In Response to a Punctual Stress Male and Female Tyrosine Hydroxylase Haploinsufficient Mice Show a Deteriorated Behavior, Immunity, and Redox State
Abstract
1. Introduction
2. Results
2.1. Behavioral Tests
2.1.1. Sensorimotor Abilities
2.1.2. Anxiety-like Behaviors
2.1.3. Exploratory Behaviors
2.2. Immune Function
2.3. Oxidative Stress
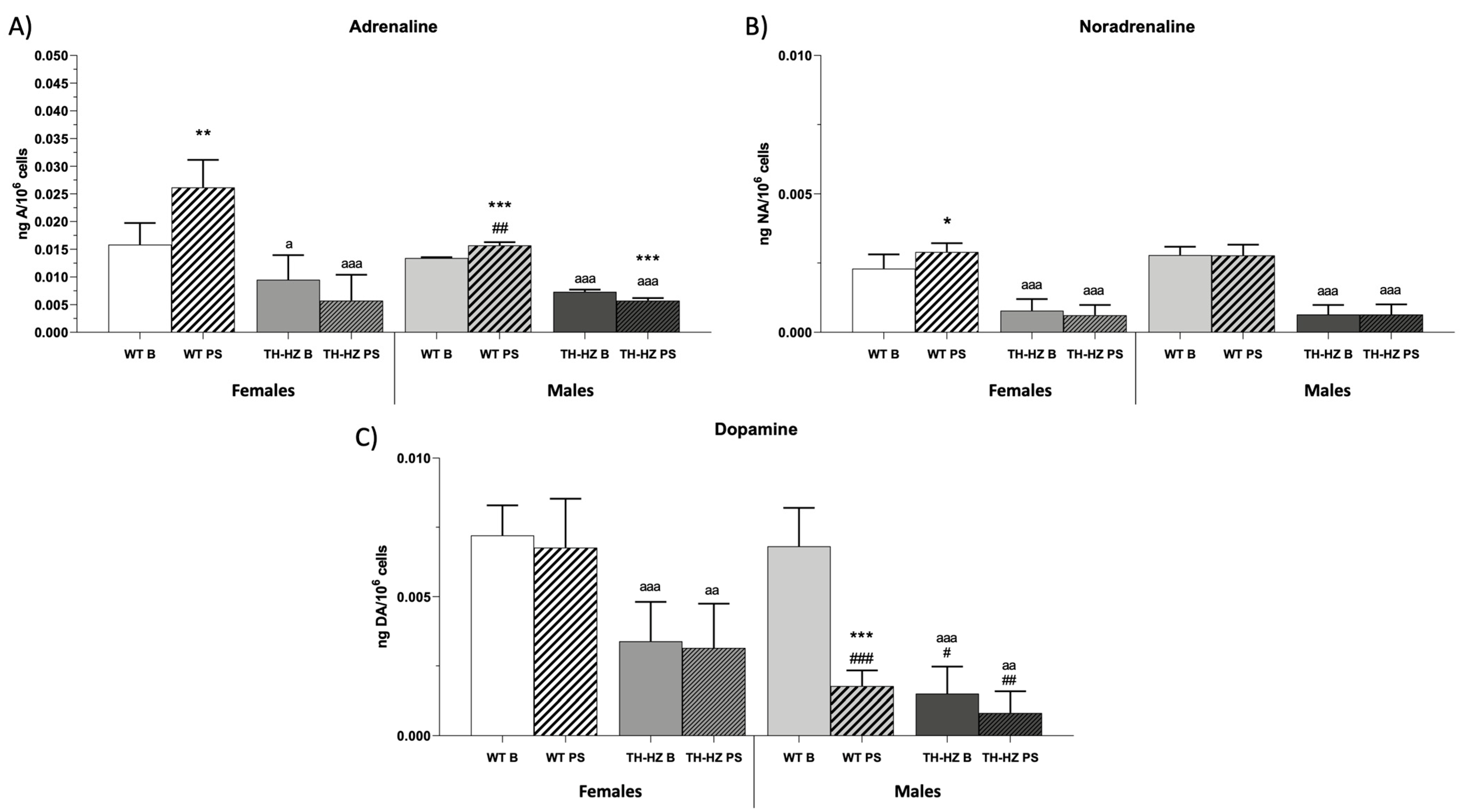
2.4. Catecholamine Concentrations
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Restriction Stress
4.4. Behavioral Tests
4.4.1. Sensorimotor Abilities
- Visual Placing and Hindlimb extensor reflex
- Wood rod test
- Tightrope test
4.4.2. Exploratory Behavioral Tests
- T-Maze test
- Corner test
- Holeboard test
4.4.3. Anxiety Behavioral Tests
- Elevated plus maze
- Marble Burying test
4.5. Collection of Peritoneal Leukocytes
4.6. Immune Function Parameters
- Chemotaxis
- Macrophage phagocytosis
- Natural Killer Activity
- Lymphoproliferative capacity
4.7. Oxidative Stress Parameters
- Catalase activity
- Glutathione reductase activity
- Glutathione peroxidase activity
- Glutathione concentration
- Xanthine oxidase activity
4.8. Concentration of Catecholamines
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sapolsky, R.M. Individual differences and the stress response. In Seminars in Neuroscience; Elsevier: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Lu, S.; Wei, F.; Li, G. The evolution of the concept of stress and the framework of the stress system. Cell Stress 2021, 5, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Seyle, H. “Stress” conducta social. Jano Med. Humanid. 1975, 11–17. [Google Scholar]
- De la Fuente, M.; Miquel, J. An update of the oxidation-inflammation theory of aging: The involvement of the immune system in oxi-inflamm-aging. Curr. Pharm. Des. 2009, 15, 3003–3026. [Google Scholar] [CrossRef]
- De la Fuente, M. Oxidation and Inflammation in the Immune and Nervous Systems, a Link between Aging and Anxiety; Springer Nature: Cham, Switzerland, 2018; pp. 1–31. [Google Scholar]
- Cruces, J.; Venero, C.; Pereda-Pérez, I.; De la Fuente, M. The effect of psychological stress and social isolation on neuroimmunoendocrine communication. Curr. Pharm. Des. 2014, 20, 4608–4628. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, D.L.; Millar, B.A.; Perez, S.; Carter, J.; Wood, C.; ThyagaRajan, S.; Molinaro, C.; Lubahn, C.; Lorton, D. Sympathetic modulation of immunity: Relevance to disease. Cell Immunol. 2008, 252, 27–56. [Google Scholar] [CrossRef]
- Elenkov, I.J.; Wilder, R.L.; Chrousos, G.P.; Vizi, E.S. The sympathetic nerve--an integrative interface between two supersystems: The brain and the immune system. Pharmacol. Rev. 2000, 52, 595–638. [Google Scholar]
- Procaccini, C.; Pucino, V.; De Rosa, V.; Marone, G.; Matarese, G. Neuro-endocrine networks controlling immune system in health and disease. Front. Immunol. 2014, 5, 143. [Google Scholar] [CrossRef]
- Borodovitsyna, O.; Flamini, M.; Chandler, D. Noradrenergic Modulation of Cognition in Health and Disease. Neural Plast. 2017, 2017, 6031478. [Google Scholar] [CrossRef]
- Gasser, P.J. Organic Cation Transporters in Brain Catecholamine Homeostasis. Handb. Exp. Pharmacol. 2021, 266, 187–197. [Google Scholar]
- Shimizu, N.; Kaizuka, Y.; Hori, T.; Nakane, H. Immobilization increases norepinephrine release and reduces NK cytotoxicity in spleen of conscious rat. Am. J. Physiol. 1996, 271, R537–R544. [Google Scholar] [CrossRef]
- Weinstein, L.I.; Revuelta, A.; Pando, R.H. Catecholamines and acetylcholine are key regulators of the interaction between microbes and the immune system. Ann. N. Y. Acad. Sci. 2015, 1351, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Nagatsu, T.; Levitt, M.; Udenfriend, S. Tyrosine hydroxylase. The initial step in norepinephrine biosynthesis. J. Biol. Chem. 1964, 239, 2910–2917. [Google Scholar] [CrossRef] [PubMed]
- Garrido, A.; Cruces, J.; Ceprián, N.; Hernández-Sánchez, C.; De la Fuente, M. Premature aging in behavior and immune functions in tyrosine hydroxylase haploinsufficient female mice. A longitudinal study. Brain Behav. Immun. 2018, 69, 440–455. [Google Scholar] [CrossRef]
- Garrido, A.; Cruces, J.; Ceprián, N.; Hernández-Sánchez, C.; De Pablo, F.; De la Fuente, M. Social Environment Ameliorates Behavioral and Immune Impairments in Tyrosine Hydroxylase Haploinsufficient Female Mice. J. Neuroimmune Pharmacol. 2021, 16, 548–566. [Google Scholar] [CrossRef] [PubMed]
- Ceprian, N.; Garrido, A.; De la Fuente, M. Improved immunity and mean lifespan in mature mice genetically deficient in catecholamine synthesis after living with wild type for two months. Rev. Esp. Geriatr. Gerontol. 2022, 58, 15–21. [Google Scholar] [CrossRef]
- Sheridan, J.F.; Padgett, D.A.; Avitsur, R.; Marucha, P.T. Experimental models of stress and wound healing. World J. Surg. 2004, 28, 327–330. [Google Scholar] [CrossRef]
- Sahin, E.; Gümüşlü, S. Stress-dependent induction of protein oxidation, lipid peroxidation and anti-oxidants in peripheral tissues of rats: Comparison of three stress models (immobilization, cold and immobilization-cold). Clin. Exp. Pharmacol. Physiol. 2007, 34, 425–431. [Google Scholar] [CrossRef]
- Imbe, H.; Iwai-Liao, Y.; Senba, E. Stress-induced hyperalgesia: Animal models and putative mechanisms. Front. Biosci. 2006, 11, 2179–2192. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Chen, Q.; Li, Z.; Liu, C.; Yin, S.; You, Z. Felbamate produces antidepressant-like actions in the chronic unpredictable mild stress and chronic social defeat stress models of depression. Fundam. Clin. Pharmacol. 2019, 33, 621–633. [Google Scholar] [CrossRef]
- Kõks, S.; Planken, A.; Luuk, H.; Vasar, E. Cat odour exposure increases the expression of wolframin gene in the amygdaloid area of rat. Neurosci. Lett. 2002, 322, 116–120. [Google Scholar] [CrossRef]
- Kõks, S.; Luuk, H.; Nelovkov, A.; Areda, T.; Vasar, E. A screen for genes induced in the amygdaloid area during cat odor exposure. Genes Brain Behav. 2004, 3, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Suarez, L.M.; Cerro, E.D.; Felix, J.; Gonzalez-Sanchez, M.; Ceprian, N.; Guerra-Perez, N.; Novelle, M.G.; Toda, I.M.; Fuente, M. Sex differences in neuroimmunoendocrine communication. Involvement on longevity. Mech. Ageing Dev. 2023, 111798. [Google Scholar] [CrossRef] [PubMed]
- Garrido, A.; Cruces, J.; Iriarte, I.; Hernández-Sánchez, C.; de Pablo, F.; de la Fuente, M. [Premature immunosenescence in catecholamines syntesis deficient mice. Effect of social environment]. Rev. Esp. Geriatr. Gerontol 2017, 52, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Garrido, A.; de la Fuente, M. Social environment improves the cytokine profile and lymphoproliferative response in chronologically old and prematurely aging mice. Mech. Ageing Dev. 2022, 206, 111692. [Google Scholar] [CrossRef]
- Liu, W.Z.; Zhang, W.H.; Zheng, Z.H.; Zou, J.X.; Liu, X.X.; Huang, S.H.; You, W.J.; He, Y.; Zhang, J.Y.; Wang, X.D.; et al. Identification of a prefrontal cortex-to-amygdala pathway for chronic stress-induced anxiety. Nat. Commun. 2020, 11, 2221. [Google Scholar] [CrossRef]
- Dragoş, D.; Tănăsescu, M.D. The effect of stress on the defense systems. J. Med. Life 2010, 3, 10–18. [Google Scholar]
- Dhabhar, F.S. Effects of stress on immune function: The good, the bad, and the beautiful. Immunol. Res. 2014, 58, 193–210. [Google Scholar] [CrossRef]
- Kanďár, R. The ratio of oxidized and reduced forms of selected antioxidants as a possible marker of oxidative stress in humans. Biomed. Chromatogr. 2016, 30, 13–28. [Google Scholar] [CrossRef]
- Inglis, A.; Shibin, S.; Ubungen, R.; Farooq, S.; Mata, P.; Thiam, J.; Al-Mohanna, F.A.; Collison, K.S. Strain and sex-based glucocentric & behavioral differences between KK/HlJ and C57BL/6J mice. Physiol. Behav. 2019, 210, 112646. [Google Scholar]
- Kobayashi, H.; Martínez de Toda, I.; Sanz-San Miguel, L.; De la Fuente, M. Sex-related differences in behavioural markers in adult mice for the prediction of lifespan. Biogerontology 2021, 22, 49–62. [Google Scholar] [CrossRef]
- Schmid, S.; Rammes, G.; Blobner, M.; Kellermann, K.; Bratke, S.; Fendl, D.; Kaichuan, Z.; Schneider, G.; Jungwirth, B. Cognitive decline in Tg2576 mice shows sex-specific differences and correlates with cerebral amyloid-beta. Behav. Brain Res. 2019, 359, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, H.; Klein, S.L. Sex Differences in Immunity to Viral Infections. Front. Immunol. 2021, 12, 720952. [Google Scholar] [CrossRef] [PubMed]
- Kander, M.C.; Cui, Y.; Liu, Z. Gender difference in oxidative stress: A new look at the mechanisms for cardiovascular diseases. J. Cell Mol. Med. 2017, 21, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Borrás, C.; Sastre, J.; García-Sala, D.; Lloret, A.; Pallardó, F.V.; Viña, J. Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radic. Biol. Med. 2003, 34, 546–552. [Google Scholar] [CrossRef] [PubMed]
- McCormick, C.M.; Linkroum, W.; Sallinen, B.J.; Miller, N.W. Peripheral and central sex steroids have differential effects on the HPA axis of male and female rats. Stress 2002, 5, 235–247. [Google Scholar] [CrossRef]
- Bronikowski, A.M.; Meisel, R.P.; Biga, P.R.; Walters, J.R.; Mank, J.E.; Larschan, E.; Wilkinson, G.S.; Valenzuela, N.; Conard, A.M.; de Magalhães, J.P.; et al. Sex-specific aging in animals: Perspective and future directions. Aging Cell 2022, 21, e13542. [Google Scholar] [CrossRef]
- Elenkov, I.J. Effects of catecholamines on the immune response. Neuroimmune Biol. 2007, 7, 189–206. [Google Scholar]
- Curtin, N.M.; Mills, K.H.; Connor, T.J. Psychological stress increases expression of IL-10 and its homolog IL-19 via beta-adrenoceptor activation: Reversal by the anxiolytic chlordiazepoxide. Brain Behav. Immun. 2009, 23, 371–379. [Google Scholar] [CrossRef]
- Laukova, M.; Vargovic, P.; Krizanova, O.; Kvetnansky, R. Repeated stress down-regulates β(2)- and α (2C)-adrenergic receptors and up-regulates gene expression of IL-6 in the rat spleen. Cell. Mol. Neurobiol. 2010, 30, 1077–1087. [Google Scholar] [CrossRef]
- Azpiroz, A.; Fano, E.; Garmendia, L.; Arregi, A.; Cacho, R.; Beitia, G.; Brain, P.F. Effects of chronic mild stress (CMS) and imipramine administration, on spleen mononuclear cell proliferative response, serum corticosterone level and brain norepinephrine content in male mice. Psychoneuroendocrinology 1999, 24, 345–361. [Google Scholar] [CrossRef]
- Haberfeld, M.; Johnson, R.O.; Ruben, L.N.; Clothier, R.H.; Shiigi, S. Adrenergic modulation of apoptosis in splenocytes of Xenopus laevis in vitro. Neuroimmunomodulation 1999, 6, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Avitsur, R.; Kavelaars, A.; Heijnen, C.; Sheridan, J.F. Social stress and the regulation of tumor necrosis factor-alpha secretion. Brain Behav. Immun. 2005, 19, 311–317. [Google Scholar] [CrossRef]
- O’Donnell, P.M.; Orshal, J.M.; Sen, D.; Sonnenfeld, G.; Aviles, H.O. Effects of exposure of mice to hindlimb unloading on leukocyte subsets and sympathetic nervous system activity. Stress 2009, 12, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Rai, U. Dual mode of catecholamine action on splenic macrophage phagocytosis in wall lizard, Hemidactylus flaviviridis. Gen. Comp. Endocrinol. 2004, 136, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Riddell, S.R. Adrenaline fuels a cytokine storm during immunotherapy. Nature 2018, 564, 194–196. [Google Scholar]
- Sofic, E.; Denisova, N.; Youdim, K.; Vatrenjak-Velagic, V.; De Filippo, C.; Mehmedagic, A.; Causevic, A.; Cao, G.; Joseph, J.A.; Prior, R.L. Antioxidant and pro-oxidant capacity of catecholamines and related compounds. Effects of hydrogen peroxide on glutathione and sphingomyelinase activity in pheochromocytoma PC12 cells: Potential relevance to age-related diseases. J. Neural. Transm. 2001, 108, 541–557. [Google Scholar]
- Irie, M.; Asami, S.; Nagata, S.; Miyata, M.; Kasai, H. Relationships between perceived workload, stress and oxidative DNA damage. Int. Arch. Occup. Environ. Health 2001, 74, 153–157. [Google Scholar] [CrossRef]
- Kõks, S.; Soosaar, A.; Võikar, V.; Bourin, M.; Vasar, E. BOC-CCK-4, CCK(B)receptor agonist, antagonizes anxiolytic-like action of morphine in elevated plus-maze. Neuropeptides 1999, 33, 63–69. [Google Scholar] [CrossRef]
- Kõks, S.; Männistö, P.T.; Bourin, M.; Shlik, J.; Vasar, V.; Vasar, E. Cholecystokinin-induced anxiety in rats: Relevance of pre-experimental stress and seasonal variations. J. Psychiatry Neurosci. 2000, 25, 33–42. [Google Scholar]
- Martínez de Toda, I.; Miguélez, L.; Siboni, L.; Vida, C.; De la Fuente, M. High perceived stress in women is linked to oxidation, inflammation and immunosenescence. Biogerontology 2019, 20, 823–835. [Google Scholar] [CrossRef]
- Beach, S.R.; Schulz, R.; Yee, J.L.; Jackson, S. Negative and positive health effects of caring for a disabled spouse: Longitudinal findings from the caregiver health effects study. Psychol. Aging 2000, 15, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, P.; Robles, A.M.; de Pablo, F.; Hernández-Sánchez, C. Non-neural tyrosine hydroxylase, via modulation of endocrine pancreatic precursors, is required for normal development of beta cells in the mouse pancreas. Diabetologia 2014, 57, 2339–2347. [Google Scholar] [CrossRef]
- Giménez-Llort, L.; Fernández-Teruel, A.; Escorihuela, R.M.; Fredholm, B.B.; Tobeña, A.; Pekny, M.; Johansson, B. Mice lacking the adenosine A1 receptor are anxious and aggressive, but are normal learners with reduced muscle strength and survival rate. Eur. J. Neurosci. 2002, 16, 547–550. [Google Scholar] [CrossRef] [PubMed]
- de Cabo de la Vega, C.; Pujol, A.; Paz Viveros, M. Neonatally administered naltrexone affects several behavioral responses in adult rats of both genders. Pharmacol. Biochem. Behav. 1995, 50, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Baeza, I.; De Castro, N.M.; Giménez-Llort, L.; De la Fuente, M. Ovariectomy, a model of menopause in rodents, causes a premature aging of the nervous and immune systems. J. Neuroimmunol. 2010, 219, 90–99. [Google Scholar] [CrossRef]
- Miquel, J.; Blasco, M. A simple technique for evaluation of vitality loss in aging mice, by testing their muscular coordination and vigor. Exp. Gerontol. 1978, 13, 389–396. [Google Scholar] [CrossRef]
- De la Fuente, M.; Miñano, M.; Manuel Victor, V.; Del Rio, M.; Ferrández, M.D.; Díez, A.; Miquel, J. Relation between exploratory activity and immune function in aged mice: A preliminary study. Mech. Ageing Dev. 1998, 102, 263–277. [Google Scholar] [CrossRef]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef]
- Prajapati, R.P.; Kalaria, M.V.; Karkare, V.P.; Parmar, S.K.; Sheth, N.R. Effect of methanolic extract of Lagenaria siceraria (Molina) Standley fruits on marble-burying behavior in mice: Implications for obsessive-compulsive disorder. Pharmacogn. Res. 2011, 3, 62–66. [Google Scholar] [CrossRef]
- Guayerbas, N.; Puerto, M.; Víctor, V.M.; Miquel, J.; De la Fuente, M. Leukocyte function and life span in a murine model of premature immunosenescence. Exp. Gerontol. 2002, 37, 249–256. [Google Scholar] [CrossRef]
- Martínez de Toda, I.; Maté, I.; Vida, C.; Cruces, J.; De la Fuente, M. Immune function parameters as markers of biological age and predictors of longevity. Aging 2016, 8, 3110–3119. [Google Scholar] [CrossRef] [PubMed]
- De La Fuente, M. Changes in the macrophage function with aging. Comp. Biochem. Physiol. A Comp. Physiol. 1985, 81, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Ferrández, M.D.; Correa, R.; Del Rio, M.; De la Fuente, M. Effects in vitro of several antioxidants on the natural killer function of aging mice. Exp. Gerontol. 1999, 34, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, C.; Alvarez, P.; Puerto, M.; Gausserès, N.; Jiménez, L.; De la Fuente, M. Dietary supplementation with antioxidants improves functions and decreases oxidative stress of leukocytes from prematurely aging mice. Nutrition 2006, 22, 767–777. [Google Scholar] [CrossRef]


| Females | Males | |||||||
|---|---|---|---|---|---|---|---|---|
| WT Basal | WT Post-Stress | TH-HZ Basal | TH-HZ Post-Stress | WT Basal | WT Post-Stress | TH-HZ Basal | TH-HZ Post-Stress | |
| Weight (g) | 39 ± 2 | 39 ± 1 | 38 ± 3 | 40 ± 2 | 41 ± 1 | 40 ± 1 | 40 ± 2 | 42 ± 1 |
| Reflex Visual placing reflex % Mice showing this response Hindlimb extensor reflex % Mice showing this response | 100 100 | 100 100 | 100 100 | 100 100 | 100 100 | 100 100 | 100 100 | 100 100 |
| Wood rod test Motor coordination Latency to leave the starting segment (s) Total number of crossing segments Time of permanence (s) | 4 ± 2 3 ± 1 16 ± 5 | 8 ± 5 5 ± 2 43 ± 18 * | 10 ± 6 5 ± 2 23 ± 6 | 9 ± 11 2 ± 2 * a 48 ± 22 * | 4 ± 1 3 ± 1 11 ± 3 | 5 ± 3 6 ± 2 * 35 ± 22 * | 14 ± 4 aa 3 ± 1 28 ± 5 aaa | 4 ± 2 *** 4 ± 3 46 ± 17 * |
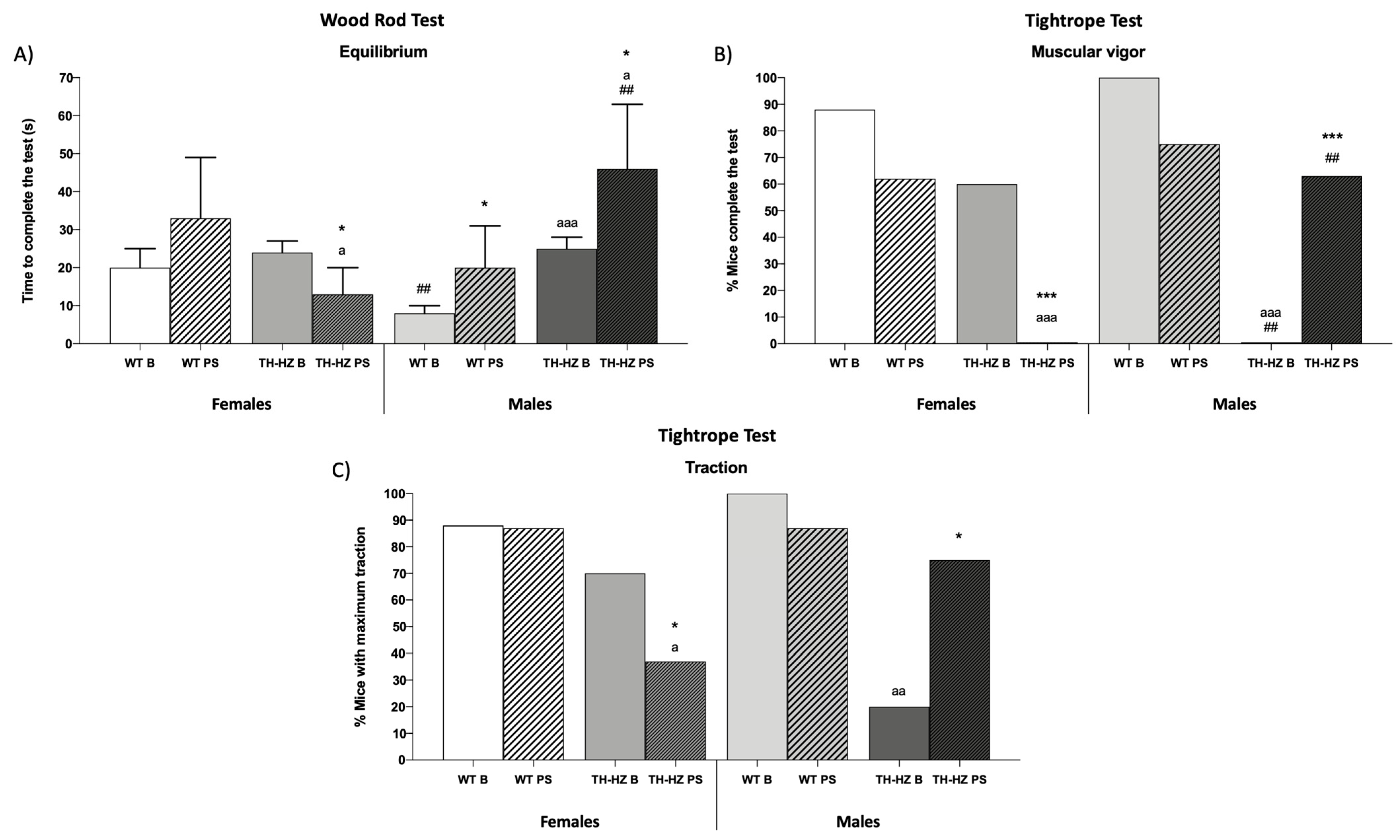
| Equilibrium % Mice falling off the wood rod Latency to fall (s) % Mice that complete the test Time to complete the test (s) | 0 0 100 (Figure 1A) | 0 0 63 | 0 0 75 | 0 0 25 ** a | 0 0 100 | 0 0 63 | 0 0 50 a | 0 0 50 |
| Other behaviors Number of freezings Time of freezing (s) % Mice performing freezing | 0 0 0 | 1 ± 1 3 ± 1 ** 25 | 0 0 0 | 2 ± 1 3 ± 1 ** 63 ** a | 0 0 0 | 0 0 0 | 0 0 0 | 1 ± 1 3 ± 1 * a 12 # |
| Tightrope test Motor coordination Total number of crossing segments Time of permanence (s) | 3 ± 1 20 ± 4 | 2 ± 1 42 ± 18 * | 3 ± 1 50 ± 8 aaa | 1 ± 1 ** 31 ± 24 | 4 ± 1 24 ± 5 | 3 ± 1 28 ± 14 | 4 ± 1 50 ± 7 aaa | 3 ± 1 ## 40 ± 16 |
| Muscular vigor % Mice falling off the tightrope Latency to fall (s) % Mice that complete the test Time to complete the test (s) | 12 29 ± 4 (Figure 1B) 21 ± 5 | 38 37 ± 5 * 10 ± 7 * | 20 17 ± 2 aaa 45 ± 12 aa | 63 * 22 ± 19 0 *** a | 0 0 ### 23 ± 5 | 13 26 ± 6 *** ## 24 ± 7 ## | 50 a 28 ± 4 aaa ### 0 aaa ### | 36 23 ± 8 34 ± 14 ** ## |
| Traction (%) Low Medium Maximum | 0 12 (Figure 1C) | 13 0 | 0 30 | 50 * 13 | 0 0 | 0 13 | 20 60 | 0 # 25 |
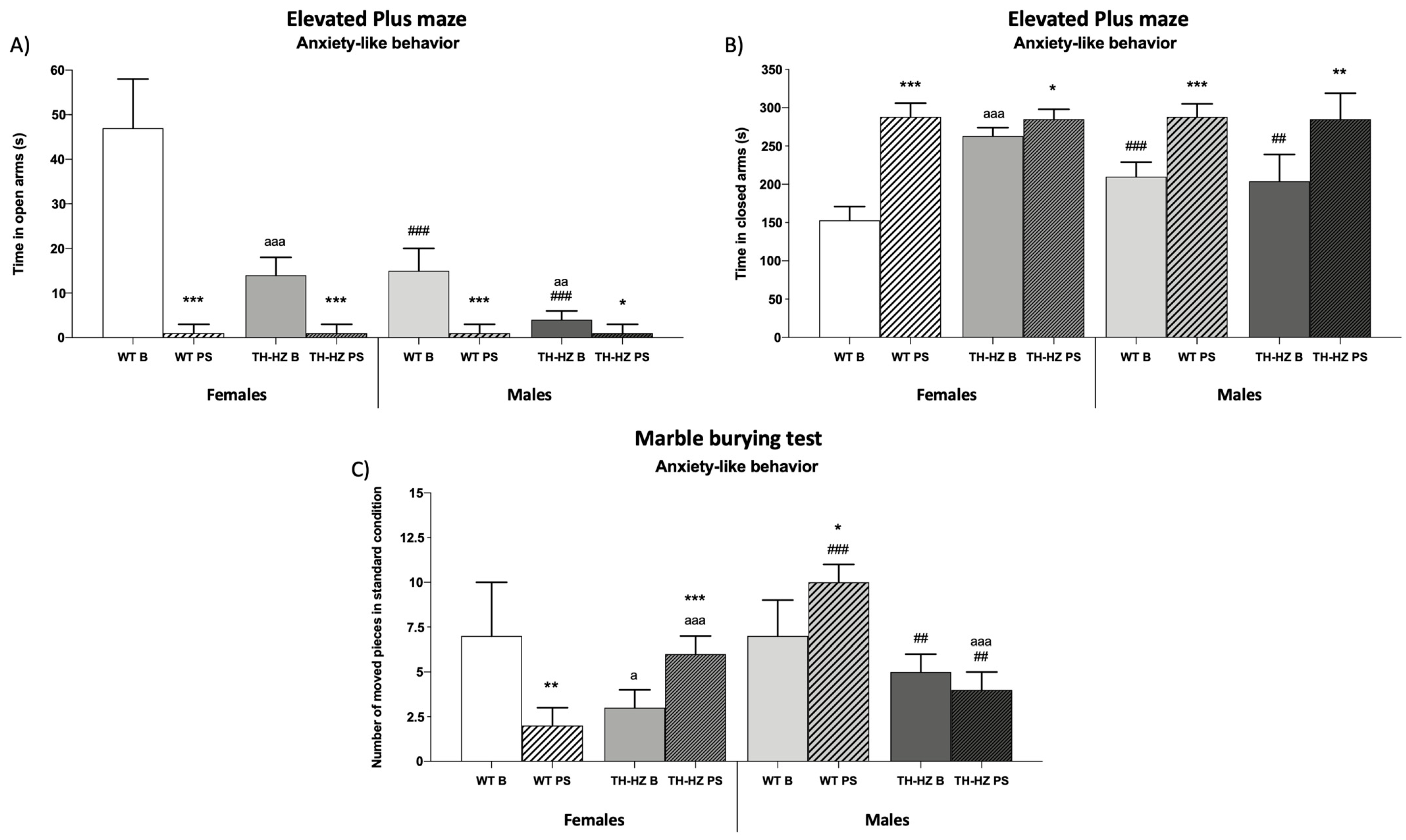
| Elevated plus maze Number of times mice explore open arms Number of times mice explore closed arms Time in open arms (s) Time in closed arms (s) Time in central platform (s) % Time in open arms % Time in closed arms % Time in central platform Number of rearings Time of rearings (s) Number of groomings Time of groomings (s) | 7 ± 3 8 ± 2 (Figure 2A) (Figure 2B) 100 ± 16 16 ± 3 51 ± 12 33 ± 9 7 ± 3 12 ± 4 1 ± 1 4 ± 2 | 1 ± 1 ** 1 ± 1 *** 11 ± 16 *** 0.3 ± 1 *** 96 ± 4 *** 3.7 ± 1 *** 5 ± 2 7 ± 4 11 ± 2 *** 15 ± 3 *** | 3 ± 2 a 17 ± 3 aaa 23 ± 8 aaa 5 ± 1 aaa 88 ± 7 aaa 8 ± 3 aaa 7 ± 2 9 ± 3 15 ± 4 aaa 17 ± 5 aaa | 1 ± 1 * 3 ± 2 *** 6 ± 2 ** 0.3 ± 1 *** 95 ± 2 * 4.7 ± 1 * 6 ± 2 9 ± 2 18 ± 3 aa 27 ± 4 ** aaa | 3 ± 1 # 14 ± 2 ### 74 ± 13 # 5 ± 2 ### 70 ± 6 ## 25 ± 3 18 ± 3 ### 25 ± 4 ### 3 ± 2 7 ± 2 # | 1 ± 1 ** 1 ± 1 *** 11 ± 16 *** 0.3 ± 1 ** 96 ± 4 *** 3.7 ± 2 *** 5 ± 2 *** 6 ± 3 *** 11 ± 3 *** 18 ± 5 ** | 1 ± 1 aa 13 ± 4 78 ± 23 ## 1 ± 1 aa ### 68 ± 4 ### 31 ± 3 aa ### 14 ± 5 # 16 ± 4 aa ## 5 ± 3 ### 9 ± 2 ## | 1 ± 1 3 ± 2 *** 14 ± 12 *** 0.3 ± 1 95 ± 3 *** 4.7 ± 1 *** 6 ± 2 ** 8 ± 3 ** 18 ± 2 *** aa 25 ± 4 *** a |
| Burial behavior Standard condition Number of intact pieces Number of moved pieces Number of buried pieces | 2 ± 1 (Figure 2C) 6 ± 3 | 10 ± 1 *** 2 ± 1 * | 5 ± 1 aaa 8 ± 2 | 6 ± 1 aaa 6 ± 1 aaa | 1 ± 1 9 ± 1 | 2 ± 1 ### 7 ± 1 ** ### | 4 ± 1 aaa 6 ± 2 a | 8 ± 1 *** aaa ## 2 ± 1 ** aaa ### |
| Bizonal condition Number of intact pieces Number of moved pieces Number of buried pieces | 3 ± 1 6 ± 2 2 ± 2 | 4 ± 1 4 ± 1 3 ± 1 | 1 ± 2 6 ± 1 6 ± 1 aa | 1 ± 1 aaa 7 ± 1 aaa 5 ± 1 aa | 1 ± 1 ## 5 ± 2 5 ± 1 # | 1 ± 1 ### 7 ± 1 ### 6 ± 1 ### | 3 ± 2 4 ± 1 ## 3 ± 1 aa ### | 3 ± 1 aa ## 5 ± 1 aa ## 3 ± 1 aaa ## |
| Females | Males | |||||||
|---|---|---|---|---|---|---|---|---|
| WT Basal | WT Post-Stress | TH-HZ Basal | TH-HZ Post-Stress | WT Basal | WT Post-Stress | TH-HZ Basal | TH-HZ Post-Stress | |
| Holeboard test Non-goal-directed behavior Vertical exploration Number of wall rearings Time of wall rearings (s) Number of central rearings Time of central rearings (s) Horizontal exploration Inner locomotion Middle locomotion External locomotion Total locomotion Inner locomotion average Middle locomotion average External locomotion average Total locomotion average % Middle locomotion % External locomotion % Total locomotion Other behaviors Number of groomings Time of groomings (s) Number of freezings Time of freezings (s) Goal-directed behavior Number of head dippings Time of head dippings (s) | 16 ± 4 19 ± 5 9 ± 4 8 ± 3 48 ± 7 139 ± 11 169 ± 12 Figure 3A 12 ± 2 12 ± 3 10 ± 2 12 ± 2 13 ± 3 39 ± 5 47 ± 5 0 0 0 0 Figure 3B 53 ± 9 | 10 ± 3 * 11 ± 4 * 2 ± 2 ** 4 ± 3 * 17 ± 12 *** 53 ± 24 *** 128 ± 28 * 4 ± 2 *** 4 ± 1 *** 7 ± 1 * 5 ± 1 *** 7 ± 6 22 ± 13 * 71 ± 18 *** 2 ± 1 ** 5 ± 2 ** 2 ± 1 ** 7 ± 3 ** 32 ± 6 ** | 38 ± 5 aaa 41 ± 5 aaa 2 ± 1 aa 4 ± 1 a 17 ± 4 aaa 63 ± 5 aaa 174 ± 18 4 ± 1 aaa 5 ± 1 aa 9 ± 1 7 ± 1 aaa 6 ± 2 aa 25 ± 3 aaa 69 ± 5 aaa 12 ± 3 aaa 17 ± 9 aa 4 ± 3 a 5 ± 2 aa 11 ± 3 aaa | 0 *** aaa 0 *** aa 0 ** 0 *** a 7 ± 6 ** 54 ± 23 144 ± 14 ** 1 ± 1 *** a 4 ± 2 7 ± 1 ** 6 ± 1 5 ± 4 21 ± 11 73 ± 15 16 ± 3 * aaa 24 ± 3 aaa 5 ± 1 aaa 6 ± 2 3 ± 1 *** aaa | 20 ± 4 22 ± 3 5 ± 2 7 ± 2 38 ± 6 # 118 ± 12 # 196 ± 22 # 10 ± 1 10 ± 1 10 ± 2 10 ± 1 11 ± 1 34 ± 3 56 ± 3 ## 0 0 0 0 49 ± 15 | 8 ± 2 *** 10 ± 5 *** 0 ** 0 *** # 13 ± 10 *** 79 ± 12 *** # 212 ± 32 ### 3 ± 1 *** 7 ± 1 *** ### 11 ± 1 ### 8 ± 1 ** ### 4 ± 2 *** 26 ± 3 *** 70 ± 3 *** 5 ± 2 ** # 9 ± 2 *** ## 4 ± 2 ** 9 ± 1 *** 16 ± 3 ** ### | 32 ± 5 aa 32 ± 5 aa # 1 ± 1 aa 1 ± 1 aaa ### 21 ± 7 aa 73 ± 11 aaa 176 ± 19 5 ± 2 aaa 6 ± 1 aaa 9 ± 1 7 ± 1 aaa 8 ± 2 a 27 ± 1 aa 65 ± 3 aaa 8 ± 5 a 8 ± 5 a 3 ± 2 a 3 ± 2 a 11 ± 3 aa | 0 *** aaa 0 *** aa 0 0 6 ± 4 ** 31 ± 8 *** aaa 133 ± 20 ** aaa 2 ± 1 * 3 ± 2 * aa 7 ± 1 ** aaa 5 ± 1 ** aaa 4 ± 2 ** 18 ± 3 *** aaa 78 ± 5 *** aa 10 ± 2 aa ## 16 ± 1 * aaa ### 8 ± 4 * 12 ± 3 *** ## 5 ± 1 ** aaa ## |
| T-Maze test Horizontal exploration Intersection time (s) Exploratory efficacy (s) Vertical exploration Number of rearings Time of rearings (s) Other behaviors Number of groomings Time of groomings (s) Number of freezings Time of freezings (s) | 7 ± 1 Figure 3C 2 ± 1 3 ± 2 0 0 0 0 | 5 ± 2 1 ± 1 3 ± 1 1 ± 1 2 ± 1 ** 0 0 | 13 ± 1 aaa 2 ± 1 2 ± 1 0 0 0 0 | 10 ± 3 aa 0 ** 0 ** aaa 3 ± 1 *** aa 8 ± 2 *** aaa 2 ± 1 ** aa 5 ± 2 ** aa | 4 ± 2 # 9 ± 2 ### 10 ± 2 ### 0 0 0 0 | 6 ± 1 1 ± 1 *** 3 ± 1 *** 1 ± 1 2 ± 1 ** 0 0 | 21 ± 3 aaa ### 2 ± 1 aaa 3 ± 1 aaa 3 ± 5 8 ± 2 aaa ### 2 ± 1 aa ## 6 ± 1 aaa ### | 8 ± 2 *** 0 ** 0 *** aaa 1 ± 1 ## 10 ± 2 aaa 5 ± 1 *** aaa ### 7 ± 2 aaa |
| Corner test Number of corners Number of wall rearings Number of groomings Number of scratches | Figure 3D 2 ± 1 1 ± 1 3 ± 2 | 5 ± 1 *** 1 ± 1 2 ± 1 | 7 ± 3 aa 1 ± 1 a 0 | 4 ± 2 1 ± 1 1 ± 1 | 5 ± 2 # 1 ± 1 2 ± 1 | 4 ± 1 3 ± 2 1 ± 1 | 6 ± 1 1 ± 1 2 ± 1 ## | 3 ± 2 * 10 ± 2 *** aaa ### 1 ± 1 |
| Females | Males | |||||||
|---|---|---|---|---|---|---|---|---|
| WT Basal | WT Post-Stress | TH-HZ Basal | TH-HZ Post-Stress | WT Basal | WT Post-Stress | TH-HZ Basal | TH-HZ Post-Stress | |
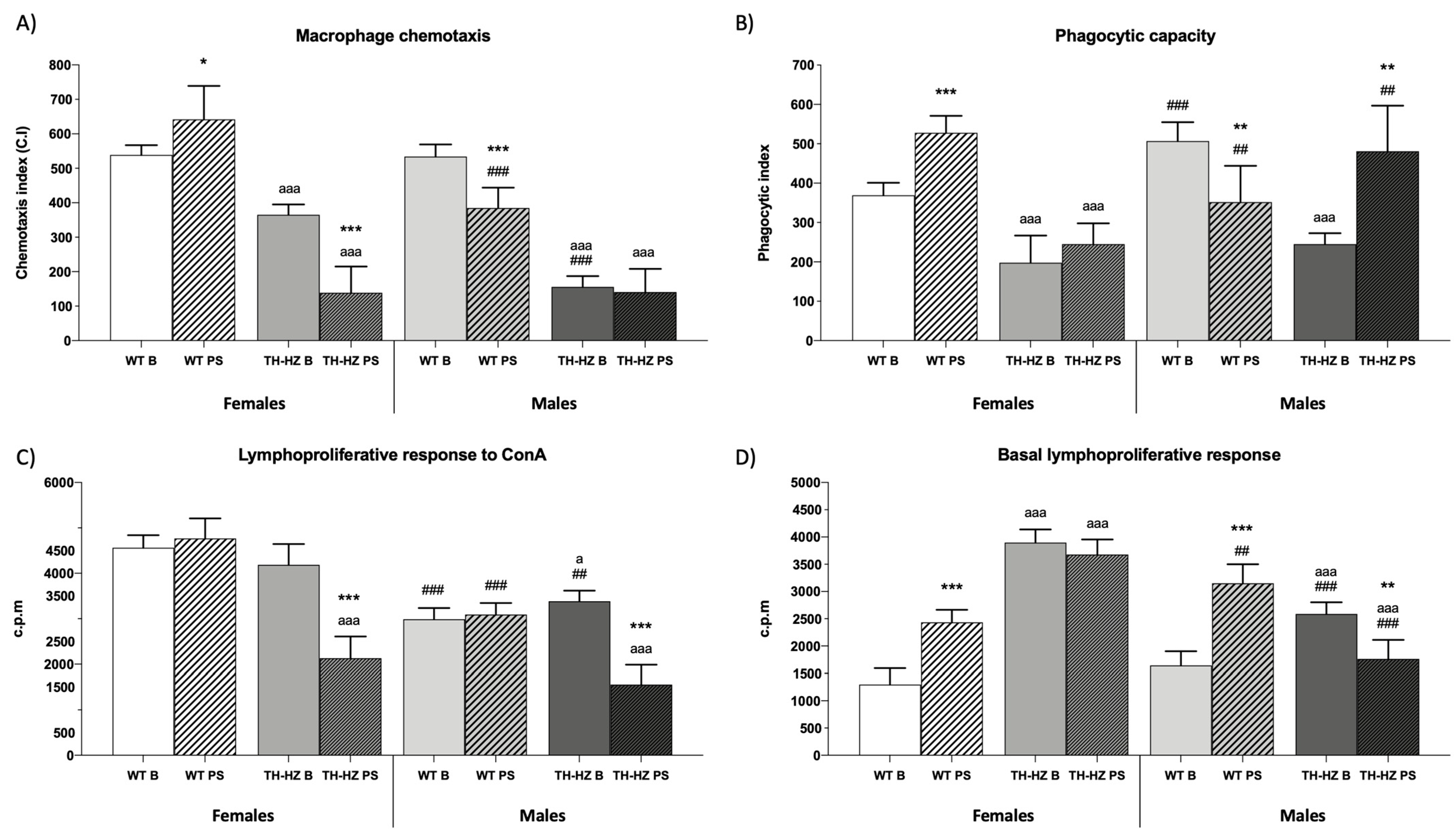
| Macrophage functions Chemotaxis index (C.I) Phagocytic efficacy (%) Phagocytic index | Figure 4A 74 ± 1 Figure 4B | 85 ± 6 ** | 49 ± 4 aaa | 54 ± 11 aaa | 75 ± 3 | 68 ± 6 * ### | 57 ± 3 aaa ## | 48 ± 4 ** aaa |
| Lymphocyte functions Chemotaxis index (C.I) Lymphoproliferation Basal response (c.p.m) LPS response (c.p.m) ConA response (c.p.m) | 1119 ± 124 Figure 4D 4336 ± 135 Figure 4C | 739 ± 128 *** 4235 ± 284 | 758 ± 67 aaa 3249 ± 294 aaa | 197 ± 54 *** aaa 1254 ± 289 *** aaa | 654 ± 76 ### 3897 ± 281 # | 586 ± 17 # 3546 ± 322 ## | 299 ± 36 aaa ### 2199 ± 255 aaa ### | 256 ± 22 * aaa # 1239 ± 233 *** aaa |
| Natural Killer activity (%) | 42 ± 2 | 67 ± 3 *** | 28 ± 3 aaa | 31 ± 4 aaa | 35 ± 3 ## | 37 ± 6 ### | 26 ± 2 aaa | 17 ± 4 ** aaa ### |
| Antioxidant compounds Catalase activity (UI CAT/106 cells) Glutathione reductase activity (mU GR/106 cells) Glutathione peroxidase activity (mU GPx/106 cells) Reduced glutathione levels (nmol GSH/106 cells) | 19 ± 3 63 ± 12 Figure 5A Figure 5B | 16 ± 3 367 ± 28 *** | 10 ± 3 aaa 39 ± 15 a | 7 ± 3 aaa 57 ± 12 * aaa | 14 ± 3 # 47 ± 10 # | 21 ± 2 ** ## 59 ± 5 * ### | 9 ± 6 27 ± 11 aa | 9 ± 4 aaa 74 ± 17 *** |
| Oxidant compounds Xanthine oxidase activity (U XAO/106 cells) Oxidized glutathione levels (nmol GSSG/106 cells) | Figure 5C 1.25 ± 0.39 | 2.47 ± 0.12 *** | 2.55 ± 0.98 a | 4.46 ± 0.23 ** aaa | 1.88 ± 0.23 ## | 3.45 ± 0.23 *** ### | 2.82 ± 0.13 aaa | 4.54 ± 0.13 *** aaa |
| Redox state indicator GSSG/GSH Ratio | Figure 5D | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Félix, J.; Garrido, A.; De la Fuente, M. In Response to a Punctual Stress Male and Female Tyrosine Hydroxylase Haploinsufficient Mice Show a Deteriorated Behavior, Immunity, and Redox State. Int. J. Mol. Sci. 2023, 24, 7335. https://doi.org/10.3390/ijms24087335
Félix J, Garrido A, De la Fuente M. In Response to a Punctual Stress Male and Female Tyrosine Hydroxylase Haploinsufficient Mice Show a Deteriorated Behavior, Immunity, and Redox State. International Journal of Molecular Sciences. 2023; 24(8):7335. https://doi.org/10.3390/ijms24087335
Chicago/Turabian StyleFélix, Judith, Antonio Garrido, and Mónica De la Fuente. 2023. "In Response to a Punctual Stress Male and Female Tyrosine Hydroxylase Haploinsufficient Mice Show a Deteriorated Behavior, Immunity, and Redox State" International Journal of Molecular Sciences 24, no. 8: 7335. https://doi.org/10.3390/ijms24087335
APA StyleFélix, J., Garrido, A., & De la Fuente, M. (2023). In Response to a Punctual Stress Male and Female Tyrosine Hydroxylase Haploinsufficient Mice Show a Deteriorated Behavior, Immunity, and Redox State. International Journal of Molecular Sciences, 24(8), 7335. https://doi.org/10.3390/ijms24087335







