Renewable Biopolymers Combined with Ionic Liquids for the Next Generation of Supercapacitor Materials
Abstract
1. Historic Perspective of IL Electrolytes for Electric Double Layer Capacitors (EDLCs)
1.1. Performance Requirements for EDLCs: The Emergence of ILs as Electrolytes
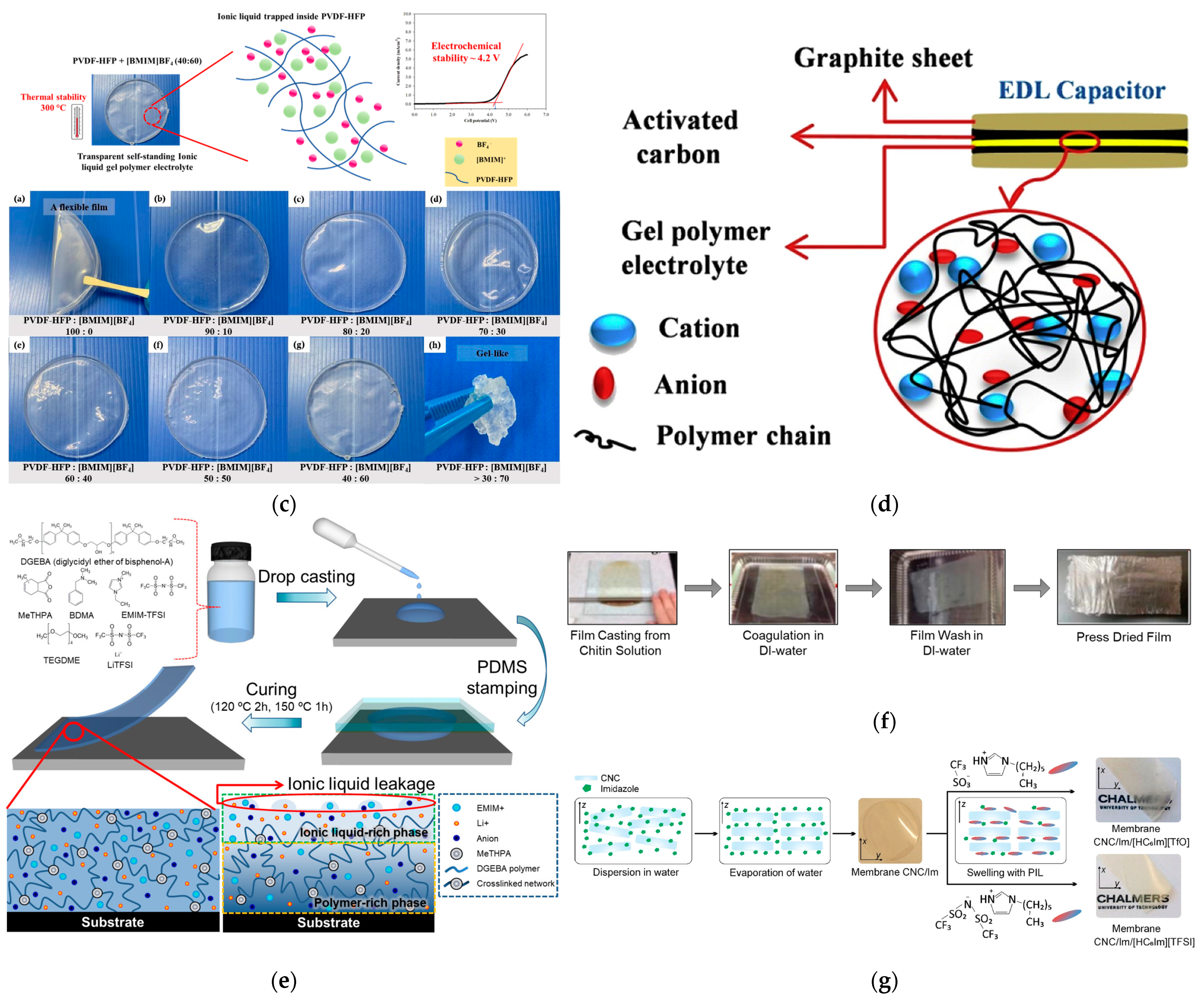
1.2. Next Generation of Electrolytes: Gel Polymer Electrolytes (GPEs) and Solid Polymer Electrolytes (SPEs)
2. Can Biopolymers Be Used in the Design and Development of IL Electrolytes?
3. Biopolymeric Carbon as Electrode and ILs as Electrolytes
4. Discussion & Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, C.; Yu, Z.; Neff, D.; Zhamu, A.; Jang, B.Z. Graphene-based supercapacitor with an ultrahigh energy density. Nano Lett. 2010, 10, 4863–4868. [Google Scholar] [CrossRef] [PubMed]
- Beidaghi, M.; Wang, C. Micro-supercapacitors based on interdigital electrodes of reduced graphene oxide and carbon nanotube composites with ultrahigh power handling performance. Adv. Funct. Mater. 2012, 22, 4501–4510. [Google Scholar] [CrossRef]
- Pech, D.; Brunet, M.; Durou, H.; Huang, P.; Mochalin, V.; Gogotsi, Y.; Taberna, P.-L.; Simon, P. Ultrahigh-power micrometre-sized supercapacitors based on onion-like carbon. Nat. Nanotechnol. 2010, 5, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-K.; Cho, S.-H.; Ok, Y.-W.; Seong, T.-Y.; Yoon, Y.S. All solid-state rechargeable thin-film microsupercapacitor fabricated with tungsten co-sputtered ruthenium oxide electrodes. J. Vac. Sci. Technol. B 2003, 21, 949–952. [Google Scholar] [CrossRef]
- Wang, K.; Zou, W.; Quan, B.; Yu, A.; Wu, H.; Jiang, P.; Wei, Z. An all-solid-state flexible micro-supercapacitor on a chip. Adv. Energy Mater. 2011, 1, 1068–1072. [Google Scholar] [CrossRef]
- Beidaghi, M.; Wang, C. Micro-supercapacitors Based on three dimensional interdigital polypyrrole/C-MEMS electrodes. Electrochim. Acta 2011, 56, 9508–9514. [Google Scholar] [CrossRef]
- Khomenko, V.; Frackowiak, E.; Béguin, F. Determination of the specific capacitance of conducting polymer/nanotubes composite electrodes using different cell configurations. Electrochim. Acta 2005, 50, 2499–2506. [Google Scholar] [CrossRef]
- Brandt, A.; Pohlmann, S.; Varzi, A.; Balducci, A.; Passerini, S. Ionic Liquids in Supercapacitors. MRS Bull. 2013, 38, 554–559. [Google Scholar] [CrossRef]
- Wilkes, J.S.; Levisky, J.A.; Wilson, R.A.; Hussey, C.L. Dialkylimidazolium chloroaluminate melts: A new class of room-temperature ionic liquids for electrochemistry, spectroscopy and synthesis. Inorg. Chem. 1982, 21, 1263–1264. [Google Scholar] [CrossRef]
- Osteryoung, R.A.; Osteryoung, J.G. Studies in ambient temperature ionic liquids. In AFOSR Report 1987; AFOSR-TR-87-0879; Order No. AD-A182723; AFOSR: Arlington, VA, USA, 1987. [Google Scholar]
- Zhang, L.; Gao, H.; Jin, G.; Liu, S.; Wu, J.; Wu, H.; Yang, Y.; Wang, Q.; Wang, S. Cellulose-Based Electrolytes for Advanced Lithium-Ion Batteries: Recent Advances and Future Perspectives. ChemNanoMat 2022, 8, e202200142. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Theerthagiri, J.; Vikraman, D.; Yim, C.J.; Hussain, S.; Sharma, R.; Maiyalagan, T.; Qin, J.; Kim, H.S. Ionic liquid-based electrolytes for energy storage devices: A brief review on their limits and applications. Polymers 2020, 12, 918. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, D.R.; Forsyth, M.; Howlett, P.C.; Kar, M.; Passerini, S.; Pringle, J.M.; Ohno, H.; Watanabe, M.; Yan, F.; Zheng, W.; et al. Ionic liquids and their solid-state analogues as materials for energy generation and storage. Nat. Rev. Mater. 2016, 1, 15005. [Google Scholar] [CrossRef]
- Varela, J.C.; Sankar, K.; Hino, A.; Lin, X.; Chang, W.-s.; Coker, D.; Grinstaff, M. Piperidinium ionic liquids as electrolyte solvents for sustained high temperature supercapacitor operation. Chem. Commun. 2018, 54, 5590–5593. [Google Scholar] [CrossRef]
- Pitawala, J.; Navarra, M.A.; Scrosati, B.; Jacobsson, P.; Matic, A. Structure and properties of Li-ion conducting polymer gel electrolytes based on ionic liquids of the pyrrolidinium cation and the bis (trifluoromethanesulfonyl) imide anion. J. Power Sources 2014, 245, 830–835. [Google Scholar] [CrossRef]
- Srour, H.; Rouault, H.; Santini, C. Imidazolium based ionic liquid electrolytes for Li-ion secondary batteries based on graphite and LiFePO4. J. Electrochem. Soc. 2012, 160, A66–A69. [Google Scholar] [CrossRef]
- Mousavi, M.P.S.; Wilson, B.E.; Kashefolgheta, S.; Anderson, E.L.; He, S.; Bühlmann, P.; Stein, A. Ionic liquids as electrolytes for electrochemical double-layer capacitors: Structures that optimize specific energy. ACS Appl. Mater. Interfaces 2016, 8, 3396–3406. [Google Scholar] [CrossRef] [PubMed]
- Galiński, M.; Lewandowski, A.; Stępniak, I. Ionic liquids as electrolytes. Electrochim. Acta 2006, 51, 5567–5580. [Google Scholar] [CrossRef]
- Nancarrow, P.; Al-Othman, A.; Mital, D.K.; Dopking, S. Comprehensive analysis and correlation of ionic liquid conductivity data for energy applications. Energy 2021, 220, 119761. [Google Scholar] [CrossRef]
- Weingärtner, H. The static dielectric permittivity of ionic liquids. J. Molec. Liquid 2014, 192, 185–190. [Google Scholar] [CrossRef]
- Kalb, R.S. Chapter 11: Toward Industrialization of Ionic Liquids. In Commercial Applications of Ionic Liquids; Shiflett, M., Ed.; Springer Nature: Cham, Switzerland, 2020. [Google Scholar]
- Matsumoto, H.; Sakaebe, H.; Tatsumi, K.; Kikuta, M.; Ishiko, E.; Kono, M. Fast cycling of Li/LiCoO2 cell with low-viscosity ionic liquids based on bis(fluorosulfonyl)imide [FSI]−. J. Power Sources 2006, 160, 1308–1313. [Google Scholar] [CrossRef]
- Zhou, Q.; Henderson, W.A.; Appetecchi, G.B.; Montanino, M.; Passerini, S. Physical and electrochemical properties of n-alkyl-n-methylpyrrolidinium bis(fluorosulfonyl)imide ionic liquids: PY13FSI and PY14FSI. J. Phys. Chem. B 2008, 112, 13577–13580. [Google Scholar] [CrossRef]
- Liu, K.; Zhou, Y.-X.; Han, H.-B.; Zhou, S.-S.; Feng, W.-F.; Nie, J.; Li, H.; Huang, X.-J.; Armand, M.; Zhou, Z.-B. Ionic liquids based on (fluorosulfonyl)(pentafluoroethanesulfonyl)imide with various oniums. Electrochim. Acta 2010, 55, 7145–7151. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Meakin, P.; Sun, J.; Amini, N.; Forsyth, M. Pyrrolidinium imides: A new family of molten salts and conductive plastic crystal phases. J. Phys. Chem. B 1999, 103, 4164–4170. [Google Scholar] [CrossRef]
- Ignat’ev, N.V.; Welz-Biermann, U.; Kucheryna, A.; Bissky, G.; Willner, H. New ionic liquids with tris(perfluoroalkyl)trifluorophosphate (FAP) anions. J. Fluor. Chem. 2005, 126, 1150–1159. [Google Scholar] [CrossRef]
- Abdallah, T.; Lemordant, D.; Claude-Montigny, B. Are room temperature ionic liquids able to improve the safety of supercapacitors organic electrolytes without degrading the performances? J. Power Sources 2012, 201, 353–359. [Google Scholar] [CrossRef]
- Yao, C.; Pitner, W.R.; Anderson, J.L. Ionic liquids containing the tris(pentafluoroethyl)trifluorophosphate anion: A new class of highly selective and ultra hydrophobic solvents for the extraction of polycyclic aromatic hydrocarbons using single drop microextraction. Anal. Chem. 2009, 81, 5054–5063. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, R.; Aldous, L.; Rogers, E.I.; Ward Jones, S.E.; Compton, R.G. A Study of the Na/Na+ redox couple in some room temperature ionic liquids. J. Phys. Chem. C 2010, 114, 3618–3626. [Google Scholar] [CrossRef]
- O’Mahony, A.M.; Silvester, D.S.; Aldous, L.; Hardacre, C.; Compton, R.G. Effect of water on the electrochemical window and potential limits of room-temperature ionic liquids. J. Chem. Eng. Data 2008, 53, 2884–2891. [Google Scholar] [CrossRef]
- Zhou, T.; Zhao, Y.; Choi, J.W.; Coskun, A. Ionic liquid functionalized gel polymer electrolytes for stable lithium metal batteries. Angew. Chem. Int. Ed. 2021, 60, 22791–22796. [Google Scholar] [CrossRef] [PubMed]
- Dzulkipli, M.Z.; Karim, J.; Ahmad, A.; Dzulkurnain, N.A.; Su’ait, M.S.; Yoshizawa-Fujita, M.; Tian-Khoon, L.; Hassan, N.H. The influences of 1-butyl-3-methylimidazolium tetrafluoroborate on electrochemical, thermal and structural studies as ionic liquid gel polymer electrolyte. Polymers 2021, 13, 1277. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Ghosh, A. Solid-state gel polymer electrolytes based on ionic liquids containing imidazolium cations and tetrafluoroborate anions for electrochemical double layer capacitors: Influence of cations size and viscosity of ionic liquids. J. Power Sources 2018, 406, 128–140. [Google Scholar] [CrossRef]
- Lee, D.; Song, Y.H.; Choi, U.H.; Kim, J. Highly flexible and stable solid-state supercapacitors based on a homogeneous thin ion gel polymer electrolyte using a poly(dimethylsiloxane) stamp. ACS Appl. Mater. Interfaces 2019, 11, 42221–42232. [Google Scholar] [CrossRef]
- Danyliv, O.; Strach, M.; Nechyporchuk, O.; Nypelö, T.; Martinelli, A. Self-standing, robust membranes made of cellulose nanocrystals (CNCs) and a protic ionic liquid: Toward sustainable electrolytes for fuel cells. ACS Appl. Energy Mater. 2021, 4, 6474–6485. [Google Scholar] [CrossRef]
- Ma, D.; Yuan, D.; Ponce de Leon, C.; Jiang, Z.; Xia, X.; Pan, J. Current progress and future perspectives of electrolytes for rechargeable aluminum-ion batteries. Energy Environ. Mater. 2023, 6, e12301. [Google Scholar] [CrossRef]
- Hopson, C.; Villar-Chavero, M.M.; Dominguez, J.C.; Alonso, M.V.; Oliet, M.; Rodriguez, F. Cellulose ionogels, a perspective of the last decade: A review. Carbohydr. Polym. 2021, 274, 118663. [Google Scholar] [CrossRef] [PubMed]
- Jamil, R.; Silvester, D.S. Ionic liquid gel polymer electrolytes for flexible supercapacitors: Challenges and prospects. Curr. Opin. Electrochem. 2022, 35, 101046. [Google Scholar] [CrossRef]
- Sun, L.; Zhuo, K.; Chen, Y.; Du, Q.; Zhang, S.; Wang, J. Ionic liquid-based redox active electrolytes for supercapacitors. Adv. Funct. Mater. 2022, 32, 2203611. [Google Scholar] [CrossRef]
- Chen, L.; Fu, J.; Lu, Q.; Shi, L.; Li, M.; Dong, L.; Xu, Y.; Jia, R. Cross-linked polymeric ionic liquids ion gel electrolytes by in situ radical polymerization. Chem. Eng. J. 2019, 378, 122245. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, H.; Girard, G.M.A.; Yunis, R.; MacFarlane, D.R.; Mecerreyes, D.; Bhattacharyya, A.J.; Howlett, P.C.; Forsyth, M. Preparation and characterization of gel polymer electrolytes using poly(ionic liquids) and high lithium salt concentration ionic liquids. J. Mater. Chem. A 2017, 5, 23844–23852. [Google Scholar] [CrossRef]
- Niu, H.; Ding, M.; Zhang, N.; Li, X.; Su, X.; Han, X.; Zhang, N.; Guan, P.; Hu, X. Preparation of imidazolium based polymerized ionic liquids gel polymer electrolytes for high-performance lithium batteries. Mater. Chem. Phys. 2023, 293, 126971. [Google Scholar] [CrossRef]
- Sen, S.; Goodwin, S.E.; Barbará, P.V.; Rance, G.A.; Wales, D.; Cameron, J.M.; Sans, V.; Mamlouk, M.; Scott, K.; Walsh, D.A. Gel–polymer electrolytes based on poly(ionic liquid)/ionic liquid networks. ACS Appl. Polym. Mater. 2021, 3, 200–208. [Google Scholar] [CrossRef]
- Balo, L.; Gupta, H.; Singh, S.K.; Singh, V.K.; Tripathi, A.K.; Srivastava, N.; Tiwari, R.K.; Mishra, R.; Meghnani, D.; Singh, R.K. Development of gel polymer electrolyte based on LiTFSI and EMIMFSI for application in rechargeable lithium metal battery with GO-LFP and NCA cathodes. J. Solid State Electrochem. 2019, 23, 2507–2518. [Google Scholar] [CrossRef]
- Mathela, S.; Kumar, S.; Singh, P.K.; Chandra, S.R.; Shukla, P.; Singh, V.; Noor, I.; Kakroo, S.; Madkhli, A.Y.; Tomar, R. Ionic liquid dispersed highly conducting polymer electrolyte for supercapacitor application: Current scenario and prospects—“ICSEM 2021”. High Perform. Polym. 2022, 34, 652–672. [Google Scholar] [CrossRef]
- Osada, I.; de Vries, H.; Scrosati, B.; Passerini, S. Ionic-liquid-based polymer electrolytes for battery applications. Angew. Chem. Int. Ed. 2016, 55, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Tamilarasan, P.; Ramaprabhu, S. Stretchable supercapacitors based on highly stretchable ionic liquid incorporated polymer electrolyte. Mater. Chem. Phys. 2014, 148, 48–56. [Google Scholar] [CrossRef]
- Liew, C.W.; Ramesh, S.; Arof, A.K. Good prospect of ionic liquid based-poly(vinyl alcohol) polymer electrolytes for supercapacitors with excellent electrical, electrochemical and thermal properties. Int. J. Hydrogen Energy 2014, 39, 2953–2963. [Google Scholar] [CrossRef]
- Shi, M.J.; Kou, S.Z.; Shen, B.S.; Lang, J.W.; Yang, Z.; Yan, X.B. Improving the performance of all-solid-state supercapacitors by modifying ionic liquid gel electrolytes with graphene nanosheets prepared by arc-discharge. Chin. Chem. Lett. 2014, 25, 859–864. [Google Scholar] [CrossRef]
- Ujjain, S.K.; Sahu, V.; Sharma, R.K.; Singh, G. High performance, all solid state, flexible supercapacitor based on Ionic liquid functionalized Graphene. Electrochim. Acta 2015, 157, 245–251. [Google Scholar] [CrossRef]
- Liew, C.; Ramesh, S.; Arof, A.K. Characterization of ionic liquid added poly(vinyl alcohol)-based proton conducting polymer electrolytes and electrochemical studies on the supercapacitors. Int. J. Hydrogen Energy 2014, 40, 852–862. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, F.; Zhang, L.; Zhang, T.; Huang, Y.; Chen, Y.A. High-performance graphene oxide-doped ion gel as gel polymer electrolyte for all-solid-state supercapacitor applications. Adv. Funct. Mater. 2013, 23, 3353–3360. [Google Scholar] [CrossRef]
- Pandey, G.P.; Hashmi, S.A.; Kumar, Y. Performance studies of activated charcoal based electrical double layer capacitors with ionic liquid gel polymer electrolytes. Energy Fuels 2010, 24, 6644–6652. [Google Scholar] [CrossRef]
- Yun, Y.S.; Kim, J.H.; Lee, S.Y.; Shim, E.G.; Kim, D.W. Cycling performance and thermal stability of lithium polymer cells assembled with ionic liquid-containing gel polymer electrolytes. J. Power Sources 2011, 196, 6750–6755. [Google Scholar] [CrossRef]
- Chinnam, P.R.; Zhang, H.; Wunder, S.L. Blends of Pegylated Polyoctahedralsilsesquioxanes (POSS-PEG) and Methyl Cellulose as Solid Polymer Electrolytes for Lithium Batteries. Electrochim. Acta 2015, 170, 191–201. [Google Scholar] [CrossRef]
- Xiao, S.Y.; Yang, Y.Q.; Li, M.X.; Wang, F.X.; Chang, Z.; Wu, Y.P.; Liu, X. A composite membrane based on a biocompatible cellulose as a host of gel polymer electrolyte for lithium ion batteries. J. Power Sources 2014, 270, 53–58. [Google Scholar] [CrossRef]
- Sudhakar, Y.N.; Selvakumar, M. Lithium perchlorate doped plasticized chitosan and starch blend as biodegradable polymer electrolyte for supercapacitors. Electrochim. Acta 2012, 78, 398–405. [Google Scholar] [CrossRef]
- Xu, D.; Jin, J.; Chen, C.; Wen, Z. From nature to energy storage: A novel sustainable 3D cross-linked chitosan–PEGGE-based gel polymer electrolyte with excellent lithium-ion transport properties for lithium batteries. ACS Appl. Mater. Interfaces 2018, 10, 38526–38537. [Google Scholar] [CrossRef]
- Song, A.; Huang, Y.; Zhong, X.; Cao, H.; Liu, B.; Lin, Y.; Wang, M.; Li, X. Gel polymer electrolyte with high performances based on pure natural polymer matrix of potato starch composite lignocellulose. Electrochim. Acta 2017, 245, 981–992. [Google Scholar] [CrossRef]
- Kasprzak, D.; Galiński, M. Chitin and chitin-cellulose composite hydrogels prepared by ionic liquid-based process as the novel electrolytes for electrochemical capacitors. J. Solid State Electrochem. 2021, 25, 2549–2563. [Google Scholar] [CrossRef]
- Mittal, N.; Ojanguren, A.; Cavasin, N.; Lizundia, E.; Niederberger, M. Transient rechargeable battery with a high lithium transport number cellulosic separator. Adv. Funct. Mat. 2021, 31, 202101827. [Google Scholar] [CrossRef]
- Liew, C.-W.; Ramesh, S. Comparing triflate and hexafluorophosphate anions of ionic liquids in polymer electrolytes for supercapacitor applications. Materials 2014, 7, 4019–4033. [Google Scholar] [CrossRef]
- Kasprzak, D.; Galiński, M. Biopolymer-based gel electrolytes with an ionic liquid for high-voltage electrochemical capacitors. Electrochem. Commun. 2022, 138, 107282. [Google Scholar] [CrossRef]
- Konwar, S.; Singh, A.; Singh, P.K.; Singh, R.C.; Rawat, S.; Dhapola, P.S.; Agarwal, D.; Yahya, M.Z.A. Highly conducting corn starch doped ionic liquid solid polymer electrolyte for energy storage devices. High Perform. Polym. 2023, 35, 63–70. [Google Scholar] [CrossRef]
- Adarsh Rag, S.; Selvakumar, M.; Bhat, S.; Chidangil, S.; De, S. Synthesis and characterization of reduced graphene oxide for supercapacitor application with a biodegradable electrolyte. J. Electron. Mater. 2020, 49, 985–994. [Google Scholar] [CrossRef]
- Adarsh Rag, S.; Selvakumar, M.; De, S.; Chidangil, S.; Bhat, S. Laser induced graphene with biopolymer electrolyte for supercapacitor applications. Mater. Today Proc. 2022, 48, 365–370. [Google Scholar]
- Ramesh, S.; Liew, C.-W.; Arof, A.K. Ion conducting corn starch biopolymer electrolytes doped with ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate. J. Non-Cryst. Sol. 2011, 357, 3654–3660. [Google Scholar] [CrossRef]
- Liew, C.-W.; Ramesh, S. Electrical, structural, thermal and electrochemical properties of corn starch-based biopolymer electrolytes. Carbohydr. Polym. 2015, 124, 222–228. [Google Scholar] [CrossRef]
- Liew, C.-W.; Ramesh, S.; Ramesh, K.; Arof, A.K. Preparation and characterization of lithium ion conducting ionic liquid-based biodegradable corn starch polymer electrolytes. J. Solid State Electrochem. 2012, 16, 1869–1875. [Google Scholar] [CrossRef]
- Ahuja, H.; Dhapola, P.S.; Rahul Sahoo, N.G.; Singh, V.; Singh, P.K. Ionic liquid (1-hexyl-3-methylimidazolium iodide)-incorporated biopolymer electrolyte for efficient supercapacitor. High Perform. Polym. 2020, 32, 220–225. [Google Scholar] [CrossRef]
- Trivedi, T.J.; Bhattacharjya, D.; Yu, J.-S.; Kumar, A. Functionalized agarose self-healing ionogels suitable for supercapacitors. ChemSusChem 2015, 8, 3294–3303. [Google Scholar] [CrossRef]
- Asnawi, A.S.F.M.; Hamsan, M.H.; Aziz, S.B.; Kadir, M.F.Z.; Matmin, J.; Yusof, Y.M. Impregnation of [Emim]Br ionic liquid as plasticizer in biopolymer electrolytes for EDLC application. Electrochim. Acta 2021, 375, 137923. [Google Scholar] [CrossRef]
- Chupp, J.; Shellikeri, A.; Palui, G.; Chatterjee, J. Chitosan-based gel film electrolytes containing ionic liquid and lithium salt for energy storage applications. J. Appl. Polym. Sci. 2015, 132, 42143. [Google Scholar] [CrossRef]
- Singh, D.; Kumar, S.; Singh, A.; Sharma, T.; Singh Dhapola, P.; Konwar, S.; Arkhipova, E.A.; Savilov, S.V.; Singh, P.K. Ionic liquid–biopolymer electrolyte for electrochemical devices. Ionics 2022, 28, 759–766. [Google Scholar] [CrossRef]
- Yamagata, M.; Soeda, K.; Ikebe, S.; Yamazaki, S.; Ishikawa, M. Chitosan-based gel electrolyte containing an ionic liquid for high-performance nonaqueous supercapacitors. Electrochim. Acta 2013, 100, 275–280. [Google Scholar] [CrossRef]
- Mantravadi, R.; Chinnam, P.R.; Dikin, D.A.; Wunder, S.L. High conductivity, high strength solid electrolytes formed by in situ encapsulation of ionic liquids in nanofibrillar methyl cellulose networks. ACS Appl. Mater. Interfaces 2016, 8, 13426–13436. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wen, Z.; Wu, D.; Wang, H.; Yanga, J.; Wang, Q. Tough BMIMCl-based ionogels exhibiting excellent and adjustable performance in high-temperature supercapacitors. J. Mater. Chem. A 2014, 2, 11569–11573. [Google Scholar] [CrossRef]
- Yamazaki, S.; Takegawa, A.; Kaneko, Y.; Kadokawa, J.-I.; Yamagata, M.; Ishikawa, M. An acidic cellulose–chitin hybrid gel as novel electrolyte for an electric double layer capacitor. Electrochem. Commun. 2009, 11, 68–70. [Google Scholar] [CrossRef]
- Lee, H.; Erwin, A.; Buxton, M.L.; Kim, M.; Stryutsky, A.V.; Shevchenko, V.V.; Sokolov, A.P.; Tsukruk, V.V. Shape persistent, highly conductive ionogels from ionic liquids reinforced with cellulose nanocrystal network. Adv. Funct. Mat. 2021, 31, 202103083. [Google Scholar] [CrossRef]
- Flouda, P.; Bukharina, D.; Pierce, K.J.; Stryutsky, A.V.; Shevchenko, V.V.; Tsukruk, V.V. Flexible sustained ionogels with ionic hyperbranched polymers for enhanced ion-conduction and energy storage. ACS Appl. Mater. Interfaces 2022, 14, 27028–27039. [Google Scholar] [CrossRef]
- Kato, R.; Lettow, J.H.; Patel, S.N.; Rowan, S.J. Ion-conducting thermoresponsive films based on polymer-grafted cellulose nanocrystals. ACS Appl. Mater. Interfaces 2020, 12, 54083–54093. [Google Scholar] [CrossRef]
- Yamazaki, S.; Takegawa, A.; Kaneko, Y.; Kadokawa, J.i.; Yamagata, M.; Ishikawa, M. High/low temperature operation of electric double layer capacitor utilizing acidic cellulose–chitin hybrid gel electrolyte. J. Power Sources 2010, 195, 6245–6249. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, J.; Deng, H.; Du, Y.; Shi, X. Chitin derived nitrogen-doped porous carbons with ultrahigh specific surface area and tailored hierarchical porosity for high performance supercapacitors. J. Bioresour. Bioprod. 2021, 6, 142–151. [Google Scholar] [CrossRef]
- Dong, D. Ternary Composite MnO2@MoS2/Polypyrrole from In-situ Synthesis for Binder-free and Flexible Supercapacitor. J. Bioresour. Bioprod. 2019, 4, 242–250. [Google Scholar]
- Wei, L.; Deng, W.; Li, S.; Wu, Z.; Cai, J.; Luo, J. Sandwich-like chitosan porous carbon Spheres/MXene composite with high specific capacitance and rate performance for supercapacitors. J. Bioresour. Bioprod. 2022, 7, 63–72. [Google Scholar] [CrossRef]
- Abdah, M.A.A.M.; Zubair, N.A.; Azman, N.H.N.; Sulaiman, Y. Fabrication of PEDOT coated PVA-GO nanofiber for supercapacitor. Mater. Chem. Phys. 2017, 192, 161. [Google Scholar] [CrossRef]
- Dong, J.; Wang, Z.; Kang, X. The synthesis of graphene/PVDF composite binder and its application in high performance MnO2 supercapacitors. Colloids Surf. A Physicochem. Eng. Asp. 2016, 489, 282–288. [Google Scholar] [CrossRef]
- Andres, B.; Dahlström, C.; Blomquist, N.; Norgren, M.; Olin, H. Cellulose binders for electric double-layer capacitor electrodes: The influence of cellulose quality on electrical properties. Mater. Des. 2018, 141, 342–349. [Google Scholar] [CrossRef]
- Rogers, R.D.; Zavgorodnya, O.; Shamshina, J.L.; Gurau, G. Graphene-Biopolymer Composite Materials and Methods of Making Thereof. U.S. Patent 15/936,056, 2018. [Google Scholar]
- Choudhury, N.A.; Northrop, P.W.; Crothers, A.C.; Jain, S.; Subramanian, V.R. Chitosan hydrogel-based electrode binder and electrolyte membrane for EDLCs: Experimental studies and model validation. J. Appl. Electrochem. 2012, 42, 935–943. [Google Scholar] [CrossRef]
- Zhao, Z.; Cannon, F.S.; Nieto-Delgado, C.; Pena, L. Lignin/collagen hybrid biomaterials as binder substitute for specialty graphites and electrodes. Carbon 2016, 108, 303–317. [Google Scholar] [CrossRef]
- Ghatee, M.H.; Moosavi, F. Physisorption of hydrophobic and hydrophilic 1-alkyl-3-methylimidazolium ionic liquids on the graphenes. J. Phys. Chem. C 2011, 115, 5626–5636. [Google Scholar] [CrossRef]
- Murashko, K.; Nevstrueva, D.; Pihlajamäki, A.; Koiranen, T.; Pyrhönen, J. Cellulose and activated carbon based flexible electrical double-layer capacitor electrode: Preparation and characterization. Energy 2017, 119, 435–441. [Google Scholar] [CrossRef]
- Ye, W.; Li, X.; Zhu, H.; Wang, X.; Wang, S.; Wang, H.; Sun, R. Green fabrication of cellulose/graphene composite in ionic liquid and its electrochemical and photothermal properties. Chem. Eng. J. 2016, 299, 45–55. [Google Scholar] [CrossRef]
- King, C.; Easton, M.E.; Rogers, R.D. Chitin for the replacement of fluoropolymers in the assembly of electrochemical devices. ChemRxiv Camb. Camb. Open Engag. 2018. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Nie, Y.; Wang, C.; Ji, X.; Zhou, L.; Pan, F.; Zhang, S. Preparation of MWCNTs-graphene-cellulose fiber with ionic liquids. ACS Sustain. Chem. Eng. 2019, 7, 20013–22002. [Google Scholar] [CrossRef]
- Lorenzo, M.; Srinivasan, G. Durable flexible supercapacitors utilizing the multifunctional role of ionic liquids. Energy Technol. 2018, 6, 196–204. [Google Scholar] [CrossRef]
- Li, J.; Wang, N.; Tian, J.; Qian, W.; Chu, W. Cross-coupled macro-mesoporous carbon network toward record high energy-power density supercapacitor at 4 V. Adv. Funct. Mater. 2018, 28, 1806153. [Google Scholar] [CrossRef]
- Nath, G.; Singh, P.K.; Dhapola, P.S.; Dohare, S.; Noor, I.M.; Sharma, T.; Singh, A. Fabrication of cornstarch biopolymer-derived nano porous carbon as electrode material for supercapacitor application. Biomass Convers. Biorefinery 2022, 1–8. [Google Scholar] [CrossRef]
- Suhas; Carrott, P.J.M.; Ribeiro Carrott, M.M.L. Lignin-from natural adsorbent to activated carbon: A review. Bioresour. Technol. 2007, 98, 2301–2312. [Google Scholar] [CrossRef] [PubMed]
- Babeł, K.; Jurewicz, K. KOH activated lignin based nanostructured carbon exhibiting high hydrogen electrosorption. Carbon 2008, 46, 1948–1956. [Google Scholar] [CrossRef]
- Kijima, M.; Hirukawa, T.; Hanawa, F.; Hata, T. Thermal conversion of alkaline lignin and its structured derivatives to porous carbonized materials. Bioresour. Technol. 2011, 102, 6279–6285. [Google Scholar] [CrossRef]
- Klose, M.; Reinhold, R.; Logsch, F.; Wolke, F.; Linnemann, J.; Stoeck, U.; Oswald, S.; Uhlemann, M.; Balach, J.; Markowski, J.; et al. Softwood lignin as a sustainable feedstock for porous carbons as active material for supercapacitors using an ionic liquid electrolyte. ACS Sustain. Chem. Eng. 2017, 5, 4094–4102. [Google Scholar] [CrossRef]
- Krishnadoss, V.; Kanjilal, B.; Hesketh, A.; Miller, C.; Mugweru, A.; Akbard, M.; Khademhosseini, A.; Leijten, J.; Noshadi, I. In situ 3D printing of implantable energy storage devices. Chem. Eng. J. 2021, 409, 128213. [Google Scholar] [CrossRef]
- Fang, A.; Smolyanitsky, A. Large variations in the composition of ionic liquid-solvent mixtures in nanoscale confinement. ACS Appl. Mater. Interfaces 2019, 11, 27243–27250. [Google Scholar] [CrossRef]
- Kislenko, S.A.; Samoylov, I.S.; Amirov, R.H. Molecular dynamics simulation of the electrochemical interface between a graphite surface and the ionic liquid [BMIM][PF6]. Phys. Chem. Chem. Phys. 2009, 11, 5584–5590. [Google Scholar] [CrossRef] [PubMed]
- Kondrat, S.; Wu, P.; Qiao, R.; Kornyshev, A.A. Accelerating charging dynamics in subnanometre pores. Nat. Mater 2014, 13, 387. [Google Scholar] [CrossRef] [PubMed]
- de Souza, J.P.; Pivnic, K.; Bazant, M.Z.; Urbakh, M.; Kornyshev, A.A. Structural forces in ionic liquids: The role of ionic size asymmetry. J. Phys. Chem. B 2022, 126, 1242–1253. [Google Scholar] [CrossRef]
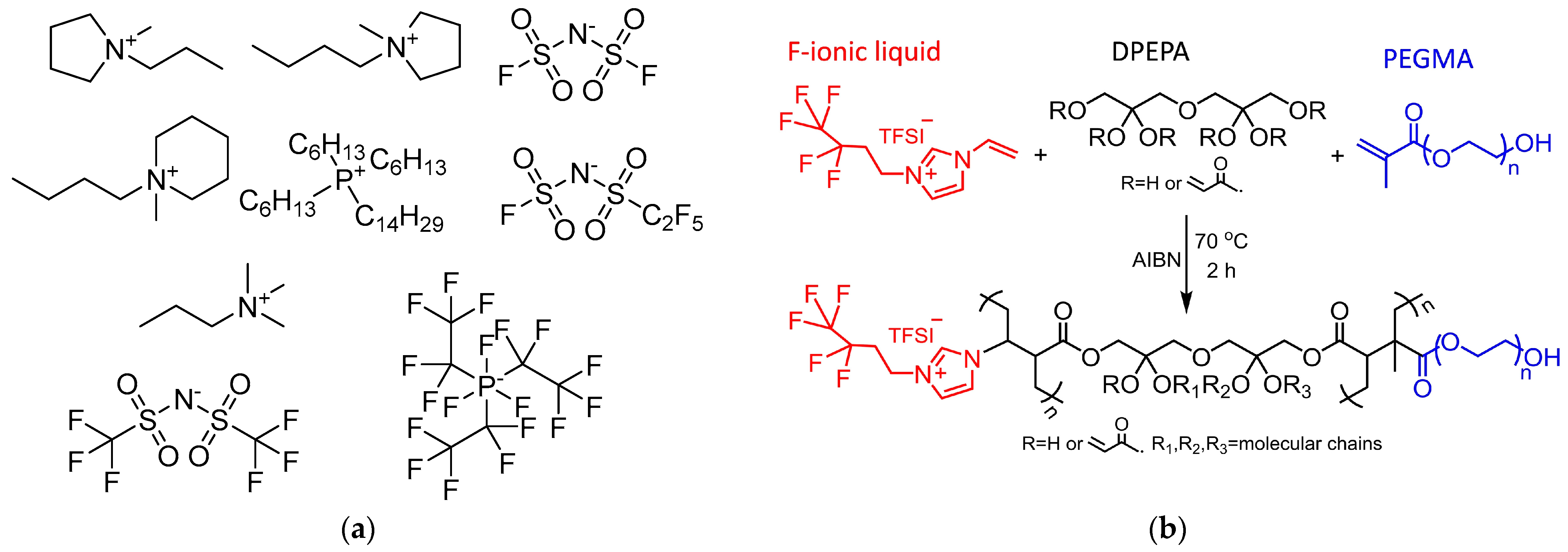
| Cation a | Anion b | Tm, °C | Tdec, °C | Density, g cm−3 | Viscosity, cP | EW, V | Conduct., mS cm−1 | Ref. |
|---|---|---|---|---|---|---|---|---|
| [Pyrr1,3] | [NTf2] | 12 | ND | 1.45 | 61 | 5.3 | 3.9 | [18,22] |
| [N(SO2F)2] | −9 | 219 | ND | 40 | 5.3 | 8.2 | [18,23] | |
| [N(SO2F)(SO2(C2F5))] | −99 | 343 | 1.44 | 56 | 6.0 | 3.5 | [24] | |
| [Pyrr1,4] | [NTf2] | −17.9 | ND | 1.41 | 85 | 6.0 | 2.2 | [25,26] |
| [NTf2] | ND | ND | 1.39 | 83 | 5.5 | 2.8 | [27] | |
| [N1113] | [NTf2] | 19 | 368 | 1.43 | 72 | 5.5 | 3.2 | [27] |
| [Pip1,4] | [NTf2] | ND | 371 | 1.38 | 155.3 | 2.2 | 1.1 | [27] |
| [N2222] | [N(SO2F)(SO2(C2F5))] | 6 | 324 | 1.44 | 104 | 6.0 | 1.5 | [24] |
| [P66614] | [(C2F5)3PF3] | −50 | ND | 1.18 | 464 | 6.3 | ND | [26,28,29] |
| [NTf2] | −50 | ND | 1.18 | 464 | 5.2 | ND | [30] |
| Biopolymeric Matrix | Dopant IL | Ionic Conductivity (mS cm−1) | Supercapacitor Electrode | Capacitance | Ref. |
|---|---|---|---|---|---|
| PVA | [C2mim][EtSO4] + [NH4][OAc] | 0.656 | Reduced graphene oxide | 138 F g−1 | [65] |
| PVA/PVP | [N4444]I | Not reported | Laser-Induced Graphene | 54.28 F g−1 | [66] |
| Corn starch | Li[PF6] + [C4mim][PF6] | 0.147 ± 0.02 (at 40 °C) | Activated carbon | 36.79 F g−1 | [62,67] |
| 0.199 ± 0.02 (at 80 °C) | [68] | ||||
| Li[PF6] + [C4mim][OTf] | 0.600 ± 0.01 (at 80 °C) | Activated carbon | 42.44 F g−1 | [69] | |
| 0.321 ± 0.01 (at 40 °C) | [62] | ||||
| NaCl + [C6mim]I | 0.34 (16 wt% [C6mim]I) | Reduced graphene oxide | 18.4 F g−1 (scan rate of 10 mV s−1); 24.8 F g−1 (low-frequency impedance) | [70] | |
| [NH4]I + [C2mim][SCN] | 0.1 (12 wt% [C2mim][SCN]) | Activated carbon | 130 F g−1 | [64] | |
| Agarose functionalized (acetylated/carbanilated) in [C4mim][OAc] | [HEA][Formate] + [C4mim]Cl | 0.848–1.200 | Activated carbon | 53 F g−1 | [71] |
| Maltodextrin–MC–[NH4]Br | [C2mim]Br | 0.339 ± 0.22 (at room temperature, 30 wt% IL) | Composite of carbon black, activated carbon, and PVdF | 9.85 F g−1 | [72] |
| Chitosan | acetic acid or adipic acid, [C4mim][BF4] + LiCl | 2.91 (chitosan/adipic acid); 2.67 (chitosan/acetic acid) | Bucky paper | Not reported | [73] |
| MC | [Pyrr1,4][NTf2] | 1.4 (at 30 °C), 6 (at 90 °C), 11.3 (at 140 °C) | Not tested | Not tested | [74] |
| MC | [C2mim][TCM] | 19.3 (at 60 wt% IL) | Paste of porous carbon | 38 F g−1 (at 5 mV S−1) | [75] |
| Cellulose or chitin/[C2mim][OAc] | [C2mim][BF4] | 21.7 ± 3.5 (cellulose/[C2mim][BF4]); 22.2 ± 3.5 (chitin/[C2mim][BF4]) | Activated carbon fiber cloth | 140–145 F g−1 (capacitance retention ca. 90% after 10,000 galvanostatic charge-discharge cycles) | [63] |
| Chitosan/acetic acid/sodium hydroxide | [C2mim][BF4] | 16.3 ± 0.2 (at 25 °C) | Activated carbon fiber cloths immersed in [C2mim][BF4] | 131 F g−1 (stable for up to 5000 cycles) | [76] |
| Chitosan/hydroxyethyl methacrylate (HEMA)/[C4mim]Cl | [C4mim]Cl | 31 to 81 (80 to 200 °C) | Activated charcoal | 165 F g−1 (at 200 °C) | [77] |
| Chitin/[AMim]Br + Cellulose/[C4mim]Cl | [AMim]Br + [C4mim]Cl + H2SO4 | 578 (at 25 °C) | Activated carbon fiber cloths immersed in H2SO4 | 162 F g−1 (at 25 °C) | [78] |
| Sulfonated CNC/hyperbranched PILs | [C2mim][NTf2] | 7.8 (95 wt% IL, at 30 °C) | Not tested | Not tested | [79] |
| CNFs/CNCs | [C2mim][NTf2] | 4.3–6.2 (88–90 wt% IL) | Reduced graphene oxide films | 34.5−44.1 F g−1 at 100 mV s−1 | [80] |
| CNC-grafted-PMMA | [C6mim][NTf2] | 5.7 ± 2.1 × 10−3 (at 30 °C) | Not tested | Not tested | [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shamshina, J.L.; Berton, P. Renewable Biopolymers Combined with Ionic Liquids for the Next Generation of Supercapacitor Materials. Int. J. Mol. Sci. 2023, 24, 7866. https://doi.org/10.3390/ijms24097866
Shamshina JL, Berton P. Renewable Biopolymers Combined with Ionic Liquids for the Next Generation of Supercapacitor Materials. International Journal of Molecular Sciences. 2023; 24(9):7866. https://doi.org/10.3390/ijms24097866
Chicago/Turabian StyleShamshina, Julia L., and Paula Berton. 2023. "Renewable Biopolymers Combined with Ionic Liquids for the Next Generation of Supercapacitor Materials" International Journal of Molecular Sciences 24, no. 9: 7866. https://doi.org/10.3390/ijms24097866
APA StyleShamshina, J. L., & Berton, P. (2023). Renewable Biopolymers Combined with Ionic Liquids for the Next Generation of Supercapacitor Materials. International Journal of Molecular Sciences, 24(9), 7866. https://doi.org/10.3390/ijms24097866