Identification of a Specific Plasma Sphingolipid Profile in a Group of Normal-Weight and Obese Subjects: A Novel Approach for a “Biochemical” Diagnosis of Metabolic Syndrome?
Abstract
1. Introduction
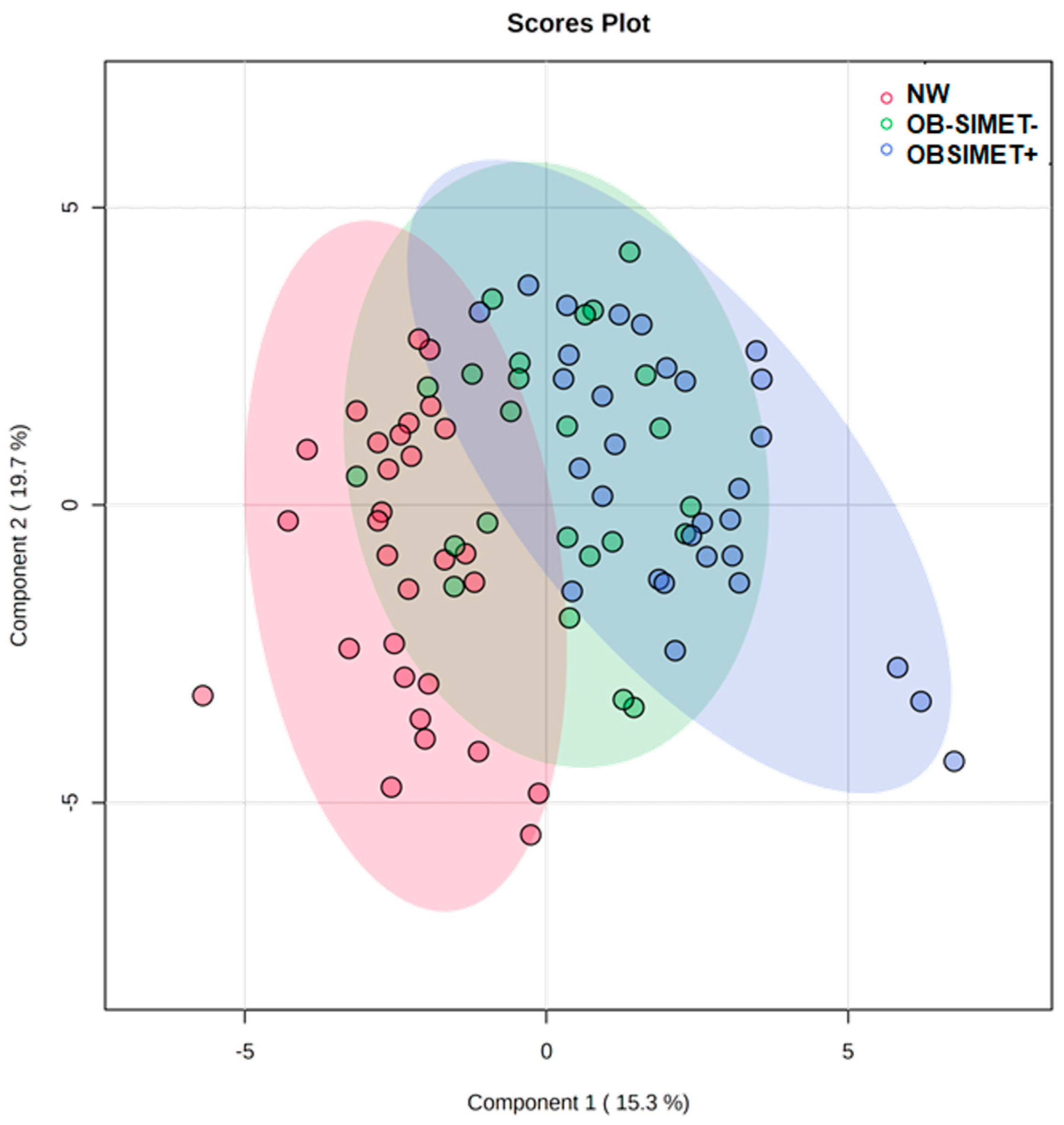
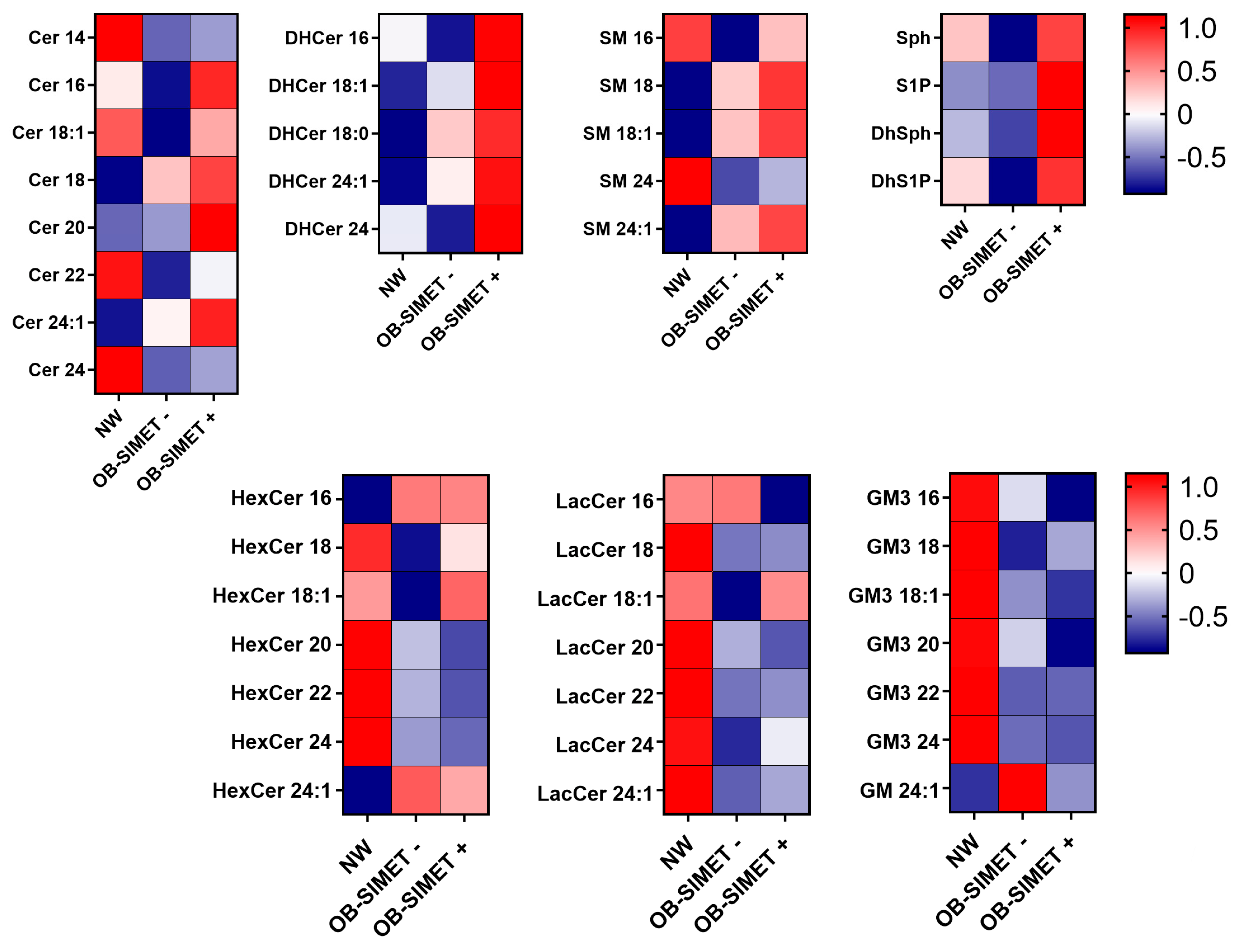
2. Results
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Anthropometric Measurements
4.3. Metabolic Variables
4.4. Blood Pressure
4.5. Definition of Metabolic Syndrome
4.6. Lipid Extraction and Sphingolipid Content Quantification
4.7. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Choi, R.H.; Tatum, S.M.; Symons, J.D.; Summers, S.A.; Holland, W.L. Ceramides and other sphingolipids as drivers of cardiovascular disease. Nat. Rev. Cardiol. 2021, 18, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Devlin, C.M.; Leventhal, A.R.; Kuriakose, G.; Schuchman, E.H.; Williams, K.J.; Tabas, I. Acid sphingomyelinase promotes lipoprotein retention within early atheromata and accelerates lesion progression. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Schissel, S.L.; Tweedie-Hardman, J.; Rapp, J.H.; Graham, G.; Williams, K.J.; Tabas, I. Rabbit aorta and human atherosclerotic lesions hydrolyze the sphingomyelin of retained low-density lipoprotein. Proposed role for arterial-wall sphingomyelinase in subendothelial retention and aggregation of atherogenic lipoproteins. J. Clin. Investig. 1996, 98, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, B.; Summers, S.A. Ceramides—Lipotoxic inducers of metabolic disorders. Trends Endocrinol. Metab. 2015, 26, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Summers, S.A.; Chaurasia, B.; Holland, W.L. Metabolic messengers: Ceramides. Nat. Metab. 2019, 1, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Peng, Y.; Hang, W.; Li, N.; Zhou, N.; Wang, D.W. Emerging roles of ceramide in cardiovascular diseases. Aging Dis. 2022, 13, 232–245. [Google Scholar] [CrossRef]
- Bikman, B.T.; Guan, Y.; Shui, G.; Siddique, M.M.; Holland, W.L.; Kim, J.Y.; Fabriàs, G.; Wenk, M.R.; Summers, S.A. Fenretinide prevents lipid-induced insulin resistance by blocking ceramide biosynthesis. J. Biol. Chem. 2012, 287, 17426–17437. [Google Scholar] [CrossRef]
- Blachnio-Zabielska, A.U.; Chacinska, M.; Vendelbo, M.H.; Zabielski, P. The crucial role of C18-Cer in fat-induced skeletal muscle insulin resistance. Cell. Physiol. Biochem. 2016, 40, 1207–1220. [Google Scholar] [CrossRef]
- Chaurasia, B.; Kaddai, V.A.; Lancaster, G.I.; Henstridge, D.C.; Sriram, S.; Galam, D.L.; Gopalan, V.; Prakash, K.N.; Velan, S.S.; Bulchand, S.; et al. Adipocyte ceramides regulate subcutaneous adipose browning, inflammation, and metabolism. Cell Metab. 2016, 24, 820–834. [Google Scholar] [CrossRef]
- Hojjati, M.R.; Li, Z.; Zhou, H.; Tang, S.; Huan, C.; Ooi, E.; Lu, S.; Jiang, X.C. Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in apoE-deficient mice. J. Biol. Chem. 2005, 280, 10284–10289. [Google Scholar] [CrossRef]
- Holland, W.L.; Brozinick, J.T.; Wang, L.P.; Hawkins, E.D.; Sargent, K.M.; Liu, Y.; Narra, K.; Hoehn, K.L.; Knotts, T.A.; Siesky, A.; et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007, 5, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Kurek, K.; Piotrowska, D.M.; Wiesiołek-Kurek, P.; Łukaszuk, B.; Chabowski, A.; Górski, J.; Zendzian-Piotrowska, M. Inhibition of ceramide de novo synthesis reduces liver lipid accumulation in rats with nonalcoholic fatty liver disease. Liver Int. 2014, 34, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Park, T.S.; Panek, R.L.; Rekhter, M.D.; Mueller, S.B.; Rosebury, W.S.; Robertson, A.; Hanselman, J.C.; Kindt, E.; Homan, R.; Karathanasis, S.K. Modulation of lipoprotein metabolism by inhibition of sphingomyelin synthesis in ApoE knockout mice. Atherosclerosis 2006, 189, 264–272. [Google Scholar] [CrossRef]
- Park, T.S.; Rosebury, W.; Kindt, E.K.; Kowala, M.C.; Panek, R.L. Serine palmitoyltransferase palmitoyltransferase inhibitor myriocin induces the regression of atherosclerotic plaques in hyperlipidemic ApoE-deficient mice. Pharmacol. Res. 2008, 58, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Turpin, S.M.; Nicholls, H.T.; Willmes, D.M.; Mourier, A.; Brodesser, S.; Wunderlich, C.M.; Mauer, J.; Xu, E.; Hammerschmidt, P.; Bronneke, H.S.; et al. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 2014, 20, 678–686. [Google Scholar] [CrossRef]
- Ussher, J.R.; Koves, T.R.; Cadete, V.J.; Zhang, L.; Jaswal, J.S.; Swyrd, S.J.; Lopaschuk, D.G.; Proctor, S.D.; Keung, W.; Muoio, D.M.; et al. Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes 2010, 59, 2453–2464. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Badeanlou, L.; Bielawski, J.; Roberts, A.J.; Hannun, Y.A.; Samad, F. Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E211–E224. [Google Scholar] [CrossRef]
- Zhang, Q.J.; Holland, W.L.; Wilson, L.; Tanner, J.M.; Kearns, D.; Cahoon, J.M.; Pettey, D.; Losee, J.; Duncan, B.; Gale, D.; et al. Ceramide mediates vascular dysfunction in diet-induced obesity by PP2A-mediated dephosphorylation of the eNOS-Akt complex. Diabetes 2012, 61, 1848–1859. [Google Scholar] [CrossRef]
- Cheng, J.M.; Suoniemi, M.; Kardys, I.; Vihervaara, T.; de Boer, S.P.; Akkerhuis, K.M.; Sysi-Aho, M.; Ekroos, K.; Garcia-Garcia, H.M.; Oemrawsingh, R.M.; et al. Plasma concentrations of molecular lipid species in relation to coronary plaque characteristics and cardiovascular outcome: Results of the ATHEROREMO-IVUS study. Atherosclerosis 2015, 243, 560–566. [Google Scholar] [CrossRef]
- Laaksonen, R.; Ekroos, K.; Sysi-Aho, M.; Hilvo, M.; Vihervaara, T.; Kauhanen, D.; Suoniemi, M.; Hurme, R.; März, W.; Scharnagl, H.; et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur. Heart J. 2016, 37, 1967–1976. [Google Scholar] [CrossRef]
- Pan, W.; Yu, J.; Shi, R.; Yan, L.; Yang, T.; Li, Y.; Zhang, Z.; Yu, G.; Bai, Y.; Schuchman, E.H.; et al. Elevation of ceramide and activation of secretory acid sphingomyelinase in patients with acute coronary syndromes. Coron. Artery Dis. 2014, 25, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Tarasov, K.; Ekroos, K.; Suoniemi, M.; Kauhanen, D.; Sylvänne, T.; Hurme, R.; Gouni-Berthold, I.; Berthold, H.K.; Kleber, M.E.; Laaksonen, R.; et al. Molecular lipids identify cardiovascular risk and are efficiently lowered by simvastatin and PCSK9 deficiency. J. Clin. Endocrinol. Metab. 2014, 99, E45–E52. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Pan, W.; Shi, R.; Yang, T.; Li, Y.; Yu, G.; Bai, Y.; Schuchman, E.H.; He, X.; Zhang, G. Ceramide is upregulated and associated with mortality in patients with chronic heart failure. Can. J. Cardiol. 2015, 31, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Bergman, B.C.; Brozinick, J.T.; Strauss, A.; Bacon, S.; Kerege, A.; Bui, H.H.; Sanders, P.; Siddall, P.; Kuo, M.S.; Perreault, L. Serum sphingolipids: Relationships to insulin sensitivity and changes with exercise in humans. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E398–E408. [Google Scholar] [CrossRef]
- Boon, J.; Hoy, A.J.; Stark, R.; Brown, R.D.; Meex, R.C.; Henstridge, D.C.; Schenk, S.; Meikle, P.J.; Horowitz, J.F.; Kingwell, B.A.; et al. Ceramides contained in LDL are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance. Diabetes 2013, 62, 401–410. [Google Scholar] [CrossRef]
- de Mello, V.D.; Lankinen, M.; Schwab, U.; Kolehmainen, M.; Lehto, S.; Seppänen-Laakso, T.; Oresic, M.; Pulkkinen, L.; Uusitupa, M.; Erkkila, A.T. Link between plasma ceramides, inflammation and insulin resistance: Association with serum IL-6 concentration in patients with coronary heart disease. Diabetologia 2009, 52, 2612–2615. [Google Scholar] [CrossRef]
- Haus, J.M.; Kashyap, S.R.; Kasumov, T.; Zhang, R.; Kelly, K.R.; Defronzo, R.A.; Kirwan, J.P. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes 2009, 58, 337–343. [Google Scholar] [CrossRef]
- Lopez, X.; Goldfine, A.B.; Holland, W.L.; Gordillo, R.; Scherer, P.E. Plasma ceramides are elevated in female children and adolescents with type 2 diabetes. J. Pediatr. Endocrinol. Metab. 2013, 26, 995–998. [Google Scholar] [CrossRef]
- Summers, S.A. Could ceramides become the new cholesterol? Cell Metab. 2018, 27, 276–280. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C.; et al. Harmonizing the metabolic syndrome. A joint interim statement of the International Diabetes Federation Task Force on epidemiology and prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Reaven, G.M. The metabolic syndrome: Is this diagnosis necessary? Am. J. Clin. Nutr. 2006, 83, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Green, C.D.; Maceyka, M.; Cowart, L.A.; Spiegel, S. Sphingolipids in metabolic disease: The good, the bad, and the unknown. Cell Metab. 2021, 33, 1293–1306. [Google Scholar] [CrossRef] [PubMed]
- Holland, W.L.; Bikman, B.T.; Wang, L.P.; Yuguang, G.; Sargent, K.M.; Bulchand, S.; Knotts, T.A.; Shui, G.; Clegg, D.J.; Wenk, M.R.; et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J. Clin. Investig. 2011, 121, 1858–1870. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Bielawski, J.; Samad, F.; Merrill, A.H.; Cowart, L.A. Palmitate increases sphingosine-1-phosphate in C2C12 myotubes via upregulation of sphingosine kinase message and activity. J. Lipid Res. 2009, 50, 1852–1862. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Ross, J.; Geng, T.; Brice, S.E.; Cowart, L.A. Differential regulation of dihydroceramide desaturase by palmitate versus monounsaturated fatty acids: Implications for insulin resistance. J. Biol. Chem. 2011, 286, 16596–16605. [Google Scholar] [CrossRef]
- Xie, C.; Yagai, T.; Luo, Y.; Liang, X.; Chen, T.; Wang, Q.; Sun, D.; Zhao, J.; Ramakrishnan, S.K.; Sun, L.; et al. Activation of intestinal hypoxia-inducible factor 2a during obesity contributes to hepatic steatosis. Nat. Med. 2017, 23, 1298–1308. [Google Scholar] [CrossRef]
- Chen, W.; Lu, H.; Yang, J.; Xiang, H.; Peng, H. Sphingosine 1-phosphate in metabolic syndrome (Review). Int. J. Mol. Med. 2016, 38, 1030–1038. [Google Scholar] [CrossRef]
- Visser, M.; Bouter, L.M.; McQuillan, G.M.; Wener, M.H.; Harris, T.B. Elevated C-reactive protein levels in overweight and obese adults. JAMA 1999, 282, 2131–2135. [Google Scholar] [CrossRef]
- Mohan, V.; Deepa, R.; Pradeepa, R.; Vimaleswaran, K.S.; Mohan, A.; Velmurugan, K.; Radha, V. Association of low adiponectin levels with the metabolic syndrome—The Chennai Urban Rural Epidemiology Study (CURES-4). Metabolism 2005, 54, 476–481. [Google Scholar] [CrossRef]
- Holland, W.L.; Scherer, P.E. PAQRs: A counteracting force to ceramides? Mol. Pharmacol. 2009, 75, 740–743. [Google Scholar] [CrossRef]
- Blachnio-Zabielska, A.U.; Koutsari, C.; Tchkonia, T.; Jensen, M.D. Sphingolipid content of human adipose tissue: Relationship to adiponectin and insulin resistance. Obesity 2012, 20, 2341–2347. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, B.; Tippetts, T.S.; Mayoral Monibas, R.; Liu, J.; Li, Y.; Wang, L.; Wilkerson, J.L.; Sweeney, C.R.; Pereira, R.F.; Sumida, D.H.; et al. Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science 2019, 365, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Obeid, L.M.; Linardic, C.M.; Karolak, L.A.; Hannun, Y.A. Programmed cell death induced by ceramide. Science 1993, 259, 1769–1771. [Google Scholar] [CrossRef]
- Kolesnick, R. Ceramide: A novel second messenger. Trends Cell Biol. 1992, 2, 232–236. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Ceramide: An intracellular signal for apoptosis. Trends Biochem. Sci. 1995, 20, 73–77. [Google Scholar] [CrossRef]
- Maceyka, M.; Spiegel, S. Sphingolipid metabolites in inflammatory disease. Nature 2014, 510, 58–67. [Google Scholar] [CrossRef]
- Geng, T.; Sutter, A.; Harland, M.D.; Law, B.A.; Ross, J.S.; Lewin, D.; Palanisamy, A.; Russo, S.B.; Chavin, K.D.; Cowart, L.A. SphK1 mediates hepatic inflammation in a mouse model of NASH induced by high saturated fat feeding and initiates proinflammatory signaling in hepatocytes. J. Lipid Res. 2015, 56, 2359–2371. [Google Scholar] [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Petrov, D.; Jucan, A.E.; Lacatusu, C.M.; Floria, M.; Tarniceriu, C.C.; Costea, C.F.; Ciocoiu, M.; Rezus, C. Involvement of ceramides in non-alcoholic fatty liver disease (NAFLD) atherosclerosis (ATS) development: Mechanisms and therapeutic targets. Diagnostics 2021, 11, 2053. [Google Scholar] [CrossRef]
- Chavez, J.A.; Knotts, T.A.; Wang, L.P.; Li, G.; Dobrowsky, R.T.; Florant, G.L.; Summers, S.A. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J. Biol. Chem. 2003, 278, 10297–10303. [Google Scholar] [CrossRef]
- Stratford, S.; Hoehn, K.L.; Liu, F.; Summers, S.A. Regulation of insulin action by ceramide: Dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J. Biol. Chem. 2004, 279, 36608–36615. [Google Scholar] [CrossRef] [PubMed]
- Powell, D.J.; Hajduch, E.; Kular, G.; Hundal, H.S. Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKCzeta-dependent mechanism. Mol. Cell. Biol. 2003, 23, 7794–7808. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Xie, C.; Li, F.; Zhang, L.; Nichols, R.G.; Krausz, K.W.; Cai, J.; Qi, Y.; Fang, Z.Z.; Takahashi, S.; et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J. Clin. Investig. 2015, 125, 386–402. [Google Scholar] [CrossRef]
- Xia, J.Y.; Holland, W.L.; Kusminski, C.M.; Sun, K.; Sharma, A.X.; Pearson, M.J.; Sifuentes, A.J.; McDonald, J.G.; Gordillo, R.; Scherer, P.E. Targeted induction of ceramide degradation leads to improved systemic metabolism and reduced hepatic steatosis. Cell Metab. 2015, 22, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Hammerschmidt, P.; Ostkotte, D.; Nolte, H.; Gerl, M.J.; Jais, A.; Brunner, H.L.; Sprenger, H.G.; Awazawa, M.; Nicholls, H.T.; Turpin-Nolan, S.M.; et al. CerS6-derived sphingolipids interact with Mff and promote mitochondrial fragmentation in obesity. Cell 2019, 177, 1536–1552. [Google Scholar] [CrossRef]
- Zigdon, H.; Kogot-Levin, A.; Park, J.W.; Goldschmidt, R.; Kelly, S.; Merrill, A.H.; Scherz, A.; Pewzner-Jung, Y.; Saada, A.; Futerman, A.H. Ablation of ceramide synthase 2 causes chronic oxidative stress due to disruption of the mitochondrial respiratory chain. J. Biol. Chem. 2013, 288, 4947–4956. [Google Scholar] [CrossRef]
- Raichur, S.; Wang, S.T.; Chan, P.W.; Li, Y.; Ching, J.; Chaurasia, B.; Dogra, S.; Ohman, M.K.; Takeda, K.; Sugii, S.; et al. CerS2 haploinsufficiency inhibits b-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab. 2014, 20, 687–695. [Google Scholar] [CrossRef]
- Hammad, S.M.; Pierce, J.S.; Soodavar, F.; Smith, K.J.; Al Gadban, M.M.; Rembiesa, B.; Klein, R.L.; Hannun, Y.A.; Bielawski, J.; Bielawska, A. Blood sphingolipidomics in healthy humans: Impact of sample collection methodology. J. Lipid Res. 2010, 51, 3074–3087. [Google Scholar] [CrossRef]
- Scherer, M.; Böttcher, A.; Schmitz, G.; Liebisch, G. Sphingolipid profiling of human plasma and FPLC-separated lipoprotein fractions by hydrophilic interaction chromatography tandem mass spectrometry. Biochim. Biophys. Acta 2011, 1811, 68–75. [Google Scholar] [CrossRef]
- Iqbal, J.; Walsh, M.T.; Hammad, S.M.; Cuchel, M.; Tarugi, P.; Hegele, R.A.; Davidson, N.O.; Rader, D.J.; Klein, R.L.; Hussain, M.M. Microsomal triglyceride transfer protein transfers and determines plasma concentrations of ceramide and sphingomyelin but not glycosylceramide. J. Biol. Chem. 2015, 290, 25863–25875. [Google Scholar] [CrossRef]
- Iqbal, J.; Walsh, M.T.; Hammad, S.M.; Hussain, M.M. Sphingolipids and Lipoproteins in Health and Metabolic Disorders. Trends Endocrinol. Metab. 2017, 28, 506–518. [Google Scholar] [CrossRef]
- Bismuth, J.; Lin, P.; Yao, Q.; Chen, C. Ceramide: A common pathway for atherosclerosis? Atherosclerosis 2008, 196, 497–504. [Google Scholar] [CrossRef]
- McGurk, K.A.; Keavney, B.D.; Nicolaou, A. Circulating ceramides as biomarkers of cardiovascular disease: Evidence from phenotypic and genomic studies. Atherosclerosis 2021, 327, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Li, W.; Wang, J.; Altura, B.T.; Altura, B.M. Sphingomyelinase and ceramide analogs induce contraction and rises in [Ca2+]i in canine cerebral vascular muscle. Am. J. Physiol.-Heart Circ. Physiol. 2000, 278, H1421–H1428. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.; Kane, K.A.; Pyne, N.J.; Pyne, S. Targeting Sphingosine-1-Phosphate Signalling for Cardioprotection. Curr. Opin. Pharmacol. 2009, 9, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Van Brocklyn, J.R.; Williams, J.B. The Control of the Balance between Ceramide and Sphingosine-1-Phosphate by Sphingosine Kinase: Oxidative Stress and the Seesaw of Cell Survival and Death. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2012, 163, 26–36. [Google Scholar] [CrossRef]
- Shalaby, Y.M.; Al Aidaros, A.; Valappil, A.; Ali, B.R.; Akawi, N. Role of ceramides in the molecular pathogenesis and potential therapeutic strategies of cardiometabolic diseases: What we know so far. Front. Cell Dev. Biol. 2022, 9, 816301. [Google Scholar] [CrossRef]
- Kayser, B.D.; Prifti, E.; Lhomme, M.; Belda, E.; Dao, M.C.; Aron-Wisnewsky, J.; MICRO-Obes Consortium; Kontush, A.; Zucker, J.D.; Rizkalla, S.W.; et al. Elevated serum ceramides are linked with obesity-associated gut dysbiosis and impaired glucose metabolism. Metabolomics 2019, 15, 140. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Merrill, A.H.; Sullards, M.C.; Allegood, J.C.; Kelly, S.; Wang, E. Sphingolipidomics: High-throughput, structure-specific, and quantitative analysis of sphingolipids by liquid chromatography tandem mass spectrometry. Methods 2005, 36, 207–224. [Google Scholar] [CrossRef]
- Platania, C.B.M.; Dei Cas, M.; Cianciolo, S.; Fidilio, A.; Lazzara, F.; Paroni, R.; Pignatello, R.; Strettoi, E.; Ghidoni, R.; Drago, F.; et al. Novel ophthalmic formulation of myriocin: Implications in retinitis pigmentosa. Drug Deliv. 2019, 26, 237–243. [Google Scholar] [CrossRef] [PubMed]

| Parameter | NW | OB-SIMET− | OB-SIMET+ |
|---|---|---|---|
| N. | 30 | 24 | 30 |
| Sex (F/M) | 19F-11M | 18F-6M | 19F-11M |
| Age (years) | 29.15 [26.46; 33.14] | 27.38 [21.35; 35.65] | 30.43 [23.98; 41.18] |
| BMI (kg/m2) | 22.85 [20.79; 24.70] | 42.88 [40.75; 119.25] a | 43.44 [41.53; 46.54] a |
| WHR | 78 [76.25; 82.75] | 110 [106; 82.75] a | 120 [113.25; 126.50] a |
| FFM (kg) | 53.06 [46.26; 59.02] | 55.64 [50.84; 63.59] | 61.86 [53.76; 66.03] a |
| FFM % | 79.45 [73.98; 82.30] | 47.40 [44.88; 53.08] a | 48.75 [43.68; 52.73] a |
| FM (kg) | 13.13 [10.83; 17.95] | 60.27 [52.80; 67.06] a | 61 [57.27; 68.08] a |
| FM % | 20.20 [17.53; 26.03] | 52.60 [46.93; 55.13] a | 51.25 [47.40; 56.53] a |
| SBP (mmHg) | 120 [110; 120] | 120 [120; 130] a | 130 [130; 140] a,b |
| DBP (mmHg) | 70 [70; 75] | 80 [77.50; 80] a | 80 [80; 90] a |
| HR (bm) | 70 [69; 72] | 80 [77.50; 90] a | 88 [84.25; 96.75] a |
| REE (kcal/24 h) | 1572.50 [1378.75; 1845.75] | 1902.50 [1804; 2245.75] a | 2070.50 [1852.25; 2308.50] a |
| Glucose (mg/dL) | 87 [82.25; 94.25] | 83 [80; 88.25] | 86 [82.25; 94.75] |
| Insulin (mU/L) | 6.65 [5.13; 8.80] | 15.85 [11; 23.55] a | 25.05 [19.08; 30.25] a |
| HOMA-IR | 1.54 [1.07; 1.84] | 3.23 [2.23; 4.68] a | 5.30 [4.32; 6.21] a |
| T-C (mg/dL) | 173 [158; 200.50] | 160.50 [133.25; 188.50] | 163 [148; 196] |
| HDL-C (mg/dL) | 65 [56.25; 70.75] | 45.50 [39.50; 50.25] a | 37.50 [32.50; 43.75] a |
| LDL-C (mg/dL) | 106.50 [86.25; 120.50] | 101.50 [77.75; 122.25] | 107.50 [96; 124.75] |
| TG (mg/dL) | 63 [53; 85.75] | 96 [85.75; 123.25] a | 125.50 [103.50; 159.25] a |
| HbA1c (mmol/L) | 5.10 [5; 5.30] | 5.10 [5; 5.40] | 5.40 [5.10; 5.60] a |
| CRP (mg/dL) | 0.10 [0; 0.20] | 0.50 [0.28; 1.03] a | 0.55 [0.40; 1.08] a |
| Sphingolipid | NW | OB-SIMET− | OB-SIMET+ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (µmol/L) | Median | 25th | 75th | Median | 25th | 75th | Median | 25th | 75th |
| Cer 14:0 | 0.0168 | 0.0140 | 0.0207 | 0.0124 | 0.0102 | 0.0174 | 0.0138 | 0.0112 | 0.0159 |
| Cer 16:0 | 0.4463 | 0.3834 | 0.5280 | 0.4359 | 0.4025 | 0.4727 | 0.4533 | 0.3815 | 0.5484 |
| Cer 18:1 | 0.0149 | 0.0132 | 0.0171 | 0.0143 | 0.0123 | 0.0164 | 0.0156 | 0.0127 | 0.0176 |
| Cer 18:0 | 0.0663 | 0.0557 | 0.0790 | 0.1121 | 0.0794 | 0.1377 | 0.1242 | 0.1053 | 0.1714 |
| Cer 20:0 | 0.0810 | 0.0640 | 0.0961 | 0.0861 | 0.0679 | 0.0968 | 0.0859 | 0.0718 | 0.1213 |
| Cer 22:0 | 0.6195 | 0.4530 | 0.7052 | 0.4103 a | 0.3459 | 0.5539 | 0.4815 | 0.3658 | 0.6725 |
| Cer 24:1 | 0.7663 | 0.5601 | 0.9362 | 0.9581 | 0.8408 | 1.1890 | 1.1021 a | 0.8999 | 1.3634 |
| Cer 24:0 | 3.7086 | 2.9225 | 4.0040 | 2.1955 a | 1.7563 | 3.1066 | 2.3265 a | 1.9734 | 3.0387 |
| DHCER 16:0 | 0.0260 | 0.0200 | 0.0356 | 0.0240 | 0.0204 | 0.0282 | 0.0335 b | 0.0250 | 0.0402 |
| DHCer 18:1 | 0.0046 | 0.0031 | 0.0075 | 0.0061 | 0.0046 | 0.0100 | 0.0084 a | 0.0069 | 0.0128 |
| DHCer 18:0 | 0.0060 | 0.0037 | 0.0078 | 0.0170 a | 0.0108 | 0.0205 | 0.0230 a | 0.0177 | 0.0323 |
| DHCER 24:1 | 0.0746 | 0.0542 | 0.1108 | 0.1415 a | 0.0931 | 0.1991 | 0.2081 a,b | 0.1541 | 0.2759 |
| DHCer 24:0 | 0.1817 | 0.1339 | 0.2618 | 0.1630 | 0.1248 | 0.2486 | 0.2486 | 0.1697 | 0.3262 |
| SM 16:0 | 120.4211 | 113.9908 | 131.7082 | 117.3254 | 101.4098 | 127.1680 | 121.3630 | 110.4303 | 133.8067 |
| SM 18:0 | 33.2488 | 24.8709 | 38.5798 | 41.0255 a | 31.5397 | 47.9056 | 47.6799 a | 39.0624 | 54.0556 |
| SM 18:1 | 21.7878 | 18.5967 | 24.0871 | 28.0090 a | 25.9735 | 31.1825 | 31.2947 a | 28.3160 | 34.2958 |
| SM 24:0 | 17.1339 | 11.9928 | 28.2404 | 13.4373 | 10.2122 | 21.6739 | 14.3467 | 10.0835 | 21.3914 |
| SM 24:1 | 40.4368 | 29.6155 | 49.6716 | 50.2870 | 37.7062 | 59.8301 | 48.4161 a | 42.1587 | 59.6591 |
| Total Cer | 5.8230 | 4.3690 | 6.3426 | 4.3506 a | 3.7056 | 5.5548 | 4.5706 | 3.8788 | 6.0811 |
| Total DHCer | 0.3034 | 0.2126 | 0.4238 | 0.3677 | 0.2625 | 0.4987 | 0.5083 a,b | 0.4007 | 0.7113 |
| Total SM | 237.6740 | 204.1032 | 265.0412 | 246.9296 | 221.2554 | 279.2727 | 270.6917 | 234.2189 | 304.6080 |
| HexCer 16:0 | 1.4962 | 1.2149 | 1.8136 | 1.3930 | 1.2467 | 1.7919 | 1.4189 | 1.1997 | 1.8053 |
| HexCer 18:0 | 0.2198 | 0.1820 | 0.2800 | 0.2269 | 0.2033 | 0.2564 | 0.2253 | 0.1839 | 0.2572 |
| HexCer 18:1 | 0.0288 | 0.0167 | 0.0451 | 0.0209 | 0.0163 | 0.0276 | 0.0347 | 0.0182 | 0.0462 |
| HexCer 20:0 | 0.2955 | 0.2324 | 0.3929 | 0.2625 | 0.2014 | 0.3078 | 0.2503 | 0.1916 | 0.2917 |
| HexCer 22:0 | 4.1124 | 3.7338 | 5.0359 | 2.8256 a | 2.3509 | 3.4540 | 2.7980 a | 2.0631 | 3.3255 |
| HexCer 24:0 | 4.6624 | 3.8590 | 5.7986 | 3.1278 a | 2.3262 | 4.0775 | 3.0659 a | 2.3020 | 4.7563 |
| HexCer 24:1 | 3.8078 | 3.3694 | 4.7813 | 5.2074 a | 4.4501 | 6.6098 | 5.0210 a | 4.0688 | 6.5814 |
| LacCer 16:0 | 8.1071 | 6.6496 | 10.3397 | 7.8664 | 6.4482 | 10.4543 | 7.0130 | 6.0758 | 8.9284 |
| LacCer 18:0 | 0.1483 | 0.1045 | 0.2188 | 0.1370 | 0.0964 | 0.1783 | 0.1199 | 0.0883 | 0.2107 |
| LacCer 18:1 | 0.0454 | 0.0361 | 0.0709 | 0.0421 | 0.0320 | 0.0632 | 0.0478 | 0.0328 | 0.0616 |
| LacCer 20:0 | 0.0519 | 0.0267 | 0.1098 | 0.0430 | 0.0243 | 0.0774 | 0.0300 | 0.0194 | 0.0648 |
| LacCer 22:0 | 0.1005 | 0.0648 | 0.4088 | 0.1216 | 0.0385 | 0.2006 | 0.0519 | 0.0276 | 0.1925 |
| LacCer 24:0 | 0.0154 | 0.0065 | 0.1763 | 0.0397 | 0.0049 | 0.1195 | 0.0089 | 0.0032 | 0.0940 |
| LacCer 24:1 | 0.2026 | 0.1410 | 1.3683 | 0.3930 | 0.1163 | 1.1021 | 0.1191 | 0.0701 | 0.8776 |
| GM3 16:0 | 2.1501 | 1.6216 | 3.0089 | 1.8378 | 1.6095 | 2.6726 | 1.7537 | 1.3393 | 2.2612 |
| GM3 18:0 | 0.4925 | 0.3363 | 0.7387 | 0.3964 | 0.2763 | 0.6066 | 0.3483 | 0.2703 | 0.4925 |
| GM3 18:1 | 0.0240 | 0.0240 | 0.0480 | 0.0240 | 0.0240 | 0.0300 | 0.0240 | 0.0000 | 0.0480 |
| GM3 20:0 | 0.1201 | 0.0541 | 0.1922 | 0.1081 | 0.0661 | 0.1682 | 0.0841 | 0.0480 | 0.1201 |
| GM3 22:0 | 1.2252 | 0.8108 | 1.6996 | 0.7447 a | 0.4985 | 0.9429 | 0.6246 a | 0.4444 | 0.7928 |
| GM3 24:0 | 0.2162 | 0.1742 | 0.3123 | 0.1201 a | 0.0480 | 0.1501 | 0.0721 a | 0.0240 | 0.1201 |
| GM3 24:1 | 1.0991 | 0.8108 | 1.3453 | 1.2132 | 0.7087 | 1.7837 | 1.0450 | 0.8288 | 1.4579 |
| Total HexCer | 14.6067 | 12.9005 | 16.8163 | 13.5736 | 11.3378 | 15.6432 | 12.7833 | 10.3120 | 16.5317 |
| Total LacCer | 8.8129 | 7.0560 | 13.4106 | 9.3226 | 6.7950 | 12.6217 | 7.3023 | 6.2825 | 10.8854 |
| Total GM3 | 5.1409 | 3.8797 | 6.4382 | 4.3722 | 3.0855 | 5.8496 | 3.9037 | 3.2011 | 4.7520 |
| Sph | 0.1182 | 0.0982 | 0.1528 | 0.1094 | 0.0977 | 0.1233 | 0.1207 | 0.1059 | 0.1483 |
| S1P | 1.6429 | 1.3007 | 1.9686 | 1.5321 | 1.3267 | 1.7749 | 1.9854 a,b | 1.7742 | 2.1176 |
| DHSph | 0.0155 | 0.0132 | 0.0204 | 0.0177 | 0.0131 | 0.0207 | 0.0187 | 0.0133 | 0.0242 |
| DHS1P | 0.3589 | 0.2552 | 0.4163 | 0.2694 | 0.2282 | 0.3673 | 0.3509 | 0.2894 | 0.4011 |
| Coefficient (×10−3) | Std. Error (×10−3) | t | p | VIF | |
|---|---|---|---|---|---|
| DHCer 18:0 | |||||
| Constant | −4.7 | 11.4 | −0.409 | 0.684 | |
| WC (cm) | 0.3 | 0.1 | 3.747 | <0.001 | 3.476 |
| SBP (mmHg) | −0.1 | 0.1 | −0.779 | 0.439 | 2.478 |
| DBP (mmHg) | −0.2 | 0.1 | −1.281 | 0.204 | 2.291 |
| HOMA-IR | 2.0 | 0.4 | 4.739 | <0.001 | 1.864 |
| HDL (mg/dL) | 0.1 | 0.1 | 0.777 | 0.44 | 2.583 |
| TG (mg/dL) | 0.0 | 0.0 | 2.122 | 0.037 | 1.567 |
| CRP (mg/dL) | 2.0 | 1.6 | 1.294 | 0.2 | 1.38 |
| DHCer 24:1 | |||||
| Constant | 19.2 | 97.4 | 0.198 | 0.844 | |
| WC (cm) | 1.5 | 0.6 | 2.408 | 0.018 | 3.476 |
| SBP (mmHg) | −0.6 | 0.8 | −0.752 | 0.454 | 2.478 |
| DBP (mmHg) | −1.0 | 1.1 | −0.943 | 0.349 | 2.291 |
| HOMA-IR | 15.2 | 3.6 | 4.183 | <0.001 | 1.864 |
| HDL (mg/dL) | 0.4 | 0.7 | 0.65 | 0.518 | 2.583 |
| TG (mg/dL) | 0.6 | 0.2 | 3.504 | <0.001 | 1.567 |
| CRP (mg/dL) | 12.8 | 13.4 | 0.953 | 0.344 | 1.38 |
| Cer 18:0 | |||||
| Constant | 90.1 | 66.4 | 1.356 | 0.179 | |
| WC (cm) | 1.0 | 0.4 | 2.459 | 0.016 | 3.476 |
| SBP (mmHg) | −1.3 | 0.6 | −2.25 | 0.027 | 2.478 |
| DBP (mmHg) | 0.6 | 0.8 | 0.801 | 0.426 | 2.291 |
| HOMA-IR | 6.4 | 2.5 | 2.571 | 0.012 | 1.864 |
| HDL (mg/dL) | 0.0 | 0.5 | −0.0872 | 0.931 | 2.583 |
| TG (mg/dL) | 0.0 | 0.1 | −0.0714 | 0.943 | 1.567 |
| CRP (mg/dL) | 15.4 | 9.1 | 1.683 | 0.096 | 1.38 |
| HexCer 22:0 | |||||
| Constant | 4314.0 | 1757.0 | 2.455 | 0.016 | |
| WC (cm) | −22.3 | 10.9 | −2.057 | 0.043 | 3.476 |
| SBP (mmHg) | −21.4 | 15.3 | −1.395 | 0.167 | 2.478 |
| DBP (mmHg) | 30.1 | 19.8 | 1.517 | 0.133 | 2.291 |
| HOMA-IR | 65.3 | 65.7 | 0.994 | 0.323 | 1.864 |
| HDL (mg/dL) | 30.7 | 12.1 | 2.536 | 0.013 | 2.583 |
| TG (mg/dL) | 0.6 | 2.9 | 0.195 | 0.846 | 1.567 |
| CRP (mg/dL) | −35.8 | 241.0 | −0.148 | 0.883 | 1.38 |
| GM3 24:0 | |||||
| Constant | 602.0 | 237.0 | 2.539 | 0.013 | |
| WC (cm) | −2.0 | 1.5 | −1.335 | 0.186 | 3.476 |
| SBP (mmHg) | −3.4 | 2.1 | −1.665 | 0.1 | 2.478 |
| DBP (mmHg) | 2.4 | 2.7 | 0.882 | 0.381 | 2.291 |
| HOMA-IR | 1.8 | 8.9 | 0.203 | 0.84 | 1.864 |
| HDL (mg/dL) | 0.8 | 1.6 | 0.466 | 0.643 | 2.583 |
| TG (mg/dL) | −0.2 | 0.4 | −0.485 | 0.629 | 1.567 |
| CRP (mg/dL) | −19.7 | 32.6 | −0.604 | 0.548 | 1.38 |
| Cer 24:1 | |||||
| Constant | 562.0 | 460.0 | 1.222 | 0.226 | |
| WC (cm) | 7.9 | 2.8 | 2.765 | 0.007 | 3.476 |
| SBP (mmHg) | −9.6 | 4.0 | −2.384 | 0.02 | 2.478 |
| DBP (mmHg) | 4.0 | 5.2 | 0.777 | 0.439 | 2.291 |
| HOMA-IR | 37.8 | 17.2 | 2.201 | 0.031 | 1.864 |
| HDL (mg/dL) | 4.0 | 3.2 | 1.274 | 0.207 | 2.583 |
| TG (mg/dL) | 0.8 | 0.8 | 1.077 | 0.285 | 1.567 |
| CRP (mg/dL) | 65.4 | 63.2 | 1.034 | 0.304 | 1.38 |
| SM 18:1 | |||||
| Constant | 5765.0 | 7455.0 | 0.773 | 0.442 | |
| WC (cm) | 167.0 | 46.1 | 3.633 | <0.001 | 3.476 |
| SBP (mmHg) | −96.1 | 65.0 | −1.478 | 0.143 | 2.478 |
| DBP (mmHg) | 90.7 | 84.2 | 1.078 | 0.285 | 2.291 |
| HOMA-IR | 801.0 | 279.0 | 2.874 | 0.005 | 1.864 |
| HDL (mg/dL) | 71.4 | 51.3 | 1.392 | 0.168 | 2.583 |
| TG (mg/dL) | 27.1 | 12.3 | 2.208 | 0.03 | 1.567 |
| CRP (mg/dL) | −1023.0 | 1024.0 | −0.999 | 0.321 | 1.38 |
| SM 18:0 | |||||
| Constant | 38,547.0 | 14,936.0 | 2.581 | 0.012 | |
| WC (cm) | 171.0 | 92.3 | 1.852 | 0.068 | 3.476 |
| SBP (mmHg) | −333.0 | 130.0 | −2.557 | 0.013 | 2.478 |
| DBP (mmHg) | 256.0 | 169.0 | 1.52 | 0.133 | 2.291 |
| HOMA-IR | 1359.0 | 558.0 | 2.435 | 0.017 | 1.864 |
| HDL (mg/dL) | −14.6 | 103.0 | −0.142 | 0.887 | 2.583 |
| TG (mg/dL) | −0.1 | 24.6 | −0.00211 | 0.998 | 1.567 |
| CRP (mg/dL) | 2107.0 | 2052.0 | 1.027 | 0.308 | 1.38 |
| DHCer 18:1 | |||||
| Constant | −5.6 | 7.9 | −0.711 | 0.48 | |
| WC (cm) | 0.0 | 0.0 | 0.996 | 0.322 | 3.476 |
| SBP (mmHg) | 0.0 | 0.1 | 0.183 | 0.855 | 2.478 |
| DBP (mmHg) | 0.0 | 0.1 | 0.092 | 0.927 | 2.291 |
| HOMA-IR | 0.5 | 0.3 | 1.623 | 0.109 | 1.864 |
| HDL (mg/dL) | 0.1 | 0.1 | 1.058 | 0.293 | 2.583 |
| TG (mg/dL) | 0.0 | 0.0 | 0.195 | 0.846 | 1.567 |
| CRP (mg/dL) | 2.3 | 1.1 | 2.123 | 0.037 | 1.38 |
| HexCer 24:0 | |||||
| Constant | 3487.0 | 2627.0 | 1.327 | 0.188 | |
| WC (cm) | −18.2 | 16.2 | −1.12 | 0.266 | 3.476 |
| SBP (mmHg) | −31.1 | 22.9 | −1.357 | 0.179 | 2.478 |
| DBP (mmHg) | 41.8 | 29.7 | 1.41 | 0.162 | 2.291 |
| HOMA-IR | 80.8 | 98.2 | 0.823 | 0.413 | 1.864 |
| HDL (mg/dL) | 45.5 | 18.1 | 2.519 | 0.014 | 2.583 |
| TG (mg/dL) | 4.6 | 4.3 | 1.051 | 0.296 | 1.567 |
| CRP (mg/dL) | 79.0 | 361.0 | 0.219 | 0.827 | 1.38 |
| SM 24:1 | |||||
| Constant | 55,470.0 | 20,366.0 | 2.724 | 0.008 | |
| WC (cm) | 81.7 | 126.0 | 0.649 | 0.518 | 3.476 |
| SBP (mmHg) | −329.0 | 178.0 | −1.853 | 0.068 | 2.478 |
| DBP (mmHg) | 255.0 | 230.0 | 1.107 | 0.272 | 2.291 |
| HOMA-IR | 1387.0 | 761.0 | 1.822 | 0.072 | 1.864 |
| HDL (mg/dL) | −67.3 | 140.0 | −0.48 | 0.632 | 2.583 |
| TG (mg/dL) | 3.9 | 33.5 | 0.116 | 0.908 | 1.567 |
| CRP (mg/dL) | 3251.0 | 2798.0 | 1.162 | 0.249 | 1.38 |
| S1P | |||||
| Constant | 282.0 | 716.0 | 0.394 | 0.695 | |
| WC (cm) | −3.0 | 4.4 | −0.676 | 0.501 | 3.476 |
| SBP (mmHg) | 8.3 | 6.2 | 1.334 | 0.186 | 2.478 |
| DBP (mmHg) | 2.7 | 8.1 | 0.331 | 0.742 | 2.291 |
| HOMA-IR | 39.1 | 26.8 | 1.46 | 0.148 | 1.864 |
| HDL (mg/dL) | 3.4 | 4.9 | 0.69 | 0.493 | 2.583 |
| TG (mg/dL) | 0.9 | 1.2 | 0.772 | 0.443 | 1.567 |
| CRP (mg/dL) | 211.0 | 98.4 | 2.145 | 0.035 | 1.38 |
| SM 16:0 | |||||
| Constant | 97,236.0 | 26,232.0 | 3.707 | <0.001 | |
| WC (cm) | 58.5 | 162.0 | 0.361 | 0.719 | 3.476 |
| SBP (mmHg) | −336.0 | 229.0 | −1.471 | 0.145 | 2.478 |
| DBP (mmHg) | 429.0 | 296.0 | 1.447 | 0.152 | 2.291 |
| HOMA-IR | 151.0 | 980.0 | 0.154 | 0.878 | 1.864 |
| HDL (mg/dL) | 330.0 | 181.0 | 1.826 | 0.072 | 2.583 |
| TG (mg/dL) | 105.0 | 43.2 | 2.434 | 0.017 | 1.567 |
| CRP (mg/dL) | −3135.0 | 3604.0 | −0.87 | 0.387 | 1.38 |
| C24:1-HexCer | |||||
| Constant | 2457.0 | 2332.0 | 1.053 | 0.296 | |
| WC (cm) | 19.3 | 14.4 | 1.339 | 0.185 | 3.476 |
| SBP (mmHg) | −35.0 | 20.3 | −1.721 | 0.089 | 2.478 |
| DBP (mmHg) | 44.0 | 26.3 | 1.669 | 0.099 | 2.291 |
| HOMA-IR | 127.0 | 87.2 | 1.463 | 0.148 | 1.864 |
| HDL (mg/dL) | 16.1 | 16.0 | 1.001 | 0.32 | 2.583 |
| TG (mg/dL) | −0.2 | 3.8 | −0.0417 | 0.967 | 1.567 |
| CRP (mg/dL) | 311.0 | 320.0 | 0.97 | 0.335 | 1.38 |
| LacCer 22:0 | |||||
| Constant | 522.0 | 334.0 | 1.56 | 0.123 | |
| WC (cm) | −5.5 | 2.1 | −2.653 | 0.01 | 3.476 |
| SBP (mmHg) | −1.2 | 2.9 | −0.415 | 0.679 | 2.478 |
| DBP (mmHg) | 5.2 | 3.8 | 1.364 | 0.176 | 2.291 |
| HOMA-IR | 12.2 | 12.5 | 0.976 | 0.332 | 1.864 |
| HDL (mg/dL) | −0.9 | 2.3 | −0.395 | 0.694 | 2.583 |
| TG (mg/dL) | −0.4 | 0.6 | −0.67 | 0.505 | 1.567 |
| CRP (mg/dL) | 46.7 | 45.9 | 1.017 | 0.312 | 1.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigamonti, A.E.; Dei Cas, M.; Caroli, D.; De Col, A.; Cella, S.G.; Paroni, R.; Sartorio, A. Identification of a Specific Plasma Sphingolipid Profile in a Group of Normal-Weight and Obese Subjects: A Novel Approach for a “Biochemical” Diagnosis of Metabolic Syndrome? Int. J. Mol. Sci. 2023, 24, 7451. https://doi.org/10.3390/ijms24087451
Rigamonti AE, Dei Cas M, Caroli D, De Col A, Cella SG, Paroni R, Sartorio A. Identification of a Specific Plasma Sphingolipid Profile in a Group of Normal-Weight and Obese Subjects: A Novel Approach for a “Biochemical” Diagnosis of Metabolic Syndrome? International Journal of Molecular Sciences. 2023; 24(8):7451. https://doi.org/10.3390/ijms24087451
Chicago/Turabian StyleRigamonti, Antonello E., Michele Dei Cas, Diana Caroli, Alessandra De Col, Silvano G. Cella, Rita Paroni, and Alessandro Sartorio. 2023. "Identification of a Specific Plasma Sphingolipid Profile in a Group of Normal-Weight and Obese Subjects: A Novel Approach for a “Biochemical” Diagnosis of Metabolic Syndrome?" International Journal of Molecular Sciences 24, no. 8: 7451. https://doi.org/10.3390/ijms24087451
APA StyleRigamonti, A. E., Dei Cas, M., Caroli, D., De Col, A., Cella, S. G., Paroni, R., & Sartorio, A. (2023). Identification of a Specific Plasma Sphingolipid Profile in a Group of Normal-Weight and Obese Subjects: A Novel Approach for a “Biochemical” Diagnosis of Metabolic Syndrome? International Journal of Molecular Sciences, 24(8), 7451. https://doi.org/10.3390/ijms24087451










