Knockdown of BAP31 Overcomes Hepatocellular Carcinoma Doxorubicin Resistance through Downregulation of Survivin
Abstract
1. Introduction
2. Results
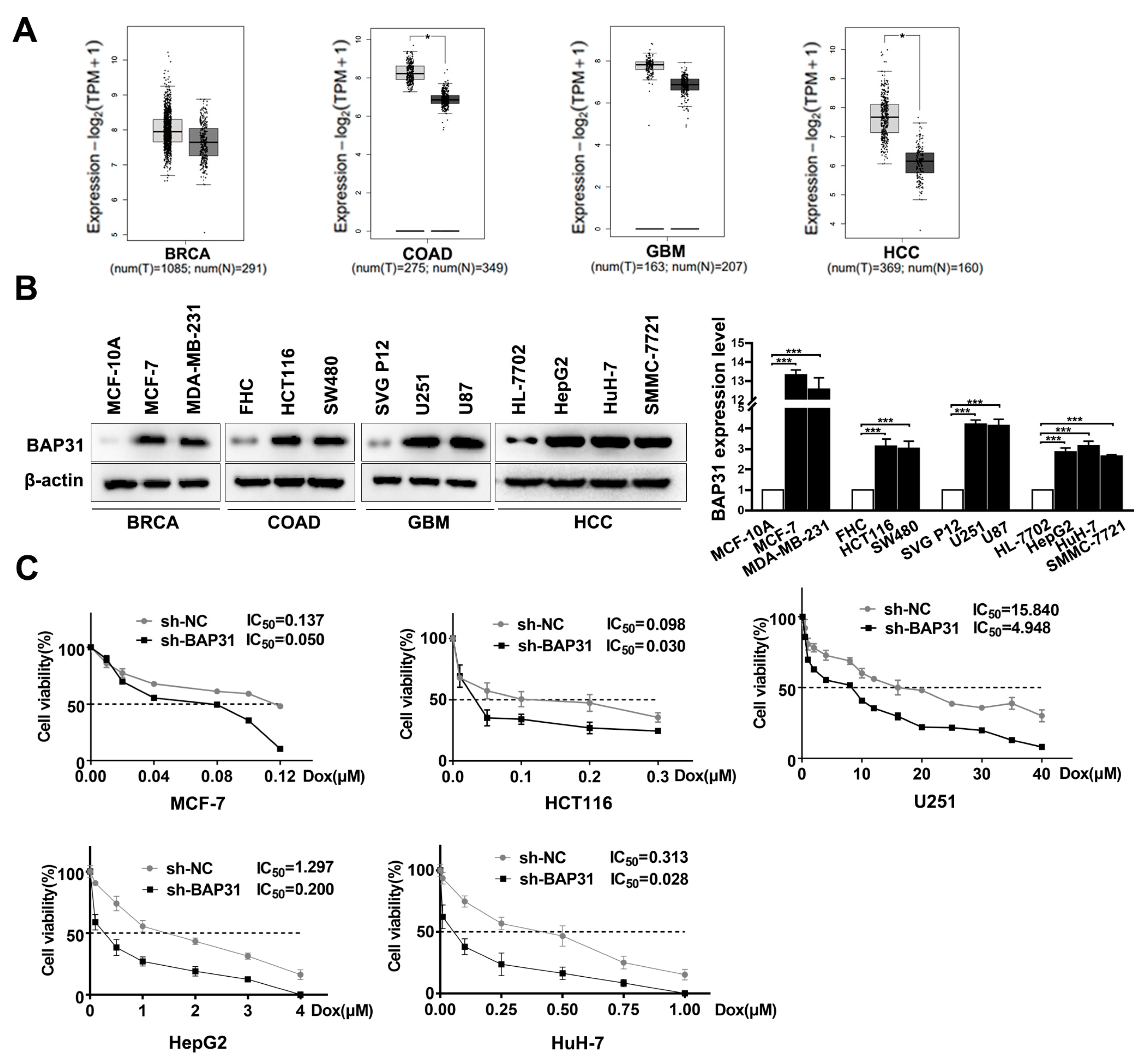
2.1. BAP31 Is Increased in Cancer Cells and Associated with Chemosensitivity to Dox
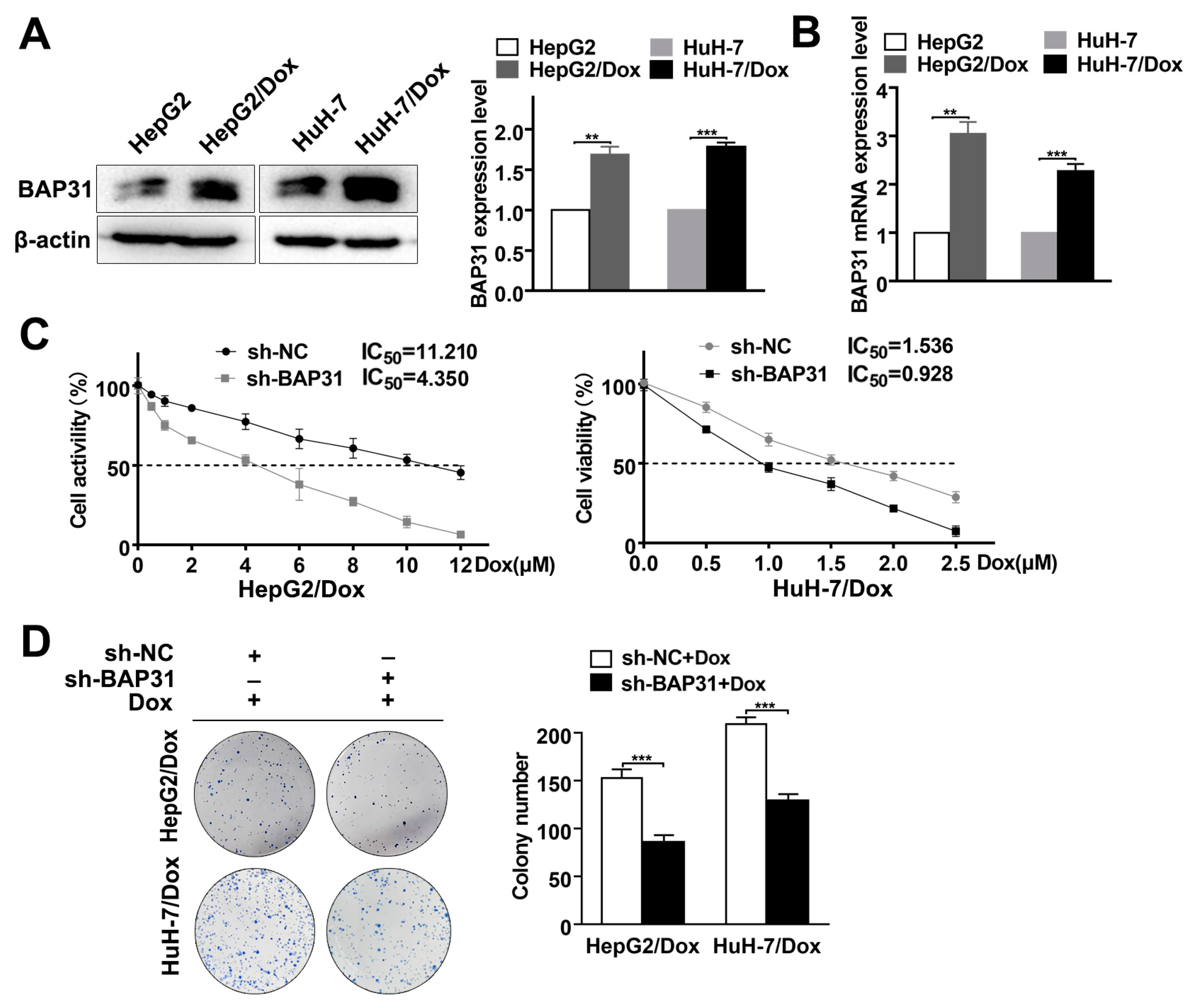
2.2. Knockdown of BAP31 Reduces Dox Resistance in HCC/Dox Cells
2.3. Knockdown of BAP31 Enhances Dox-Induced Apoptosis in HCC Cells
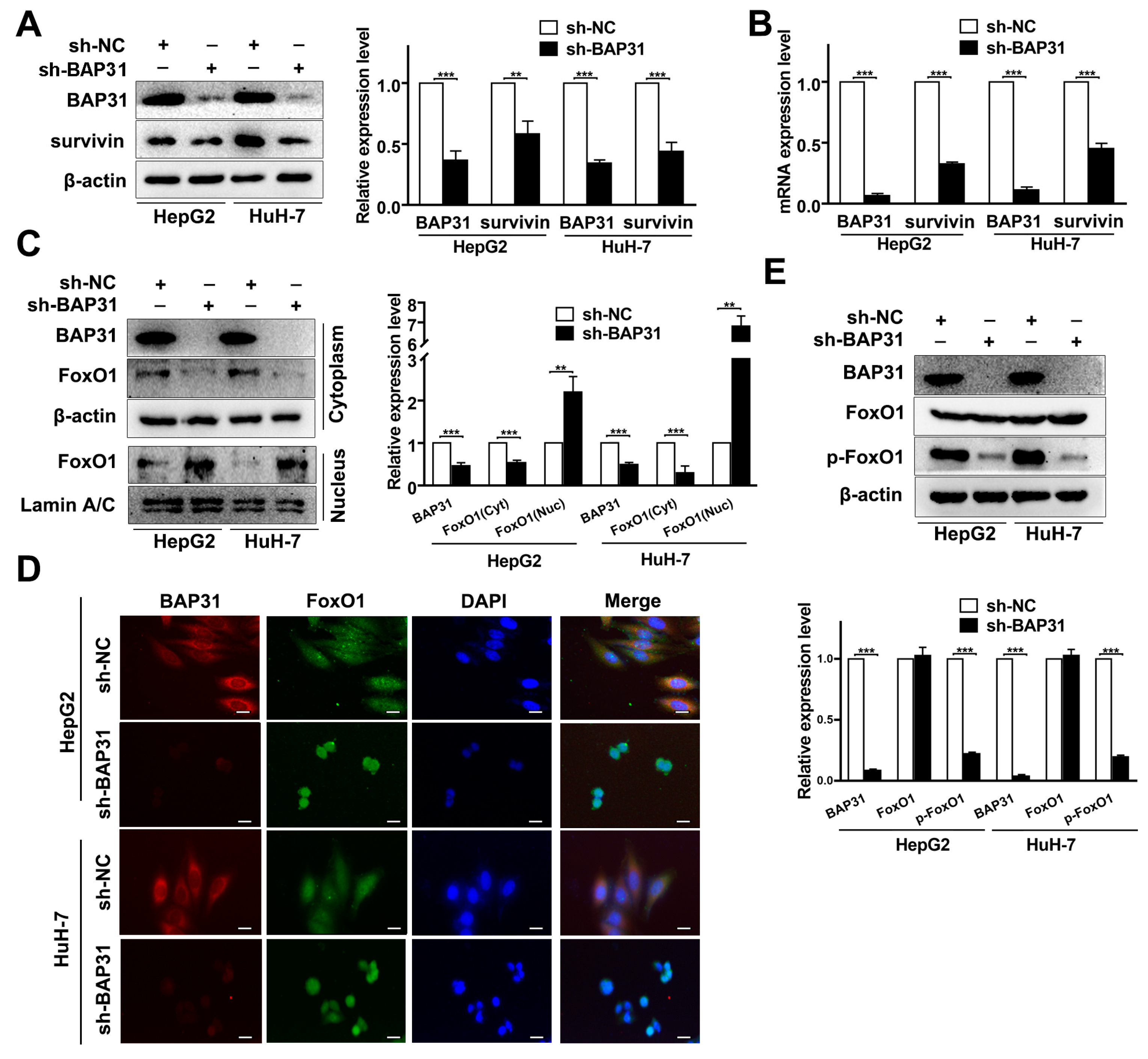
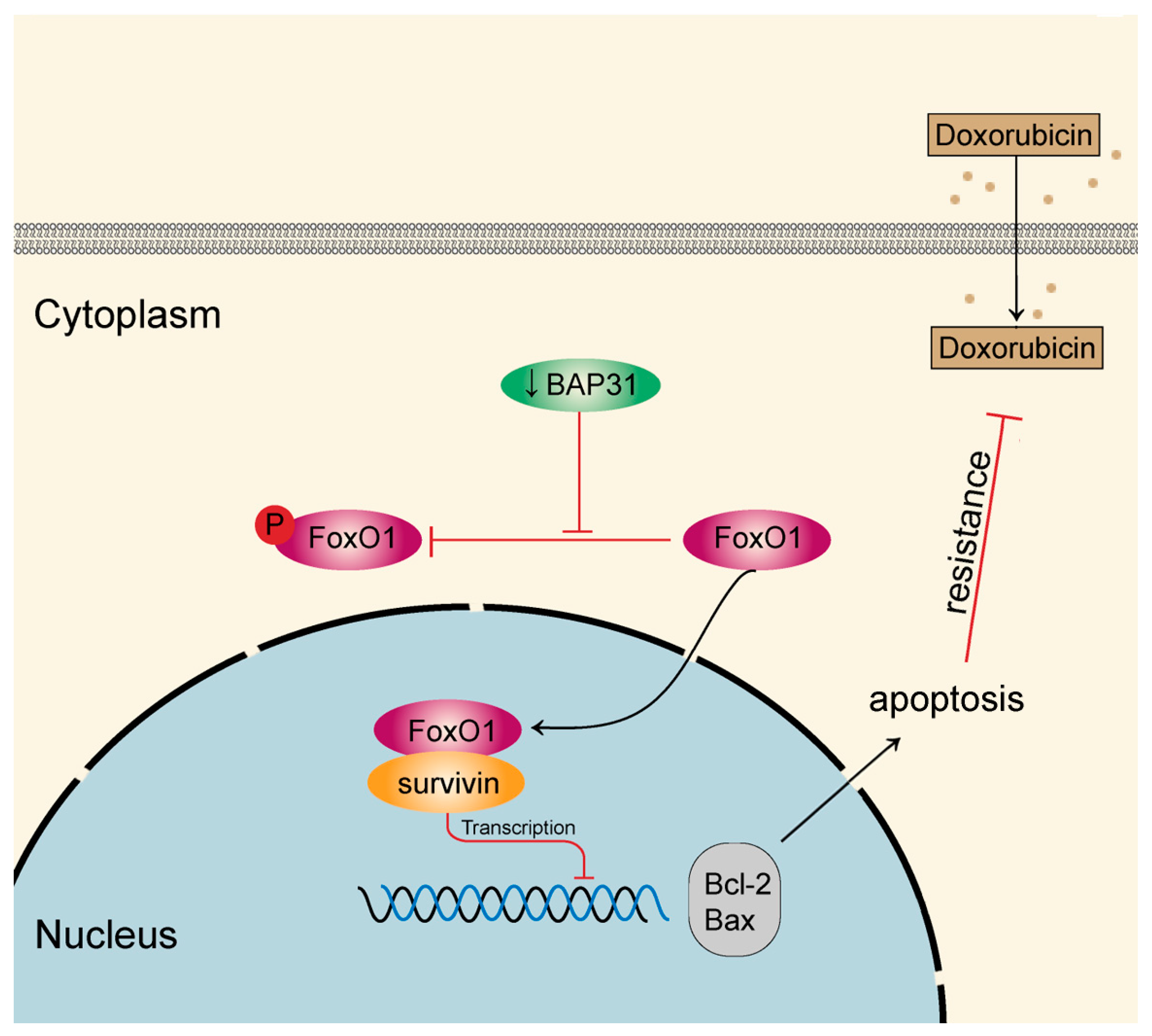
2.4. BAP31 Regulates Survivin through the Nucleus-Cytoplasm Translocation of FoxO1
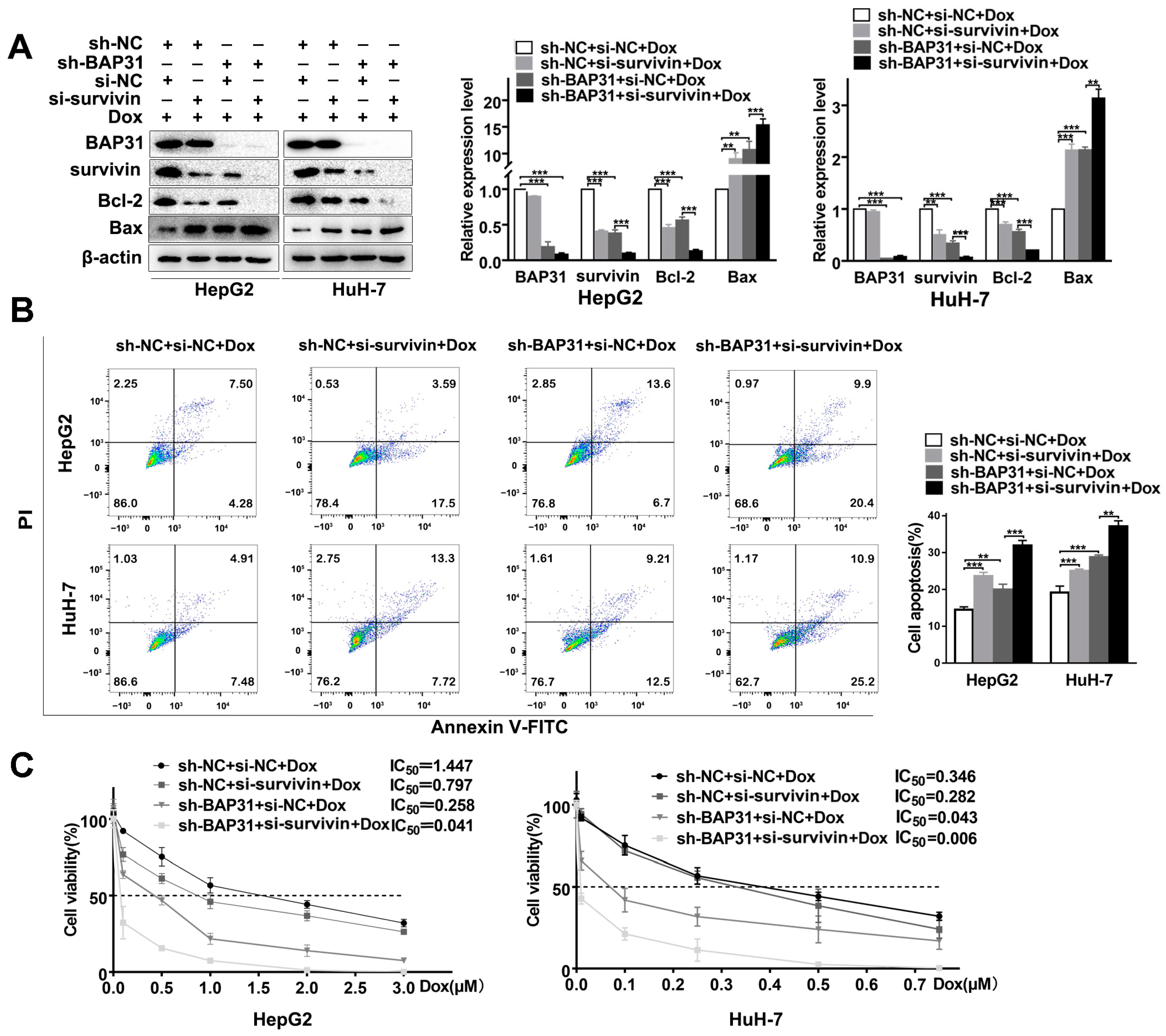
2.5. BAP31 and Survivin Have a Synergistic Effect on Chemosensitivity of Dox in HCC Cells
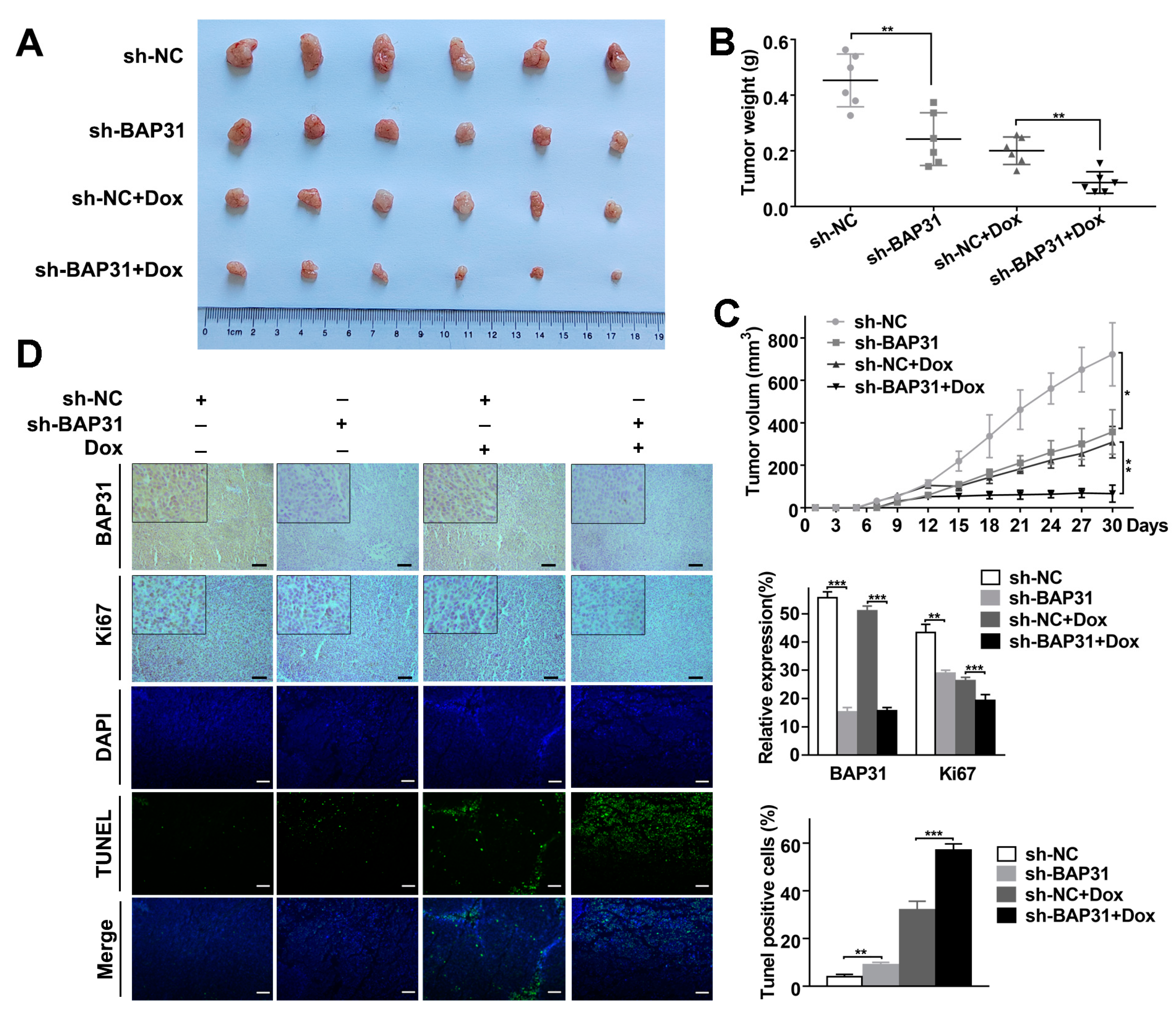
2.6. Knockdown of BAP31 Enhances the Antitumor Effect of Dox In Vivo
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Chemicals
4.2. Cell Viability Assay
4.3. Western Blot Analysis
4.4. qRT-PCR Assay
4.5. Flow Cytometry Analysis
4.6. Immunofluorescence Analysis
4.7. Animal Experiments
4.8. IHC and TUNEL Assay
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef]
- Salehan, M.R.; Morse, H.R. DNA damage repair and tolerance: A role in chemotherapeutic drug resistance. Br. J. Biomed. Sci. 2013, 70, 31–40. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef] [PubMed]
- Schmid, I.; von Schweinitz, D. Pediatric hepatocellular carcinoma: Challenges and solutions. J. Hepatocell. Carcinoma 2017, 4, 15–21. [Google Scholar] [CrossRef]
- Nicoletto, R.E.; Ofner, C.M., 3rd. Cytotoxic mechanisms of doxorubicin at clinically relevant concentrations in breast cancer cells. Cancer Chemother. Pharmacol. 2022, 89, 285–311. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, C.; He, R.; Zhou, M.; Liu, Y.; Guo, X.; Wang, M.; Zhu, F.; Qin, R.; Li, X. Danthron suppresses autophagy and sensitizes pancreatic cancer cells to doxorubicin. Toxicol. In Vitr. 2019, 54, 345–353. [Google Scholar] [CrossRef]
- Rivankar, S. An overview of doxorubicin formulations in cancer therapy. J. Cancer Res. Ther. 2014, 10, 853–858. [Google Scholar] [CrossRef]
- Kciuk, M.; Gielecinska, A.; Mujwar, S.; Kolat, D.; Kaluzinska-Kolat, Z.; Celik, I.; Kontek, R. Doxorubicin-An Agent with Multiple Mechanisms of Anticancer Activity. Cells 2023, 12, 659. [Google Scholar] [CrossRef]
- Shi, Y.; Moon, M.; Dawood, S.; McManus, B.; Liu, P.P. Mechanisms and management of doxorubicin cardiotoxicity. Herz 2011, 36, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Kitakata, H.; Endo, J.; Ikura, H.; Moriyama, H.; Shirakawa, K.; Katsumata, Y.; Sano, M. Therapeutic Targets for DOX-Induced Cardiomyopathy: Role of Apoptosis vs. Ferroptosis. Int. J. Mol. Sci. 2022, 23, 1414. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Heath-Engel, H.; Zhang, D.; Nguyen, N.; Thomas, D.Y.; Hanrahan, J.W.; Shore, G.C. BAP31 interacts with Sec61 translocons and promotes retrotranslocation of CFTRDeltaF508 via the derlin-1 complex. Cell 2008, 133, 1080–1092. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yu, J.; Ge, C.; Zhao, F.; Miao, C.; Jin, W.; Su, Y.; Geng, Q.; Chen, T.; Xie, H.; et al. B-Cell Receptor-Associated Protein 31 Promotes Metastasis via AKT/beta-Catenin/Snail Pathway in Hepatocellular Carcinoma. Front. Mol. Biosci. 2021, 8, 656151. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, D.; Yang, S.; Sun, Y.; Liu, Y.; Shi, J.; Hu, C.; Pan, J.; Liu, T.; Jin, B.; et al. BAP31 Promotes Tumor Cell Proliferation by Stabilizing SERPINE2 in Hepatocellular Carcinoma. Front. Cell Dev. Biol. 2020, 8, 607906. [Google Scholar] [CrossRef]
- Li, T.; Hao, Z.; Tang, Z.; Li, C.; Cheng, L.; Wang, T.; Zhu, X.; He, Y.; Huang, Y.; Wang, B. BAP31 Regulates Wnt Signaling to Modulate Cell Migration in Lung Cancer. Front. Oncol. 2022, 12, 859195. [Google Scholar] [CrossRef]
- Dang, E.; Yang, S.; Song, C.; Jiang, D.; Li, Z.; Fan, W.; Sun, Y.; Tao, L.; Wang, J.; Liu, T.; et al. BAP31, a newly defined cancer/testis antigen, regulates proliferation, migration, and invasion to promote cervical cancer progression. Cell Death Dis. 2018, 9, 791. [Google Scholar] [CrossRef]
- Sun, M.; Liu, X.; Wei, W.; Ge, N.; Luo, S.; Shen, S.; Ge, R. BAP31 Promotes Proliferation, Invasion, and Metastasis of Liver Cancer Cells via Activating PI3K/AKT Pathway. J. Healthc. Eng. 2022, 2022, 7686728. [Google Scholar] [CrossRef]
- Machihara, K.; Namba, T. BAP31 Inhibits Cell Adaptation to ER Stress Conditions, Negatively Regulating Autophagy Induction by Interaction with STX17. Cells 2019, 8, 1350. [Google Scholar] [CrossRef]
- Liang, H.; Dong, J.; Cheng, Z.; Li, Q.; Feng, D.; Ling, B. B-cell receptor-associated protein 31 promotes migration and invasion in ovarian cancer cells. Exp. Ther. Med. 2021, 22, 858. [Google Scholar] [CrossRef]
- Chen, J.; Guo, H.; Jiang, H.; Namusamba, M.; Wang, C.; Lan, T.; Wang, T.; Wang, B. A BAP31 intrabody induces gastric cancer cell death by inhibiting p27(kip1) proteasome degradation. Int. J. Cancer 2019, 144, 2051–2062. [Google Scholar] [CrossRef] [PubMed]
- Mikubo, M.; Inoue, Y.; Liu, G.; Tsao, M.S. Mechanism of Drug Tolerant Persister Cancer Cells: The Landscape and Clinical Implication for Therapy. J. Thorac. Oncol. 2021, 16, 1798–1809. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Bai, L.; Liao, W.; Feng, M.; Zhang, M.; Wu, Q.; Zhou, K.; Wen, F.; Lei, W.; Zhang, N.; et al. The role of non-apoptotic cell death in the treatment and drug-resistance of digestive tumors. Exp. Cell Res. 2021, 405, 112678. [Google Scholar] [CrossRef] [PubMed]
- Bergman, P.J.; Harris, D. Radioresistance, chemoresistance, and apoptosis resistance. The past, present, and future. Vet. Clin. N. Am. Small Anim. Pract. 1997, 27, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, W.; Yang, J.; Edwards, M.; Jiang, S. Survivin as a biological biomarker for diagnosis and therapy. Expert Opin. Biol. Ther. 2021, 21, 1429–1441. [Google Scholar] [CrossRef]
- Warrier, N.M.; Krishnan, R.K.; Prabhu, V.; Hariharapura, R.C.; Agarwal, P.; Kumar, P. Survivin Inhibition by Piperine Sensitizes Glioblastoma Cancer Stem Cells and Leads to Better Drug Response. Int. J. Mol. Sci. 2022, 23, 7604. [Google Scholar] [CrossRef]
- Sun, X.P.; Dong, X.; Lin, L.; Jiang, X.; Wei, Z.; Zhai, B.; Sun, B.; Zhang, Q.; Wang, X.; Jiang, H.; et al. Up-regulation of survivin by AKT and hypoxia-inducible factor 1alpha contributes to cisplatin resistance in gastric cancer. FEBS J. 2014, 281, 115–128. [Google Scholar] [CrossRef]
- Nan, X.W.; Gong, L.H.; Chen, X.; Zhou, H.H.; Ye, P.P.; Yang, Y.; Xing, Z.H.; Wei, M.N.; Li, Y.; Wang, S.T.; et al. Survivin Promotes Piperlongumine Resistance in Ovarian Cancer. Front. Oncol. 2019, 9, 1345. [Google Scholar] [CrossRef]
- Ku, J.H.; Seo, S.Y.; Kwak, C.; Kim, H.H. The role of survivin and Bcl-2 in zinc-induced apoptosis in prostate cancer cells. Urol. Oncol. 2012, 30, 562–568. [Google Scholar] [CrossRef]
- Meredith, A.M.; Dass, C.R. Increasing role of the cancer chemotherapeutic doxorubicin in cellular metabolism. J. Pharm. Pharmacol. 2016, 68, 729–741. [Google Scholar] [CrossRef]
- Zhao, Y.; Huan, M.L.; Liu, M.; Cheng, Y.; Sun, Y.; Cui, H.; Liu, D.Z.; Mei, Q.B.; Zhou, S.Y. Doxorubicin and resveratrol co-delivery nanoparticle to overcome doxorubicin resistance. Sci. Rep. 2016, 6, 35267. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Zhang, Y.; Cao, P. Inhibition of BAP31 expression inhibits cervical cancer progression by suppressing metastasis and inducing intrinsic and extrinsic apoptosis. Biochem. Biophys. Res. Commun. 2019, 508, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Voss, A.K.; Strasser, A. The essentials of developmental apoptosis. F1000Research 2020, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- Goldar, S.; Khaniani, M.S.; Derakhshan, S.M.; Baradaran, B. Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac. J. Cancer Prev. 2015, 16, 2129–2144. [Google Scholar] [CrossRef] [PubMed]
- Quistgaard, E.M. BAP31: Physiological functions and roles in disease. Biochimie 2021, 186, 105–129. [Google Scholar] [CrossRef]
- Yan, D.; An, G.; Kuo, M.T. C-Jun N-terminal kinase signalling pathway in response to cisplatin. J. Cell Mol. Med. 2016, 20, 2013–2019. [Google Scholar] [CrossRef]
- Al-Odat, O.S.; Guirguis, D.A.; Schmalbach, N.K.; Yao, G.; Budak-Alpdogan, T.; Jonnalagadda, S.C.; Pandey, M.K. Autophagy and Apoptosis: Current Challenges of Treatment and Drug Resistance in Multiple Myeloma. Int. J. Mol. Sci. 2022, 24, 644. [Google Scholar] [CrossRef]
- Sun, C.; Gao, W.; Liu, J.; Cheng, H.; Hao, J. FGL1 regulates acquired resistance to Gefitinib by inhibiting apoptosis in non-small cell lung cancer. Respir. Res. 2020, 21, 210. [Google Scholar] [CrossRef]
- Altieri, D.C. Targeting survivin in cancer. Cancer Lett. 2013, 332, 225–228. [Google Scholar] [CrossRef]
- Liu, X.; Jiao, K.; Jia, C.C.; Li, G.X.; Yuan, Q.; Xu, J.K.; Hou, Y.; Wang, B. BAP31 regulates IRAK1-dependent neuroinflammation in microglia. J. Neuroinflamm. 2019, 16, 281. [Google Scholar] [CrossRef]
- Oh, J.; Lee, B.S.; Lim, G.; Lim, H.; Lee, C.J.; Park, S.; Lee, S.H.; Chung, J.H.; Kang, S.M. Atorvastatin protects cardiomyocyte from doxorubicin toxicity by modulating survivin expression through FOXO1 inhibition. J. Mol. Cell Cardiol. 2020, 138, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Guha, M.; Plescia, J.; Leav, I.; Li, J.; Languino, L.R.; Altieri, D.C. Endogenous tumor suppression mediated by PTEN involves survivin gene silencing. Cancer Res. 2009, 69, 4954–4958. [Google Scholar] [CrossRef] [PubMed]
- Tabata, M.; Tsubaki, M.; Takeda, T.; Tateishi, K.; Tsurushima, K.; Imano, M.; Satou, T.; Ishizaka, T.; Nishida, S. Dasatinib reverses drug resistance by downregulating MDR1 and Survivin in Burkitt lymphoma cells. BMC Complement. Med. Ther. 2020, 20, 84. [Google Scholar] [CrossRef] [PubMed]
- Nestal de Moraes, G.; Vasconcelos, F.C.; Delbue, D.; Mognol, G.P.; Sternberg, C.; Viola, J.P.; Maia, R.C. Doxorubicin induces cell death in breast cancer cells regardless of Survivin and XIAP expression levels. Eur. J. Cell Biol. 2013, 92, 247–256. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhang, Q.; Wang, C.; Yang, J.; Yang, S.; Wang, T.; Wang, B. Knockdown of BAP31 Overcomes Hepatocellular Carcinoma Doxorubicin Resistance through Downregulation of Survivin. Int. J. Mol. Sci. 2023, 24, 7622. https://doi.org/10.3390/ijms24087622
Liu J, Zhang Q, Wang C, Yang J, Yang S, Wang T, Wang B. Knockdown of BAP31 Overcomes Hepatocellular Carcinoma Doxorubicin Resistance through Downregulation of Survivin. International Journal of Molecular Sciences. 2023; 24(8):7622. https://doi.org/10.3390/ijms24087622
Chicago/Turabian StyleLiu, Jingjing, Qi Zhang, Changli Wang, Jiaying Yang, Sheng Yang, Tianyi Wang, and Bing Wang. 2023. "Knockdown of BAP31 Overcomes Hepatocellular Carcinoma Doxorubicin Resistance through Downregulation of Survivin" International Journal of Molecular Sciences 24, no. 8: 7622. https://doi.org/10.3390/ijms24087622
APA StyleLiu, J., Zhang, Q., Wang, C., Yang, J., Yang, S., Wang, T., & Wang, B. (2023). Knockdown of BAP31 Overcomes Hepatocellular Carcinoma Doxorubicin Resistance through Downregulation of Survivin. International Journal of Molecular Sciences, 24(8), 7622. https://doi.org/10.3390/ijms24087622




