The Effect of Hypertension on the Recovery of Renal Dysfunction following Reversal of Unilateral Ureteral Obstruction in the Rat
Abstract
1. Introduction
2. Results
2.1. Glomerular and Tubular Functions
2.2. Urinary Albumin Creatinine Ratio
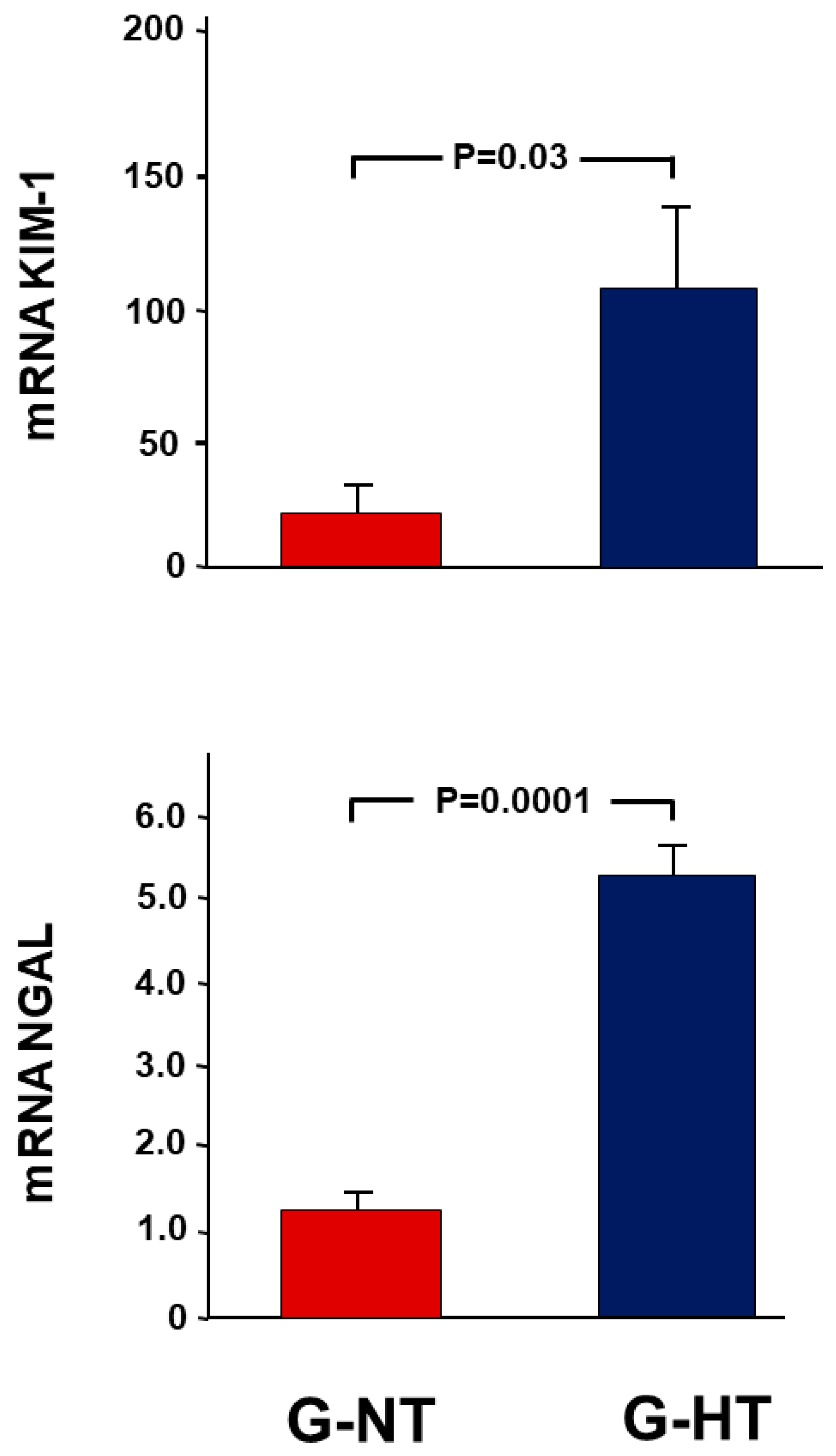
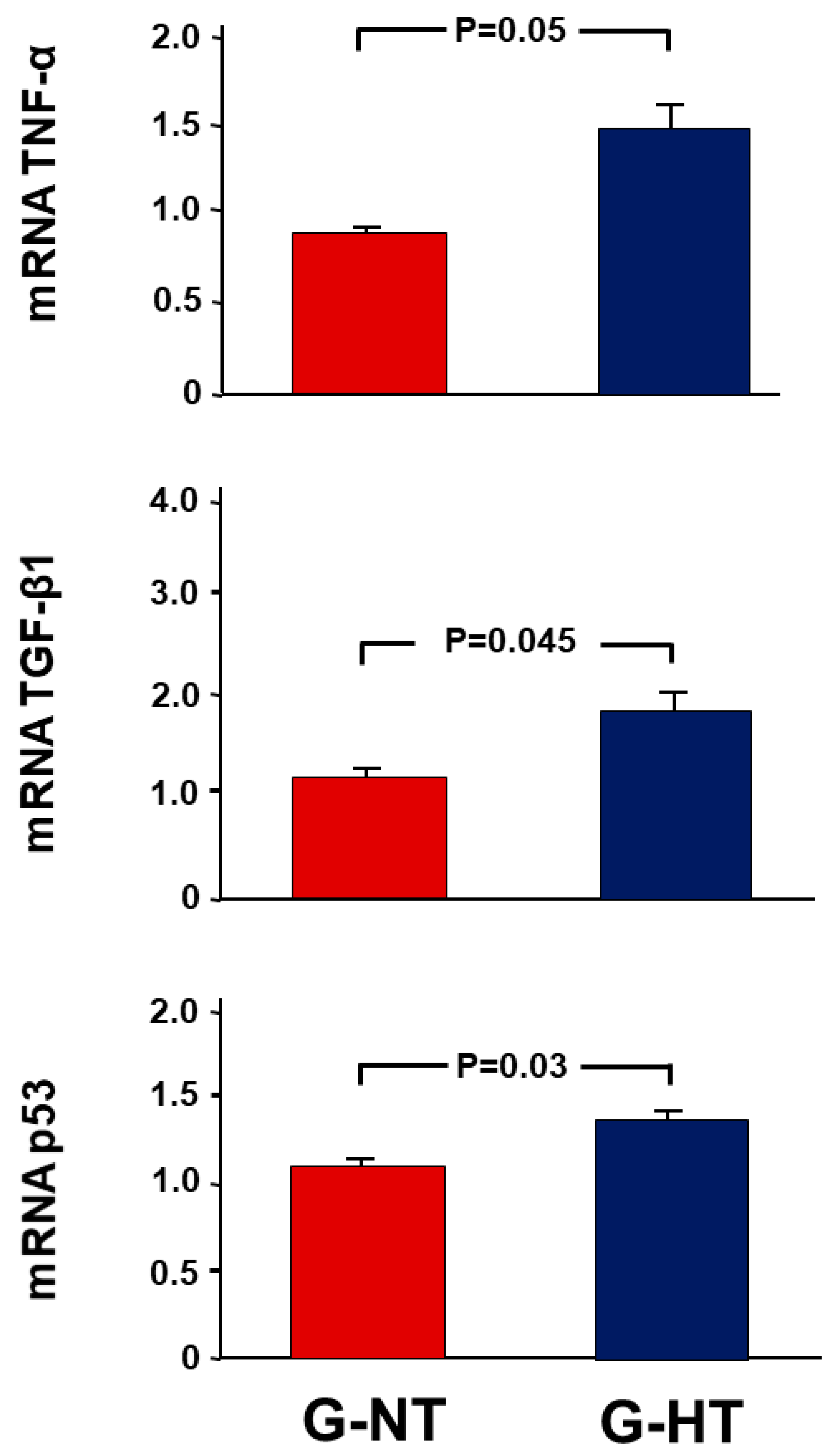
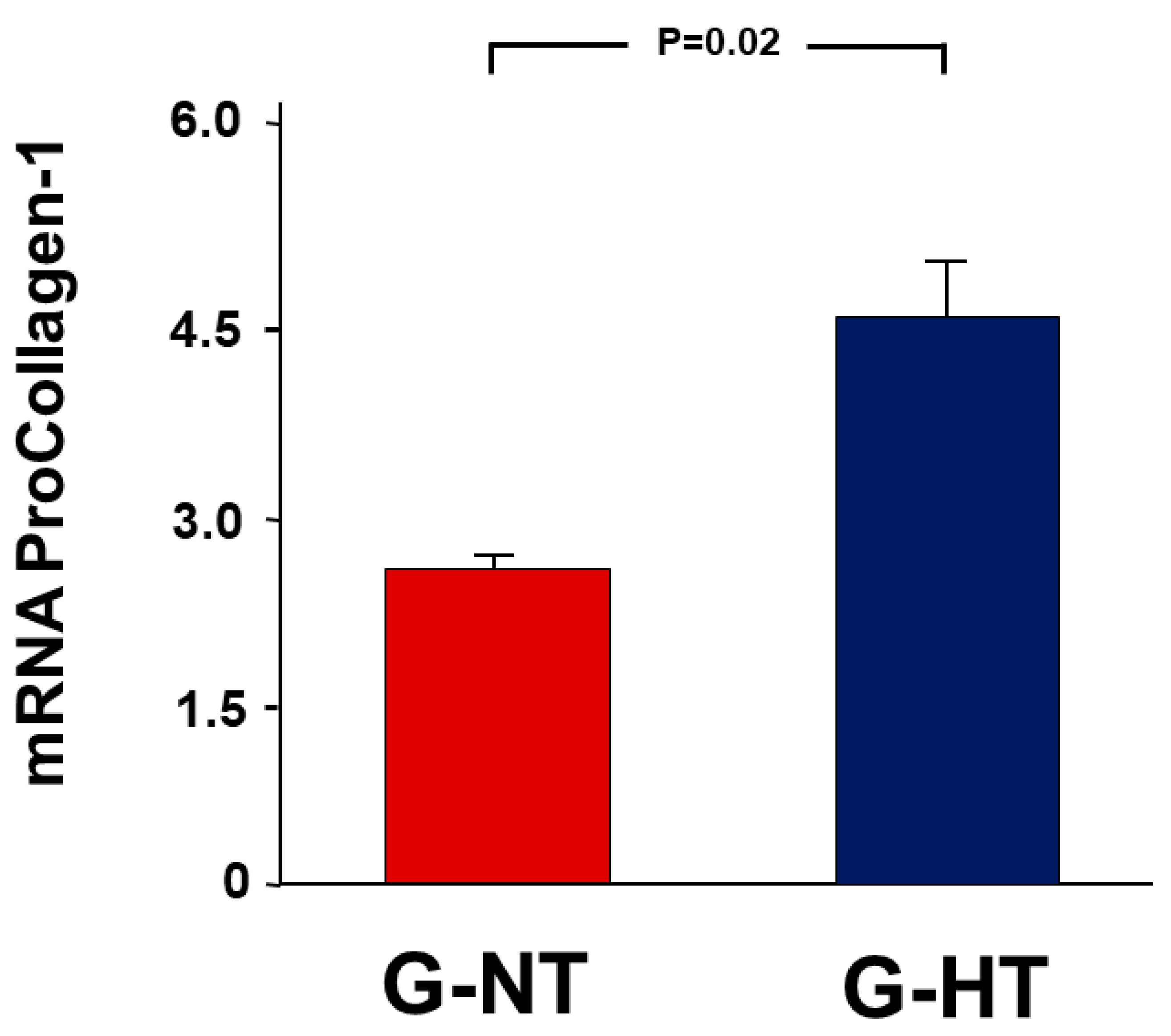
2.3. Gene Expression Analysis Results
2.4. Western Blot Analysis
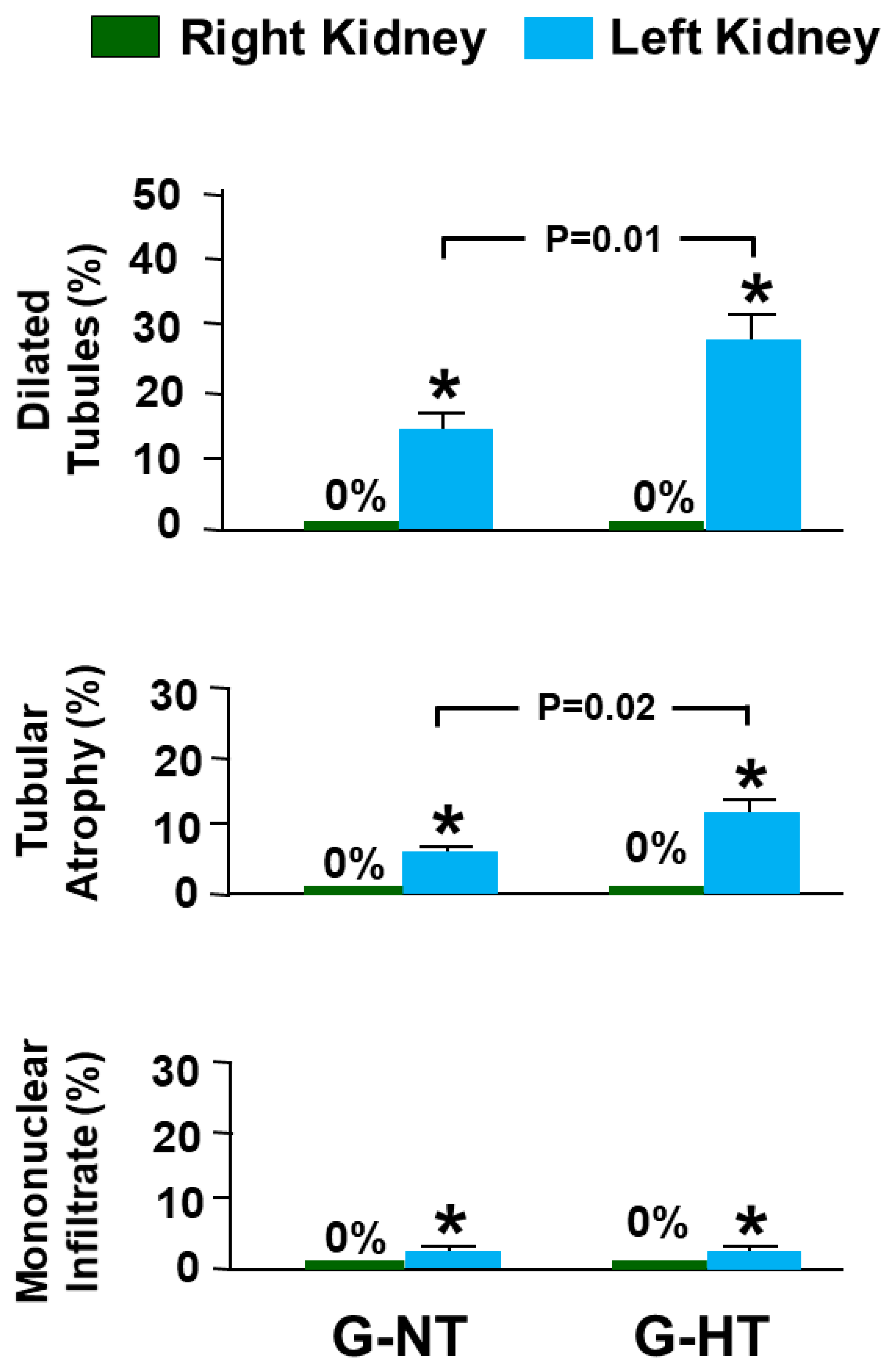
2.5. Histological Studies
2.6. Gene Expression Analysis Results
3. Discussion
4. Materials and Methods
4.1. Experimental Groups
4.2. Ureteral Occlusion and Reversal
4.3. Surgical Procedure in the Terminal Experiment
4.4. Experimental Protocol and Assays
4.5. Urine Collection and Measurement of Albumin Creatinine Ratio
4.6. Gene Expression Analysis
- Markers of acute kidney injury: kidney injury molecule-1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL).
- Pro-inflammatory and pro-fibrotic cytokines: tumour necrosis factor-alpha (TNF-α) and transforming growth factor-β (TGF-β1).
- The pro-apoptotic gene p53.
- Procollagen type-1 (COL1A).
4.7. Western Blot Analysis
4.8. Histological Studies
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bander, S.J.; Buerkert, J.E.; Martin, D.; Klahr, S. Long-term effects of 24-hr unilateral ureteral obstruction on renal function in the rat. Kidney Int. 1985, 28, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Hammad, F.T.; Lubbad, L. Does curcumin protect against renal dysfunction following reversible unilateral ureteric obstruction in the rat? Eur. Surg. Res. 2011, 46, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Hammad, F.T.; Wheatley, A.M.; Davis, G. Long-term renal effects of unilateral ureteral obstruction and the role of endothelin. Kidney Int. 2000, 58, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Klahr, S. Pathophysiology of obstructive nephropathy. Kidney Int. 1983, 23, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; McCulloch, C.E.; Darbinian, J.; Go, A.S.; Iribarren, C. Elevated blood pressure and risk of end-stage renal disease in subjects without baseline kidney disease. Arch. Intern. Med. 2005, 165, 923–928. [Google Scholar] [CrossRef]
- Iseki, K.; Ikemiya, Y.; Fukiyama, K. Blood pressure and risk of end-stage renal disease in a screened cohort. Kidney Int. Suppl. 1996, 55, S69–S71. [Google Scholar] [PubMed]
- Klag, M.J.; Whelton, P.K.; Randall, B.L.; Neaton, J.D.; Brancati, F.L.; Ford, C.E.; Shulman, N.B.; Stamler, J. Blood pressure and end-stage renal disease in men. N. Engl. J. Med. 1996, 334, 13–18. [Google Scholar] [CrossRef]
- Siewert-Delle, A. Long-term renal function in primary hypertension. An epidemiological and pathophysiological study. Scand. J. Urol. Nephrol. Suppl. 1999, 199, 1–36. [Google Scholar] [PubMed]
- Tozawa, M.; Iseki, K.; Iseki, C.; Kinjo, K.; Ikemiya, Y.; Takishita, S. Blood pressure predicts risk of developing end-stage renal disease in men and women. Hypertension 2003, 41, 1341–1345. [Google Scholar] [CrossRef]
- Carlström, M.; Wåhlin, N.; Skøtt, O.; Persson, A.E. Relief of chronic partial ureteral obstruction attenuates salt-sensitive hypertension in rats. Acta Physiol. 2007, 189, 67–75. [Google Scholar] [CrossRef]
- Inada, Y.; Terashita, Z.; Shibouta, Y.; Nishikawa, K.; Shino, A.; Kikuchi, S.; Shimamoto, K. Acceleration of hypotension and development of stroke in the spontaneously hypertensive rat by unilateral ureteral obstruction. Clin. Exp. Hypertens. 1980, 2, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Inada, Y.; Terashita, Z.; Shibouta, Y.; Shimakawa, H.; Nishikawa, K.; Kikuchi, S.; Shimamoto, K. Facilitated hypertension and development of stroke in the spontaneously hypertensive rat by a unilateral ureteral obstruction [proceedings]. Jpn. Heart J. 1979, 20, 711. [Google Scholar] [CrossRef]
- Lucarelli, G.; Ditonno, P.; Bettocchi, C.; Grandaliano, G.; Gesualdo, L.; Selvaggi, F.P.; Battaglia, M. Delayed relief of ureteral obstruction is implicated in the long-term development of renal damage and arterial hypertension in patients with unilateral ureteral injury. J. Urol. 2013, 189, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Fukuda, N.; Matsuda, H.; Endo, M.; Wang, X.; Saito, K.; Ueno, T.; Matsumoto, T.; Matsumoto, K.; Soma, M.; et al. Complement 3 activates the renal renin-angiotensin system by induction of epithelial-to-mesenchymal transition of the nephrotubulus in mice. Am. J. Physiol. Ren. Physiol. 2013, 305, F957–F967. [Google Scholar] [CrossRef] [PubMed]
- Berecek, K.H.; Schwertschlag, U.; Gross, F. Alterations in renal vascular resistance and reactivity in spontaneous hypertension of rats. Am. J. Physiol. 1980, 238, H287–H293. [Google Scholar] [CrossRef]
- Sorensen, C.M.; Leyssac, P.P.; Skott, O.; Holstein-Rathlou, N.H. NO mediates downregulation of RBF after a prolonged reduction of renal perfusion pressure in SHR. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R329–R338. [Google Scholar] [CrossRef]
- Dai, F.X.; Skopec, J.; Diederich, A.; Diederich, D. Prostaglandin H2 and thromboxane A2 are contractile factors in intrarenal arteries of spontaneously hypertensive rats. Hypertension 1992, 19, 795–798. [Google Scholar] [CrossRef]
- Limas, C.J.; Limas, C. Vascular prostaglandin synthesis in the spontaneously hypertensive rat. Am. J. Physiol. 1977, 233, H493–H499. [Google Scholar] [CrossRef]
- Schirner, M.; Taube, C. Different effects of aspirin on blood pressure of spontaneously hypertensive rats (SHR) with high and spontaneously low levels of blood pressure. Br. J. Pharmacol. 1993, 109, 900–901. [Google Scholar] [CrossRef]
- Morrison, A.R.; Nishikawa, K.; Needleman, P. Unmasking of thromboxane A2 synthesis by ureteral obstruction in the rabbit kidney. Nature 1977, 267, 259–260. [Google Scholar] [CrossRef]
- Purkerson, M.L.; Klahr, S. Prior inhibition of vasoconstrictors normalizes GFR in postobstructed kidneys. Kidney Int. 1989, 35, 1305–1314. [Google Scholar] [CrossRef]
- Frokiaer, J.; Knudsen, L.; Nielsen, A.S.; Pedersen, E.B.; Djurhuus, J.C. Enhanced intrarenal angiotensin II generation in response to obstruction of the pig ureter. Am. J. Physiol. 1992, 263 Pt 2, F527–F533. [Google Scholar] [CrossRef]
- Hammad, F.T.; Lubbad, L. The effect of aliskiren on the renal dysfunction following unilateral ureteral obstruction in the rat. Int. J. Physiol. Pathophysiol. Pharmacol. 2016, 8, 70–77. [Google Scholar] [PubMed]
- Hammad, F.T.; Wheatley, A.M.; Davis, G. Role of endothelin ET(A) receptor antagonism in the post-transplant renal response to angiotensin II in the rat. Exp. Physiol. 2001, 86, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, H.; Morrissey, J.; Morrison, A.R.; Purkerson, M.L.; Klahr, S. Role of ANG II in eicosanoid production by isolated glomeruli from rats with bilateral ureteral obstruction. Am. J. Physiol. 1990, 258 Pt 2, F85–F93. [Google Scholar] [CrossRef]
- Yarger, W.E.; Schocken, D.D.; Harris, R.H. Obstructive nephropathy in the rat: Possible roles for the renin-angiotensin system, prostaglandins, and thromboxanes in postobstructive renal function. J. Clin. Investig. 1980, 65, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.M.; Chen, J.; Silver, R.B.; Poppas, D.P.; Vaughan, E.D., Jr.; Felsen, D. Effect of UUO on D1aR expression reveals a link among dopamine, transforming growth factor-beta, and nitric oxide. Am. J. Physiol. Renal. Physiol. 2004, 286, F509–F515. [Google Scholar] [CrossRef]
- Xuan, M.Y.; Piao, S.G.; Ding, J.; Nan, Q.Y.; Piao, M.H.; Jiang, Y.J.; Zheng, H.L.; Jin, J.Z.; Li, C. Dapagliflozin Alleviates Renal Fibrosis by Inhibiting RIP1-RIP3-MLKL-Mediated Necroinflammation in Unilateral Ureteral Obstruction. Front. Pharmacol. 2021, 12, 798381. [Google Scholar] [CrossRef]
- Knight, S.; Johns, E.J. Renal functional responses to ischaemia-reperfusion injury in normotensive and hypertensive rats following non-selective and selective cyclo-oxygenase inhibition with nitric oxide donation. Clin. Exp. Pharmacol. Physiol. 2008, 35, 11–16. [Google Scholar] [CrossRef]
- Bakoush, O.; Lubbad, L.; Öberg, C.M.; Hammad, F.T. Effect of diabetes mellitus on the recovery of changes in renal functions and glomerular permeability following reversible 24-hour unilateral ureteral obstruction. J. Diabetes 2019, 11, 674–683. [Google Scholar] [CrossRef]
- Rocha, N.N. Are Wistar Rats the Most Suitable Normotensive Controls for Spontaneously Hypertensive Rats to Assess Blood Pressure and Cardiac Structure and Function? Int. J. Cardiovasc. Sci. 2022, 35, 172–173. [Google Scholar] [CrossRef]
- Kurtz, T.W.; Morris, R.C., Jr. Biological variability in Wistar-Kyoto rats. Implications for research with the spontaneously hypertensive rat. Hypertension 1987, 10, 127–131. [Google Scholar] [CrossRef]
- Rezende, L.M.T.; Soares, L.L.; Drummond, F.R.; Suarez, P.Z.; Leite, L.; Rodrigues, J.A.; Leal, T.; Favarato, L.; Reis, E.C.C.; Favarato, E.; et al. Is the Wistar Rat a more Suitable Normotensive Control for SHR to Test Blood Pressure and Cardiac Structure and Function? Int. J. Cardiovasc. Sci. 2022, 35, 161–171. [Google Scholar] [CrossRef]
- Moreira, N.J.D.; Dos Santos, F.; Moreira, E.D.; Farah, D.; de Souza, L.E.; da Silva, M.B.; Moraes-Silva, I.C.; Lincevicius, G.S.; Caldini, E.G.; Irigoyen, M.C.C. Acute renal denervation normalizes aortic function and decreases blood pressure in spontaneously hypertensive rats. Sci. Rep. 2020, 10, 21826. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Johns, E.J. Renal nerves, renin, and angiotensinogen gene expression in spontaneously hypertensive rats. Hypertension 1995, 25, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Guo, L.; Liu, G.; Peng, T.; Li, H.; Xie, T.; Li, D.; Zhen, J.; Wang, Y.; Yang, H.; et al. Protective effect of calcitriol on podocytes in spontaneously hypertensive rat. J. Chin. Med. Assoc. JCMA 2018, 81, 691–698. [Google Scholar] [CrossRef]
- Hammad, F.T.; Lubbad, L. The effect of diclofenac sodium on renal function in reversible unilateral ureteric obstruction. Urol. Res. 2011, 39, 351–356. [Google Scholar] [CrossRef]
- Hammad, F.T.; Salam, S.A.; Nemmar, A.; Ali, M.; Lubbad, L. The Effect of Arabic Gum on Renal Function in Reversible Unilateral Ureteric Obstruction. Biomolecules 2019, 9, 25. [Google Scholar] [CrossRef] [PubMed]



| RBF | GFR | FENa | |||||||
|---|---|---|---|---|---|---|---|---|---|
| NOK | POK | % Diff. | NOK | POK | % Diff. | NOK | POK | % Diff. | |
| G-NT | 5.98 ± 0.86 | 4.19 ± 0.69 * | −40 ± 11 | 0.95 ± 0.12 | 0.56 ± 0.09 * | −55 ± 8 | 0.031 ± 0.004 | 0.055 ± 0.010 * | 21 ± 10 |
| G-HT | 3.88 ± 0.67 # | 1.08 ± 0.29 * | −107 ± 18 $ | 0.48 ± 0.07 # | 0.11 ± 0.02 * | −132 ± 13 $ | 0.027 ± 0.004 | 0.069 ± 0.013 * | 54 ± 16 |
| Kim1 (NM_173149.2) | Forward | CTCACACTCAGATCATCTTCTC |
| Reverse | CCGCTTGGTGGTTTGCTAC | |
| Probe | FAM-CTCGAGTGACAAGCCCGTAGCC-BHQ-1 | |
| NGAL (Lcn2) (NM_130741.1) | Forward | CTGTTCCCACCGACCAATGC |
| Reverse | CCACTGCACATCCCAGTCA | |
| Probe | FAM-TGACAACTGAACAGACGGTGAGCG-BHQ-1 | |
| TNF-α (NM_012675.3) | Forward | CTCACACTCAGATCATCTTCTC |
| Reverse | CCGCTTGGTGGTTTGCTAC | |
| Probe | FAM-CTCGAGTGACAAGCCCGTAGCC-BHQ-1 | |
|
TGF-β1 (NM_021578.2) | Forward | GTGGCTGAACCAAGGAGACG |
| Reverse | CGTGGAGTACATTATCTTTGCTGTC | |
| Probe | FAM-ACAGGGCTTTCGCTTCAGTGCTC-BHQ-1 | |
| p53 (NM_030989.3) | Forward | CGAGATGTTCCGAGAGCTGAATG |
| Reverse | GTCTTCGGGTAGCTGGAGTG | |
| Probe | FAM-CCTTGGAATTAAAGGATGCCCGTGC-BHQ-1 | |
| COL1A (NM_053304.1) | Forward | CTGACTGGAAGAGCGGAGAGT |
| Reverse | CCTGTCTCCATGTTGCAGTAGAC | |
| Probe | FAM-ACTGGATCGACCCTAACCAAGGC-BHQ-1 | |
| PPIA (NM_017101.1) | Forward | GCGTCTGCTTCGAGCTGT |
| Reverse | CACCCTGGCACATGAATCC | |
| Probe | Quasar 670-TGCAGACAAAGTTCCAAAGACAGCA-BHQ-2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammad, F.T.; Lubbad, L.; Al-Salam, S.; Yasin, J.; Meeran, M.F.N.; Ojha, S.; Hammad, W.F. The Effect of Hypertension on the Recovery of Renal Dysfunction following Reversal of Unilateral Ureteral Obstruction in the Rat. Int. J. Mol. Sci. 2023, 24, 7365. https://doi.org/10.3390/ijms24087365
Hammad FT, Lubbad L, Al-Salam S, Yasin J, Meeran MFN, Ojha S, Hammad WF. The Effect of Hypertension on the Recovery of Renal Dysfunction following Reversal of Unilateral Ureteral Obstruction in the Rat. International Journal of Molecular Sciences. 2023; 24(8):7365. https://doi.org/10.3390/ijms24087365
Chicago/Turabian StyleHammad, Fayez T., Loay Lubbad, Suhail Al-Salam, Javed Yasin, Mohamed Fizur Nagoor Meeran, Shreesh Ojha, and Waheed F. Hammad. 2023. "The Effect of Hypertension on the Recovery of Renal Dysfunction following Reversal of Unilateral Ureteral Obstruction in the Rat" International Journal of Molecular Sciences 24, no. 8: 7365. https://doi.org/10.3390/ijms24087365
APA StyleHammad, F. T., Lubbad, L., Al-Salam, S., Yasin, J., Meeran, M. F. N., Ojha, S., & Hammad, W. F. (2023). The Effect of Hypertension on the Recovery of Renal Dysfunction following Reversal of Unilateral Ureteral Obstruction in the Rat. International Journal of Molecular Sciences, 24(8), 7365. https://doi.org/10.3390/ijms24087365








