Genetic Research Progress: Heat Tolerance in Rice
Abstract
1. Introduction
2. QTL Identification of Heat Tolerance in Rice
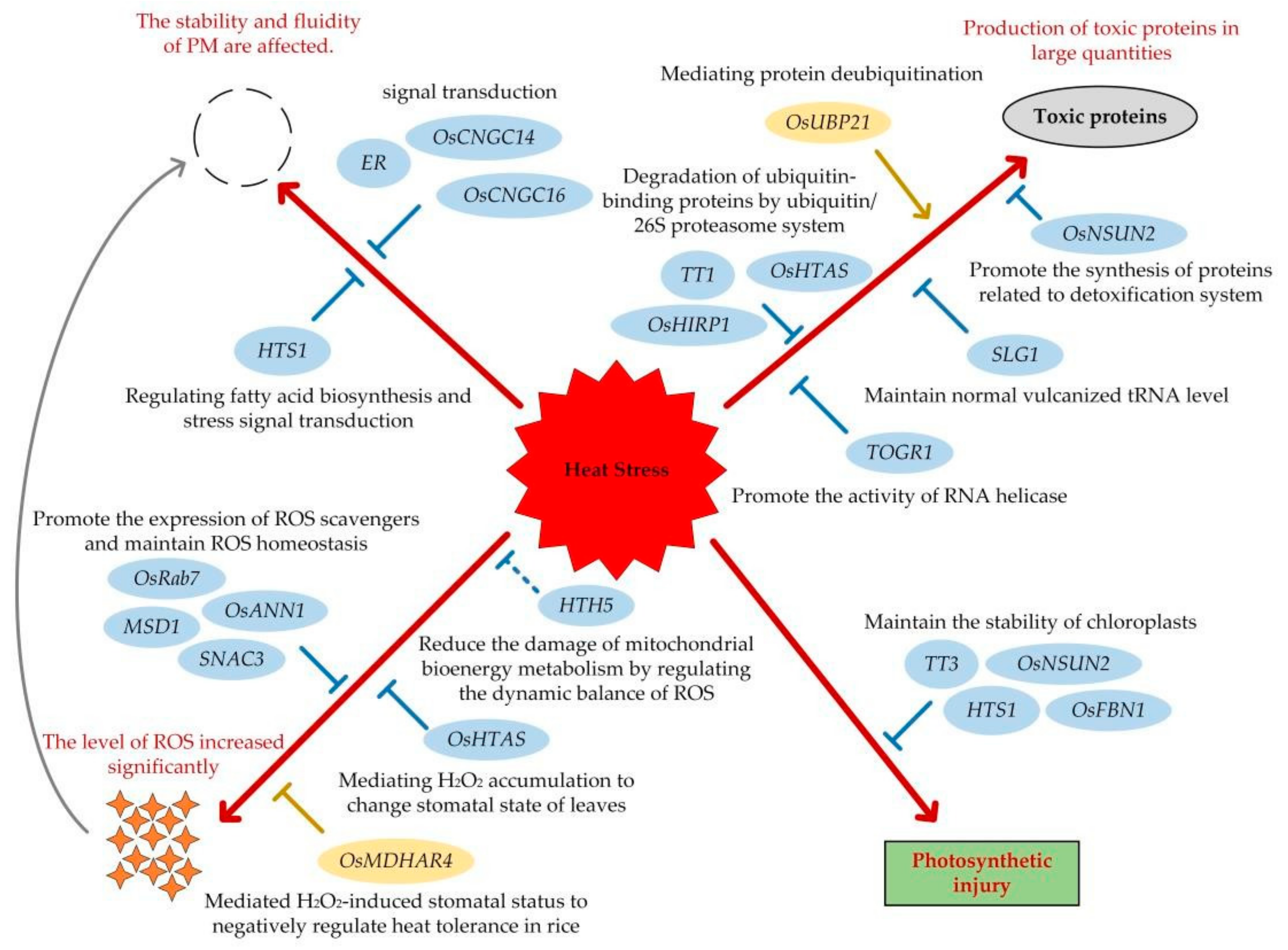
3. The Cloning of Functional Genes Related to Heat Tolerance in Rice
3.1. Functional Genes That Play a Positive Regulatory Role
3.2. Functional Genes That Play a Reverse Regulatory Role
| Gene | Gene Characteristics | Mechanism | Subcellular Localization | Expression Pattern | Function (Research) Period | Regulative Effect * | Reference |
|---|---|---|---|---|---|---|---|
| TT1 | α2 subunit of the 26S proteasome | The degradation of ubiquitinated proteins | Seedling stage, flowering stage, and filling stage | + | [34] | ||
| ER (ERECTA) | Receptor-like kinase | Confers thermotolerance independent of water loss | Seedling and flowering stage | + | [35] | ||
| OsHTAS | RING finger ubiquitin E3 ligase | Through modulating ROS homeostasis to regulate stomatal aperture status | Nucleus and cytoplasm | All tissues surveyed and peaked in leaf blade | Seedling stage | + | [37] |
| TOGR1 | DEAD box RNA helicase | Maintains pre-rRNA homeostasis under high temperatures by securing a proper pre-rRNA structure by elevating its helicase activity | Ducleolus | Regulated by both temperature and the circadian clock | Seedling stage | + | [38] |
| Sus3 | Sucrose synthase | The increase in Sus3 expression leads to tolerance of high temperatures | Ripening stage | + | [39] | ||
| SLG1 | Cytosolic tRNA 2-thiolation protein 2 (RCTU2) | Plays a key role in the response of rice plants to high temperature stress | Nucleus and cytoplasm | Universal expression | seedling and reproductive stages | + | [40] |
| HTS1 | β-ketoacyl carrider protein re-reductase | Via the regulation of fatty acid biosynthesis and stress signaling | Thylakoid membrane | Predominantly expressed in green tissues and strongly induced by HS | Seedling stage | + | [41] |
| TT3 | E3 ligase, chloroplast; and precursor protein | Protects chloroplasts to enhance thermotolerance | PM, endosomes, and chloroplast | Seedling stage, heading stage, and filling stage | + | [42] | |
| HTH5 | Pyridoxal phosphate homeostasis protein (PLPHP) | Reduces reactive oxygen species accumulation by increasing the heat-induced pyridoxal 5′-phosphate (PLP) content | Mitochondrion | Widely expressed | Heading stage | + | [43] |
| MSD1 | Golgi/plastid-type manganese superoxide dismutase | Induced the expression of ROS scavengers, molecular chaperones, and the quality control system in developing seeds | Golgi apparatus and plastids | Actively expressed throughout the rice plant | Heading stage and filling stage | + | [44] |
| OsANN1 | Rice annexin | By modulating the production of H2O2 | Cell periphery and cytosol | Highly expressed in seeds and panicles | Seedling stage | + | [45] |
| SNAC3 | NAC transcription factor | Through modulation of reactive oxygen species | Nucleus | Expressed ubiquitously | Seedling stage | + | [46] |
| OsNTL3 | Membrane-associated NAC transcription factor | Through relaying HS signals/effects from PM to nucleus | PM and nuclues | Seedling stage | + | [47] | |
| OsHIRP1 | Heat-induced RING finger protein | OsHIRP1 is an E3 ligase that acts as a positive regulator in the plant response to HS | Cytoplasm and nucleus | Highly expressed under HS conditions | Germination stage | + | [48] |
| OsRab7 | Small GTP-binding protein | By modulating osmolytes, antioxidants, and abiotic stress responsive genes expression | Seedling stage | + | [49] | ||
| OsRGB1 | Heterotrimeric G protein beta subunit | Overexpression of OsRGB1 confers HS tolerance in rice | Germination and seedling stage | + | [50] | ||
| Os-CNGC14, OsCNGC16 | Cyclic nucleotide-gated ion channel protein | The modulators of calcium signals in response to temperature stress | PM | Expressed in most organs | Seedling stage | + | [51] |
| OsNSUN2 | RNA 5-methylatesine (M5C) me-methyltransferase | Plays essential roles in the maintenance of chloroplast function during heat acclimation | Nucleus | The highest expression level at the shoot tip | Seedling stage | + | [52] |
| DPB3-1 | transcriptional regulator DNA polymerase II subunit B3-1 | Increase HS tolerance in crops without negative effects on vegetative and reproductive growth | + | [53] | |||
| TT2 | Gγ subunit | Through SCT1-dependent alteration of wax biosynthesis | Nucleus | Vegetative and reproductive growth period | − | [54] | |
| Os-MDHAR4 | Monodehydroascorbate reductase (MDHAR) | By mediating H2O2-induced stomatal closure | Chloroplasts | Expressed in all tissues surveyed and peaked in leaf blade | Seedling stage | − | [55] |
| OsFBN1 | Fibrillin | Plays essential roles in plastoglobule formation and lipid metabolism in chloroplasts | Chloroplasts | highly expressed in green tissues | Seedling stage and reproductive growth stage | − | [56] |
| OsUBP21 | Ubiquitin-specific protease | mediated protein de-ubiquitination plays a negative role in regulating basal thermotoleranance in rice | Intracellular | Mainly expressed in inflorescences, pistils, embryos, and shoots | Seedling stage | − | [57] |
| OsNRT2.3 | Nitrate transporter | Required to maintain high yield and high nitrogen use efficiency | − | [58] |
4. The Molecular Mechanism of The Rice Response to HS
4.1. Stability and Fluidity of PM
4.2. The Dynamic Balance of Protein
4.3. The Accumulation of ROS
4.4. Photosynthesis and Chloroplast Stability
5. Ways to Improve The Heat Tolerance of Rice
5.1. Agronomic Management
5.2. Conventional Breeding
5.3. Molecular Marker-Assisted Breeding
5.4. Transgenic Methods and Genome Editing Technology
6. Prospect
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P.; et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef] [PubMed]
- Pingali, P.L. Green revolution: Impacts, limits, and the path ahead. Proc. Natl. Acad. Sci. USA 2012, 109, 12302–12308. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Huang, J.; Sheehy, J.E.; Laza, R.C.; Visperas, R.M.; Zhong, X.; Centeno, G.S.; Khush, G.S.; Cassman, K.G. Rice yields decline with higher night temperature from global warming. Proc. Natl. Acad. Sci. USA 2004, 101, 9971–9975. [Google Scholar] [CrossRef] [PubMed]
- Lobell, D.B.; Schlenker, W.; Costa-Roberts, J. Climate trends and global crop production since 1980. Science 2011, 333, 616–620. [Google Scholar] [CrossRef]
- Challinor, A.J.; Watson, J.; Lobell, D.B.; Howden, S.M.; Smith, D.R.; Chhetri, N. A meta-analysis of crop yield under climate change and adaptation. Nat. Clim. Chang. 2014, 4, 287–291. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather. Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Mittler, R.; Finka, A.; Goloubinoff, P. How do plants feel the heat? Trends Biochem. Sci. 2012, 37, 118–125. [Google Scholar] [CrossRef]
- Pan, Y.; Liang, H.; Gao, L.; Dai, G.; Chen, W.; Yang, X.; Qing, D.; Gao, J.; Wu, H.; Huang, J.; et al. Transcriptomic profiling of germinating seeds under cold stress and characterization of the cold-tolerant gene LTG5 in rice. BMC Plant Biol. 2020, 20, 371. [Google Scholar] [CrossRef]
- Nubankoh, P.; Wanchana, S.; Saensuk, C.; Ruanjaichon, V.; Cheabu, S.; Vanavichit, A.; Toojinda, T.; Malumpong, C.; Arikit, S. QTL-seq reveals genomic regions associated with spikelet fertility in response to a high temperature in rice (Oryza sativa L.). Plant Cell Rep. 2020, 39, 149–162. [Google Scholar] [CrossRef]
- Jagadish, S.V.K.; Septiningsih, E.M.; Kohli, A.; Thomson, M.J.; Ye, C.; Redoña, E.; Kumar, A.; Gregorio, G.B.; Wassmann, R.; Ismail, A.M.; et al. Genetic Advances in Adapting Rice to a Rapidly Changing Climate. J. Agron. Crop Sci. 2012, 198, 360–373. [Google Scholar] [CrossRef]
- Zhu, S.; Huang, R.; Wai, H.P.; Xiong, H.; Shen, X.H.; He, H.H.; Yan, S. Mapping quantitative trait loci for heat tolerance at the booting stage using chromosomal segment substitution lines in rice. Physiol. Mol. Biol. Plants 2017, 23, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Satake, T.; Yoshida, S. High tenperature induced sterility in indica rices at flowering. Jpn. J. Crop Sci. 1978, 47, 6–17. [Google Scholar] [CrossRef]
- Zhu, C.L.; Xiao, Y.H.; Wang, C.M.; Jiang, L.; Zhai, H.Q.; Wan, J.M. Mapping QTL for heat-tolerance at grain filling stage in rice. Rice Sci. 2005, 12, 33. [Google Scholar]
- Welch, J.R.; Vincent, J.R.; Auffhammer, M.; Moya, P.F.; Dobermann, A.; Dawe, D. Rice yields in tropical/subtropical Asia exhibit large but opposing sensitivities to minimum and maximum temperatures. Proc. Natl. Acad. Sci. USA 2010, 107, 14562–14567. [Google Scholar] [CrossRef]
- Shi, W.; Li, X.; Schmidt, R.C.; Struik, P.C.; Yin, X.; Jagadish, S.V.K. Pollen germination and in vivo fertilization in response to high temperature during flowering in hybrid and inbred rice. Plant Cell Environ. 2018, 41, 1287–1297. [Google Scholar] [CrossRef]
- Driedonks, N.; Rieu, I.; Vriezen, W.H. Breeding for plant heat tolerance at vegetative and reproductive stages. Plant Reprod. 2016, 29, 67–79. [Google Scholar] [CrossRef]
- Ye, C.; Tenorio, F.A.; Argayoso, M.A.; Laza, M.A.; Koh, H.-J.; Redoña, E.D.; Jagadish, K.S.V.; Gregorio, G.B. Identifyingx and confirming quantitative trait loci associated with heat tolerance at flowering stage in different rice populations. BMC Genet. 2015, 16, 41. [Google Scholar] [CrossRef]
- Ye, C.; Tenorio, F.A.; Redoña, E.D.; Morales Cortezano, P.S.; Cabrega, G.A.; Jagadish, K.S.V.; Gregorio, G.B. Fine-mapping and validating qHTSF4.1 to increase spikelet fertility under heat stress at flowering in rice. Theor. Appl. Genet. 2015, 128, 1507–1517. [Google Scholar] [CrossRef]
- Kilasi, N.L.; Singh, J.; Vallejos, C.E.; Ye, C.; Jagadish, S.V.K.; Kusolwa, P.; Rathinasabapathi, B. Heat Stress Tolerance in Rice (Oryza sativa L.): Identification of Quantitative Trait Loci and Candidate Genes for Seedling Growth Under Heat Stress. Front. Plant Sci. 2018, 9, 1578. [Google Scholar] [CrossRef]
- Ishimaru, T.; Hirabayashi, H.; Ida, M.; Takai, T.; San-Oh, Y.A.; Yoshinaga, S.; Ando, I.; Ogawa, T.; Kondo, M. A genetic resource for early-morning flowering trait of wild rice Oryza officinalis to mitigate high temperature-induced spikelet sterility at anthesis. Ann. Bot. 2010, 106, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Grandillo, S.; Ahn, S.N.; McCouch, S.R.; Tanksley, S.D.; Li, J.; Yuan, L. Genes from wild rice improve yield. Nature 1996, 384, 223–224. [Google Scholar] [CrossRef]
- Cao, Z.; Tang, X.; Xiao, W.; Mao, L.; Nie, Y.; Li, Y.; Xie, H.; Cai, Y.; Yuan, L.; Xie, H.; et al. Mapping a QTL (qHTH5) for Heat Tolerance at the Heading Stage on Rice Chromosome 5 and Its Genetic Effect Analysis. Chin. J. Rice Sci. 2015, 29, 119–125. [Google Scholar] [CrossRef]
- Cao, Z.; Tang, X.; Xiao, W.; Mao, L.; Nie, Y.; Li, Y.; Xie, H.; Cai, Y.; Yuan, L. Identification and Genetic Effect Analysis of QTL (qHTH10) for Heat Tolerance at Heading and Flowering Stage of Rice. Mol. Plant Breed. 2019, 17, 2223–2230. [Google Scholar] [CrossRef]
- Cao, Z.; Li, Y.; Tang, H.; Zeng, B.; Tang, X.; Long, Q.; Wu, X.; Cai, Y.; Yuan, L.; Wan, J. Fine mapping of the qHTB1-1QTL, which confers heat tolerance at the booting stage, using an Oryza rufipogon Griff. introgression line. Theor. Appl. Genet. 2020, 133, 1161–1175. [Google Scholar] [CrossRef]
- Ye, C.; Argayoso, M.A.; Redoña, E.D.; Sierra, S.N.; Laza, M.A.; Dilla, C.J.; Mo, Y.; Thomson, M.J.; Chin, J.; Delaviña, C.B.; et al. Mapping QTL for heat tolerance at flowering stage in rice using SNP markers. Plant Breed. 2012, 131, 33–41. [Google Scholar] [CrossRef]
- Ps, S.; Sv, A.M.; Prakash, C.; Mk, R.; Tiwari, R.; Mohapatra, T.; Singh, N.K. High Resolution Mapping of QTLs for Heat Tolerance in Rice Using a 5K SNP Array. Rice 2017, 10, 28. [Google Scholar] [CrossRef]
- Zhao, L.; Lei, J.; Huang, Y.; Zhu, S.; Chen, H.; Huang, R.; Peng, Z.; Tu, Q.; Shen, X.; Yan, S. Mapping quantitative trait loci for heat tolerance at anthesis in rice using chromosomal segment substitution lines. Breed. Sci. 2016, 66, 358–366. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, T.; Yu, T.; Zhang, S.; Mao, X.; Zhao, J.; Wang, X.; Dong, J.; Liu, B. Integrating Small RNA Sequencing with QTL Mapping for Identification of miRNAs and Their Target Genes Associated with Heat Tolerance at the Flowering Stage in Rice. Front. Plant Sci. 2017, 8, 43. [Google Scholar] [CrossRef]
- Li, M.-M.; Li, X.; Yu, L.-Q.; Wu, J.-W.; Li, H.; Liu, J.; Ma, X.-D.; Jo, S.-M.; Park, D.-S.; Song, Y.; et al. Identification of QTLs associated with heat tolerance at the heading and flowering stage in rice (Oryza sativa L.). Euphytica 2018, 214, 70. [Google Scholar] [CrossRef]
- Park, J.-R.; Yang, W.-T.; Kim, D.-H.; Kim, K.-M. Identification of a Novel Gene, Osbht, in Response to High Temperature Tolerance at Booting Stage in Rice. Int. J. Mol. Sci. 2020, 21, 5862. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, Q.; Tang, M.; Zhang, X.; Pan, Y.; Yang, X.; Gao, G.; Lv, R.; Tao, W.; Jiang, L.; et al. QTL Mapping and Identification of Candidate Genes for Heat Tolerance at the Flowering Stage in Rice. Front. Genet. 2021, 11, 621871. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Jiang, J.; Li, Y.; Song, S.; Zou, Y.; Jing, C.; Zhang, Y.; Wang, D.; He, Q.; Dang, X. QTL mapping and identification of candidate genes using a genome-wide association study for heat tolerance at anthesis in rice (Oryza sativa L.). Front. Genet. 2022, 13, 983525. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-M.; Chao, D.-Y.; Wu, Y.; Huang, X.; Chen, K.; Cui, L.-G.; Su, L.; Ye, W.-W.; Chen, H.; Chen, H.-C.; et al. Natural alleles of a proteasome α2 subunit gene contribute to thermotolerance and adaptation of African rice. Nat. Genet. 2015, 47, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zhong, X.; Zhao, F.; Wang, Y.; Yan, B.; Li, Q.; Chen, G.; Mao, B.; Wang, J.; Li, Y.; et al. Overexpression of receptor-like kinase ERECTA improves thermotolerance in rice and tomato. Nat. Biotechnol. 2015, 33, 996–1003. [Google Scholar] [CrossRef]
- Wei, H.; Liu, J.; Wang, Y.; Huang, N.; Zhang, X.; Wang, L.; Zhang, J.; Tu, J.; Zhong, X. A Dominant Major Locus in Chromo-some 9 of Rice (Oryza sativa L.) Confers Tolerance to 48 ℃ High Temperature at Seedling Stage. J. Hered. 2013, 104, 287–294. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, C.; Wei, C.; Liu, X.; Wang, M.; Yu, F.; Xie, Q.; Tu, J. The RING Finger Ubiquitin E3 Ligase OsHTAS Enhances Heat Tolerance by Promoting H2O2-Induced Stomatal Closure in Rice. Plant Physiol. 2016, 170, 429–443. [Google Scholar] [CrossRef]
- Wang, D.; Qin, B.; Li, X.; Tang, D.; Zhang, Y.E.; Cheng, Z.; Xue, Y. Nucleolar DEAD-Box RNA Helicase TOGR1 Regulates Thermotolerant Growth as a Pre-rRNA Chaperone in Rice. PLoS Genet. 2016, 12, e1005844. [Google Scholar] [CrossRef]
- Takehara, K.; Murata, K.; Yamaguchi, T.; Yamaguchi, K.; Chaya, G.; Kido, S.; Iwasaki, Y.; Ogiwara, H.; Ebitani, T.; Miura, K. Thermo-responsive allele of sucrose synthase 3 (Sus3) provides high-temperature tolerance during the ripening stage in rice (Oryza sativa L.). Breed. Sci. 2018, 68, 336–342. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, L.; Ou, S.; Wang, R.; Wang, Y.; Chu, C.; Yao, S. Natural variations of SLG1 confer high-temperature tolerance in indica rice. Nat. Commun. 2020, 11, 5441. [Google Scholar] [CrossRef]
- Chen, F.; Dong, G.; Wang, F.; Shi, Y.; Zhu, J.; Zhang, Y.; Ruan, B.; Wu, Y.; Feng, X.; Zhao, C.; et al. A β-ketoacyl carrier protein reductase confers heat tolerance via the regulation of fatty acid biosynthesis and stress signaling in rice. New Phytol. 2021, 232, 655–672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, J.-F.; Kan, Y.; Shan, J.-X.; Ye, W.-W.; Dong, N.-Q.; Guo, T.; Xiang, Y.-H.; Yang, Y.-B.; Li, Y.-C.; et al. A genetic module at one locus in rice protects chloroplasts to enhance thermotolerance. Science 2022, 376, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Tang, H.; Cai, Y.; Zeng, B.; Zhao, J.; Tang, X.; Lu, M.; Wang, H.; Zhu, X.; Wu, X.; et al. Natural variation of HTH5 from wild rice, Oryza rufipogon Griff., is involved in conferring high-temperature tolerance at the heading stage. Plant Biotechnol. J. 2022, 20, 1591–1605. [Google Scholar] [CrossRef] [PubMed]
- Shiraya, T.; Mori, T.; Maruyama, T.; Sasaki, M.; Takamatsu, T.; Oikawa, K.; Itoh, K.; Kaneko, K.; Ichikawa, H.; Mitsui, T. Golgi/plastid-type manganese superoxide dismutase involved in heat-stress tolerance during grain filling of rice. Plant Biotechnol. J. 2015, 13, 1251–1263. [Google Scholar] [CrossRef]
- Qiao, B.; Zhang, Q.; Liu, D.; Wang, H.; Yin, J.; Wang, R.; He, M.; Cui, M.; Shang, Z.; Wang, D.; et al. A calcium-binding protein, rice annexin OsANN1, enhances heat stress tolerance by modulating the production of H2O2. J. Exp. Bot. 2015, 66, 5853–5866. [Google Scholar] [CrossRef]
- Fang, Y.; Liao, K.; Du, H.; Xu, Y.; Song, H.; Li, X.; Xiong, L. A stress-responsive NAC transcription factor SNAC3 confers heat and drought tolerance through modulation of reactive oxygen species in rice. J. Exp. Bot. 2015, 66, 6803–6817. [Google Scholar] [CrossRef]
- Liu, X.H.; Lyu, Y.S.; Yang, W.; Yang, Z.T.; Lu, S.J.; Liu, J.X. A membrane-associated NAC transcription factor OsNTL3 is involved in thermotolerance in rice. Plant Biotechnol. J. 2020, 18, 1317–1329. [Google Scholar] [CrossRef]
- Kim, J.H.; Lim, S.D.; Jang, C.S. Oryza sativa heat-induced RING finger protein 1 (OsHIRP1) positively regulates plant response to heat stress. Plant Mol. Biol. 2019, 99, 545–559. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Alayafi, A.A. Overexpression of Rice Rab7 Gene Improves Drought and Heat Tolerance and Increases Grain Yield in Rice (Oryza sativa L.). Genes 2019, 10, 56. [Google Scholar] [CrossRef]
- Biswas, S.; Islam, M.N.; Sarker, S.; Tuteja, N.; Seraj, Z.I. Overexpression of heterotrimeric G protein beta subunit gene (OsRGB1) confers both heat and salinity stress tolerance in rice. Plant Physiol. Biochem. 2019, 144, 334–344. [Google Scholar] [CrossRef]
- Cui, Y.; Lu, S.; Li, Z.; Cheng, J.; Hu, P.; Zhu, T.; Wang, X.; Jin, M.; Wang, X.; Li, L.; et al. Cyclic nucleotide-gated ion channels 14 and 16 Promote Tolerance to Heat and Chilling in Rice. Plant Physiol. 2020, 183, 1794–1808. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Gao, C.C.; Gao, Y.; Yang, Y.; Shi, B.; Yu, J.L.; Lyu, C.; Sun, B.F.; Wang, H.L.; Xu, Y.; et al. OsNSUN2-Mediated 5-Methylcytosine mRNA Modification Enhances Rice Adaptation to High Temperature. Dev. Cell 2020, 53, 272–286.e277. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Todaka, D.; Kudo, M.; Mizoi, J.; Kidokoro, S.; Zhao, Y.; Shinozaki, K.; Yamaguchi Shinozaki, K. The Arabidopsis transcriptional regulator DPB3-1 enhances heat stress tolerance without growth retardation in rice. Plant Biotechnol. J. 2016, 14, 1756–1767. [Google Scholar] [CrossRef] [PubMed]
- Kan, Y.; Mu, X.-R.; Zhang, H.; Gao, J.; Shan, J.-X.; Ye, W.-W.; Lin, H.-X. TT2 controls rice thermotolerance through SCT1-dependent alteration of wax biosynthesis. Nat. Plants 2022, 8, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, X.; Xu, F.; Zhang, Y.; Zhang, Q.; Miao, R.; Zhang, J.; Liang, J.; Xu, W. Suppression of OsMDHAR4 enhances heat tolerance by mediating H2O2-induced stomatal closure in rice plants. Rice 2018, 11, 38. [Google Scholar] [CrossRef]
- Li, J.; Yang, J.; Zhu, B.; Xie, G. Overexpressing OsFBN1 enhances plastoglobule formation, reduces grain-filling percent and jasmonate levels under heat stress in rice. Plant Sci. 2019, 285, 230–238. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, X.; Huo, C.; Wang, H.; An, Z.; Sun, D.; Liu, J.; Tang, W.; Zhang, B. A Quantitative Proteomics Study of Early Heat-Regulated Proteins by Two-Dimensional Difference Gel Electrophoresis Identified OsUBP21 as a Negative Regulator of Heat Stress Responses in Rice. Proteomics 2019, 19, 1900153. [Google Scholar] [CrossRef]
- Zhang, Y.; Tateishi-Karimata, H.; Endoh, T.; Jin, Q.; Li, K.; Fan, X.; Ma, Y.; Gao, L.; Lu, H.; Wang, Z.; et al. High-temperature adaptation of an OsNRT2.3 allele is thermoregulated by small RNAs. Sci. Adv. 2022, 8, eadc9785. [Google Scholar] [CrossRef]
- Barkla, B.J.; Pantoja, O. Plasma Membrane and Abiotic Stress; Springer: Berlin/Heidelberg, Germany, 2011; pp. 457–470. [Google Scholar]
- Murata, N.; Los, D.A. Membrane Fluidity and Temperature Perception. Plant Physiol. 1997, 115, 875–879. [Google Scholar] [CrossRef]
- Lamers, J.; Van Der Meer, T.; Testerink, C. How plants sense and respond to stressful environments. Plant Physiol. 2020, 182, 1624–1635. [Google Scholar] [CrossRef]
- Niu, Y.; Xiang, Y. An Overview of Biomembrane Functions in Plant Responses to High-Temperature Stress. Front. Plant Sci. 2018, 9, 915. [Google Scholar] [CrossRef]
- Finka, A.; Cuendet, A.F.; Maathuis, F.J.; Saidi, Y.; Goloubinoff, P. Plasma membrane cyclic nucleotide gated calcium chan-nels control land plant thermal sensing and acquired thermotolerance. Plant. Cell 2012, 24, 3333–3348. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Du, H.; Wang, Y.; Wang, H.; Yang, S.; Li, C.; Chen, N.; Yang, H.; Zhang, Y.; Zhu, Y. The calcium-dependent protein kinase ZmCDPK7 functions in heat-stress tolerance in maize. J. Integr. Plant Biol. 2021, 63, 510–527. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhou, L.; Liu, J.; Cao, Z.; Du, X.; Huang, F.; Pan, G.; Cheng, F. Involvement of CAT in the detoxification of HT-induced ROS burst in rice anther and its relation to pollen fertility. Plant Cell Rep. 2018, 37, 741–757. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhou, L.; Liu, J.; Du, X.; Asad, M.A.; Huang, F.; Pan, G.; Cheng, F. Relationship of ROS accumulation and superoxide dismutase isozymes in developing anther with floret fertility of rice under heat stress. Plant Physiol. Biochem. 2018, 122, 90–101. [Google Scholar] [CrossRef]
- Chakraborty, A.; Bhattacharjee, S. Differential competence of redox-regulatory mechanism under extremes of temperature determines growth performances and cross tolerance in two indica rice cultivars. J. Plant Physiol. 2015, 176, 65–77. [Google Scholar] [CrossRef]
- Bahuguna, R.N.; Jha, J.; Pal, M.; Shah, D.; Lawas, L.M.F.; Khetarpal, S.; Jagadish, K.S.V. Physiological and biochemical characterization of NERICA-L-44: A novel source of heat tolerance at the vegetative and reproductive stages in rice. Physiol. Plant. 2015, 154, 543–559. [Google Scholar] [CrossRef]
- Sailaja, B.; Subrahmanyam, D.; Neelamraju, S.; Vishnukiran, T.; Rao, Y.V.; Vijayalakshmi, P.; Voleti, S.R.; Bhadana, V.P.; Mangrauthia, S.K. Integrated Physiological, Biochemical, and Molecular Analysis Identifies Important Traits and Mechanisms Associated with Differential Response of Rice Genotypes to Elevated Temperature. Front. Plant Sci. 2015, 6, 1044. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive Oxygen Species: Metabolism, Oxidative Stress, and Signaling Transduction. Annu. Rev. Plant Biol. 2004, 55, 373. [Google Scholar] [CrossRef]
- Slimen, I.B.; Najar, T.; Ghram, A.; Dabbebi, H.; Ben Mrad, M.; Abdrabbah, M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int. J. Hyperth. 2014, 30, 513–523. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Lee, D.J.; Cheema, S.A.; Aziz, T. Comparative Time Course Action of the Foliar Applied Glycinebetaine, Salicylic Acid, Nitrous Oxide, Brassinosteroids and Spermine in Improving Drought Resistance of Rice. J. Agron. Crop Sci. 2010, 196, 336–345. [Google Scholar] [CrossRef]
- Wang, X.; Xu, C.; Cai, X.; Wang, Q.; Dai, S. Heat-Responsive Photosynthetic and Signaling Pathways in Plants: Insight from Proteomics. Int. J. Mol. Sci. 2017, 18, 2191. [Google Scholar] [CrossRef] [PubMed]
- Hueve, K.; Bichele, I.; Rasulov, B.; Niinemets, Ü. When it is too hot for photosynthesis: Heat-induced instability of photosynthesis in relation to respiratory burst, cell permeability changes and H2O2 formation. Plant Cell Environ. 2011, 34, 113–126. [Google Scholar] [CrossRef]
- Wang, Q.L.; Chen, J.H.; He, N.Y.; Guo, F.Q. Metabolic Reprogramming in Chloroplasts under Heat Stress in Plants. Int. J. Mol. Sci. 2018, 19, 849. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Shigeoka, S. Recent advances in ascorbate biosynthesis and the physiological significance of ascorbate peroxidase in photosynthesizing organisms. Biosci. Biotechnol. Biochem. 2008, 72, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Maruta, T.; Noshi, M.; Tanouchi, A.; Tamoi, M.; Yabuta, Y.; Yoshimura, K.; Ishikawa, T.; Shigeoka, S. H2O2-triggered retrograde signaling from chloroplasts to nucleus plays specific role in response to stress. J. Biol. Chem. 2012, 287, 11717–11729. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Li, T.; Yang, Y.; Qu, Z.; Ouyang, L.; Jiang, Z.; Lin, X.; Zhu, C.; Peng, L.; Fu, J. OsPLS4 is involved in cuticular wax biosynthesis and affects leaf senescence in rice. Front. Plant Sci. 2020, 11, 782. [Google Scholar] [CrossRef]
- Camejo, D.; Rodriguez, P.; Morales, M.A.; Dell’Amico, J.M.; Torrecillas, A.; Alarcon, J.J. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J. Plant Physiol. 2005, 162, 281–282. [Google Scholar] [CrossRef]
- Krishnan, P.; Ramakrishnan, B.; Reddy, K.R.; Reddy, V.R. High-temperature effects on rice growth, yield, and grain quality. Adv. Agron. 2011, 111, 87–206. [Google Scholar]
- Oh-e, I.; Saitoh, K.; Kuroda, T. Effects of high temperature on growth, yield and dry-matter production of rice grown in the paddy field. Plant Prod. Sci. 2007, 10, 412–422. [Google Scholar] [CrossRef]
- Liu, K.; Deng, J.; Lu, J.; Wang, X.; Lu, B.; Tian, X.; Zhang, Y. High Nitrogen Levels Alleviate Yield Loss of Super Hybrid Rice Caused by High Temperatures During the Flowering Stage. Front. Plant Sci. 2019, 10, 357. [Google Scholar] [CrossRef] [PubMed]
- Fahad, S.; Hussain, S.; Saud, S.; Hassan, S.; Tanveer, M.; Ihsan, M.Z.; Shah, A.N.; Ullah, A.; Nasrullah; Khan, F.; et al. A combined application of biochar and phosphorus alleviates heat-induced adversities on physiological, agronomical and quality attributes of rice. Plant Physiol. Biochem. 2016, 103, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Hua, M.; Yang, X.; Hu, N.; Qiu, R.; Yang, S. Impacts of Mist Spray on Rice Field Micrometeorology and Rice Yield under Heat Stress Condition. Sci. Rep. 2020, 10, 1579. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Cui, K.; Wang, W.; Li, Q.; Fahad, S.; Hu, Q.; Huang, J.; Nie, L.; Peng, S. Heat-induced phytohormone changes are associated with disrupted early reproductive development and reduced yield in rice. Sci. Rep. 2016, 6, 34978. [Google Scholar] [CrossRef]
- Feng, B.; Zhang, C.; Chen, T.; Zhang, X.; Tao, L.; Fu, G. Salicylic acid reverses pollen abortion of rice caused by heat stress. BMC Plant Biol. 2018, 18, 245. [Google Scholar] [CrossRef]
- Wu, C.; Cui, K.; Wang, W.; Li, Q.; Fahad, S.; Hu, Q.; Huang, J.; Nie, L.; Mohapatra, P.K.; Peng, S. Heat-Induced Cytokinin Transportation and Degradation Are Associated with Reduced Panicle Cytokinin Expression and Fewer Spikelets per Panicle in Rice. Front. Plant Sci. 2017, 8, 371. [Google Scholar] [CrossRef]
- Chandrakala, J.U.; Chaturvedi, A.K.; Ramesh, K.V.; Rai, P.; Khetarpal, S.; Pal, M. Acclimation response of signalling molecules for high temperature stress on photosynthetic characteristics in rice genotypes. Indian J. Plant Physiol. 2013, 18, 142–150. [Google Scholar] [CrossRef]
- Wu, Y.S.; Yang, C.Y. Ethylene-mediated signaling confers thermotolerance and regulates transcript levels of heat shock factors in rice seedlings under heat stress. Bot. Stud. 2019, 60, 23. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Wei, Y.X.; Peng, C.L. Effects of endogenous ascorbic acid on resistance to high-temperature stress in excised rice leaves. Photosynthetica 2018, 56, 1453–1458. [Google Scholar] [CrossRef]
- Xu, J.; Henry, A.; Sreenivasulu, N. Rice yield formation under high day and night temperatures—A prerequisite to ensure future food security. Plant Cell Environ. 2020, 43, 1595–1608. [Google Scholar] [CrossRef]
- Arshad, M.S.; Farooq, M.; Asch, F.; Krishna, J.S.V.; Prasad, P.V.V.; Siddique, K.H.M. Thermal stress impacts reproductive development and grain yield in rice. Plant Physiol. Biochem. 2017, 115, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Massel, K.; Godwin, I.D.; Gao, C. Applications and potential of genome editing in crop improvement. Genome Biol. 2018, 19, 210. [Google Scholar] [CrossRef] [PubMed]
- Gao, C. Precision plant breeding using genome editing technologies. Transgenic Res. 2019, 28, 53–55. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Zeng, B.; Zhao, J.; Yan, S.; Wan, J.; Cao, Z. Genetic Research Progress: Heat Tolerance in Rice. Int. J. Mol. Sci. 2023, 24, 7140. https://doi.org/10.3390/ijms24087140
Liu H, Zeng B, Zhao J, Yan S, Wan J, Cao Z. Genetic Research Progress: Heat Tolerance in Rice. International Journal of Molecular Sciences. 2023; 24(8):7140. https://doi.org/10.3390/ijms24087140
Chicago/Turabian StyleLiu, Huaqing, Bohong Zeng, Jialiang Zhao, Song Yan, Jianlin Wan, and Zhibin Cao. 2023. "Genetic Research Progress: Heat Tolerance in Rice" International Journal of Molecular Sciences 24, no. 8: 7140. https://doi.org/10.3390/ijms24087140
APA StyleLiu, H., Zeng, B., Zhao, J., Yan, S., Wan, J., & Cao, Z. (2023). Genetic Research Progress: Heat Tolerance in Rice. International Journal of Molecular Sciences, 24(8), 7140. https://doi.org/10.3390/ijms24087140




