Chronic N-Acetyl-Cysteine Treatment Enhances the Expression of the Immediate Early Gene Nr4a1 in Response to an Acute Challenge in Male Rats: Comparison with the Antidepressant Venlafaxine
Abstract
1. Introduction
2. Results
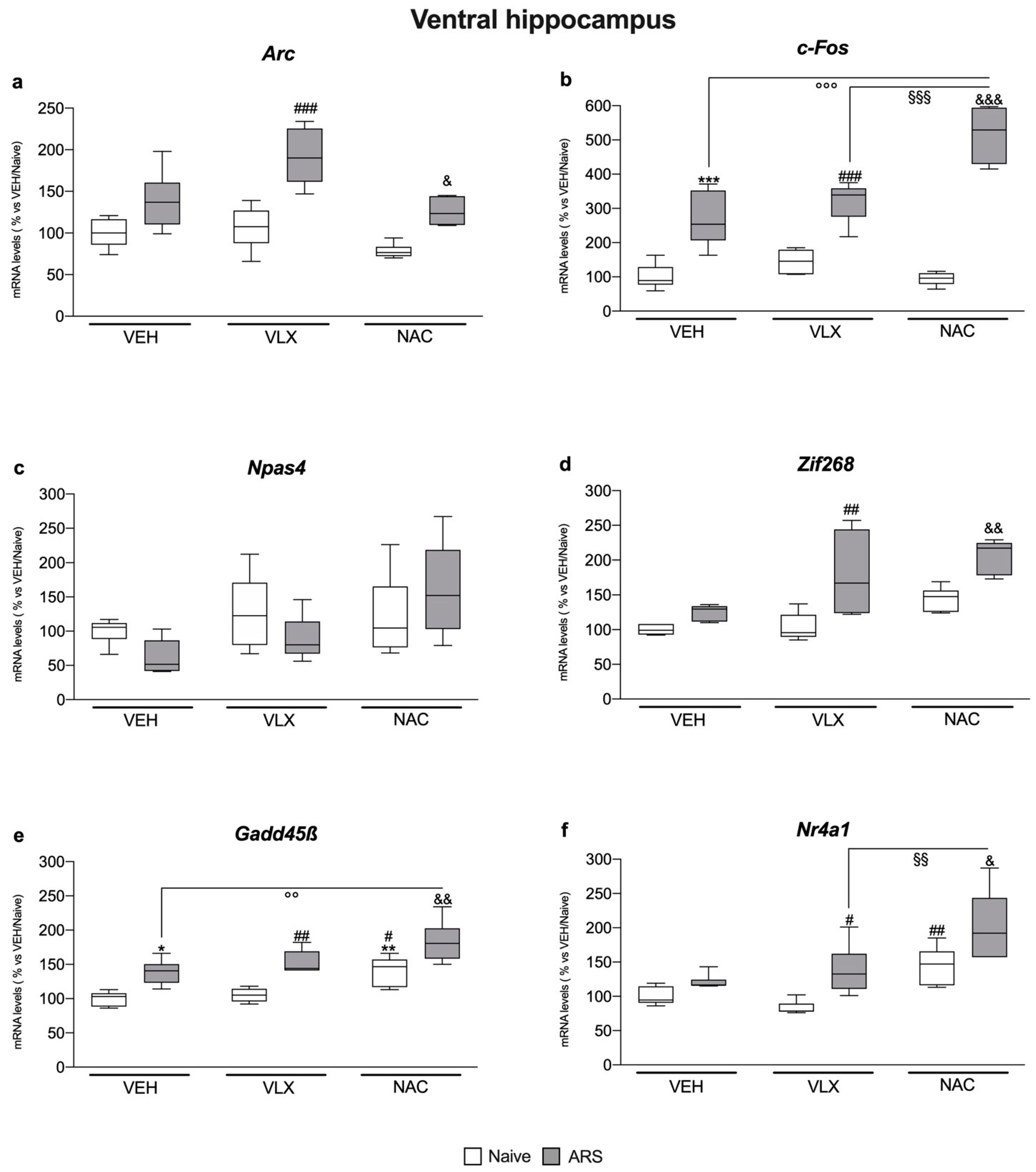
2.1. Chronic NAC Treatment Enhanced c-Fos and Nr4a1 Gene Expression More Than VLX in the Ventral Hippocampus of Acutely Stressed Rats
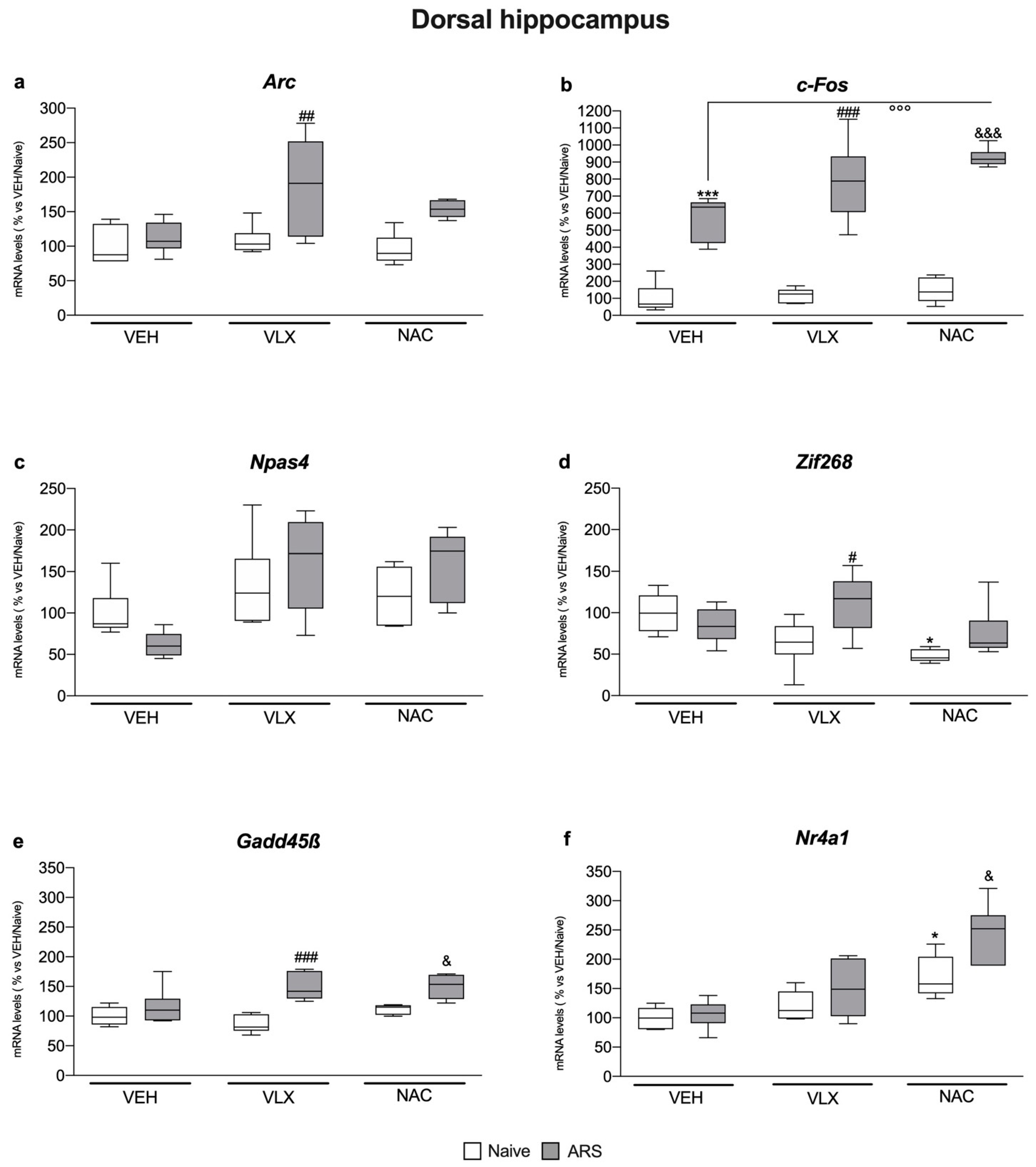
2.2. IEG Expression in Dorsal Hippocampus Was Differently Modulated by ARS and the Treatments: Comparison with Ventral Hippocampus
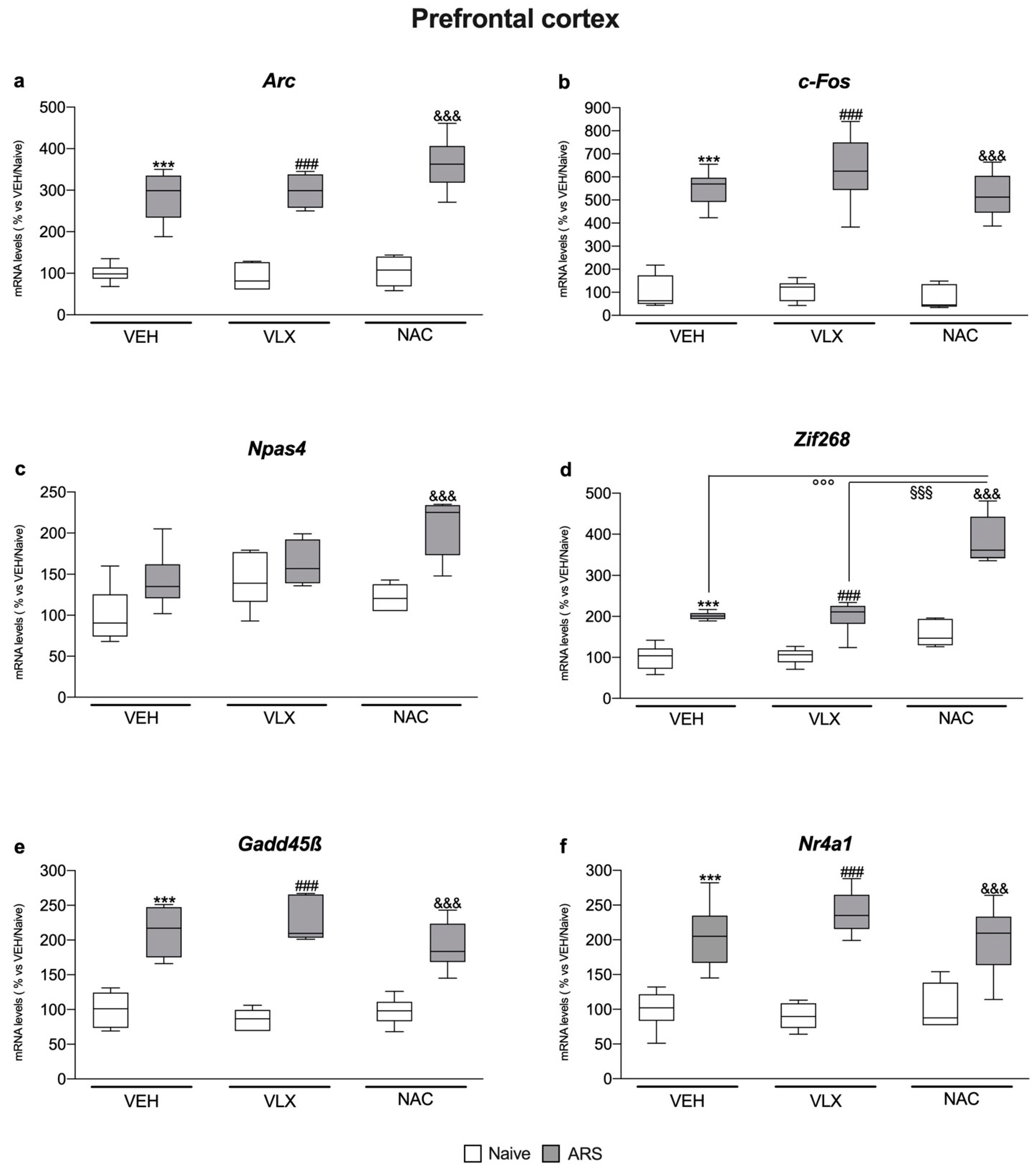
2.3. In the Prefrontal Cortex, Acute Stress Modulated IEG Expression Independently from Pretreatments
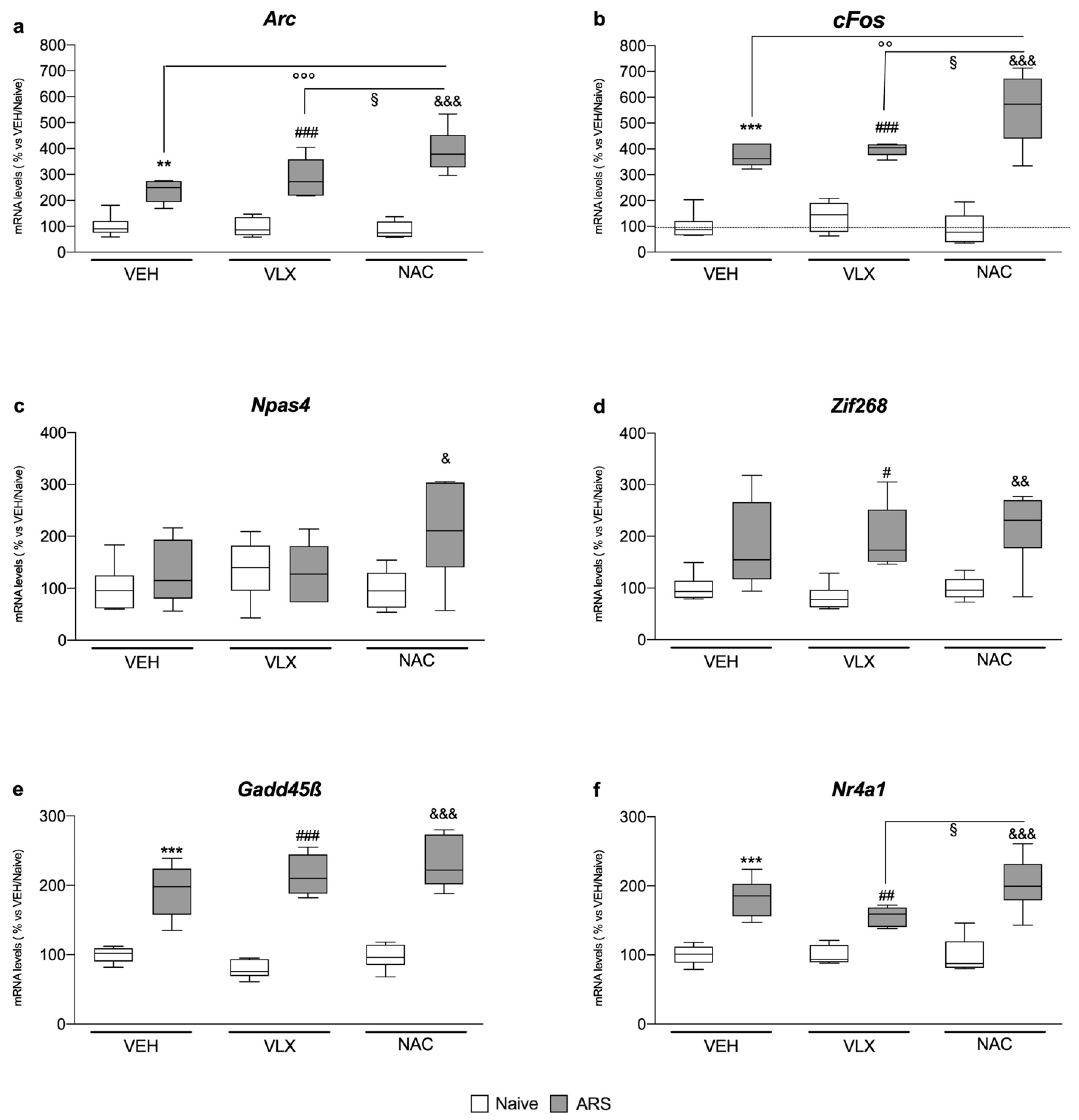
2.4. Chronic NAC Treatment Induced Higher IEG Expression in Comparison to VLX in the Amygdala of Acutely Stressed Rats
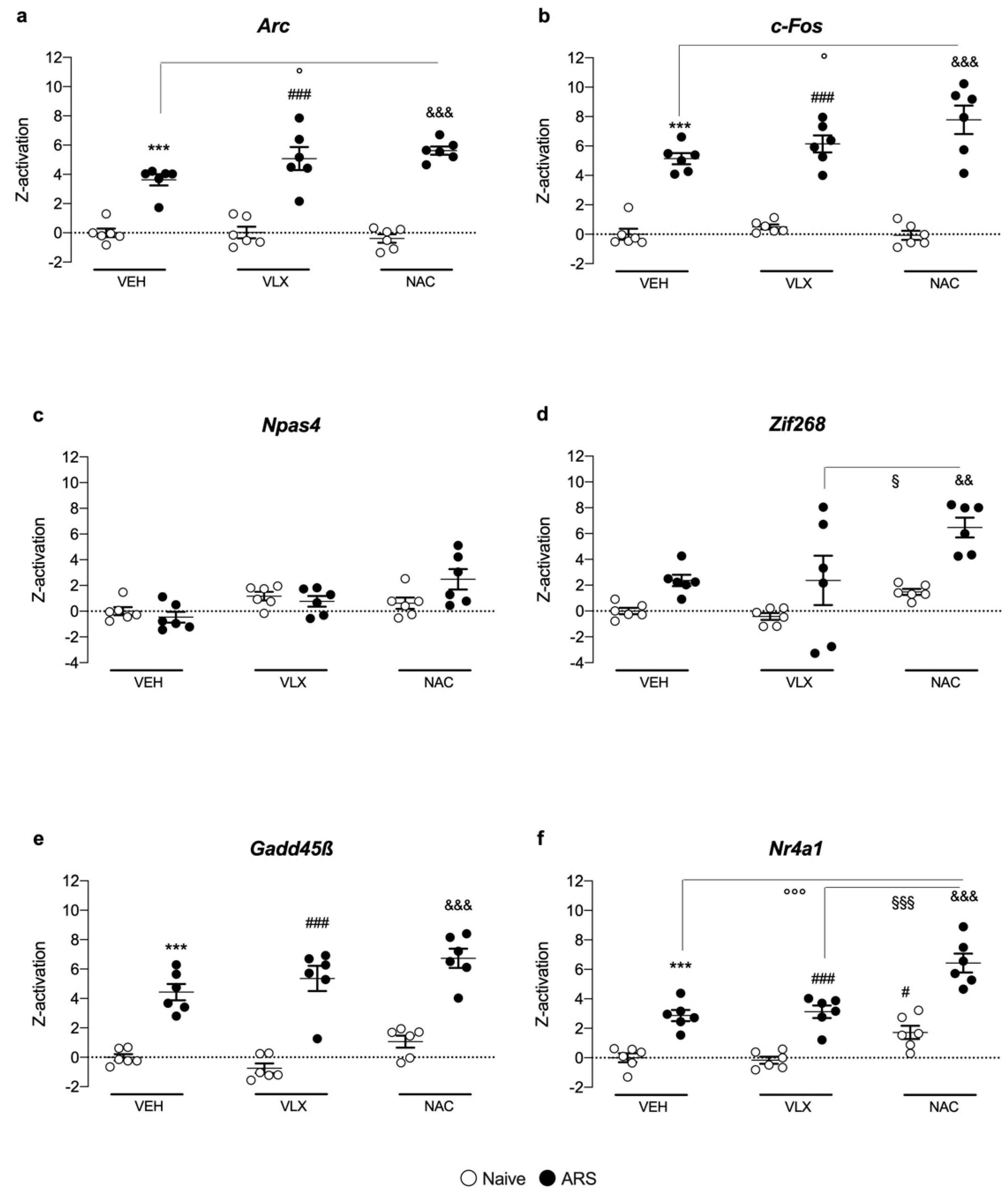
2.5. Z-Score Activation Underlines the Role of NAC in Enhancing Nr4a1 Gene Expression Specifically
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Pharmacological Treatment and Stress Procedure
- (1)
- VEH/Naïve: rats treated with VEH and not exposed to the ARS (n = 6).
- (2)
- VEH/ARS: rats treated with VEH and exposed to the ARS (n = 6).
- (3)
- VLX/Naïve: rats treated with VLX (10 mg/kg) and not exposed to the ARS (n = 6).
- (4)
- VLX/ARS: rats treated with VLX (10 mg/kg) and exposed to the ARS (n = 6).
- (5)
- NAC/Naïve: rats treated with NAC (300 mg/kg) and not exposed to the ARS (n = 6).
- (6)
- NAC/ARS: rats treated with NAC (300 mg/kg) and exposed to the ARS (n = 6).
4.3. RNA Preparation and Gene Expression by Quantitative Real-Time PCR
4.4. Z-Score
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hasin, D.S.; Sarvet, A.L.; Meyers, J.L.; Saha, T.D.; Ruan, W.J.; Stohl, M.; Grant, B.F. Epidemiology of Adult DSM-5 Major Depressive Disorder and Its Specifiers in the United States. JAMA Psychiatry 2018, 75, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Fava, M. Diagnosis and definition of treatment-resistant depression. Biol. Psychiatry 2003, 53, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.B. Past, Present, and Future Directions for Defining Optimal Treatment Outcome in Depression. JAMA 2003, 289, 3152. [Google Scholar] [CrossRef]
- Dodd, S.; Dean, O.; Copolov, D.L.; Malhi, G.S.; Berk, M. N -acetylcysteine for antioxidant therapy: Pharmacology and clinical utility. Expert Opin. Biol. Ther. 2008, 8, 1955–1962. [Google Scholar] [CrossRef]
- Berk, M.; Dean, O.M.; Cotton, S.M.; Jeavons, S.; Tanious, M.; Kohlmann, K.; Hewitt, K.; Moss, K.; Allwang, C.; Schapkaitz, I.; et al. The Efficacy of Adjunctive N -Acetylcysteine in Major Depressive Disorder. J. Clin. Psychiatry 2014, 75, 628–636. [Google Scholar] [CrossRef]
- Dean, O.; Giorlando, F.; Berk, M. N-acetylcysteine in psychiatry: Current therapeutic evidence and potential mechanisms of action. J. Psychiatry Neurosci. 2011, 36, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Ng, F.; Berk, M.; Dean, O.; Bush, A.I. Oxidative stress in psychiatric disorders: Evidence base and therapeutic implications. Int. J. Neuropsychopharmacol. 2008, 11, 851–876. [Google Scholar] [CrossRef]
- Coutens, B.; Yrondi, A.; Rampon, C.; Guiard, B.P. Psychopharmacological properties and therapeutic profile of the antidepressant venlafaxine. Psychopharmacology 2022, 239, 2735–2752. [Google Scholar] [CrossRef] [PubMed]
- Flati, T.; Gioiosa, S.; Chillemi, G.; Mele, A.; Oliverio, A.; Mannironi, C.; Rinaldi, A.; Castrignanò, T. A gene expression atlas for different kinds of stress in the mouse brain. Sci. Data 2020, 7, 437. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H.; Um, J.W. Synapse development organized by neuronal activity-regulated immediate-early genes. Exp. Mol. Med. 2018, 50, 1–7. [Google Scholar] [CrossRef]
- Brivio, P.; Sbrini, G.; Riva, M.A.; Calabrese, F. Acute Stress Induces Cognitive Improvement in the Novel Object Recognition Task by Transiently Modulating Bdnf in the Prefrontal Cortex of Male Rats. Cell. Mol. Neurobiol. 2020, 40, 1037–1047. [Google Scholar] [CrossRef]
- Brivio, P.; Sbrini, G.; Corsini, G.; Paladini, M.S.; Racagni, G.; Molteni, R.; Calabrese, F. Chronic Restraint Stress Inhibits the Response to a Second Hit in Adult Male Rats: A Role for BDNF Signaling. Int. J. Mol. Sci. 2020, 21, 6261. [Google Scholar] [CrossRef]
- Brivio, P.; Gallo, M.T.; Gruca, P.; Lason, M.; Litwa, E.; Fumagalli, F.; Papp, M.; Calabrese, F. Resilience to chronic mild stress-induced anhedonia preserves the ability of the ventral hippocampus to respond to an acute challenge. Eur. Arch. Psychiatry Clin. Neurosci. 2022, in press. [Google Scholar] [CrossRef]
- Brivio, P.; Corsini, G.; Riva, M.A.; Calabrese, F. Chronic vortioxetine treatment improves the responsiveness to an acute stress acting through the ventral hippocampus in a glucocorticoid-dependent way. Pharmacol. Res. 2019, 142, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Marchisella, F.; Paladini, M.S.; Guidi, A.; Begni, V.; Brivio, P.; Spero, V.; Calabrese, F.; Molteni, R.; Riva, M.A. Chronic treatment with the antipsychotic drug blonanserin modulates the responsiveness to acute stress with anatomical selectivity. Psychopharmacology 2020, 237, 1783–1793. [Google Scholar] [CrossRef]
- Fumagalli, F.; Calabrese, F.; Luoni, A.; Bolis, F.; Racagni, G.; Riva, M.A. Modulation of BDNF expression by repeated treatment with the novel antipsychotic lurasidone under basal condition and in response to acute stress. Int. J. Neuropsychopharmacol. 2012, 15, 235–246. [Google Scholar] [CrossRef]
- Molteni, R.; Calabrese, F.; Cattaneo, A.; Mancini, M.; Gennarelli, M.; Racagni, G.; Riva, M.A. Acute Stress Responsiveness of the Neurotrophin BDNF in the Rat Hippocampus is Modulated by Chronic Treatment with the Antidepressant Duloxetine. Neuropsychopharmacology 2009, 34, 1523–1532. [Google Scholar] [CrossRef]
- Guzowski, J.F.; Timlin, J.A.; Roysam, B.; McNaughton, B.L.; Worley, P.F.; Barnes, C.A. Mapping behaviorally relevant neural circuits with immediate-early gene expression. Curr. Opin. Neurobiol. 2005, 15, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Janak, P.H.; Tye, K.M. From circuits to behaviour in the amygdala. Nature 2015, 517, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Fanselow, M.S.; Dong, H.W. Are the Dorsal and Ventral Hippocampus Functionally Distinct Structures? Neuron 2010, 65, 7–19. [Google Scholar] [CrossRef]
- Bridi, M.S.; Abel, T. The NR4A orphan nuclear receptors mediate transcription-dependent hippocampal synaptic plasticity. Neurobiol. Learn. Mem. 2013, 105, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Y.; Ertürk, A.; Kallop, D.; Jiang, Z.; Weimer, R.M.; Kaminker, J.; Sheng, M. Activity-induced Nr4a1 regulates spine density and distribution pattern of excitatory synapses in pyramidal neurons. Neuron 2014, 83, 431–443. [Google Scholar] [CrossRef]
- Close, A.F.; Rouillard, C.; Buteau, J. NR4A orphan nuclear receptors in glucose homeostasis: A minireview. Diabetes Metab. 2013, 39, 478–484. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, B.; Zhang, X.; Sun, G.; Sun, X. Targeting Orphan Nuclear Receptors NR4As for Energy Homeostasis and Diabetes. Front. Pharmacol. 2020, 11, 587457. [Google Scholar] [CrossRef]
- Jeanneteau, F.; Barrère, C.; Vos, M.; De Vries, C.J.M.; Rouillard, C.; Levesque, D.; Dromard, Y.; Moisan, M.-P.; Duric, V.; Franklin, T.C.; et al. The Stress-Induced Transcription Factor NR4A1 Adjusts Mitochondrial Function and Synapse Number in Prefrontal Cortex. J. Neurosci. 2018, 38, 1335–1350. [Google Scholar] [CrossRef] [PubMed]
- Hawk, J.D.; Abel, T. The role of NR4A transcription factors in memory formation. Brain Res. Bull. 2011, 85, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Hawk, J.D.; Bookout, A.L.; Poplawski, S.G.; Bridi, M.; Rao, A.J.; Sulewski, M.E.; Kroener, B.T.; Manglesdorf, D.J.; Abel, T. NR4A nuclear receptors support memory enhancement by histone deacetylase inhibitors. J. Clin. Investig. 2012, 122, 3593–3602. [Google Scholar] [CrossRef] [PubMed]
- Bridi, M.S.; Hawk, J.D.; Chatterjee, S.; Safe, S.; Abel, T. Pharmacological Activators of the NR4A Nuclear Receptors Enhance LTP in a CREB/CBP-Dependent Manner. Neuropsychopharmacology 2017, 42, 1243–1253. [Google Scholar] [CrossRef]
- Abraham, W.C.; Dragunow, M.; Tate, W.P. The role of immediate early genes in the stabilization of long-term potentiation. Mol. Neurobiol. 1991, 5, 297–314. [Google Scholar] [CrossRef]
- Tyssowski, K.M.; DeStefino, N.R.; Cho, J.-H.; Dunn, C.J.; Poston, R.G.; Carty, C.E.; Jones, R.D.; Chang, S.M.; Romeo, P.; Wurzelmann, M.K.; et al. Different Neuronal Activity Patterns Induce Different Gene Expression Programs. Neuron 2018, 98, 530–546.e11. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, F.; Qin, D.; Zong, C.; Han, F.; Pu, Z.; Liu, D.; Li, X.; Zhang, Y.; Liu, Y.; et al. The orphan nuclear receptor NR4A1 attenuates oxidative stress-induced β cells apoptosis via up-regulation of glutathione peroxidase 1. Life Sci. 2018, 203, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Moll, U.M.; Marchenko, N.; Zhang, X.-K. p53 and Nur77/TR3-transcription factors that directly target mitochondria for cell death induction. Oncogene 2006, 25, 4725–4743. [Google Scholar] [CrossRef]
- Rogóż, Z.; Kamińska, K.; Lech, M.A.; Lorenc-Koci, E. N-Acetylcysteine and Aripiprazole Improve Social Behavior and Cognition and Modulate Brain BDNF Levels in a Rat Model of Schizophrenia. Int. J. Mol. Sci. 2022, 23, 2125. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, E.; Nikbakht, F.; Vazifekhah, S.; Babae, J.F.; Jogataei, M.T. Evaluation the cognition-improvement effects of N-acetyl cysteine in experimental temporal lobe epilepsy in rat. Behav. Brain Res. 2023, 440, 114263. [Google Scholar] [CrossRef] [PubMed]
- Joëls, M.; Pu, Z.; Wiegert, O.; Oitzl, M.S.; Krugers, H.J. Learning under stress: How does it work? Trends Cogn. Sci. 2006, 10, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.C.; Gorgievski, V.; Ortiz-Teba, P.; Belzeaux, R.; Turecki, G.; Sibille, E.; Charbonnier, G.; Tzavara, E.T. Transcriptomic Studies of Antidepressant Action in Rodent Models of Depression: A First Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 13543. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Brivio, P.; Paladini, M.S.; Racagni, G.; Riva, M.A.; Calabrese, F.; Molteni, R. From Healthy Aging to Frailty: In Search of the Underlying Mechanisms. Curr. Med. Chem. 2019, 26, 3685–3701. [Google Scholar] [CrossRef]
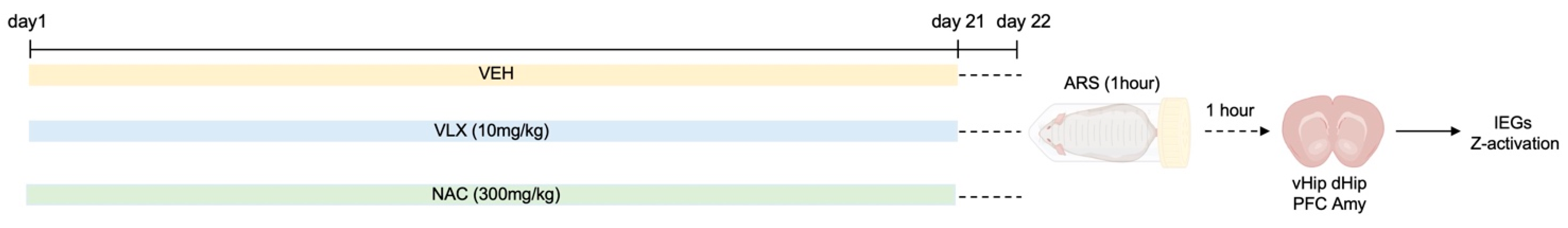
| Gene | Forward Primer | Reverse Primer | Probe |
|---|---|---|---|
| Arc | GGTGGGTGGCTCTGAAGAAT | ACTCCACCCAGTTCTTCACC | GATCCAGAACCACATGAATGGG |
| c-Fos | TCCTTACGGACTCCCCAC | CTCCGTTTCTCTTCCTCTTCAG | TGCTCTACTTTGCCCCTTCTGCC |
| Npas4 | TCATTGACCCTGCTGACCAT | AAGCACCAGTTTGTTGCCTG | TGATCGCCTTTTCCGTTGTC |
| Zif268 | GAGCGAACAACCCTACGAG | GTATAGGTGATGGGAGGCAAC | TCTGAATAACGAGAAGGCGCTGGTG |
| 36B4 | TCAGTGCCTCACTCCATCAT | AGGAAGGCCTTGACCTTTTC | TGGATACAAAAGGGTCCTGG |
| Gene | Accession Number | Assay ID |
|---|---|---|
| Gadd45β | BC085337.1 | Rn01452520_g1 |
| Nr4a1 | BC097313.1 | Rn01533237_m1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brivio, P.; Gallo, M.T.; Gruca, P.; Lason, M.; Litwa, E.; Fumagalli, F.; Papp, M.; Calabrese, F. Chronic N-Acetyl-Cysteine Treatment Enhances the Expression of the Immediate Early Gene Nr4a1 in Response to an Acute Challenge in Male Rats: Comparison with the Antidepressant Venlafaxine. Int. J. Mol. Sci. 2023, 24, 7321. https://doi.org/10.3390/ijms24087321
Brivio P, Gallo MT, Gruca P, Lason M, Litwa E, Fumagalli F, Papp M, Calabrese F. Chronic N-Acetyl-Cysteine Treatment Enhances the Expression of the Immediate Early Gene Nr4a1 in Response to an Acute Challenge in Male Rats: Comparison with the Antidepressant Venlafaxine. International Journal of Molecular Sciences. 2023; 24(8):7321. https://doi.org/10.3390/ijms24087321
Chicago/Turabian StyleBrivio, Paola, Maria Teresa Gallo, Piotr Gruca, Magdalena Lason, Ewa Litwa, Fabio Fumagalli, Mariusz Papp, and Francesca Calabrese. 2023. "Chronic N-Acetyl-Cysteine Treatment Enhances the Expression of the Immediate Early Gene Nr4a1 in Response to an Acute Challenge in Male Rats: Comparison with the Antidepressant Venlafaxine" International Journal of Molecular Sciences 24, no. 8: 7321. https://doi.org/10.3390/ijms24087321
APA StyleBrivio, P., Gallo, M. T., Gruca, P., Lason, M., Litwa, E., Fumagalli, F., Papp, M., & Calabrese, F. (2023). Chronic N-Acetyl-Cysteine Treatment Enhances the Expression of the Immediate Early Gene Nr4a1 in Response to an Acute Challenge in Male Rats: Comparison with the Antidepressant Venlafaxine. International Journal of Molecular Sciences, 24(8), 7321. https://doi.org/10.3390/ijms24087321







