sci. 2023, 24, 7870. https://doi.org/10.3390/ijms24097870
Nuchtavorn N, Leanpolchareanchai J, Visansirikul S, Bunsupa S. Optimization of Magnetic and Paper-Based Molecularly Imprinted Polymers for Selective Extraction of Charantin in Momordica charantia. International Journal of Molecular Sciences. 2023; 24(9):7870. https://doi.org/10.3390/ijms24097870
Chicago/Turabian StyleNuchtavorn, Nantana, Jiraporn Leanpolchareanchai, Satsawat Visansirikul, and Somnuk Bunsupa. 2023. "Optimization of Magnetic and Paper-Based Molecularly Imprinted Polymers for Selective Extraction of Charantin in Momordica charantia" International Journal of Molecular Sciences 24, no. 9: 7870. https://doi.org/10.3390/ijms24097870
APA StyleNuchtavorn, N., Leanpolchareanchai, J., Visansirikul, S., & Bunsupa, S. (2023). Optimization of Magnetic and Paper-Based Molecularly Imprinted Polymers for Selective Extraction of Charantin in Momordica charantia. International Journal of Molecular Sciences, 24(9), 7870. https://doi.org/10.3390/ijms24097870






