Heat Shock Proteins in Tooth Development and Injury Repair
Abstract
1. Introduction
2. HSP25
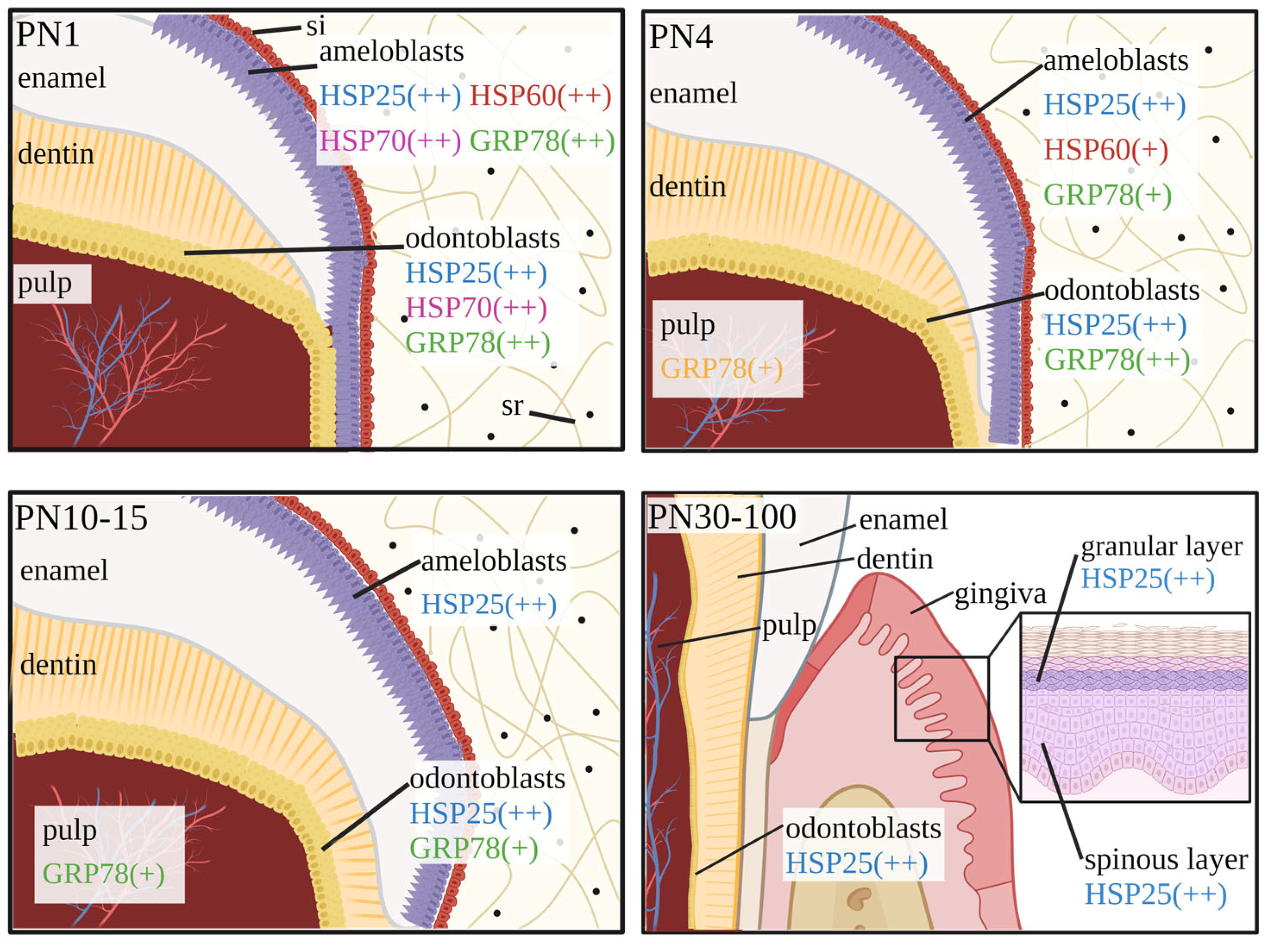
2.1. Expression of HSP25 in the Developing Molar
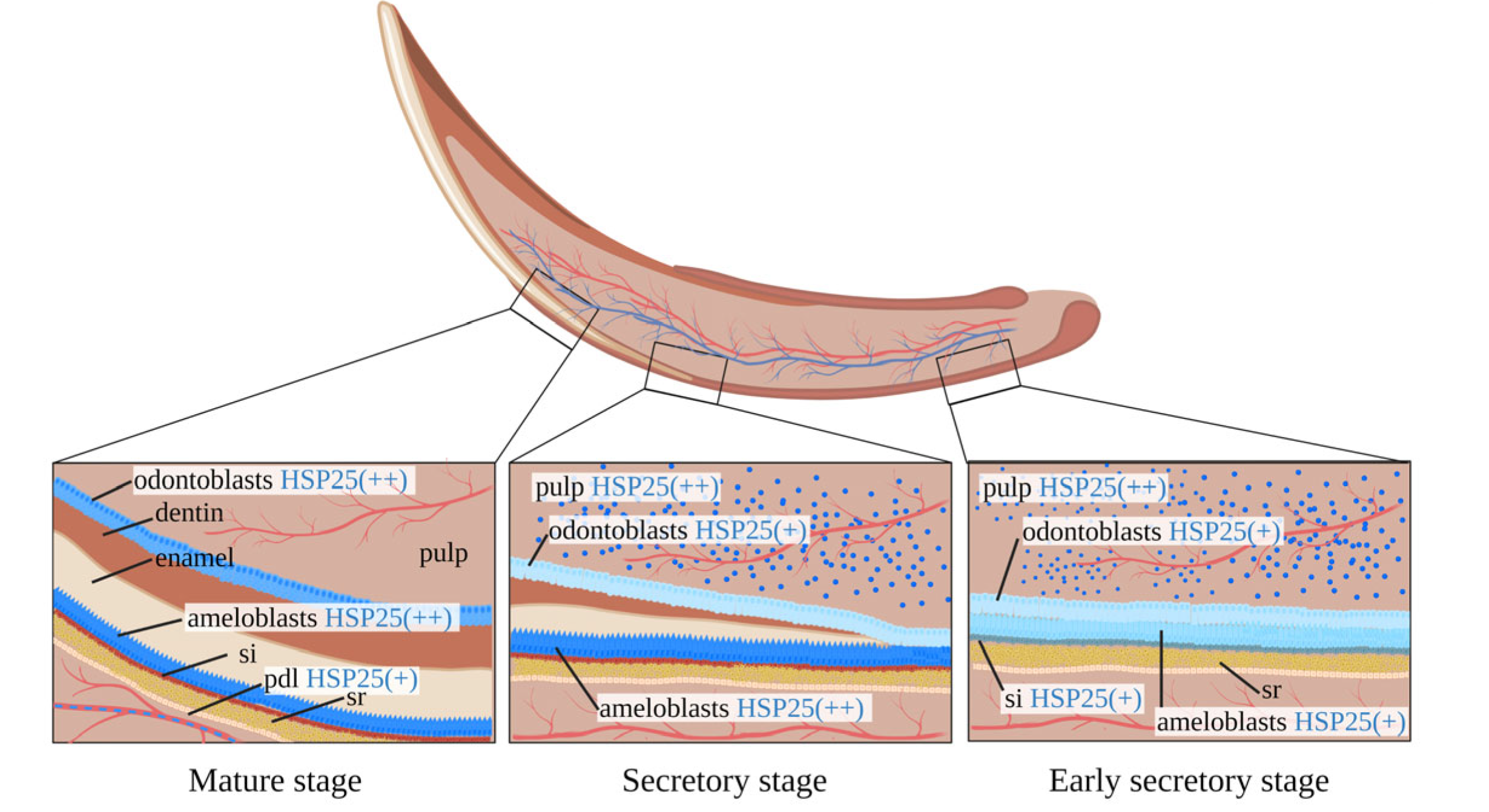
2.2. The Expression of HSP25 in the Developing Incisor
2.3. Expression of HSP25 in the Gingiva during Tooth Eruption
2.4. Expression of HSP25 during Tooth Injury
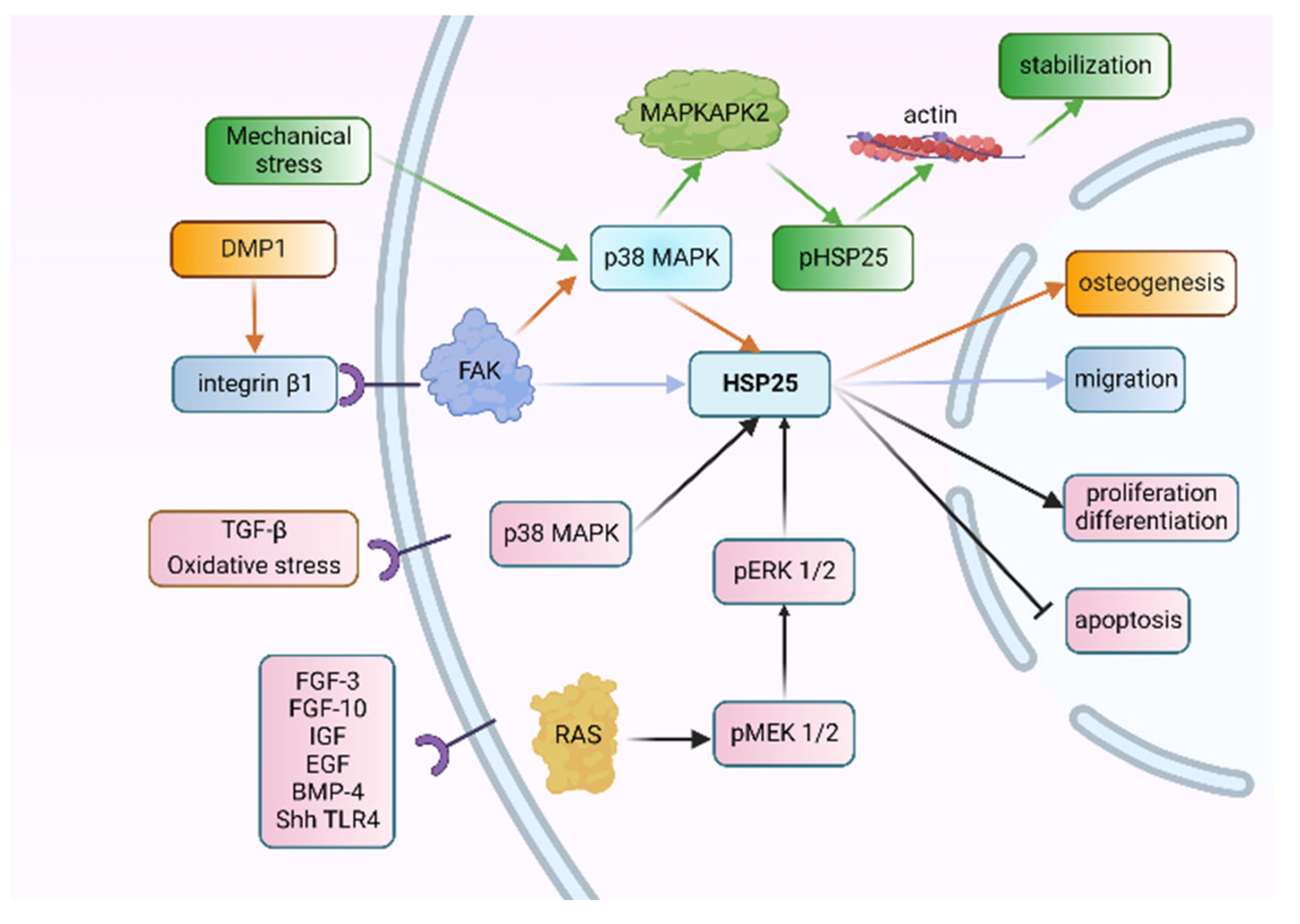
2.5. HSP25-Related Signaling Pathways during Tooth Development
3. HSP60
3.1. Expression of HSP60 in the Developing Tooth
3.2. HSP60-Associated Signaling Pathways during Tooth Development
4. HSP70
4.1. Expression of HSP70 in the Developing Tooth
4.2. Expression of HSP70 in Tooth Injury
4.3. HSP70-Related Signaling Pathways during Tooth Development
5. Research Prospect of HSPs in Tooth Development and Injury Repair
6. Possible Roles of HSP10, HSPB5, HSPB6, HSPB8, HSP90 and HSP110 in Tooth Development
7. Expression Patterns of HSP25, HSP60 and HSP70 in Tooth Development and Injury Repair
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feder, M.E.; Hofmann, G.E. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and ecological physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef]
- Kampinga, H.H.; Hageman, J.; Vos, M.J.; Kubota, H.; Tanguay, R.M.; Bruford, E.A.; Cheetham, M.E.; Chen, B.; Hightower, L.E. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 2009, 14, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Tai-Nagara, I.; Matsuoka, S.; Ariga, H.; Suda, T. Mortalin and DJ-1 coordinately regulate hematopoietic stem cell function through the control of oxidative stress. Blood 2014, 123, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.J.; Carra, S.; Kanon, B.; Bosveld, F.; Klauke, K.; Sibon, O.C.; Kampinga, H.H. Specific protein homeostatic functions of small heat-shock proteins increase lifespan. Aging Cell 2016, 15, 217–226. [Google Scholar] [CrossRef]
- Jee, B.; Dhar, R.; Singh, S.; Karmakar, S. Heat Shock Proteins and Their Role in Pregnancy: Redefining the Function of “Old Rum in a New Bottle”. Front. Cell Dev. Biol. 2021, 9, 648463. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.M.; Abdelmalek, D.H.; Elfiky, A.A. GRP78: A cell’s response to stress. Life Sci. 2019, 226, 156–163. [Google Scholar] [CrossRef]
- Rodriguez, A.; Von Salzen, D.; Holguin, B.A.; Bernal, R.A. Complex Destabilization in the Mitochondrial Chaperonin Hsp60 Leads to Disease. Front. Mol. Biosci. 2020, 7, 159. [Google Scholar] [CrossRef]
- Rahman, M.; Steuer, J.; Gillgren, P.; Hayderi, A.; Liu, A.; Frostegård, J. Induction of Dendritic Cell-Mediated Activation of T Cells From Atherosclerotic Plaques by Human Heat Shock Protein 60. J. Am. Heart Assoc. 2017, 6, e006778. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Wang, Y.; Zhang, X.; Kong, Q.; Li, C.; Li, Y.; Ding, Z.; Liu, L. HSP27 Alleviates Cardiac Aging in Mice via a Mechanism Involving Antioxidation and Mitophagy Activation. Oxidative Med. Cell. Longev. 2016, 2016, 2586706. [Google Scholar] [CrossRef]
- Stacchiotti, A.; Morandini, F.; Bettoni, F.; Schena, I.; Lavazza, A.; Grigolato, P.G.; Apostoli, P.; Rezzani, R.; Aleo, M.F. Stress proteins and oxidative damage in a renal derived cell line exposed to inorganic mercury and lead. Toxicology 2009, 264, 215–224. [Google Scholar] [CrossRef]
- Collier, M.P.; Alderson, T.R.; de Villiers, C.P.; Nicholls, D.; Gastall, H.Y.; Allison, T.M.; Degiacomi, M.T.; Jiang, H.; Mlynek, G.; Fürst, D.O.; et al. HspB1 phosphorylation regulates its intramolecular dynamics and mechanosensitive molecular chaperone interaction with filamin C. Sci. Adv. 2019, 5, eaav8421. [Google Scholar] [CrossRef] [PubMed]
- Ojha, J.; Masilamoni, G.; Dunlap, D.; Udoff, R.A.; Cashikar, A.G. Sequestration of toxic oligomers by HspB1 as a cytoprotective mechanism. Mol. Cell. Biol. 2011, 31, 3146–3157. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; Shi, C.; Deng, J.; Diao, C.; Maarouf, N.; Rosin, M.; Shrivastava, V.; Hu, A.A.; Bharadwa, S.; Adijiang, A.; et al. HSP25 Vaccination Attenuates Atherogenesis via Upregulation of LDLR Expression, Lowering of PCSK9 Levels and Curbing of Inflammation. Arterioscler. Thromb. Vasc. Biol. 2021, 41, e338–e353. [Google Scholar] [CrossRef]
- Shen, L.; Qi, Z.; Zhu, Y.; Song, X.; Xuan, C.; Ben, P.; Lan, L.; Luo, L.; Yin, Z. Phosphorylated heat shock protein 27 promotes lipid clearance in hepatic cells through interacting with STAT3 and activating autophagy. Cell. Signal. 2016, 28, 1086–1098. [Google Scholar] [CrossRef] [PubMed]
- Nakasone, N.; Yoshie, H.; Ohshima, H. The relationship between the termination of cell proliferation and expression of heat-shock protein-25 in the rat developing tooth germ. Eur. J. Oral Sci. 2006, 114, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Nakasone, N.; Yoshie, H.; Ohshima, H. An immunohistochemical study of the expression of heat-shock protein-25 and cell proliferation in the dental pulp and enamel organ during odontogenesis in rat molars. Arch. Oral Biol. 2006, 51, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Biz, M.T.; Marques, M.R.; Crema, V.O.; Moriscot, A.S.; dos Santos, M.F. GTPases RhoA and Rac1 are important for amelogenin and DSPP expression during differentiation of ameloblasts and odontoblasts. Cell Tissue Res. 2010, 340, 459–470. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Yan, G.X.; Liu, C.W.; Zhang, X.; Hu, Y.; Hao, X.Q.; Zhao, H.; Shi, C.; Sun, H.C. Polarity of ameloblasts and odontoblasts and their related regulators. West China J. Stomatol. 2019, 37, 309–313. [Google Scholar] [CrossRef]
- Onishi, T.; Tsubone, H.; Ooshima, T.; Sobue, S.; El-Sharaby, A.; Wakisaka, S. Immunohistochemical localization of heat shock protein 25 (HSP 25) during root formation of the rat molar. Anat. Rec. 2002, 267, 321–329. [Google Scholar] [CrossRef]
- Huang, X.; Bringas, P., Jr.; Slavkin, H.C.; Chai, Y. Fate of HERS during tooth root development. Dev. Biol. 2009, 334, 22–30. [Google Scholar] [CrossRef]
- Du, Y.; Gu, H.J.; Gong, Q.M.; Yang, F.; Ling, J.Q. HSP25 affects the proliferation and differentiation of rat dental follicle cells. Int. J. Oral Sci. 2009, 1, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Yoon, J.H.; Lim, Y.S.; Hwang, H.K.; Kim, S.A.; Ahn, S.G.; Yoon, J.H. TAT-Hsp27 promotes adhesion and migration of murine dental papilla-derived MDPC-23 cells through beta1 integrin-mediated signaling. Int. J. Mol. Med. 2010, 26, 373–378. [Google Scholar] [PubMed]
- Krivanek, J.; Soldatov, R.A.; Kastriti, M.E.; Chontorotzea, T.; Herdina, A.N.; Petersen, J.; Szarowska, B.; Landova, M.; Matejova, V.K.; Holla, L.I.; et al. Dental cell type atlas reveals stem and differentiated cell types in mouse and human teeth. Nat. Commun. 2020, 11, 4816. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, H.; Ajima, H.; Kawano, Y.; Nozawa-Inoue, K.; Wakisaka, S.; Maeda, T. Transient expression of heat shock protein (Hsp)25 in the dental pulp and enamel organ during odontogenesis in the rat incisor. Arch. Histol. Cytol. 2000, 63, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Domínguez, A.; El-Yazbi, A.F.; Zhu, H.L.; Colinas, O.; Zhong, X.Z.; Walsh, E.J.; Cole, D.M.; Kargacin, G.J.; Walsh, M.P.; Cole, W.C. Cytoskeletal reorganization evoked by Rho-associated kinase- and protein kinase C-catalyzed phosphorylation of cofilin and heat shock protein 27, respectively, contributes to myogenic constriction of rat cerebral arteries. J. Biol. Chem. 2014, 289, 20939–20952. [Google Scholar] [CrossRef] [PubMed]
- Smoyer, W.E.; Ransom, R.; Harris, R.C.; Welsh, M.J.; Lutsch, G.; Benndorf, R. Ischemic acute renal failure induces differential expression of small heat shock proteins. J. Am. Soc. Nephrol. JASN 2000, 11, 211–221. [Google Scholar] [CrossRef]
- Ropeleski, M.J.; Tang, J.; Walsh-Reitz, M.M.; Musch, M.W.; Chang, E.B. Interleukin-11-induced heat shock protein 25 confers intestinal epithelial-specific cytoprotection from oxidant stress. Gastroenterology 2003, 124, 1358–1368. [Google Scholar] [CrossRef]
- Yuan, X.; Nishikawa, S. Angular distribution of cross-sectioned cell boundaries at the distal terminal web in differentiating preameloblasts, inner enamel secretory ameloblasts and outer enamel secretory ameloblasts. Microscopy 2014, 63, 33–39. [Google Scholar] [CrossRef]
- Sasaki, A.; Yamada, T.; Inoue, K.; Momoi, T.; Tokunaga, H.; Sakiyama, K.; Kanegae, H.; Suda, N.; Amano, O. Localization of heat shock protein 27 (hsp27) in the rat gingiva and its changes with tooth eruption. Acta Histochem. Cytochem. 2011, 44, 17–24. [Google Scholar] [CrossRef]
- Duverger, O.; Morange, M. Heat shock protein 25 plays multiple roles during mouse skin development. Cell Stress Chaperones 2005, 10, 268–277. [Google Scholar] [CrossRef]
- Duverger, O.; Paslaru, L.; Morange, M. HSP25 is involved in two steps of the differentiation of PAM212 keratinocytes. J. Biol. Chem. 2004, 279, 10252–10260. [Google Scholar] [CrossRef] [PubMed]
- Honoré, B.; Rasmussen, H.H.; Celis, A.; Leffers, H.; Madsen, P.; Celis, J.E. The molecular chaperones HSP28, GRP78, endoplasmin, and calnexin exhibit strikingly different levels in quiescent keratinocytes as compared to their proliferating normal and transformed counterparts: cDNA cloning and expression of calnexin. Electrophoresis 1994, 15, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, P.; Hu, P.; Warren, S.; Liu, Z.; Diaz, L.A.; Rubenstein, D.S. p38MAPK inhibition prevents disease in pemphigus vulgaris mice. Proc. Natl. Acad. Sci. USA 2006, 103, 12855–12860. [Google Scholar] [CrossRef] [PubMed]
- Bier, C.A.; Shemesh, H.; Tanomaru-Filho, M.; Wesselink, P.R.; Wu, M.K. The ability of different nickel-titanium rotary instruments to induce dentinal damage during canal preparation. J. Endod. 2009, 35, 236–238. [Google Scholar] [CrossRef]
- Ohshima, H.; Nakakura-Ohshima, K.; Takeuchi, K.; Hoshino, M.; Takano, Y.; Maeda, T. Pulpal regeneration after cavity preparation, with special reference to close spatio-relationships between odontoblasts and immunocompetent cells. Microsc. Res. Tech. 2003, 60, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Nomura, S.; Maeda, T.; Ohshima, H. An immunocytochemical study of pulpal responses to cavity preparation by laser ablation in rat molars by using antibodies to heat shock protein (Hsp) 25 and class II MHC antigen. Cell Tissue Res. 2004, 315, 311–319. [Google Scholar] [CrossRef]
- Kawagishi, E.; Nakakura-Ohshima, K.; Nomura, S.; Ohshima, H. Pulpal responses to cavity preparation in aged rat molars. Cell Tissue Res. 2006, 326, 111–122. [Google Scholar] [CrossRef]
- Balic, A.; Thesleff, I. Tissue Interactions Regulating Tooth Development and Renewal. Curr. Top. Dev. Biol. 2015, 115, 157–186. [Google Scholar] [CrossRef]
- Safa, A.; Abak, A.; Shoorei, H.; Taheri, M.; Ghafouri-Fard, S. MicroRNAs as regulators of ERK/MAPK pathway: A comprehensive review. Biomed. Pharmacother. 2020, 132, 110853. [Google Scholar] [CrossRef]
- Apps, J.R.; Carreno, G.; Gonzalez-Meljem, J.M.; Haston, S.; Guiho, R.; Cooper, J.E.; Manshaei, S.; Jani, N.; Hölsken, A.; Pettorini, B.; et al. Tumour compartment transcriptomics demonstrates the activation of inflammatory and odontogenic programmes in human adamantinomatous craniopharyngioma and identifies the MAPK/ERK pathway as a novel therapeutic target. Acta Neuropathol. 2018, 135, 757–777. [Google Scholar] [CrossRef]
- Lee, M.J.; Cai, J.; Kwak, S.W.; Cho, S.W.; Harada, H.; Jung, H.S. MAPK mediates Hsp25 signaling in incisor development. Histochem. Cell Biol. 2009, 131, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Han, J.; Ito, Y.; Bringas, P., Jr.; Deng, C.; Chai, Y. Ectodermal Smad4 and p38 MAPK are functionally redundant in mediating TGF-beta/BMP signaling during tooth and palate development. Dev. Cell 2008, 15, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.L.; Wang, Z.; Nan, X.; Zhang, Q.C.; Liu, W. Downregulation of microRNA-143-5p is required for the promotion of odontoblasts differentiation of human dental pulp stem cells through the activation of the mitogen-activated protein kinases 14-dependent p38 mitogen-activated protein kinases signaling pathway. J. Cell. Physiol. 2019, 234, 4840–4850. [Google Scholar] [CrossRef]
- Hoffman, L.; Jensen, C.C.; Yoshigi, M.; Beckerle, M. Mechanical signals activate p38 MAPK pathway-dependent reinforcement of actin via mechanosensitive HspB1. Mol. Biol. Cell 2017, 28, 2661–2675. [Google Scholar] [CrossRef] [PubMed]
- Eapen, A.; Sundivakkam, P.; Song, Y.; Ravindran, S.; Ramachandran, A.; Tiruppathi, C.; George, A. Calcium-mediated stress kinase activation by DMP1 promotes osteoblast differentiation. J. Biol. Chem. 2010, 285, 36339–36351. [Google Scholar] [CrossRef]
- Seo, N.H.; Lee, E.H.; Seo, J.H.; Song, H.R.; Han, M.K. HSP60 is required for stemness and proper differentiation of mouse embryonic stem cells. Exp. Mol. Med. 2018, 50, e459. [Google Scholar] [CrossRef]
- Aluksanasuwan, S.; Sueksakit, K.; Fong-Ngern, K.; Thongboonkerd, V. Role of HSP60 (HSPD1) in diabetes-induced renal tubular dysfunction: Regulation of intracellular protein aggregation, ATP production, and oxidative stress. FASEB J. 2017, 31, 2157–2167. [Google Scholar] [CrossRef]
- Duan, Y.; Tang, H.; Mitchell-Silbaugh, K.; Fang, X.; Han, Z.; Ouyang, K. Heat Shock Protein 60 in Cardiovascular Physiology and Diseases. Front. Mol. Biosci. 2020, 7, 73. [Google Scholar] [CrossRef]
- Guo, J.; Li, X.; Zhang, W.; Chen, Y.; Zhu, S.; Chen, L.; Xu, R.; Lv, Y.; Wu, D.; Guo, M.; et al. HSP60-regulated Mitochondrial Proteostasis and Protein Translation Promote Tumor Growth of Ovarian Cancer. Sci. Rep. 2019, 9, 12628. [Google Scholar] [CrossRef]
- Papp, T.; Polyak, A.; Papp, K.; Meszar, Z.; Zakany, R.; Meszar-Katona, E.; Tünde, P.T.; Ham, C.H.; Felszeghy, S. Modification of tooth development by heat shock protein 60. Int. J. Oral Sci. 2016, 8, 24–31. [Google Scholar] [CrossRef]
- Pandya, M.; Liu, H.; Dangaria, S.J.; Zhu, W.; Li, L.L.; Pan, S.; Abufarwa, M.; Davis, R.G.; Guggenheim, S.; Keiderling, T.; et al. Integrative Temporo-Spatial, Mineralogic, Spectroscopic, and Proteomic Analysis of Postnatal Enamel Development in Teeth with Limited Growth. Front. Physiol. 2017, 8, 793. [Google Scholar] [CrossRef] [PubMed]
- Alfaqeeh, S.A.; Gaete, M.; Tucker, A.S. Interactions of the tooth and bone during development. J. Dent. Res. 2013, 92, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.M.; Lee, Y.S.; Kim, Y.S.; Park, S.H.; Lee, S.H.; Kim, H.H.; Lee, M.S.; Lee, K.U.; Kim, G.S. Heat shock protein 60 causes osteoclastic bone resorption via toll-like receptor-2 in estrogen deficiency. Bone 2009, 45, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Sun, W.; Liang, Y.; Chen, T.; Guo, W.; Tian, W. Maternal diabetes modulates offspring cell proliferation and apoptosis during odontogenesis via the TLR4/NF-κB signalling pathway. Cell Prolif. 2017, 50, e12324. [Google Scholar] [CrossRef]
- Heiserman, J.P.; Chen, L.; Kim, B.S.; Kim, S.C.; Tran, A.L.; Siebenborn, N.; Knowlton, A.A. TLR4 mutation and HSP60-induced cell death in adult mouse cardiac myocytes. Cell Stress Chaperones 2015, 20, 527–535. [Google Scholar] [CrossRef]
- Min, S.; Kim, J.Y.; Cho, H.M.; Park, S.; Hwang, J.M.; You, H.; Chan Chae, Y.; Lee, W.J.; Sun, W.; Kang, D.; et al. Heat shock protein 60 couples an oxidative stress-responsive p38/MK2 signaling and NF-κB survival machinery in cancer cells. Redox Biol. 2022, 51, 102293. [Google Scholar] [CrossRef]
- Ohazama, A.; Hu, Y.; Schmidt-Ullrich, R.; Cao, Y.; Scheidereit, C.; Karin, M.; Sharpe, P.T. A dual role for Ikk alpha in tooth development. Dev. Cell 2004, 6, 219–227. [Google Scholar] [CrossRef]
- Clerico, E.M.; Meng, W.; Pozhidaeva, A.; Bhasne, K.; Petridis, C.; Gierasch, L.M. Hsp70 molecular chaperones: Multifunctional allosteric holding and unfolding machines. Biochem. J. 2019, 476, 1653–1677. [Google Scholar] [CrossRef]
- Liu, P.Y.; Shen, H.H.; Kung, C.W.; Chen, S.Y.; Lu, C.H.; Lee, Y.M. The Role of HSP70 in the Protective Effects of NVP-AUY922 on Multiple Organ Dysfunction Syndrome in Endotoxemic Rats. Front. Pharmacol. 2021, 12, 724515. [Google Scholar] [CrossRef]
- Rosenzweig, R.; Nillegoda, N.B.; Mayer, M.P.; Bukau, B. The Hsp70 chaperone network. Nat. Rev. Mol. Cell Biol. 2019, 20, 665–680. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, X.; Zhao, G.; Liu, Z.; Yu, M.; Fang, Z.; Li, X. Hepatocellular iNOS protects liver from ischemia/reperfusion injury through HSF1-dependent activation of HSP70. Biochem. Biophys. Res. Commun. 2019, 512, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Sreedharan, R.; Van Why, S.K. Heat shock proteins in the kidney. Pediatr. Nephrol. 2016, 31, 1561–1570. [Google Scholar] [CrossRef]
- Balogi, Z.; Multhoff, G.; Jensen, T.K.; Lloyd-Evans, E.; Yamashima, T.; Jäättelä, M.; Harwood, J.L.; Vígh, L. Hsp70 interactions with membrane lipids regulate cellular functions in health and disease. Prog. Lipid Res. 2019, 74, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.K.; Tahrir, F.G.; Knezevic, T.; White, M.K.; Gordon, J.; Cheung, J.Y.; Khalili, K.; Feldman, A.M. GRP78 Interacting Partner Bag5 Responds to ER Stress and Protects Cardiomyocytes From ER Stress-Induced Apoptosis. J. Cell. Biochem. 2016, 117, 1813–1821. [Google Scholar] [CrossRef]
- Lebeau, P.F.; Wassef, H.; Byun, J.H.; Platko, K.; Ason, B.; Jackson, S.; Dobroff, J.; Shetterly, S.; Richards, W.G.; Al-Hashimi, A.A.; et al. The loss-of-function PCSK9Q152H variant increases ER chaperones GRP78 and GRP94 and protects against liver injury. J. Clin. Investig. 2021, 131, e128650. [Google Scholar] [CrossRef] [PubMed]
- Bonam, S.R.; Ruff, M.; Muller, S. HSPA8/HSC70 in Immune Disorders: A Molecular Rheostat that Adjusts Chaperone-Mediated Autophagy Substrates. Cells 2019, 8, 849. [Google Scholar] [CrossRef]
- Stricher, F.; Macri, C.; Ruff, M.; Muller, S. HSPA8/HSC70 chaperone protein: Structure, function, and chemical targeting. Autophagy 2013, 9, 1937–1954. [Google Scholar] [CrossRef]
- Chen, C.; Wang, D.; Yu, Y.; Zhao, T.; Min, N.; Wu, Y.; Kang, L.; Zhao, Y.; Du, L.; Zhang, M.; et al. Legumain promotes tubular ferroptosis by facilitating chaperone-mediated autophagy of GPX4 in AKI. Cell Death Dis. 2021, 12, 65. [Google Scholar] [CrossRef]
- Kero, D.; Kalibovic Govorko, D.; Medvedec Mikic, I.; Vukojevic, K.; Cigic, L.; Saraga-Babic, M. Analysis of expression patterns of IGF-1, caspase-3 and HSP-70 in developing human tooth germs. Arch. Oral Biol. 2015, 60, 1533–1544. [Google Scholar] [CrossRef]
- Ravindran, S.; Gao, Q.; Ramachandran, A.; Sundivakkam, P.; Tiruppathi, C.; George, A. Expression and distribution of grp-78/bip in mineralizing tissues and mesenchymal cells. Histochem. Cell Biol. 2012, 138, 113–125. [Google Scholar] [CrossRef]
- Sens, D.A.; McGuirt, J.P.; Khan, W.; Howell, R.M.; Todd, J.H. Expression of hsc 70, but not hsp 70, in human third molar dental pulp. Eur. J. Oral Sci. 1997, 105, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Pileggi, R.; Holland, G.R. The expression of heat shock protein 70 in the dental pulp following trauma. Dent. Traumatol. 2009, 25, 426–428. [Google Scholar] [CrossRef]
- Austin, C.; Smith, T.M.; Farahani, R.M.; Hinde, K.; Carter, E.A.; Lee, J.; Lay, P.A.; Kennedy, B.J.; Sarrafpour, B.; Wright, R.J.; et al. Uncovering system-specific stress signatures in primate teeth with multimodal imaging. Sci. Rep. 2016, 6, 18802. [Google Scholar] [CrossRef] [PubMed]
- Diamanti, E.; Mathieu, S.; Jeanneau, C.; Kitraki, E.; Panopoulos, P.; Spyrou, G.; About, I. Endoplasmic reticulum stress and mineralization inhibition mechanism by the resinous monomer HEMA. Int. Endod. J. 2013, 46, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Tomasello, L.; Mauceri, R.; Coppola, A.; Pitrone, M.; Pizzo, G.; Campisi, G.; Pizzolanti, G.; Giordano, C. Mesenchymal stem cells derived from inflamed dental pulpal and gingival tissue: A potential application for bone formation. Stem Cell Res. Ther. 2017, 8, 179. [Google Scholar] [CrossRef]
- Ravindran, S.; Narayanan, K.; Eapen, A.S.; Hao, J.; Ramachandran, A.; Blond, S.; George, A. Endoplasmic reticulum chaperone protein GRP-78 mediates endocytosis of dentin matrix protein 1. J. Biol. Chem. 2008, 283, 29658–29670. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Liu, H.; Sun, Q.; Yuan, G.; Zhang, L.; Chen, Z. KLF4 promoted odontoblastic differentiation of mouse dental papilla cells via regulation of DMP1. J. Cell. Physiol. 2013, 228, 2076–2085. [Google Scholar] [CrossRef]
- Sagomonyants, K.; Kalajzic, I.; Maye, P.; Mina, M. FGF Signaling Prevents the Terminal Differentiation of Odontoblasts. J. Dent. Res. 2017, 96, 663–670. [Google Scholar] [CrossRef]
- Chen, E.; Xue, D.; Zhang, W.; Lin, F.; Pan, Z. Extracellular heat shock protein 70 promotes osteogenesis of human mesenchymal stem cells through activation of the ERK signaling pathway. FEBS Lett. 2015, 589 Pt B, 4088–4096. [Google Scholar] [CrossRef]
- Zhang, W.; Xue, D.; Yin, H.; Wang, S.; Li, C.; Chen, E.; Hu, D.; Tao, Y.; Yu, J.; Zheng, Q.; et al. Overexpression of HSPA1A enhances the osteogenic differentiation of bone marrow mesenchymal stem cells via activation of the Wnt/β-catenin signaling pathway. Sci. Rep. 2016, 6, 27622. [Google Scholar] [CrossRef]
- Fan, G.C. Role of heat shock proteins in stem cell behavior. Prog. Mol. Biol. Transl. Sci. 2012, 111, 305–322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Jiang, L.; He, Y.; Fan, W.; Guan, X.; Deng, Q.; Huang, F.; He, H. Changes of mitochondrial respiratory function during odontogenic differentiation of rat dental papilla cells. J. Mol. Histol. 2018, 49, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Nefedova, V.V.; Sudnitsyna, M.V.; Gusev, N.B. Interaction of small heat shock proteins with light component of neurofilaments (NFL). Cell Stress Chaperones 2017, 22, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Faust, O.; Abayev-Avraham, M.; Wentink, A.S.; Maurer, M.; Nillegoda, N.B.; London, N.; Bukau, B.; Rosenzweig, R. HSP40 proteins use class-specific regulation to drive HSP70 functional diversity. Nature 2020, 587, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Biebl, M.M.; Buchner, J. Structure, Function, and Regulation of the Hsp90 Machinery. Cold Spring Harb. Perspect. Biol. 2019, 11, a034017. [Google Scholar] [CrossRef]
- Gozzi, G.J.; Gonzalez, D.; Boudesco, C.; Dias, A.M.M.; Gotthard, G.; Uyanik, B.; Dondaine, L.; Marcion, G.; Hermetet, F.; Denis, C.; et al. Selecting the first chemical molecule inhibitor of HSP110 for colorectal cancer therapy. Cell Death Differ. 2020, 27, 117–129. [Google Scholar] [CrossRef]
- Yu, N.; Kakunda, M.; Pham, V.; Lill, J.R.; Du, P.; Wongchenko, M.; Yan, Y.; Firestein, R.; Huang, X. HSP105 recruits protein phosphatase 2A to dephosphorylate β-catenin. Mol. Cell. Biol. 2015, 35, 1390–1400. [Google Scholar] [CrossRef]


| HSP25 | Components of the Molar Tooth Germ | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Epithelium | Interstitial Tissue | ||||||||
| Stage | iee | oee | sr | si | pab | ab | mc | pob | ob |
| Bud stage | / | / | / | / | / | / | / | / | / |
| Cap stage | - | - | - | / | - | - | / | / | / |
| Early bell stage | - | - | - | - | - | - | - | - | - |
| Late bell stage | - | - | + | - | + | + | + | + | + |
| HSP25 | Components of the Molar Tooth Germ | |||||||
|---|---|---|---|---|---|---|---|---|
| Stage (Day) | eo | dp | mc | pab | ab | pob | ob | oe |
| PN1 | - | ph (++) | dp (-) | + | + | + | ++ | |
| sob (+) | ||||||||
| PN5 | ph (+) | ++ | ++ | |||||
| PN10 | dp (+) | ++ | ++ | |||||
| PN15 | ph (+) | ++ | ++ | + | ||||
| tr (+) | ||||||||
| PN30 | ra (+) | tc (++) | ||||||
| tc (+) | tr (+/-) | |||||||
| pf (+/-) | ||||||||
| PN60–100 | tc (++) | |||||||
| rt (++) | ||||||||
| pf (++) | ||||||||
| HSP25 | Components of the Incisors | ||||
|---|---|---|---|---|---|
| Stage | pdl | dp | si | ab | ob |
| Early secretory stage | - | ++ | + | + | + |
| Secretory stage | - | ++ | - | ++ | |
| Mature stage | blood vessels (+) | - | ra (++) sa (+) | ++ | |
| HSP60 | Components of the Incisors Germ | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epithelium | Interstitial Tissue | |||||||||||
| Stage | oe | eb | iee | oee | ek | si | pab | ab | dp | df | pob | ob |
| Initial Stage | + | + | ||||||||||
| Bud Stage | ++ | ++ | ++ | + | + | |||||||
| Cap stage | ++ | ++ | ++ | + | + | |||||||
| Bell Stage | ++ | ++ | ++ | ++ | ++ | + | + | + | ||||
| HSP70 | Tooth Germ Parts | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epithelial | Epithelial | |||||||||||
| Age (Weeks) | tb | dl | iee | oee | ek | cl | sr | si | pa | dp | df | pob |
| 9 | +++ | +++ | +++ | +++ | + | / | / | / | / | - | - | / |
| 12 | / | +++ | +++ | +++ | +++ | ++ | ++ | / | / | - | - | / |
| 14 | / | ++ | ++/+++ | +++ | / | ++ | + | + | ++ | + | - | / |
| 20 | / | + | ++ | + | / | +++ | ++ | ++ | +++ | + | - | ++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.; Yang, H.; Liu, J.; Meng, Z.; Sui, L. Heat Shock Proteins in Tooth Development and Injury Repair. Int. J. Mol. Sci. 2023, 24, 7455. https://doi.org/10.3390/ijms24087455
Guo S, Yang H, Liu J, Meng Z, Sui L. Heat Shock Proteins in Tooth Development and Injury Repair. International Journal of Molecular Sciences. 2023; 24(8):7455. https://doi.org/10.3390/ijms24087455
Chicago/Turabian StyleGuo, Shuling, Haosun Yang, Jiacheng Liu, Zhaosong Meng, and Lei Sui. 2023. "Heat Shock Proteins in Tooth Development and Injury Repair" International Journal of Molecular Sciences 24, no. 8: 7455. https://doi.org/10.3390/ijms24087455
APA StyleGuo, S., Yang, H., Liu, J., Meng, Z., & Sui, L. (2023). Heat Shock Proteins in Tooth Development and Injury Repair. International Journal of Molecular Sciences, 24(8), 7455. https://doi.org/10.3390/ijms24087455







