Metabolic Reprogramming of HCC: A New Microenvironment for Immune Responses
Abstract
1. Introduction
2. The Immune Landscape of HCC
3. A Comprehensive Picture of Liver Cancer Cell Metabolism
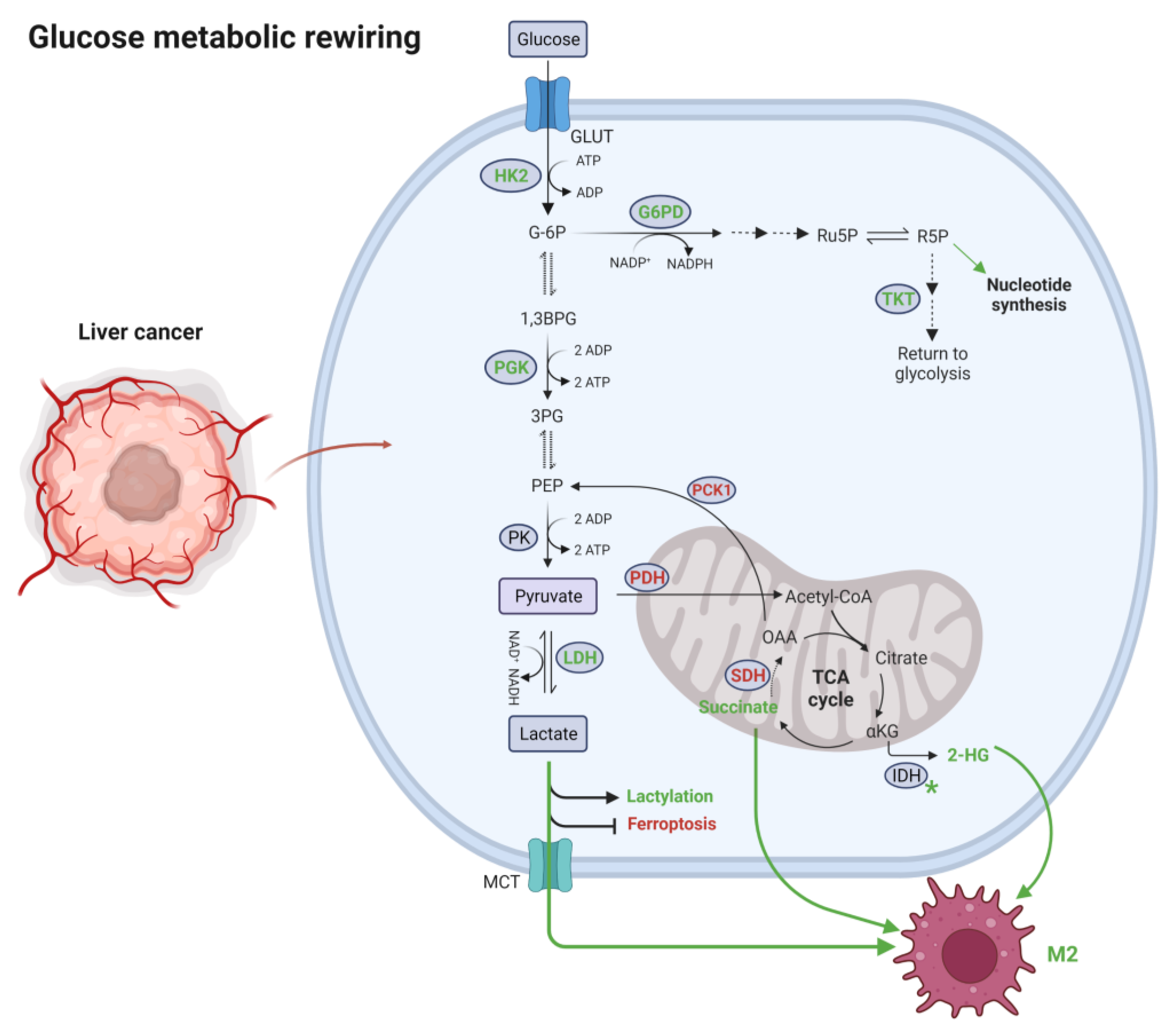
3.1. Alteration of Glucose Metabolism
3.1.1. Glycolysis and Gluconeogenesis
3.1.2. Pentose Phosphate Pathway
3.1.3. Tricarboxylic Acid Cycle
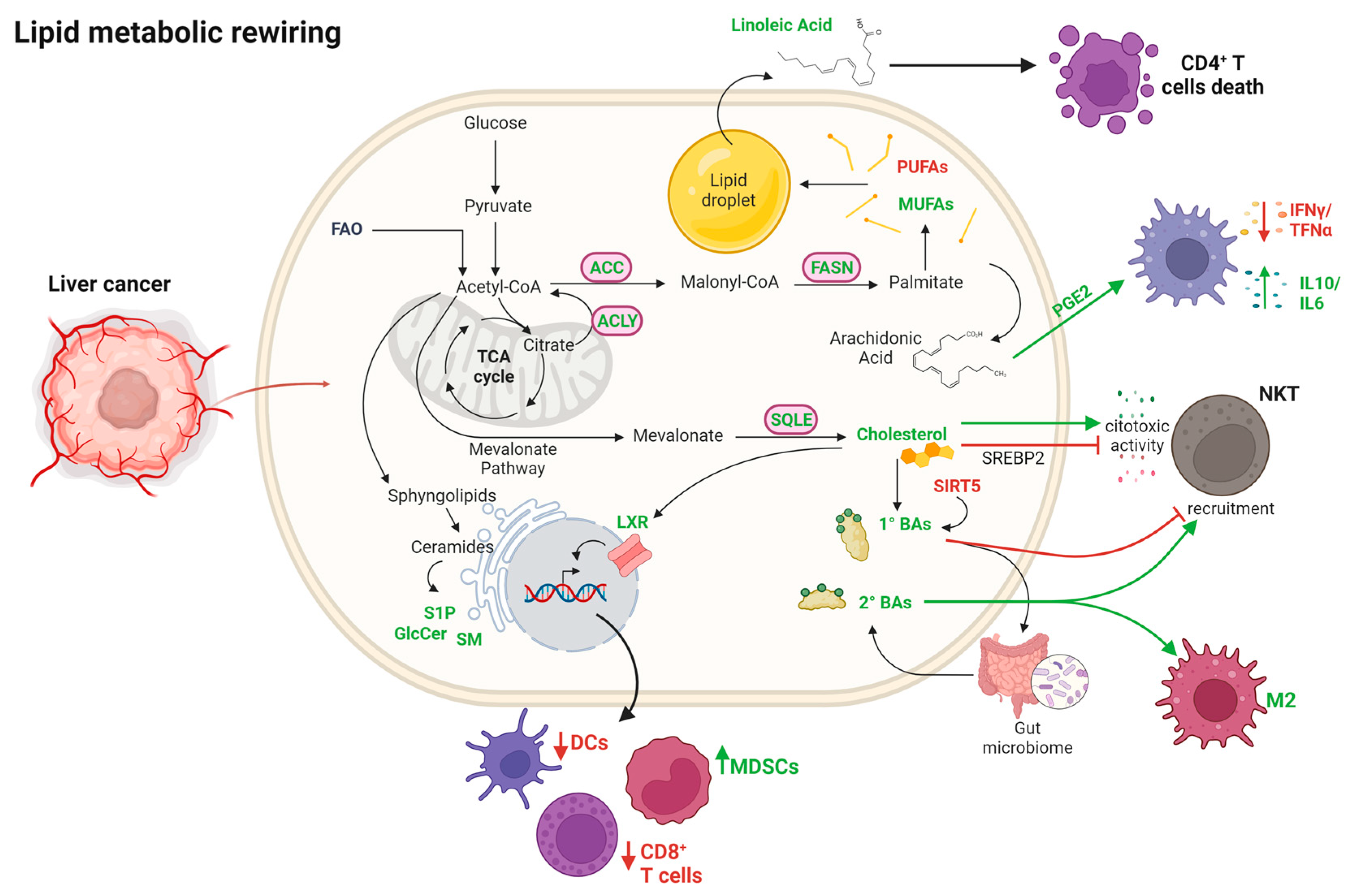
3.2. Alteration of Fatty Acid Metabolism
3.2.1. Fatty Acid Synthesis
3.2.2. MUFAs and PUFAs
3.2.3. Cholesterol and Bile Acids
3.2.4. Sphingolipids
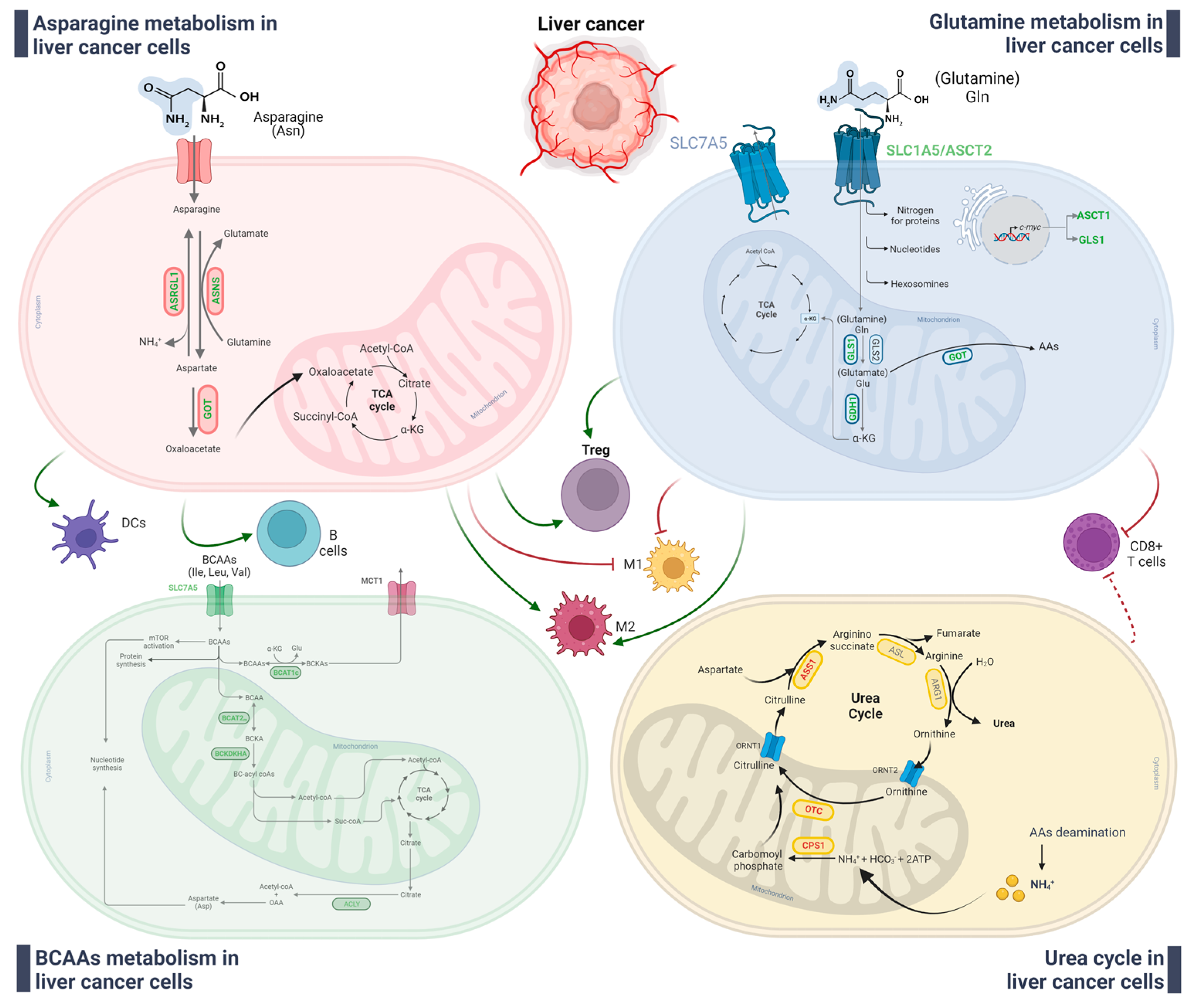
3.3. Alteration of Amino Acid and Glutamine Metabolisms
3.3.1. Glutamine Metabolism
3.3.2. Urea Cycle
3.3.3. Branched-Chain Amino Acid
3.3.4. Asparagine Metabolism
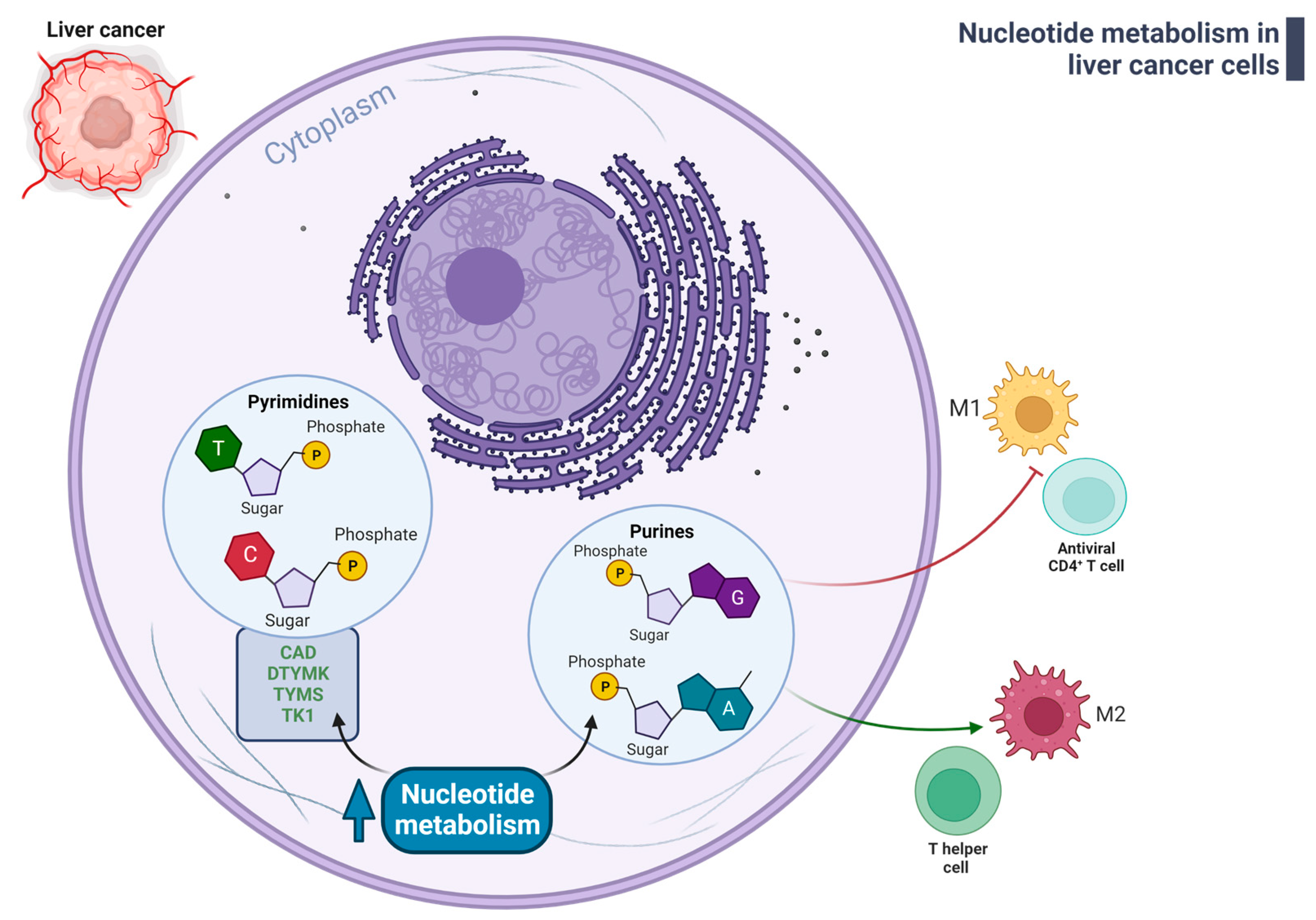
3.4. Nucleotide Metabolism
4. Metabolic Changes as a Result of HCC Etiology
5. Effect of HCC Metabolic Reprogramming on Immunity
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ringelhan, M.; Pfister, D.; O’Connor, T.; Pikarsky, E.; Heikenwalder, M. The Immunology of Hepatocellular Carcinoma. Nat. Immunol. 2018, 19, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, E.; Sarkar, D. Emerging Therapies for Hepatocellular Carcinoma (HCC). Cancers 2022, 14, 2798. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular Carcinoma. Nat. Rev. Dis. Prim. 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Meyer, T.; Sapisochin, G.; Salem, R.; Saborowski, A. Hepatocellular Carcinoma. Lancet 2022, 400, 1345–1362. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Tacke, F.; Arrese, M.; Chander Sharma, B.; Mostafa, I.; Bugianesi, E.; Wai-Sun Wong, V.; Yilmaz, Y.; George, J.; Fan, J.; et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2019, 69, 2672–2682. [Google Scholar] [CrossRef]
- Calderaro, J.; Couchy, G.; Imbeaud, S.; Amaddeo, G.; Letouzé, E.; Blanc, J.F.; Laurent, C.; Hajji, Y.; Azoulay, D.; Bioulac-Sage, P.; et al. Histological Subtypes of Hepatocellular Carcinoma Are Related to Gene Mutations and Molecular Tumour Classification. J. Hepatol. 2017, 67, 727–738. [Google Scholar] [CrossRef]
- Cucarull, B.; Tutusaus, A.; Rider, P.; Hernáez-Alsina, T.; Cuño, C.; de Frutos, P.G.; Colell, A.; Marí, M.; Morales, A. Hepatocellular Carcinoma: Molecular Pathogenesis and Therapeutic Advances. Cancers 2022, 14, 621. [Google Scholar] [CrossRef]
- Craig, A.J.; von Felden, J.; Garcia-Lezana, T.; Sarcognato, S.; Villanueva, A. Tumour Evolution in Hepatocellular Carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 139–152. [Google Scholar] [CrossRef]
- Lunt, S.Y.; Fendt, S.M. Metabolism—A Cornerstone of Cancer Initiation, Progression, Immune Evasion and Treatment Response. Curr. Opin. Syst. Biol. 2018, 8, 67–72. [Google Scholar] [CrossRef]
- Tenen, D.G.; Chai, L.; Tan, J.L. Metabolic Alterations and Vulnerabilities in Hepatocellular Carcinoma. Gastroenterol. Rep. 2021, 9, 1–13. [Google Scholar] [CrossRef]
- Sharma, A.; Seow, J.J.W.; Dutertre, C.A.; Pai, R.; Blériot, C.; Mishra, A.; Wong, R.M.M.; Singh, G.S.N.; Sudhagar, S.; Khalilnezhad, S.; et al. Onco-Fetal Reprogramming of Endothelial Cells Drives Immunosuppressive Macrophages in Hepatocellular Carcinoma. Cell 2020, 183, 377–394. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Zucman-Rossi, J.; Pikarsky, E.; Sangro, B.; Schwartz, M.; Sherman, M.; Gores, G. Hepatocellular Carcinoma. Nat. Rev. Dis. Prim. 2016, 2, 16018. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Gea, V.; Toffanin, S.; Friedman, S.L.; Llovet, J.M. Role of the Microenvironment in the Pathogenesis and Treatment of Hepatocellular Carcinoma. Gastroenterology 2013, 144, 512–527. [Google Scholar] [CrossRef] [PubMed]
- Fattovich, G.; Stroffolini, T.; Zagni, I.; Donato, F. Hepatocellular Carcinoma in Cirrhosis: Incidence and Risk Factors. Gastroenterology 2004, 127 (Suppl. S1), S35–S50. [Google Scholar] [CrossRef]
- Cannito, S.; Dianzani, U.; Parola, M.; Albano, E.; Sutti, S. Inflammatory Processes Involved in NASH-Related Hepatocellular Carcinoma. Biosci. Rep. 2023, 43, BSR20221271. [Google Scholar] [CrossRef]
- Sutti, S.; Albano, E. Adaptive Immunity: An Emerging Player in the Progression of NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 81–92. [Google Scholar] [CrossRef]
- Sprinzl, M.F.; Reisinger, F.; Puschnik, A.; Ringelhan, M.; Ackermann, K.; Hartmann, D.; Schiemann, M.; Weinmann, A.; Galle, P.R.; Schuchmann, M.; et al. Sorafenib Perpetuates Cellular Anticancer Effector Functions by Modulating the Crosstalk between Macrophages and Natural Killer Cells. Hepatology 2013, 57, 2358–2368. [Google Scholar] [CrossRef]
- Pfister, D.; Núñez, N.G.; Pinyol, R.; Govaere, O.; Pinter, M.; Szydlowska, M.; Gupta, R.; Qiu, M.; Deczkowska, A.; Weiner, A.; et al. NASH Limits Anti-Tumour Surveillance in Immunotherapy-Treated HCC. Nature 2021, 592, 450–456. [Google Scholar] [CrossRef]
- Kurebayashi, Y.; Ojima, H.; Tsujikawa, H.; Kubota, N.; Maehara, J.; Abe, Y.; Kitago, M.; Shinoda, M.; Kitagawa, Y.; Sakamoto, M. Landscape of Immune Microenvironment in Hepatocellular Carcinoma and Its Additional Impact on Histological and Molecular Classification. Hepatology 2018, 68, 1025–1041. [Google Scholar] [CrossRef]
- Chew, V.; Lai, L.; Pan, L.; Lim, C.J.; Li, J.; Ong, R.; Chua, C.; Leong, J.Y.; Lim, K.H.; Toh, H.C.; et al. Delineation of an Immunosuppressive Gradient in Hepatocellular Carcinoma Using High-Dimensional Proteomic and Transcriptomic Analyses. Proc. Natl. Acad. Sci. USA 2017, 114, E5900–E5909. [Google Scholar] [CrossRef]
- Sia, D.; Jiao, Y.; Martinez-Quetglas, I.; Kuchuk, O.; Villacorta-Martin, C.; Castro de Moura, M.; Putra, J.; Camprecios, G.; Bassaganyas, L.; Akers, N.; et al. Identification of an Immune-Specific Class of Hepatocellular Carcinoma, Based on Molecular Features. Gastroenterology 2017, 153, 812–826. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; He, Y.; Luo, N.; Patel, S.J.; Han, Y.; Gao, R.; Modak, M.; Carotta, S.; Haslinger, C.; Kind, D.; et al. Landscape and Dynamics of Single Immune Cells in Hepatocellular Carcinoma. Cell 2019, 179, 829–845.e20. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.; Lalor, P.F.; Adams, D.H. Liver Sinusoidal Endothelial Cells—Gatekeepers of Hepatic Immunity. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; He, J.; Pan, Q.Z.; Yang, J.; Zhao, J.; Zhang, Y.J.; Huang, Y.; Tang, Y.; Wang, Q.; He, J.; et al. Cancer-Associated Fibroblast-Mediated Cellular Crosstalk Supports Hepatocellular Carcinoma Progression. Hepatology 2021, 73, 1717–1735. [Google Scholar] [CrossRef]
- Chen, S.; Morine, Y.; Tokuda, K.; Yamada, S.; Saito, Y.; Nishi, M.; Ikemoto, T.; Shimada, M. Cancer-associated Fibroblast-induced M2-polarized Macrophages Promote Hepatocellular Carcinoma Progression via the Plasminogen Activator Inhibitor-1 Pathway. Int. J. Oncol. 2021, 59, 59. [Google Scholar] [CrossRef]
- Zhou, Z.; Hu, Y.; Wu, Y.; Qi, Q.; Wang, J.; Chen, L.; Wang, F. The Immunosuppressive Tumor Microenvironment in Hepatocellular Carcinoma-Current Situation and Outlook. Mol. Immunol. 2022, 151, 218–230. [Google Scholar] [CrossRef]
- Di Blasi, D.; Boldanova, T.; Mori, L.; Terracciano, L.; Heim, M.H.; De Libero, G. Unique T-Cell Populations Define Immune-Inflamed Hepatocellular Carcinoma. Cell. Mol. Gastroenterol. Hepatol. 2020, 9, 195–218. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-Derived Suppressor Cells as Regulators of the Immune System. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Ostrand-Rosenberg, S.; Fenselau, C. Myeloid-Derived Suppressor Cells: Immune-Suppressive Cells That Impair Antitumor Immunity and Are Sculpted by Their Environment. J. Immunol. 2018, 200, 422–431. [Google Scholar] [CrossRef]
- Li, Z.; Wu, T.; Zheng, B.; Chen, L. Individualized Precision Treatment: Targeting TAM in HCC. Cancer Lett. 2019, 458, 86–91. [Google Scholar] [CrossRef]
- Sica, A.; Schioppa, T.; Mantovani, A.; Allavena, P. Tumour-Associated Macrophages Are a Distinct M2 Polarised Population Promoting Tumour Progression: Potential Targets of Anti-Cancer Therapy. Eur. J. Cancer 2006, 42, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Sanchez, E.; Vaquero, J.; Férnandez-Barrena, M.G.; Lasarte, J.J.; Avila, M.A.; Sarobe, P.; Reig, M.; Calvo, M.; Fabregat, I. The TGF-β Pathway: A Pharmacological Target in Hepatocellular Carcinoma? Cancers 2021, 13, 3248. [Google Scholar] [CrossRef] [PubMed]
- Park, D.J.; Sung, P.S.; Lee, G.W.; Cho, S.; Kim, S.M.; Kang, B.Y.; Hur, W.; Yang, H.; Lee, S.K.; Lee, S.H.; et al. Preferential Expression of Programmed Death Ligand 1 Protein in Tumor-Associated Macrophages and Its Potential Role in Immunotherapy for Hepatocellular Carcinoma. Int. J. Mol. Sci. 2021, 22, 4710. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.Z.; Pollard, J.W. Macrophage Diversity Enhances Tumor Progression and Metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef]
- Wan, S.; Kuo, N.; Kryczek, I.; Zou, W.; Welling, T.H. Myeloid Cells in Hepatocellular Carcinoma. Hepatology 2015, 62, 1304–1312. [Google Scholar] [CrossRef]
- Wang, D.; Yang, L.; Yue, D.; Cao, L.; Li, L.; Wang, D.; Ping, Y.; Shen, Z.; Zheng, Y.; Wang, L.; et al. Macrophage-Derived CCL22 Promotes an Immunosuppressive Tumor Microenvironment via IL-8 in Malignant Pleural Effusion. Cancer Lett. 2019, 452, 244–253. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, Y.C.; Chen, Y.; Zhao, J.L.; Gao, C.C.; Han, H.; Liu, W.C.; Qin, H.Y. Crosstalk between Hepatic Tumor Cells and Macrophages via Wnt/β-Catenin Signaling Promotes M2-like Macrophage Polarization and Reinforces Tumor Malignant Behaviors. Cell Death Dis. 2018, 9, 793. [Google Scholar] [CrossRef]
- Peng, S.H.; Deng, H.; Yang, J.F.; Xie, P.P.; Li, C.; Li, H.; Feng, D.Y. Significance and Relationship between Infiltrating Inflammatory Cell and Tumor Angiogenesis in Hepatocellular Carcinoma Tissues. World J. Gastroenterol. 2005, 11, 6521–6524. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q.; Lou, Y.; Fu, Q.; Chen, Q.; Wei, T.; Yang, J.; Tang, J.; Wang, J.; Chen, Y.; et al. Hypoxia-Inducible Factor-1α/Interleukin-1β Signaling Enhances Hepatoma Epithelial-Mesenchymal Transition through Macrophages in a Hypoxic-Inflammatory Microenvironment. Hepatology 2018, 67, 1872–1889. [Google Scholar] [CrossRef]
- Peng, Z.P.; Jiang, Z.Z.; Guo, H.F.; Zhou, M.M.; Huang, Y.F.; Ning, W.R.; Huang, J.H.; Zheng, L.; Wu, Y. Glycolytic Activation of Monocytes Regulates the Accumulation and Function of Neutrophils in Human Hepatocellular Carcinoma. J. Hepatol. 2020, 73, 906–917. [Google Scholar] [CrossRef]
- Geh, D.; Leslie, J.; Rumney, R.; Reeves, H.L.; Bird, T.G.; Mann, D.A. Neutrophils as Potential Therapeutic Targets in Hepatocellular Carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Arvanitakis, K.; Mitroulis, I.; Germanidis, G. Tumor-Associated Neutrophils in Hepatocellular Carcinoma Pathogenesis, Prognosis, and Therapy. Cancers 2021, 13, 2899. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Zhang, H.; Zhou, J.; Wang, B.; Chen, Y.; Kong, Y.; Xie, X.; Wang, X.; Fei, R.; Wei, L.; et al. Peritumoural Neutrophils Negatively Regulate Adaptive Immunity via the PD-L1/PD-1 Signalling Pathway in Hepatocellular Carcinoma. J. Exp. Clin. Cancer Res. 2015, 34, 141. [Google Scholar] [CrossRef] [PubMed]
- Di Maira, G.; Foglia, B.; Napione, L.; Turato, C.; Maggiora, M.; Sutti, S.; Novo, E.; Alvaro, M.; Autelli, R.; Colombatto, S.; et al. Oncostatin M Is Overexpressed in NASH-Related Hepatocellular Carcinoma and Promotes Cancer Cell Invasiveness and Angiogenesis. J. Pathol. 2022, 257, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, H.; Wang, Y.; Brown, Z.J.; Xia, Y.; Huang, Z.; Shen, C.; Hu, Z.; Beane, J.; Ansa-Addo, E.A.; et al. Regulatory T-Cell and Neutrophil Extracellular Trap Interaction Contributes to Carcinogenesis in Non-Alcoholic Steatohepatitis. J. Hepatol. 2021, 75, 1271–1283. [Google Scholar] [CrossRef]
- Langhans, B.; Nischalke, H.D.; Krämer, B.; Dold, L.; Lutz, P.; Mohr, R.; Vogt, A.; Toma, M.; Eis-Hübinger, A.M.; Nattermann, J.; et al. Role of Regulatory T Cells and Checkpoint Inhibition in Hepatocellular Carcinoma. Cancer Immunol. Immunother. 2019, 68, 2055–2066. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jiang, P.; Wei, S.; Xu, X.; Wang, J. Regulatory T Cells in Tumor Microenvironment: New Mechanisms, Potential Therapeutic Strategies and Future Prospects. Mol. Cancer 2020, 19, 116. [Google Scholar] [CrossRef]
- Zheng, C.; Zheng, L.; Yoo, J.K.; Guo, H.; Zhang, Y.; Guo, X.; Kang, B.; Hu, R.; Huang, J.Y.; Zhang, Q.; et al. Landscape of Infiltrating T Cells in Liver Cancer Revealed by Single-Cell Sequencing. Cell 2017, 169, 1342–1356.e16. [Google Scholar] [CrossRef]
- Liu, F.; Liu, W.; Sanin, D.E.; Jia, G.; Tian, M.; Wang, H.; Zhu, B.; Lu, Y.; Qiao, T.; Wang, X.; et al. Heterogeneity of Exhausted T Cells in the Tumor Microenvironment Is Linked to Patient Survival Following Resection in Hepatocellular Carcinoma. Oncoimmunology 2020, 9, 1746573. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, Z.; Zhou, L.; Qi, Z.; Xing, S.; Lv, J.; Shi, J.; Fu, B.; Liu, Z.; Zhang, J.Y.; et al. Impairment of CD4+ Cytotoxic T Cells Predicts Poor Survival and High Recurrence Rates in Patients with Hepatocellular Carcinoma. Hepatology 2013, 58, 139–149. [Google Scholar] [CrossRef]
- Tan, S.; Xu, Y.; Wang, Z.; Wang, T.; Du, X.; Song, X.; Guo, X.; Peng, J.; Zhang, J.; Liang, Y.; et al. Tim-3 Hampers Tumor Surveillance of Liver-Resident and Conventional NK Cells by Disrupting PI3K Signaling. Cancer Res. 2020, 80, 1130–1142. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zhou, J.; Yang, W.; Feng, Y.; Wu, H.; Mok, M.T.S.; Zhang, L.; Liang, Z.; Liu, X.; Xiong, Z.; et al. Aberrant Cholesterol Metabolic Signaling Impairs Antitumor Immunosurveillance through Natural Killer T Cell Dysfunction in Obese Liver. Cell. Mol. Immunol. 2022, 19, 834–847. [Google Scholar] [CrossRef] [PubMed]
- Shalapour, S.; Lin, X.J.; Bastian, I.N.; Brain, J.; Burt, A.D.; Aksenov, A.A.; Vrbanac, A.F.; Li, W.; Perkins, A.; Matsutani, T.; et al. Inflammation-Induced IgA+ Cells Dismantle Anti-Liver Cancer Immunity. Nature 2017, 551, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Z.; Wu, D.; Chen, L.; Xie, L. Single-Cell RNA-Seq Analysis Reveals Microenvironmental Infiltration of Plasma Cells and Hepatocytic Prognostic Markers in HCC With Cirrhosis. Front. Oncol. 2020, 10, 596318. [Google Scholar] [CrossRef] [PubMed]
- Faggioli, F.; Palagano, E.; Di Tommaso, L.; Donadon, M.; Marrella, V.; Recordati, C.; Mantero, S.; Villa, A.; Vezzoni, P.; Cassani, B. B Lymphocytes Limit Senescence-Driven Fibrosis Resolution and Favor Hepatocarcinogenesis in Mouse Liver Injury. Hepatology 2018, 67, 1970–1985. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Dai, Y.; Wang, D.; Wang, X.; Cao, Y.; Liu, W.; Tao, Z. Glycolysis-Related Gene Expression Profiling Serves as a Novel Prognosis Risk Predictor for Human Hepatocellular Carcinoma. Sci. Rep. 2021, 11, 18875. [Google Scholar] [CrossRef]
- Lee, N.C.W.; Carella, M.A.; Papa, S.; Bubici, C. High Expression of Glycolytic Genes in Cirrhosis Correlates With the Risk of Developing Liver Cancer. Front. Cell Dev. Biol. 2018, 6, 138. [Google Scholar] [CrossRef]
- Li, W.; Hao, J.; Zhang, L.; Cheng, Z.; Deng, X.; Shu, G. Astragalin Reduces Hexokinase 2 through Increasing MiR-125b to Inhibit the Proliferation of Hepatocellular Carcinoma Cells in Vitro and in Vivo. J. Agric. Food Chem. 2017, 65, 5961–5972. [Google Scholar] [CrossRef]
- Xu, D.; Jin, J.; Yu, H.; Zhao, Z.; Ma, D.; Zhang, C.; Jiang, H. Chrysin Inhibited Tumor Glycolysis and Induced Apoptosis in Hepatocellular Carcinoma by Targeting Hexokinase-2. J. Exp. Clin. Cancer Res. 2017, 36, 44. [Google Scholar] [CrossRef]
- DeWaal, D.; Nogueira, V.; Terry, A.R.; Patra, K.C.; Jeon, S.-M.; Guzman, G.; Au, J.; Long, C.P.; Antoniewicz, M.R.; Hay, N. Hexokinase-2 Depletion Inhibits Glycolysis and Induces Oxidative Phosphorylation in Hepatocellular Carcinoma and Sensitizes to Metformin. Nat. Commun. 2018, 9, 446. [Google Scholar] [CrossRef]
- Li, M.; Shao, J.; Guo, Z.; Jin, C.; Wang, L.; Wang, F.; Jia, Y.; Zhu, Z.; Zhang, Z.; Zhang, F.; et al. Novel Mitochondrion-Targeting Copper(II) Complex Induces HK2 Malfunction and Inhibits Glycolysis via Drp1-Mediating Mitophagy in HCC. J. Cell. Mol. Med. 2020, 24, 3091–3107. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Pan, L.; Gao, C.; Xu, H.; Li, Y.; Zhang, L.; Ma, L.; Meng, L.; Sun, X.; Qin, H. Quercetin Inhibits the Proliferation of Glycolysis-Addicted HCC Cells by Reducing Hexokinase 2 and Akt-MTOR Pathway. Molecules 2019, 24, 1993. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, A.; Qi, X.; Yu, R.; Li, J. A Novel Inhibitor of PGK1 Suppresses the Aerobic Glycolysis and Proliferation of Hepatocellular Carcinoma. Biomed. Pharmacother. 2023, 158, 114115. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does It Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, M.; Yao, X.; Fei, Y.; Lin, Z.; Li, Z.; Cai, K.; Zhao, Y.; Luo, Z. HCAR1/MCT1 Regulates Tumor Ferroptosis through the Lactate-Mediated AMPK-SCD1 Activity and Its Therapeutic Implications. Cell Rep. 2020, 33, 108487. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Z.; Huang, H.; Zhou, G.; Cui, C.; Weng, Y.; Liu, W.; Kim, S.; Lee, S.; Perez-Neut, M.; et al. Metabolic Regulation of Gene Expression by Histone Lactylation. Nature 2019, 574, 575–580. [Google Scholar] [CrossRef]
- Yang, Z.; Yan, C.; Ma, J.; Peng, P.; Ren, X.; Cai, S.; Shen, X.; Wu, Y.; Zhang, S.; Wang, X.; et al. Lactylome Analysis Suggests Lactylation-Dependent Mechanisms of Metabolic Adaptation in Hepatocellular Carcinoma. Nat. Metab. 2023, 5, 61–79. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, S.; Chen, J.; Su, Z. Unraveling the Regulation of Hepatic Gluconeogenesis. Front. Endocrinol. 2018, 9, 802. [Google Scholar] [CrossRef]
- Liu, M.-X.; Jin, L.; Sun, S.-J.; Liu, P.; Feng, X.; Cheng, Z.-L.; Liu, W.-R.; Guan, K.-L.; Shi, Y.-H.; Yuan, H.-X.; et al. Metabolic Reprogramming by PCK1 Promotes TCA Cataplerosis, Oxidative Stress and Apoptosis in Liver Cancer Cells and Suppresses Hepatocellular Carcinoma. Oncogene 2018, 37, 1637–1653. [Google Scholar] [CrossRef]
- Li, F.; Huangyang, P.; Burrows, M.; Guo, K.; Riscal, R.; Godfrey, J.; Lee, K.E.; Lin, N.; Lee, P.; Blair, I.A.; et al. FBP1 Loss Disrupts Liver Metabolism and Promotes Tumorigenesis through a Hepatic Stellate Cell Senescence Secretome. Nat. Cell Biol. 2020, 22, 728–739. [Google Scholar] [CrossRef]
- Ghanem, N.; El-Baba, C.; Araji, K.; El-Khoury, R.; Usta, J.; Darwiche, N. The Pentose Phosphate Pathway in Cancer: Regulation and Therapeutic Opportunities. Chemotherapy 2021, 66, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wu, D.; Bao, L.; Yin, T.; Lei, D.; Yu, J.; Tong, X. 6PGD Inhibition Sensitizes Hepatocellular Carcinoma to Chemotherapy via AMPK Activation and Metabolic Reprogramming. Biomed. Pharmacother. 2019, 111, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Fang, M.; He, Z.; Cui, D.; Jia, S.; Lin, X.; Xu, X.; Zhou, T.; Liu, W. Hepatitis B Virus Stimulates G6PD Expression through HBx-Mediated Nrf2 Activation. Cell Death Dis. 2015, 6, e1980. [Google Scholar] [CrossRef] [PubMed]
- Xu, I.M.-J.; Lai, R.K.-H.; Lin, S.-H.; Tse, A.P.-W.; Chiu, D.K.-C.; Koh, H.-Y.; Law, C.-T.; Wong, C.-M.; Cai, Z.; Wong, C.C.-L.; et al. Transketolase Counteracts Oxidative Stress to Drive Cancer Development. Proc. Natl. Acad. Sci. USA 2016, 113, E725–E734. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Tang, B.; Li, J.-H.; Wang, Y.; Zhang, L.; Xie, X.-Y.; Zhang, B.-H.; Qiu, S.-J.; Wu, W.-Z.; Ren, Z.-G. ID1 Promotes Hepatocellular Carcinoma Proliferation and Confers Chemoresistance to Oxaliplatin by Activating Pentose Phosphate Pathway. J. Exp. Clin. Cancer Res. 2017, 36, 166. [Google Scholar] [CrossRef]
- Sun, J.; Li, J.; Guo, Z.; Sun, L.; Juan, C.; Zhou, Y.; Gu, H.; Yu, Y.; Hu, Q.; Kan, Q.; et al. Overexpression of Pyruvate Dehydrogenase E1α Subunit Inhibits Warburg Effect and Induces Cell Apoptosis Through Mitochondria-Mediated Pathway in Hepatocellular Carcinoma. Oncol. Res. 2019, 27, 407–414. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. We Need to Talk about the Warburg Effect. Nat. Metab. 2020, 2, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Tseng, P.-L.; Wu, W.-H.; Hu, T.-H.; Chen, C.-W.; Cheng, H.-C.; Li, C.-F.; Tsai, W.-H.; Tsai, H.-J.; Hsieh, M.-C.; Chuang, J.-H.; et al. Decreased Succinate Dehydrogenase B in Human Hepatocellular Carcinoma Accelerates Tumor Malignancy by Inducing the Warburg Effect. Sci. Rep. 2018, 8, 3081. [Google Scholar] [CrossRef]
- Yuan, T.; Zhou, T.; Qian, M.; Du, J.; Liu, Y.; Wang, J.; Li, Y.; Fan, G.; Yan, F.; Dai, X.; et al. SDHA/B Reduction Promotes Hepatocellular Carcinoma by Facilitating the DeNEDDylation of Cullin1 and Stabilizing YAP/TAZ. Hepatology 2022, 10, 1002. [Google Scholar] [CrossRef]
- Cheng, G.; Zielonka, J.; Dranka, B.P.; McAllister, D.; Mackinnon, A.C.; Joseph, J.; Kalyanaraman, B. Mitochondria-Targeted Drugs Synergize with 2-Deoxyglucose to Trigger Breast Cancer Cell Death. Cancer Res. 2012, 72, 2634–2644. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Wu, L.-C.; Hsia, C.-Y.; Yin, P.-H.; Chi, C.-W.; Yeh, T.-S.; Lee, H.-C. Energy Metabolism Determines the Sensitivity of Human Hepatocellular Carcinoma Cells to Mitochondrial Inhibitors and Biguanide Drugs. Oncol. Rep. 2015, 34, 1620–1628. [Google Scholar] [CrossRef]
- Budhu, A.; Roessler, S.; Zhao, X.; Yu, Z.; Forgues, M.; Ji, J.; Karoly, E.; Qin, L.X.; Ye, Q.H.; Jia, H.L.; et al. Integrated Metabolite and Gene Expression Profiles Identify Lipid Biomarkers Associated with Progression of Hepatocellular Carcinoma and Patient Outcomes. Gastroenterology 2013, 144, 1066–1075.e1. [Google Scholar] [CrossRef] [PubMed]
- Alannan, M.; Fayyad-Kazan, H.; Trézéguet, V.; Merched, A. Targeting Lipid Metabolism in Liver Cancer. Biochemistry 2020, 59, 3951–3964. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.M.; Kleczko, E.K.; Nemenoff, R.A. Eicosanoids in Cancer: New Roles in Immunoregulation. Front. Pharmacol. 2020, 11, 595498. [Google Scholar] [CrossRef]
- Björnson, E.; Mukhopadhyay, B.; Asplund, A.; Pristovsek, N.; Cinar, R.; Romeo, S.; Uhlen, M.; Kunos, G.; Nielsen, J.; Mardinoglu, A. Stratification of Hepatocellular Carcinoma Patients Based on Acetate Utilization. Cell Rep. 2015, 13, 2014–2026. [Google Scholar] [CrossRef]
- Ning, Z.; Guo, X.; Liu, X.; Lu, C.; Wang, A.; Wang, X.; Wang, W.; Chen, H.; Qin, W.; Liu, X.; et al. USP22 Regulates Lipidome Accumulation by Stabilizing PPARγ in Hepatocellular Carcinoma. Nat. Commun. 2022, 13, 2187. [Google Scholar] [CrossRef]
- Wang, M.D.; Wu, H.; Fu, G.B.; Zhang, H.L.; Zhou, X.; Tang, L.; Dong, L.W.; Qin, C.J.; Huang, S.; Zhao, L.H.; et al. Acetyl-Coenzyme A Carboxylase Alpha Promotion of Glucose-Mediated Fatty Acid Synthesis Enhances Survival of Hepatocellular Carcinoma in Mice and Patients. Hepatology 2016, 63, 1272–1286. [Google Scholar] [CrossRef]
- Raeisi, M.; Hassanbeigi, L.; Khalili, F.; Kharrati-Shishavan, H.; Yousefi, M.; Mehdizadeh, A. Stearoyl-CoA Desaturase 1 as a Therapeutic Target for Cancer: A Focus on Hepatocellular Carcinoma. Mol. Biol. Rep. 2022, 49, 8871–8882. [Google Scholar] [CrossRef]
- Lin, H.P.; Cheng, Z.L.; He, R.Y.; Song, L.; Tian, M.X.; Zhou, L.S.; Groh, B.S.; Liu, W.R.; Ji, M.B.; Ding, C.; et al. Destabilization of Fatty Acid Synthase by Acetylation Inhibits De Novo Lipogenesis and Tumor Cell Growth. Cancer Res. 2016, 76, 6924–6936. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.M.; Shi, X.H.; Ye, K.; Fu, X.L.; Wang, X.; Guo, S.M.; Ma, J.Q.; Xu, F.F.; Sun, H.M.; et al. Sorafenib Triggers Ferroptosis via Inhibition of HBXIP/SCD Axis in Hepatocellular Carcinoma. Acta Pharmacol. Sin. 2022, 44, 622–634. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Honda, M.; Takatori, H.; Nishino, R.; Minato, H.; Takamura, H.; Ohta, T.; Kaneko, S. Activation of Lipogenic Pathway Correlates with Cell Proliferation and Poor Prognosis in Hepatocellular Carcinoma. J. Hepatol. 2009, 50, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.L.W.; Israeli, E.; Ericksen, R.E.; Chow, P.K.H.; Han, W. The Altered Lipidome of Hepatocellular Carcinoma. Semin. Cancer Biol. 2022, 86, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 Dictates Ferroptosis Sensitivity by Shaping Cellular Lipid Composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.K.F.; Lau, E.Y.T.; Leung, D.H.W.; Lo, J.; Ho, N.P.Y.; Cheng, L.K.W.; Ma, S.; Lin, C.H.; Copland, J.A.; Ding, J.; et al. Stearoyl-CoA Desaturase Regulates Sorafenib Resistance via Modulation of ER Stress-Induced Differentiation. J. Hepatol. 2017, 67, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.K.Y.; Kweon, S.M.; Chi, F.; Hwang, E.; Kabe, Y.; Higashiyama, R.; Qin, L.; Yan, R.; Wu, R.P.; Lai, K.; et al. Stearoyl-CoA Desaturase Promotes Liver Fibrosis and Tumor Development in Mice via a Wnt Positive-Signaling Loop by Stabilization of Low-Density Lipoprotein-Receptor-Related Proteins 5 and 6. Gastroenterology 2017, 152, 1477–1491. [Google Scholar] [CrossRef] [PubMed]
- Vriens, K.; Christen, S.; Parik, S.; Broekaert, D.; Yoshinaga, K.; Talebi, A.; Dehairs, J.; Escalona-Noguero, C.; Schmieder, R.; Cornfield, T.; et al. Evidence for an Alternative Fatty Acid Desaturation Pathway Increasing Cancer Plasticity. Nature 2019, 566, 403–406. [Google Scholar] [CrossRef]
- Lewinska, M.; Santos-Laso, A.; Arretxe, E.; Alonso, C.; Zhuravleva, E.; Jimenez-Agüero, R.; Eizaguirre, E.; Pareja, M.J.; Romero-Gómez, M.; Jimenez, M.A.; et al. The Altered Serum Lipidome and Its Diagnostic Potential for Non-Alcoholic Fatty Liver (NAFL)-Associated Hepatocellular Carcinoma. EBioMedicine 2021, 73, 103661. [Google Scholar] [CrossRef]
- Peng, L.; Yan, Q.; Chen, Z.; Hu, Y.; Sun, Y.; Miao, Y.; Wu, Y.; Yao, Y.; Tao, L.; Chen, F.; et al. Research Progress on the Role of Cholesterol in Hepatocellular Carcinoma. Eur. J. Pharmacol. 2023, 938, 175410. [Google Scholar] [CrossRef]
- Liang, J.Q.; Teoh, N.; Xu, L.; Pok, S.; Li, X.; Chu, E.S.H.; Chiu, J.; Dong, L.; Arfianti, E.; Haigh, W.G.; et al. Dietary Cholesterol Promotes Steatohepatitis Related Hepatocellular Carcinoma through Dysregulated Metabolism and Calcium Signaling. Nat. Commun. 2018, 9, 4490. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, X.; Wu, L.; Yang, M.; Li, Z.; Gao, Y.; Liu, S.; Zhou, G.; Zhao, J.; Liu, Y.; et al. Lipid Rafts Promote Liver Cancer Cell Proliferation and Migration by Up-Regulation of TLR7 Expression. Oncotarget 2016, 7, 63856–63869. [Google Scholar] [CrossRef] [PubMed]
- Calvisi, D.F.; Wang, C.; Ho, C.; Ladu, S.; Lee, S.A.; Mattu, S.; Destefanis, G.; Delogu, S.; Zimmermann, A.; Ericsson, J.; et al. Increased Lipogenesis, Induced by AKT-MTORC1-RPS6 Signaling, Promotes Development of Human Hepatocellular Carcinoma. Gastroenterology 2011, 140, 1071–1083.e5. [Google Scholar] [CrossRef]
- Liu, D.; Wong, C.C.; Fu, L.; Chen, H.; Zhao, L.; Li, C.; Zhou, Y.; Zhang, Y.; Xu, W.; Yang, Y.; et al. Squalene Epoxidase Drives NAFLD-Induced Hepatocellular Carcinoma and Is a Pharmaceutical Target. Sci. Transl. Med. 2018, 10, eaap9840. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Sun, A.; Zhao, Y.; Ying, W.; Sun, H.; Yang, X.; Xing, B.; Sun, W.; Ren, L.; Hu, B.; et al. Proteomics Identifies New Therapeutic Targets of Early-Stage Hepatocellular Carcinoma. Nature 2019, 567, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wu, W.; Jiao, H.; Chen, Y.; Ji, X.; Cao, J.; Yin, F.; Yin, W. Squalene Epoxidase Promotes Hepatocellular Carcinoma Development by Activating STRAP Transcription and TGF-β/SMAD Signalling. Br. J. Pharmacol. 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Zhu, Y.; Gu, L.; Lin, X.; Zhou, X.; Lu, B.; Liu, C.; Li, Y.; Prochownik, E.V.; Karin, M.; Wang, F.; et al. P53 Deficiency Affects Cholesterol Esterification to Exacerbate Hepatocarcinogenesis. Hepatology, 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Guo, Y.; Peng, Q.; Hao, L.; Ji, J.; Zhang, Z.; Xue, Y.; Liu, Y.; Gao, Y.; Li, C.; Shi, X. Dihydroartemisinin Promoted FXR Expression Independent of YAP1 in Hepatocellular Carcinoma. FASEB J. 2022, 36, e22361. [Google Scholar] [CrossRef]
- Hassan, H.M.; Onabote, O.; Isovic, M.; Passos, D.T.; Dick, F.A.; Torchia, J. Regulation of Chromatin Accessibility by the Farnesoid X Receptor Is Essential for Circadian and Bile Acid Homeostasis In Vivo. Cancers 2022, 14, 6191. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Wang, J.; Zhou, L.; Hu, B.; Chen, Y.; Zhu, Z. GPBAR1 Is Associated with Asynchronous Bone Metastasis and Poor Prognosis of Hepatocellular Carcinoma. Front. Oncol. 2023, 12, 1113785. [Google Scholar] [CrossRef]
- Kim, I.; Morimura, K.; Shah, Y.; Yang, Q.; Ward, J.M.; Gonzalez, F.J. Spontaneous Hepatocarcinogenesis in Farnesoid X Receptor-Null Mice. Carcinogenesis 2007, 28, 940–946. [Google Scholar] [CrossRef]
- Han, L.Y.; Fan, Y.C.; Mu, N.N.; Gao, S.; Li, F.; Ji, X.F.; Dou, C.Y.; Wang, K. Aberrant DNA Methylation of G-Protein-Coupled Bile Acid Receptor Gpbar1 (TGR5) Is a Potential Biomarker for Hepatitis B Virus Associated Hepatocellular Carcinoma. Int. J. Med. Sci. 2014, 11, 164–171. [Google Scholar] [CrossRef]
- Attia, Y.M.; Tawfiq, R.A.; Gibriel, A.A.; Ali, A.A.; Kassem, D.H.; Hammam, O.A.; Elmazar, M.M. Activation of FXR Modulates SOCS3/Jak2/STAT3 Signaling Axis in a NASH-Dependent Hepatocellular Carcinoma Animal Model. Biochem. Pharmacol. 2021, 186, 114497. [Google Scholar] [CrossRef]
- Jiao, N.; Baker, S.S.; Chapa-Rodriguez, A.; Liu, W.; Nugent, C.A.; Tsompana, M.; Mastrandrea, L.; Buck, M.J.; Baker, R.D.; Genco, R.J.; et al. Suppressed Hepatic Bile Acid Signalling despite Elevated Production of Primary and Secondary Bile Acids in NAFLD. Gut 2018, 67, 1881–1891. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, L.; Wang, H.; Cai, W.; Xie, Q. Modulation of Bile Acid Profile by Gut Microbiota in Chronic Hepatitis B. J. Cell. Mol. Med. 2020, 24, 2573–2581. [Google Scholar] [CrossRef] [PubMed]
- Sydor, S.; Best, J.; Messerschmidt, I.; Manka, P.; Vilchez-Vargas, R.; Brodesser, S.; Lucas, C.; Wegehaupt, A.; Wenning, C.; Aßmuth, S.; et al. Altered Microbiota Diversity and Bile Acid Signaling in Cirrhotic and Noncirrhotic NASH-HCC. Clin. Transl. Gastroenterol. 2020, 11, e00131. [Google Scholar] [CrossRef] [PubMed]
- Green, C.D.; Maceyka, M.; Cowart, L.A.; Spiegel, S. Sphingolipids in Metabolic Disease: The Good, the Bad, and the Unknown. Cell Metab. 2021, 33, 1293. [Google Scholar] [CrossRef] [PubMed]
- Morad, S.A.F.; Cabot, M.C. Ceramide-Orchestrated Signalling in Cancer Cells. Nat. Rev. Cancer 2013, 13, 51–65. [Google Scholar] [CrossRef]
- Ogretmen, B. Sphingolipid Metabolism in Cancer Signalling and Therapy. Nat. Rev. Cancer 2018, 18, 33–50. [Google Scholar] [CrossRef]
- Liu, X.T.; Chung, L.H.; Liu, D.; Chen, J.; Huang, Y.; Teo, J.D.; Han, X.D.; Zhao, Y.; Guan, F.H.X.; Tran, C.; et al. Ablation of Sphingosine Kinase 2 Suppresses Fatty Liver-Associated Hepatocellular Carcinoma via Downregulation of Ceramide Transfer Protein. Oncogenesis 2022, 11, 67. [Google Scholar] [CrossRef]
- Lin, M.; Liao, W.; Dong, M.; Zhu, R.; Xiao, J.; Sun, T.; Chen, Z.; Wu, B.; Jin, J. Exosomal Neutral Sphingomyelinase 1 Suppresses Hepatocellular Carcinoma via Decreasing the Ratio of Sphingomyelin/Ceramide. FEBS J. 2018, 285, 3835–3848. [Google Scholar] [CrossRef]
- Krautbauer, S.; Meier, E.M.; Rein-Fischboeck, L.; Pohl, R.; Weiss, T.S.; Sigruener, A.; Aslanidis, C.; Liebisch, G.; Buechler, C. Ceramide and Polyunsaturated Phospholipids Are Strongly Reduced in Human Hepatocellular Carcinoma. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2016, 1861, 1767–1774. [Google Scholar] [CrossRef]
- Li, Z.; Guan, M.; Lin, Y.; Cui, X.; Zhang, Y.; Zhao, Z.; Zhu, J. Aberrant Lipid Metabolism in Hepatocellular Carcinoma Revealed by Liver Lipidomics. Int. J. Mol. Sci. 2017, 18, 2550. [Google Scholar] [CrossRef]
- Asano, S.; Kitatani, K.; Taniguchi, M.; Hashimoto, M.; Zama, K.; Mitsutake, S.; Igarashi, Y.; Takeya, H.; Kigawa, J.; Hayashi, A.; et al. Regulation of Cell Migration by Sphingomyelin Synthases: Sphingomyelin in Lipid Rafts Decreases Responsiveness to Signaling by the CXCL12/CXCR4 Pathway. Mol. Cell. Biol. 2012, 32, 3242. [Google Scholar] [CrossRef] [PubMed]
- Wesley, U.V.; Hatcher, J.F.; Dempsey, R.J. Sphingomyelin Synthase 1 Regulates Neuro-2a Cell Proliferation and Cell Cycle Progression Through Modulation of P27 Expression and Akt Signaling. Mol. Neurobiol. 2015, 51, 1530–1541. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Chen, Z.; Feng, H.; Chen, Y.; Zhang, C.; Yu, J.; Luo, Y.; Zhao, L.; Jiang, X.; Shi, F. Sphingomyelin Synthase 2 Promotes an Aggressive Breast Cancer Phenotype by Disrupting the Homoeostasis of Ceramide and Sphingomyelin. Cell Death Dis. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Nagahashi, M.; Prasoon, P.; Hirose, Y.; Kobayashi, T.; Sakata, J.; Abe, M.; Sakimura, K.; Matsuda, Y.; Butash, A.L.; et al. Dysregulation of Sphingolipid Metabolic Enzymes Leads to High Levels of Sphingosine-1-Phosphate and Ceramide in Human Hepatocellular Carcinoma. Hepatol. Res. 2021, 51, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Grammatikos, G.; Schoell, N.; Ferreirós, N.; Bon, D.; Herrmann, E.; Farnik, H.; Köberle, V.; Piiper, A.; Zeuzem, S.; Kronenberger, B.; et al. Serum Sphingolipidomic Analyses Reveal an Upregulation of C16- Ceramide and Sphingosine-1-Phosphate in Hepatocellular Carcinoma. Oncotarget 2016, 7, 18095–18105. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.W.; Wu, G.F.; Wang, C. Generalized Likelihood Block Detection for SPAD-Based Underwater VLC System. IEEE Photonics J. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Zeng, Y.; Yao, X.; Chen, L.; Yan, Z.; Liu, J.; Zhang, Y.; Feng, T.; Wu, J.; Liu, X.; Zeng, Y.; et al. Sphingosine-1-Phosphate Induced Epithelial-Mesenchymal Transition of Hepatocellular Carcinoma via an MMP-7/ Syndecan-1/TGF-β Autocrine Loop. Oncotarget 2016, 7, 63324–63337. [Google Scholar] [CrossRef]
- Mücke, V.T.; Thomas, D.; Mücke, M.M.; Waidmann, O.; Zeuzem, S.; Sarrazin, C.; Pfeilschifter, J.; Vermehren, J.; Finkelmeier, F.; Grammatikos, G. Serum Sphingolipids Predict de Novo Hepatocellular Carcinoma in Hepatitis C Cirrhotic Patients with Sustained Virologic Response. Liver Int. 2019, 39, 2174–2183. [Google Scholar] [CrossRef]
- Cheng, J.C.; Wang, E.Y.; Yi, Y.; Thakur, A.; Tsai, S.H.; Hoodless, P.A. S1P Stimulates Proliferation by Upregulating CTGF Expression through S1PR2-Mediated YAP Activation. Mol. Cancer Res. 2018, 16, 1543–1555. [Google Scholar] [CrossRef]
- Bao, M.; Chen, Z.; Xu, Y.; Zhao, Y.; Zha, R.; Huang, S.; Liu, L.; Chen, T.; Li, J.; Tu, H.; et al. Sphingosine Kinase 1 Promotes Tumour Cell Migration and Invasion via the S1P/EDG1 Axis in Hepatocellular Carcinoma. Liver Int. 2012, 32, 331–338. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J.; Wang, S.; Jiang, Q.; Xu, K. Identification and Validation of a Nine-Gene Amino Acid Metabolism-Related Risk Signature in HCC. Front. Cell Dev. Biol. 2021, 9, 731790. [Google Scholar] [CrossRef] [PubMed]
- Ullah Khan, S.; Ullah Khan, M. The Role of Amino Acid Metabolic Reprogramming in Tumor Development and Immunotherapy. Biochem. Mol. Biol. 2022, 7, 6–12. [Google Scholar] [CrossRef]
- Golonka, R.M.; Vijay-Kumar, M. Chapter Five—Atypical Immunometabolism and Metabolic Reprogramming in Liver Cancer: Deciphering the Role of Gut Microbiome. Adv. Cancer Res. 2021, 149, 171–255. [Google Scholar] [PubMed]
- Bai, J.; Tang, R.; Zhou, K.; Chang, J.; Wang, H.; Zhang, Q.; Shi, J.; Sun, C. An Asparagine Metabolism-Based Classification Reveals the Metabolic and Immune Heterogeneity of Hepatocellular Carcinoma. BMC Med. Genom. 2022, 15, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, K.; Shimizu, Y. Branched-Chain Amino Acids in Liver Diseases. Transl. Gastroenterol. Hepatol. 2018, 3, 47. [Google Scholar] [CrossRef]
- Du, D.; Liu, C.; Qin, M.; Zhang, X.; Xi, T.; Yuan, S.; Hao, H.; Xiong, J. Metabolic Dysregulation and Emerging Therapeutical Targets for Hepatocellular Carcinoma. Acta Pharm. Sin. B 2022, 12, 558. [Google Scholar] [CrossRef]
- Zhu, W.W.; Lu, M.; Wang, X.Y.; Zhou, X.; Gao, C.; Qin, L.X. The Fuel and Engine: The Roles of Reprogrammed Metabolism in Metastasis of Primary Liver Cancer. Genes Dis. 2020, 7, 299–307. [Google Scholar] [CrossRef]
- Yu, D.; Shi, X.; Meng, G.; Chen, J.; Yan, C.; Jiang, Y.; Wei, J.; Ding, Y. Kidney-Type Glutaminase (GLS1) Is a Biomarker for Pathologic Diagnosis and Prognosis of Hepatocellular Carcinoma. Oncotarget 2015, 6, 7619–7631. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, F.; Fan, N.; Zhou, C.; Li, D.; Macvicar, T.; Dong, Q.; Bruns, C.J.; Zhao, Y. Targeting Glutaminolysis: New Perspectives to Understand Cancer Development and Novel Strategies for Potential Target Therapies. Front. Ooncol. 2020, 10, 589508. [Google Scholar] [CrossRef]
- Mungamuri, S.K.; Sinha, S.N.; Javvadi, Y. Understanding the Alterations in Lipid Metabolism in NAFLD Progression: Current Trends and Future Directions. Crit. Rev. Oncog. 2021, 26, 35–49. [Google Scholar] [CrossRef]
- He, L.; Cai, X.; Cheng, S.; Zhou, H.; Zhang, Z.; Ren, J.; Ren, F.; Yang, Q.; Tao, N.; Chen, J. Ornithine Transcarbamylase Downregulation Is Associated with Poor Prognosis in Hepatocellular Carcinoma. Oncol. Lett. 2019, 17, 5030–5038. [Google Scholar] [CrossRef] [PubMed]
- Hajaj, E.; Sciacovelli, M.; Frezza, C.; Erez, A. The Context-Specific Roles of Urea Cycle Enzymes in Tumorigenesis. Mol. Cell 2021, 81, 3749–3759. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Chen, W.; Chen, J.; Zheng, Q.; Dong, J.; Zhu, Y. The Oncogenic Role of ARG1 in Progression and Metastasis of Hepatocellular Carcinoma. Biomed Res. Int. 2018, 2018, 2109865. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, A.L.; Leslie, N.D.; Gupta, A.; Geller, J.I. Acquired Ornithine Transcarbamylase Deficiency in Pediatric and Adolescent Patients with Fibrolamellar Hepatocellular Carcinoma. Pediatr. Blood Cancer 2018, 65, e27392. [Google Scholar] [CrossRef]
- Sun, L.; Suo, C.; Li, S.T.; Zhang, H.; Gao, P. Metabolic Reprogramming for Cancer Cells and Their Microenvironment: Beyond the Warburg Effect. Biochim. Biophys. Acta-Rev. Cancer 2018, 1870, 51–66. [Google Scholar] [CrossRef]
- Yang, D.; Liu, H.; Cai, Y.; Lu, K.; Zhong, X.; Xing, S.; Song, W.; Zhang, Y.; Ye, L.; Zhu, X.; et al. Branched-Chain Amino Acid Catabolism Breaks Glutamine Addiction to Sustain Hepatocellular Carcinoma Progression. Cell Rep. 2022, 41, 111691. [Google Scholar] [CrossRef]
- Lynch, C.J.; Adams, S.H. Branched-Chain Amino Acids in Metabolic Signalling and Insulin Resistance. Nat. Rev. Endocrinol. 2014, 10, 723. [Google Scholar] [CrossRef]
- Ananieva, E.A.; Wilkinson, A.C. Branched-Chain Amino Acid Metabolism in Cancer. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 64. [Google Scholar] [CrossRef]
- Hayaishi, S.; Chung, H.; Kudo, M.; Ishikawa, E.; Takita, M.; Ueda, T.; Kitai, S.; Inoue, T.; Yada, N.; Hagiwara, S.; et al. Oral Branched-Chain Amino Acid Granules Reduce the Incidence of Hepatocellular Carcinoma and Improve Event-Free Survival in Patients with Liver Cirrhosis. Dig. Dis. 2011, 29, 326–332. [Google Scholar] [CrossRef]
- Plauth, M.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schütz, T.; Bischoff, S.C. ESPEN Guideline on Clinical Nutrition in Liver Disease. Clin. Nutr. 2019, 38, 485–521. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Hiroshima, Y.; Matsuo, K.; Kawaguchi, D.; Murakami, T.; Yabushita, Y.; Endo, I.; Taguri, M.; Koda, K.; Tanaka, K. A Randomized Clinical Trial of Preoperative Administration of Branched-Chain Amino Acids to Prevent Postoperative Ascites in Patients with Liver Resection for Hepatocellular Carcinoma. Ann. Surg. Oncol. 2016, 23, 3727–3735. [Google Scholar] [CrossRef] [PubMed]
- Wubetu, G.Y.; Utsunomiya, T.; Ishikawa, D.; Ikemoto, T.; Yamada, S.; Morine, Y.; Iwahashi, S.; Saito, Y.; Arakawa, Y.; Imura, S.; et al. Branched Chain Amino Acid Suppressed Insulin-Initiated Proliferation of Human Cancer Cells through Induction of Autophagy. Anticancer Res. 2014, 34, 4789–4796. [Google Scholar] [PubMed]
- Miuma, S.; Ichikawa, T.; Arima, K.; Takeshita, S.; Muraoka, T.; Matsuzaki, T.; Ootani, M.; Shibata, H.; Akiyama, M.; Ozawa, E.; et al. Branched-Chain Amino Acid Deficiency Stabilizes Insulin-Induced Vascular Endothelial Growth Factor MRNA in Hepatocellular Carcinoma Cells. J. Cell. Biochem. 2012, 113, 3113–3121. [Google Scholar] [CrossRef] [PubMed]
- Takegoshi, K.; Honda, M.; Okada, H.; Takabatake, R.; Matsuzawa-Nagata, N.; Campbell, J.S.; Nishikawa, M.; Shimakami, T.; Shirasaki, T.; Sakai, Y.; et al. Branched-Chain Amino Acids Prevent Hepatic Fibrosis and Development of Hepatocellular Carcinoma in a Non-Alcoholic Steatohepatitis Mouse Model. Oncotarget 2017, 8, 18191–18205. [Google Scholar] [CrossRef]
- Ericksen, R.E.; Lim, S.L.; McDonnell, E.; Shuen, W.H.; Vadiveloo, M.; White, P.J.; Ding, Z.; Kwok, R.; Lee, P.; Radda, G.K.; et al. Loss of BCAA Catabolism during Carcinogenesis Enhances MTORC1 Activity and Promotes Tumor Development and Progression. Cell Metab. 2019, 29, 1151–1165.e6. [Google Scholar] [CrossRef]
- Zhang, B.; Dong, L.-W.; Tan, Y.-X.; Zhang, J.; Pan, Y.-F.; Yang, C.; Li, M.-H.; Ding, Z.-W.; Liu, L.-J.; Jiang, T.-Y.; et al. Asparagine Synthetase Is an Independent Predictor of Surgical Survival and a Potential Therapeutic Target in Hepatocellular Carcinoma. Br. J. Cancer 2013, 9, 14–23. [Google Scholar] [CrossRef]
- Xue, C.; Gao, P.; Cui, X.; Zhang, X.; Lei, J.; Li, R.; Zhu, C.; Qin, X. ASRGL1 Correlates With Immune Cell Infiltration in Hepatocellular Carcinoma and Can Serve as a Prognostic Biomarker. Front. Oncol. 2021, 11, 680070. [Google Scholar] [CrossRef]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjöstedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A Pathology Atlas of the Human Cancer Transcriptome. Science 2017, 357, eaan2507. [Google Scholar] [CrossRef]
- Nwosu, Z.C.; Megger, D.A.; Hammad, S.; Sitek, B.; Roessler, S.; Ebert, M.P.; Meyer, C.; Dooley, S. Identification of the Consistently Altered Metabolic Targets in Human Hepatocellular Carcinoma. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 303. [Google Scholar] [CrossRef]
- Peng, X.; Chen, Z.; Farshidfar, F.; Xu, X.; Philip, L.; Wang, Y.; Cheng, F.; Tan, L.; Mojumdar, K.; Du, D.; et al. Molecular characterization and clinical relevance of metabolic expression subtypes in human cancers. HHS Public Access. 2018, 23, 255–269. [Google Scholar] [CrossRef]
- Ally, A.; Balasundaram, M.; Carlsen, R.; Chuah, E.; Clarke, A.; Dhalla, N.; Holt, R.A.; Jones, S.J.M.; Lee, D.; Ma, Y.; et al. Comprehensive and Integrative Genomic Characterization of Hepatocellular Carcinoma. Cell 2017, 169, 1327–1341.e23. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Dong, H.; Robertson, K.; Liu, C. DNA Methylation Suppresses Expression of the Urea Cycle Enzyme Carbamoyl Phosphate Synthetase 1 (CPS1) in Human Hepatocellular Carcinoma. Am. J. Pathol. 2011, 178, 652. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.-W.; Lee, S.-S.; Chang, C.-Y.; Hu, C.-M.; Jou, Y.-S. Pyrimidine Metabolic Rate Limiting Enzymes in Poorly-Differentiated Hepatocellular Carcinoma Are Signature Genes of Cancer Stemness and Associated with Poor Prognosis. Oncotarget 2017, 8, 77734–77751. [Google Scholar] [CrossRef]
- Beudeker, B.J.B.; Groothuismink, Z.M.A.; van der Eijk, A.A.; Debes, J.D.; Boonstra, A. Circulating Cytokines Reflect the Etiology-Specific Immune Environment in Cirrhosis and HCC. Cancers 2022, 14, 4900. [Google Scholar] [CrossRef]
- Hwan Kim, K.; Shin, H.; Kim, K.; Choi, H.M.; Hoon Rhee, S.; Moon, H.; Hoe Kim, H.; Suk Yang, U.; Cheong, J. Hepatitis B Virus X Protein Induces Hepatic Steatosis Via Transcriptional Activation of SREBP1 and PPAR. Gastroenterology 2007, 132, 1955–1967. [Google Scholar]
- Schoeman, J.C.; Hou, J.; Harms, A.C.; Vreeken, R.J.; Berger, R.; Hankemeier, T.; Boonstra, A. Metabolic Characterization of the Natural Progression of Chronic Hepatitis B. Genome Med. 2016, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Levy, G.; Habib, N.; Guzzardi, M.A.; Kitsberg, D.; Bomze, D.; Ezra, E.; Uygun, B.E.; Uygun, K.; Trippler, M.; Schlaak, J.F.; et al. Nuclear Receptors Control Pro-Viral and Antiviral Metabolic Responses to Hepatitis C Virus Infection. Nat. Chem. Biol. 2016, 12, 1037. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Tsukamoto, H.; Liu, J.C.; Kashiwabara, C.; Feldman, D.; Sher, L.; Dooley, S.; French, S.W.; Mishra, L.; Petrovic, L.; et al. Reciprocal Regulation by TLR4 and TGF-β in Tumor-Initiating Stem-like Cells. J. Clin. Investig. 2013, 123, 2832. [Google Scholar] [CrossRef]
- Gerresheim, G.K.; Roeb, E.; Michel, A.M.; Niepmann, M. Hepatitis C Virus Downregulates Core Subunits of Oxidative Phosphorylation, Reminiscent of the Warburg Effect in Cancer Cells. Cells 2019, 8, 1410. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Z.; Wang, N.; Guo, M.; Chi, X.; Pan, Y.; Jiang, J.; Niu, J.; Ksimu, S.; Li, J.Z.; et al. Role of HDAC9-FoxO1 Axis in the Transcriptional Program Associated with Hepatic Gluconeogenesis. Sci. Rep. 2017, 7, 6102. [Google Scholar] [CrossRef]
- Clugston, R.D.; Jiang, H.; Lee, M.X.; Piantedosi, R.; Yuen, J.J.; Ramakrishnan, R.; Lewis, M.J.; Gottesman, M.E.; Huang, L.S.; Goldberg, I.J.; et al. Altered Hepatic Lipid Metabolism in C57BL/6 Mice Fed Alcohol: A Targeted Lipidomic and Gene Expression Study. J. Lipid Res. 2011, 52, 2021–2031. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The Diagnosis and Management of Nonalcoholic Fatty Liver Disease: Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Gaggini, M.; Carli, F.; Rosso, C.; Buzzigoli, E.; Marietti, M.; Della Latta, V.; Ciociaro, D.; Abate, M.L.; Gambino, R.; Cassader, M.; et al. Altered Amino Acid Concentrations in NAFLD: Impact of Obesity and Insulin Resistance. Hepatology 2018, 67, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, M.V.; Neuschwander-Tetri, B.A. The Metabolic Basis of Nonalcoholic Steatohepatitis. Endocrinol. Diabetes Metab. 2020, 3, e00112. [Google Scholar] [CrossRef] [PubMed]
- Dodd, K.M.; Tee, A.R. Leucine and MTORC1: A Complex Relationship. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1329–E1342. [Google Scholar] [CrossRef] [PubMed]
- Sunny, N.E.; Parks, E.J.; Browning, J.D.; Burgess, S.C. Excessive Hepatic Mitochondrial TCA Cycle and Gluconeogenesis in Humans with Nonalcoholic Fatty Liver Disease. Cell Metab. 2011, 14, 804–810. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Campbell-Sargent, C.; Mirshahi, F.; Rizzo, W.B.; Contos, M.J.; Sterling, R.K.; Luketic, V.A.; Shiffman, M.L.; Clore, J.N. Nonalcoholic Steatohepatitis: Association of Insulin Resistance and Mitochondrial Abnormalities. Gastroenterology 2001, 120, 1183–1192. [Google Scholar] [CrossRef]
- Xia, L.; Oyang, L.; Lin, J.; Tan, S.; Han, Y.; Wu, N.; Yi, P.; Tang, L.; Pan, Q.; Rao, S.; et al. The Cancer Metabolic Reprogramming and Immune Response. Mol. Cancer 2021, 20, 1–21. [Google Scholar] [CrossRef]
- Ho, P.C.; Liu, P.S. Metabolic Communication in Tumors: A New Layer of Immunoregulation for Immune Evasion. J. Immunother. Cancer 2016, 4, 1–9. [Google Scholar] [CrossRef]
- Colegio, O.R.; Chu, N.-Q.; Szabo, A.L.; Chu, T.; Rhebergen, A.M.; Jairam, V.; Cyrus, N.; Brokowski, C.E.; Eisenbarth, S.C.; Phillips, G.M.; et al. Functional Polarization of Tumour-Associated Macrophages by Tumour-Derived Lactic Acid. Nature 2014, 513, 559–563. [Google Scholar] [CrossRef]
- Chen, P.; Zuo, H.; Xiong, H.; Kolar, M.J.; Chu, Q.; Saghatelian, A.; Siegwart, D.J.; Wan, Y. Gpr132 Sensing of Lactate Mediates Tumor-Macrophage Interplay to Promote Breast Cancer Metastasis. Proc. Natl. Acad. Sci. USA 2017, 114, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Faubert, B.; Li, K.Y.; Cai, L.; Hensley, C.T.; Kim, J.; Zacharias, L.G.; Yang, C.; Do, Q.N.; Doucette, S.; Burguete, D.; et al. Lactate Metabolism in Human Lung Tumors. Cell 2017, 171, 358–371.e9. [Google Scholar] [CrossRef] [PubMed]
- Littlewood-Evans, A.; Sarret, S.; Apfel, V.; Loesle, P.; Dawson, J.; Zhang, J.; Muller, A.; Tigani, B.; Kneuer, R.; Patel, S.; et al. GPR91 Senses Extracellular Succinate Released from Inflammatory Macrophages and Exacerbates Rheumatoid Arthritis. J. Exp. Med. 2016, 213, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-Y.; Huang, T.-W.; Hsieh, Y.-T.; Wang, Y.-F.; Yen, C.-C.; Lee, G.-L.; Yeh, C.-C.; Peng, Y.-J.; Kuo, Y.-Y.; Wen, H.-T.; et al. Cancer-Derived Succinate Promotes Macrophage Polarization and Cancer Metastasis via Succinate Receptor. Mol. Cell 2020, 77, 213–227.e5. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.-H. Succinate as a Regulator of Hepatic Stellate Cells in Liver Fibrosis. Front. Endocrinol. 2018, 9, 455. [Google Scholar] [CrossRef]
- Shen, D.; Zhang, J.; Yuan, K.; Zhao, J.; Zhao, Z.; Cui, L.; Zhang, Y.; Wang, G.; Cai, S.; Bai, Y.; et al. Landscape of IDH1/2 Mutations in Chinese Patients with Solid Tumors: A Pan-Cancer Analysis. Mol. Genet. Genom. Med. 2021, 9, e1697. [Google Scholar] [CrossRef]
- Ježek, P. 2-Hydroxyglutarate in Cancer Cells. Antioxid. Redox Signal. 2020, 33, 903–926. [Google Scholar] [CrossRef]
- Borger, D.R.; Goyal, L.; Yau, T.; Poon, R.T.; Ancukiewicz, M.; Deshpande, V.; Christiani, D.C.; Liebman, H.M.; Yang, H.; Kim, H.; et al. Circulating Oncometabolite 2-Hydroxyglutarate Is a Potential Surrogate Biomarker in Patients with Isocitrate Dehydrogenase-Mutant Intrahepatic Cholangiocarcinoma. Clin. Cancer Res. 2014, 20, 1884–1890. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Lai, Y.-S.; Tsai, H.-J.; Kuo, C.-C.; Yen, B.L.; Yeh, S.-P.; Sun, H.S.; Hung, W.-C. The Oncometabolite R-2-Hydroxyglutarate Activates NF-ΚB-Dependent Tumor-Promoting Stromal Niche for Acute Myeloid Leukemia Cells. Sci. Rep. 2016, 6, 32428. [Google Scholar] [CrossRef]
- Ma, C.; Kesarwala, A.H.; Eggert, T.; Medina-Echeverz, J.; Kleiner, D.E.; Jin, P.; Stroncek, D.F.; Terabe, M.; Kapoor, V.; ElGindi, M.; et al. NAFLD Causes Selective CD4+ T Lymphocyte Loss and Promotes Hepatocarcinogenesis. Nature 2016, 531, 253–257. [Google Scholar] [CrossRef]
- Zang, S.; Ma, X.; Wu, Y.; Liu, W.; Cheng, H.; Li, J.; Liu, J.; Huang, A. PGE2 Synthesis and Signaling in Malignant Transformation and Progression of Human Hepatocellular Carcinoma. Hum. Pathol. 2017, 63, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Loo, T.M.; Kamachi, F.; Watanabe, Y.; Yoshimoto, S.; Kanda, H.; Arai, Y.; Nakajima-Takagi, Y.; Iwama, A.; Koga, T.; Sugimoto, Y.; et al. Gut Microbiota Promotes Obesity-Associated Liver Cancer through Pge2-Mediated Suppression of Antitumor Immunity. Cancer Discov. 2017, 7, 522–538. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.H.; Yang, Z.S.; Li, M.; Chen, Y.; Zhao, X.F.; Qin, Y.Y.; Song, J.Q.; Wang, B.B.; Yuan, B.; Cui, X.L.; et al. High Serum Levels of Cholesterol Increase Antitumor Functions of Nature Killer Cells and Reduce Growth of Liver Tumors in Mice. Gastroenterology 2020, 158, 1713–1727. [Google Scholar] [CrossRef] [PubMed]
- He, X.Y.; Zhu, M.M.; Zheng, J.; Wang, C.Y.; Zhao, X.K.; Zhang, B.T.; Zhou, D.C.; Zhang, S.; Yang, X.X.; Duan, Y.J.; et al. Liver X Receptor Agonists Exert Antitumor Effects against Hepatocellular Carcinoma via Inducing REPS2 Expression. Acta Pharmacol. Sin. 2023, 44, 635–646. [Google Scholar] [CrossRef]
- Xie, Y.; Sun, R.; Gao, L.; Guan, J.; Wang, J.; Bell, A.; Zhu, J.; Zhang, M.; Xu, M.; Lu, P.; et al. Chronic Activation of LXRα Sensitizes Mice to Hepatocellular Carcinoma. Hepatol. Commun. 2022, 6, 1123–1139. [Google Scholar] [CrossRef]
- Ma, C.; Han, M.; Heinrich, B.; Fu, Q.; Zhang, Q.; Sandhu, M.; Agdashian, D.; Terabe, M.; Berzofsky, J.A.; Fako, V.; et al. Gut Microbiome-Mediated Bile Acid Metabolism Regulates Liver Cancer via NKT Cells. Science 2018, 360, eaan5931. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, Z.; Bao, R.; Guo, X.; Gu, Y.; Yang, W.; Wei, J.; Chen, X.; Tong, L.; Meng, J.; et al. Loss of SIRT5 Promotes Bile Acid-Induced Immunosuppressive Microenvironment and Hepatocarcinogenesis. J. Hepatol. 2022, 77, 453–466. [Google Scholar] [CrossRef]
- Chen, J.; Wang, R.; Liu, Z.; Fan, J.; Liu, S.; Tan, S.; Li, X.; Li, B.; Yang, X. Unbalanced Glutamine Partitioning between CD8T Cells and Cancer Cells Accompanied by Immune Cell Dysfunction in Hepatocellular Carcinoma. Cells 2022, 11, 3924. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, Z.; Tu, M.; Meng, W.; Gao, H.; Li, M.D.; Li, L. Correlation Between Prognostic Biomarker SLC1A5 and Immune Infiltrates in Various Types of Cancers Including Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Zhang, Q.; Lou, Y.; Yang, J.; Wang, J.; Feng, J.; Zhao, Y.; Wang, L.; Huang, X.; Fu, Q.; Ye, M.; et al. Integrated Multiomic Analysis Reveals Comprehensive Tumour Heterogeneity and Novel Immunophenotypic Classification in Hepatocellular Carcinomas. Gut 2019, 68, 2019–2031. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, B.; Tan, W.; Qi, L.; Ma, X.; Wang, X. A Novel Purine and Uric Metabolism Signature Predicting the Prognosis of Hepatocellular Carcinoma. Front. Genet. 2022, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Jiang, W.; Ma, L.; Sun, J.; Yan, X.; Qian, J.; Wang, Y.; Shi, Y.; Ni, S.; Yao, N. A Metabolism-Related Gene Signature for Predicting the Prognosis and Therapeutic Responses in Patients with Hepatocellular Carcinoma. Ann. Transl. Med. 2021, 9, 500. [Google Scholar] [CrossRef] [PubMed]
| Metabolic Pathway | Metabolite | Receptor/Enzyme | Function | References |
|---|---|---|---|---|
| Glycolysis | Lactate | Favor M1 to M2 phenotypic switch | [183,184,185] | |
| Tricarboxylic acid cycle | Succinate | Promote TAM differentiation and cancer cell migration, invasion and metastasis | [187] | |
| 2-Hydroxyglutarate | Promote macrophages differentiation to M2 phenotype | [190,191,192] | ||
| Lipid metabolism | Linoleic acid | Mediate selective intrahepatic CD4+ T lymphocytes cell-death | [204,193] | |
| Prostaglandin E2 | Reduce pro-inflammatory mediators (IFNγ and TNFα) and stimulate the production of anti-inflammatory cytokines (IL-10) | [195] | ||
| Cholesterol | NK cell activation | [196] | ||
| Cholesterol | Reduce cytotoxic activity against liver cancer cells | [52] | ||
| Primary bile acids | Induce the recruitment of NKT cells | [200] | ||
| Secondary bile acids | Reduce NKT cells recruitment favoring a pro-tumorigenic milieu | [200] | ||
| ↑ activity of oxysterol liver X receptor (LXRα) | Induce MDSCs and suppress of cytotoxic T cells and DCs | [198] | ||
| Asparagine metabolism | ↑ ASCT2 ↓ GLS2 | Create immune-suppressive TME: high levels of Treg, Th follicular cells, MΦ macrophage and memory B cells infiltration; low levels of M1 macrophages, T γδ cells, resting memory CD4+ T cells, mast cell and naïve B cells. | [136] | |
| ↑ ASRGL1 | Correlate with TAMs, macrophages, Treg cells, CD8+ T cells, B cells, monocytes, dendritic cells (DCs) and Th1 infiltration | [159] | ||
| Glutamine metabolism | ↑ SLC1A5 | Correlate positively with M2 and Tregs phenotypical markers and negatively with M1 macrophages markers | [201] | |
| Nucleotide metabolism | ↑ purine biosynthesis and metabolism | Correlate M1 macrophages and CD4+ T cells as well as high infiltration of helper T cells and M2 macrophages | [204] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foglia, B.; Beltrà, M.; Sutti, S.; Cannito, S. Metabolic Reprogramming of HCC: A New Microenvironment for Immune Responses. Int. J. Mol. Sci. 2023, 24, 7463. https://doi.org/10.3390/ijms24087463
Foglia B, Beltrà M, Sutti S, Cannito S. Metabolic Reprogramming of HCC: A New Microenvironment for Immune Responses. International Journal of Molecular Sciences. 2023; 24(8):7463. https://doi.org/10.3390/ijms24087463
Chicago/Turabian StyleFoglia, Beatrice, Marc Beltrà, Salvatore Sutti, and Stefania Cannito. 2023. "Metabolic Reprogramming of HCC: A New Microenvironment for Immune Responses" International Journal of Molecular Sciences 24, no. 8: 7463. https://doi.org/10.3390/ijms24087463
APA StyleFoglia, B., Beltrà, M., Sutti, S., & Cannito, S. (2023). Metabolic Reprogramming of HCC: A New Microenvironment for Immune Responses. International Journal of Molecular Sciences, 24(8), 7463. https://doi.org/10.3390/ijms24087463







