Shotgun Proteomics Analysis, Functional Networks, and Peptide Biomarkers for Seafood-Originating Biogenic-Amine-Producing Bacteria
Abstract
1. Introduction
2. Results and Discussion
2.1. Shotgun Proteomics Data Repository
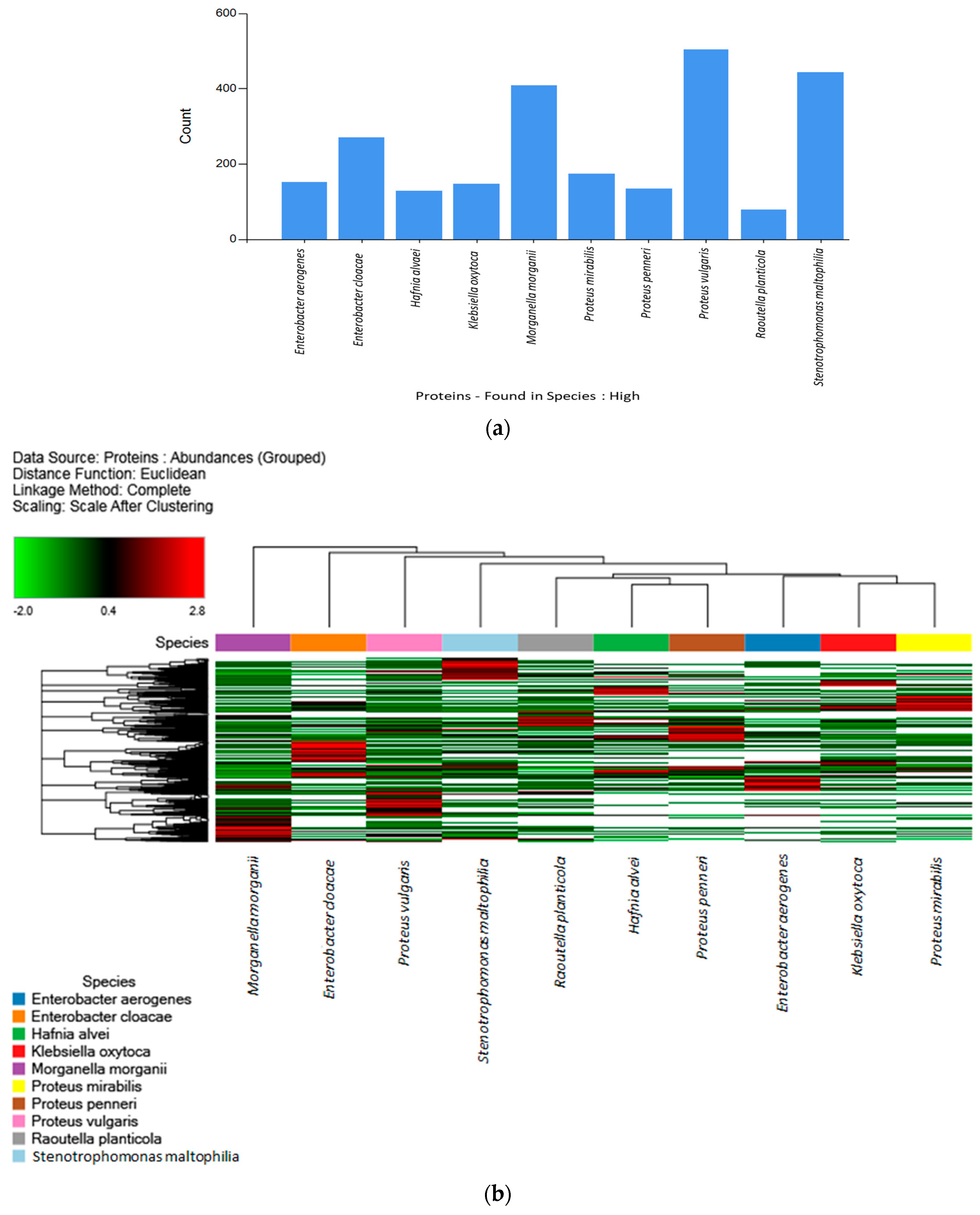
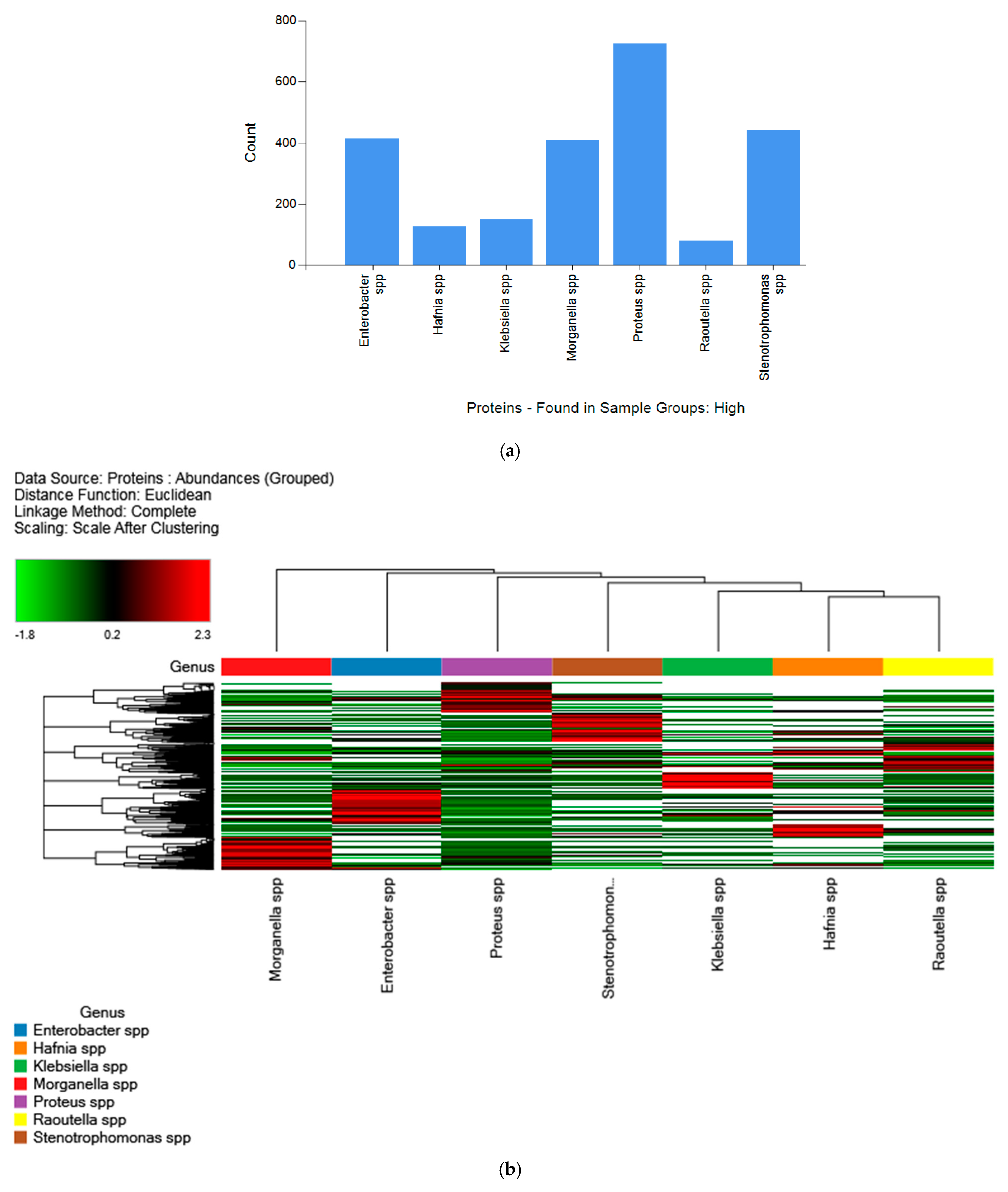
2.2. Label-Free Quantification (LFQ) of Biogenic-Amine-Producing Bacteria and Hierarchical Clustering
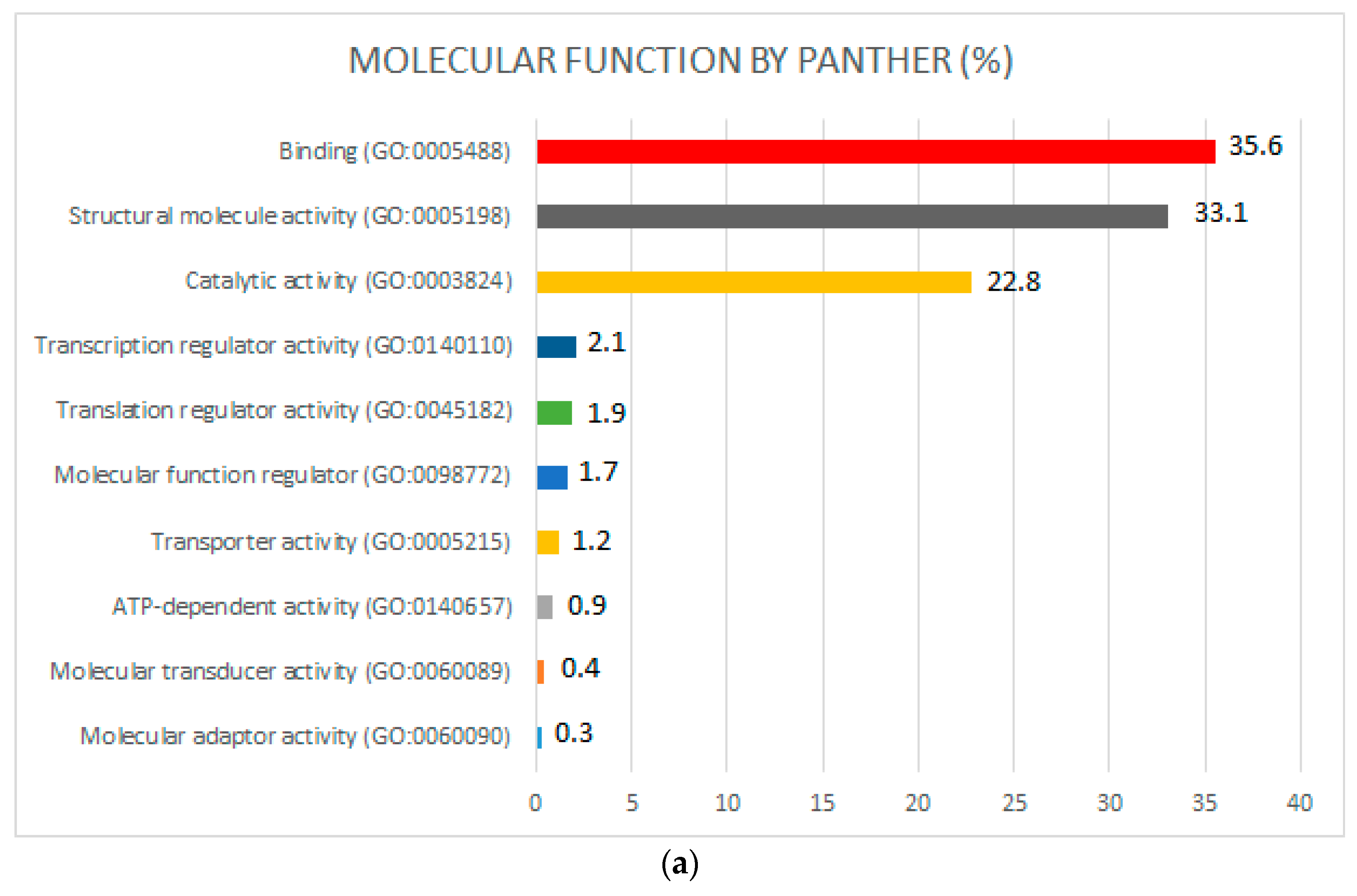
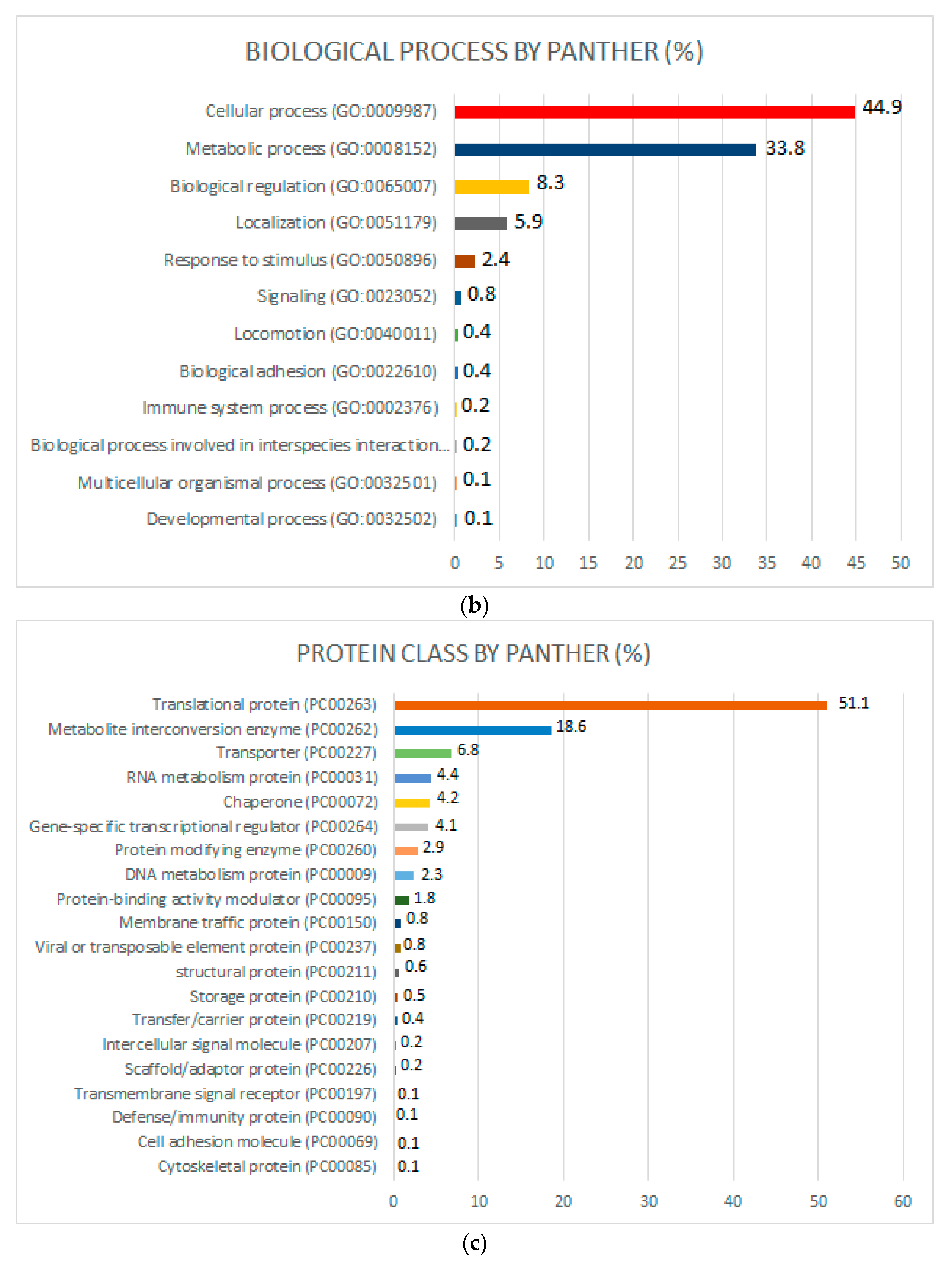
2.3. Functional Pathways and Gene Ontology (GO)
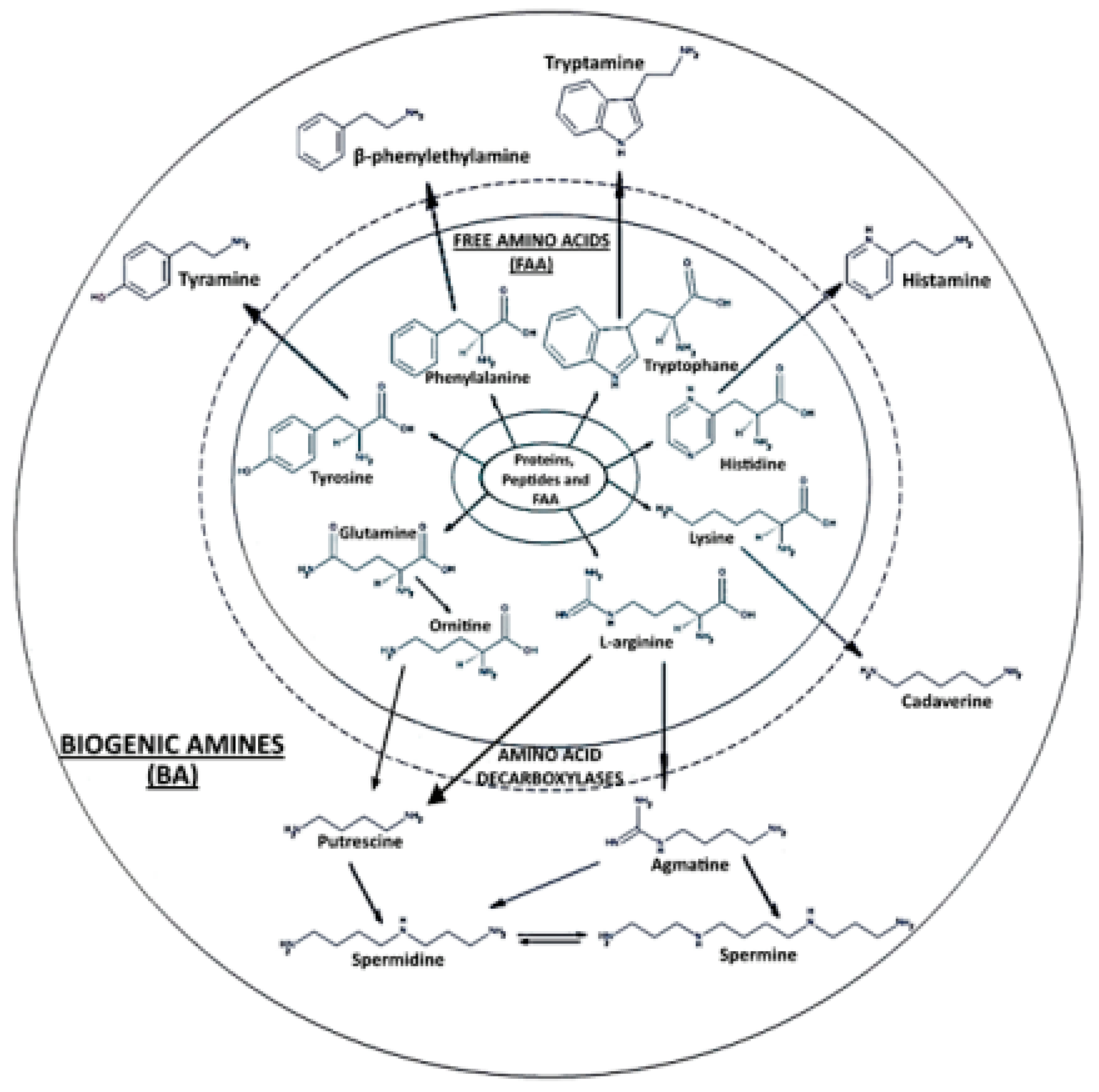
2.4. Biogenic Amine-Related Proteins and Peptides Detected via LC-ESI-MS/MS
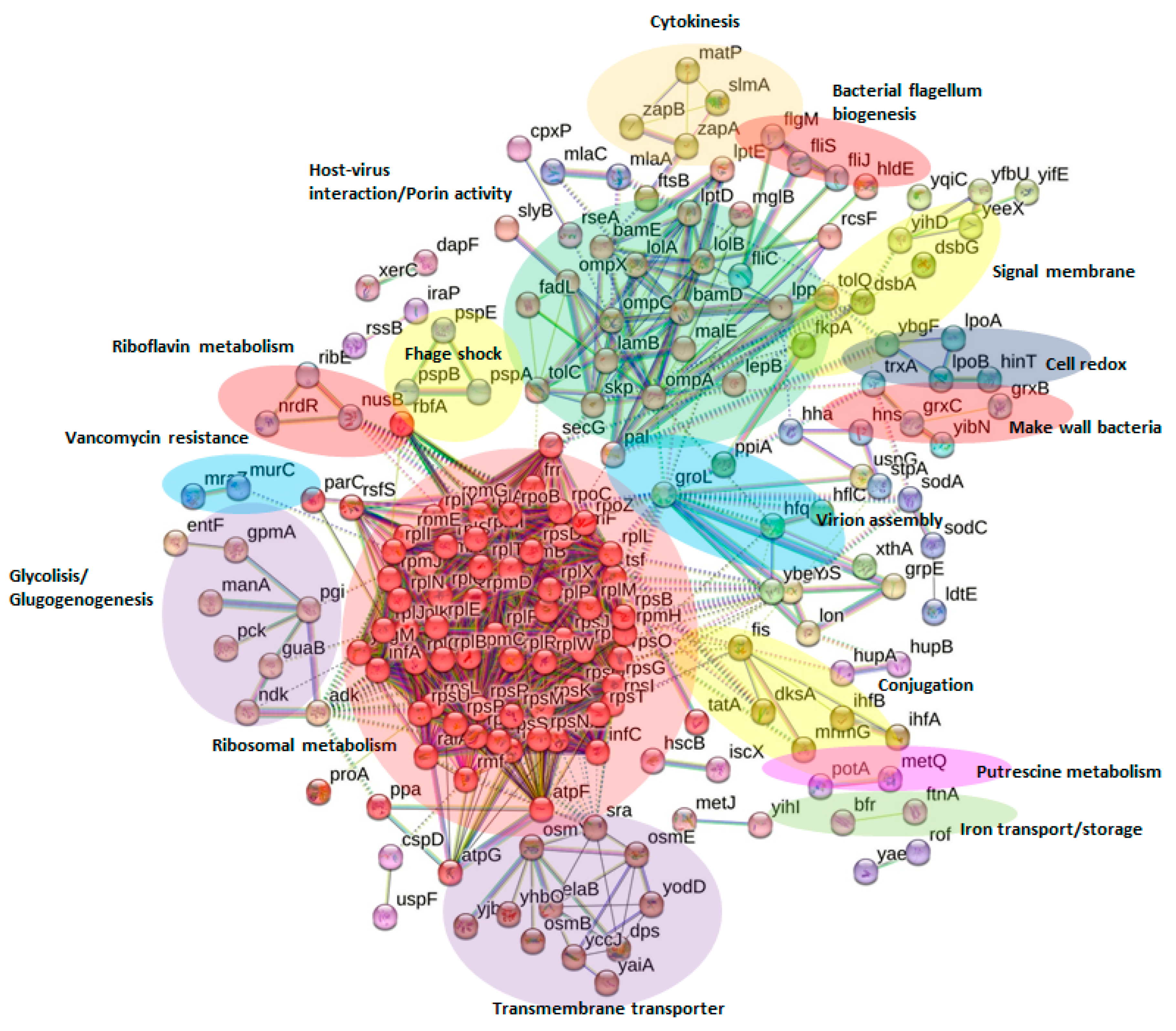
2.5. Network Analysis
2.6. Virulence Factors
2.7. Potential Species-Specific Peptide Biomarkers
3. Materials and Methods
3.1. Bacterial Strains
3.2. Protein Extraction
3.3. Peptide Sample Preparation
3.4. Shotgun LC-ESI-MS/MS Analysis in a LTQ-Orbitrap Instrument
3.5. LC-ESI-MS/MS Data Processing
3.6. Euclidean Hierarchical Clustering
3.7. Functional Analysis: Gene Ontology (GO) and Pathways Analysis
3.8. Network Analysis
3.9. Virulence Factors
3.10. Selection of Potential Peptide Biomarkers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spano, G.; Russo, P.; Lonvaud-Funel, A.; Lucas, P.; Alexandre, H.; Grandvalet, C.; Coton, E.; Coton, M.; Barnavon, L.; Bach, B.; et al. Biogenic amines in fermented foods. Eur. J. Clin. Nutr. 2010, 64, S95–S100. [Google Scholar] [CrossRef]
- Silla Santos, M.H. Biogenic amines: Their importance in foods. Int. J. Food Microbiol. 1996, 29, 213–231. [Google Scholar] [CrossRef]
- Feddern, V.; Mazzuco, H.; Fonseca, F.N.; De Lima, G.J.M.M. A review on biogenic amines in food and feed: Toxicological aspects, impact on health and control measures. Anim. Prod. Sci. 2019, 59, 608–618. [Google Scholar] [CrossRef]
- Trinki, M.; Herrero, A.M.; Jiménez-Colmenero, F.; Ruiz-Capillas, C. Quality assessment of fresh meat from several species based on free amino acid and biogenic amine contents during chilling storage. Foods 2018, 7, 132. [Google Scholar] [CrossRef] [PubMed]
- Dale, H.H.; Laidlaw, P.P. The physiological action of β-iminazolylethylamine. J. Physiol. 1910, 41, 318–344. [Google Scholar] [CrossRef]
- Maintz, L.; Novak, N. Histamine and histamine intolerance. Am. J. Clin. Nutr. 2007, 85, 1185–1196. [Google Scholar] [CrossRef]
- del Rio, B.; Redruello, B.; Linares, D.M.; Ladero, V.; Ruas-Madiedo, P.; Fernandez, M.; Martin, M.C.; Alvarez, M.A. The biogenic amines putrescine and cadaverine show in vitro cytotoxicity at concentrations that can be found in foods. Sci. Rep. 2019, 9, 120. [Google Scholar] [CrossRef] [PubMed]
- Ben-Gigirey, B.; Vieites Baptista De Sousa, J.M.; Villa, T.G.; Barros-Velazquez, J. Changes in Biogenic Amines and Microbiological Analysis in Albacore (Thunnus alalunga) Muscle during Frozen Storage. J. Food Prot. 1998, 61, 608–615. [Google Scholar] [CrossRef]
- Ben-Gigirey, B.; Vieites Baptista De Sousa, J.M.; Villa, T.G.; Barros-Velazquez, J. Histamine and Cadaverine Production by Bacteria Isolated from Fresh and Frozen Albacore (Thunnus alalunga). J. Food Prot. 1999, 62, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Fernández-No, I.C.; Böhme, K.; Calo-Mata, P.; Barros-Velázquez, J. Characterisation of histamine-producing bacteria from farmed blackspot seabream (Pagellus bogaraveo) and turbot (Psetta maxima). Int. J. Food Microbiol. 2011, 151, 182–189. [Google Scholar] [CrossRef]
- Ridolo, E.; Martignago, I.; Senna, G.; Ricci, G. Scombroid syndrome: It seems to be fish allergy but. it isn’t. Curr. Opin. Allergy Clin. Immunol. 2016, 16, 516–521. [Google Scholar] [CrossRef]
- Gardini, F.; Özogul, Y.; Suzzi, G.; Tabanelli, G.; Özogul, F. Technological factors affecting biogenic amine content in foods: A review. Front. Microbiol. 2016, 7, 1218. [Google Scholar] [CrossRef]
- Bodmer, S.; Imark, C.; Kneubühl, M. Biogenic amines in foods: Histamine and food processing. Inflamm. Res. 1999, 48, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Linares, D.M.; Martĺn, M.C.; Ladero, V.; Alvarez, M.A.; Fernández, M. Biogenic Amines in Dairy Products. Crit. Rev. Food Sci. Nutr. 2011, 51, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Kimura, B.; Yoshikawa, M.; Fujii, T. Cloning and sequencing of the histidine decarboxylase genes of gram-negative, histamine-producing bacteria and their application in detection and identification of these organisms in fish. Appl. Environ. Microbiol. 2003, 69, 2568–2579. [Google Scholar] [CrossRef] [PubMed]
- Coton, E.; Coton, M. Multiplex PCR for colony direct detection of Gram-positive histamine- and tyramine-producing bacteria. J. Microbiol. Methods 2005, 63, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Ladero, V.; Fernández, M.; Calles-Enríquez, M.; Sánchez-Llana, E.; Cañedo, E.; Martín, M.C.; Alvarez, M.A. Is the production of the biogenic amines tyramine and putrescine a species-level trait in enterococci? Food Microbiol. 2012, 30, 132–138. [Google Scholar] [CrossRef]
- Linares, D.M.; Del Río, B.; Ladero, V.; Martínez, N.; Fernández, M.; Martín, M.C.; Álvarez, M.A. Factors influencing biogenic amines accumulation in dairy products. Front. Microbiol. 2012, 3, 180. [Google Scholar] [CrossRef]
- Costa, M.P.; Balthazar, C.F.; Rodrigues, B.L.; Lazaro, C.A.; Silva, A.C.O.; Cruz, A.G.; Conte Junior, C.A. Determination of biogenic amines by high-performance liquid chromatography (HPLC-DAD) in probiotic cow’s and goat’s fermented milks and acceptance. Food Sci. Nutr. 2015, 3, 172–178. [Google Scholar] [CrossRef]
- Coton, E.; Rollan, G.; Bertrand, A.; Lonvaud-Funel, A. Histamine-Producing Lactic Acid Bacteria in Wines: Early Detection, Frequency, and Distribution. Am. J. Enol. Vitic. 1998, 49, 199–204. [Google Scholar] [CrossRef]
- Fernández, M.; Del Río, B.; Linares, D.M.; Martín, M.C.; Alvarez, M.A. Real-time polymerase chain reaction for quantitative detection of histamine-producing bacteria: Use in cheese production. J. Dairy Sci. 2006, 89, 3763–3769. [Google Scholar] [CrossRef] [PubMed]
- Abril, A.G.; Carrera, M.; Böhme, K.; Barros-Velázquez, J.; Cañas, B.; Rama, J.L.R.; Villa, T.G.; Calo-Mata, P. Characterization of Bacteriophage Peptides of Pathogenic Streptococcus by LC-ESI-MS/MS: Bacteriophage Phylogenomics and Their Relationship to Their Host. Front. Microbiol. 2020, 11, 1241. [Google Scholar] [CrossRef]
- Abril, A.G.; Carrera, M.; Böhme, K.; Barros-Velázquez, J.; Cañas, B.; Rama, J.L.R.; Villa, T.G.; Calo-Mata, P. Proteomic Characterization of Bacteriophage Peptides from the Mastitis Producer Staphylococcus aureus by LC-ESI-MS/MS and the Bacteriophage Phylogenomic Analysis. Foods 2021, 10, 799. [Google Scholar] [CrossRef] [PubMed]
- Carrera, M.; Böhme, K.; Gallardo, J.M.; Barros-Velázquez, J.; Cañas, B.; Calo-Mata, P. Characterization of foodborne strains of Staphylococcus aureus by shotgun proteomics: Functional networks, virulence factors and species-specific peptide biomarkers. Front. Microbiol. 2017, 8, 2458. [Google Scholar] [CrossRef]
- Calo-Mata, P.; Abril, A.G.; Velázquez, J.B.; Villa, T.G. Omics for the Investigation of Bacteriophages in Foodborne Bacteria; Foodomics: Omics strategies and applications in food science; Chapter 6; The Royal Society of Chemistry: London, UK, 2021. [Google Scholar]
- Abril, A.G.; Carrera, M.; Böhme, K.; Barros-Velázquez, J.; Rama, J.L.R.; Calo-Mata, P.; Sánchez-Pérez, A.; Villa, T.G. Proteomic Characterization of Antibiotic Resistance, and Production of Antimicrobial and Virulence Factors in Streptococcus Species Associated with Bovine Mastitis. Could Enzybiotics Represent Novel Therapeutic Agents Against These Pathogens? Antibiotics 2020, 9, 302. [Google Scholar] [CrossRef] [PubMed]
- Abril, A.G.; Carrera, M.; Böhme, K.; Barros-Velázquez, J.; Calo-Mata, P.; Sánchez-Pérez, A.; Villa, T.G. Proteomic characterization of antibiotic resistance in Listeria and production of antimicrobial and virulence factors. Int. J. Mol. Sci. 2021, 22, 8141. [Google Scholar] [CrossRef]
- Landete, J.M.; Ferrer, S.; Pardo, I. Improved enzymatic method for the rapid determination of histamine in wine. Food Addit. Contam. 2004, 21, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Sato, M.; Abe, N.; Yamaguchi, T.; Nakano, T. Simple and rapid detection of histamine-forming bacteria by differential agar medium. Food Control 2009, 20, 903–906. [Google Scholar] [CrossRef]
- Abril, A.G.; Quintela-Baluja, M.; Villa, T.G.; Calo-Mata, P.; Barros-Velázquez, J.; Carrera, M. Proteomic Characterization of Virulence Factors and Related Proteins in Enterococcus Strains from Dairy and Fermented Food Products. Int. J. Mol. Sci. 2022, 23, 10971. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; García-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef] [PubMed]
- Karovičová, J.; Kohajdová, Z. Biogenic Amines in Food. Chem. Pap. 2005, 59, 70–79. [Google Scholar]
- Prester, L. Biogenic amines in fish, fish products and shellfish: A review. Food Addit. Contam.—Part A 2011, 28, 1157–1560. [Google Scholar] [CrossRef]
- Hungerford, J.M. Histamine and Scombrotoxins. Toxicon 2021, 201, 115–126. [Google Scholar] [CrossRef]
- Kyriakidis, D.A.; Theodorou, M.C.; Tiligada, E. Histamine in two component system-mediated bacterial signaling. Front. Biosci. 2012, 17, 1108–1119. [Google Scholar]
- ten Brink, B.; Damink, C.; Joosten, H.M.L.J.; Huis in ′t Veld, J.H.J. Occurrence and formation of biologically active amines in foods. Int. J. Food Microbiol. 1990, 11, 73–84. [Google Scholar] [CrossRef]
- Taylor, S.L.; World Health Organization. Histamine Poisoning Associated with Fish, Cheese, and Other Foods/by Steve L. Taylor; World Health Organization: Geneva, Switzerland, 1985. [Google Scholar]
- Hu, Y.; Huang, Z.; Li, J.; Yang, H. Concentrations of biogenic amines in fish, squid and octopus and their changes during storage. Food Chem. 2012, 135, 2604–2611. [Google Scholar] [CrossRef]
- Pegg, A.E. S-Adenosylmethionine decarboxylase. Essays Biochem. 2009, 46, 25–46. [Google Scholar] [PubMed]
- Van Poelje, P.D.; Snell, E.E. Pyruvoyl-dependent enzymes. Annu. Rev. Biochem. 1990, 59, 29–59. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E.; Xiong, H.; Feith, D.J.; Shantz, L.M. S-adenosylmethionine decarboxylase: Structure, function and regulation by polyamines. Biochem. Soc. Trans. 1998, 26, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Najafzadeh, H.; Enayati, A.; Pashmforoush, M. Biogenic Amines Content of Canned Tuna Fish Marketed in Iran. J. Toxicol. Sci. 2011, 3, 190–193. [Google Scholar]
- Hungerford, J.M.; Arefyev, A.A. Flow-injection assay of enzyme inhibition in fish using immobilized diamine oxidase. Anal. Chim. Acta 1992, 261, 351–359. [Google Scholar] [CrossRef]
- Kurihara, S.; Suzuki, H.; Oshida, M.; Benno, Y. A novel putrescine importer required for type 1 pili-driven surface motility induced by extracellular putrescine in Escherichia coli K-12. J. Biol. Chem. 2011, 286, 10185–10192. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.C.; Sirisoma, N.S.; Kuppusamy, P.; Zweier, J.L.; Woster, P.M.; Casero, R.A. The natural polyamine spermine functions directly as a free radical scavenger. Proc. Natl. Acad. Sci. USA 1998, 95, 11140–11145. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Panda, S.; Barik, S.; Sarkar, A.; Singh, D.V.; Mohapatra, H. Antibiotic Resistance Profile, Outer Membrane Proteins, Virulence Factors and Genome Sequence Analysis Reveal Clinical Isolates of Enterobacter Are Potential Pathogens Compared to Environmental Isolates. Front. Cell. Infect. Microbiol. 2020, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Long, H.; Hu, Y.; Feng, Y.; McNally, A.; Zong, Z. Klebsiella oxytoca Complex: Update on Taxonomy, Antimicrobial Resistance, and Virulence. Clin. Microbiol. Rev. 2022, 35, e00006-21. [Google Scholar] [CrossRef]
- Li, T.; Mei, Y.; He, B.; Sun, X.; Li, J. Reducing quorum sensing-mediated virulence factor expression and biofilm formation in Hafnia alvei by using the potential quorum sensing inhibitor L-carvone. Front. Microbiol. 2019, 10, 3324. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, J.; Hu, Q.; Rao, X. Morganella morganii, a non-negligent opportunistic pathogen. Int. J. Infect. Dis. 2016, 50, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.; Elkamel, A.A. Proteus vulgaris, an emergin fish pathogen in Egypt. Assiut Vet. Med. J. 2006, 52, 36–50. [Google Scholar]
- Yang, Q.; Li, W.; Du, C.; Liu, Y.; Ma, S.; Yu, X.; Yao, W.; Wu, Z. Emerging pathogens caused disease and mortality in freshwater mussels, Hyriopsis cumingii, in China. Aquac. Res. 2020, 51, 5096–5105. [Google Scholar] [CrossRef]
- Hajjar, R.; Ambaraghassi, G.; Sebajang, H.; Schwenter, F.; Su, S.H. Raoultella ornithinolytica: Emergence and resistance. Infect. Drug Resist. 2020, 13, 1091–1104. [Google Scholar] [CrossRef]
- Vetting, M.W.; Luiz, L.P.; Yu, M.; Hegde, S.S.; Magnet, S.; Roderick, S.L.; Blanchard, J.S. Structure and functions of the GNAT superfamily of acetyltransferases. Arch. Biochem. Biophys. 2005, 433, 212–226. [Google Scholar] [CrossRef]
- Ermolenko, D.N.; Makhatadze, G.I. Bacterial cold-shock proteins. Cell. Mol. Life Sci. C 2002, 59, 1902–1913. [Google Scholar] [CrossRef] [PubMed]
- Barcarolo, M.V.; Gottig, N.; Ottado, J.; Garavaglia, B.S. Participation of two general stress response proteins from Xanthomonas citri subsp. citri in environmental stress adaptation and virulence. FEMS Microbiol. Ecol. 2020, 96, fiaa138. [Google Scholar] [CrossRef]
- Nagy, Z.A.; Szakács, D.; Boros, E.; Héja, D.; Vígh, E.; Sándor, N.; Józsi, M.; Oroszlán, G.; Dobó, J.; Gál, P.; et al. Ecotin, a microbial inhibitor of serine proteases, blocks multiple complement dependent and independent microbicidal activities of human serum. PLoS Pathog. 2019, 15, e1008232. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamed, H.; Ramachandran, R.; Narayanan, L.; Ozdemir, O.; Cooper, A.; Olivier, A.K.; Karsi, A.; Lawrence, M.L. Contributions of a LysR transcriptional regulator to Listeria monocytogenes virulence and identification of its regulons. J. Bacteriol. 2020, 202, 300087-20. [Google Scholar] [CrossRef]
- Michaux, C.; Sanguinetti, M.; Reffuveille, F.; Auffray, Y.; Posteraro, B.; Gilmore, M.S.; Hartke, A.; Giard, J.C. SlyA is a transcriptional regulator involved in the virulence of Enterococcus faecalis. Infect. Immun. 2011, 79, 2638–2645. [Google Scholar] [CrossRef]
- Epler Barbercheck, C.R.; Bullitt, E.; Andersson, M. Bacterial adhesion pili. Subcell. Biochem. 2018, 87, 1–18. [Google Scholar]
- Bateman, A.; Bycroft, M. The structure of a LysM domain from E. coli membrane-bound lytic murein transglycosylase D (MltD). J. Mol. Biol. 2000, 299, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Broxton, C.N.; Culotta, V.C. SOD Enzymes and Microbial Pathogens: Surviving the Oxidative Storm of Infection. PLoS Pathog. 2016, 12, e1005295. [Google Scholar] [CrossRef] [PubMed]
- Walton, T.A.; Sandoval, C.M.; Fowler, C.A.; Pardi, A.; Sousa, M.C. The cavity-chaperone Skp protects its substrate from aggregation but allows independent folding of substrate domains. Proc. Natl. Acad. Sci. USA 2009, 106, 1772–1777. [Google Scholar] [CrossRef]
- Jin, X.; Kightlinger, W.; Kwon, Y.C.; Hong, S.H. Rapid production and characterization of antimicrobial colicins using Escherichia coli-based cell-free protein synthesis. Synth. Biol. 2018, 3, ysy004. [Google Scholar] [CrossRef]
- Tanaka, K.J.; Song, S.; Mason, K.; Pinkett, H.W. Selective substrate uptake: The role of ATP-binding cassette (ABC) importers in pathogenesis. BBA—Biomembr. 2018, 1860, 868–877. [Google Scholar] [CrossRef]
- Palmer, K.L.; Kos, V.N.; Gilmore, M.S. Horizontal gene transfer and the genomics of enterococcal antibiotic resistance. Curr. Opin. Microbiol. 2010, 13, 632–639. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Fernández-No, I.C.; Böhme, K.; Gallardo, J.M.; Barros-Velázquez, J.; Cañas, B.; Calo-Mata, P. Differential characterization of biogenic amine-producing bacteria involved in food poisoning using MALDI-TOF mass fingerprinting. Electrophoresis 2010, 31, 1116–1127. [Google Scholar]
- Böhme, K.; Fernández-No, I.C.; Barros-Velázquez, J.; Gallardo, J.M.; Calo-Mata, P.; Cañas, B. Species differentiation of seafood spoilage and pathogenic gram-negative bacteria by MALDI-TOF mass fingerprinting. J. Proteome Res. 2010, 9, 3169–3183. [Google Scholar] [CrossRef] [PubMed]
- Böhme, K.; Fernández-No, I.C.; Barros-Velázquez, J.; Gallardo, J.M.; Cañas, B.; Calo-Mata, P. Comparative analysis of protein extraction methods for the identification of seafood-borne pathogenic and spoilage bacteria by MALDI-TOF mass spectrometry. Anal. Methods 2010, 2, 1941–1947. [Google Scholar] [CrossRef]
- Carrera, M.; Cañas, B.; Gallardo, J.M. The sarcoplasmic fish proteome: Pathways, metabolic networks and potential bioactive peptides for nutritional inferences. J. Proteom. 2013, 78, 211–220. [Google Scholar] [CrossRef]
- Käll, L.; Canterbury, J.D.; Weston, J.; Noble, W.S.; MacCoss, M.J. Semi-supervised learning for peptide identification from shotgun proteomics datasets. Nat. Methods 2007, 4, 923–925. [Google Scholar] [CrossRef] [PubMed]
| Sample | Bacterial Strain | Code | Source | GenBank |
|---|---|---|---|---|
| H1 | Enterobacter aerogenes | EbAe1 | ATCC 13048 | FJ971882 |
| H2 | Enterobacter cloacae | EbCl1 | ATCC 13047 | FJ971883 |
| H3 | Hafnia alvei | HaAl2 | ATCC 9760 | FJ971884 |
| H4 | Klebsiella oxytoca | KlOx1 | ATCC 13182 | FJ971867 |
| H5 | Morganella morganii | MoMo1 | BM 65 | FJ971858 |
| H6 | Morganella morganii | MoMo2 | ATCC 8076 | FJ971868 |
| H7 | Proteus mirabilis | PrMi1 | ATCC 14153 | FJ971887 |
| H8 | Proteus penneri | PrPe1 | ATCC 33519 | FJ971869 |
| H9 | Proteus vulgaris | PrVu1 | ATCC 9484 | FJ971888 |
| H10 | Proteus vulgaris | Sard1 | Sardine | JN630885 |
| H11 | Proteus vulgaris | Sard2 | Sardine | JN630886 |
| H12 | Raoultella planticola | RaPl2 | ATCC 33531 | FJ971885 |
| H13 | Stenotrophomonas maltophilia | 25MC6 | Albacore tuna | FJ971861 |
| H14 | Stenotrophomonas maltophilia | 5PC6 | Albacore tuna | FJ971863 |
| H15 | Stenotrophomonas maltophilia | StMa2 | 15MF | FJ971862 |
| Biogenic Amine | Precursor | Proteins Identified by LC-ESI-MS/MS | Peptides | Sample | Bacterial Strain |
|---|---|---|---|---|---|
| Agmatine | Arginine | Arginine ABC transporter substrate-binding protein | IDAVFGDTAVVTEWLK | H4 | K. oxytoca |
| Lysine-arginine-ornithine-binding periplasmic protein | C*TWVGSDFDSLIPSLK | H4 | K. oxytoca | ||
| “ | IGTDATYAPFSSK | H4 | K. oxytoca | ||
| Cadaverine | Lysine | Lysine-arginine-ornithine-binding periplasmic protein | C*TWVGSDFDSLIPSLK | H4 | K. oxytoca |
| “ | IGTDATYAPFSSK | H4 | K. oxytoca | ||
| Histamine | Histidine | Histidine kinase | IDSEDLPHVRASVAR | H6 | M. morganii |
| “ | LAM*NLRTRLFLSISALITVALLGLLLGLVSVM*QM*AGSQEILIR | H13 | S. maltophilia | ||
| “ | M*IAEAANADSKQAQR | H4, H12 | K. oxytoca, R. planticola | ||
| “ | TIDQINQQKIQLEQEIADRK | H8, H9 | P. penneri, P. vulgaris | ||
| “ | GEADATLDSEVSAWRAVAR | H11 | P. vulgaris | ||
| “ | LSSELWNC*KIDPTQAEM*AM*INILANAR | H7 | P. mirabilis | ||
| “ | SEASENTVDLIVEDEGSGIPK | H7 | P. mirabilis | ||
| “ | NEEARDNLKLISELTAR | H11 | P. vulgaris | ||
| “ | RYAYSEQLGDLLQR | H15 | S. maltophilia | ||
| Histidine phosphatase | HAQASEYGSALFVAVGQAKQVK | H3 | H. alvei | ||
| Histidine-binding periplasmic protein | IGVLQGTTQETYGNEHWAPK | H4 | K. oxytoca | ||
| Histidine triad nucleotide-binding protein | EIPSDIVYQDELVTAFR | H4 | K. oxytoca | ||
| “ | IAEQEGIAEDGYR | H2 | E. cloacae | ||
| Histidine ammonia-lyase | LAAM*QQALGAQIAAVEEDR | H2 | E. cloacae | ||
| H6 | M. morganii | ||||
| Putrescine | Arginine | Lysine-arginine-ornithine-binding periplasmic protein | C*TWVGSDFDSLIPSLK | H4 | K. oxytoca |
| “ | IGTDATYAPFSSK | H4 | K. oxytoca | ||
| Glutamine | Glutamine ABC transporter periplasmic protein | AVGDSIEAQQYGIAFPK | H4 | K. oxytoca | |
| Glutamine-fructose-6-phosphate aminotransferase | IDAAQEAELIKALFEAPR | H4 | K. oxytoca | ||
| N-acetylglutaminylglutamine amidotransferase | SGANAAVDKALRLDSTVM*LVDDPVK | H3 | H. alvei | ||
| Methionine | Type 1 glutamine amidotransferase domain-containing protein | IFRTLALM*LLVTSATAFAASK | H7 | P. mirabilis | |
| L-glutamine-binding protein | ADAVIHDTPNILYFIK | H4 | K. oxytoca | ||
| Ornithine | “ | AVGDSLEAQQYGIAFPK | H3 | H. alvei | |
| S-adenosylmethionine decarboxylase proenzyme | ALSFNIYDVC*YAR | H15 | S. maltophilia | ||
| Spermidine | Agmatine | Spermidine/putrescine import ATP-binding protein | VDEVHDNAEAEGLIGYIR | H9 | P. vulgaris |
| Methionine | S-adenosylmethionine decarboxylase proenzyme | ALSFNIYDVC*YAR | H15 | S. maltophilia | |
| Putrescine | |||||
| Spermine | |||||
| Spermine | Agmatine | ||||
| Methionine | S-adenosylmethionine decarboxylase proenzyme | ALSFNIYDVC*YAR | H15 | S. maltophilia | |
| Spermidine |
| Funtion | Protein | Sample |
|---|---|---|
| Toxins | Entericidin A/B family lipoprotein | H1, H2, H3, H4 |
| Addiction module toxin, GnsA/GnsB family | H12 | |
| Antitoxin ParD | H15, H13 | |
| Ecotin | H1, H3, H4 | |
| Antimicrobial compounds production | Bacteriocin immunity protein | H9 |
| Colicin immunity protein/pyocin immunity protein | H1 | |
| Antimicrobial resistance | Penicillin-binding protein activator LpoB | H4 |
| TetR family transcriptional regulator | H5, H15 | |
| Acriflavine resistance protein B | H61 | |
| Methicillin resistance protein | H15 | |
| GNAT family N-acetyltransferase | H1, H5 | |
| Additional resistances and tolerances | Acid stress chaperone HdeB | H5, H6 |
| Cold shock protein CspA | H4, H14 | |
| Cold shock protein CspC | H4 | |
| Cold shock protein CspD | H14, H6, H8 | |
| Cold shock protein CspE | H4 | |
| Copper resistance protein | H5, H6 | |
| Envelope stress response membrane protein PspB | H1, H3, H5, H8, H9 | |
| General stress protein | H4, H13, H15 | |
| Heat shock survival AAA family ATPase ClpK | H2, H4 | |
| Stress response protein ElaB | H13 | |
| Stress response translation initiation inhibitor YciH | H5 | |
| Stress-induced bacterial acidophilic repeat motif | H2 | |
| Universal stress protein | H2, H3, H4, H12 | |
| YdeI family stress tolerance OB fold protein | H2 | |
| CsbD family protein | H2, H3, H9, H15 | |
| Peroxide/acid resistance protein YodD | H2 | |
| Putative ‘Cold-shock’ DNA-binding domain protein | H15 | |
| L,D-transpeptidase YnhG | H4 | |
| Protein sufA | H5 | |
| Spy/CpxP family protein refolding chaperone | H4 | |
| Host colonization and immune evasion | Beta-aspartyl-peptidase | H9 |
| Chaperone protein Skp | H1, H2, H3, H4, H5, H7, H8, H9, H12 | |
| Chemotaxis protein CheY | H13 | |
| Cytosol nonspecific dipeptidase | H2 | |
| Filamentous hemagglutinin | H2 | |
| Fimbrial protein FimV | H15 | |
| Type 1 fimbrial protein | H8 | |
| Flagellar biosynthesis protein FliC | H14 | |
| Flagellar hook protein FlgE | H13 | |
| Flagellar secretion chaperone FliS | H15 | |
| Flagellin | H8, H10, H11, H13, H14, H15 | |
| Hemagluttinin | H1 | |
| Hemolysin expression modulator Hha | H3, H7 | |
| Inhibitor of vertebrate lysozyme | H3 | |
| Isoaspartyl peptidase/L-asparaginase | H7, H11, H13, H15 | |
| Lipoprotein involved in copper homeostasis and adhesion | H4 | |
| Lon protease | H1 | |
| LysM | H2, H4, H13, H15 | |
| LysR family transcriptional regulator | H12 | |
| Maltoporin | H4 | |
| Ferrichrome porin FhuA | H4 | |
| MipA/OmpV family protein | H4 | |
| Molecular chaperone OsmY | H2, H3, H4 | |
| OmpA | H1, H2, H3, H4, H8, H13 | |
| OmpC | H4, H10 | |
| OmpD | H4 | |
| OmpF | H4 | |
| OmpK36 | H4 | |
| Omptin family outer membrane protease | H4 | |
| OmpX | H4 | |
| Phosphate-selective porin OprO/OprP | H15 | |
| OsmC family peroxiredoxin | H4 | |
| Outer membrane lipoprotein RcsF | H4 | |
| Peptidase S74 | H15 | |
| Peptidase S8 and S53 subtilisin kexin sedolisin | H13 | |
| Periplasmic serine endoprotease DegP-like | H15 | |
| IAP aminopeptidase MEROPS family M28C | H1 | |
| Membrane-bound metallopeptidase | H9 | |
| Metalloprotease | H4 | |
| Alkaline serine protease | H14 | |
| Signal peptidase I | H12 | |
| Superoxide dismutase | H4, H7, H9, H15, H13, H2 | |
| Tautomerase PptA | H2 | |
| Tautomerase ydcE | H12 | |
| TonB | H4, H14, H15 | |
| Transcriptional regulator SlyA | H1, H2, H3, H4, H12 | |
| Twitching motility protein PilH | H14 | |
| Type I restriction enzyme endonuclease | H4 | |
| Type VI secretion protein | H15 | |
| VacJ family lipoprotein | H15 | |
| ABC transporters | Amino acid ABC transporter | H2 |
| Arginine ABC transporter substrate-binding protein | H4 | |
| Glutamine ABC transporter periplasmic protein | H3, H4 | |
| Manganese ABC transporter | H4 | |
| Iron ABC transporter | H4 | |
| Oligopeptide ABC transporter | H5, H6 | |
| Putative ABC-type sugar transport system | H1 | |
| Ribose ABC transporter substrate-binding protein RbsB | H4 | |
| Xylose ABC transporter, periplasmic xylose-binding protein XylF | H2 | |
| Phage proteins | Presumed capsid scaffolding protein (GpO) | H10 |
| Bacteriophage CI repressor | H1 | |
| Beta_helix domain-containing protein (Klebsiella phage vB_KpM_FBKp24) | H7 | |
| Phage portal protein, HK97 family | H5 | |
| Phage shock protein PspA | H1, H2, H3, H4, H5, H6, H7, H9, H11, H12 | |
| Uncharacterized protein (Stenotrophomonas phage BUCT608) | H12 | |
| Terminase (Klebsiella phage vB_KppS-Storm) | H11 | |
| Uncharacterized protein (Stenotrophomonas phage Marzo) | H11 | |
| Alternative virulence factors and proteins involved in horizontal transfer | Pilus assembly protein, pilin FimA | H5 |
| IS3 family transposase | H5 | |
| Major type 1 subunit fimbrin (Pilin) | H12 | |
| Plasmid stability protein | H3 | |
| Plasmid-related protein | H7 | |
| Rop family plasmid primer RNA-binding protein | H5 | |
| Transposase | H15 | |
| Tyrosine recombinase XerC | H5 | |
| MobC family plasmid mobilization relaxosome protein | H5 |
| Protein | Peptide | Sample | Specific by Blastp |
|---|---|---|---|
| DUF883 domain-containing protein | NLADTLEEVLNSSTDKSKEELGK | H1 | E. aerogenes |
| Uncharacterized protein | LLQLALAAIDSADEAGVSHEDIDNQHTEEEASPLTPK | H1 | E. aerogenes |
| Hemagluttinin domain-containing protein | SVAQNAAAITDTR | H1 | E. aerogenes |
| Anti-adapter protein IraP | LIQDIETAMEQVKPGPLVDDRDTQLLQQYIK | H1 | E. aerogenes |
| Cell division protein ZapB | NTTLAQEVQSAQHGREELERENSQLR | H1 | E. aerogenes |
| Der GTPase-activating protein YihI | KPIPLGVTESTPAVK | H1 | E. aerogenes |
| DUF1471 domain-containing protein | AREEGAKGFVVNSAGGDNHMYGTATIYK | H2 | E. cloacae |
| DUF2511 domain-containing protein | SSGQPISVIQIDDPSSPGQK | H2 | E. cloacae |
| Uncharacterized protein | ESGFEGELTDLSDDILIYHLK | H2 | E. cloacae |
| Universal stress protein UspF | M*FNSILVPVDISESR | H2 | E. cloacae |
| DNA-binding protein | IKDNNAEYVEPLDMLAELC*EDNKLLAAELR | H3 | Hafnia alvei |
| ATP synthase subunit b | KAQIIDEAKVEAEQER | H3 | Hafnia alvei |
| DUF883_C domain-containing protein | GVANEAAGQVEESYGEATNSHQHRLEGQAR | H3 | Hafnia alvei |
| Exodeoxyribonuclease 7 small subunit | APAAPSFEQALSELEQIVTHLESGELPLEDALNEFER | H3 | Hafnia alvei |
| ATP synthase subunit b | AQIIDEAKVEAEQERNK | H3 | Hafnia alvei |
| Cell division protein ZapB | ESLVRENEQLKEEQTAWQER | H3 | Hafnia alvei |
| 50S ribosomal protein L10 | IVEGTPFEC*LKDTFVGPTLVAFSMEHPGAAAR | H3 | Hafnia alvei |
| Uncharacterized protein | RKLSPAEELALGK | H3 | Hafnia alvei |
| Major outer membrane lipoprotein Lpp | VDQLSNDVNAM*RADVQTAKDDAAR | H3 | Hafnia alvei |
| DUF1471 domain-containing protein | IGDVSAEVRDGTM*DDIVK | H3 | Hafnia alvei |
| Uncharacterized protein | ESDAEREEKTFTWKPSAVR | H3 | Hafnia alvei |
| Cell division protein ZapB | ENEQLKEEQTAWQER | H3 | Hafnia alvei |
| Membrane protein | NGVPESGFTLDVVPNDQADASGGQVVGHC*ENDTQK | H3 | Hafnia alvei |
| Chaperone protein Skp | ATELQGQERDLQSK | H3 | Hafnia alvei |
| Outer membrane lipoprotein SlyB | AVQIQGGDESNAIGAIGGAVLGGFLGNTIGGGTGR | H4 | K. oxytoca |
| Putative porin | NYVEANGGISWTPLTPLTIK | H4 | K. oxytoca |
| TolC-like protein | QAGIQDVTYQTDQQTLILNTATAYFK | H4 | K. oxytoca |
| Maltoporin | SSESGGSGTFADRDQFGNR | H4 | K. oxytoca |
| Glutamate/aspartate ABC transporter substrate-binding protein | LIPVTSQNRIPLLQNGTFDFEC*GSTTNNLAR | H6 | M. morganii |
| Integration host factor subunit alpha | AEM*SENLSEKLDLSKR | H5 | M. morganii |
| FAD assembly factor SdhE | GRPDDEALYQIIR | H5 | M. morganii |
| Cell division protein ZapB | VQQALDTITLLQM*EIEELKEKNDALNQEVQGAR | H7 | P. mirabilis |
| Outer membrane protein assembly factor BamE | VRQETLTLTFDNNGILTK | H7 | P. mirabilis |
| DUF2594 family protein | AISEVADEQQAETFRNTLNQIK | H7 | P. mirabilis |
| DNA-binding protein | IIADFLGVAPSEIWPSRYFHPETGELLER | H7 | P. mirabilis |
| Inner membrane protein YhcB | SSNELMPDMPEQENPFNYR | H8 | P. penneri |
| DUF1043 family protein | SSNELM*PDM*PAQDNPFNYR | H9 | P. vulgaris |
| Lipoprotein | QQAQETENQALDKADQLTDQAK | H9 | P. vulgaris |
| Arsenate reductase | SLELADPQLSEDALIQAIVDNPK | H12 | R. planticola |
| Uncharacterized protein | SEHAAQGKSDSVGSQVSEGAQKTWNK | H12 | R. planticola |
| Uncharacterized protein | EAEQLDNDKNFYYQEAK | H12 | R. planticola |
| Exported protein | VGTISSTGQTAPGDARAELLK | H12 | R. planticola |
| Uncharacterized protein | TPLSDTDFANKILASQANQEYVR | H12 | R. planticola |
| DUF1311 domain-containing protein | SRDGELDTALYDDSQPGNLQGELNDVMR | H15 | S. maltophilia |
| Type VI secretion protein | NAPAADTQNFYNAPAPR | H15 | S. maltophilia |
| Uncharacterized protein | VLAAGGTAAQALAASQAAAR | H15 | S. maltophilia |
| Uncharacterized protein | GSTTVAGQDISLNQDFK | H15 | S. maltophilia |
| Uncharacterized protein | DQKDLPHPDAEAQRPDPVSPLQAK | H15 | S. maltophilia |
| DNA-binding protein | SLIAQAEKQQSK | H15 | S. maltophilia |
| Uncharacterized protein | AQSTQDLGLHTSC*R | H15 | S. maltophilia |
| Uncharacterized protein | TYFDYSEEQPFIR | H15 | S. maltophilia |
| 30S ribosomal protein S16 | VGFYNPVAQGGEK | H15 | S. maltophilia |
| Cu(I)-responsive transcriptional regulator | LGEDDQTPVPVDTAK | H15 | S. maltophilia |
| Antitoxin ParD | QLIAEGLASGPSAPLAPDHFDKLR | H15 | S. maltophilia |
| DUF3606 domain-containing protein | AAVAEVGPTAAAVR | H15 | S. maltophilia |
| TonB-dependent receptor | NASSGPGAVVLSPTHPDNPIPGQASR | H15 | S. maltophilia |
| RidA family protein | AFDNLKAVAEAAGGSLDQVVR | H13 | S. maltophilia |
| LysM peptidoglycan-binding domain-containing protein | KADFSGVSGSVDSTAEQVPK | H13 | S. maltophilia |
| Uncharacterized protein | SANAAATAAQEAADAAAAK | H13 | S. maltophilia |
| DUF4124 domain-containing protein | ANLALLDGGGQVMQDTDGDGKADTPLAPEQR | H13 | S. maltophilia |
| LysM peptidoglycan-binding domain-containing protein | ADFSGVSGSVDSTAEQVPK | H13 | S. maltophilia |
| DUF4124 domain-containing protein | SPQAAASAETPAAPVPEQC*STAR | H13 | S. maltophilia |
| Flagellin | FTSTIANLNTNSENLSAAR | H13 | S. maltophilia |
| Uncharacterized protein | DAADKTAAASEQAAADTQQALDKAADATANAADQAK | H13 | S. maltophilia |
| Attachment protein | LLGDIAKDLTNAPLEDIQK | H13 | S. maltophilia |
| Antitoxin ParD | QLIAEGLASGPAVPVTAATFER | H13 | S. maltophilia |
| Uncharacterized protein | TQADAILADSAANEYDKSLAAQLASQAAYQTDDTPAAVAYLK | H13 | S. maltophilia |
| Excinuclease ATPase subunit | VEQSLQELISSQAAK | H13 | S. maltophilia |
| Uncharacterized conserved protein, DUF2147 | SIVEISQAANGTLTGK | H13 | S. maltophilia |
| 50S ribosomal protein L31 type B | STM*GTKETIQWEDGNEYPLVK | H13 | S. maltophilia |
| LysM peptidoglycan-binding domain-containing protein | ADFSGVSASVDSTADVVSGGTYTVQKGDSLSK | H13 | S. maltophilia |
| DUF3613 domain-containing protein | SFEHEIPDFFEADVAK | H13 | S. maltophilia |
| Poly(Hydroxyalkanoate) granule-associated protein | LHVPTADEVTALEARIDALQAR | H13 | S. maltophilia |
| Heme exporter protein D | AVKQDAAAPLSTELER | H13 | S. maltophilia |
| RNA polymerase-binding transcription factor DksA | TDEATGRPILPTGYKPGSEEEYM*SPLQQEYFR | H13 | S. maltophilia |
| Transcriptional regulator | LEALDALLPSDSPNPIDLLER | H13 | S. maltophilia |
| CsbD-like protein | IQKGVGEVQSDVGKAR | H13 | S. maltophilia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abril, A.G.; Calo-Mata, P.; Böhme, K.; Villa, T.G.; Barros-Velázquez, J.; Pazos, M.; Carrera, M. Shotgun Proteomics Analysis, Functional Networks, and Peptide Biomarkers for Seafood-Originating Biogenic-Amine-Producing Bacteria. Int. J. Mol. Sci. 2023, 24, 7704. https://doi.org/10.3390/ijms24097704
Abril AG, Calo-Mata P, Böhme K, Villa TG, Barros-Velázquez J, Pazos M, Carrera M. Shotgun Proteomics Analysis, Functional Networks, and Peptide Biomarkers for Seafood-Originating Biogenic-Amine-Producing Bacteria. International Journal of Molecular Sciences. 2023; 24(9):7704. https://doi.org/10.3390/ijms24097704
Chicago/Turabian StyleAbril, Ana González, Pilar Calo-Mata, Karola Böhme, Tomás G. Villa, Jorge Barros-Velázquez, Manuel Pazos, and Mónica Carrera. 2023. "Shotgun Proteomics Analysis, Functional Networks, and Peptide Biomarkers for Seafood-Originating Biogenic-Amine-Producing Bacteria" International Journal of Molecular Sciences 24, no. 9: 7704. https://doi.org/10.3390/ijms24097704
APA StyleAbril, A. G., Calo-Mata, P., Böhme, K., Villa, T. G., Barros-Velázquez, J., Pazos, M., & Carrera, M. (2023). Shotgun Proteomics Analysis, Functional Networks, and Peptide Biomarkers for Seafood-Originating Biogenic-Amine-Producing Bacteria. International Journal of Molecular Sciences, 24(9), 7704. https://doi.org/10.3390/ijms24097704








