Dickkopf-1 Acts as a Profibrotic Mediator in Progressive Chronic Kidney Disease
Abstract
1. Introduction
2. Results
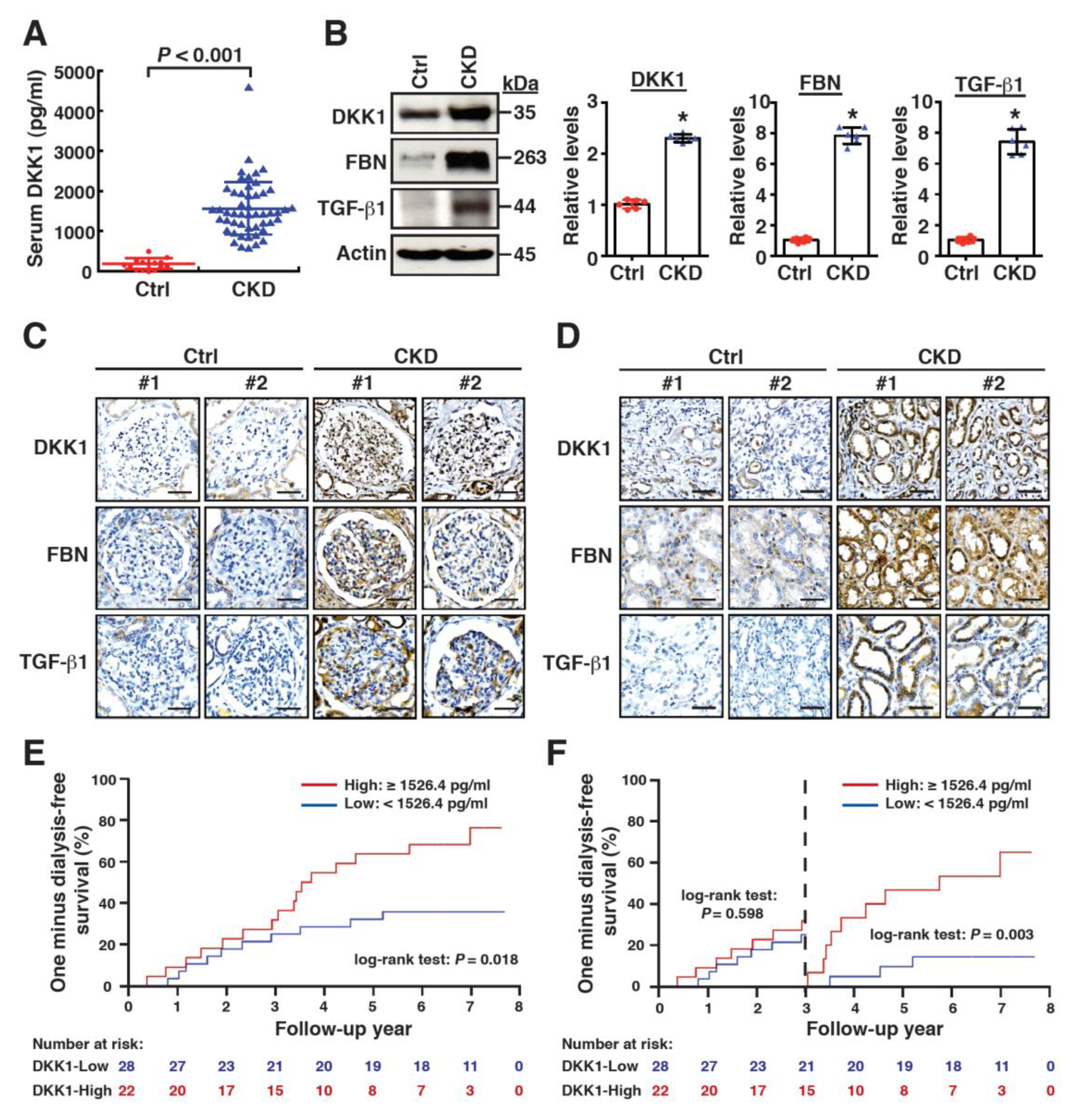
2.1. High Levels of Serum DKK1 Are Associated with CKD Progression
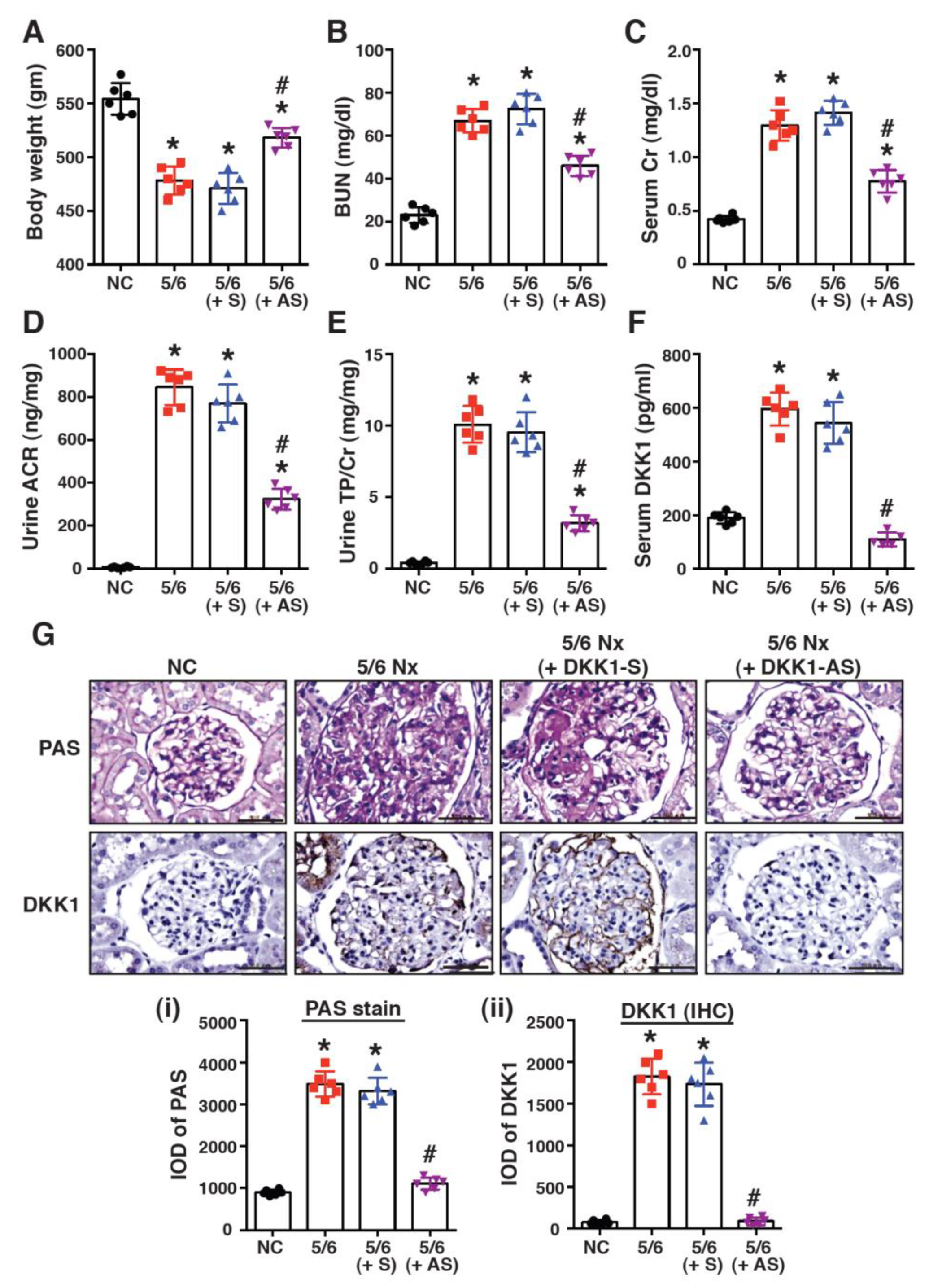
2.2. Treatment with DKK1-Antisense Oligonucleotides Attenuates CKD-Associated Phenotypes in a 5/6-Nephrectomized Rat Model
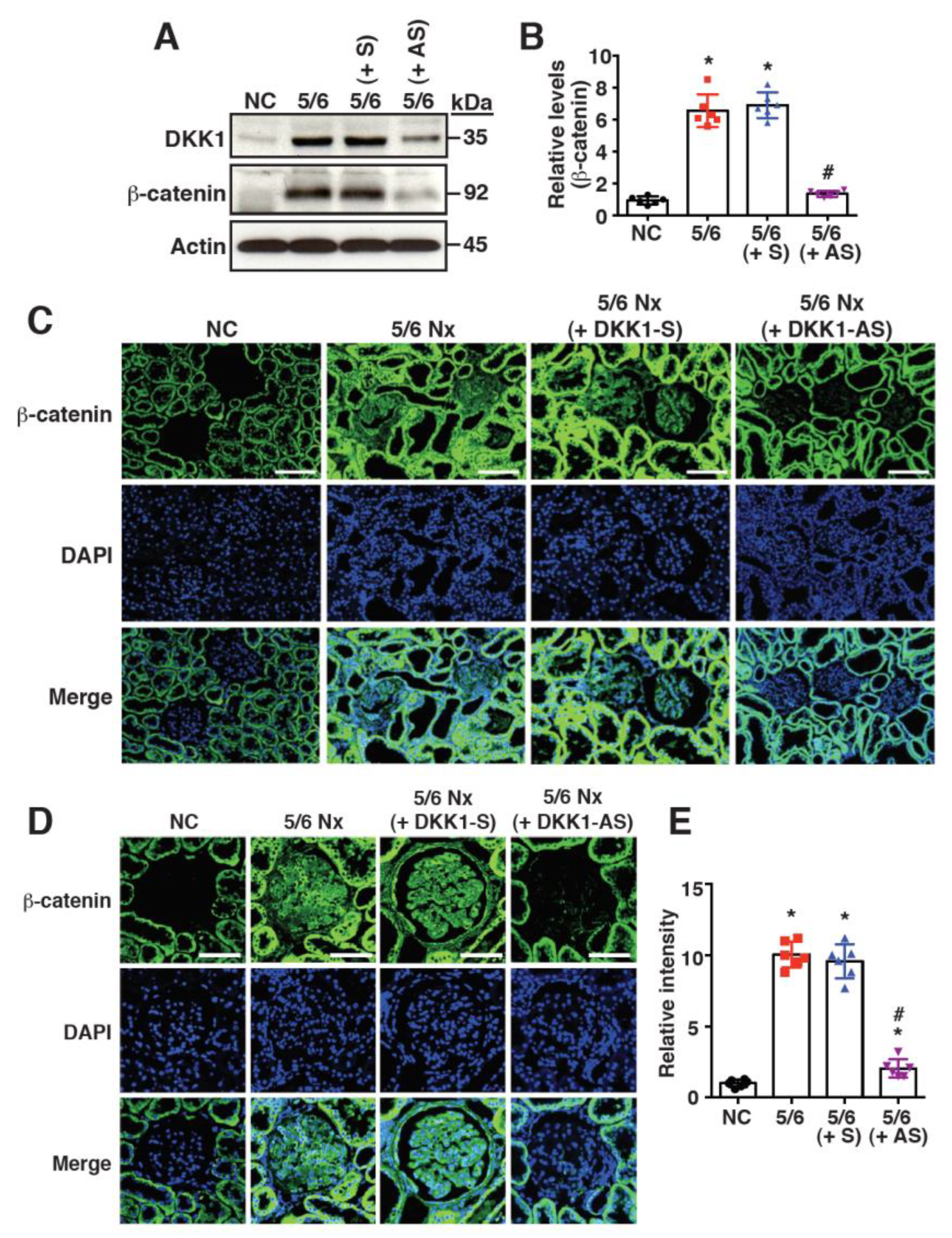
2.3. Increased DKK1 Expression Correlates with Accumulation of β-Catenin in Renal Tissues of 5/6 Nx Rats
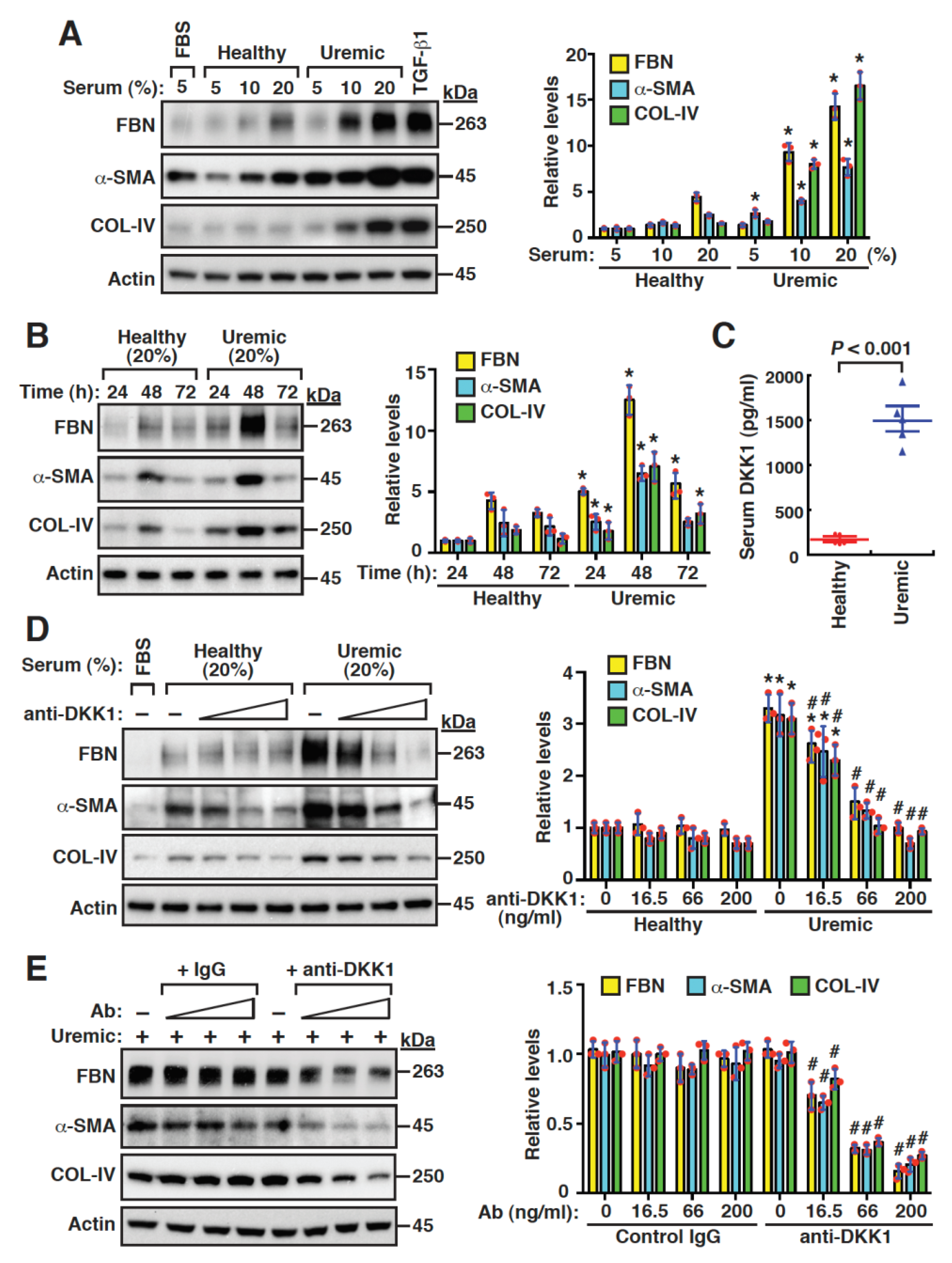
2.4. Uremic Serum Stimulates Fibrogenesis of Mouse Mesangial Cells, at Least Partly, through DKK1-Mediated Signaling
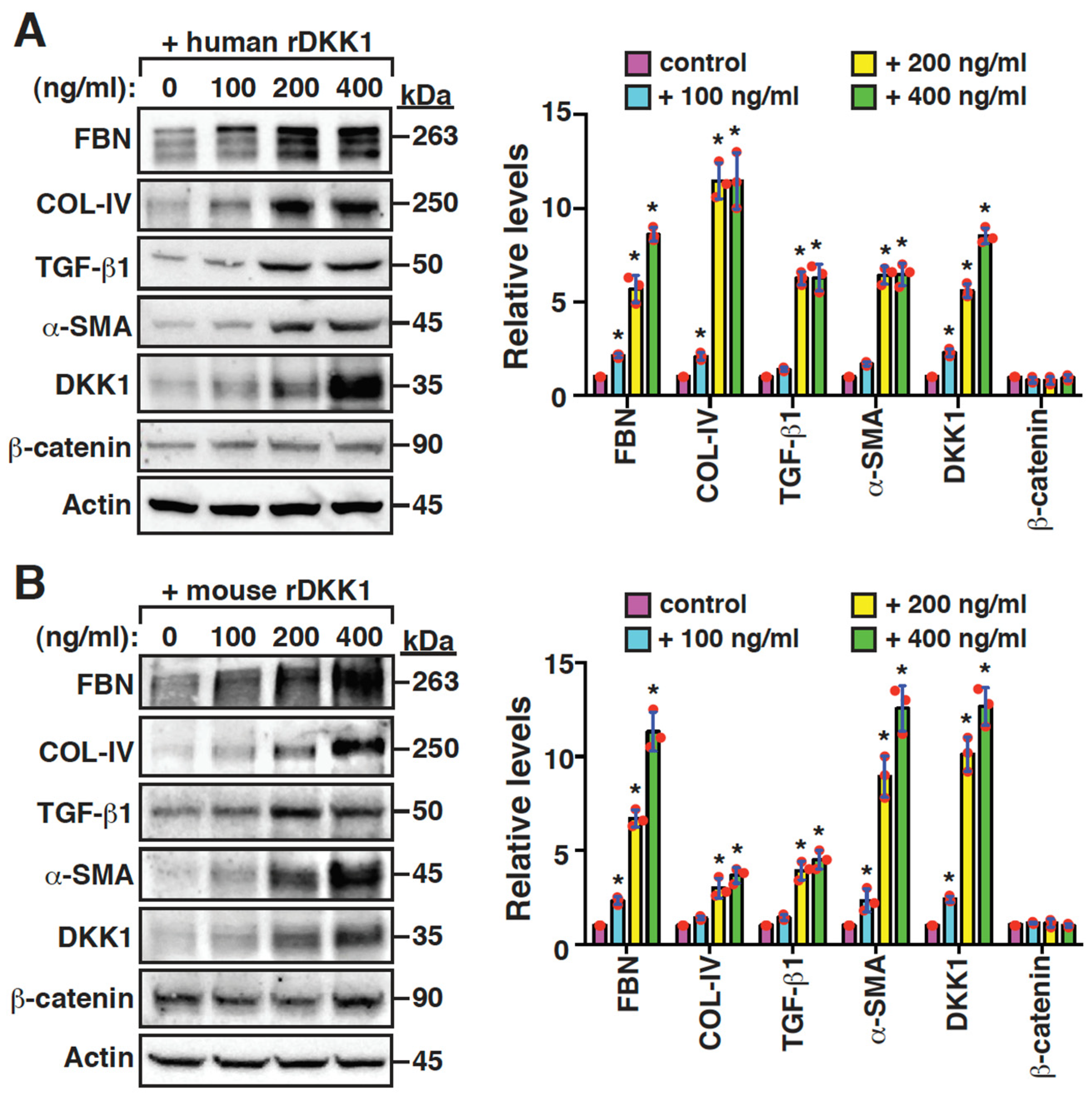
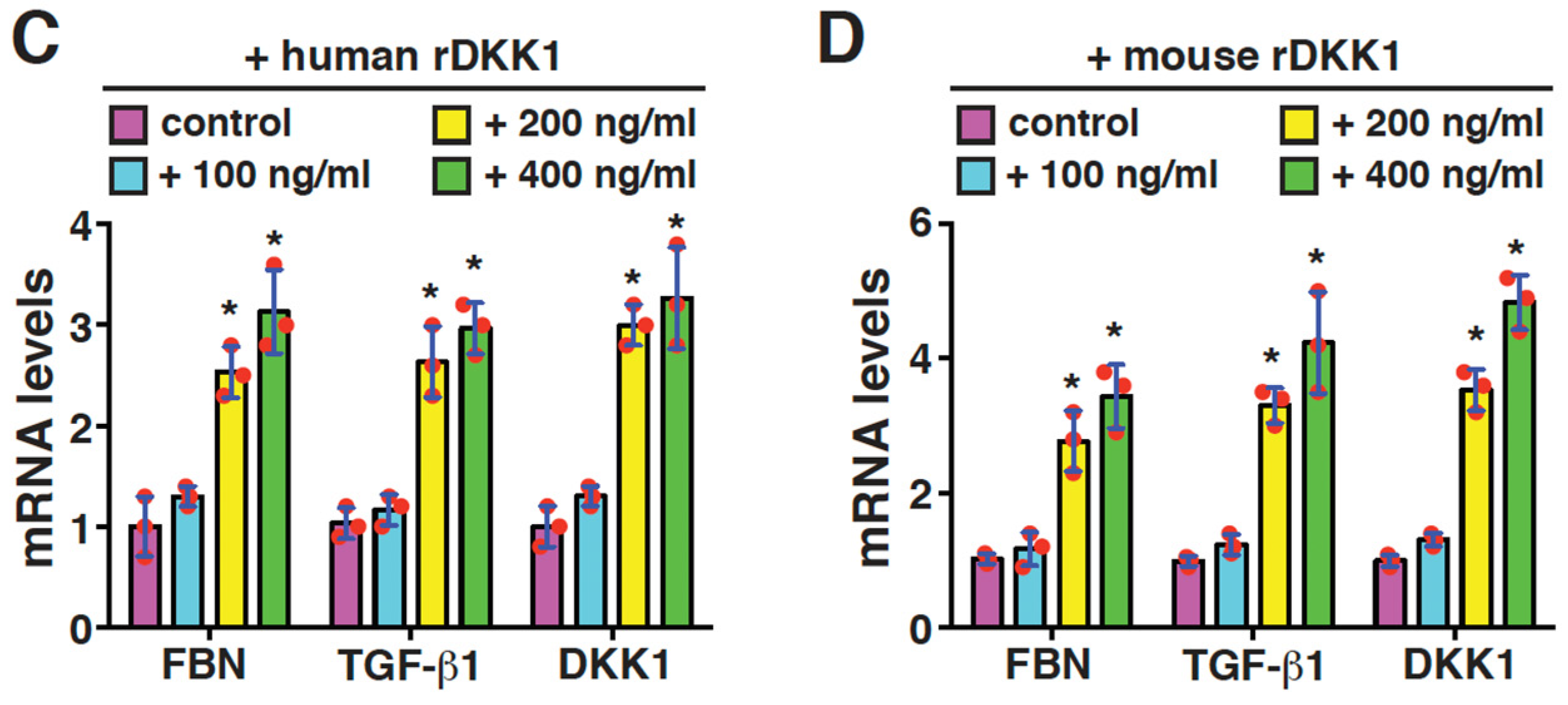
2.5. DKK1 Is a Profibrotic Mediator and Can Reinforce Its Own Expression in Mesangial Cells
3. Discussion
4. Materials and Methods
4.1. Study Design and Population
4.2. Human Serum and Kidney Samples
4.3. Western Blot Analysis
4.4. In Vivo CKD Animal Model
4.5. Biochemical Assays of Blood and Urine
4.6. Periodic Acid-Schiff (PAS) Staining, Immunohistochemistry and Immunofluorescence
4.7. In Vitro Mesangial Cell Cultures
4.8. Quantitative Reverse Transcription-PCR (RT-qPCR)
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bruck, K.; Stel, V.S.; Gambaro, G.; Hallan, S.; Volzke, H.; Arnlov, J.; Kastarinen, M.; Guessous, I.; Vinhas, J.; Stengel, B.; et al. CKD Prevalence Varies across the European General Population. J. Am. Soc. Nephrol. 2016, 27, 2135–2147. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.K.; Knicely, D.H.; Grams, M.E. Chronic kidney disease diagnosis and management: A revew. JAMA 2019, 322, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Lewis, R.D.; Morgan, A.R.; Whyte, M.B.; Hanif, W.; Bain, S.C.; Davies, S.; Dashora, U.; Yousef, Z.; Patel, D.C.; et al. A narrative review of chronic kidney disease in clinical pratice: Current challenges and future perspectives. Adv. Ther. 2022, 39, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D. Global prevalence of chronic kidney disease—A systematic review and meta-analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef]
- Shabaka, A.; Cases-Corona, C.; Fernandez-Juarez, G. Therapeutic insights in chronic kidney disease progresssion. Front. Med. 2021, 8, 645187. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Iribarren, C.; McCulloch, C.E.; Darbinian, J.; Go, A.S. Risk factors for end-stage renal disease: 25-year follow-up. Arch. Intern. Med. 2009, 169, 342–350. [Google Scholar] [CrossRef]
- Luyckx, V.A.; Tuttle, K.R.; Garcia-Garcia, G.; Gharbi, M.B.; Heerspink, H.J.L.; Johnson, D.W.; Liu, Z.H.; Massy, Z.A.; Moe, O.; Nelson, R.G.; et al. Reducing major risk factors for chronic kidney disease. Kidney Int. Suppl. 2017, 7, 71–87. [Google Scholar] [CrossRef]
- Tuttle, K.R.; Alicic, R.Z.; Duru, O.K.; Jones, C.R.; Daratha, K.B.; Nicholas, S.B.; McPherson, S.M.; Neumiller, J.J.; Bell, D.S.; Mangione, C.M.; et al. Clinical characteristics of and risk factors for chronic kidney disease among adults and children: An analysis of the CURE-CKD registry. JAMA Netw. Open. 2019, 2, e1918169. [Google Scholar] [CrossRef]
- Tsai, W.C.; Wu, H.Y.; Peng, Y.S.; Ko, M.J.; Wu, M.S.; Hung, K.Y.; Wu, K.D.; Chu, T.S.; Chien, K.L. Risk Factors for Development and Progression of Chronic Kidney Disease: A Systematic Review and Exploratory Meta-Analysis. Medicine 2016, 95, e3013. [Google Scholar] [CrossRef]
- Ren, L.L.; Li, X.J.; Duan, T.T.; Li, Z.H.; Yang, J.Z.; Zhang, Y.M.; Zou, L.; Miao, H.; Zhao, Y.Y. Transforming growth factor-beta signaling: From tissue fibrosis to therapeutic opportunities. Chem. Biol. Interact. 2023, 369, 110289. [Google Scholar] [CrossRef]
- Wang, Y.N.; Zhang, Z.H.; Liu, H.J.; Guo, Z.Y.; Zou, L.; Zhang, Y.M.; Zhao, Y.Y. Integrative phosphatidylcholine metabolism through phospholipase A(2) in rats with chronic kidney disease. Acta Pharmacol. Sin. 2023, 44, 393–405. [Google Scholar] [CrossRef]
- Hayat, R.; Manzoor, M.; Hussain, A. Wnt signaling pathway: A comprehensive review. Cell. Biol. Int. 2022, 46, 863–877. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/beta-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal. Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Nusse, R.; Clevers, H. Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell. 2017, 169, 985–999. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, C.J.; Liu, Y. Wnt Signaling in Kidney Development and Disease. Prog. Mol. Biol. Transl. Sci. 2018, 153, 181–207. [Google Scholar]
- Hu, L.; Ding, M.; He, W. Emerging Therapeutic Strategies for Attenuating Tubular EMT and Kidney Fibrosis by Targeting Wnt/beta-Catenin Signaling. Front. Pharmacol. 2021, 12, 830340. [Google Scholar] [CrossRef]
- Wang, M.; Chen, D.Q.; Chen, L.; Cao, G.; Zhao, H.; Liu, D.; Vaziri, N.D.; Guo, Y.; Zhao, Y.Y. Novel inhibitors of the cellular renin-angiotensin system components, poricoic acids, target Smad3 phosphorylation and Wnt/beta-catenin pathway against renal fibrosis. Br. J. Pharmacol. 2018, 175, 2689–2708. [Google Scholar] [CrossRef]
- Tan, R.J.; Zhou, D.; Zhou, L.; Liu, Y. Wnt/beta-catenin signaling and kidney fibrosis. Kidney Int. Suppl. 2014, 4, 84–90. [Google Scholar] [CrossRef]
- Meng, P.; Zhu, M.; Ling, X.; Zhou, L. Wnt signaling in kidney: The initiator or terminator? J. Mol. Med. 2020, 98, 1511–1523. [Google Scholar] [CrossRef]
- Zhou, D.; Li, Y.; Lin, L.; Zhou, L.; Igarashi, P.; Liu, Y. Tubule-specific ablation of endogenous beta-catenin aggravates acute kidney injury in mice. Kidney Int. 2012, 82, 537–547. [Google Scholar] [CrossRef]
- Lin, S.L.; Li, B.; Rao, S.; Yeo, E.J.; Hudson, T.E.; Nowlin, B.T.; Pei, H.; Chen, L.; Zheng, J.J.; Carroll, T.J.; et al. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc. Natl. Acad. Sci. USA 2010, 107, 4194–4199. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Tan, R.J.; Fu, H.; Liu, Y. Wnt/beta-catenin signaling in kidney injury and repair: A double-edged sword. Lab. Investig. 2016, 96, 156–167. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Dai, C.; Li, Y.; Zeng, G.; Monga, S.P.; Liu, Y. Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J. Am. Soc. Nephrol. 2009, 20, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Stolz, D.B.; Kiss, L.P.; Monga, S.P.; Holzman, L.B.; Liu, Y. Wnt/beta-catenin signaling promotes podocyte dysfunction and albuminuria. J. Am. Soc. Nephrol. 2009, 20, 1997–2008. [Google Scholar] [CrossRef]
- Surendran, K.; Schiavi, S.; Hruska, K.A. Wnt-dependent beta-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J. Am. Soc. Nephrol. 2005, 16, 2373–2384. [Google Scholar] [CrossRef]
- He, W.; Kang, Y.S.; Dai, C.; Liu, Y. Blockade of Wnt/beta-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J. Am. Soc. Nephrol. 2011, 22, 90–103. [Google Scholar] [CrossRef]
- Hao, S.; He, W.; Li, Y.; Ding, H.; Hou, Y.; Nie, J.; Hou, F.F.; Kahn, M.; Liu, Y. Targeted inhibition of beta-catenin/CBP signaling ameliorates renal interstitial fibrosis. J. Am. Soc. Nephrol. 2011, 22, 1642–1653. [Google Scholar] [CrossRef]
- Zuo, Y.; Liu, Y. New insights into the role and mechanism of Wnt/beta-catenin signalling in kidney fibrosis. Nephrology 2018, 23 (Suppl. 4), 38–43. [Google Scholar] [CrossRef]
- Li, S.S.; Sun, Q.; Hua, M.R.; Suo, P.; Chen, J.R.; Yu, X.Y.; Zhao, Y.Y. Targeting the Wnt/beta-Catenin Signaling Pathway as a Potential Therapeutic Strategy in Renal Tubulointerstitial Fibrosis. Front. Pharmacol. 2021, 12, 719880. [Google Scholar] [CrossRef]
- Giralt, I.; Gallo-Oller, G.; Navarro, N.; Zarzosa, P.; Pons, G.; Magdaleno, A.; Segura, M.F.; Sanchez de Toledo, J.; Moreno, L.; Gallego, S.; et al. Dickkopf proteins and their role in cancer: A family of Wnt antagonists with a dual role. Pharmaceuticals 2021, 14, 810. [Google Scholar] [CrossRef]
- Kim, J.; Han, W.; Park, T.; Kim, E.J.; Bang, I.; Lee, H.S.; Jeong, Y.; Roh, K.; Kim, J.; Kim, J.S.; et al. Sclerostin inhibits Wnt signaling through tandem interaction with two LRP6 ectodomains. Nat. Commun. 2020, 11, 5357. [Google Scholar] [CrossRef]
- Chu, H.Y.; Chen, Z.; Wang, L.; Zhang, Z.K.; Tan, X.; Liu, S.; Zhang, B.T.; Lu, A.; Yu, Y.; Zhang, G. Dickkopf-1: A Promising Target for Cancer Immunotherapy. Front. Immunol. 2021, 12, 658097. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Y.; Li, X.; Chen, L.; Wang, H.; Wu, J.; Zheng, J.; Wu, D. Characterization of the Kremen-binding site on Dkk1 and elucidation of the role of Kremen in Dkk-mediated Wnt antagonism. J. Biol. Chem. 2008, 283, 23371–23375. [Google Scholar] [CrossRef]
- Mishra, S.K.; Funair, L.; Cressley, A.; Gittes, G.K.; Burns, R.C. High-affinity Dkk1 receptor Kremen1 is internalized by clathrin-mediated endocytosis. PLoS ONE 2012, 7, e52190. [Google Scholar] [CrossRef]
- Kimura, H.; Fumoto, K.; Shojima, K.; Nojima, S.; Osugi, Y.; Tomihara, H.; Eguchi, H.; Shintani, Y.; Endo, H.; Inoue, M.; et al. CKAP4 is a Dickkopf1 receptor and is involved in tumor progression. J. Clin. Investig. 2016, 126, 2689–2705. [Google Scholar] [CrossRef]
- Bhavanasi, D.; Speer, K.F.; Klein, P.S. CKAP4 is identified as a receptor for Dickkopf in cancer cells. J. Clin. Investig. 2016, 126, 2419–2421. [Google Scholar] [CrossRef]
- Niida, A.; Hiroko, T.; Kasai, M.; Furukawa, Y.; Nakamura, Y.; Suzuki, Y.; Sugano, S.; Akiyama, T. DKK1, a negative regulator of Wnt signaling, is a target of the beta-catenin/TCF pathway. Oncogene 2004, 23, 8520–8526. [Google Scholar] [CrossRef]
- Fang, Y.; Ginsberg, C.; Seifert, M.; Agapova, O.; Sugatani, T.; Register, T.C.; Freedman, B.I.; Monier-Faugere, M.C.; Malluche, H.; Hruska, K.A. CKD-induced wingless/integration1 inhibitors and phosphorus cause the CKD-mineral and bone disorder. J. Am. Soc. Nephrol. 2014, 25, 1760–1773. [Google Scholar] [CrossRef]
- Lin, C.L.; Wang, J.Y.; Ko, J.Y.; Huang, Y.T.; Kuo, Y.H.; Wang, F.S. Dickkopf-1 promotes hyperglycemia-induced accumulation of mesangial matrix and renal dysfunction. J. Am. Soc. Nephrol. 2010, 21, 124–135. [Google Scholar] [CrossRef]
- Behets, G.J.; Viaene, L.; Meijers, B.; Blocki, F.; Brandenburg, V.M.; Verhulst, A.; D’Haese, P.C.; Evenepoel, P. Circulating levels of sclerostin but not DKK1 associate with laboratory parameters of CKD-MBD. PLoS ONE 2017, 12, e0176411. [Google Scholar] [CrossRef]
- Hamada-Ode, K.; Taniguchi, Y.; Shimamura, Y.; Fujimoto, S.; Terada, Y. Serum dickkopf-related protein 1 and sclerostin may predict the progression of chronic kidney disease in Japanese patients. Nephrol. Dial. Transplant. 2019, 34, 1426–1427. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhou, H.; Zhang, X.; Ma, X.; Liu, Z.; Liu, X. Elevated levels of Dickkopf-1 are associated with beta-catenin accumulation and poor prognosis in patients with chondrosarcoma. PLoS ONE 2014, 9, e105414. [Google Scholar]
- Yu, B.; Yang, X.; Xu, Y.; Yao, G.; Shu, H.; Lin, B.; Hood, L.; Wang, H.; Yang, S.; Gu, J.; et al. Elevated expression of DKK1 is associated with cytoplasmic/nuclear beta-catenin accumulation and poor prognosis in hepatocellular carcinomas. J. Hepatol. 2009, 50, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Lin, H.M.; Broering, R.; Shi, X.D.; Yu, X.H.; Xu, L.B.; Wu, W.R.; Liu, C. Dickkopf-1 contributes to hepatocellular carcinoma tumorigenesis by activating the Wnt/beta-catenin signaling pathway. Signal Transduct. Target. Ther. 2019, 4, 54. [Google Scholar] [CrossRef]
- Zhu, G.; Song, J.; Chen, W.; Yuan, D.; Wang, W.; Chen, X.; Liu, H.; Su, H.; Zhu, J. Expression and Role of Dickkopf-1 (Dkk1) in Tumors: From the Cells to the Patients. Cancer Manag. Res. 2021, 13, 659–675. [Google Scholar] [CrossRef]
- Lin, C.L.; Wang, F.S.; Hsu, Y.C.; Chen, C.N.; Tseng, M.J.; Saleem, M.A.; Chang, P.J.; Wang, J.Y. Modulation of notch-1 signaling alleviates vascular endothelial growth factor-mediated diabetic nephropathy. Diabetes 2010, 59, 1915–1925. [Google Scholar] [CrossRef]
- Griffin, K.A.; Picken, M.; Bidani, A.K. Method of renal mass reduction is a critical modulator of subsequent hypertension and glomerular injury. J. Am. Soc. Nephrol. 1994, 4, 2023–2031. [Google Scholar] [CrossRef]
- Ware, K.; Brodsky, P.; Satoskar, A.A.; Nadasdy, T.; Nadasdy, G.; Wu, H.; Rovin, B.H.; Bhatt, U.; Von Visger, J.; Hebert, L.A.; et al. Warfarin-related nephropathy modeled by nephron reduction and excessive anticoagulation. J. Am. Soc. Nephrol. 2011, 22, 1856–1862. [Google Scholar] [CrossRef]
- Lin, C.L.; Hsu, Y.C.; Huang, Y.T.; Shih, Y.H.; Wang, C.J.; Chiang, W.C.; Chang, P.J. A KDM6A-KLF10 reinforcing feedback mechanism aggravates diabetic podocyte dysfunction. EMBO Mol. Med. 2019, 11, e9828. [Google Scholar] [CrossRef]


| Variable | Total (n = 50) | ≥1526.4 pg/mL (n = 22) | <1526.4 pg/mL (n = 28) | p Value |
|---|---|---|---|---|
| Male, n (%) | 36 (72.0) | 13 (59.1) | 23 (82.1) | 0.113 |
| Age, year | 60.7 ± 13.5 | 63.0 ± 11.1 | 59.0 ± 15.1 | 0.308 |
| Body mass index, kg/m2 | 25.2 ± 3.6 | 25.3 ± 3.1 | 25.1 ± 4.0 | 0.814 |
| Diabetes, n (%) | 21 (42.0) | 10 (45.5) | 11 (39.3) | 0.775 |
| Hypertension, n (%) | 46 (92.0) | 19 (86.4) | 27 (96.4) | 0.308 |
| Baseline eGFR, mL/min/1.73 m2 | 17.1 ± 8.5 | 16.4 ± 9.0 | 17.7 ± 8.1 | 0.614 |
| Albumin, mg/dL | 3.67 ± 0.36 | 3.66 ± 0.41 | 3.72 ± 0.31 | 0.515 |
| Creatinine clearance rate, mL/min | 24.2 ± 13.5 | 22.2 ± 12.5 | 25.7 ± 14.3 | 0.374 |
| Blood urea nitrogen, mg/dL | 47.8 ± 23.6 | 54.8 ± 27.0 | 42.3 ± 19.3 | 0.063 |
| Hemoglobin, g/dL | 11.3 ± 2.1 | 10.8 ± 2.4 | 11.7 ± 1.8 | 0.105 |
| hs-CRP, mg/dL | 2.0 ± 3.1 | 2.1 ± 3.1 | 1.9 ± 3.2 | 0.838 |
| HbA1c, % | 6.1 ± 1.3 | 6.0 ± 1.0 | 6.2 ± 1.5 | 0.608 |
| Daily urine output, mL | 2189 ± 645 | 1944 ± 491 | 2382 ± 693 | 0.015 |
| Total protein (urine), mg/day | 75.9 ± 88.5 | 96.0 ± 100.9 | 60.1 ± 75.6 | 0.156 |
| Total protein intake, kg | 0.84 ± 0.25 | 0.89 ± 0.22 | 0.80 ± 0.27 | 0.196 |
| Outcome/Model | Adjusted HR (95% CI) | p Value |
|---|---|---|
| Dialysis | ||
| Unadjusted model | 1.046 (0.998–1.097) | 0.059 |
| Model 1 | 1.04 (0.99–1.10) | 0.145 |
| Model 2 | 1.07 (1.01–1.15) | 0.031 |
| Model 3 | 1.08 (1.01–1.15) | 0.026 |
| Model 4 | 1.08 (1.01–1.15) | 0.035 |
| Mortality | ||
| Unadjusted model | 1.05 (0.98–1.12) | 0.138 |
| Model 1 | 1.05 (0.96–1.15) | 0.257 |
| Model 2 | 1.06 (0.96–1.17) | 0.233 |
| Model 3 | 1.06 (0.96–1.17) | 0.231 |
| Model 4 | 1.06 (0.96–1.17) | 0.237 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, Y.-C.; Chang, C.-C.; Hsieh, C.-C.; Huang, Y.-T.; Shih, Y.-H.; Chang, H.-C.; Chang, P.-J.; Lin, C.-L. Dickkopf-1 Acts as a Profibrotic Mediator in Progressive Chronic Kidney Disease. Int. J. Mol. Sci. 2023, 24, 7679. https://doi.org/10.3390/ijms24087679
Hsu Y-C, Chang C-C, Hsieh C-C, Huang Y-T, Shih Y-H, Chang H-C, Chang P-J, Lin C-L. Dickkopf-1 Acts as a Profibrotic Mediator in Progressive Chronic Kidney Disease. International Journal of Molecular Sciences. 2023; 24(8):7679. https://doi.org/10.3390/ijms24087679
Chicago/Turabian StyleHsu, Yung-Chien, Cheng-Chih Chang, Ching-Chuan Hsieh, Yu-Ting Huang, Ya-Hsueh Shih, Hsiu-Ching Chang, Pey-Jium Chang, and Chun-Liang Lin. 2023. "Dickkopf-1 Acts as a Profibrotic Mediator in Progressive Chronic Kidney Disease" International Journal of Molecular Sciences 24, no. 8: 7679. https://doi.org/10.3390/ijms24087679
APA StyleHsu, Y.-C., Chang, C.-C., Hsieh, C.-C., Huang, Y.-T., Shih, Y.-H., Chang, H.-C., Chang, P.-J., & Lin, C.-L. (2023). Dickkopf-1 Acts as a Profibrotic Mediator in Progressive Chronic Kidney Disease. International Journal of Molecular Sciences, 24(8), 7679. https://doi.org/10.3390/ijms24087679






