Mineralocorticoid Receptor Antagonists for Preventing Chronic Kidney Disease Progression: Current Evidence and Future Challenges
Abstract
1. Introduction: Overview of MR Research
2. Physiological and Pathophysiological Roles of MR in the Kidney
2.1. Electrolyte Handling in Renal Tubules
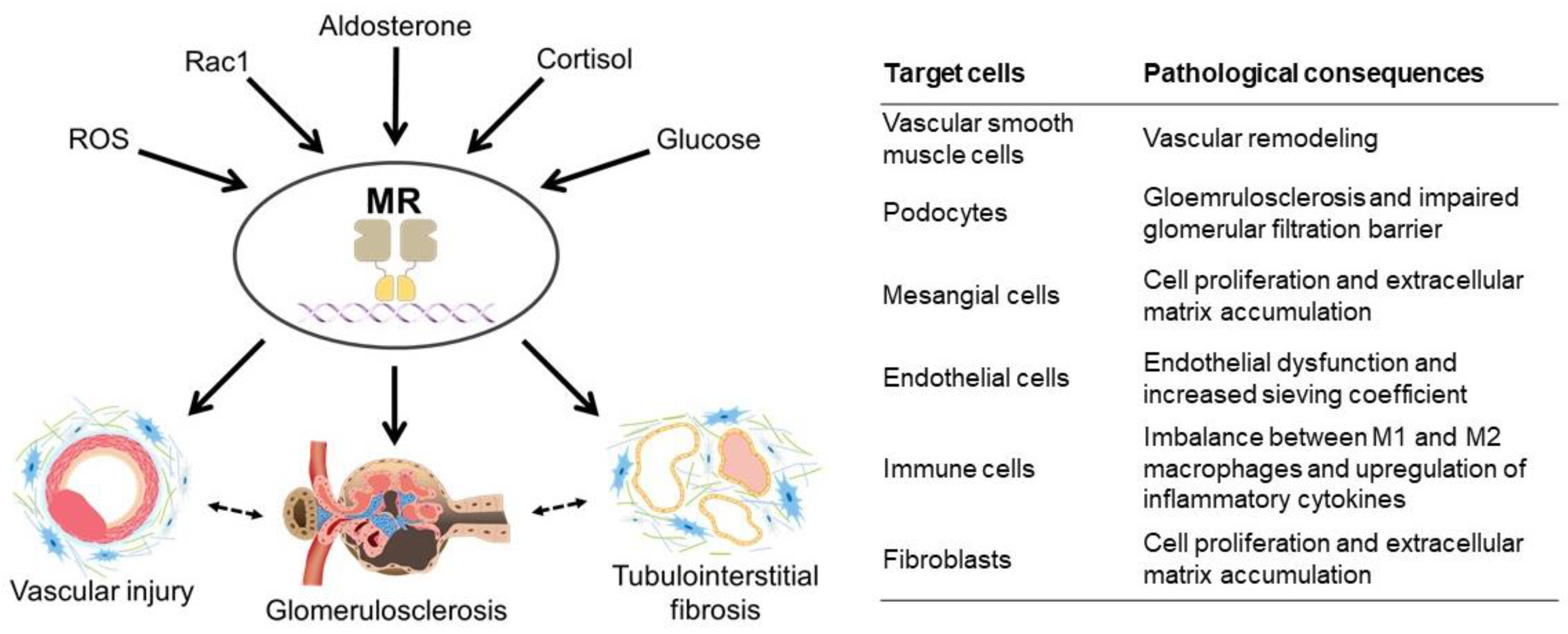
2.2. Kidney Disease and MR
2.3. Vascular Injury
2.4. Glomerular Damage
2.5. Inflammation and Fibrosis
3. Renal Injury Induced by Aldosterone Excess in Humans
4. Renoprotective Effects of MR Antagonists in CKD Patients
4.1. Steroidal MR Antagonists
4.2. Esaxerenone
4.3. Finerenone
| Frist Author, Study Name (year) | Design | Participants | MR Antagonists and Dosages | Length | Primary Endpoint | Results |
|---|---|---|---|---|---|---|
| Bianchi (2006) [150] | Open-label RCT | n = 165, chronic glomerulonephritis | Spironolactone 25 mg/day | 12 months | Urinary protein-to-creatinine ratio | Spironolactone, 2.1 ± 0.08 to 0.89 ± 0.06 g/gCr (p < 0.001 from baseline); conventional therapy, 2.0 ± 0.07 to 2.11 ± 0.08 g/gCr |
| Epstein (2006) [153] | Double-blind RCT | n = 240, type 2 DM with albuminuria | Eplerenone 50 mg/day or 100 mg/day | 12 weeks | Change in the urinary albumin to creatinine ratio (UACR) from baseline (%) | Eplerenone 50 mg/day, −41.0% from baseline; eplerenone 100 mg/day, −48.4% from baseline; placebo, −7.4% from baseline (both p < 0.001 vs. placebo) |
| Ando, EVALUATE (2014) [155] | Double-blind RCT | n = 314, non-diabetic CKD with albuminuria | Eplerenone 50 mg/day | 52 weeks | Change in the UACR from baseline (%) | Eplerenone 50 mg/day, −17.3% (95%CI: −33.54 to −0.94%) from baseline; placebo, +10.3% (95%CI: −6.75 to 22.3%) from baseline (p = 0.02 vs. placebo) |
| Ito, ESAX-DN (2020) [163] | Double-blind RCT | n = 449, type 2 DM with hypertension and albuminuria | Esaxerenone 1.25–2.5 mg/day | 52 weeks | UACR remission rate * | Remission rate was 22% in the esaxerenone group and 4% in the placebo group (p < 0.001 vs. placebo) |
| Bakris, ARTS-DN (2015) [167] | Double-blind RCT | n = 764, type 2 DM and CKD with albuminuria | Finerenone 1.25–20 mg/day | 90 days | Ratio of UACR at day 90 vs. baseline | Placebo-corrected mean ratio of UACR at day 90 relative to baseline was 0.79 for finerenone 7.5 mg/day, 0.76 for 10 mg/day, 0.67 for 15 mg/day, and 0.62 for 20 mg/day |
| Bakris, FIDELIO-DKD (2020) [168] | Double-blind RCT | n = 5674, type 2 DM and CKD with albuminuria | Finerenone 10 or 20 mg/day | 2.6 years | Composite of kidney failure, >57% decrease in eGFR from baseline, death from renal causes | Primary outcome occurred 17.8% in finerenone group and 21.1% in placebo group (hazard ratio, 0.82; 95%CI 0.73 to 0.93; p = 0.001) |
4.4. Meta-Analysis and Real-World Evidence of MR Antagonists on Kidney Protection
4.5. Ongoing Clinical Studies and MR Antagonists in Development
5. Summary and Areas of Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steiger, M.; Reichstein, T. Partial Synthesis of a Crystallized Compound with the Biological Activity of the Adrenal-cortical Hormone. Nature 1937, 139, 925–926. [Google Scholar] [CrossRef]
- Selye, H.; Jensen, H. The chemistry of the hormones. Annu. Rev. Biochem. 1946, 15, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Thorn, G.W.; Engel, L.L.; Eisenberg, H. The effect of corticosterone and related compounds on the renal excretion of electrolytes. J. Exp. Med. 1938, 68, 161–171. [Google Scholar] [CrossRef]
- Thorn, G.W.; Howard, R.P.; Emerson, K. Treatment of addison’s disease with desoxy-corticosterone acetate, a synthetic adrenal cortical hormone (preliminary report). J. Clin. Investig. 1939, 18, 449–467. [Google Scholar] [CrossRef]
- Cleghorn, R.A.; Fowler, J.L.; Wenzel, J.S. The treatment of addison’s disease by a synthetic adrenal cortical hormone (desoxycorticosterone acetate). Can. Med. Assoc. J. 1939, 41, 226–231. [Google Scholar] [PubMed]
- Dunlop, D.M. Desoxycorticosterone in Addison’s Disease. BMJ 1943, 1, 557–558. [Google Scholar] [CrossRef] [PubMed]
- Vinson, G.P. The mislabelling of deoxycorticosterone: Making sense of corticosteroid structure and function. J. Endocrinol. 2011, 211, 3–16. [Google Scholar] [CrossRef]
- Kuhlmann, D.; Ragan, C.; Ferrebee, J.W.; Atchley, D.W.; Loeb, R.F. Toxic Effects of Desoxycorticosterone Esters in Dogs. Science 1939, 90, 496–497. [Google Scholar] [CrossRef]
- Perera, G.A.; Knowlton, A.I.; Lowell, A.; Loeb, R.F. Effect of desoxycorticosterone acetate on the blood pressure of man. JAMA 1944, 125, 1030–1035. [Google Scholar] [CrossRef]
- Selye, H.; Hall, C.E.; Rowley, E.M. Malignant Hypertension Produced by Treatment with Desoxycorticosterone Acetate and Sodium Chloride. Can. Med. Assoc. J. 1943, 49, 88–92. [Google Scholar]
- Grundy, H.M.; Simpson, S.A.; Tait, J.F. Isolation of a Highly Active Mineralocorticoid from Beef Adrenal Extract. Nature 1952, 169, 795–796. [Google Scholar] [CrossRef]
- Simpson, S.A.; Tait, J.F.; Wettstein, A.; Neher, R.; Von Euw, J.; Reichstein, T. Isolation from the adrenals of a new crystalline hormone with especially high effectiveness on mineral metabolism. Experientia 1953, 9, 333–335. [Google Scholar] [CrossRef]
- Simpson, S.A.; Tait, J.F.; Wettstein, A.; Neher, R.; Euw, J.V.; Schindler, O.; Reichstein, T. Constitution of aldosterone, a new mineralocorticoid. Experientia 1954, 10, 132–133. [Google Scholar] [CrossRef]
- Conn, J.W. Presidential address. I. Painting background. II. Primary aldosteronism, a new clinical syndrome. J. Lab. Clin. Med. 1955, 45, 3–17. [Google Scholar] [PubMed]
- Conn, J.W.; Louis, L.H. Primary aldosteronism: A new clinical entity. Trans. Assoc. Am. Physicians 1955, 68, 215–231; discussion 231–233. [Google Scholar] [PubMed]
- Gordon, R.D.; Geddes, R.A.; Pawsey, C.G.; O’Halloran, M.W. Hypertension and severe hyperkalaemia associated with suppression of renin and aldosterone and completely reversed by dietary sodium restriction. Australas. Ann. Med. 1970, 19, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, D.J.; Ruse, J.L.; Laidlaw, J.C. Hypertension, increased aldosterone secretion and low plasma renin activity relieved by dexamethasone. Can. Med. Assoc. J. 1966, 95, 1109–1119. [Google Scholar]
- Gw, L. A familial renal disorder simulating primary aldosteronism but with negligible aldosterone secretion. Trans. Assoc. Am. Physicians 1963, 76, 199–213. [Google Scholar]
- Kagawa, C.M.; Cella, J.A.; Van Arman, C.G. Action of new steroids in blocking effects of aldosterone and desoxycorticosterone on salt. Science 1957, 126, 1015–1016. [Google Scholar] [CrossRef]
- Liddle, G.W. Sodium Diuresis Induced by Steroidal Antagonists of Aldosterone. Science 1957, 126, 1016–1018. [Google Scholar] [CrossRef]
- Taylor, F.F.; Faloon, W.W. The role of potassium in the natriuretic response to a steroidal lactone (sc-9420). J. Clin. Endocrinol. Metab. 1959, 19, 1683–1687. [Google Scholar] [CrossRef] [PubMed]
- The Spirolactones. Br. Med. J. 1960, 2, 364–365. [CrossRef]
- Arriza, J.L.; Weinberger, C.; Cerelli, G.; Glaser, T.M.; Handelin, B.L.; Housman, D.E.; Evans, R.M. Cloning of Human Mineralocorticoid Receptor Complementary DNA: Structural and Functional Kinship with the Glucocorticoid Receptor. Science 1987, 237, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Canessa, C.M.; Horisberger, J.-D.; Rossier, B.C. Epithelial sodium channel related to proteins involved in neurodegeneration. Nature 1993, 361, 467–470. [Google Scholar] [CrossRef]
- Canessa, C.M.; Schild, L.; Buell, G.; Thorens, B.; Gautschi, I.; Horisberger, J.-D.; Rossier, B.C. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 1994, 367, 463–467. [Google Scholar] [CrossRef]
- Lifton, R.P.; Gharavi, A.G.; Geller, D.S. Molecular Mechanisms of Human Hypertension. Cell 2001, 104, 545–556. [Google Scholar] [CrossRef]
- LIfton, R.P.; Dluhy, R.G.; Powers, M.; Rich, G.M.; Cook, S.; Ulick, S.; Lalouel, J.M. A chimaeric 11 beta-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature 1992, 355, 262–265. [Google Scholar] [CrossRef]
- Geller, D.S.; Farhi, A.; Pinkerton, N.; Fradley, M.; Moritz, M.; Spitzer, A.; Meinke, G.; Tsai, F.T.F.; Sigler, P.B.; Lifton, R.P. Activating Mineralocorticoid Receptor Mutation in Hypertension Exacerbated by Pregnancy. Science 2000, 289, 119–123. [Google Scholar] [CrossRef]
- Shimkets, R.A.; Warnock, D.G.; Bositis, C.M.; Nelson-Williams, C.; Hansson, J.H.; Schambelan, M.; Gill, J.R., Jr.; Ulick, K.; Milora, R.V.; Findling, J.W.; et al. Liddle’s syndrome: Heritable human hypertension caused by mutations in the beta subunit of the epithelial sodium channel. Cell 1994, 79, 407–414. [Google Scholar] [CrossRef]
- Chang, S.S.; Gründer, S.; Hanukoglu, A.; Rösler, A.; Mathew, P.; Hanukoglu, I.; Schild, L.; Lu, Y.; Shimkets, R.A.; Nelson-Williams, C.; et al. Mutations in subunits of the epithelial sodium channel cause salt wasting with hyperkalaemic acidosis, pseudohypoaldosteronism type 1. Nat. Genet. 1996, 12, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Strautnieks, S.S.; Thompson, R.J.; Gardiner, R.M.; Chung, E. A novel splice-site mutation in the gamma subunit of the epithelial sodium channel gene in three pseudohypoaldosteronism type 1 families. Nat. Genet. 1996, 13, 248–250. [Google Scholar] [CrossRef]
- Mitsuuchi, Y.; Kawamoto, T.; Rösler, A.; Naiki, Y.; Miyahara, K.; Toda, K.; Kuribayashi, I.; Orii, T.; Yasuda, K.; Miura, K.; et al. Congenitally defective aldosterone biosynthesis in humans: The involvement of point mutations of the P-450C18 gene (CYP11B2) in CMO II deficient patients. Biochem. Biophys. Res. Commun. 1992, 182, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Pascoe, L.; Curnow, K.M.; Slutsker, L.; Rösler, A.; White, P.C. Mutations in the human CYP11B2 (aldosterone synthase) gene causing corticosterone methyloxidase II deficiency. Proc. Natl. Acad. Sci. USA 1992, 89, 4996–5000. [Google Scholar] [CrossRef]
- Geller, D.S.; Rodriguez-Soriano, J.; Boado, A.V.; Schifter, S.; Bayer, M.; Chang, S.S.; Lifton, R.P. Mutations in the mineralocorticoid receptor gene cause autosomal dominant pseudohypoaldosteronism type I. Nat. Genet. 1998, 19, 279–281. [Google Scholar] [CrossRef]
- Choi, M.; Scholl, U.I.; Yue, P.; Björklund, P.; Zhao, B.; Nelson-Williams, C.; Ji, W.; Cho, Y.; Patel, A.; Men, C.J.; et al. K+ Channel Mutations in Adrenal Aldosterone-Producing Adenomas and Hereditary Hypertension. Science 2011, 331, 768–772. [Google Scholar] [CrossRef]
- Scholl, U.I.; Goh, G.; Stölting, G.; De Oliveira, R.C.; Choi, M.; Overton, J.D.; Fonseca, A.L.; Korah, R.; Starker, L.F.; Kunstman, J.; et al. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat. Genet. 2013, 45, 1050–1054. [Google Scholar] [CrossRef]
- Azizan, E.A.B.; Poulsen, H.; Tuluc, P.; Zhou, J.; Clausen, M.V.; Lieb, A.; Maniero, C.; Garg, S.; Bochukova, E.; Zhao, W.; et al. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat. Genet. 2013, 45, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Beuschlein, F.; Boulkroun, S.; Osswald, A.; Wieland, T.; Nielsen, H.N.; Lichtenauer, U.D.; Penton, D.; Schack, V.R.; Amar, L.; Fischer, E.; et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat. Genet. 2013, 45, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Scholl, U.I.; Stölting, G.; Nelson-Williams, C.; Vichot, A.A.; Choi, M.; Loring, E.; Prasad, M.L.; Goh, G.; Carling, T.; Juhlin, C.C.; et al. Recurrent gain of function mutation in calcium channel CACNA1H causes early-onset hypertension with primary aldosteronism. eLife 2015, 4, e06315. [Google Scholar] [CrossRef] [PubMed]
- Teo, A.E.; Garg, S.; Shaikh, L.H.; Zhou, J.; Frankl, F.E.K.; Gurnell, M.; Happerfield, L.; Marker, A.; Bienz, M.; Azizan, E.A.; et al. Pregnancy, Primary Aldosteronism, and Adrenal CTNNB1 Mutations. N. Engl. J. Med. 2015, 373, 1429–1436. [Google Scholar] [CrossRef]
- Scholl, U.I.; Stölting, G.; Schewe, J.; Thiel, A.; Tan, H.; Nelson-Williams, C.; Vichot, A.A.; Jin, S.C.; Loring, E.; Untiet, V.; et al. CLCN2 chloride channel mutations in familial hyperaldosteronism type II. Nat. Genet. 2018, 50, 349–354. [Google Scholar] [CrossRef]
- Zhou, J.; Azizan, E.A.B.; Cabrera, C.P.; Fernandes-Rosa, F.L.; Boulkroun, S.; Argentesi, G.; Cottrell, E.; Amar, L.; Wu, X.; O’toole, S.; et al. Somatic mutations of GNA11 and GNAQ in CTNNB1-mutant aldosterone-producing adenomas presenting in puberty, pregnancy or menopause. Nat. Genet. 2021, 53, 1360–1372. [Google Scholar] [CrossRef]
- Pitt, B.; Zannad, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; Palensky, J.; Wittes, J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N. Engl. J. Med. 1999, 341, 709–717. [Google Scholar] [CrossRef]
- Pitt, B.; Remme, W.; Zannad, F.; Neaton, J.; Martinez, F.; Roniker, B.; Bittman, R.; Hurley, S.; Kleiman, J.; Gatlin, M. Eplerenone, a Selective Aldosterone Blocker, in Patients with Left Ventricular Dysfunction after Myocardial Infarction. N. Engl. J. Med. 2003, 348, 1309–1321. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, 1757–1780. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Rinehart, J.; Zhang, J.; Moeckel, G.; Castañeda-Bueno, M.; Stiegler, A.L.; Boggon, T.J.; Gamba, G.; Lifton, R.P. Mineralocorticoid Receptor Phosphorylation Regulates Ligand Binding and Renal Response to Volume Depletion and Hyperkalemia. Cell Metab. 2013, 18, 660–671. [Google Scholar] [CrossRef]
- Shibata, S. 30 years of the mineralocorticoid receptor: Mineralocorticoid receptor and NaCl transport mechanisms in the renal distal nephron. J. Endocrinol. 2017, 234, T35–T47. [Google Scholar] [CrossRef] [PubMed]
- Hirohama, D.; Ayuzawa, N.; Ueda, K.; Nishimoto, M.; Kawarazaki, W.; Watanabe, A.; Shimosawa, T.; Marumo, T.; Shibata, S.; Fujita, T. Aldosterone Is Essential for Angiotensin II-Induced Upregulation of Pendrin. J. Am. Soc. Nephrol. 2017, 29, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Ishizawa, K.; Wang, Q.; Xu, N.; Fujita, T.; Uchida, S.; Lifton, R.P. ULK1 Phosphorylates and Regulates Mineralocorticoid Receptor. Cell Rep. 2018, 24, 569–576. [Google Scholar] [CrossRef]
- Yamazaki, O.; Ishizawa, K.; Hirohama, D.; Fujita, T.; Shibata, S. Electrolyte transport in the renal collecting duct and its regulation by the renin–angiotensin–aldosterone system. Clin. Sci. 2019, 133, 75–82. [Google Scholar] [CrossRef]
- Ayuzawa, N.; Nishimoto, M.; Ueda, K.; Hirohama, D.; Kawarazaki, W.; Shimosawa, T.; Marumo, T.; Fujita, T. Two Mineralocorticoid Receptor–Mediated Mechanisms of Pendrin Activation in Distal Nephrons. J. Am. Soc. Nephrol. 2020, 31, 748–764. [Google Scholar] [CrossRef]
- Ellison, D.H.; Welling, P. Insights into Salt Handling and Blood Pressure. N. Engl. J. Med. 2021, 385, 1981–1993. [Google Scholar] [CrossRef]
- Yamazaki, O.; Hirohama, D.; Ishizawa, K.; Shibata, S. Role of the Ubiquitin Proteasome System in the Regulation of Blood Pressure: A Review. Int. J. Mol. Sci. 2020, 21, 5358. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Arroyo, J.P.; Castañeda-Bueno, M.; Puthumana, J.; Zhang, J.; Uchida, S.; Stone, K.L.; Lam, T.T.; Lifton, R.P. Angiotensin II signaling via protein kinase C phosphorylates Kelch-like 3, preventing WNK4 degradation. Proc. Natl. Acad. Sci. USA 2014, 111, 15556–15561. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Bueno, M.; Arroyo, J.P.; Zhang, J.; Puthumana, J.; Yarborough, O.; Shibata, S.; Rojas-Vega, L.; Gamba, G.; Rinehart, J.; Lifton, R.P. Phosphorylation by PKC and PKA regulate the kinase activity and downstream signaling of WNK4. Proc. Natl. Acad. Sci. USA 2017, 114, E879–E886. [Google Scholar] [CrossRef]
- Ishizawa, K.; Wang, Q.; Li, J.; Yamazaki, O.; Tamura, Y.; Fujigaki, Y.; Uchida, S.; Lifton, R.P.; Shibata, S. Calcineurin dephosphorylates Kelch-like 3, reversing phosphorylation by angiotensin II and regulating renal electrolyte handling. Proc. Natl. Acad. Sci. USA 2019, 116, 3155–3160. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, S.B.; Fenton, R.A. K+ and the renin–angiotensin–aldosterone system: New insights into their role in blood pressure control and hypertension treatment. J. Physiol. 2019, 597, 4451–4464. [Google Scholar] [CrossRef]
- Maeoka, Y.; Su, X.-T.; Wang, W.-H.; Duan, X.-P.; Sharma, A.; Li, N.; Staub, O.; McCormick, J.A.; Ellison, D.H. Mineralocorticoid Receptor Antagonists Cause Natriuresis in the Absence of Aldosterone. Hypertension 2022, 79, 1423–1434. [Google Scholar] [CrossRef]
- Pearce, D.; Manis, A.D.; Nesterov, V.; Korbmacher, C. Regulation of distal tubule sodium transport: Mechanisms and roles in homeostasis and pathophysiology. Pflügers Arch. 2022, 474, 869–884. [Google Scholar] [CrossRef]
- Wall, S.M. Regulation of Blood Pressure and Salt Balance by Pendrin-Positive Intercalated Cells: Donald Seldin Lecture 2020. Hypertension 2022, 79, 706–716. [Google Scholar] [CrossRef]
- Knowlton, A.I.; Stoerk, H.; Seegal, B.C.; Loeb, E.N. Influence of adrenal cortical steroids upon the blood pressure and the rate of progression of experimental nephritis in rats. Endocrinology 1946, 38, 315–324. [Google Scholar] [CrossRef]
- Gavras, H.; Brunner, H.R.; Laragh, J.H. Renin and aldosterone and the pathogenesis of hypertensive vascular damage. Prog. Cardiovasc. Dis. 1974, 17, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Tikkanen, I.; Törnroth, T.; Miettinen, A.; Fyhrquist, F. Heymann nephritis-DOCA-NaCl hypertension in the rat. Role of nephritis, DOCA, NaCl, and vascular lesions in the development of hypertension. Nephron 1981, 28, 90–95. [Google Scholar] [CrossRef]
- Lewis, E.J.; Hunsicker, L.G.; Bain, R.P.; Rohde, R.D. The Effect of Angiotensin-Converting-Enzyme Inhibition on Diabetic Nephropathy. N. Engl. J. Med. 1993, 329, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Lafayette, R.A.; Mayer, G.; Park, S.K.; Meyer, T.W. Angiotensin II receptor blockade limits glomerular injury in rats with reduced renal mass. J. Clin. Investig. 1992, 90, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B.M.; Cooper, M.E.; De Zeeuw, D.; Keane, W.F.; Mitch, W.E.; Parving, H.-H.; Remuzzi, G.; Snapinn, S.M.; Zhang, Z.; Shahinfar, S. Effects of Losartan on Renal and Cardiovascular Outcomes in Patients with Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2001, 345, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Greene, E.L.; Kren, S.; Hostetter, T.H. Role of aldosterone in the remnant kidney model in the rat. J. Clin. Investig. 1996, 98, 1063–1068. [Google Scholar] [CrossRef]
- Todd-Turla, K.M.; Schnermann, J.; Fejes-Toth, G.; Náray-Fejes-Tóth, A.; Smart, A.; Killen, P.D.; Briggs, J.P. Distribution of mineralocorticoid and glucocorticoid receptor mRNA along the nephron. Am. J. Physiol. 1993, 264 Pt 2, F781–F791. [Google Scholar] [CrossRef]
- Aldigier, J.C.; Kanjanbuch, T.; Ma, L.-J.; Brown, N.J.; Fogo, A.B. Regression of Existing Glomerulosclerosis by Inhibition of Aldosterone. J. Am. Soc. Nephrol. 2005, 16, 3306–3314. [Google Scholar] [CrossRef]
- Shibata, S.; Nagase, M.; Yoshida, S.; Kawarazaki, W.; Kurihara, H.; Tanaka, H.; Miyoshi, J.; Takai, Y.; Fujita, T. Modification of mineralocorticoid receptor function by Rac1 GTPase: Implication in proteinuric kidney disease. Nat. Med. 2008, 14, 1370–1376. [Google Scholar] [CrossRef]
- Gupta, I.R.; Baldwin, C.; Auguste, D.; Ha, K.C.H.; El Andalousi, J.; Fahiminiya, S.; Bitzan, M.; Bernard, C.; Akbari, M.R.; Narod, S.A.; et al. ARHGDIA: A novel gene implicated in nephrotic syndrome. J. Med. Genet. 2013, 50, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Gee, H.Y.; Saisawat, P.; Ashraf, S.; Hurd, T.W.; Vega-Warner, V.; Fang, H.; Beck, B.B.; Gribouval, O.; Zhou, W.; Diaz, K.A.; et al. ARHGDIA mutations cause nephrotic syndrome via defective RHO GTPase signaling. J. Clin. Investig. 2013, 123, 3243–3253. [Google Scholar] [CrossRef]
- Guo, C.; Martinez-Vasquez, D.; Mendez, G.P.; Toniolo, M.F.; Yao, T.M.; Oestreicher, E.M.; Kikuchi, T.; Lapointe, N.; Pojoga, L.; Williams, G.H.; et al. Mineralocorticoid Receptor Antagonist Reduces Renal Injury in Rodent Models of Types 1 and 2 Diabetes Mellitus. Endocrinology 2006, 147, 5363–5373. [Google Scholar] [CrossRef] [PubMed]
- Hirohama, D.; Nishimoto, M.; Ayuzawa, N.; Kawarazaki, W.; Fujii, W.; Oba, S.; Shibata, S.; Marumo, T.; Fujita, T. Activation of Rac1-Mineralocorticoid Receptor Pathway Contributes to Renal Injury in Salt-Loaded db/db Mice. Hypertension 2021, 78, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Zitt, E.; Eller, K.; Huber, J.M.; Kirsch, A.H.; Tagwerker, A.; Mayer, G.; Rosenkranz, A.R. The selective mineralocorticoid receptor antagonist eplerenone is protective in mild anti-GBM glomeru-lonephritis. Int. J. Clin. Exp. Pathol. 2011, 4, 606–615. [Google Scholar] [PubMed]
- Huang, L.L.; Nikolic-Paterson, D.J.; Han, Y.; Ozols, E.; Ma, F.Y.; Young, M.J.; Tesch, G.H. Myeloid Mineralocorticoid Receptor Activation Contributes to Progressive Kidney Disease. J. Am. Soc. Nephrol. 2014, 25, 2231–2240. [Google Scholar] [CrossRef]
- Trachtman, H.; Weiser, A.C.; Valderrama, E.; Morgado, M.; Palmer, L.S. Prevention of renal fibrosis by spironolactone in mice with complete unilateral ureteral obstruction. J. Urol. 2004, 172 Pt 2, 1590–1594. [Google Scholar] [CrossRef]
- Wang, C.-H.; Wang, Z.; Liang, L.-J.; Wang, X.-T.; Ma, X.-L.; Liu, B.-B.; He, J.-Q.; Shimosawa, T.; Xu, Q.-Y. The Inhibitory Effect of Eplerenone on Cell Proliferation in the Contralateral Kidneys of Rats with Unilateral Ureteral Obstruction. Nephron 2017, 136, 328–338. [Google Scholar] [CrossRef]
- Droebner, K.; Pavkovic, M.; Grundmann, M.; Hartmann, E.; Goea, L.; Nordlohne, J.; Klar, J.; Eitner, F.; Kolkhof, P. Direct Blood Pressure-Independent Anti-Fibrotic Effects by the Selective Nonsteroidal Mineralocorticoid Receptor Antagonist Finerenone in Progressive Models of Kidney Fibrosis. Am. J. Nephrol. 2021, 52, 588–601. [Google Scholar] [CrossRef]
- Barrera-Chimal, J.; André-Grégoire, G.; Cat, A.N.D.; Lechner, S.M.; Cau, J.; Prince, S.; Kolkhof, P.; Loirand, G.; Sauzeau, V.; Hauet, T.; et al. Benefit of Mineralocorticoid Receptor Antagonism in AKI: Role of Vascular Smooth Muscle Rac1. J. Am. Soc. Nephrol. 2017, 28, 1216–1226. [Google Scholar] [CrossRef]
- Barrera-Chimal, J.; Pérez-Villalva, R.; Rodríguez-Romo, R.; Reyna, J.; Uribe, N.; Gamba, G.; Bobadilla, N.A. Spironolactone prevents chronic kidney disease caused by ischemic acute kidney injury. Kidney Int. 2013, 83, 93–103. [Google Scholar] [CrossRef]
- Lattenist, L.; Lechner, S.M.; Messaoudi, S.; Le Mercier, A.; El Moghrabi, S.; Prince, S.; Bobadilla, N.A.; Kolkhof, P.; Jaisser, F.; Barrera-Chimal, J. Nonsteroidal Mineralocorticoid Receptor Antagonist Finerenone Protects Against Acute Kidney Injury-Mediated Chronic Kidney Disease: Role of Oxidative Stress. Hypertension 2017, 69, 870–878. [Google Scholar] [CrossRef]
- Yokota, K.; Shibata, H.; Kurihara, I.; Kobayashi, S.; Suda, N.; Murai-Takeda, A.; Saito, I.; Kitagawa, H.; Kato, S.; Saruta, T.; et al. Coactivation of the N-terminal transactivation of mineralocorticoid receptor by Ubc9. J. Biol. Chem. 2007, 282, 1998–2010. [Google Scholar] [CrossRef] [PubMed]
- Young, M.J.; Morgan, J.; Brolin, K.; Fuller, P.; Funder, J.W. Activation of Mineralocorticoid Receptors by Exogenous Glucocorticoids and the Development of Cardiovascular Inflammatory Responses in Adrenalectomized Rats. Endocrinology 2010, 151, 2622–2628. [Google Scholar] [CrossRef]
- Nagase, M.; Ayuzawa, N.; Kawarazaki, W.; Ishizawa, K.; Ueda, K.; Yoshida, S.; Fujita, T. Oxidative stress causes mineralocorticoid receptor activation in rat cardiomyocytes: Role of small GTPase Rac1. Hypertension 2012, 59, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Shibata, H.; Kurihara, I.; Yokota, K.; Mitsuishi, Y.; Ohashi, K.; Murai-Takeda, A.; Jo, R.; Ohyama, T.; Sakamoto, M.; et al. High Glucose Stimulates Mineralocorticoid Receptor Transcriptional Activity Through the Protein Kinase C β Signaling. Int. Heart J. 2017, 58, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Mitsuishi, Y.; Shibata, H.; Kurihara, I.; Kobayashi, S.; Yokota, K.; Murai-Takeda, A.; Hayashi, T.; Jo, R.; Nakamura, T.; Morisaki, M.; et al. Epidermal growth factor receptor/extracellular signal-regulated kinase pathway enhances mineralocorticoid receptor transcriptional activity through protein stabilization. Mol. Cell. Endocrinol. 2018, 473, 89–99. [Google Scholar] [CrossRef]
- Rocha, R.; Stier, C.T., Jr.; Kifor, I.; Ochoa-Maya, M.R.; Rennke, H.G.; Williams, G.H.; Adler, G.K. Aldosterone: A Mediator of Myocardial Necrosis and Renal Arteriopathy. Endocrinology 2000, 141, 3871–3878. [Google Scholar] [CrossRef] [PubMed]
- Rocha, R.; Chander, P.N.; Zuckerman, A.; Stier, C.T., Jr. Role of Aldosterone in Renal Vascular Injury in Stroke-Prone Hypertensive Rats. Hypertension 1999, 33 Pt 2, 232–237. [Google Scholar] [CrossRef]
- Jaisser, F.; Farman, N. Emerging Roles of the Mineralocorticoid Receptor in Pathology: Toward New Paradigms in Clinical Pharmacology. Pharmacol. Rev. 2016, 68, 49–75. [Google Scholar] [CrossRef]
- Wilson, P.C.; Wu, H.; Kirita, Y.; Uchimura, K.; Ledru, N.; Rennke, H.G.; Welling, P.A.; Waikar, S.S.; Humphreys, B.D. The single-cell transcriptomic landscape of early human diabetic nephropathy. Proc. Natl. Acad. Sci. USA 2019, 116, 19619–19625. [Google Scholar] [CrossRef] [PubMed]
- Barrera-Chimal, J.; Lima-Posada, I.; Bakris, G.L.; Jaisser, F. Mineralocorticoid receptor antagonists in diabetic kidney disease—Mechanistic and therapeutic effects. Nat. Rev. Nephrol. 2022, 18, 56–70. [Google Scholar] [CrossRef]
- Pruthi, D.; McCurley, A.; Aronovitz, M.; Galayda, C.; Karumanchi, S.A.; Jaffe, I.Z. Aldosterone Promotes Vascular Remodeling by Direct Effects on Smooth Muscle Cell Mineralocorticoid Receptors. Arter. Thromb. Vasc. Biol. 2014, 34, 355–364. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Li, C.; Shen, Z.-X.; Zhang, W.-C.; Ai, T.-J.; Du, L.-J.; Zhang, Y.-Y.; Yao, G.-F.; Liu, Y.; Sun, S.; et al. Mineralocorticoid Receptor Deficiency in Macrophages Inhibits Neointimal Hyperplasia and Suppresses Macrophage Inflammation through SGK1-AP1/NF-κB Pathways. Arter. Thromb. Vasc. Biol. 2016, 36, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Belden, Z.; Deiuliis, J.A.; Dobre, M.; Rajagopalan, S. The Role of the Mineralocorticoid Receptor in Inflammation: Focus on Kidney and Vasculature. Am. J. Nephrol. 2017, 46, 298–314. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.-X.; Chen, X.-Q.; Sun, X.-N.; Sun, J.-Y.; Zhang, W.-C.; Zheng, X.-J.; Zhang, Y.-Y.; Shi, H.-J.; Zhang, J.-W.; Li, C.; et al. Mineralocorticoid Receptor Deficiency in Macrophages Inhibits Atherosclerosis by Affecting Foam Cell Formation and Efferocytosis. J. Biol. Chem. 2017, 292, 925–935. [Google Scholar] [CrossRef]
- Moss, M.E.; Lu, Q.; Iyer, S.L.; Engelbertsen, D.; Marzolla, V.; Caprio, M.; Lichtman, A.H.; Jaffe, I.Z. Endothelial Mineralocorticoid Receptors Contribute to Vascular Inflammation in Atherosclerosis in a Sex-Specific Manner. Arter. Thromb. Vasc. Biol. 2019, 39, 1588–1601. [Google Scholar] [CrossRef] [PubMed]
- Kretzler, M.; Koeppen-Hagemann, I.; Kriz, W. Podocyte damage is a critical step in the development of glomerulosclerosis in the uninephrectomised-desoxycorticosterone hypertensive rat. Virchows Arch. 1994, 425, 181–193. [Google Scholar] [CrossRef]
- Shibata, S.; Nagase, M.; Yoshida, S.; Kawachi, H.; Fujita, T. Podocyte as the target for aldosterone: Roles of oxidative stress and Sgk1. Hypertension 2007, 49, 355–364. [Google Scholar] [CrossRef]
- Chen, C.; Liang, W.; Jia, J.; van Goor, H.; Singhal, P.C.; Ding, G. Aldosterone Induces Apoptosis in Rat Podocytes: Role of PI3-K/Akt and p38MAPK Signaling Pathways. Nephron Exp. Nephrol. 2009, 113, e26–e34. [Google Scholar] [CrossRef]
- Li, D.; Lu, Z.; Xu, Z.; Ji, J.; Zheng, Z.; Lin, S.; Yan, T. Spironolactone promotes autophagy via inhibiting PI3K/AKT/mTOR signalling pathway and reduce adhesive capacity damage in podocytes under mechanical stress. Biosci. Rep. 2016, 36, e00355. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Chen, Y.; Zhao, M.; Zhang, Y.; He, J.C.-J.; Huang, S.; Jia, Z.; Zhang, A. NLRP3 inflammasome activation contributes to aldosterone-induced podocyte injury. Am. J. Physiol. Physiol. 2017, 312, F556–F564. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Xu, X.; Zhao, C.; Zhao, M.; Wang, H.; Zhang, B.; Wang, N.; Mao, H.; Zhang, A.; Xing, C. The roles of oxidative stress, endoplasmic reticulum stress, and autophagy in aldosterone/mineralocorticoid receptor-induced podocyte injury. Lab. Investig. 2015, 95, 1374–1386. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, Z.; Kokeny, G.; Godo, M.; Mózes, M.; Rosivall, L.; Gross, M.-L.; Ritz, E.; Hamar, P. Increased renoprotection with ACE inhibitor plus aldosterone antagonist as compared to monotherapies—The effect on podocytes. Nephrol. Dial. Transplant. 2009, 24, 3640–3651. [Google Scholar] [CrossRef]
- Takagi, N.; Tanizawa, T.; Kon, V.; Fogo, A.B.; Ichikawa, I.; Ma, J. Mineralocorticoid Receptor Blocker Protects against Podocyte-Dependent Glomerulosclerosis. Nephron Extra 2012, 2, 17–26. [Google Scholar] [CrossRef]
- Nagase, M.; Shibata, S.; Yoshida, S.; Nagase, T.; Gotoda, T.; Fujita, T. Podocyte Injury Underlies the Glomerulopathy of Dahl Salt-Hypertensive Rats and Is Reversed by Aldosterone Blocker. Hypertension 2006, 47, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Itoh, H. Mineralocorticoid Receptor-Associated Hypertension and Its Organ Damage: Clinical Relevance for Resistant Hypertension. Am. J. Hypertens. 2012, 25, 514–523. [Google Scholar] [CrossRef]
- Akilesh, S.; Suleiman, H.; Yu, H.; Stander, M.C.; Lavin, P.; Gbadegesin, R.; Antignac, C.; Pollak, M.; Kopp, J.B.; Winn, M.P.; et al. Arhgap24 inactivates Rac1 in mouse podocytes, and a mutant form is associated with familial focal segmental glomerulosclerosis. J. Clin. Investig. 2011, 121, 4127–4137. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Suleiman, H.; Kim, A.H.J.; Miner, J.H.; Dani, A.; Shaw, A.S.; Akilesh, S. Rac1 Activation in Podocytes Induces Rapid Foot Process Effacement and Proteinuria. Mol. Cell. Biol. 2013, 33, 4755–4764. [Google Scholar] [CrossRef]
- Robins, R.; Baldwin, C.; Aoudjit, L.; Côté, J.-F.; Gupta, I.R.; Takano, T. Rac1 activation in podocytes induces the spectrum of nephrotic syndrome. Kidney Int. 2017, 92, 349–364. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, C.; Ma, P.; Lai, Y.; Yang, F.; Cai, J.; Cheng, Z.; Zhang, K.; Liu, Z.; Tian, Y.; et al. Kindlin-2 Association with Rho GDP-Dissociation Inhibitor α Suppresses Rac1 Activation and Podocyte Injury. J. Am. Soc. Nephrol. 2017, 28, 3545–3562. [Google Scholar] [CrossRef]
- Hall, G.; Lane, B.M.; Khan, K.; Pediaditakis, I.; Xiao, J.; Wu, G.; Wang, L.; Kovalik, M.E.; Chryst-Stangl, M.; Davis, E.E.; et al. The Human FSGS-Causing ANLN R431C Mutation Induces Dysregulated PI3K/AKT/mTOR/Rac1 Signaling in Podocytes. J. Am. Soc. Nephrol. 2018, 29, 2110–2122. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Castonguay, P.; Sidhom, E.-H.; Clark, A.R.; Dvela-Levitt, M.; Kim, S.; Sieber, J.; Wieder, N.; Jung, J.Y.; Andreeva, S.; et al. A small-molecule inhibitor of TRPC5 ion channels suppresses progressive kidney disease in animal models. Science 2017, 358, 1332–1336. [Google Scholar] [CrossRef] [PubMed]
- Walsh, L.; Reilly, J.F.; Cornwall, C.; Gaich, G.A.; Gipson, D.S.; Heerspink, H.J.; Johnson, L.; Trachtman, H.; Tuttle, K.R.; Farag, Y.M.; et al. Safety and Efficacy of GFB-887, a TRPC5 Channel Inhibitor, in Patients with Focal Segmental Glomerulosclerosis, Treatment-Resistant Minimal Change Disease, or Diabetic Nephropathy: TRACTION-2 Trial Design. Kidney Int. Rep. 2021, 6, 2575–2584. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Mu, S.; Kawarazaki, H.; Muraoka, K.; Ishizawa, K.-I.; Yoshida, S.; Kawarazaki, W.; Takeuchi, M.; Ayuzawa, N.; Miyoshi, J.; et al. Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor–dependent pathway. J. Clin. Investig. 2011, 121, 3233–3243. [Google Scholar] [CrossRef]
- Shibata, S.; Fujita, T. Mineralocorticoid receptors in the pathophysiology of chronic kidney diseases and the metabolic syndrome. Mol. Cell. Endocrinol. 2012, 350, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Ishizawa, K.; Uchida, S. Mineralocorticoid receptor as a therapeutic target in chronic kidney disease and hypertension. Hypertens. Res. 2017, 40, 221–225. [Google Scholar] [CrossRef]
- Ayuzawa, N.; Nagase, M.; Ueda, K.; Nishimoto, M.; Kawarazaki, W.; Marumo, T.; Aiba, A.; Sakurai, T.; Shindo, T.; Fujita, T. Rac1-Mediated Activation of Mineralocorticoid Receptor in Pressure Overload–Induced Cardiac Injury. Hypertension 2016, 67, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Lavall, D.; Schuster, P.; Jacobs, N.; Kazakov, A.; Böhm, M.; Laufs, U. Rac1 GTPase regulates 11β hydroxysteroid dehydrogenase type 2 and fibrotic remodeling. J. Biol. Chem. 2017, 292, 7542–7553. [Google Scholar] [CrossRef]
- Wynne, B.M.; Samson, T.K.; Moyer, H.C.; van Elst, H.J.; Moseley, A.S.; Hecht, G.G.; Paul, O.; Al-Khalili, O.; Gomez-Sanchez, C.E.; Ko, B.; et al. Interleukin 6 mediated activation of the mineralocorticoid receptor in the aldosterone-sensitive distal nephron. Am. J. Physiol. Cell Physiol. 2022, 323, C1512–C1523. [Google Scholar] [CrossRef]
- González-Blázquez, R.; Somoza, B.; Gil-Ortega, M.; Ramos, M.M.; Cortijo, D.R.; Vega-Martín, E.; Schulz, A.; Ruilope, L.M.; Kolkhof, P.; Kreutz, R.; et al. Finerenone Attenuates Endothelial Dysfunction and Albuminuria in a Chronic Kidney Disease Model by a Reduction in Oxidative Stress. Front. Pharmacol. 2018, 9, 1131. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.J.; Ramnath, R.; Kadoya, H.; Desposito, D.; Riquier-Brison, A.; Ferguson, J.K.; Onions, K.L.; Ogier, A.S.; ElHegni, H.; Coward, R.J.; et al. Aldosterone induces albuminuria via matrix metalloproteinase–dependent damage of the endothelial glycocalyx. Kidney Int. 2019, 95, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, A.; Yao, L.; Fan, Y.; Kyaw, M.; Kataoka, N.; Hashimoto, K.; Nagai, Y.; Nakamura, E.; Yoshizumi, M.; Shokoji, T.; et al. Involvement of Aldosterone and Mineralocorticoid Receptors in Rat Mesangial Cell Proliferation and Deformability. Hypertension 2005, 45, 710–716. [Google Scholar] [CrossRef]
- Terada, Y.; Kobayashi, T.; Kuwana, H.; Tanaka, H.; Inoshita, S.; Kuwahara, M.; Sasaki, S. Aldosterone stimulates proliferation of mesangial cells by activating mitogen-activated protein kinase 1/2, cyclin D1, and cyclin A. J. Am. Soc. Nephrol. 2005, 16, 2296–2305. [Google Scholar] [CrossRef]
- Lai, L.; Chen, J.; Hao, C.-M.; Lin, S.; Gu, Y. Aldosterone promotes fibronectin production through a Smad2-dependent TGF-beta1 pathway in mesangial cells. Biochem. Biophys. Res. Commun. 2006, 348, 70–75. [Google Scholar] [CrossRef]
- Yuan, J.; Jia, R.; Bao, Y. Aldosterone up-regulates production of plasminogen activator inhibitor-1 by renal mesangial cells. J. Biochem. Mol. Biol. 2007, 40, 180–188. [Google Scholar] [CrossRef]
- Gauer, S.; Segitz, V.; Goppelt-Struebe, M. Aldosterone induces CTGF in mesangial cells by activation of the glucocorticoid receptor. Nephrol. Dial. Transplant. 2007, 22, 3154–3159. [Google Scholar] [CrossRef]
- Huang, W.; Xu, C.; Kahng, K.W.; Noble, N.A.; Border, W.A.; Huang, Y. Aldosterone and TGF-beta1 synergistically increase PAI-1 and decrease matrix degradation in rat renal mesangial and fibroblast cells. Am. J. Physiol. Renal. Physiol. 2008, 294, F1287–F1295. [Google Scholar] [CrossRef]
- Ma, J.; Weisberg, A.; Griffin, J.; Vaughan, D.; Fogo, A.; Brown, N. Plasminogen activator inhibitor-1 deficiency protects against aldosterone-induced glomerular injury. Kidney Int. 2006, 69, 1064–1072. [Google Scholar] [CrossRef]
- Sogawa, Y.; Nagasu, H.; Itano, S.; Kidokoro, K.; Taniguchi, S.; Takahashi, M.; Kadoya, H.; Satoh, M.; Sasaki, T.; Kashihara, N. The eNOS-NO pathway attenuates kidney dysfunction via suppression of inflammasome activation in aldosterone-induced renal injury model mice. PLoS ONE 2018, 13, e0203823. [Google Scholar] [CrossRef] [PubMed]
- Barrera-Chimal, J.; Estrela, G.R.; Lechner, S.M.; Giraud, S.; El Moghrabi, S.; Kaaki, S.; Kolkhof, P.; Hauet, T.; Jaisser, F. The myeloid mineralocorticoid receptor controls inflammatory and fibrotic responses after renal injury via macrophage interleukin-4 receptor signaling. Kidney Int. 2018, 93, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Nikolic-Paterson, D.; Ma, F.; Tesch, G. Aldosterone Induces Kidney Fibroblast Proliferation via Activation of Growth Factor Receptors and PI3K/MAPK Signalling. Nephron Exp. Nephrol. 2012, 120, e115–e122. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Girerd, S.; Jaisser, F.; Barrera-Chimal, J. Nonepithelial mineralocorticoid receptor activation as a determinant of kidney disease. Kidney Int. Suppl. 2022, 12, 12–18. [Google Scholar] [CrossRef]
- Luther, J.M.; Fogo, A.B. The role of mineralocorticoid receptor activation in kidney inflammation and fibrosis. Kidney Int. Suppl. 2022, 12, 63–68. [Google Scholar] [CrossRef]
- Leroy, V.A.A.; De Seigneux, S.; Agassiz, V.; Hasler, U.; Rafestin-Oblin, M.-E.; Vinciguerra, M.; Martin, P.-Y.; Raille, E.F.A. Aldosterone activates NF-kappaB in the collecting duct. J. Am. Soc. Nephrol. 2009, 20, 131–144. [Google Scholar] [CrossRef]
- Martín-Fernandez, B.; Rubio-Navarro, A.; Cortegano, I.; Ballesteros, S.; Alia, M.; Cannata-Ortiz, P.; Olivares-Alvaro, E.; Egido, J.; De Andrés, B.; Gaspar, M.L.; et al. Aldosterone Induces Renal Fibrosis and Inflammatory M1-Macrophage Subtype via Mineralocorticoid Receptor in Rats. PLoS ONE 2016, 11, e0145946. [Google Scholar] [CrossRef] [PubMed]
- Bonnard, B.; Ibarrola, J.; Lima-Posada, I.; Fernández-Celis, A.; Durand, M.; Genty, M.; Lopez-Andreés, N.; Jaisser, F. Neutrophil Gelatinase-Associated Lipocalin from Macrophages Plays a Critical Role in Renal Fibrosis Via the CCL5 (Chemokine Ligand 5)-Th2 Cells-IL4 (Interleukin 4) Pathway. Hypertension 2022, 79, 352–364. [Google Scholar] [CrossRef]
- Beevers, D.G.; Brown, J.J.; Ferriss, J.B.; Fraser, R.; Lever, A.F.; Robertson, J.I.; Tree, M. Renal abnormalities and vascular complications in primary hyperaldosteronism. Evidence on tertiary hyperaldosteronism. QJM Int. J. Med. 1976, 45, 401–410. [Google Scholar]
- Rossi, G.P.; Bernini, G.; Desideri, G.; Fabris, B.; Ferri, C.; Giacchetti, G.; Letizia, C.; Maccario, M.; Mannelli, M.; Matterello, M.-J.; et al. Renal damage in primary aldosteronism: Results of the PAPY Study. Hypertension 2006, 48, 232–238. [Google Scholar] [CrossRef]
- Kawashima, A.; Sone, M.; Inagaki, N.; Takeda, Y.; Itoh, H.; Kurihara, I.; Umakoshi, H.; Ichijo, T.; Katabami, T.; Wada, N.; et al. Renal impairment is closely associated with plasma aldosterone concentration in patients with primary aldosteronism. Eur. J. Endocrinol. 2019, 181, 339–350. [Google Scholar] [CrossRef]
- Brown, S.; Foster, W. Localization of Occult Bronchogenic Carcinoma by Bronchography. Chest 1991, 100, 1160–1162. [Google Scholar] [CrossRef] [PubMed]
- Ribstein, J.; Du Cailar, G.; Fesler, P.; Mimran, A. Relative Glomerular Hyperfiltration in Primary Aldosteronism. J. Am. Soc. Nephrol. 2005, 16, 1320–1325. [Google Scholar] [CrossRef] [PubMed]
- Iwakura, Y.; Morimoto, R.; Kudo, M.; Ono, Y.; Takase, K.; Seiji, K.; Arai, Y.; Nakamura, Y.; Sasano, H.; Ito, S.; et al. Predictors of Decreasing Glomerular Filtration Rate and Prevalence of Chronic Kidney Disease after Treatment of Primary Aldosteronism: Renal Outcome of 213 Cases. J. Clin. Endocrinol. Metab. 2014, 99, 1593–1598. [Google Scholar] [CrossRef] [PubMed]
- Tanase-Nakao, K.; Naruse, M.; Nanba, K.; Tsuiki, M.; Tagami, T.; Usui, T.; Okuno, H.; Shimatsu, A.; Hashimoto, S.; Katabami, T.; et al. Chronic kidney disease score for predicting postoperative masked renal insufficiency in patients with primary aldosteronism. Clin. Endocrinol. 2014, 81, 665–670. [Google Scholar] [CrossRef]
- Reincke, M.; Rump, L.C.; Quinkler, M.; Hahner, S.; Diederich, S.; Lorenz, R.; Seufert, J.; Schirpenbach, C.; Beuschlein, F.; Bidlingmaier, M.; et al. Risk Factors Associated with a Low Glomerular Filtration Rate in Primary Aldosteronism. J. Clin. Endocrinol. Metab. 2009, 94, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Ogata, H.; Yamazaki, Y.; Tezuka, Y.; Gao, X.; Omata, K.; Ono, Y.; Kawasaki, Y.; Tanaka, T.; Nagano, H.; Wada, N.; et al. Renal Injuries in Primary Aldosteronism: Quantitative Histopathological Analysis of 19 Patients with Primary Adosteronism. Hypertension 2021, 78, 411–421. [Google Scholar] [CrossRef]
- Chrysostomou, A.; Becker, G. Spironolactone in Addition to ACE Inhibition to Reduce Proteinuria in Patients with Chronic Renal Disease. N. Engl. J. Med. 2001, 345, 925–926. [Google Scholar] [CrossRef]
- Schjoedt, K.J.; Rossing, K.; Juhl, T.R.; Boomsma, F.; Rossing, P.; Tarnow, L.; Parving, H.-H. Beneficial impact of spironolactone in diabetic nephropathy. Kidney Int. 2005, 68, 2829–2836. [Google Scholar] [CrossRef]
- Schjoedt, K.; Rossing, P.; Juhl, T.; Boomsma, F.; Tarnow, L.; Parving, H. Beneficial impact of spironolactone on nephrotic range albuminuria in diabetic nephropathy. Kidney Int. 2006, 70, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, S.; Bigazzi, R.; Campese, V. Long-term effects of spironolactone on proteinuria and kidney function in patients with chronic kidney disease. Kidney Int. 2006, 70, 2116–2123. [Google Scholar] [CrossRef]
- Mehdi, U.F.; Adams-Huet, B.; Raskin, P.; Vega, G.L.; Toto, R.D. Addition of Angiotensin Receptor Blockade or Mineralocorticoid Antagonism to Maximal Angiotensin-Converting Enzyme Inhibition in Diabetic Nephropathy. J. Am. Soc. Nephrol. 2009, 20, 2641–2650. [Google Scholar] [CrossRef]
- Hou, J.; Xiong, W.; Cao, L.; Wen, X.; Li, A. Spironolactone Add-on for Preventing or Slowing the Progression of Diabetic Nephropathy: A Meta-analysis. Clin. Ther. 2015, 37, 2086–2103.e10. [Google Scholar] [CrossRef]
- Epstein, M.; Williams, G.H.; Weinberger, M.; Lewin, A.; Krause, S.; Mukherjee, R.; Patni, R.; Beckerman, B. Selective Aldosterone Blockade with Eplerenone Reduces Albuminuria in Patients with Type 2 Diabetes. Clin. J. Am. Soc. Nephrol. 2006, 1, 940–951. [Google Scholar] [CrossRef]
- Agarwal, R.; Rossignol, P.; Budden, J.; Mayo, M.R.; Arthur, S.; Williams, B.; White, W.B. Patiromer and Spironolactone in Resistant Hypertension and Advanced CKD: Analysis of the Randomized AMBER Trial. Kidney360 2021, 2, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Ohtsu, H.; Uchida, S.; Kaname, S.; Arakawa, Y.; Fujita, T. Anti-albuminuric effect of the aldosterone blocker eplerenone in non-diabetic hypertensive patients with albuminuria: A double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014, 2, 944–953. [Google Scholar] [CrossRef]
- Nishimoto, M.; Ohtsu, H.; Marumo, T.; Kawarazaki, W.; Ayuzawa, N.; Ueda, K.; Hirohama, D.; Kawakami-Mori, F.; Shibata, S.; Nagase, M.; et al. Mineralocorticoid receptor blockade suppresses dietary salt-induced ACEI/ARB-resistant albuminuria in non-diabetic hypertension: A sub-analysis of evaluate study. Hypertens. Res. 2019, 42, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, M.; Jongs, N.; Vart, P.; Stefánsson, B.V.; Chertow, G.M.; Langkilde, A.M.; McMurray, J.J.; Correa-Rotter, R.; Rossing, P.; Sjöström, C.D.; et al. The Kidney Protective Effects of the Sodium–Glucose Cotransporter-2 Inhibitor, Dapagliflozin, Are Present in Patients with CKD Treated with Mineralocorticoid Receptor Antagonists. Kidney Int. Rep. 2021, 7, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, M.; Puchades, M.J.; Garofalo, C.; Jongs, N.; D’marco, L.; Andreucci, M.; De Nicola, L.; Gorriz, J.L.; Heerspink, H.J. Albuminuria-Lowering Effect of Dapagliflozin, Eplerenone, and Their Combination in Patients with Chronic Kidney Disease: A Randomized Crossover Clinical Trial. J. Am. Soc. Nephrol. 2022, 33, 1569–1580. [Google Scholar] [CrossRef]
- Ito, S.; Itoh, H.; Rakugi, H.; Okuda, Y.; Yamakawa, S. Efficacy and safety of esaxerenone (CS-3150) for the treatment of essential hypertension: A phase 2 randomized, placebo-controlled, double-blind study. J. Hum. Hypertens. 2019, 33, 542–551. [Google Scholar] [CrossRef]
- Itoh, H.; Ito, S.; Rakugi, H.; Okuda, Y.; Nishioka, S. Efficacy and safety of dosage-escalation of low-dosage esaxerenone added to a RAS inhibitor in hypertensive patients with type 2 diabetes and albuminuria: A single-arm, open-label study. Hypertens. Res. 2019, 42, 1572–1581. [Google Scholar] [CrossRef]
- Rakugi, H.; Ito, S.; Itoh, H.; Okuda, Y.; Yamakawa, S. Long-term phase 3 study of esaxerenone as mono or combination therapy with other antihypertensive drugs in patients with essential hypertension. Hypertens. Res. 2019, 42, 1932–1941. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Kashihara, N.; Shikata, K.; Nangaku, M.; Wada, T.; Okuda, Y.; Sawanobori, T. Efficacy and safety of esaxerenone (CS-3150) in Japanese patients with type 2 diabetes and macroalbuminuria: A multicenter, single-arm, open-label phase III study. Clin. Exp. Nephrol. 2021, 25, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Kashihara, N.; Shikata, K.; Nangaku, M.; Wada, T.; Okuda, Y.; Sawanobori, T. Esaxerenone (CS-3150) in Patients with Type 2 Diabetes and Microalbuminuria (ESAX-DN): Phase 3 Randomized Controlled Clinical Trial. Clin. J. Am. Soc. Nephrol. 2020, 15, 1715–1727. [Google Scholar] [CrossRef] [PubMed]
- Okuda, Y.; Ito, S.; Kashihara, N.; Shikata, K.; Nangaku, M.; Wada, T.; Sawanobori, T.; Taguri, M. The renoprotective effect of esaxerenone independent of blood pressure lowering: A post hoc mediation analysis of the ESAX-DN trial. Hypertens. Res. 2023, 46, 437–444. [Google Scholar] [CrossRef]
- Rakugi, H.; Yamakawa, S.; Sugimoto, K. Management of hyperkalemia during treatment with mineralocorticoid receptor blockers: Findings from esaxerenone. Hypertens. Res. 2020, 44, 371–385. [Google Scholar] [CrossRef] [PubMed]
- Shikata, K.; Ito, S.; Kashihara, N.; Nangaku, M.; Wada, T.; Okuda, Y.; Sawanobori, T.; Sugimoto, K. Reduction in the magnitude of serum potassium elevation in combination therapy with esaxerenone (CS-3150) and sodium–glucose cotransporter 2 inhibitor in patients with diabetic kidney disease: Subanalysis of two phase III studies. J. Diabetes Investig. 2022, 13, 1190–1202. [Google Scholar] [CrossRef]
- Bakris, G.L.; Agarwal, R.; Chan, J.C.; Cooper, M.E.; Gansevoort, R.T.; Haller, H.; Remuzzi, G.; Rossing, P.; Schmieder, R.E.; Nowack, C.; et al. Effect of Finerenone on Albuminuria in Patients with Diabetic Nephropathy: A Randomized Clinical Trial. JAMA 2015, 314, 884–894. [Google Scholar] [CrossRef]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 2219–2229. [Google Scholar] [CrossRef]
- Pitt, B.; Filippatos, G.; Agarwal, R.; Anker, S.D.; Bakris, G.L.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Schloemer, P.; et al. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 2252–2263. [Google Scholar] [CrossRef]
- Agarwal, R.; Filippatos, G.; Pitt, B.; Anker, S.D.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Gebel, M.; Ruilope, L.M.; et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: The FIDELITY pooled analysis. Eur. Heart J. 2021, 43, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Rossing, P.; Anker, S.D.; Filippatos, G.; Pitt, B.; Ruilope, L.M.; Birkenfeld, A.L.; McGill, J.B.; Rosas, S.E.; Joseph, A.; Gebel, M.; et al. Finerenone in Patients with Chronic Kidney Disease and Type 2 Diabetes by Sodium–Glucose Cotransporter 2 Inhibitor Treatment: The FIDELITY Analysis. Diabetes Care 2022, 45, 2991–2998. [Google Scholar] [CrossRef] [PubMed]
- McGill, J.B.; Agarwal, R.; Anker, S.D.; Bakris, G.L.; Filippatos, G.; Pitt, B.; Ruilope, L.M.; Birkenfeld, A.L.; Caramori, M.L.; Brinker, M.; et al. Effects of finerenone in persons with CKD and T2D are independent of HbA1c at baseline, HbA1c variability, diabetes duration and insulin use at baseline. Diabetes Obes. Metab. 2023. [Google Scholar] [CrossRef] [PubMed]
- Ruilope, L.M.; Agarwal, R.; Anker, S.D.; Filippatos, G.; Pitt, B.; Rossing, P.; Sarafidis, P.; Schmieder, R.E.; Joseph, A.; Rethemeier, N.; et al. Blood Pressure and Cardiorenal Outcomes with Finerenone in Chronic Kidney Disease in Type 2 Diabetes. Hypertension 2022, 79, 2685–2695. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Ruilope, L.M.; Ruiz-Hurtado, G.; Haller, H.; Schmieder, R.E.; Anker, S.D.; Filippatos, G.; Pitt, B.; Rossing, P.; Lambelet, M.; et al. Effect of finerenone on ambulatory blood pressure in chronic kidney disease in type 2 diabetes. J. Hypertens. 2022, 41, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Joseph, A.; Anker, S.D.; Filippatos, G.; Rossing, P.; Ruilope, L.M.; Pitt, B.; Kolkhof, P.; Scott, C.; Lawatscheck, R.; et al. Hyperkalemia Risk with Finerenone: Results from the FIDELIO-DKD Trial. J. Am. Soc. Nephrol. 2022, 33, 225–237. [Google Scholar] [CrossRef]
- Alexandrou, M.E.; Sarafidis, P.; Loutradis, C.; Tsapas, A.; Boutou, A.; Piperidou, A.; Papadopoulou, D.; Papagianni, A.; Ruilope, L.; Bakris, G. Effects of mineralocorticoid receptor antagonists in proteinuric kidney disease: A systematic review and meta-analysis of randomized controlled trials. J Hypertens. 2019, 37, 2307–2324. [Google Scholar] [CrossRef]
- Chen, Q.; Liang, Y.; Yan, J.; Du, Y.; Li, M.; Chen, Z.; Zhou, J. Efficacy and safety of non-steroidal mineralocorticoid receptor antagonists for renal outcomes: A systematic review and meta-analysis. Diabetes Res. Clin. Pr. 2022, 195, 110210. [Google Scholar] [CrossRef]
- Morita, R.; Tsukamoto, S.; Obata, S.; Yamada, T.; Uneda, K.; Uehara, T.; Rehman, M.E.; Azushima, K.; Wakui, H.; Tamura, K. Effects of sodium-glucose cotransporter 2 inhibitors, mineralocorticoid receptor antagonists, and their combination on albuminuria in diabetic patients. Diabetes Obes. Metab. 2023, 25, 1271–1279. [Google Scholar] [CrossRef]
- Oka, T.; Sakaguchi, Y.; Hattori, K.; Asahina, Y.; Kajimoto, S.; Doi, Y.; Kaimori, J.-Y.; Isaka, Y. Mineralocorticoid Receptor Antagonist Use and Hard Renal Outcomes in Real-World Patients with Chronic Kidney Disease. Hypertension 2022, 79, 679–689. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2022, 102, S1–S127. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 11. Chronic Kidney Disease and Risk Management: Standards of Care in Diabetes—2023. Diabetes Care 2022, 46 (Suppl. 1), S191–S202. [Google Scholar] [CrossRef]
- A Trial to Learn How Well Finerenone Works and How Safe it is in Adult Participants with Non-diabetic Chronic Kidney Disease. Available online: https://clinicaltrials.gov/ct2/show/NCT05047263 (accessed on 27 February 2023).
- Green, J.B.; Mottl, A.K.; Bakris, G.; Heerspink, H.J.L.; Mann, J.F.E.; McGill, J.B.; Nangaku, M.; Rossing, P.; Scott, C.; Gay, A.; et al. Design of the COmbinatioN effect of FInerenone anD EmpaglifloziN in participants with chronic kidney disease and type 2 diabetes using a UACR Endpoint study (CONFIDENCE). Nephrol. Dial. Transplant. 2022, 38, 894–903. [Google Scholar] [CrossRef]
- Cp, C. Pharmacological Profile of KBP-5074, a Novel NonSteroidal Mineralocorticoid Receptor Antagonist for the Treatment of Cardiorenal Diseases. J. Drug Res. Dev. 2017, 3, 1–9. [Google Scholar] [CrossRef]
- Wada, T.; Inagaki, M.; Yoshinari, T.; Terata, R.; Totsuka, N.; Gotou, M.; Hashimoto, G. Apararenone in patients with diabetic nephropathy: Results of a randomized, double-blind, placebo-controlled phase 2 dose-response study and open-label extension study. Clin. Exp. Nephrol. 2021, 25, 120–130. [Google Scholar] [CrossRef]
- Bakris, G.; Pergola, P.E.; Delgado, B.; Genov, D.; Doliashvili, T.; Vo, N.; Yang, Y.F.; McCabe, J.; Benn, V.; Pitt, B.; et al. Effect of KBP-5074 on Blood Pressure in Advanced Chronic Kidney Disease: Results of the BLOCK-CKD Study. Hypertension 2021, 78, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Gansevoort, R.T.; Coresh, J.; Inker, L.A.; Heerspink, H.L.; Grams, M.E.; Greene, T.; Tighiouart, H.; Matsushita, K.; Ballew, S.H.; et al. Change in Albuminuria and GFR as End Points for Clinical Trials in Early Stages of CKD: A Scientific Workshop Sponsored by the National Kidney Foundation in Collaboration with the US Food and Drug Administration and European Medicines Agency. Am. J. Kidney Dis. 2020, 75, 84–104. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.R.; Lasserson, D.; Thompson, B.; Perera-Salazar, R.; Wolstenholme, J.; Bower, P.; Blakeman, T.; Fitzmaurice, D.; Little, P.; Feder, G.; et al. Benefits of Aldosterone Receptor Antagonism in Chronic Kidney Disease (BARACK D) trial–a multi-centre, prospective, randomised, open, blinded end-point, 36-month study of 2616 patients within primary care with stage 3b chronic kidney disease to compare the efficacy of spironolactone 25 mg once daily in addition to routine care on mortality and cardiovascular outcomes versus routine care alone: Study protocol for a randomized controlled trial. Trials 2014, 15, 160. [Google Scholar] [CrossRef] [PubMed]
- Girerd, S.; Frimat, L.; Ducloux, D.; Le Meur, Y.; Mariat, C.; Moulin, B.; Mousson, C.; Rieu, P.; Dali-Youcef, N.; Merckle, L.; et al. EPURE Transplant (Eplerenone in Patients Undergoing Renal Transplant) study: Study protocol for a randomized controlled trial. Trials 2018, 19, 595. [Google Scholar] [CrossRef]
- Barrera-Chimal, J.; Gerarduzzi, C.; Rossignol, P.; Jaisser, F. The non-steroidal mineralocorticoid receptor antagonist finerenone is a novel therapeutic option for patients with Type 2 diabetes and chronic kidney disease. Clin. Sci. 2022, 136, 1005–1017. [Google Scholar] [CrossRef]
- Kario, K.; Ito, S.; Itoh, H.; Rakugi, H.; Okuda, Y.; Yamakawa, S. Effect of esaxerenone on nocturnal blood pressure and natriuretic peptide in different dipping phenotypes. Hypertens. Res. 2022, 45, 97–105. [Google Scholar] [CrossRef]
- Canaud, B.; Kooman, J.; Selby, N.M.; Taal, M.; Francis, S.; Kopperschmidt, P.; Maierhofer, A.; Kotanko, P.; Titze, J. Sodium and water handling during hemodialysis: New pathophysiologic insights and management approaches for improving outcomes in end-stage kidney disease. Kidney Int. 2019, 95, 296–309. [Google Scholar] [CrossRef]
- Takizawa, K.; Ueda, K.; Sekiguchi, M.; Nakano, E.; Nishimura, T.; Kajiho, Y.; Kanda, S.; Miura, K.; Hattori, M.; Hashimoto, J.; et al. Urinary extracellular vesicles signature for diagnosis of kidney disease. iScience 2022, 25, 105416. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, N.; Hayashi, K.; Hishikawa, A.; Hashiguchi, A.; Nakamichi, R.; Sugita-Nishimura, E.; Yoshida-Hama, E.; Azegami, T.; Nakayama, T.; Itoh, H. Significance of podocyte DNA damage and glomerular DNA methylation in CKD patients with proteinuria. Hypertens. Res. 2023, 46, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Ochiai-Homma, F.; Kuribayashi-Okuma, E.; Tsurutani, Y.; Ishizawa, K.; Fujii, W.; Odajima, K.; Kawagoe, M.; Tomomitsu, Y.; Murakawa, M.; Asakawa, S.; et al. Characterization of pendrin in urinary extracellular vesicles in a rat model of aldosterone excess and in human primary aldosteronism. Hypertens. Res. 2021, 44, 1557–1567. [Google Scholar] [CrossRef]
- Tofte, N.; Lindhardt, M.; Adamova, K.; Bakker, S.J.L.; Beige, J.; Beulens, J.W.J.; Birkenfeld, A.L.; Currie, G.; Delles, C.; Dimos, I.; et al. Early detection of diabetic kidney disease by urinary proteomics and subsequent intervention with spironolactone to delay progression (PRIORITY): A prospective observational study and embedded randomised placebo-controlled trial. Lancet Diabetes Endocrinol. 2020, 8, 301–312. [Google Scholar] [CrossRef]
- Marumo, T.; Hoshino, J.; Kawarazaki, W.; Nishimoto, M.; Ayuzawa, N.; Hirohama, D.; Yamanouchi, M.; Ubara, Y.; Okaneya, T.; Fujii, T.; et al. Methylation pattern of urinary DNA as a marker of kidney function decline in diabetes. BMJ Open Diabetes Res. Care 2020, 8, e001501. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujii, W.; Shibata, S. Mineralocorticoid Receptor Antagonists for Preventing Chronic Kidney Disease Progression: Current Evidence and Future Challenges. Int. J. Mol. Sci. 2023, 24, 7719. https://doi.org/10.3390/ijms24097719
Fujii W, Shibata S. Mineralocorticoid Receptor Antagonists for Preventing Chronic Kidney Disease Progression: Current Evidence and Future Challenges. International Journal of Molecular Sciences. 2023; 24(9):7719. https://doi.org/10.3390/ijms24097719
Chicago/Turabian StyleFujii, Wataru, and Shigeru Shibata. 2023. "Mineralocorticoid Receptor Antagonists for Preventing Chronic Kidney Disease Progression: Current Evidence and Future Challenges" International Journal of Molecular Sciences 24, no. 9: 7719. https://doi.org/10.3390/ijms24097719
APA StyleFujii, W., & Shibata, S. (2023). Mineralocorticoid Receptor Antagonists for Preventing Chronic Kidney Disease Progression: Current Evidence and Future Challenges. International Journal of Molecular Sciences, 24(9), 7719. https://doi.org/10.3390/ijms24097719





